- 1Graduate School of Engineering, Nagaoka University of Technology, Nagaoka, Niigata, Japan
- 2Department of Industrial Management, Faculty of Applied Sciences, Wayamba University of Sri Lanka, Kuliyapitiya, Sri Lanka
- 3R&D, Sunstar Inc., Takatsuki, Osaka, Japan
Background: Mouthwashes play a pivotal role in oral care, and their efficacy has been explored extensively across various dimensions. As a contribution to the development of novel oral care products, this study aims to investigate the psychophysiological effects of aromatic mouthwashes during the resilience period from a short-term cognitive stressor utilizing biological signals and subjective evaluations.
Methods: A within-participant experimental design with 22 healthy females was conducted with four mouthwashes; peppermint (Mint), peppermint + bergamot (MB), peppermint + sweet orange (MO), and peppermint + lavender (ML), and water as the control (Ctl), after a 20-min calculation task. Subjective evaluations and physiological responses including skin conductance level and electrocardiogram were recorded throughout the experiment.
Results: Citrus mouthwashes (MO and MB) showed a greater decrease in heart rate and a significant increase in the high-frequency component of heart rate variability. The participants indicated a significant effect in terms of “flavor preference” and “refreshing sensation” for mouthwash use compared to the Ctl.
Conclusion: The results suggest that rinsing with citrus-flavored mouthwashes has a positive impact in alleviating the physiological stress response (in terms of cardiac activity). These findings may have implications for the development of innovative, novel oral care products that promote stress reduction and improve oral health.
1 Introduction
Bio-signals, measured via human-machine interaction are indispensable for detecting acute stress and are broadly categorized into physical and physiological signals (1). Physical bio-signals encompass measurements of bodily deformations arising from muscle activities, including pupil size, eye movements, blinks, and various postures and movements. Physiological bio-signals are more directly connected to essential bodily functions, involving cardiac activities [electrocardiogram (ECG), blood volume pulse], brain operations (electroencephalogram), exocrine activities [evaluated through electrodermal activity (EDA)], and muscle excitability gauged via electromyography (2). EDA also referred to as galvanic skin response (GSR) or skin conductance (SC), manifests because of constant changes in the skin's electrical property fluctuations caused by sweating. This dynamic electrical change, measurable through placing electrodes on the skin, offers insights into emotional, psychological, or physiological arousal (3). Heart rate (HR) stands as the extensively utilized and direct metric for assessing acute stress, and heart rate variability (HRV), indicating fluctuations in time intervals between successive heartbeats (4–6), serves as a non-invasive tool for assessing psychological states like fatigue, stress, and anxiety (7). Numerous studies across psychology and neuroscience have affirmed that HRV measurements reflect the continuous interaction between the sympathetic and parasympathetic branches of the autonomic nervous system (ANS) (8, 9).
Research has extensively employed various bio-signals to evaluate acute stress response. Notably, studies utilizing ECG and SC have shown promising results (10–12). For instance, a study found that listening to music before a standardized stressor affected the ANS (in terms of a faster recovery), with an increase in HR during the psychosocial stress test followed by a decrease in HR in the resilience period (13). Another study indicated that olfactory stimulation by Hinoki cypress leaf oil significantly increased the high frequency (HF) component of HRV, inducing physiological relaxation (14). Additionally, a study on citrus mint-flavored mouthwash showed a significant decrease in skin conductance level (SCL) during the resilience period, after a calculation task (15). These findings underscore the potential of ECG and SCL in assessing acute stress responses.
Mouthwashes, integral to oral care, are liquid products designed to enhance oral hygiene. These solutions are used by individuals to supplement their regular dental care routines, targeting various oral concerns such as bad breath, bacteria control, and gum health (16, 17). They are formulated with active ingredients, including antimicrobial agents and fluoride, alongside flavoring agents like menthol, mint, citrus, or herbal extracts, providing a refreshing sensation (18). In this context, studies evaluated the efficacy of various mouthwash formulations, aiming to establish their effects on oral health and well-being. Research demonstrated that mouthwash rinsing holds significant potential in alleviating xerostomia, ameliorating dry mouth symptoms, and enhancing salivary production (19–21), preventing dental caries (22, 23), addressing dentinal sensitivity (24), and inhibiting the buildup of plaque and the development of gingivitis (25–27).
Conversely, concerning aromatherapy, many studies have investigated the psychophysiological effects of different aromas. Lavender alleviated job-related stress in nurses (28), bergamot essential oil exerted psychological and physiological effects in a relatively short time (29), and peppermint oil was found to be beneficial in relieving stress (30) and enhancing sleep quality (31). Our previous study suggested that inhalation of orange essential oil inhibited the physiological stress response (32). Given that these aroma studies have demonstrated diverse psychophysiological efficacies of aromas on ANS, it is reasonable to anticipate a certain level of psychophysiological impact from the utilization of aromatic mouthwashes.
Aromatic mouthwashes, a subset integrating sensory experiences with oral hygiene, not only offer functional benefits but also sensory pleasures. However, research about the effects of aromatic mouthwashes remains relatively limited. One study examined an aromatic oral care solution incorporating peppermint, tea tree, and lemon essential oils, and found its potential to enhance the oral health of elderly dementia patients within long-term care settings (33). Another study reported that an aroma gargle comprising peppermint, lemon, tea tree, and ylang-ylang was effective in relieving stress, decreasing xerostomia, and reducing the objective halitosis of the nurses (34).
Despite extensive research on mouthwash efficacy, few studies have evaluated their impact through bio-signals. One study suggested that mouthwashes, carbohydrates, or a placebo, improve running endurance compared to the control condition in a heat-stress environment, with no significant difference in HR, rectal temperature, skin temperature, or skin blood flow between the three trials (35). Jeffries et al. (36) showed that the L-menthol mouth rinse improved the performance of athletes during exercise compared to the placebo, whilst there were no significant differences found in bio-signals such as HR and skin temperature (36). Another study that investigated the physiological effects of a mint-flavored mouthwash at bedtime to enhance subsequent sleep quality using a wristwatch-type HR monitoring device found that the use of mouthwashes before sleep promotes a positive mood and potentially improves physiological sleep (37). Moreover, studies that focused on the physiological effects of citrus mint flavored mouthwash (15), and mouthwashes with varying alcohol concentrations (38), revealed a significant decrease in SCL during the resilience phase after a cognitive stressor. Another study utilizing mouthwashes with different types of binders (thickening agents) suggested that with higher thickness levels, an increased HF component of HRV can be exhibited with a potential recovery from the physiological stress response (39, 40).
