- Department of Periodontics and Dental Hygiene, School of Dentistry, University of Texas Health Science Center at Houston, Houston, TX, United States
Periodontitis is a chronic inflammatory disease induced by dysbiotic dental biofilms. Management of periodontitis is primarily anti-bacterial via mechanical removal of bacterial biofilm. The successful resolution requires wound healing and tissue regeneration, which are not always achieved with these traditional methods. The discovery of specialized pro-resolving mediators (SPMs), a class of lipid mediators that induce the resolution of inflammation and promote local tissue homeostasis, creates another option for the treatment of periodontitis and other diseases of chronic inflammation. In this mini-review, we discuss the host-modulatory effects of SPMs on periodontal tissues and changes in the taxonomic composition of the gut and oral microbiome in the presence of SPMs and SPM precursor lipids. Further research into the relationship between host SPM production and microbiome-SPM modification has the potential to unveil new diagnostic markers of inflammation and wound healing. Expanding this field may drive the discovery of microbial-derived bioactive therapeutics to modulate immune responses.
Epidemiology of periodontitis
Oral health is an essential public health issue. Oral diseases cause pain, discomfort, masticatory malfunction, esthetically unacceptable appearance, and overall lower quality of life. Oral health is also highly associated with systemic health. Uncontrolled oral diseases can worsen systemic conditions. According to the 2022 World Health Organization (WHO) Global Oral Health Status Report, oral diseases are estimated to affect approximately 3.5 billion people worldwide, with 3 out of 4 affected people living in middle-income countries. The three most prevalent oral conditions are untreated caries, severe periodontitis, and extensive tooth loss (1). Among the prevalent oral diseases, periodontitis has one of the most complicated mechanisms of pathogenesis. Many researchers are studying its pathogenesis and developing novel diagnostic methods and therapy.
Periodontitis is a biofilm-induced chronic inflammatory disease characterized by gingival inflammation and alveolar bone loss around teeth. Untreated periodontitis leads to gingival bleeding, suppuration, pain, and progressive bone loss, eventually causing tooth loss. According to the 2009–2014 National Health and Nutrition Examination Survey (NHANES), in the United States, around 61 million adults over 30 years old have periodontitis (42.2%), with 7.8% having severe periodontitis (2). The global cost of lost productivity from severe periodontitis alone was $39 billion yearly, based on 2015 data (1). A recent report demonstrated that periodontal disease generally resulted in indirect and direct costs of $154.06 billion in the United States and €158.64 billion in Europe in 2018 (3).
Pathogenesis of periodontitis
The disproportionate inflammatory host response and microbiota dysbiosis are the two major etiologic factors in the pathogenesis of periodontitis (4, 5). These two factors do not always exist simultaneously, but they influence each other. The coexistence of these two factors results in the development of periodontitis. A pathogenic biofilm is a prerequisite for periodontitis initiation. Several bacterial species, such as the core red complex species Porphyromonas gingivalis (P. gingivalis), Tannerella forsythia (T.forsythia), and Treponema denticola (T.denticola), are significantly associated with the severity and progression of periodontitis (6). Their virulence factors causing periodontal tissue destruction are also identified. However, these species are usually present in small amounts. The concept of keystone pathogens was proposed (7). The keystone pathogens, such as P. gingivalis, have a relatively small proportion of the overall microbial biomass but can dysregulate immune responses and induce microbiota dysbiosis, accelerating periodontal inflammation. This concept explains that the presence of specific species can be critical for the progression of periodontitis. While technologies advance, more and more species are identified as being associated with periodontitis and promoting disease progression. The antagonistic and synergistic interactions between multiple species as a network will be worth studying to elucidate the complexity of oral microbiota (8).
Although the biofilm is required to initiate periodontitis, the destruction of periodontal tissues in periodontitis are mainly caused by inflammation induced by bacteria (9). Persistent inflammation and tissue destruction create an environment leading to dysbiotic microbiota (5, 10). Local inflammation induced by periodontitis can further influence systemic immune responses. The status of periodontal health and systemic health condition are mutually affected (11). Studies demonstrated that periodontitis is associated with increased systemic levels of cytokines, such as interleukin-1α (12), interleukin-1β (12–14), interleukin-6 (IL-6) (12, 15, 16), C-reactive protein (CRP) (16, 17), interferon-γ and tumor necrosis factor-α (12, 13) in serum or plasma. Periodontal therapy can reduce systemic levels of inflammatory mediators, such as CRP (18, 19) and IL-6 (16, 20). Systemic level changes of cytokines in periodontitis patients indicate that periodontitis may play a role in the etiologic mechanisms of systemic inflammatory diseases, and inflammation drives the pathogenesis of periodontitis.
