- 1Private Practitioner, Waukesha, WI, United States
- 2Department of Surgical and Diagnostic Sciences, Marquette University School of Dentistry, Milwaukee, WI, United States
- 3Department of General Dental Sciences, Marquette University School of Dentistry, Milwaukee, WI, United States
Introduction: Attachment loss due to periodontal diseases is associated with functional limitations as well as physical pain and psychological discomfort, which may lead to a reduced quality of life. The purpose of this study is to determine if the oral health status, specifically the periodontal status, influences oral health–related quality of life.
Materials and methods: Survey data were collected in a US dental school clinical setting in a cross-sectional study. Quality of life related to oral health was assessed with the Oral Health Impact Profile-49 (OHIP-49). In addition, DMFT index, periodontal status, and health literacy scores (dental and medical health literacy) were recorded, and the data of n = 97 subjects were statistically analyzed.
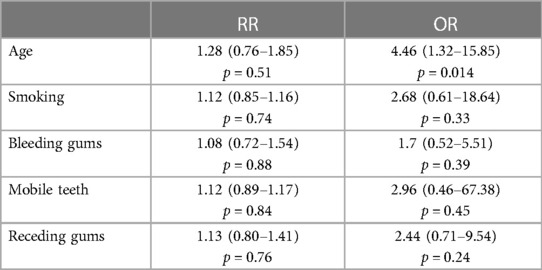
Results: The DMFT index of the study population was 14.98 ± 6.21 (D: 4.72 ± 4.77; M: 3.19 ± 3.46; F: 7.12 ± 4.62). Of the subjects, 44% were identified as periodontitis cases. These periodontitis cases demonstrated significantly higher OHIP-49 scores (66.93 ± 30.72) than subjects without signs of periodontal diseases (NP) (32.40 ± 19.27, p < 0.05). There was also a significant difference between NP patients and patients with gingivitis (66.24 ± 46.12, p < 0.05). It was found that there was a statistically significant difference between Stage 3 (severe) periodontitis and periodontal health (p = 0.003). Pearson correlations were completed, and positive relationships were found with OHIP-49 and DMFT (0.206, p < 0.05), and periodontal risk self-assessment (0.237, p < 0.05). Age [odds ratio (OR) 4.46], smoking (OR 2.67), and the presence of mobile teeth (OR 2.96) are associated with periodontitis.
Conclusions: Periodontal diseases may negatively impact the oral health–related quality of life. Patients suffering from periodontitis also showed more missing teeth, which might influence function. Age and smoking are associated with a higher prevalence of periodontitis. A good general health literacy was no guarantee for having an adequate oral literacy.
1 Introduction
When patients are asked to evaluate their overall quality of life, it is not uncommon for them to provide an answer based primarily on how they feel from a strictly physical and psychological perspective (1). It is also not uncommon for patients to completely overlook their oral health condition, regardless of its condition, and not attribute their oral health status to their overall quality of life (2). Several studies have found that oral health and overall quality of life tend to go together and that poor oral health conditions have a negative effect on the overall quality of life (3, 4). In addition, it was suggested that oral health problems impair the physical functioning, the social standing, and the wellbeing of individuals, which underlines the association of oral health and general health in terms of impacts on the quality of life (2, 5). The oral health–related quality of life can be evaluated using the Oral Health Impact Profile-49 (OHIP-49) questionnaire (6). The OHIP-49 assesses seven domains, including functional limitation, physical pain, psychological discomfort, physical, psychological, and social disability as well as handicap. The higher the score, the lower the oral health–related quality of life (2).
In the United States alone, much of the population deals with gingivitis and nearly half of the population have periodontitis (7). As we know with periodontitis, the loss of attachment and tooth support leads to discomfort of mobile teeth, further progression of disease, and many times tooth loss. Although replacement of missing teeth is no longer a difficulty with many different available treatment options, it is the destruction of the sites where those teeth once resided and their adjacent conditions that makes the replacement difficult (8). Because of this difficulty, patients are typically put in a position that requires them to manage their predisposing oral health condition, namely, periodontal disease. While gingivitis is reversible and limited to an inflammation of the gingiva, periodontitis is a chronic inflammatory process in which attachment and bone loss occur (9, 10). When bone loss is severe enough, it leads to significant loss of support of the teeth (11). For many patients, this disease process does not happen suddenly and is a result of a lack of awareness and a lack of routine care with their dentist. It has been documented that the strongest risk factor for poor oral health–related quality of life, which was obtained from NHANES (National Health and Nutrition Examination Survey) data, was the perceived need to relieve dental pain (12). While many non-compliant patients would present to their dentist when they experience tooth pain, periodontitis often progresses silently and therefore results in severe damage, which is often too late to address appropriately.