So far, previous studies have evaluated the efficacy of different mouthwash formulations for various purposes, and some studies have explored the usage of mouthwashes utilizing bio-signals. However, the exploration of aroma-infused mouthwashes in the resilience period after a short-term stressor using biological signals has not been extensively pursued. Addressing this gap, the current study strives to contribute to the development of innovative oral care products by examining the psychophysiological effects of aromatic mouthwashes during the resilience period following a short-term cognitive stressor. The research hypothesis posits that the administration of aromatic mouthwashes will alleviate physiological stress responses within the ANS. Specifically, during the resilience phase, it is anticipated to observe a greater decrease in HR and SCL compared to the mouthwashes without aroma, indicative of a sympathetic nervous system (SNS) response. Simultaneously, it is hypothesized that there will be a greater increase in the HF component of HRV, reflecting a parasympathetic nervous system (PSNS) response.
Following the working hypothesis, as a part of new oral care product development, this research aims to investigate the psychophysiological potential of aromatic mouthwashes incorporating peppermint, lavender, bergamot, and orange aromas in alleviating acute stress response, utilizing an array of biological signals including HR, HRV, and SCL. The research methodology of the study employed a within-participant experimental design involving 22 females in a laboratory setting. The experimental conditions included four different types of mouthwash, and water served as the control (Ctl) condition. Biological signals, including HR, SCL, and HRV, were assessed to evaluate the impact of aromatic mouthwashes on the ANS during the resilience period.
2 Materials and methods
2.1 Participants
Twenty-two healthy female university students with a mean [±standard deviation (SD)] age of 22.32 (±0.92) years participated in the study. All participants had normal gustation, and based on their self-reports, none of them had a history of gustatory dysfunction. The study excluded the participants who were habitual smokers. The participants were provided with compensation in the form of monetary incentives for their involvement in the study, aiming to acknowledge and reciprocate their contribution to the research endeavor. They were instructed to abstain from consuming food, beverages (except water), vigorous physical activities, or oral care routines for 1 h before each experimental trial. Detailed prior information regarding the exact composition of the mouthwashes was not provided to the participants.
The research adhered to the ethical principles outlined in the Helsinki Declaration. Informed consent was obtained from all participants before their involvement. The experimental design was approved by the ethics review committee of the Nagaoka University of Technology.
2.2 Experimental protocol
The study employed a within-participant experimental design, including an initial 10-min period of rest (referred to as R1), followed by a 20-min calculation task (T), a 20-s mouthwash rinsing period (MW), and a subsequent resilience period for 20 min (R2) as shown in Figure 1.
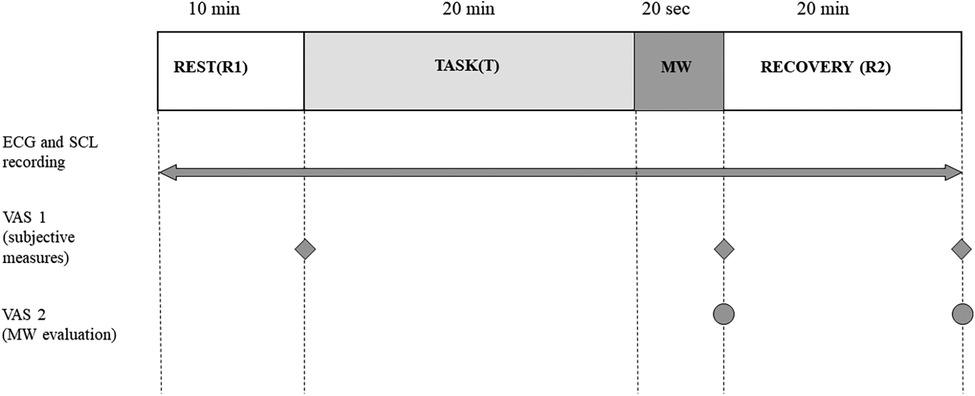
Figure 1. Schematic diagram of the experimental design. MW, rinsing with the mouthwash; ECG, electrocardiogram; SCL, skin conductance level; VAS, visual analog scale.
The experiment incorporated four distinct aromatic mouthwashes: peppermint (Mint), peppermint + bergamot (MB), peppermint + sweet orange (MO), and peppermint + lavender (ML), and mineral water with pH value 7.6 was used as the Ctl condition. Each participant was instructed to rinse their mouth for 20 s using 10 ml of the designated mouthwash or water. After rinsing, participants were directed to spit out the mouthwash into a paper cup. The participants received the mouthwashes in a counterbalanced sequence over five separate days, with all sessions conducted at approximately the same time of day.
All experimental procedures were conducted within an air-controlled laboratory environment, maintaining an average temperature of 23.68°C (±2.12°C) and a humidity level of 51.30% (±6.13%).
2.2.1 Cognitive stressor
The calculation task, referred to as the Kraepelin test was used as the cognitive stressor in the study. It involved performing continuous mental calculations that consisted of adding single-digit numbers displayed on a computer screen. Participants were instructed to complete this task with both speed and accuracy. The Kraepelin test is a standardized cognitive stressor commonly utilized to evaluate participants' concentration and mental workload interacting with the computer (15, 32, 39, 40).
2.3 Mouthwashes formula
The experiment utilized four types of non-alcoholic mouthwashes with a similar general composition. These mouthwashes were formulated with components such as a surfactant (to enhance dispersion within the oral cavity), glycerine, and other typical ingredients commonly found in mouthwash products.