Treating periodontitis through host-modulation
For decades, the standard treatment has been biofilm removal via mechanical debridement, such as scaling and root planing (SRP). However, this approach has limited effects in patients with aggressive forms of periodontitis associated with dysregulated immune response. To address this issue, host-modulation has been considered. Host-modulation therapy aims to modify the host response by reducing those damaging aspects of the inflammatory response leading to tissue destruction (21). Generally, there are two categories of host-modulation therapy: (1) modulating the host's inflammatory response by inhibition or resolution; (2) modulating the host's pathologic collagenolytic response in periodontal tissues (22). The first category includes the use of anti-inflammatory agents, such as non-steroidal anti-inflammatory drugs (NSAIDs), in addition to conventional periodontal treatment (e.g., SRP). The second category includes the use of subantimicrobial-dose doxycycline, which reduces collagenase activity to inhibit disease progression.
Restoration of a proper host immune response is crucial in treating periodontitis, but no effective host-modulatory approach currently exists. NSAIDs have been used to treat periodontitis, with positive clinical outcomes (23, 24). However, there are significant concerns regarding the long-term use of NSAIDs due to their adverse effects on the renal, cardiovascular, gastrointestinal, and hepatic systems (25). The clinical effects of anti-cytokine therapies used to treat rheumatoid arthritis and other immune diseases have also been investigated. Although these therapies can control inflammation, their clinical effects on periodontitis patients without immune disorders are not clear, and the adverse effects of systemic use, such as the increased risk of infection and malignancy, are concerning (21, 26). Although, statistically, subantimicrobial-dose doxycycline has beneficial clinical effects, but the absolute changes in pocket depth and clinical attachment level are limited. Also, the compliance with the long-term use can be challenging for patients (27). Host-modulation therapy for periodontitis is a promising approach, but more studies are required to make it practical and effective.
Specialized pro-resolving mediators promote the resolution of inflammation and tissue regeneration
Anti-inflammation has been the central concept of treating periodontitis for years. Another option was presented with the discovery of specialized pro-resolving mediators (SPMs), a class of lipid mediators derived from omega-3 or omega-6 polyunsaturated fatty acids (PUFAs) that induce the resolution of inflammation and promote local tissue homeostasis (28). The resolution of inflammation is a proactive process induced by SPMs, including lipoxins, resolvins, protectins, and maresins. These SPMs are produced by enzymatic activation of membrane phospholipids and bind to specific G protein-coupled receptors on a variety of cells to regulate the immune response. In the resolution phase of inflammation, there are decreased infiltration of neutrophils, reduced levels of pro-inflammatory cytokines and lipid mediators, and increased recruitment of resolving macrophages, such as M2 macrophages, that clear the lesion by efferocytosis without immune suppression (29, 30). SPMs also stimulate the phagocytosis and killing of microbes (31). SPMs possess dual anti-inflammatory and pro-resolution properties.
Initial inflammation is required to defend against bacterial challenge. Neutrophils and macrophages play important roles in innate immunity. However, if acute inflammation is not properly resolved (e.g., excessive neutrophil infiltration and pro-inflammatory cytokine production), it leads to fibrosis, decreased apoptosis, impaired phagocytosis, and cellular senescence, resulting in chronic inflammation and tissue damage (32). Resolution of inflammation not only mitigates inflammation but also promotes tissue healing, regeneration, and reduction of pain. Due to the aforementioned characteristics, it is feasible to treat inflammatory diseases with SPMs. SPMs can control inflammation in many preclinical inflammatory-disease models, such as peritonitis (33), inflammatory bowel disease (34), diabetes (35), and periodontitis (36).
Recently, some newly identified conjugates of SPMs in tissue regeneration (CTRs) were identified, and these conjugates can promote tissue regeneration (30, 37). The novel cysteinyl-resolvin significantly accelerates tissue regeneration with planaria and inhibits human granuloma formation (37). A recent study demonstrated that porcine periodontal ligament stem cells (pPDLSCs) can synthesize cysteinyl-containing SPMs (cys-SPMs), specifically, maresin 3 conjugates in tissue regeneration (MCTR3), and pretreatment of pPDLSCs with MCTR3 reduced the production of acute and chronic proinflammatory cytokines and chemokines in an inflammatory environment (38).
