- 1Community Health Center, Angara, India
- 2Department of Oral Medicine and Radiology, Manipal College of Dental Sciences Mangalore, Manipal Academy of Higher Education, Manipal, India
- 3Department of Prosthodontics and Crown & Bridge, Awadh Dental College & Hospital, Jamshedpur, India
Inflammation of the gingiva is one of the most common and routine findings in dental practice. These routine appearances of inflammatory gingivae can show peculiarity when associated with an underlying systemic condition or because of reactive, benign, or malignant pathologies. This case highlights minute clinical signs of the gingiva that deviate from the routine presentation and warrant further investigations. A 63-year-old woman presented with a chief complaint of severe pain in relation to the lower front teeth region for 1 month. Intraoral examination revealed a gingival lesion on the labial aspect of 41, 42, and 43, and an intraoral periapical radiograph showed mild bone loss. The lesion persisted despite oral prophylaxis, and a biopsy was advised. The final diagnosis was stage 1 gingival squamous cell carcinoma (GSCC). It is important to note that the non-descript presentation of GSCC in early stages often mimics benign traumatic or inflammatory lesions of the gingiva. Peculiar clinical features of GSCC of note include the lack of traditionally associated risk factors and localized red or ulcerative lesions with increased bleeding tendencies that do not respond to routine periodontal treatment within 2 weeks.
1. Introduction
Inflammation of the gingiva is one of the most common and routine findings in dental practice. Morphological changes associated with chronic gingivitis and periodontitis present as redness of the gingiva, enlargement, loss of stippling, bleeding on probing, etc. These routine appearances of inflammatory gingiva can show peculiarity when they are associated with an underlying systemic condition (pregnancy, diabetes, etc.) or when there are local pathologies that are reactive, benign, or malignant in nature (1). Dentists need to identify minute changes in clinical appearance that deviate from the routine inflammatory conditions or benign lesions to pursue further investigations. We present one such case of a common finding of a red gingival lesion with a concerning diagnostic outcome.
2. Case description
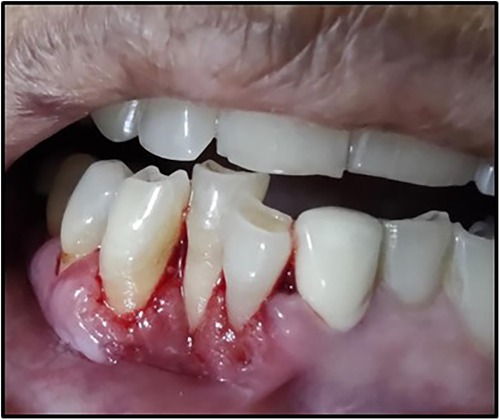
A 63-year-old woman presented with a chief complaint of severe pain in the lower front teeth for 1 month (Figure 1). The patient reported severe pain upon mechanical stimulation in the region and avoided biting food. She reported occasional bleeding gums when brushing that area, which had persisted for 10 days. The patient had no history of any oral abusive habits, such as tobacco consumption or use of toothpicks. There was no history of trauma reported. Her medical history was positive for hypothyroidism and hypertension, for which the patient was on medication that controlled the conditions. Her family history was positive with her brother having undergone treatment for throat cancer. The patient did not report any previous consultation or use of home remedies for the treatment of the lesion.
