- 1Faculty of Dentistry, Oral and Craniofacial Sciences, Centre for Host-Microbiome Interactions, King’s College London, London, United Kingdom
- 2Department of Anaesthesia, King’s College Hospital NHS Foundation Trust, London, United Kingdom
- 3Department of Periodontology, Guy’s and St Thomas’ NHS Foundation Trust, London, United Kingdom
- 4Department of Cardiology, King’s College Hospital NHS Foundation Trust, London, United Kingdom
- 5Department of Cardiothoracic Surgery, King’s College Hospital NHS Foundation Trust, London, United Kingdom
- 6National Heart and Lung Institute, Imperial College London, London, United Kingdom
- 7Imperial College Healthcare NHS Trust, London, United Kingdom
Introduction: Infective Endocarditis (IE) is a rare, life-threatening infection of the endocardium with multisystem effects. Culprit microorganisms derived from different niches circulate through the bloodstream and attach to the endocardium, particularly the heart valves. This study aimed to investigate culprit microorganisms among a cross-sectional cohort of IE patients, their associated factors, and to explore the potential relationship to the oral microbiome.
Methods: In this observational study, we undertook a cross-sectional analysis of 392 medical records from patients diagnosed with IE. The primary outcome of this study was to analyse the association between the IE culprit microorganisms and the underlying anatomical types of IE (native valve (NVE), prosthetic valve (PVE), or cardiac device-related (CDE)). Secondary outcomes encompassed a comparative analysis of additional factors, including: the treatment approaches for IE, and the categorisation of blood cultures, extending to both genus and species levels. Additionally, we cross-referenced and compared the species-level identification of IE bacteraemia outcome measures with data from the expanded Human Oral Microbiome Database (eHOMD).
Results: A culprit microorganism was identified in 299 (76.28%) case participants. Staphylococcal infections were the most common (p < 0.001), responsible for 130 (33.16%) hospitalisations. There were 277 (70.66%) cases of NVE, 104 (26.53%) cases of PVE, and 11 (2.81%) cases of CDE. The majority of PVE occurred on prosthetic aortic valves (78/104, 75%), of which 72 (93.5%) were surgical aortic valve replacements (SAVR), 6 (7.8%) were transcatheter aortic valve implants, and one transcatheter pulmonary valve implant. Overall, underlying anatomy (p = 0.042) as well as the treatment approaches for IE (p < 0.001) were significantly associated with IE culprit microorganisms. Cross-reference between IE bacteraemia outcomes with the eHOMD was observed in 267/392 (68.11%) cases.
Conclusions: This study demonstrated that IE patients with a history of stroke, smoking, intravenous drug use, or dialysis were more likely to be infected with Staphylococcus aureus. CDE case participants and patients who had previous SAVR were most associated with Staphylococcus epidermidis. IE patients aged 78+ were more likely to develop enterococci IE than other age groups. Oral microorganisms indicated by the eHOMD are significantly observed in the IE population. Further research, through enhanced dental and medical collaboration, is required to correlate the presence of oral microbiota as causative factor for IE.
1 Introduction
Infective Endocarditis (IE) is a rare, life-threatening infection of the heart, particularly the heart valves, with multisystem effects (1). It affects up to 1:10,000 of the population, and has a high mortality rate that may reach up to 30% at 30 days post-infection (1). Culprit pathogens circulate in the blood and attach to cardiac tissue. These microorganisms can damage surrounding tissue and lead to vegetation and/or abscess formation, potentially causing life-threatening complications, including valvular insufficiency, heart failure and septic emboli to distal organs including the brain, lungs, spleen, and others (1).
Risk factors for IE encompass a broad range of medical and social determinants. Patients with prosthetic heart valves or implanted cardiac devices, including permanent pacemakers and cardioverter defibrillators, are at increased risk (1). Additionally, pre-existing conditions such as underlying structural heart disease, congenital heart disease, and various comorbidities significantly elevate the risk. Other factors, for example intravenous drug use (IVDU) and frequent contact with the healthcare system, also contribute to a heightened risk of developing IE (1). These factors collectively identify individuals as high-risk for IE, underscoring the importance of targeted preventive measures and vigilant monitoring.
Further, the oral microbiome represents an important source of recurrent bacteraemia, and recent literature has established that common dental procedures, frequently cause bacteraemia by oral commensals (2). Patients with active unstable periodontal disease are particularly at risk of bacteraemia even after routine tooth-brushing (2). Within the context of bacteraemia involving a pathogenic agent, the formation of infected vegetation can occur as the ultimate consequence of the complex interplay between the invading microorganisms and the immune response of the host. The involvement of oral pathogens in the onset of IE is attributed to the hematogenous dissemination of oral microorganisms (3). It has been reported that infections caused by the oral viridans group of streptococci (such as Streptococcus mutans, Streptococcus mitis, Streptococcus sanguinis) are responsible for nearly 20% of IE cases (3).
The oral microbiome is home to more than 700 bacterial species. Resources such as the expanded Human Oral Microbiome Database (eHOMD) offer a wealth of taxonomic and genomic information on oral microbes (3, 4). This data can be instrumental in analysing bacteraemia cases and investigating their potential origins in the oral microbiome (3, 4). The eHOMD Taxonomic Level page offers a detailed enumeration of taxa across the seven hierarchical levels of taxonomy: Domain, Phylum, Class, Order, Family, Genus, and Species. For instance, within the Genus level, it catalogues the 169 genera found in the HOMD, providing a count of species for each genus along with a link to the corresponding family (4). It utilises 16S rRNA RefSeq to enable comprehensive curated information on bacterial identification (4).