Specialized pro-resolving mediators in periodontitis
In periodontitis, preclinical studies have demonstrated that SPMs prevent and treat experimental periodontitis (36, 39). Topical application of RvE1 can prevent bone loss, regenerate the lost bone, change gene expression patterns in gingiva, and result in shifts of the oral microbiota (39, 40) and immune cellular components (41) in animals affected with experimental periodontitis. SPMs, including resolvins, lipoxins, and maresins, are now studied to understand their impact on periodontal inflammation and tissue healing. In the in vitro inflammatory condition, resolvin D1 (RvD1) can promote periodontal ligament fibroblasts (PDLF) proliferation (42, 43), reduce proinflammatory cytokine productions in gingival fibroblasts (44), and maresin-1 (MaR1) and resolvin E1 (RvE1) restore the regenerative properties of human PDLSCs (45, 46).
The impact of SPMs on periodontal pathogens was also investigated. An in vitro study showed that MaR1 enhanced intracellular antimicrobial reactive oxygen species production and restored impaired phagocytosis of P.gingivalis and Aggregatibacter actinomycetemcomitans (A.actinomycetemcomitans) in macrophages of localized aggressive periodontitis patients (47). This finding can be one of the mechanisms of oral microbiota shifts induced by SPMs.
For clinical applications, an effective vehicle to deliver SPMs is important to maintain high concentration and prevent lipid peroxidation. Membrane-shed vesicles, termed microparticles, have been used to deliver SPMs to treat experimental periodontitis (48, 49). Compared to SPM alone, SPM delivered in microparticles can increase treatment efficacy by targeting tissues without dilution or inactivation of the mediator. In a clinical trial, a formulated-mouthwash with methyl ester-benzo-lipoxin A4, one type of SPM, has been approved safe and could reduce local inflammation and increase the abundance of pro-resolution molecules in serum of human participants (46). Using SPMs to treat periodontitis in the clinic has the potential but more clinical studies are required.
Lipid profiles and inflammatory diseases
Humans cannot efficiently produce the precursors for SPMs de novo. Instead, SPMs are derived from the ingestion of dietary omega-3 PUFA: alpha-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) (Figure 1) (50). ALA, EPA, and DHA are incorporated as phospholipids into cellular membranes throughout the body, and SPMs are enzymatically released from these lipids to resolve inflammation (50). SPMs have been identified in many human samples, including milk (51), serum, lymphoid tissue (52), saliva, and gingival crevicular fluid (53, 54). These SPM levels have been shown to be involved in regulating the resolution of inflammation throughout the body, including the inflammatory status of mammary glands (51), the stability of atherosclerotic plaques (55), the severity of tuberculous meningitis (56), and the disease status of periodontitis (53, 54, 57). The action of omega-3 PUFA is in direct competition with dietary omega-6 PUFA, linoleic acid, which is the precursor to arachidonic acid (ARA), and the proinflammatory lipid mediators, prostaglandins and leukotrienes (58, 59). The relative ratios of dietary omega-3 and omega-6 PUFA are believed to contribute to homeostasis in the initiation and resolution of inflammation throughout the body (60, 61).
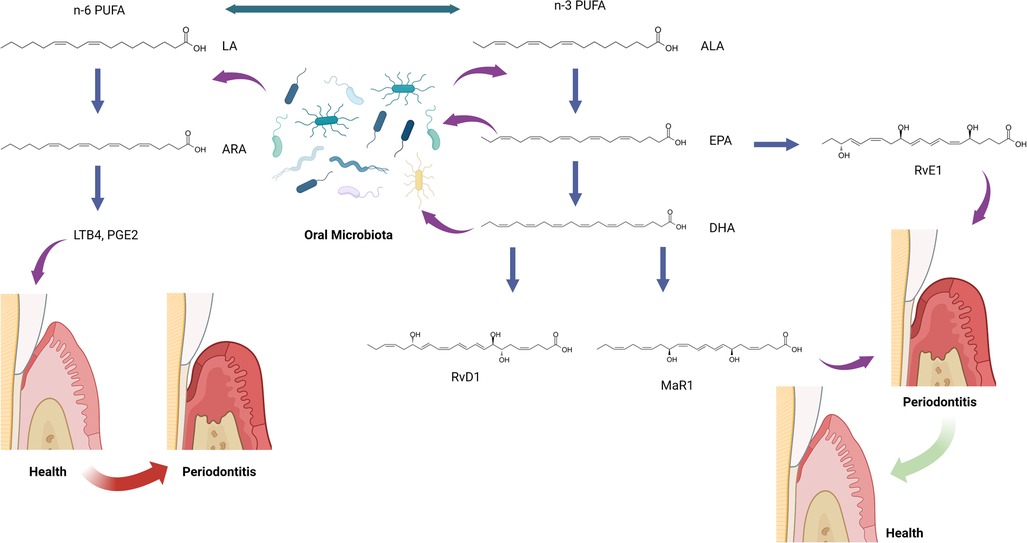
Figure 1. The hypothesis of oral microbiota and specialized pro-resolving mediator (SPM) interactions. ALA, alpha-linolenic acid; ARA, arachidonic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; LA, linoleic acid; LTB4, leukotriene B4; MaR1, maresin-1; n-3 PUFA, omega-3 polyunsaturated fatty acid; n-6 PUFA, omega-6 polyunsaturated fatty acid; PGE2, prostaglandin E2; RvD1, resolvin D1; RvE1, resolvin E1. This figure was created with BioRender.com.