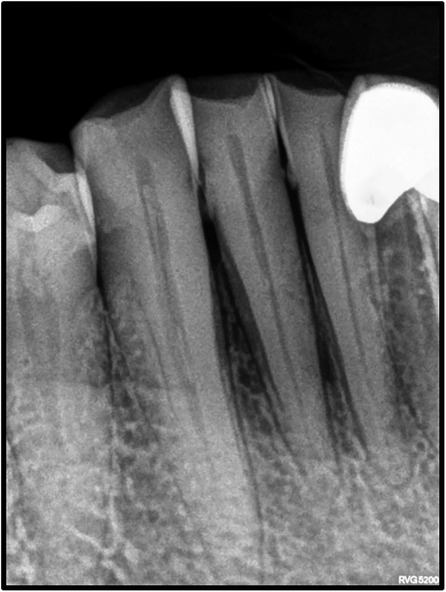
On intraoral examination, there was a gingival lesion on the labial aspects of 41, 42, and 43, extending from the mesial aspect of 41 to the distal aspect of 43. The lesion involved marginal, interdental, and attached gingiva. The gingival lesion appeared to be erythematous, and the surface was irregular with erosions. The margins of the lesion were well-defined with raised edges. It was soft in consistency and tender on palpation. On periodontal examination, there was the presence of bleeding upon probing, with no exudation. There was a moderate deposition of calculi. There was an absence of mobility i.r.t 41, 42, and 43. There was no tenderness on lateral and vertical percussions on teeth i.r.t 41, 42, and 43. The rest of the oral mucosa had no significant findings. On extraoral palpation, submandibular lymph nodes on the right side were palpable, firm, non-tender, and mobile. An intraoral periapical radiograph (Figure 2) was taken and revealed mild horizontal interdental bone loss in the regions 42 and 43, which suggested chronic periodontitis.
2.1. Differential diagnosis
Considering the gingival lesion with a tendency for bleeding and the presence of interdental bone loss, chronic periodontitis exacerbated by an underlying systemic condition such as diabetes mellitus could be considered as a diagnosis in this case (2). The other commonly associated systemic conditions are vitamin C deficiency (3) and hormonal dysfunction (estrogen and progesterone) (4), which can lead to a friable and inflamed gingiva. However, such a systemic condition would cause a more generalized gingival involvement rather than a localized lesion.
Localized lesions involving gingivae may occur because of chronic trauma. Additionally, toothbrush injury, the use of toothpicks, abrasive dentifrices, and rare self-inflicted injuries may cause gingival erosions and ulcerations (5). However, a lack of relevant history and the overall irregular surface texture of the lesions made this an unlikely possibility. Erythroplakia and oral squamous cell carcinoma (OSCC) can present as localized lesions on the gingiva. Erythroplakia is a potentially malignant disorder that appears as a well-defined red lesion that, unlike leukoplakia, can appear as a mucosal depression. The surface of the lesion may be smooth, velvety, or granular. Erythroplakia is usually not associated with habits and could not be ruled out in this case (6). Considering a positive family history of malignancy, associated pain, and increased bleeding tendency of the lesion, OSCC can also be considered in this case (1, 7). Furthermore, apart from erythroplakia and OSCC, granulomatous conditions, such as tuberculosis (8), sarcoidosis (9), Wegner's granulomatosis (10), and deep fungal infections (11), can present similarly and can be ruled out with further investigation.
2.2. Management
Initially, a round of oral prophylaxis was carried out, and she was prescribed 0.2% chlorhexidine mouthwash. Additionally, she was advised to have a complete blood count and a random blood glucose test and recall after 2 weeks. The blood report was within normal range.