In the unfortunate cases where patients have lost teeth due to periodontal disease or caries, they are subjected to adapting to a new reality. They encounter reduced function, less esthetics, and sometimes comorbidities (13). It is not unknown that there have been associations made between periodontal disease and cardiovascular and mental health (14). Although the connection is very complex and requires more research, correlations are present to provide better answers. For example, diabetes has been shown to have a two-way relationship with periodontal disease. In patients with uncontrolled diabetes, the body's inflammatory process leads to faster and more significant destruction of the periodontium in the presence of bacterial plaque. It is also known that due to the same pathophysiological problems (i.e., RAGE-AGE) with diabetes, wound healing is significantly hindered. The ongoing discomfort and lack of proper healing requires patients to have more frequent visits to their dental provider and longer healing time before addressing other areas of concern (15). With diabetes as an example, it is no surprise that there are several factors that influence quality of life. In addition, numerous chronic systemic diseases are associated with periodontitis, and the prevalence of most chronic diseases increases with age (16). It is suggested that upregulated inflammatory mediators, cytokines, and other pathological reactions are the principal mechanisms linking oral infections to a number of systemic diseases, such as pneumonia, osteoarthritis, rheumatic diseases, inflammatory bowel diseases, kidney diseases, liver diseases, metabolic syndrome and diabetes, cancer, and Alzheimer's disease (17).
The aim of this study was to assess the prevalence of periodontitis in an US-based dental school sample, to identify correlations with the quality of life and health literacy scores, and to determine the oral health literacy of the investigated population. The null-hypothesis is that there is no correlation between the presence of periodontitis and quality of life.
2 Methods
2.1 Study design
This cross-sectional study was approved by the Institutional Review Board of Marquette University (HR#: 3148). All participants in this study were newly accepted patients at the Marquette University School of Dentistry (Milwaukee, WI) who were scheduled for comprehensive dental examinations. These patients were admitted to the school through initial screening to ensure they qualified as patients and were approached during their radiology appointment prior to their comprehensive examination. The participant would be brought back for clinical examination to confirm periodontal diagnosis. A total of 115 participants were interviewed between 2017 and 2018. Of the 115 analyzed, 97 participants showed comprehensive data for complete analysis.
2.2 Inclusion and exclusion criteria
Participants were at least 18 years of age and required to be literate in English. To be included, the participants were required to finish the questionnaire and have a comprehensive evaluation. Participants were excluded if they could not read or write in English. Participants who were evaluated but did not return for clinical examination were also excluded. Further exclusion criteria were mental, vision, or hearing impairments.
2.3 Questionnaires
The session involved surveys and questions from Rapid Estimate of Adult Literacy in Dentistry-30 (REALD-30), Short Assessment of Healthy Literacy (SAHL), Periodontal Self-Risk Assessment, OHIP-49, Modified Dental Anxiety Scale, and general demographic information. Three calibrated periodontists (KA, JG, and AG) performed the interviews in a standardized manner.
2.3.1 OHIP-49
The OHIP-49 is a questionnaire that was developed to assess oral health–related quality of life from a patient perspective (6). The questionnaire is comprised of 49 questions with answers on a scale of 0–4. The answers are then tallied for a grand total (maximum of 196). The greater the number, the lower assessed oral health–related quality of life.
2.3.2 Health literacy
2.3.2.1 REALD-30
Oral health literacy was tested using a validated dental word recognition instrument (18). The interviewer provided the participant a sheet of the 30 words. Participants were asked to read each one out loud, and the investigator marked if they were able to correctly read the word. Participants were asked not to guess and say “pass” in the event they needed to guess or did not know the pronunciation of the word. To ensure no non-verbal cues were given, the investigator stood behind the patient for the duration of the questionnaire.
2.3.2.2 SAHL
The SAHL test illustrated medical literacy via word association (19). Participants were given a list of 18 words. Participants were asked to read the words out loud and waited for the investigator to say two words. One of the words was directly related, while the other word was relevant but not as closely associated. The participant was advised to select the word that was most directly related. In the event the participant did not know which option to choose, they were asked to simply state that they did not know. The threshold score was 14, and anything below this score was considered low risk.