It is important to note that high doses of dietary PUFAs does not guarantee high production of SPMs. Actions of lipoxygenases are required to produce SPMs from PUFAs. Also, SPM corresponding receptors must be present on cells to bind SPMs inducing the resolution of inflammation.
Effects of dietary lipids on Microbiota
As dietary compounds, omega-3 PUFAs are directly exposed to the microbiome of the oral cavity and digestive tract, and multiple studies in animals and humans describe significant changes in gut inflammation and the composition of the gut microbiome based on dietary PUFA quality and quantity (58, 62, 63). For example, in a comparison of mice fed with lard vs. fish oil (high in omega-3 PUFAs) as the major dietary fat, significant increases in Lactobacilli, Bifidobacterium, and Akkermansia muciniphila were detected in the fish oil diet, and transfer of A. muciniphila to the lard-fed mice could partially reduce the diet-induced intestinal inflammation and improve mucosal barrier function (64). The increase in Bifidobacterium in response to dietary EPA and DHA was confirmed in another mouse study, which also demonstrated that a diet high in omega-3 PUFAs from flax-seed and fish oil increases the bacterial diversity of the mouse gut (65). The same study determined that the flaxseed/fish oil diet increased the levels of ALA, EPA, and DHA in multiple tissues with a parallel decrease in ARA, indicating that the lipid membranes of host tissues are the ultimate destination for dietary omega-3 PUFAs. Lipids play important roles in microbial physiology; as structural components of the cell membrane, as energy storage modules, and for cell signaling and regulation of cellular activities (66). There is considerable overlap in lipid metabolic activities between eukaryotes and prokaryotes, providing opportunities for inter-kingdom cross-talk via lipid modification (58). In the gut microbiome, dietary lipids can be biotransformed by bacterial enzymes, resulting in downstream effects on host lipid physiology (58). Bifidobacterium and Lactobacilli are associated with reduced intestinal inflammation and diets high in omega-3 PUFAs. These bacteria have been shown to produce enzymes that modify omega-6 linoleic acid to a conjugated linoleic acid (CLA) that has anti-inflammatory effects and blocks the production of ARA-derived lipids (67). These same microorganisms produce a conjugated omega-3 alpha-linolenic acid (CLNA) with significant antioxidant effects (68).
These commensal bacteria utilize host lipids as an energy source and metabolize some lipids to new isoforms to enhance mucosal barrier function. Conversely, gut bacteria have also been associated with detrimental changes to dietary lipids, as demonstrated by studies of the microbiome associated with irritable bowel syndrome (69, 70). These studies begin to shed light on the potential circular relationship between dietary lipids, changes in the local host environment, and selection for bacteria that metabolize those same nutrients and benefit from changes to the environment (71). It is important to note that gut bacteria primarily influence the production of pro-inflammatory or pro-resolution lipids at the level of precursor molecules by manipulation of linoleic and alpha-linolenic acids. Identification of novel bioactive lipid molecules produced by gut bacteria has the potential to create new therapeutics for the regulation of the host immune system (72).
There are a few studies investigating the impact of omega-3 PUFAs on oral microbiota. An in vitro study showed that omega-3 PUFA, including EPA, DHA, and ALA, and their ester derivatives inhibited the growth of various oral bacteria, including Streptococcus mutans, Candida albicans, A.actinomycetemcomitans, Fusobacterium nucleatum (F.nucleatum), and P. gingivalis (73). The other in vitro study also showed that DHA and EPA possessed antibacterial activities against planktonic and biofilm forms of periodontal pathogens, P.gingivalis and F.nucleatum (74). High-dose omega-3 PUFA intake during non-surgical treatment in stage III or IV periodontitis patients was associated with reduced counts of periodontal pathogens, including P.gingivalis, T.forsythia, T.denticola and A.actinomycetemcomitans in a randomized clinical trial (75). The antimicrobial property of omega-3 PUFA indicates its potential effects on the composition of the oral microbiota. Potentially, gut and oral microbiota influenced by dietary lipids can affect each other (76, 77). More research is needed to investigate the impact of dietary lipids on oral microbiota and the interactions between oral and gut microbiota.