The management of IE usually necessitates the collaboration of a multidisciplinary team. Antimicrobial therapy (AT) is essential for all patients, while cardiovascular surgery may be advantageous for a specific subset (5). A positive blood culture is fundamental to diagnosing infections microbiologically. Obtaining three sets of blood cultures can identify 96%–98% of bacteraemia cases. Samples are routinely collected for culture prior to initiating antibiotic treatment in patients (5). Approximately 10% of patients with IE have blood cultures that yield no growth [blood culture negative endocarditis (BCNE)], leading to challenges in diagnosis. This phenomenon may be attributed to several factors, including the administration of antibiotics prior to taking blood cultures, infection with fastidious bacteria or fungi, or other conditions such as nonbacterial thrombotic endocarditis (5, 6).
The American Heart Association (AHA) and the European Society of Cardiology (ESC) reserve antibiotic prophylaxis (AP) for high-risk patients who undergo invasive dental procedures (7). This is in contrast to the National Institute for Health and Clinical Excellence (NICE guideline CG64 2008, and the 2015 update) in the United Kingdom. It advised the total discontinuation of AP to prevent IE in individuals with heart conditions who were scheduled for dental procedures (8). There remains uncertainty over the relationship between the oral microbiome and IE, and further insight is warranted.
We conducted an observational study to investigate culprit microorganisms among a cross-sectional cohort of IE patients, their associated factors, and to explore the potential relationship to the oral microbiome.
2 Materials and methods
2.1 Study design
This was an observational study focused on a cross-sectional cohort of adult patients diagnosed with IE (9). The study was approved as a service evaluation by King's College Hospital NHS Foundation Trust, Department of Cardiology (CV003-2022). Anonymised medical records of patients, who were admitted to the Cardiology Department at King's College Hospital (KCH) between December 2013 and February 2021, were assessed. This study was conducted in accordance with the Helsinki Declaration of 1975. Adult (≥18-years-old) patients with a diagnosis of definite or possible IE described by Modified Duke criteria were included (10).
2.2 Data collection
The electronic medical records of the included patients were analysed to extract anonymised data in relation to (i) demographics, (ii) comorbidities, (iii) other medical and social history factors, (iv) IE diagnosis confirmation (10), (v) bacteremia (at a genus and species level), (vi) underlying anatomy [native valve endocarditis (NVE), prosthetic valve endocarditis (PVE), cardiac device endocarditis (CDE)], (vii) type of IE treatment [AT, or surgical valve replacement (SVR)], and (viii) pre-existing cardiac conditions in adult patients known to increase the risk of developing IE (8, 11). All anonymised data were entered into a dedicated encrypted database. Access was restricted to data entry personnel and study investigators. Upon completion, the data were proofed for entry errors.
2.3 Outcome measures
The primary outcome of this study was to analyse the association between the IE culprit microorganisms and the underlying anatomical types of IE (native valve, prosthetic valve, or cardiac device-related). Secondary outcomes encompassed a comparative analysis of additional factors, including: the treatment approaches for IE, and the categorisation of blood cultures, extending to both genus and species levels. Additionally, we cross-referenced and compared the species-level identification of IE bacteraemia with data from the expanded Human Oral Microbiome Database (eHOMD, 16S rRNA RefSeq, Version 15.23).
2.4 Statistical analysis
Patient ages were categorised into four groups: 18–37, 38–57, 58–77 and 78+. Culprit microorganisms were categorised into groups by genus and analysed for trends. Staphylococcus, Streptococcus and Enterococcus were genus groups of focus, as they are cumulatively responsible for up to 90% of all IE (1). Microorganisms not belonging to these genus groups were defined as “Other”. In cases of BCNE the term “None” was used. For genus groups returning an association with analysed factors, species within the genus were then investigated.
A normality test was conducted using Q-Q Plots and the Kolmogorov–Smirnov test. The analysis of the microorganisms responsible IE and their association with specific factors–namely, the most prevalent culprits, the underlying anatomical types of IE, and the IE treatment methods–was carried out using the χ2 test (p < 0.05). Descriptive statistics were employed to identify and compare the infection characteristics across different groups of microorganisms. IE culprit microbiology results were cross-referenced with the eHOMD at a descriptive level (12). Analyses were performed IBM SPSS Statistics [Version 29.0.2.0 (20), IBM Corp, New York, United States, 2023].