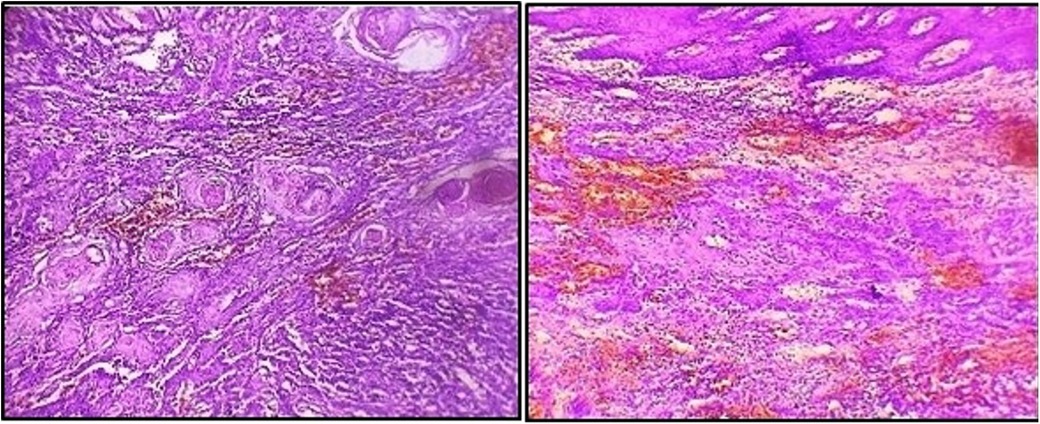
As the lesion persisted along with bleeding tendency during the follow-up visit, an incisional biopsy was performed. A histopathological report revealed a squamous epithelium overlying the connective tissue with tumors irregularly proliferating in the form of sheets and islands in the connective tissue stroma. Additionally, keratin pearls were evident within the tumor islands. Squamous tumor islands showed dysplastic features such as hyperchromatism, pleomorphism, an altered nuclear-cytoplasmic ratio, individual cell keratinization, and a few mitoses. The connective tissue stroma comprised bundles of collagen fibers with spindle-shaped fibroblasts, chronic inflammatory infiltrate, and endothelial-lined blood vessels with RBCs (Figure 3). Thus, the final diagnosis was well-differentiated OSCC.
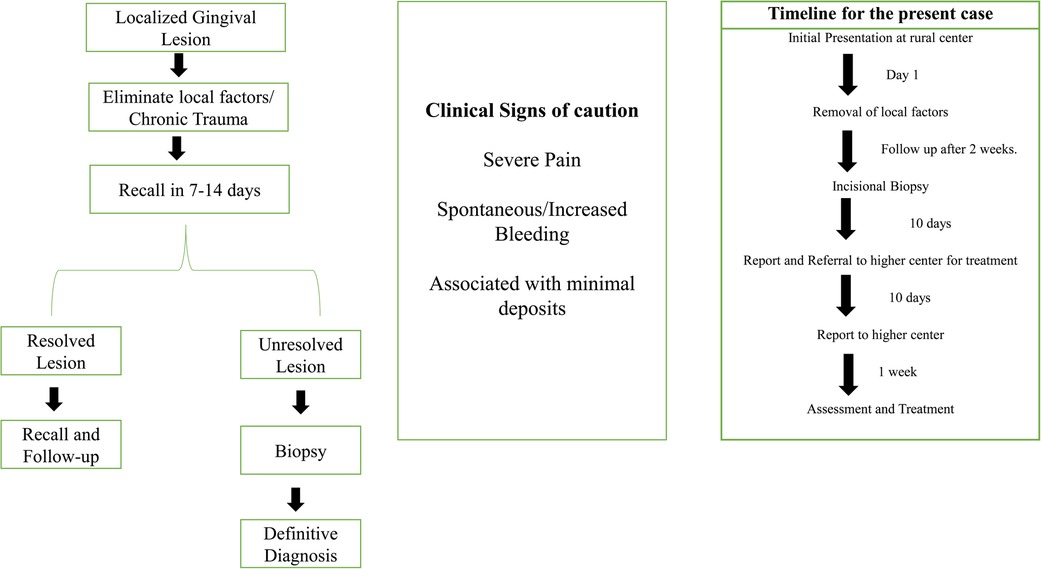
The patient was referred to a higher center for surgical intervention. Further investigations, such as contrast CT, were performed, and the patient was diagnosed with gingival squamous cell carcinoma (GSCC) Stage 1 (T1, N0, M X). The patient underwent surgery and radiotherapy and is currently in follow-up. Figure 4 shows the timeline from the episode through the administration of care.