2.3.3 Periodontal risk self-assessment
The periodontal risk self-assessment (PRSA) is a 13-item questionnaire, including age, gender, family history of periodontal disease, oral hygiene habits, clinical symptoms of periodontal diseases, smoking habits, and dental history. The answer options are weighted, and scores from 1 to 3 are given. High total scores correlate with the presence of periodontitis (20).
2.3.4 Oral health
All participants were diagnosed based on comprehensive exam information including full mouth radiographs as well as periodontal charting. This exercise was completed by two investigators (AG and KA) independently to ensure consistency. Periodontal status was diagnosed based on the World Workshop of Periodontal Classification (21–23) as periodontal health, gingivitis, and periodontitis. Periodontal health was characterized by <10% of bleeding on probing (BoP), the absence of bone loss, and normal gingival sulcus depths (24). A gingivitis case was diagnosed when BoP scores were ≥10% and the absence of bone loss (25). A periodontitis case was defined as interdental clinical attachment loss (CAL) at ≥2 mm non-adjacent teeth, or buccal or oral CAL ≥3 mm with pocketing >3 mm was detectable at ≥2 teeth (23). The decayed, missing, and filled teeth were recorded as DMFT (26).
2.4 Statistical analysis
All data were transferred to a spreadsheet for data organization and analysis. All the variables were described using appropriate statistics. For example, categorical variables were described as frequency and percentage, whereas all continuous variables were described as means and standard deviations. Two-sample t-test was used for comparison of other variables (OHIP, DMFT, PRSA, Age, SAHL). The two-sample t-test was used for comparison of the OHIP score for two groups (disease and no disease). The one-way ANOVA was used for comparison of the OHIP score of different periodontal stages. Relative risk (RR) and odds ratio (OR) were calculated between healthy and diseased individuals across different covariates. Chi-square test was used to compare SAHL and REALD-30. For all statistical tests, the alpha was set at 0.05 and all statistical analyses were done using statistical software (R version 4.2.2). Using GPower 3.1, a sample size of 97 with effect size of 0.40, the computed achieved power for using ANOVA fixed effect with alpha being 0.05 was 89% (27).
3 Results
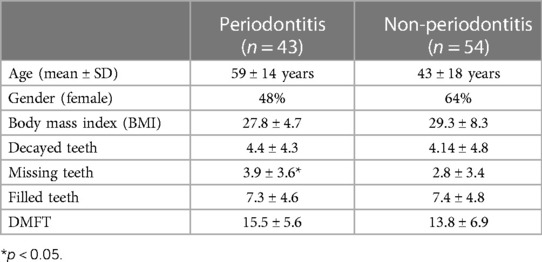
The demographics and oral health status of the participants are presented in Table 1. Of the included n = 97 subjects, n = 22 were diagnosed with periodontal health, n = 32 with gingivitis, n = 21 with stage 1 or 2 periodontitis, and n = 22 with stage 3 or 4 periodontitis. The DMFT index of the study population was 14.98 ± 6.21 (D: 4.72 ± 4.77; M: 3.19 ± 3.46; F: 7.12 ± 4.62). The average age of the investigated population was 49 years, with a range from 18 to 84; 56.7% identified as female. When asking about participant race, 62.4% of the participants indicated they were “White” with 19.4% indicating they were “Black.”
3.1 Periodontal disease and quality of life
Comparing the OHIP-49 scores of patients with periodontal disease and periodontal health, scores for patients with disease was 63.6, which is significantly higher, compared to the OHIP scores for the patient without disease (35.6; p < 0.001).
Periodontally healthy patients had an OHIP-49 score of 34.2 ± 20.5, which was significantly lower than for patients with gingivitis with 66.7 ± 47.4 (p = 0.015) and patients with stage 3 and 4 periodontitis with 72.9 ± 31.9 (p = 0.006). Patients with stage 1 and stage 2 periodontitis had an OHIP-49 score of 60.7 ± 29.0 that was tentatively higher than for patients with periodontal health (p = 0.12; Figure 1).