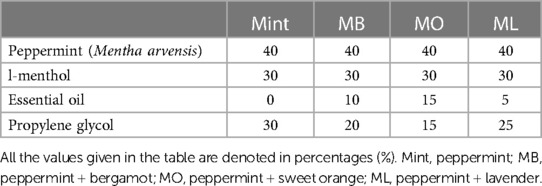
Each of these four mouthwash variants was prepared by infusing an aroma (Mint, MB, MO, and ML) into the basal mouthwash at a concentration of 0.1%. The specific composition of the aroma blend is detailed in Table 1. Peppermint essential oil base was utilized in all mouthwash formulations as it is an essential prerequisite in oral care product development attributing to its beneficial effects on oral health and overall sensory experience. The aromatic compositions, including specific aromas, their corresponding concentrations, and textures, were developed by expert aroma practitioners exclusively for research purposes.
2.4 Measurements
2.4.1 Physiological measurements
Throughout the experiment (R1-T-MW-R2; as illustrated in Figure 1), ECG and SCL measurements were recorded using a bio-amplifier (MP 150, BIOPAC Systems Inc., USA) operating at a sampling rate of 500 Hz and a resolution of 16 bits. For ECG measurements, electrodes were placed beneath the right clavicle and on the lower left abdomen, following the “Lead II” configuration (ECG sensor: TSD155C, BIOPAC Systems Inc., USA). For SCL measurements, electrodes were attached to the palmar side of the middle phalanges of the second and fourth fingers of the participant's non-dominant hand (sensor: TSD203, BIOPAC Systems Inc., USA). The acquired signals underwent filtration processes, including a 35 Hz low-pass pulse, a 50 Hz notch filter, and a 0.05 Hz low-cut filter for ECG. In the case of SCL, a low-pass filter set at 10 Hz was applied.
ECG data were collected for the analysis of both HR and HRV. HRV specifically focused on the high-frequency (HF) component within the frequency range of 0.15–0.40 Hz. This HF component serves as an indicator of activity within the cardiac PSNS (4).
Conversely, SCL measurements were employed as an indicator of SNS activity at peripheral sites and can reflect changes in physiological arousal or responses to stress (41).
2.4.2 Psychological measurements
The participants' subjective evaluations were assessed using a visual analog scale (VAS 1) comprising five attributes: “fatigue”, “nervousness”, “refreshing feeling”, “drowsiness”, and “relaxation”. This assessment was conducted three times: after R1, following the MW, and after R2 as depicted in Figure 1. Additionally, a VAS 2 questionnaire was administered to determine preference among the mouthwashes after MW and at the end of R2. This questionnaire consisted of four items: “flavor preference”, “stimulative sensation (tingling sensation)”, “cooling sensation”, and “refreshing sensation”. In the VAS, a calibrated line was presented with endpoints at 0% and 100%, instructing participants to mark their perception on the line corresponding to each attribute based on their experience (39, 40, 42).
2.5 Data analysis
The raw data for HR, HF, and SCL were standardized (converted to z-score) to account for individual variations across participants and conditions. Based on the study hypothesis, the mean difference during R2 was calculated for each physiological parameter as the variable for analysis. The Shapiro-Wilk test was conducted to assess the normality of the data distribution. Given that the datasets followed a normal distribution, a repeated measure one-way analysis of variance (ANOVA) followed by Dunnett's post-hoc tests were performed to compare the effects of five conditions on the physiological parameters. Additionally, a two-way ANOVA was employed to assess the effect of both time and conditions on the psychological parameters. The statistical significance level for p was set at 0.05. The statistical analysis was carried out using R software (Version 4.2.2, R Foundation for Statistical Computing, Vienna, Austria).
3 Results
3.1 Physiological assessment
3.1.1 Heart rate and heart rate variability
Figures 2, 3 show the box plots for the mean value of the HR and HF components of HRV during the resilience period, R2, respectively. In Figure 2, a one-way ANOVA showed that there was a statistically significant difference in mean HR value between the conditions [F (4, 90) = 5.923, p < 0.001]. Performing post hoc analysis, MB, ML, and MO mouthwashes exhibited a significantly greater decrease in HR compared to Ctl (vs. MB: p < 0.001, 95% CI: [−0.53, −0.12], vs. ML: p = 0.0008, 95% CI: [−0.53, −0.11], vs. MO: p < 0.001, 95% CI: [−0.54, −0.12]).

Figure 2. Box plot for the changes in the mean value of HR (z score) during R2 for all five conditions (n = 22). Within each box, horizontal black lines denote median values; boxes extend from the first and third quartile; vertical extending lines denote minimum and maximum values without outliers; dots denote the outliers. HR, heart rate; R2, recovery period; Ctl, control condition; MB, peppermint + bergamot; Mint, peppermint; ML, peppermint + lavender; MO, peppermint + sweet orange.
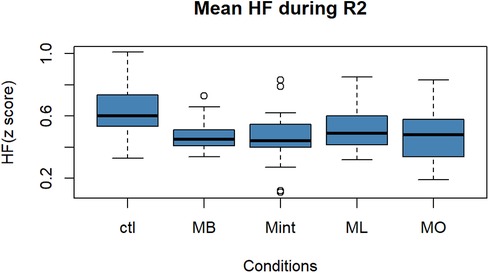
Figure 3. Box plot for the changes in the mean value of the HF component of HRV (z score) during R2 for all five conditions (n = 22). HF, high-frequency component of heart rate variability; R2, recovery period; Ctl, control condition; MB, peppermint + bergamot; Mint, peppermint; ML, peppermint + lavender; MO, peppermint + sweet orange.
As for HF in Figure 3, the ANOVA revealed that there was a significant difference among the five conditions in the HF component of HRV [F (4, 90) = 3.246, p = 0.015]. Performing post hoc analysis, Mint, MB, and MO mouthwashes showed a statistically significant increase in HF compared to Ctl (vs. Mint: p = 0.014, 95% CI: [−0.29, −0.25], vs. MB: p = 0.012, 95% CI: [−0.29, −0.28], vs. MO: p = 0.02, 95% CI: [−0.28, −0.18]).