Profiles of SPMs and Microbiota in periodontitis
The role of SPMs and relevant lipids in oral microbiota in periodontitis has been rarely investigated. Recently, SPMs, SPM pathway markers, and SPM corresponding receptor genes have been identified in human gingival tissues (57). A follow-up study aimed to analyze and integrate data on lipid mediator level (SPMs and SPM pathway markers), SPM receptor gene expression, and subgingival microbiome in subjects with periodontitis and healthy controls (78). The study included 13 periodontally healthy and 15 periodontitis subjects examined before or after non-surgical periodontal therapy. Gingival tissue and subgingival plaque samples were collected prior to and 8 weeks after non-surgical treatment, but these samples were only collected once in the healthy group before any prophylaxis. Correlations between lipid mediator levels, receptor gene expression, and bacterial abundance were analyzed using the Data Integration Analysis for Biomarker discovery using Latent components (DIABLO) and Sparse Partial Least Squares (SPLS) methods. The study demonstrated that specific bacterial species were significantly associated with lipid mediators in different inflammatory conditions. When comparing these correlated species in periodontitis before treatment to after treatment, a bacterial species, Anaeroglobus geminatus, was identified in both conditions and positively correlated with different lipid mediators. Both states (before and after treatment) had four lipid mediators, 5(S),12(S)-dihydroxy-6E,8Z,11E,14Z-eicosatetraenoic acid (5(S)12(S)-DiHETE), RvD1, MaR1, and leukotriene B4 (LTB4), correlated with different bacteria species. Among the nine bacterial species identified in the periodontitis after the SRP group, four Selenomonas species (Selenomonas sp._oral_taxon_136, Selenomonas sp._oral_taxon_137, Selenomonas sp._oral_taxon_138, Selenomonas sp._oral_taxon_479) were highly correlated with multiple lipid mediators. These identified bacteria are not considered periodontal pathogens in literature. Similar to the gut microbes described above, both A. geminatus and Selenomonas spp. encode enzymes capable of transforming linoleic and ALA-derived lipids, implying that they may play a similar role to gut bacteria in modifying oral lipids (79). It is also possible that the change in the local environment, including the inflammatory condition and lipid profiles, results in the presence of these bacteria, as we discussed in the other sections. These findings indicate the potential interactions between lipids, microbiota, and inflammation in periodontitis which has not been deeply investigated (Figure 1).
Conclusion
These findings demonstrate that, similar to the influence of diet on the gut microbiome, the resolution of inflammation induced by SPMs is associated with shifts in the taxonomic composition of the oral microbiota. Potentially, SPMs and subgingival bacterial species may have interactions that open new possibilities for the identification of diagnostic biomarkers and the development of therapeutics for periodontitis.
Author contributions
CTL conceived the topic, and CTL and GDT wrote the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Righolt AJ, Jevdjevic M, Marcenes W, Listl S. Global-, regional-, and country-level economic impacts of dental diseases in 2015. J Dent Res. (2018) 97(5):501–7. doi: 10.1177/0022034517750572
2. Eke PI, Thornton-Evans GO, Wei L, Borgnakke WS, Dye BA, Genco RJ. Periodontitis in US adults national health and nutrition examination survey 2009–2014. J Am Dent Assoc. (2018) 149(7):576–88.e6. doi: 10.1016/j.adaj.2018.04.023
3. Botelho J, Machado V, Leira Y, Proenca L, Chambrone L, Mendes JJ. Economic burden of periodontitis in the United States and Europe: an updated estimation. J Periodontol. (2022) 93(3):373–9.doi: 10.1002/JPER.21-0111