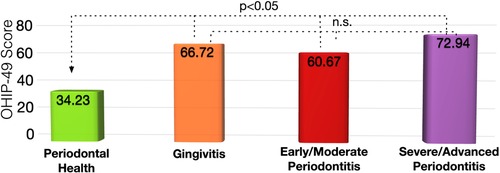
Figure 1. Oral health impact profile-49 scores for patients with different periodontal disease status. The higher the OHIP-49 score, the lower the oral-health related quality of life.
3.2 Health literacy
The subjects of this cohort had a significant discrepancy between health (SAHL) and oral health (REALD-30) literacy (p < 0.001). While 92% showed adequate health literacy, only 57% of the participants were found to be having adequate oral health literacy (Figure 2). Patients with inadequate oral health literacy had a higher risk for severe periodontitis (OR: 1.6).
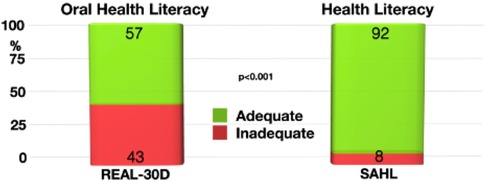
Figure 2. Discrepancy between oral health literacy (REAL-30D) and health literacy (SAHL). Significantly more study participants had adequate health literacy than being literate in oral health aspects.
3.3 Periodontal self-risk assessment
Patients with periodontal health reported lower PRSA scores (16.2 ± 1.6) than those suffering from periodontal disease (17.6 ± 2.4; p < 0.05). However, this was driven by patients with stage 3 and stage 4 periodontitis (19.1 ± 2.3), who had a significantly higher PRSA score than patients with periodontal health (p < 0.001). The scores for patients with gingivitis (17.1 ± 2.4) or periodontitis stages 1 and 2 (17.4 ± 2.1) were not significant from those of the participants with periodontal health.
Patient reported that factors such as smoking (r = 0.23, p < 0.05), having loose teeth (r = 0.28, p < 0.01), and gingival recession (r = 0.20, p < 0.05) were significantly correlated with the diagnosis of periodontitis.
Age and smoking were determined as being risk factors for periodontal diseases. While the relative risk for periodontitis was for age 1.28, it was 1.12 for smoking. Relative risks and odds ratios are presented in Table 2.
4 Discussion
The patient pool in a dental school typically presents with a specific background in terms of socioeconomic status, education level, and overall health condition. Because of these characteristics, patients may not experience the most ideal oral health status nor the most ideal or controlled overall health quality (28). The study was completed to determine if there was an association between periodontal health and quality of life patients experienced and attempted to identify factors that predispose or highlight possible risk associations. Our findings indicate that, in fact, there are significant associations with periodontal diseases and reduced overall quality of life. Patients with gingivitis and periodontitis had worse overall quality of life scores than those with periodontal health. This is consistent with the existing literature (29).
Especially patients with gingivitis and severe/advanced periodontitis reported a lower oral health–related quality of life. This may indicate that a patient with gingivitis is more aware of the periodontal changes occurring, whereas those in stages 1 and 2 typically are more silent to a patient who has progressed past gingivitis. However, when a patient reaches stage 3, the awareness increases due to possible discomfort of the gingival tissues and teeth as well as mobility of teeth (30). Patients with an advanced stage of periodontitis are also more likely to have general health issues (e.g., diabetes, cardiovascular disease) that may be poorly controlled and contributing to the patient's poor oral health and/or poor overall quality of life (31). The periodontitis risk self-assessment scores were higher in patients with severe/advanced periodontitis, which suggests that patients are typically aware of their condition and may be able to perceive what may be affecting their overall condition as well (32). Combined with the higher OHIP scores in this patient group, this may also indicate that it is at this stage of periodontitis a patient might be aware of their oral health condition and thus is more adept to being self-aware of their overall quality of life. Nisanci Yilmaz et al. reported that the highest OHIP scores and with that lower quality of life were found in patients with stage 4 grade C periodontitis (33). They also found that OHIP scores were significantly related to symptoms of periodontal disease such as bleeding gums, bad odor, and loose or drifting teeth. In a 65-year-old Norwegian population, a researcher found that reduced oral health–related quality came with increased severity of periodontitis (34), which confirmed earlier findings that the severity and progression rate of periodontitis are associated with poor quality of life (35). The good news for those patients is that when they undergo periodontal treatment and participate in a well-structured periodontal maintenance program, the quality of life can be improved and retained (36). This is especially important since the loss of natural teeth due to periodontitis impacts the chewing function, which is associated with diminished nutritional intake (37).