3.1.2 Skin conductance level
Figure 4 depicts the box plot diagram for the changes in the average SCL during the R2. There was no significant difference among the five conditions in terms of SCL during R2 [F (4, 90) = 1.6, p = 0.18].
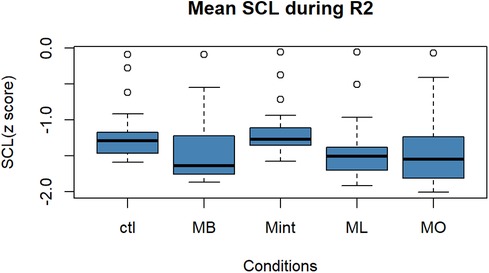
Figure 4. Box plot for the changes in the mean value of SCL (z score) during R2 for all five conditions (n = 22). SCL, skin conductance level; R2, recovery period; Ctl, control condition; MB, peppermint + bergamot; Mint, peppermint; ML, peppermint + lavender; MO, peppermint + sweet orange.
3.2 Psychological assessment
As per the results of VAS 1, a two-way ANOVA revealed that there was no statistically significant difference between the conditions in any VAS score. Regardless of the experimental conditions, there was a statistically significant increase in VAS scores from after R1 to after MW (p < 0.01—p < 0.001; since the statistical results are extensive, the details will be omitted). This indicates that the computational task introduced in this study functioned as a short-term stressor (32, 37).
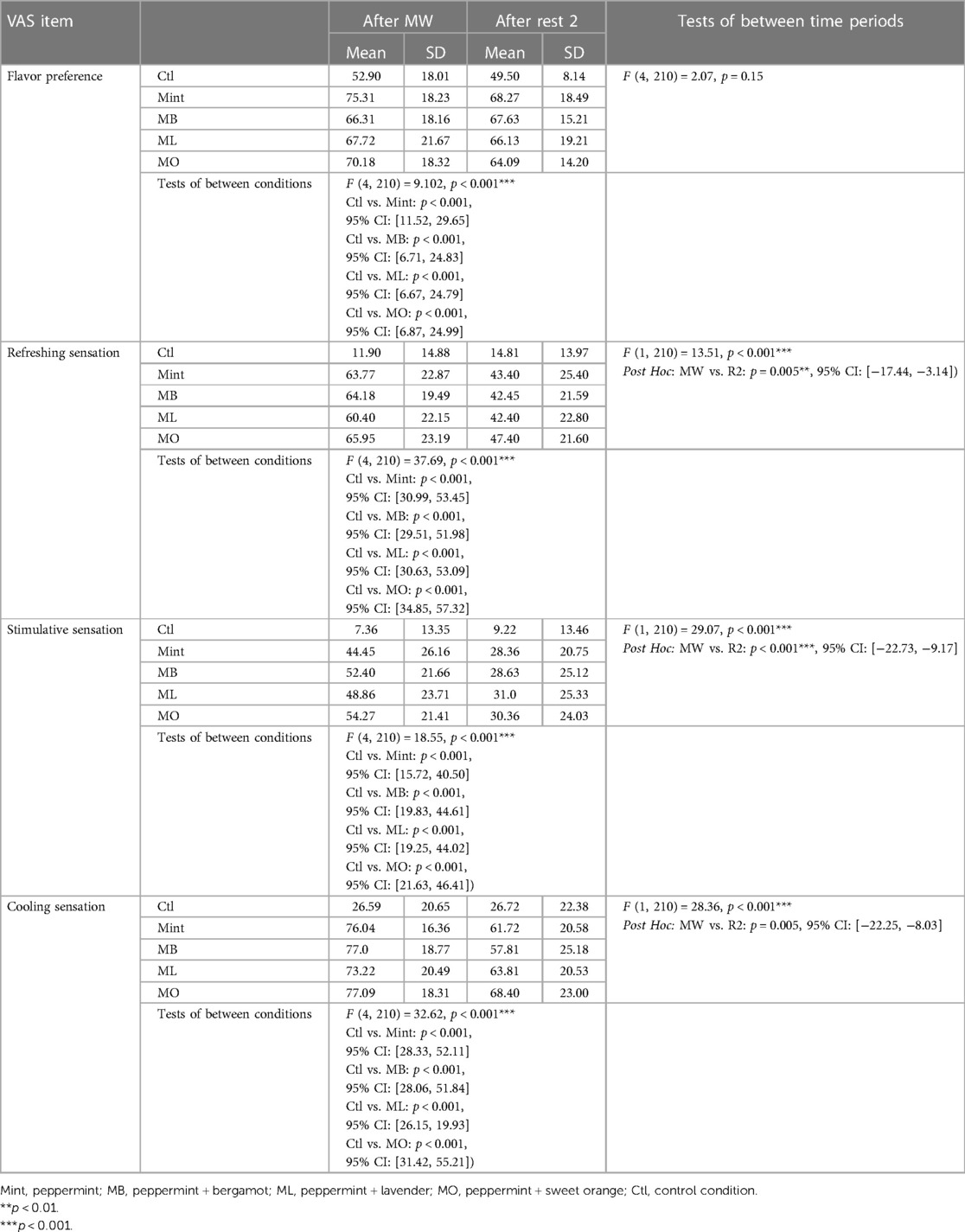
The evaluation results of subjective impressions for each condition (VAS 2) are given in Figures 5–8. Table 2 summarizes the statistical results of VAS 2. According to the results, there was no significant interaction between time × condition [F (4, 210) = 0.42, p = 0.79], and a significant effect between the conditions [F (4, 210) = 9.102, p < 0.001] was found for “flavor preference”. The post hoc test revealed significant differences among all the mouthwashes compared to the Ctl as given in Table 2. For “refreshing sensation”, there was no significant interaction between time × condition [F (4, 210) = 1.32, p = 0.26], and a significant effect between conditions [F (4, 210) = 37.69, p < 0.001], and the time [F (1, 210) = 13.51, p < 0.001] was found. The post hoc test revealed significant differences among all the mouthwashes compared to the Ctl, and a significant decrease in the “refreshing sensation” score in the period of R2. There was a significant interaction between time × condition [F (4, 210) = 2.54, p = 0.04], and a significant effect of condition [F (4, 210) = 18.55, p < 0.001], and the time [F (1, 210) = 29.07, p < 0.001] for “stimulative sensation”. The post hoc test revealed significant differences among all the mouthwashes compared to the Ctl, and a significant decrease in “stimulative sensation” in the period of R2. Furthermore, there was a significant interaction between time × condition [F (4, 210) = 2.57, p = 0.03], a significant effect of condition [F (4, 210) = 32.62, p < 0.001], and the time [F (1, 210) = 28.36, p < 0.001] for “cooling sensation”. The post hoc test revealed significant differences among all the mouthwashes compared to the Ctl, and a significant decrease in the “cooling sensation” score in the period of R2.