4. Meyle J, Chapple I. Molecular aspects of the pathogenesis of periodontitis. Periodontol 2000. (2015) 69(1):7–17. doi: 10.1111/prd.12104
5. Dyke TEV, Bartold PM, Reynolds EC. The nexus between periodontal inflammation and dysbiosis. Front Immunol. (2020) 11:511. doi: 10.3389/fimmu.2020.00511
6. Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL. Microbial complexes in subgingival plaque. J Clin Periodontol. (1998) 25(2):134–44. doi: 10.1111/j.1600-051X.1998.tb02419.x
7. Hajishengallis G, Darveau RP, Curtis MA. The keystone-pathogen hypothesis. Nat Rev Microbiol. (2012) 10(10):717–25. doi: 10.1038/nrmicro2873
8. Sedghi LM, Bacino M, Kapila YL. Periodontal disease: the good, the bad, and the unknown. Front Cell Infect Microbiol. (2021) 11:766944. doi: 10.3389/fcimb.2021.766944
9. Dyke TEV. Commentary: periodontitis is characterized by an immuno-inflammatory host-mediated destruction of bone and connective tissues that support the teeth. J Periodontol. (2014) 85(4):509–11. doi: 10.1902/jop.2014.130701
10. Marsh PD. Microbial ecology of dental plaque and its significance in health and disease. Adv Dent Res. (1994) 8(2):263–71. doi: 10.1177/08959374940080022001
11. Genco RJ, Sanz M. Clinical and public health implications of periodontal and systemic diseases: an overview. Periodontol 2000. (2020) 83(1):7–13. doi: 10.1111/prd.12344
12. Loo WT, Fan CB, Bai LJ, Yue Y, Dou YD, Wang M, et al. Gene polymorphism and protein of human pro- and anti-inflammatory cytokines in Chinese healthy subjects and chronic periodontitis patients. J Transl Med. (2012) 10(Suppl 1):S8. doi: 10.1186/1479-5876-10-S1-S8
13. Górska R, Gregorek H, Kowalski J, Laskus-Perendyk A, Syczewska M, Madaliński K. Relationship between clinical parameters and cytokine profiles in inflamed gingival tissue and serum samples from patients with chronic periodontitis. J Clin Periodontol. (2003) 30(12):1046–52. doi: 10.1046/j.0303-6979.2003.00425.x
14. Mesa F, Lanza E, García L, Marfil-Alvarez R, Magan-Fernandez A. Polymorphism IL-1RN rs419598 reduces the susceptibility to generalized periodontitis in a population of European descent. PLoS ONE. (2017) 12(10):e0186366. doi: 10.1371/journal.pone.0186366
15. Loos BG, Craandijk J, Hoek FJ, Dillen PMEW, Velden UVD. Elevation of systemic markers related to cardiovascular diseases in the peripheral blood of periodontitis patients. J Periodontol. (2000) 71(10):1528–34. doi: 10.1902/jop.2000.71.10.1528
16. Shimada Y, Komatsu Y, Ikezawa-Suzuki I, Tai H, Sugita N, Yoshie H. The effect of periodontal treatment on Serum leptin, interleukin-6, and C-reactive protein. J Periodontol. (2010) 81(8):1118–23. Available at: https://www.ncbi.nlm.nih.gov/pubmed/20370420 doi: 10.1902/jop.2010.090741
17. Paraskevas S, Huizinga JD, Loos BG. A systematic review and meta-analyses on C-reactive protein in relation to periodontitis. J Clin Periodontol. (2008) 35(4):277–90. doi: 10.1111/j.1600-051X.2007.01173.x
18. Demmer RT, Trinquart L, Zuk A, Fu BC, Blomkvist J, Michalowicz BS, et al. The influence of anti-infective periodontal treatment on C-reactive protein: a systematic review and meta-analysis of randomized controlled trials. PLoS One. (2013) 8(10):e77441. Available at: https://www.ncbi.nlm.nih.gov/pubmed/24155956 doi: 10.1371/journal.pone.0077441
19. de Souza AB, Okawa RT, Silva CO, Araujo MG. Short-term changes on C-reactive protein (CRP) levels after non-surgical periodontal treatment in systemically healthy individuals. Clin Oral Investig. (2017) 21(1):477–84. doi: 10.1007/s00784-016-1817-0
20. D’Aiuto F, Parkar M, Andreou G, Suvan J, Brett PM, Ready D, et al. Periodontitis and systemic inflammation: control of the local infection is associated with a reduction in serum inflammatory markers. J Dent Res. (2004) 83(2):156–60. doi: 10.1177/154405910408300214