The patients included in this study showed better general health literacy and were less literate in oral health matters. Wehmeyer et al. reported that despite a high level of education of the participants in their cross-sectional study, lower oral health literacy was associated with more severe periodontitis among new and referred patients to their periodontics clinic (38). The present findings suggest that patients with an inadequate REALD-30 score had 1.6 times more severe periodontitis than patients with adequate oral health literacy. Impaired oral health impacts general health and negatively impacts quality of life, and low oral health literacy is associated with reduced quality of life (39). Increasing oral health literacy in educating our patients is critical in addressing poor oral health to prevent oral diseases (40). Nouri and Rudd recommend to use plain language and teach-back by providers as well as the incorporation of oral and aural literacy into community programs and healthcare provider (e.g., dentist, dental assistance, dental hygienists) education and training (41). In the medical field, it is known that the patient awareness of general health concerns is critical in self-care and helping patients seek care when they suspect ailments (42). The same could be said for the dental world, and it may be critical for the dental community to be more of an advocate for the patient to help them self-screen even in cases where symptoms are not as apparent. A possible manner to enhance this is to include our colleagues in medicine to also advocate the oral–systemic connection and educate the patients accordingly. A recent study showed for instance that oral hygiene measures such as brushing teeth are related to the outcome of cardiovascular disease (43). It was also suggested that tooth loss due to periodontal disease or caries caused by oral bacteria impairs the chewing function and health (44), and the disruption of intestinal bacteria can also impair health (45).
Nevertheless, some limitations of this study must be acknowledged. Not all subjects who consented into the study and completed the questionnaires returned for the comprehensive oral exam and were not included in the full analysis. Financial stress, dental anxiety, occupational stress, and perceptions of needs among others might have presented as barriers for patients accessing dental care (46). The selection of questionnaires might also represent a limitation. There are numerous versions of the OHIP questionnaire. John et al. found that the 5-, 14-, 19-, and 49-item versions correlated highly, indicating that these versions measure oral health–related quality of life equally well, with the best being the OHIP-49 (47). However, they also suggest that the OHIP5 is a practical tool for general dentists to assess the oral health–related quality of life, which was also confirmed by others (48). The used oral health literacy tool measures word recognition (18). But there are more ways to assess health literacy (49), including test reading comprehension (50), testing the understanding of medical information (51), or testing numeracy and locate-the-information skills (52). However, several studies found a correlation between the REALD-30 scores and the status of periodontal health (38, 49, 53).The PRSA questionnaire was able to distinguish between periodontal health and periodontitis but failed to discriminate between gingivitis and periodontitis. Other self-reporting tools that are more sophisticated and include questions about systemic health, dietary intake, or psychological stress were shown to be able to assess the individual risk, the need for periodontal treatment, and can differentiate between gingivitis and periodontitis (54). This tool and others such as the periodontal screening score (32) can be helpful screening tools on a population level. In terms of risk or prognosis determination, tooth-level prognostic systems provide better information. Saleh et al. reported recently that the periodontal risk score (PRS), which includes parameters such as age, smoking, diabetes, tooth type, mobility, probing depth, and furcation involvement, was able to predict long-term tooth loss (55).
5 Conclusion
Periodontal diseases may negatively impact the oral health–related quality of life. Patients suffering from periodontitis also showed more missing, filled, and decayed teeth, which may have an effect on function and comfort. Age and smoking are associated with a higher prevalence of periodontitis. Good general health literacy was no guarantee for having an adequate oral literacy.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Office of Research Compliance, Marquette University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
KA: Data curation, Investigation, Writing – original draft. JG: Data curation, Investigation, Writing – review & editing. SH: Formal Analysis, Investigation, Methodology, Software, Validation, Writing – review & editing. AG: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article.
This study was funded through an internal Faculty Research Grant of Marquette University School of Dentistry granted to AG.