3 Results
3.1 Baseline characteristics
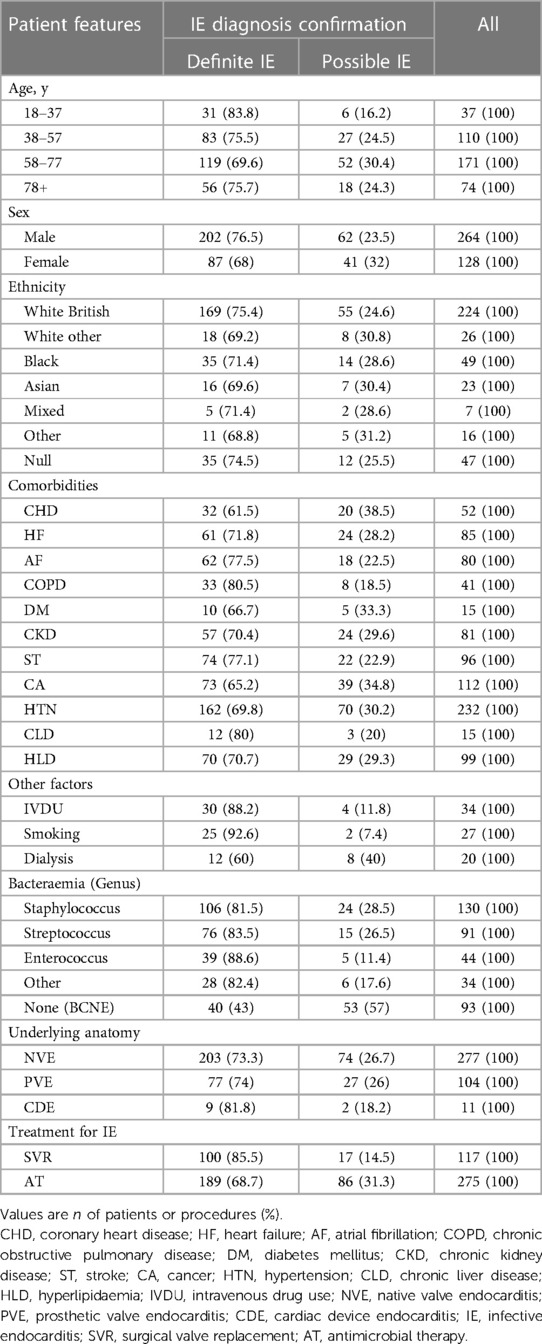
A total of 392 cases were included (Table 1). The mean age of patients with IE was 61.4 years (range 20–93), comprising predominantly males (264/392, 67.35%). The ethnic demographic of this cross-sectional cohort was 63.78% (n = 250) white, 12.50% (n = 49) black, 1.79% (n = 7) mixed, 5.87% (n = 23) Asian, 4.08% (n = 16) other ethnicity and 11.99% (n = 47) with null information. Eleven associated comorbidities were identified including Coronary Heart Disease (CHD), Heart Failure (HF), Atrial Fibrillation (AF), Chronic Obstructive Pulmonary Disease (COPD), Diabetes Mellitus (DM), Chronic Kidney Disease (CKD), Stroke (ST), Cancer (CA), Hypertension (HTN), Chronic Liver Disease (CLD) and Hyperlipidaemia (HLD). Comorbidities identified in this population are summarised in Table 1. Each patient had at least two comorbidities (n = 908), with the most common conditions being HTN (n = 232, 59.18%), CA (n = 112, 28.57%) and HLD (n = 99, 25.26%). Other associated factors for IE identified in this patient population included intravenous drug use (IVDU) (n = 34, 8.7%), smoking (n = 27, 6.9%), and dialysis (n = 20, 5.1%).
In terms of the underlying anatomy, there were 277 (70.66%) cases of NVE, 104 (26.53%) cases of PVE and 11 (2.81%) cases of CDE. Of PVE patients, the majority had SVR (n = 97, 93.3%), rather than transcatheter implanted valves (n = 7, 6.7%). The majority of PVE occurred on prosthetic aortic valves (78/104, 75%), of which 72 were surgical aortic valve replacements (SAVR) and 6 were transcatheter aortic valve implants (TAVI). Patients affected at the site of either an implantable cardioverter defibrillator (ICD) or permanent pacemaker (PPM) constituted CDE. Valve regurgitation was observed in higher proportion in Definite IE (n = 49, 86%) patients in comparison to Possible IE (n = 8, 14%). The majority of patients underwent medical treatment (n = 275, 70.2%), rather than surgical management (n = 117, 29.8%).
3.2 Microbiological outcomes
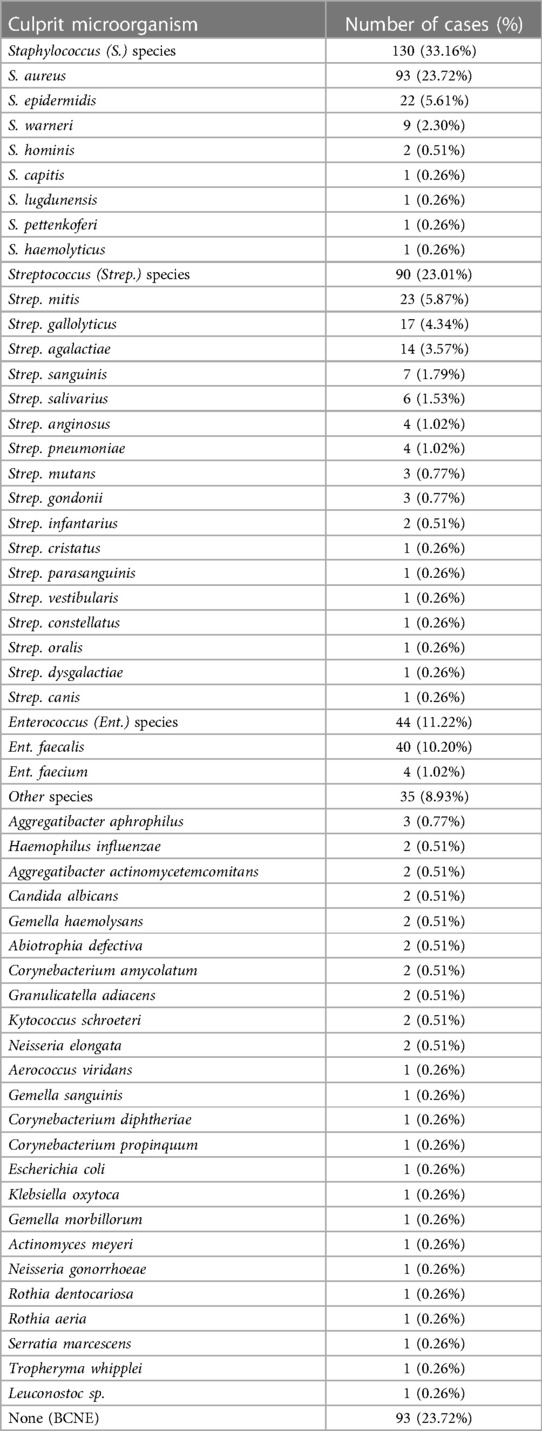
The most abundant IE culprit microorganism corresponded to the Staphylococcus (33.16%), followed by Streptococcus (23.01%), and Enterococcus (11.22%), genera [χ2(4) = 78.944, p < 0.001]. Other microorganisms (8.93%) were mainly characterised by HACEK organisms [Haemophilus influenzae (n = 2), Aggregatibacter aphrophilus (n = 3), Aggregatibacter actinomycetemcomitans (n = 2)] (13). There were 93/392 (23.72%) cases of BCNE.