21. Preshaw PM. Host modulation therapy with anti-inflammatory agents. Periodontol 2000. (2018) 76(1):131–49. doi: 10.1111/prd.12148
22. Golub LM, Lee H. Periodontal therapeutics: current host-modulation agents and future directions. Periodontol 2000. (2020) 82(1):186–204. doi: 10.1111/prd.12315
23. Howell TH. Blocking periodontal disease progression with anti-inflammatory agents. J Periodontol. (1993) 64(8 Suppl):828–33. doi: 10.1902/jop.1993.64.8s.828
24. Williams RC, Jeffcoat MK, Howell TH, Rolla A, Stubbs D, Teoh KW, et al. Altering the progression of human alveolar bone loss with the non-steroidal anti-inflammatory drug flurbiprofen. J Periodontol. (1989) 60(9):485–90. doi: 10.1902/jop.1989.60.9.485
25. Vonkeman HE, van de Laar MA. Nonsteroidal anti-inflammatory drugs: adverse effects and their prevention. Semin Arthritis Rheum. (2010) 39(4):294–312. doi: 10.1016/j.semarthrit.2008.08.001
26. Mayer Y, Elimelech R, Balbir-Gurman A, Braun-Moscovici Y, Machtei EE. Periodontal condition of patients with autoimmune diseases and the effect of anti-tumor necrosis factor-alpha therapy. J Periodontol. (2013) 84(2):136–42. doi: 10.1902/jop.2012.120009
27. Sgolastra F, Petrucci A, Gatto R, Giannoni M, Monaco A. Long-term efficacy of subantimicrobial-dose doxycycline as an adjunctive treatment to scaling and root planing: a systematic review and meta-analysis. J Periodontol. (2011) 82(11):1570–81. doi: 10.1902/jop.2011.110026
28. Fullerton JN, Gilroy DW. Resolution of inflammation: a new therapeutic frontier. Nat Rev Drug Discov. (2016) 15(8):551–67. doi: 10.1038/nrd.2016.39
29. Dyke TEV. Pro-resolving mediators in the regulation of periodontal disease. Mol Aspects Med. (2017) 58:21–36. doi: 10.1016/j.mam.2017.04.006
30. Serhan CN, Chiang N, Dalli J. The resolution code of acute inflammation: novel pro-resolving lipid mediators in resolution. Semin Immunol. (2015) 27(3):200–15. doi: 10.1016/j.smim.2015.03.004
31. Chiang N, Fredman G, Bäckhed F, Oh SF, Vickery T, Schmidt BA, et al. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature. (2012) 484(7395):524–8. doi: 10.1038/nature11042
32. Serhan CN, Sulciner ML. Resolution medicine in cancer, infection, pain and inflammation: are we on track to address the next pandemic? Cancer Metastasis Rev. (2023) 42(1):13–7. doi: 10.1007/s10555-023-10091-5
33. Krishnamoorthy S, Recchiuti A, Chiang N, Fredman G, Serhan CN. Resolvin D1 receptor stereoselectivity and regulation of inflammation and proresolving microRNAs. Am J Pathol. (2012) 180(5):2018–27. doi: 10.1016/j.ajpath.2012.01.028
34. Gewirtz AT, Collier-Hyams LS, Young AN, Kucharzik T, Guilford WJ, Parkinson JF, et al. Lipoxin a4 analogs attenuate induction of intestinal epithelial proinflammatory gene expression and reduce the severity of dextran sodium sulfate-induced colitis. J Immunol. (2002) 168(10):5260–7. doi: 10.4049/jimmunol.168.10.5260
35. Tang Y, Zhang MJ, Hellmann J, Kosuri M, Bhatnagar A, Spite M. Proresolution therapy for the treatment of delayed healing of diabetic wounds. Diabetes. (2013) 62(2):618–27. doi: 10.2337/db12-0684
36. Parra MMO, Elangovan S, Lee C. Specialized pro-resolving lipid mediators in experimental periodontitis: a systematic review. Oral Dis. (2019) 25(5):1265–76. doi: 10.1111/odi.12979
37. Serhan CN, Chiang N. Resolvins and cysteinyl-containing pro-resolving mediators activate resolution of infectious inflammation and tissue regeneration. Prostaglandins Other Lipid Mediat. (2023) 166:106718. doi: 10.1016/j.prostaglandins.2023.106718
38. Rakian A, Rakian R, Shay AE, Serhan CN, Dyke TEV. Periodontal stem cells synthesize maresin conjugate in tissue regeneration 3. J Dent Res. (2022) 101(10):1205–13. doi: 10.1177/00220345221090879
39. Lee CT, Teles R, Kantarci A, Chen T, McCafferty J, Starr JR, et al. Resolvin E1 reverses experimental periodontitis and dysbiosis. J Immunol. (2016) 197(7):2796–806. doi: 10.4049/jimmunol.1600859