Acknowledgments
The authors thank the volunteers that participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Borges TF, Regalo SC, Taba M, Siéssere S, Mestriner W, Semprini M. Changes in masticatory performance and quality of life in individuals with chronic periodontitis. J Periodontol. (2013) 84:325–31. doi: 10.1902/jop.2012.120069
2. Irani FC, Wassall RR, Preshaw PM. Impact of periodontal status on oral health-related quality of life in patients with and without type 2 diabetes. J Dent. (2015) 43:506–11. doi: 10.1016/j.jdent.2015.03.001
3. Ng SK, Leung WK. Oral health-related quality of life and periodontal status. Community Dent Oral Epidemiol. (2006) 34:114–22. doi: 10.1111/j.1600-0528.2006.00267.x
4. Sanders AE, Slade GD, Lim S, Reisine ST. Impact of oral disease on quality of life in the US and Australian populations. Community Dent Oral Epidemiol. (2009) 37:171–81. doi: 10.1111/j.1600-0528.2008.00457.x
5. Fontanive V, Abegg C, Tsakos G, Oliveira M. The association between clinical oral health and general quality of life: a population-based study of individuals aged 50-74 in southern Brazil. Community Dent Oral Epidemiol. (2013) 41:154–62. doi: 10.1111/j.1600-0528.2012.00742.x
6. Slade GD, Spencer AJ. Development and evaluation of the oral health impact profile. Community Dent Health. (1994) 11:3–11.8193981
7. Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. (2012) 91:914–20. doi: 10.1177/0022034512457373
8. Acharya A, Hao J, Mattheos N, Chau A, Shirke P, Lang NP. Residual ridge dimensions at edentulous maxillary first molar sites and periodontal bone loss among two ethnic cohorts seeking tooth replacement. Clin Oral Implants Res. (2014) 25:1386–94. doi: 10.1111/clr.12292
9. Eke PI, Page RC, Wei L, Thornton-Evans G, Genco RJ. Update of the case definitions for population-based surveillance of periodontitis. J Periodontol. (2012) 83:1449–54. doi: 10.1902/jop.2012.110664
10. Eke PI, Dye BA, Wei L, Slade GD, Thornton-Evans GO, Borgnakke WS, et al. Update on prevalence of periodontitis in adults in the United States: NHANES 2009 to 2012. J Periodontol. (2015) 86:611–22. doi: 10.1902/jop.2015.140520
11. Dederichs M, Joedecke P, Weber CT, Guentsch A. Functional load capacity of teeth with reduced periodontal support: a finite element analysis. Bioengineering (Basel). (2023) 10.38002454
12. Seirawan H, Sundaresan S, Mulligan R. Oral health-related quality of life and perceived dental needs in the United States. J Public Health Dent. (2011) 71:194–201.21972459
13. Dye B, Thornton-Evans G, Li X, Iafolla T. Dental caries and tooth loss in adults in the United States, 2011–2012. NCHS Data Brief. (2015) 197:197.
14. Allen NB, Badon S, Greenlund KJ, Huffman M, Hong Y, Lloyd-Jones DM. The association between cardiovascular health and health-related quality of life and health status measures among U.S. adults: a cross-sectional study of the National Health and Nutrition Examination Surveys, 2001–2010. Health Qual Life Outcomes. (2015) 13:152. doi: 10.1186/s12955-015-0352-z
15. Mealey BL, Oates TW; American Academy of Periodontology. Diabetes mellitus and periodontal diseases. J Periodontol. (2006) 77:1289–303. doi: 10.1902/jop.2006.050459
16. Persson GR. Dental geriatrics and periodontitis. Periodontol 2000. (2017) 74:102–15. doi: 10.1111/prd.12192
17. Meurman JH, Bascones-Martinez A. Oral infections and systemic health—more than just links to cardiovascular diseases. Oral Health Prev Dent. (2021) 19:441–8. doi: 10.3290/j.ohpd.b1993965
18. Lee JY, Rozier RG, Lee SY, Bender D, Ruiz RE. Development of a word recognition instrument to test health literacy in dentistry: the REALD-30—a brief communication. J Public Health Dent. (2007) 67:94–8. doi: 10.1111/j.1752-7325.2007.00021.x
19. Lee SY, Stucky BD, Lee JY, Rozier RG, Bender DE. Short assessment of health literacy-Spanish and English: a comparable test of health literacy for Spanish and English speakers. Health Serv Res. (2010) 45:1105–20. doi: 10.1111/j.1475-6773.2010.01119.x