The most prevalent species was Staphylococcus aureus (n = 93, 23.72%). The full range of culprit microorganisms is presented in Table 2.
A significant association was observed between the microorganisms responsible for IE and the underlying anatomical types of IE [χ2(4) = 9.926, p = 0.042]. For patients with NVE, Staphylococcus (86/277, 31.04%) and Streptococcus (70/277, 25.27%) species were both more prevalent than Enterococcus (29/277, 10.46%) and Other organisms (23/277, 8.30%). BCNE (69/277, 24.91%) was also more prevalent than Enterococcus and Other organisms. In PVE patients, although S. aureus remained the leading cause of Staphylococcal infection (15/36, 41.66%), S. epidermidis (11/36, 30.55%) and S. warneri (7/36, 19.44%) were greater causes in the PVE population than the NVE population (8.14% and 0% respectively). Patients who have had a TAVI had a higher frequency to develop Enterococcus endocarditis (3/6, 50%) than those who have had a SAVR (8/72, 11.11%). The most common culprit species of endocarditis in the TAVI population was Enterococcus faecalis. Staphylococcus epidermidis was the most represented species in the SAVR population (9/72, 12.5%), while Strep. mitis (4/20, 20%) and Strep. gallolyticus (4/20, 20%) were the most represented Streptococcus species in patients with SAVR. IE patients were more likely to have Staphylococcus infection than another genus, if they had the associated factors of stroke (42/96, 43.75%), smoking (15/27, 55.55%), intravenous drug use (IVDU) (19/34, 55.88%) or dialysis (8/20, 40%), of which Staphylococcus aureus was the most represented species for each of these patient groups. CDE cases were most associated with Staphylococcus epidermidis (4/11, 36.36%). There were two cases (18.18%) of BCNE affecting patients with cardiac devices; the rest were all caused by Staphylococci.
IE patients who were in the eldest category (aged 78–97 years) were more likely to have been infected with Enterococci (18/44, 24.32%) compared to those in the other age categories (26/318, 8.18%). Enterococci affected older ages compared to Staphylococci, Other and None genus organisms. The mean (±SD) age of patients with Staphylococcal infection was 59.59 (±16.47) years, Streptococcal was 63.60 (±15.88) years, Enterococcal was 68.82 (±15.87) years, Other genus was 54.26 (±20.21) years and No genus was 60.83 (±14.49) years. Additionally, a significant association was found between the types of microorganisms causing IE and the treatment strategies implemented for IE. Antibiotic therapy emerged as the predominant treatment strategy for IE [χ2(2) = 15.154, p < 0.001].
A secondary descriptive comparison sub-analysis to understand the cross-reference between IE culprit bacteraemia microbiological outcomes with the oral microbiome was performed. The eHOMD, provides comprehensive and curated information on almost 800 oral bacterial species reliably identified in the oral cavity (eHOMD, 16S rRNA RefSeq, Version 15.23) (12). This cross-reference sub-analysis showed that 68.11% (267/392) of patients in the cross-sectional cohort tested positive for bacterial species that have been reliably identified in the oral microbiome via the eHOMD dataabase (Table 3).