3. Discussion and conclusions
Oral cancer is a broad term that includes various malignant conditions involving the oral tissues. It is the sixth most common cancer in the world and OSCC accounts for 90% of all oral cancers (12). South Asian countries, particularly India, show the highest prevalence of OSCC. This is largely related to the widespread use of tobacco, betel quid, and alcohol, which are established risk factors (13). Approximately 10% of all OSCCs are known to be caused by the malignant transformation of potentially malignant disorders (12, 14), many of which are habit associated. In recent times, there has been an increase in the prevalence of oral cancer among populations that have no habit history, especially among younger individuals (15). The majority of oral cancers, especially in countries such as India, are diagnosed in the advanced stages. The treatment for the advanced stages is aggressive, which results in severe morbidity and poor health-related quality of life. The outcomes of treatment are poor, as 5-year survival rates for advanced stages are approximately 20%. Thus, early diagnosis and prompt treatment are essential for favorable patient outcomes. This case highlights several atypical features that need to be considered to identify OSCC during the initial stages and ensure prompt treatment.
In the present case, the patient did not report any deleterious habits that are usually associated with oral cancer. There are several risk factors associated with non-habit-associated malignancies (Figure 4). Although these risk factors are not well established, unlike tobacco and alcohol, their role in oral cancer has been reported in a few studies or case reports. Several authors have shown an increased risk of OSCC in first-degree relatives of patients previously diagnosed with malignancy (16). Genetic changes that are linked to an increased incidence of OSCC include TP53 mutations and CDKN2A inactivation, the expression of certain proto-oncogenes, and frequent copy number alterations (17, 18). Consumption of hot spicy food, presence of sharp teeth, poor oral hygiene, systemic inflammation, and conditions causing prolonged irritation are considered risk factors for oral cancer. However, these factors lack sufficient scientific evidence to establish a causal relationship (19, 20). Specific microorganisms, such as Treponema pallidum (syphilis), Candida, and human papillomavirus (HPV), have been associated with an increased risk of malignancy (12, 19). HPV has been isolated in approximately 23.5% of oral cancers (20) and sexual practices have been considered contributory to the higher incidence of HPV (21). Environmental carcinogens, such as ultraviolet rays, can contribute as risk factors. Dietary factors, such as iron deficiency and a poor diet with a lack of micronutrients and antioxidants, have been implicated in the pathogenesis of oral cancer (22). It should be noted that the causal relationship with oral cancer related to the abovementioned factors needs more clinical and experimental evidence. Unusual oral lesions coupled with the presence of risk factors should warrant further investigation. In the present case, the patient's immediate relative had been diagnosed with throat cancer; however, genetic testing was not feasible.
The most common sites of OSCC are the tongue and the floor of the mouth, followed by the soft palate, gingiva, and buccal mucosa. OSCC of the gingiva is normally painless and its incidence ranges from 2 to 27% (23, 24). Gender predilection is ambiguous with respect to GSCC, with some studies reporting female predilection (25) and others reporting male predilection (26). Mandibular involvement is usually more common than the maxilla. Additionally, GCSS is generally associated with non-traditional risk factors and is found in non-smokers and non-drinkers (1, 27). It is an insidious disease that is present without the typical clinical features attributable to malignancy and is frequently misdiagnosed as a periodontal inflammatory lesion. In such cases, presentations that could indicate malignancy include erythema with non-healing ulcers and red lesions with a granular surface texture that bleed profusely (1). It usually begins with the keratinized mucosa, quickly progressing to involve the underlying bone structure with ensuing tooth mobility (28). As the thickness of the attached gingiva is 2–3 mm, gingival carcinomas tend to involve the bone at early stages and can spread easily (29, 30). Dental extractions are usually carried out in cases that are misdiagnosed as periodontitis. Dental extractions lead to bone invasion and further spread, worsening the prognosis of the patient (31). It is essential to evaluate bony involvement in GSCC cases. Radiographic features that help distinguish malignant involvement as opposed to bone loss related to periodontitis include irregular bone loss with an associated moth-eaten appearance, non-uniform widening of the PDL space, and the absence of sclerosis of trabeculae that is associated with bone loss secondary to periodontitis. Thus, clinical and radiographic features should be carefully examined to diagnose gingival carcinoma at an early stage. This case had an insidious clinical presentation of redness of the gingiva that could be mistaken for a benign lesion. Additionally, there was an absence of typical malignant changes on the radiograph, with bone loss and accompanying sclerosis indicating chronic periodontitis. Furthermore, the lesion was associated with severe pain, which is unusual for a benign lesion. Although early stages of OSCC may be asymptomatic, pain is often an indicator of the transition of a potentially malignant lesion into a cancerous lesion and often the first presenting symptom in oral cancers. In this case, the severe pain and a lack of response of the lesion to local therapy was the main reason for suspicion and warranted further investigation (32, 33). A similar case of GSCC was reported by Brooks et al., in which ulcerations confined to the palatal marginal gingiva confined to the maxillary molars associated with profuse bleeding were observed.