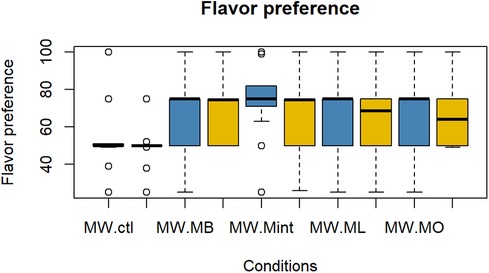
Figure 5. Box plot for the VAS scores of “flavor preference” for all five conditions (n = 22). MW, mouthwash administration; R2, recovery period; blue color: after MW, Yellow color: after R2.
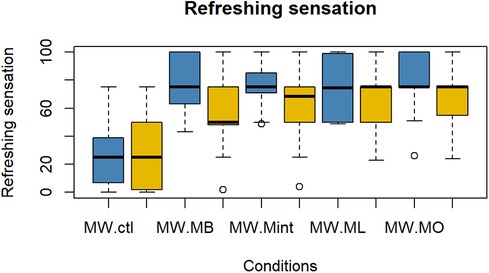
Figure 6. Box plot for the VAS scores of “refreshing sensations” for all five conditions (n = 22). MW, mouthwash administration; blue color: after MW, Yellow color: after R2.
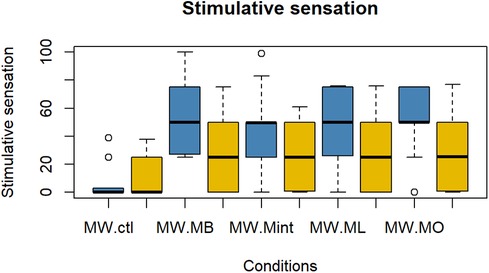
Figure 7. Box plot for the VAS scores of “refreshing sensations” for all five conditions (n = 22). MW, mouthwash administration; blue color: after MW, Yellow color: after R2.

Figure 8. Box plot for the VAS scores of “cooling sensation” for all five conditions (n = 22). MW, mouthwash administration; blue color: after MW, Yellow color: after R2.
4 Discussions
This study investigated the psychophysiological effects of aromatic mouthwashes during the resilience phase after a short-term cognitive stressor, utilizing bio-signals as indicators. In this study, we hypothesized that the administration of aromatic mouthwashes will alleviate physiological stress responses within the ANS, with a greater decrease in HR and SCL and a greater increase in the HF component of HRV. The findings supported the expected working hypothesis by demonstrating a significantly greater reduction in HR in MB, ML, and MO mouthwashes compared to the Ctl condition, suggesting a potential suppression of the cardiac SNS during R2. Mint, MB, and MO mouthwashes demonstrated a significant increase in the HF component of HRV, indicating a suppression of cardiac PSNS activity. These findings imply that citrus mouthwashes such as MB and MO have the potential to alleviate the physiological stress response (in terms of cardiac activity) compared to the control condition. The results of the psychological assessment align with these physiological measures, indicating a positive subjective experience among participants using aromatic mouthwashes compared to the control condition. The integration of both bio-signals and psychological assessments in the study is pivotal for achieving a comprehensive understanding of an individual's response to aromatic mouthwashes, encompassing both physiological and psychological dimensions, enhancing the reliability and validity of study outcomes.
Prior research has consistently demonstrated the stress-alleviating properties of citrus essential oils (29, 32, 43, 44). In line with this premise, the present study findings revealed that mouthwash infused with orange essential oil and bergamot essential oil demonstrated effectiveness in resilience from acute stress response, as manifested by a reduction in HR and enhancement in the HF component of HRV during R2. These findings are consistent with previous study findings, where inhalation of mild orange essential oil influenced cardiac nervous system activity but had no significant effect on the peripheral nervous system (32), and the inhalation of bergamot essential oil showed a similar effect on PSNS activity, indicating stress-reducing effects (29). This implies the plausible psychophysiological impacts of orange and bergamot essential oils when administered through mouthwashes. Furthermore, Ishikawa et al. (15, 38) reported that the citrus mint mouthwash exhibited a greater ability to promote recovery from physiological stress response compared to the spice mint mouthwash (15), and the use of non- or low-alcohol mouthwashes promotes recovery from peripheral stress response (38). Such mouthwashes seem particularly suited for situations requiring relaxation, evident in the decrease in SCL during R2 (38). Similarly, another study, utilizing a wristwatch-type HR monitoring device, explored the effects of mouthwash use on sleep. It revealed that mint-flavoured mouthwash induced a relaxing effect, reflected in subjective scores and a positive mood before sleep (37). These findings collectively emphasize the potential therapeutic implications of specific mouthwash formulations, particularly those incorporating citrus essential oils, in managing stress and promoting relaxation.