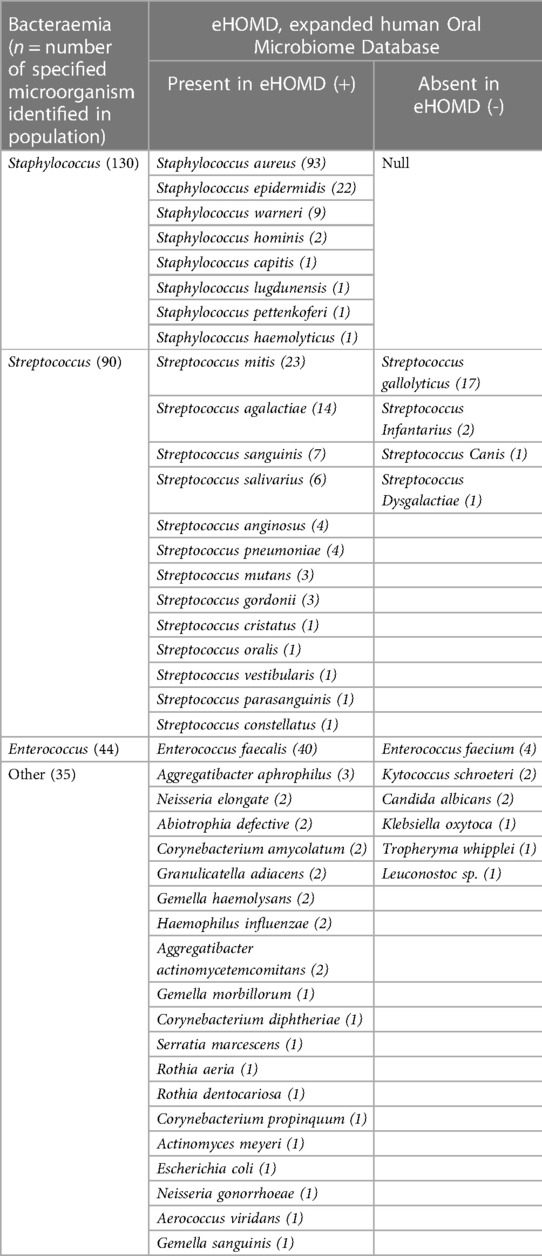
Table 3. Cross-referenced descriptive results by genus and species level between IE microbiological outcomes and the oral microbiome via eHOMD.
4 Discussion
4.1 Main findings
The mean age of infection in the present studied population IE was 61.4 years old, consistent with other studies in the literature (14, 15). Men were more affected than women, in a 2:1 ratio, although the spread of culprit organisms did not differ between the two sexes. This is consistent with other literature that reports the male:female ratio of IE between 2:1 and 9:1 (16). Studies attribute the differences in infection rate to a variety of factors including variable comorbidities, treatment biases, or inherent physiologic differences (17).
Staphylococcus aureus was a leading culprit of IE among patients with a history of stroke, smokers, IVDU or on dialysis. It has been reported that the association between S. aureus and stroke is bidirectional, as both can act as a risk factor for the other; stroke induces immunosuppression which increases the risk of infection and S. aureus has demonstrated the ability to form vegetations on heart valves, which can embolise to cause cerebral infarctions (18, 19). Cigarette smoking heightens bacterial virulence, promoting S. aureus to a virulence profile associated with persistent infection, causing increased biofilm formation, invasiveness and intracellular persistence (20). Literature highlights S. aureus as an especially prevalent culprit organism among IVDU patients (21). This is supported by our findings where all IVDU status IE patients with Staphylococci infection were caused by S. aureus. Improved education and implementation of infection control and prevention measures from healthcare providers to service users will assist patients most at risk. Staphylococci normally reside on the skin without causing issues; however, it can cause infection when there is any disruption of the skin barrier, for example with IVDU or dialysis. These bacteria are highly contagious and can easily spread through contact. Good hand hygiene precautions, cross-contamination awareness and thorough sterilisation of medical equipment are important preventative measures.
Cardiac devices, such as an ICD or PPM, can act as attachment sites for circulating bacteria. Coagulase-negative Staphylococci represent the majority of culprit organisms responsible for CDE, consistent with other reports (22), although, in recent years, the prevalence of Staphylococcus aureus among CDE patients appears to be increasing (23). The reason for S. aureus increasing prevalence among CDE cases is not well described in the literature, nonetheless, this transition is concerning as S. aureus is associated with a worse prognosis than S. epidermidis (24).
Streptococci are typically located in the oral cavity and respiratory tract, contributing approximately 20% of the global IE burden, with greater incidence in resource-limited and developing regions (25). The largest group of these bacteria, viridans group Streptococci (VGS), are commonly found among those with poor dental health and conditions such as dental caries and periodontal disease. Strep. sanguinis is particularly important as it is one of the most common causative agents of IE, comprising approximately 30% of VGS cases (26, 27). Normally within the oral environment, Strep. sanguinis is a primary coloniser of the tooth surface, protecting the human host against the damaging effects of another microorganism, Strep. mutans, which is responsible for tooth decay and caries (28). Interestingly, for this cross-sectional cohort of IE patients, the frequency of infection from Strep. sanguinis was far lower than expected (n = 7, 1.79%). Protective measures against Streptococcal infection might include regular toothbrushing, frequent professional dental cleaning and infection control. Antibiotic prophylaxis before dental procedures might offer protection to patients at high risk of IE. In the UK, the National Institute for Health and Care Excellence (NICE) concluded that there is insufficient evidence to recommend routine antibiotic prophylaxis for dental treatment for at-risk patients, despite incidence rates of IE observing an increase since this recommendation (29). Further evidence and studies regarding the efficacy of antibiotic prophylaxis are needed (30).
This study reports that the origin of organisms identified from microbiology are greatly cross-referenced with the oral microbiome, via eHOMD cross-referencing. However, this is not definitive evidence that the route of infection was from the oral cavity, as many organisms have various distinct origins, with various modes of entry into the bloodstream. For example, Staphylococci are ubiquitously found in normal skin flora, whilst Enterococci originate from the gastrointestinal flora, although organisms from both genera have been identified in the oral microbiome, thereby offering an alternative bacterial port of entry to consider (12). Furthermore, there were two cases of fungal IE due to Candida albicans infection and their presence in the oral cavity is well documented (31, 32). Collectively, these findings underscore the critical importance of rigorous oral hygiene and overall oral health for patients at risk for IE. As a result, it is recommended that dentists collaboratively integrate with the multidisciplinary medical team to ensure that IE patients receive a comprehensive dental examination and a detailed dental history at the time of admission, particularly before undergoing cardiac surgery. Whenever feasible, and the patient is stable enough to be treated in a dental chair, these examinations/management should be conducted under the vigilant oversight and guidance of the medical team.