20. Abbood HM, Hinz J, Cherukara G, Macfarlane TV. Validity of self-reported periodontal disease: a systematic review and meta-analysis. J Periodontol. (2016) 87:1474–83. doi: 10.1902/jop.2016.160196
21. Caton JG, Armitage G, Berglundh T, Chapple ILC, Jepsen S, Kornman KS, et al. A new classification scheme for periodontal and peri-implant diseases and conditions—introduction and key changes from the 1999 classification. J Clin Periodontol. (2018) 45(Suppl 20):S1–8. doi: 10.1111/jcpe.12935
22. Papapanou PN, Sanz M, Buduneli N, Dietrich T, Feres M, Fine DH, et al. Periodontitis: consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J Clin Periodontol. (2018) 45(Suppl 20):S162–70. doi: 10.1111/jcpe.12946
23. Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: framework and proposal of a new classification and case definition. J Periodontol. (2018) 89(Suppl 1):S159–72. doi: 10.1002/JPER.18-0006
24. Lang NP, Bartold PM. Periodontal health. J Periodontol. (2018) 89(Suppl 1):S9–16. doi: 10.1002/JPER.16-0517
25. Trombelli L, Farina R, Silva CO, Tatakis DN. Plaque-induced gingivitis: case definition and diagnostic considerations. J Periodontol. (2018) 89(Suppl 1):S46–73. doi: 10.1002/JPER.17-0576
27. Faul F, Erdfelder E, Buchner A, Lang A-G. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. (2009) 41:1149–60. doi: 10.3758/BRM.41.4.1149
28. Maida CA, Marcus M, Spolsky VW, Wang Y, Liu H. Socio-behavioral predictors of self-reported oral health-related quality of life. Qual Life Res. (2013) 22:559–66. doi: 10.1007/s11136-012-0173-z
29. Karaaslan F, Dikilitaş A. The association between stage-grade of periodontitis and sleep quality and oral health-related quality of life. J Periodontol. (2019) 90:1133–41. doi: 10.1002/JPER.19-0034
30. Buset SL, Walter C, Friedmann A, Weiger R, Borgnakke WS, Zitzmann NU. Are periodontal diseases really silent? A systematic review of their effect on quality of life. J Clin Periodontol. (2016) 43:333–44. doi: 10.1111/jcpe.12517
31. Naito M, Yuasa H, Nomura Y, Nakayama T, Hamajima N, Hanada N. Oral health status and health-related quality of life: a systematic review. J Oral Sci. (2006) 48:1–7. doi: 10.2334/josnusd.48.1
32. Carra MC, Gueguen A, Thomas F, Pannier B, Caligiuri G, Steg PG, et al. Self-report assessment of severe periodontitis: periodontal screening score development. J Clin Periodontol. (2018) 45:818–31. doi: 10.1111/jcpe.12899
33. Nisanci Yilmaz MN, Bulut S, Bakirarar B. Impact of stage-grade of periodontitis and self-reported symptoms on oral health-related quality of life. Int J Dent Hyg. (2022) 20:291–300. doi: 10.1111/idh.12551
34. Sodal ATT, Skudutyte-Rysstad R, Diep MT, Koldsland OC, Hove LH. Periodontitis in a 65-year-old population: risk indicators and impact on oral health-related quality of life. BMC Oral Health. (2022) 22:640. doi: 10.1186/s12903-022-02662-9
35. Goergen J, Albandar JM, Oppermann RV, Rosing CK, Susin C, Haas AN. Periodontitis stage and grade are associated with poor oral-health-related quality of life: findings from the Porto Alegre cohort study. J Clin Periodontol. (2021) 48:1333–43. doi: 10.1111/jcpe.13527
36. El Sayed N, Baeumer A, El Sayed S, Wieland L, Weber D, Eickholz P, et al. Twenty years later: oral health-related quality of life and standard of treatment in patients with chronic periodontitis. J Periodontol. (2019) 90:323–30. doi: 10.1002/JPER.18-0417
37. Yan GLK, Tan MN, Wong ML, Tay CM, Allen PF. Functional dentition, chronic periodontal disease and frailty in older adults-a narrative review. Int J Environ Res Public Health. (2022) 20:502. doi: 10.3390/ijerph20010502
38. Wehmeyer MM, Corwin CL, Guthmiller JM, Lee JY. The impact of oral health literacy on periodontal health status. J Public Health Dent. (2014) 74:80–7. doi: 10.1111/j.1752-7325.2012.00375.x