A delay in the diagnosis of oral cancer can significantly impact the health care outcomes for the patient. Survival rates of patients negatively correlate with the stage of diagnosis. Treatment for advanced stages is aggressive and is related to a poor quality of life for survivors. Multiple factors, including patient-related delays, professional delays, and healthcare system-related delays, contribute to diagnostic delays (34). Patients may delay seeking medical care due to a lack of awareness of symptoms, the nature of the disease process, or phobias and anxiety related to cancer diagnosis. Additionally, socioeconomic constraints and cultural beliefs may contribute to a delay in diagnosis. There is poor public awareness related to oral health and the work scope of dental practitioners. Thus, patients do not opt for regular dental check-ups. Infrequent or lack of practice of regular dental check-ups excessively contributes to delays in diagnosis (35, 36). In our case, unusual pain and bleeding gums prompted the patient to seek medical care. A familial history of malignancy can also contribute to patient awareness of symptoms associated with malignancy.
Once the patient reports to a health care professional, it is the responsibility of the professional to aid in early diagnosis and treatment. Professional delays are known to occur due to a lack of awareness of oral cancer symptoms and misdiagnosis. Here, the dentist may not be the first point of contact of the patient, and patients may likely present initially to a non-dental health care professional. Thus, it is essential that all health care professionals and patients are aware of the signs and symptoms related to oral cancer (36). This is particularly true of GSCC with its innocuous presentation, as observed in the present case. Additionally, understaffing and resource constraints in the healthcare system can contribute to delays in the diagnosis and treatment of cancers. There is a wide disparity in the distribution of health care professionals between rural and urban areas. The at-risk population largely resides in rural areas with socioeconomic constraints. Our patient also lived in a rural area and reported to the community health center to seek a consultation with a dentist due to tooth-related pain. The presence of an oral medicine specialist at the rural hospital significantly contributed to early diagnosis in this case. Considering these factors, it is essential to raise awareness, train health care professionals, and strengthen healthcare systems to aid in the early diagnosis of oral cancer.
Currently, there are no screening protocols for individuals who do not exhibit traditional risk factors but may still be at risk for GSCC; routine oral examination is key to early diagnosis for such cases. Additionally, toluidine blue staining (TBS), oral cytology (OC), and light-based detection (LBD) devices, such as Velscope, ViziLite plus (37), and ultrasound (38), are known to aid the early diagnosis of oral cancer. However, the cost of implementing these chair-side investigations has hindered their widespread use. Indicators of poor prognosis in GSCC are the presence and extent of mandibular involvement and the advanced stage of diagnosis. The incidence of cervical lymph node metastasis associated with GSCC is higher in relation to the lesion on the posterior of the mandible (39). These points must be considered while evaluating the patient with GSCC.