The study's insights into the psychophysiological effects of specific aromas, such as peppermint, bergamot, sweet orange, and lavender, provide a foundation for developing tailored oral care formulations. This study which incorporated a variety of aromas in the mouthwash formulations introduced diverse flavours, stimulative sensations, and refreshing experiences to the users. This variation not only facilitated the recovery from acute stress responses but also provided participants with a range of sensory encounters throughout the experiment. Companies can leverage this knowledge to create products with carefully balanced aromatic profiles that maximize stress-alleviating properties. By integrating biological signals with psychological assessments, this study has expanded our understanding of mouthwashes, particularly their psychophysiological effects during post-acute stress resilience. This approach, encompassing both bio-signals and psychological parameters, enhanced the accuracy and depth of the research findings. The insights gained from this study offer potential avenues for the development of innovative oral care products that not only enhance oral health but also promote stress reduction. The incorporation of stress-alleviating aromas in oral care products opens the door for promoting stress reduction as part of daily hygiene routines.
However, it is essential to acknowledge certain limitations in this study. The intentional recruitment of a homogeneous sample comprising female university students aligns with several considerations related to the research objectives and the characteristics of the target market of the developed oral care product. This homogeneity can enhance the internal validity of the study by minimizing the influence of gender-related physiological and psychological differences. This deliberate selection might introduce biases into the results due to potential variations in gustation based on gender (45, 46) and different phases of the menstrual cycle (47), which could influence the outcomes. Additionally, it is worth noting that bio-signals such as SCL might exhibit fluctuations influenced by hormonal imbalances during various menstrual cycle phases in women (48). Moreover, it is noteworthy that all the mouthwashes utilized in this study were formulated with a base of peppermint essential oil. The specific combination and concentrations of these aromas could also contribute to the observed variations. Further research incorporating diverse aroma compositions could provide a more profound understanding of the effects of aromatic mouthwashes.
4.1 Future implications
In the future, the integration of bio-signals using wearable technology (37, 49), facial expression capturing (50), and other advanced physiological and psychological measurements (1) could offer a more comprehensive understanding of the intricate relationship between aromatic mouthwashes and physiological stress reduction. This could potentially lead to the development of personalized oral care interventions that cater to individual preferences and physiological responses, ultimately enhancing the overall well-being and oral health of users.
5 Conclusion
This study investigated the psychophysiological effects of aromatic mouthwashes in the resilience period following a short-term cognitive stressor. The experimental results suggested that rinsing with citrus-flavored mouthwashes has a positive impact in alleviating the physiological stress response (in terms of cardiac activity). These findings may have practical implications for the development of innovative, novel oral care products that promote stress reduction and improve oral health.
Data availability statement
The original contributions presented in the study are publicly available. This data can be found here: https://osf.io/j9n8t/ doi:10.17605/OSF.IO/J9N8T.
Ethics statement
The studies involving humans were approved by Nagaoka University of Technology. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
CD: Data curation, Formal Analysis, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. TO: Data curation, Formal Analysis, Methodology, Writing – review & editing. MI: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Writing – review & editing. HK: Validation, Visualization, Writing – review & editing. SN: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article.
This study was funded by Nagaoka University of Technology (Grant No. 622101) and Sunstar Inc., Japan. The aroma mouthwashes were prepared and provided by the company.
Acknowledgments
The authors are incredibly thankful to the participants of this study. This research is a collaborative work with Sunstar Inc., Japan, and includes company employees in the author list.
Conflict of interest
MI was employed by Sunstar Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Giannakakis G, Grigoriadis D, Giannakaki K, Simantiraki O, Roniotis A, Tsiknakis M. Review on psychological stress detection using biosignals. IEEE Trans Affect Comput. (2019) 13:440–60. doi: 10.1109/TAFFC.2019.2927337
2. Wijsman J, Grundlehner B, Liu H, Hermens H, Penders J. Towards mental stress detection using wearable physiological sensors. Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS. (2011). p. 1798–1801. doi: 10.1109/IEMBS.2011.6090512
3. Kurniawan H, Maslov AV, Pechenizkiy M. Stress detection from speech and galvanic skin response signals. Proceedings of CBMS 2013—26th IEEE International Symposium on Computer-Based Medical Systems (2013). p. 209–14. doi: 10.1109/CBMS.2013.6627790
4. Berntson GG, Bigger JT Jr, Eckberg DL, Grossman P, Kaufmann PG, Malik M, et al. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. (1997) 34(6):623–48. doi: 10.1111/j.1469-8986.1997.tb02140.x