Enterococci were a leading culprit of IE among patients with advancing age and have undergone TAVI procedure. The prevalence of IE in the elderly population is steadily increasing, notably due to the rising number of invasive procedures and cardiac devices implanted in these patients. Our findings are consistent with other reports of Enterococci as the most common culprits of TAVI, followed by S. aureus (28, 33). Femoral access for TAVI could be an independent risk factor for Enterococci infection due to its close proximity to gastrointestinal output. Improving our knowledge of the aetiology of IE in the elderly is essential to disentangle any confounding factors and whether this is an issue of co-linearity or independent risk. E. faecalis infection can occur through faecal-oral transmission and spread can be mitigated through effective hygiene practices. Enterococci have become a major cause of nosocomial infection (34). Improperly cleaned catheters, dialysis ports, and other medical devices can also carry E. faecalis. Thus, people who undergo organ transplants, kidney dialysis, or cancer treatment are at increased risk for developing infections due to immunosuppression or contamination through their catheters. Patients who undergo TAVI instead of SAVR are typically already at high-risk (35), and therefore, could be more susceptible to E. faecalis infection due to other factors, rather than TAVI as an independent cause. However, there are various confounding factors regarding the relationship between TAVI and E. faecalis infection, whereby future research will be crucial in identifying methods to potentially improve patient outcomes who have had a TAVI.
BCNE accounts for approximately 20% of IE and can be caused by a variety of microorganisms, including Coxiella burnetii, Bartonella spp., Brucella spp., Tropheryma whipplei, Mycoplasma spp., Legionella spp., and non-candida fungi (6). Pre-emptive administration of antibiotic therapy is the most prevalent reason for BCNE (6). Approximately half of patients with IE undergo early surgery and 16S ribosomal ribonucleic acid (rRNA) polymerase chain reaction (PCR) of excised tissue can be vitally important to secure a diagnosis (6). 16S rRNA sequencing represents a form of next generation sequencing (NGS), which yields greater sensitivity than traditional culture methods and involves library preparation, fragmenting DNA/RNA into clusters, sequencing, and reassembling to form a genomic sequence for analysis (36). Precision medicine (e.g., pharmacogenomics) is likely to become a key tool in improving outcomes from BCNE and will contribute to an improved aetiological diagnosis and prognosis moving forwards, but this is yet to become a routine element of clinical practice.
4.2 Limitations
Although our patient population is primarily derived from the London Boroughs of Lambeth, Southwark and Bromley, the department operates as a tertiary referral centre for millions. In order to reduce selection bias, a large patient population was included. The retrospective nature of this study provides inherent limitations when compared to prospective longitudinal studies. Prospective studies, with an analysis of the patient's oral health status, would provide a more robust insight into the association between the oral microbiome and IE. Confounding factors such as the clinical variation of periodontal disease among the study population resulted in difficulty in drawing firm conclusions. Furthermore, due to the nature of the study, we were only able to cross-reference culprit microorganisms of IE and the oral microbiome using the eHOMD. Whilst the eHOMD is a reliable database detailing bacteria taxonomy that has been identified in the oral microbiome, these bacterial species could have also instigated disease through other routes and future prospective studies are required by a multidisciplinary team of dentists and doctors to clarify any association.
5 Conclusions
Infective endocarditis patients with a history of stroke, smoking, IVDU or dialysis were more likely to be infected with Staphylococcus aureus. CDE cases and patients who had previously undergone SAVR were most associated with Staphylococcus epidermidis. Infective endocarditis patients who were elderly were more likely to have been infected with Enterococcus faecalis. Patients who underwent TAVI instead of SAVR were more likely to have been infected with Enterococcus faecalis. From the study population, 68.11% of cases were caused by organisms that have been identified in the oral microbiome via the eHOMD. Nevertheless, additional research is needed to better understand the connection between the oral microbiome and infective endocarditis.
Data availability statement
The datasets for this article are not publicly available. Requests to access the datasets should be directed to the corresponding author.
Ethics statement
The study was approved as a service evaluation by King's College Hospital NHS Foundation Trust, Department of Cardiology (CV003-2022). It was conducted in accordance with the Helsinki Declaration of 1975, as revised in 2013.