This case report highlights the non-descript presentation of GSCC at early stages, which often mimics benign traumatic or inflammatory lesions of the gingiva. Peculiar clinical features of GSCC of note include the lack of traditional risk factors and localized red or ulcerative lesions with increased bleeding tendencies that do not respond to routine periodontal treatment of oral prophylaxis within 2 weeks. The affinity of GSCC to invade the bone and spread at an early stage makes early diagnosis and prompt treatment critical for improving therapeutic outcomes for the patient.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SK: Conceptualization, Data curation, Investigation, Resources, Validation, Writing – original draft. NS: Conceptualization, Methodology, Writing – original draft. AS: Validation, Visualization, Writing – original draft. JA: Conceptualization, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing. PB: Data curation, Investigation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We are grateful to the patient and her family for their permission to use clinical data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Brooks JK, Kleinman JW, Lubek JE, Price JB, Ghita I, Scurnick SA, et al. Gingival squamous cell carcinoma: an unexpected clinical presentation. Quintessence Int. (2019) 50:50–7. doi: 10.3290/j.qi.a41334
2. Casanova L, Hughes FJ, Preshaw PM. Diabetes and periodontal disease. BDJ Team. (2015) 1:15007. doi: 10.1038/bdjteam.2015.7
3. Kakade A, Raut MS, Santosh A, Nagar S, Bansal S, Desai RS. Gingival enlargement caused by vitamin c deficiency (scurvy) in a boy. J Dent Child. (2018) 85:40–2.
4. Boyapati R, Cherukuri SA, Bodduru R, Kiranmaye A. Influence of female sex hormones in different stages of women on periodontium. J Midlife Health. (2021) 12:263–6. doi: 10.4103/jmh.jmh_142_21
5. Aljadeff L, Fisher CA, Wolf SL, Byrd KM, Curtis W, Ward BB, et al. Red exophytic mass of the maxillary anterior gingiva. Oral Surg Oral Med Oral Pathol Oral Radiol. (2016) 122:379–84. doi: 10.1016/j.oooo.2015.11.007
6. Reichart PA, Philipsen HP. Oral erythroplakia—a review. Oral Oncol. (2005) 41:551–61. doi: 10.1016/j.oraloncology.2004.12.003
7. Tosios KI, Kalogirou EM, Nikitakis NG. A solitary, red, papillary–verrucous lesion on the mandibular alveolar mucosa. Oral Surg Oral Med Oral Pathol Oral Radiol. (2021) 131:41–4. doi: 10.1016/j.oooo.2019.11.004
8. Jain S, Vipin B, Khurana P. Gingival tuberculosis. J Indian Soc Periodontol. (2009) 13:106–8. doi: 10.4103/0972-124X.55836
9. Kadiwala SA, Dixit MB. Gingival enlargement unveiling sarcoidosis: report of a rare case. Contemp Clin Dent. (2013) 4:551. doi: 10.4103/0976-237X.123087
10. Vineetha R, Pai KM, Vidyasagar S. An unusual red lesion of the gingiva: differential diagnosis and management. J Cant Dent Assoc. (2011) 77:b77.
11. Alawi F. An update on granulomatous diseases of the oral tissues. Dent Clin. (2013) 57:657–71. doi: 10.1016/j.cden.2013.07.004
12. Watters AL, Hansen HJ, Patel AA, Epstein J. Head and neck cancer. In: Glick M, Greenberg MS, Lockhart PB, Challacombe SJ, editors. Burket’s oral medicine. USA: PMPH (2021). p. 212.