5. Kobayashi H, Ishibashi K, Noguchi H. Heart rate variability; an index for monitoring and analyzing human autonomic activities. J Physiol Anthropol Appl Human Sci. (1999) 18:53–9. doi: 10.2114/jpa.18.53
6. McCraty R, Shaffer F. Heart rate variability: new perspectives on physiological mechanisms, assessment of self-regulatory capacity, and health risk. Glob Adv Health Med. (2015) 4(1):46–61. doi: 10.7453/gahmj.2014.073
7. Gonzalez K, Sasangohar F, Mehta RK, Lawley M, Erraguntla M. Measuring Fatigue through Heart Rate Variability and Activity Recognition: A Scoping Literature Review of Machine Learning Techniques. Proc Hum Factors Ergon Soc Annu Meet. (2017) 61: 1748–52. doi: 10.1177/1541931213601918
8. Guzzetti S, Borroni E, Garbelli PE, Ceriani E, Della Bella P, Montano N, et al. Symbolic dynamics of heart rate variability: a probe to investigate cardiac autonomic modulation. Circulation. (2005) 112(4):465–70. doi: 10.1161/CIRCULATIONAHA.104.518449
9. Hughson RL, Yamamoto Y, McCullough RE, Sutton JR, Reeves JT. Sympathetic and parasympathetic indicators of heart rate control at altitude studied by spectral analysis. J Appl Physiol. (1994) 77(6):2537–42. doi: 10.1152/jappl.1994.77.6.2537
10. Arza A, Garzón-Rey JM, Lázaro J, Gil E, Lopez-Anton R, de la Camara C, et al. Measuring acute stress response through physiological signals: towards a quantitative assessment of stress. Med Biol Eng Comput. (2019) 57(1):271–87. doi: 10.1007/s11517-018-1879-z
11. Patania VM, Padulo J, Iuliano E, Ardigò LP, Čular D, Miletić A, et al. The psychophysiological effects of different tempo music on endurance versus high-intensity performances. Front Psychol. (2020) 11. doi: 10.3389/fpsyg.2020.00074
12. Sierra A, Snchez-Avila C, Guerra-Casanova J, Bailador-Del G. Real-time stress detection by means of physiological signals. In: Yang J, Poh N, editors. Recent Application in Biometrics. Vol. 2. Rijeka: IntechOpen. (2011). p. 23–44. doi: 10.5772/18246
13. Thoma MV, La Marca R, Brönnimann R, Finkel L, Ehlert U, Nater UM. The effect of music on the human stress response. PLoS One. (2013) 8(8):e70156. doi: 10.1371/journal.pone.0070156
14. Ikei H, Song C, Miyazaki Y. Physiological effect of olfactory stimulation by Hinoki Cypress (Chamaecyparis obtusa) leaf oil. J Physiol Anthropol. (2015) 34:44. doi: 10.1186/s40101-015-0082-2
15. Ishikawa M, Okawa Y, Wakabayashi E, Hirono A, Oshima Y, Nomura S. Physiological effects of using citrus mint flavored mouthwash. Trans Japan Soc Kansei Eng. (2023) 22(1):51–55. doi: 10.5057/jjske.TJSKE-D-22-00034
16. Barnett ML. The rationale for the daily use of an antimicrobial mouth rinse. J Am Dent Assoc. (2006) 137:S16–21. doi: 10.14219/jada.archive.2006.0408
17. Krishnamurthy S, Vijayasarathy S. Role of nanomaterials in clinical dentistry. In: Grumezescu AM, editor. Nanobiomaterials in Dentistry: Applications of Nanobiomaterials. Bucharest, Romania: William Andrew Publishing (2016). p. 211–40. doi: 10.1016/B978-0-323-42867-5.00009-6
18. Choulis NH. Dermatological drugs, topical agents, and cosmetics. In: Ray SD, editor. Side Effects of Drugs Annual. New York: Elsevier (2014). p. 203–31. doi: 10.1016/B978-0-444-63407-8.00014-9
19. Asakawa R, Suzuki H, Yagi T, Sakanashi K, Iwasaki S, Shimada T, et al. Pilot study to assess the potential of new moisturizing agents for oral dryness. Int J Oral Health Sci. (2017) 16(2):25–30. doi: 10.5466/ijoms.16.25
20. Pérez-Nicolás C, Pecci-Lloret MP, Guerrero-Gironés J. Use and efficacy of mouthwashes in elderly patients: a systematic review of randomized clinical trials. Ann Anat. (2023) 246:152026. doi: 10.1016/j.aanat.2022.152026
21. Tanigawa T, Yamashita JI, Sato T, Shinohara A, Shibata R, Ueda H, et al. Efficacy and safety of pilocarpine mouthwash in elderly patients with xerostomia. Spec Care Dentist. (2015) 35(4):164–9. doi: 10.1111/scd.12105
22. Kim YR, Nam SH. Effects of a mouthwash containing Lespedeza cuneata extract on risk of dental caries: a randomized, placebo-controlled clinical trial. Sci Rep. (2022) 12:20761. doi: 10.1038/s41598-022-25162-w
23. Kim YR, Nam SH. Effect of oral gargle containing Lespedeza cuneata extract on periodontal health improvement and disease prevention: a randomized, controlled clinical trial. BMC Oral Health. (2023) 23:116. doi: 10.1186/s12903-023-02816-3
24. Sharma D, Mcguire JA, Gallob JT, Amini P. Randomised clinical efficacy trial of potassium oxalate mouthrinse in relieving dentinal sensitivity. J Dent. (2013) 41(S4):S40–8. doi: 10.1016/S0300-5712(13)70005-8
25. Alshehri FA. The use of mouthwash containing essential oils (LISTERINE®) to improve oral health: a systematic review. Saudi Dent J. (2018) 30(1):2–6. doi: 10.1016/j.sdentj.2017.12.004
26. Gordon JM, Lamster IB, Seiger MC. Efficacy of Listerine antiseptic in inhibiting the development of plaque and gingivitis. J Clin Periodontol. (1985) 12(8):697–704. doi: 10.1111/j.1600-051x.1985.tb00941.x
27. Ren X, Zhang Y, Xiang Y, Hu T, Cheng R, Cai H. The efficacy of mouthwashes on oral microorganisms and gingivitis in patients undergoing orthodontic treatment: a systematic review and meta-analysis. BMC Oral Health. (2023) 23:204. doi: 10.1186/s12903-023-02920-4
28. Chen MC, Fang SH, Fang L. The effects of aromatherapy in relieving symptoms related to job stress among nurses. Int J Nurs Pract. (2015) 21(1):87–93. doi: 10.1111/ijn.12229
29. Watanabe E, Kuchta K, Kimura M, Rauwald HW, Kamei T, Imanishi J. Effects of bergamot (Citrus bergamia (risso) wright & arn.) essential oil aromatherapy on mood states, parasympathetic nervous system activity, and salivary cortisol levels in 41 healthy females. Complement Med Res. (2015) 22:43–9. doi: 10.1159/000380989