Author contributions
AI: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. AY: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. JLF: Formal Analysis, Methodology, Writing – review & editing. RD: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Resources, Writing – review & editing. SW: Data curation, Investigation, Methodology, Resources, Writing – review & editing. JB: Data curation, Investigation, Methodology, Project administration, Resources, Writing – review & editing. MG: Data curation, Methodology, Project administration, Resources, Writing – review & editing. HK: Conceptualization, Investigation, Methodology, Project administration, Resources, Writing – review & editing. AH: Investigation, Methodology, Writing – review & editing. JPH: Conceptualization, Investigation, Methodology, Writing – review & editing. PC: Investigation, Methodology, Writing – review & editing, Formal Analysis. MS: Investigation, Methodology, Writing – review & editing. LN: Methodology, Writing – review & editing, Supervision. VS: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
This research line has significantly benefited from discussions with numerous clinicians and researchers. We are particularly grateful to R. Rajani (Guy's and St Thomas' Hospital), M. Arias (King's College Hospital), L. Sherif, D. Moyes and S. Shoaie (King's College London), L. Nanayakkara (Barts and the London School of Medicine and Dentistry), among others, for their invaluable insights and contributions to our work. We express our gratitude to each individual who has generously shared their expertise and knowledge, significantly enriching the depth and quality of our research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rajani R, Klein JL. Infective endocarditis: a contemporary update. Clin Med. (2020) 20:31–5. doi: 10.7861/clinmed.cme.20.1.1
2. Lockhart PB, Brennan MT, Thornhill M, Michalowicz BS, Noll J, Bahrani-Mougeot FK, et al. Poor oral hygiene as a risk factor for infective endocarditis-related bacteremia. J Am Dent Assoc. (2009) 140:1238–44. doi: 10.14219/jada.archive.2009.0046
3. Bumm CV, Folwaczny M. Infective endocarditis and oral health-a narrative review. Cardiovasc Diagn Ther. (2021) 11(6):1403–15. doi: 10.21037/cdt-20-908
4. Chen T, Yu W-H, Izard J, Baranova OV, Lakshmann A, Dewhirst FE. The human oral microbiome database: a web accessible resource for investigating oral microbe taxonomic and genomic information. Database J Biol Databases Curation. (2010) 2010:baq013. doi: 10.1093/database/baq013
5. Cahill TJ, Prendergast BD. Infective endocarditis. Lancet. (2016) 387:882–93. doi: 10.1016/S0140-6736(15)00067-7
6. Godfrey R, Curtis S, Schilling WH, James PR. Blood culture negative endocarditis in the modern era of 16S rRNA sequencing. Clin Med. (2020) 20:412–6. doi: 10.7861/clinmed.2019-0342
7. Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta J-P, Zotti FD, et al. 2015 ESC guidelines for the management of infective endocarditis: the task force for the management of infective endocarditis of the European society of cardiology (ESC) endorsed by: european association for cardio-thoracic surgery (EACTS), the European association of nuclear medicine (EANM). Eur Heart J. (2015) 36:3075–128. doi: 10.1093/eurheartj/ehv319
8. NICE (National Institute for Care Excellence). Overview Prophylaxis against infective endocarditis: antimicrobial prophylaxis against infective endocarditis in adults and children undergoing interventional procedures Guidance NICE (2008). Available online at: https://www.nice.org.uk/guidance/cg64 (accessed July 15, 2023).
9. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzche PC, Vandenbroucke JP, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. (2008) 61:344–9. doi: 10.1016/j.jclinepi.2007.11.008
10. Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG Jr, Ryan T, et al. Proposed modifications to the duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. (2000) 30:633–8. doi: 10.1086/313753
11. Kaya’, “Cansın Tulunay & Erol”, ‘Cetin. How to achieve infective endocarditis prophylaxis (2018). vol. 16 Available online at: https://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-16/vol16no33 (accessed July 15, 2023).
12. Verma D, Garg PK, Dubey AK. Insights into the human oral microbiome. Arch Microbiol. (2018) 200:525–40. doi: 10.1007/s00203-018-1505-3
13. Chambers ST, Murdoch D, Morris A, Holland D, Pappas P, Almena M, et al. HACEK infective endocarditis: characteristics and outcomes from a large, multi-national cohort. PLoS One. (2013) 8:e63181. doi: 10.1371/journal.pone.0063181
14. Babes EE, Lucuta DA, Petchesi CD, Zaha AA, Iyes C, Jurca AD, et al. Clinical features and outcome of infective endocarditis in a university hospital in Romania. Medicina. (2021) 57:158. doi: 10.3390/medicina57020158
15. Armiñanzas C, Fariñas-Alvarez C, Zarauza J, Muñoz P, Ramallo VG, Sellés MM, et al. Role of age and comorbidities in mortality of patients with infective endocarditis. Eur J Intern Med. (2019) 64:63–71. doi: 10.1016/j.ejim.2019.03.006
16. Elamragy AA, Meshaal MS, El-Kholy AA, Rizk HH. Gender differences in clinical features and complications of infective endocarditis: 11-year experience of a single institute in Egypt. Egypt Heart J. (2020) 72(1):5. doi: 10.1186/s43044-020-0039-6