13. Sankaranarayanan R, Duffy SW, Padmakumary G, Day NE, Nair MK. Risk factors for cancer of the buccal and labial mucosa in Kerala, Southern India. J Epidemiol Community Health. (1990) 44:286–92. doi: 10.1136/jech.44.4.286
14. Warnakulasuriya S, Kujan O, Aguirre-Urizar JM, Bagan JV, González-Moles MÁ, Kerr AR, et al. Oral potentially malignant disorders: a consensus report from an international seminar on nomenclature and classification, convened by the WHO collaborating centre for oral cancer. Oral Dis. (2021) 27:1862–80. doi: 10.1111/odi.13704
15. Sujir N, Ahmed J, Pai K, Denny C, Shenoy N. Challenges in early diagnosis of oral cancer: cases series. Acta Stomatol Croat. (2019) 53:174–80. doi: 10.15644/asc53/2/10
16. Fantozzi PJ, Bavarian R, Tamayo I, Bind MA, Woo SB, Villa A. The role of family history of cancer in oral cavity cancer. Head Face Med. (2021) 17:1–6. doi: 10.1186/s13005-021-00298-8
17. Scully C, Field JK, Tanzawa H. Genetic aberrations in oral or head and neck squamous cell carcinoma (SCCHN): 1. Carcinogen metabolism, DNA repair and cell cycle control. Oral Oncol. (2000) 36:256–63. doi: 10.1016/S1368-8375(00)00007-5
18. Scully C, Field JK, Tanzawa H. Genetic aberrations in oral or head and neck squamous cell carcinoma 2: chromosomal aberrations. Oral Oncol. (2000) 36:311–27. doi: 10.1016/S1368-8375(00)00021-X
19. Shetty SS, Vishal Rao US. 8 “S” in oral cancer. Oral Oncol. (2019) 88:27–8. doi: 10.1016/j.oraloncology.2018.11.016
20. Ram H, Sarkar J, Kumar H, Konwar R, Bhatt ML, Mohammad S. Oral cancer: risk factors and molecular pathogenesis. J Maxillofac Oral Surg. (2011) 10:132–7. doi: 10.1007/s12663-011-0195-z
21. Shetty SS, Shetty P. Oral cancer: link with early coitus. Br Dent J. (2016) 220(6):279–80. doi: 10.1038/sj.bdj.2016.206
22. Rodríguez-Molinero J, Migueláñez-Medrán BD, Puente-Gutiérrez C, Delgado-Somolinos E, Martín Carreras-Presas C, Fernández-Farhall J, et al. Association between oral cancer and diet: an update. Nutrients. (2021) 13:1299. doi: 10.3390/nu13041299
23. Makridis SD, Mellado JR, Freedman AL, Salkin LM, Stein MD, Leal K, et al. Squamous cell carcinoma of gingiva and edentulous alveolar ridge: a clinicopathologic study. Int J Periodontics Restorative Dent. (1998) 18:292–8.9728112
24. Bezerra NV, Leite KL, de Medeiros MM, Martins ML, Cardoso AM, Alves PM, et al. Impact of the anatomical location, alcoholism and smoking on the prevalence of advanced oral cancer in Brazil. Med Oral Patol Oral Cir Bucal. (2018) 23:295–301. doi: 10.4317/medoral.22318
25. Kimura Y, Sumi M, Sumi T, Ariji Y, Ariji E, Nakamura T. Deep extension from carcinoma arising from the gingiva: CT and MR imaging features. AJNR Am J Neuroradiol. (2002) 23:468–72.11901020
26. Gomez D, Faucher A, Picot V, Siberchicot F, Renaud-Salis JL, Bussières E, et al. Outcome of squamous cell carcinoma of the gingiva: a follow-up study of 83 cases. J Craniomaxillofac Surg. (2000) 28:331–5. doi: 10.1054/jcms.2000.0177
27. Dahlstrom KR, Little JA, Zafereo ME, Lung M, Wei Q, Sturgis EM. Squamous cell carcinoma of the head and neck in never smoker-never drinkers: a descriptive epidemiologic study. Head Neck. (2008) 30:75–84. doi: 10.1002/hed.20664