30. Toda M, Morimoto K. Evaluation of effects of lavender and peppermint aromatherapy using sensitive salivary endocrinological stress markers. Stress Health. (2011) 27(5):430–5. doi: 10.1002/smi.1402
31. Hamzeh S, Safari-Faramani R, Khatony A. Effects of aromatherapy with lavender and peppermint essential oils on the sleep quality of cancer patients: a randomized controlled trial. Evid Based Complement Alternat Med. (2020) 2020:7480204. doi: 10.1155/2020/7480204
32. Lekamge S, Nakachi M, Sato S, Ito K, Nomura S. Alleviation of the acute stress response following mild orange essential oil administration. IEEJ Trans Electr Electron Eng. (2017) 12:S158–63. doi: 10.1002/tee.22428
33. Chang AK, Kim BK, Kim AY. The impact of aromatherapy-based oral care on oral conditions, salivary pH, and halitosis in older adults with dementia: pilot study. Geriatr Nurs (Minneap). (2023) 53:109–15. doi: 10.1016/j.gerinurse.2023.07.010
34. Seo EY, Song JA, Hur MH, Kyoung LM, Lee MS. Effects of aroma mouthwash on stress level, xerostomia, and halitosis in healthy nurses: a non-randomized controlled clinical trial. Eur J Integr Med. (2017) 10:82–9. doi: 10.1016/j.eujim.2017.03.001
35. Kamaruddin HK, Farah NMF, Aziz AR, Mündel T, Che Muhamed AM. Carbohydrate mouth rinse is no more effective than placebo on running endurance of dehydrated and heat-acclimated athletes. Eur J Appl Physiol. (2023) 123:1507–18. doi: 10.1007/s00421-023-05170-y
36. Jeffries O, Goldsmith M, Waldron M. L-Menthol mouth rinse or ice slurry ingestion during the latter stages of exercise in the heat provide a novel stimulus to enhance performance despite elevation in mean body temperature. Eur J Appl Physiol. (2018) 118(11):2435–42. doi: 10.1007/s00421-018-3970-4
37. Ishikawa M, Mon-Nai Y, Hirono A, Oshima Y, Nomura S. Effects of bedtime mouthwash use on sleep: a field sleep study using wristwatch-type heart rate monitoring devise. IEEJ Trans Electron Inform Syst. (2023) 143:383–9. doi: 10.1541/ieejeiss.143.383
38. Ishikawa M, Dilrukshi EAC, Hirono A, Oshima Y, Nomura S. Physiological effects of alcohol concentration in mouthwash in recovering from short-term cognitive stressor. Trans Japan Soc Kansei Eng. (2023) 22(3):317–23. doi: 10.5057/jjske.TJSKE-D-22-00077
39. Dilrukshi EAC, Nishiyama Y, Ito K, Nomura S. Alleviation of acute stress response by black pepper aroma administration. J Physiol Anthropol. (2024) 43:3. doi: 10.1186/s40101-023-00352-1
40. Dilrukshi EAC, Sato K, Ishikawa M, Nishiyama Y, Nomura S. Efficacy of mouthwashes in the recovery period following a short-term cognitive stressor: contribution of thickening agents. Int J Affect Eng. (2024). doi: 10.5057/ijae.IJAE-D-23-00017
41. Turpin G, Grandfield T. Electrodermal activity. In: Fink G, editor. Encyclopedia of Stress. Academic Press (2007). p. 899–902. doi: 10.1016/B978-012373947-6.00139-2
42. Maeneja R, Silva CR, Ferreira IS, Abreu AM. Aerobic physical exercise versus dual-task cognitive walking in cognitive rehabilitation of people with stroke: a randomized clinical trial. Front Psychol. (2023) 14:1–17. doi: 10.3389/fpsyg.2023.1258262
43. Pour F, Arman S, Jaafarzadeh M. Effect of aromatherapy with orange essential oil on salivary cortisol and pulse rate in children during dental treatment: a randomized controlled clinical trial. Adv Biomed Res. (2013) 2:10. doi: 10.4103/2277-9175.107968
44. Rashidi-Fakari F, Tabatabaeichehr M, Mortazavi H. The effect of aromatherapy by essential oil of orange on anxiety during labor: a randomized clinical trial. Iran J Nurs Midwifery Res. (2015) 20:661–4. doi: 10.4103/1735-9066.170001
45. Kuyama K, Yamamoto H. A Study of effects of mouthwash on the human oral mucosae: with special references to sites, sex differences, and smoking. J Nihon Univ Sch Dent. (1997) 39(4):202–10. doi:10.2334/josnusd1959.39.202
46. Robin O, Rousmans S, Dittmar A, Vernet-Maury E. Gender influence on emotional responses to primary tastes. Physiol Behav. (2003) 78(3):385–93. doi: 10.1016/S0031-9384(02)00981-2
47. Saperova EV, Filippova I. Heart rate variability during different phases of menstrual cycle. FASEB J. (2022) 36(S1). doi: 10.1096/fasebj.2022.36.S1.L7996
48. Gómez-Amor J, Martínez-Selva JM, Román F, Zamora S. Electrodermal activity in menstrual cycle phases: a comparison of within- and between-subjects designs. Int J Psychophysiol. (1990) 9(1):39–47. doi: 10.1016/0167-8760(90)90006-Y
49. Almadhor A, Sampedro GA, Abisado M, Abbas S, Kim YJ, Khan MA, et al. Wrist-based electrodermal activity monitoring for stress detection using federated learning. Sensors. (2023) 23(8):3984. doi: 10.3390/s23083984
Keywords: aroma, mouthwash, bio-signals, heart rate, subjective impressions, physiological stress response, oral care
Citation: Dilrukshi EAC, Ogino T, Ishikawa M, Kuroda H and Nomura S (2024) Assessing the usability of aromatic mouthwashes in alleviating physiological stress responses. Front. Oral. Health 5:1343937. doi: 10.3389/froh.2024.1343937
Received: 24 November 2023; Accepted: 18 March 2024;
Published: 4 April 2024.
Edited by:
Fawad Javed, University of Rochester Medical Center, United StatesReviewed by:
Sanjei Chitravel, University of British Columbia, CanadaEsra Ülker, Selcuk University, Türkiye
Farhad Atassi, King Saud University, Saudi Arabia
© 2024 Dilrukshi, Ogino, Ishikawa, Kuroda and Nomura. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shusaku Nomura bm9tdXJhQHZvcy5uYWdhb2thdXQuYWMuanA=
 E. A. Chayani Dilrukshi
E. A. Chayani Dilrukshi Tatsuki Ogino1
Tatsuki Ogino1 Hiroki Kuroda
Hiroki Kuroda Shusaku Nomura
Shusaku Nomura