17. Aksoy O, Meyer LT, Cabell CH, Kournay WM, Pappas PA, Sexton DJ. Gender differences in infective endocarditis: pre- and co-morbid conditions lead to different management and outcomes in female patients. Scand J Infect Dis. (2007) 39:101–7. doi: 10.1080/00365540600993285
18. Elkind MSV, Boehme AK, Smith CJ, Meisel A, Buckwalter MS. Infection as a stroke risk factor and determinant of outcome after stroke. Stroke. (2020) 51:3156–68. doi: 10.1161/STROKEAHA.120.030429
19. Mishra AK, Sahu KK, Baddam V, Sargent J. Stroke and infective endocarditis. QJM. (2020) 113:515–6. doi: 10.1093/qjmed/hcaa098
20. Lacoma A, Edwards AM, Young BC, Domínguez J, Prat C, Laabei M. Cigarette smoke exposure redirects Staphylococcus aureus to a virulence profile associated with persistent infection. Sci Rep. (2019) 9:10798. doi: 10.1038/s41598-019-47258-6
21. McDonald JR. Acute infective endocarditis. Infect Dis Clin North Am. (2009) 23:643–64. doi: 10.1016/j.idc.2009.04.013
22. Urien J-M, Camus C, Leclercq C, Dejoies L, Mabo P, Martins R, et al. The emergence of Staphylococcus aureus as the primary cause of cardiac device-related infective endocarditis. Infection. (2021) 49:999–1006. doi: 10.1007/s15010-021-01634-5
23. Yew HS, Murdoch DR. Global trends in infective endocarditis epidemiology. Curr Infect Dis Rep. (2012) 14:367–72. doi: 10.1007/s11908-012-0265-5
24. Douglas CW, Heath J, Hampton KK, Preston FE. Identity of viridans streptococci isolated from cases of infective endocarditis. J Med Microbiol. (1993) 39:179–82. doi: 10.1099/00222615-39-3-179
25. Chamat-Hedemand S, Dahl A, Østergaard L, Arpi M, Fosbøl E, Boel J, et al. Prevalence of infective endocarditis in streptococcal bloodstream infections is dependent on streptococcal Species. Circulation. (2020) 142:720–30. doi: 10.1161/CIRCULATIONAHA.120.046723
26. Kreth J, Zhang Y, Herzberg MC. Streptococcal antagonism in oral biofilms: Streptococcus sanguinis and Streptococcus gordonii interference with Streptococcus mutans. J Bacteriol. (2008) 190:4632–40. doi: 10.1128/JB.00276-08
27. Martini AM, Moricz BS, Ripperger AK, Tran PM, Sharp ME, Forsythe AN, et al. Association of novel Streptococcus sanguinis virulence factors with pathogenesis in a native valve infective endocarditis model. Front Microbiol. (2020) 11:10. doi: 10.3389/fmicb.2020.00010
28. Khan A, Aslam A, Satti KN, Ashiq S. Infective endocarditis post-transcatheter aortic valve implantation (TAVI), microbiological profile and clinical outcomes: a systematic review. PLoS One. (2020) 15:e0225077. doi: 10.1371/journal.pone.0225077
29. Dayer MJ, Jones S, Prendergast B, Baddour LM, Lockhart PB, Thornhill MH. Incidence of infective endocarditis in England, 2000–2013: a secular trend, interrupted time-series analysis. Lancet. (2015) 385:1219–28. doi: 10.1016/S0140-6736(14)62007-9
30. Thornhill MH, Gibson TB, Yoon F, Dayer MJ, Prendergast BD, Lockhart PB, et al. Antibiotic prophylaxis against infective endocarditis before invasive dental procedures. J Am Coll Cardiol. (2022) 80:1029–41. doi: 10.1016/j.jacc.2022.06.030
31. Bertolini M, Dongari-Bagtzoglou A. The relationship of Candida albicans with the oral bacterial microbiome in health and disease. Adv Exp Med Biol. (2019) 1197:69–78. doi: 10.1007/978-3-030-28524-1_6
32. Montelongo-Jauregui D, Lopez-Ribot JL. Candida interactions with the oral bacterial Microbiota. J Fungi. (2018) 4:122. doi: 10.3390/jof4040122
33. Olsen NT, De Backer O, Thyregod HGH, Vejlstrup N, Bundgaard H, Søndergaard L, et al. Prosthetic valve endocarditis after transcatheter aortic valve implantation. Circ Cardiovasc Interv. (2015) 8:e001939. doi: 10.1161/CIRCINTERVENTIONS.114.001939
34. Hunt CP. The emergence of enterococci as a cause of nosocomial infection. Br J Biomed Sci. (1998) 55:149–56.10198473
35. Howard C, Jillian L, Joshi M, Noshirwani A, Bashir M, Harky A. TAVI and the future of aortic valve replacement. J Card Surg. (2019) 34:1577–90. doi: 10.1111/jocs.14226
Keywords: endocarditis, oral microbiome, diagnostics, oral health, risk factor
Citation: Ismail A, Yogarajah A, Falconer JL, Dworakowski R, Watson S, Breeze J, Gunning M, Khan H, Hussain A, Howard JP, Cheong P, Shah M, Nibali L and Sousa V (2024) Insights into microorganisms, associated factors, and the oral microbiome in infective endocarditis patients. Front. Oral. Health 5:1270492. doi: 10.3389/froh.2024.1270492
Received: 31 July 2023; Accepted: 14 March 2024;
Published: 11 April 2024.
Edited by:
Georgios N. Belibasakis, Karolinska Institutet (KI), SwedenReviewed by:
Srinivas Sulugodu Ramachandra, Gulf Medical University, United Arab EmiratesSarhang Sarwat Gul, Sulaimani Polytechnic University, Iraq
© 2024 Ismail, Yogarajah, Falconer, Dworakowski, Watson, Breeze, Gunning, Khan, Hussain, Howard, Cheong, Shah, Nibali and Sousa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vanessa Sousa dmFuZXNzYS5zb3VzYUBrY2wuYWMudWs=
 Ayden Ismail
Ayden Ismail Amieth Yogarajah2
Amieth Yogarajah2 Joseph Luke Falconer
Joseph Luke Falconer Habib Khan
Habib Khan Luigi Nibali
Luigi Nibali Vanessa Sousa
Vanessa Sousa