39. Kwon SR, Lee S, Oyoyo U, Wiafe S, De Guia S, Pedersen C, et al. Oral health knowledge and oral health related quality of life of older adults. Clin Exp Dent Res. (2021) 7:211–8. doi: 10.1002/cre2.350
40. Bress LE. Improving oral health literacy—the new standard in dental hygiene practice. J Dent Hyg. (2013) 87:322–9.24357560
41. Nouri SS, Rudd RE. Health literacy in the “oral exchange”: an important element of patient-provider communication. Patient Educ Couns. (2015) 98:565–71. doi: 10.1016/j.pec.2014.12.002
42. Eltas A, Uslu MO, Eltas SD. Association of oral health-related quality of life with periodontal status and treatment needs. Oral Health Prev Dent. (2016) 14:339–47. doi: 10.3290/j.ohpd.a35613
43. Isomura ET, Suna S, Kurakami H, Hikoso S, Uchihashi T, Yokota Y, et al. Not brushing teeth at night may increase the risk of cardiovascular disease. Sci Rep. (2023) 13:10467. doi: 10.1038/s41598-023-37738-1
44. Tanaka T, Takahashi K, Hirano H, Kikutani T, Watanabe Y, Ohara Y, et al. Oral frailty as a risk factor for physical frailty and mortality in community-dwelling elderly. J Gerontol A Biol Sci Med Sci. (2018) 73:1661–7. doi: 10.1093/gerona/glx225
45. Lewis CV, Taylor WR. Intestinal barrier dysfunction as a therapeutic target for cardiovascular disease. Am J Physiol Heart Circ Physiol. (2020) 319:H1227–33. doi: 10.1152/ajpheart.00612.2020
46. Freeman R. Barriers to accessing dental care: dental health professional factors. Br Dent J. (1999) 187:197–200. doi: 10.1038/sj.bdj.4800238a
47. John MT, Omara M, Su N, List T, Sekulic S, Haggman-Henrikson B, et al. Recommendations for use and scoring of oral health impact profile versions. J Evid Based Dent Pract. (2022) 22:101619. doi: 10.1016/j.jebdp.2021.101619
48. Naik A, John MT, Kohli N, Self K, Flynn P. Validation of the English-language version of 5-item oral health impact profile. J Prosthodont Res. (2016) 60:85–91. doi: 10.1016/j.jpor.2015.12.003
49. Holtzman JS, Atchison KA, Macek MD, Markovic D. Oral health literacy and measures of periodontal disease. J Periodontol. (2017) 88:78–88. doi: 10.1902/jop.2016.160203
50. Baker DW, Williams MV, Parker RM, Gazmararian JA, Nurss J. Development of a brief test to measure functional health literacy. Patient Educ Couns. (1999) 38:33–42. doi: 10.1016/S0738-3991(98)00116-5
51. Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Fam Med. (2004) 36:588–94.15343421
52. Weiss BD, Mays MZ, Martz W, Castro KM, DeWalt DA, Pignone MP, et al. Quick assessment of literacy in primary care: the newest vital sign. Ann Fam Med. (2005) 3:514–22. doi: 10.1370/afm.405
53. Haridas R, Supreetha S, Ajagannanavar SL, Tikare S, Maliyil MJ, Kalappa AA. Oral health literacy and oral health status among adults attending dental college hospital in India. J Int Oral Health. (2014) 6:61–6.25628486
54. Renatus A, Kottmann T, Schwarzenberger F, Jentsch H. Evaluation of a new self-reported tool for periodontitis screening. J Clin Diagn Res. (2016) 10:ZC107–12. doi: 10.7860/JCDR/2016/19518.8063
Keywords: oral health, quality of life, health literacy, periodontitis, periodontal health
Citation: Al-Bitar KM, Garcia JM, Han S and Guentsch A (2024) Association between periodontal health status and quality of life: a cross-sectional study. Front. Oral. Health 5:1346814. doi: 10.3389/froh.2024.1346814
Received: 30 November 2023; Accepted: 3 January 2024;
Published: 25 January 2024.
Edited by:
Georgios N. Belibasakis, Karolinska Institutet (KI), SwedenReviewed by:
Sarhang Sarwat Gul, University of Sulaymaniyah, IraqFlorence Carrouel, Université Claude Bernard Lyon 1, France
© 2024 Al-Bitar, Garcia, Han and Guentsch. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Arndt Guentsch YXJuZHQuZ3VlbnRzY2hAbWFycXVldHRlLmVkdQ==
 Kinan M. Al-Bitar1,2
Kinan M. Al-Bitar1,2 Arndt Guentsch
Arndt Guentsch