28. Lubek J, El-Hakim M, Salama AR, Liu X, Ord RA. Gingival carcinoma: retrospective analysis of 72 patients and indications for elective neck dissection. Br J Oral and Maxillofac Surg. (2011) 49:182–5. doi: 10.1016/j.bjoms.2010.04.005
29. Cabral LA, de Carvalho LF, Salgado JA, Brandão AA, Almeida JD. Gingival squamous cell carcinoma: a case report. J Oral Maxillofac Res. (2010) 1:e6. doi: 10.5037/jomr.2010.1306
30. Seoane J, Varela-Centelles PI, Walsh TF, Lopez-Cedrun JL, Vazquez I. Gingival squamous cell carcinoma: diagnostic delay or rapid invasion? J Periodontol. (2006) 77:1229–33. doi: 10.1902/jop.2006.050408
31. Yamagata K, Ito H, Onizawa K, Yamatoji M, Yanagawa T, Bukawa H. Prognosis for gingival carcinomas with a delayed diagnosis after dental extraction. J Oral Maxillofac Surg. (2013) 71:2189–94. doi: 10.1016/j.joms.2013.05.008
32. Schmidt BL. What pain tells us about cancer. Pain. (2015) 156(Suppl 1):S32–34. doi: 10.1097/j.pain.0000000000000099
33. Lam DK, Schmidt BL. Orofacial pain onset predicts transition to head and neck cancer. Pain. (2011) 152:1206–9. doi: 10.1016/j.pain.2011.02.009
34. Stefanuto P, Doucet JC, Robertson C. Delays in treatment of oral cancer: a review of the current literature. Oral Surg Oral Med Oral Pathol Oral Radiol. (2014) 117:424–9. doi: 10.1016/j.oooo.2013.12.407
35. Banavali SD. Delivery of cancer care in rural India: experiences of establishing a rural comprehensive cancer care facility. Indian J Med Paediatr Oncol. (2015) 36:128–31. doi: 10.4103/0971-5851.158848
36. Sankaranarayanan R, Ramadas K, Thomas G, Muwonge R, Thara S, Mathew B, et al., Trivandrum Oral Cancer Screening Study Group. Effect of screening on oral cancer mortality in Kerala, India: a cluster-randomised controlled trial. Lancet. (2005) 365:1927–33. doi: 10.1016/S0140-6736(05)66658-5
37. International Agency for Research on Cancer. A digital manual for the early diagnosis of oral neoplasia. Ramadas K, Lucas E, Thomas G, et al., editors. Lyon France: World Health Organization (2008). Available at: https://screening.iarc.fr/atlasoral.php (Accessed October 31, 2023).
38. Minami Y, Okada Y, Ogura I. Intraoral ultrasonography for gingival squamous cell carcinoma: tumor thickness and bone invasion. Oral Sci Int. (2023) 20:104–8. doi: 10.1002/osi2.1153
Keywords: gingival carcinoma, red lesion, early diagnosis, case report, oral cancer
Citation: Kumar S, Sujir N, Saha A, Ahmed J and Bhushan P (2023) Unusual localized gingival redness: a case report. Front. Oral. Health 4:1292332. doi: 10.3389/froh.2023.1292332
Received: 11 September 2023; Accepted: 9 November 2023;
Published: 28 November 2023.
Edited by:
Ibrahim O. Bello, King Saud University, Saudi ArabiaReviewed by:
Karthikeyan Ramalingam, Saveetha University, IndiaRenato Assis Machado, Universidade Estadual de Campinas, Brazil
© 2023 Kumar, Sujir, Saha, Ahmed and Bhushan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nanditha Sujir bmFuZGl0aGEuc3VqaXJAbWFuaXBhbC5lZHU=
Abbreviations GSCC, gingival squamous cell carcinoma; IOPA, intraoral periapical radiograph; OSCC, oral squamous cell carcinoma.
 Swati Kumar1
Swati Kumar1 Nanditha Sujir
Nanditha Sujir Junaid Ahmed
Junaid Ahmed