40. Hasturk H, Kantarci A, Goguet-Surmenian E, Blackwood A, Andry C, Serhan CN, et al. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J Immunol. (2007) 179(10):7021–9. doi: 10.4049/jimmunol.179.10.7021
41. Alvarez C, Abdalla H, Suliman S, Rojas P, Wu YC, Almarhoumi R, et al. Rve1 impacts the gingival inflammatory infiltrate by inhibiting the T cell response in experimental periodontitis. Front Immunol. (2021) 12:664756. doi: 10.3389/fimmu.2021.664756
42. Zarrough AE, Hasturk H, Stephens DN, Dyke TEV, Kantarci A. Resolvin D1 modulates periodontal ligament fibroblast function. J Periodontol. (2023) 94(5):683–93. doi: 10.1002/JPER.22-0462
43. Mustafa M, Zarrough A, Bolstad AI, Lygre H, Mustafa K, Hasturk H, et al. Resolvin D1 protects periodontal ligament. Am J Physiol Cell Physiol. (2013) 305(6):C673–9. doi: 10.1152/ajpcell.00242.2012
44. Khaled M, Shibani NA, Labban N, Batarseh G, Song F, Ruby J, et al. Effects of resolvin D1 on cell survival and cytokine expression of human gingival fibroblasts. J Periodontol. (2013) 84(12):1838–46. doi: 10.1902/jop.2013.120388
45. Albuquerque-Souza E, Schulte F, Chen T, Hardt M, Hasturk H, Dyke TEV, et al. Maresin-1 and resolvin E1 promote regenerative properties of periodontal ligament stem cells under inflammatory conditions. Front Immunol. (2020) 11:585530. doi: 10.3389/fimmu.2020.585530
46. Hasturk H, Schulte F, Martins M, Sherzai H, Floros C, Cugini M, et al. Safety and preliminary efficacy of a novel host-modulatory therapy for reducing gingival inflammation. Front Immunol. (2021) 12:704163. doi: 10.3389/fimmu.2021.704163
47. Wang CW, Colas RA, Dalli J, Arnardottir HH, Nguyen D, Hasturk H, et al. Maresin 1 biosynthesis and proresolving anti-infective functions with human-localized aggressive periodontitis leukocytes. Infect Immun. (2016) 84(3):658–65. doi: 10.1128/IAI.01131-15
48. Norling LV, Spite M, Yang R, Flower RJ, Perretti M, Serhan CN. Cutting edge: humanized nano-proresolving medicines mimic inflammation-resolution and enhance wound healing. J Immunol. (2011) 186(10):5543–7. doi: 10.4049/jimmunol.1003865
49. Dyke TEV, Hasturk H, Kantarci A, Freire MO, Nguyen D, Dalli J, et al. Proresolving nanomedicines activate bone regeneration in periodontitis. J Dent Res. (2015) 94(1):148–56. doi: 10.1177/0022034514557331
50. Spite M, Clària J, Serhan CN. Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell Metab. (2014) 19(1):21–36. doi: 10.1016/j.cmet.2013.10.006
51. Arnardottir H, Orr SK, Dalli J, Serhan CN. Human milk proresolving mediators stimulate resolution of acute inflammation. Mucosal Immunol. (2016) 9(3):757–66. doi: 10.1038/mi.2015.99
52. Colas RA, Shinohara M, Dalli J, Chiang N, Serhan CN. Identification and signature profiles for pro-resolving and inflammatory lipid mediators in human tissue. Am J Physiol-Cell Ph. (2014) 307(1):C39–C54. doi: 10.1152/ajpcell.00024.2014
53. Elabdeen HR, Mustafa M, Szklenar M, Ruhl R, Ali R, Bolstad AI. Ratio of pro-resolving and pro-inflammatory lipid mediator precursors as potential markers for aggressive periodontitis. PLoS One. (2013) 8(8):e70838. doi: 10.1371/journal.pone.0070838
54. Tobon-Arroyave SI, Isaza-Guzman DM, Gomez-Ortega J, Florez-Alzate AA. Salivary levels of specialized pro-resolving lipid mediators as indicators of periodontal health/disease status. J Clin Periodontol. (2019) 46(10):978–90. doi: 10.1111/jcpe.13173
55. Fredman G, Hellmann J, Proto JD, Kuriakose G, Colas RA, Dorweiler B, et al. An imbalance between specialized pro-resolving lipid mediators and pro-inflammatory leukotrienes promotes instability of atherosclerotic plaques. Nat Commun. (2016) 7:12859. doi: 10.1038/ncomms12859
56. Colas RA, Nhat LTH, Thuong NTT, Gómez EA, Ly L, Thanh HH, et al. Proresolving mediator profiles in cerebrospinal fluid are linked with disease severity and outcome in adults with tuberculous meningitis. Faseb J. (2019) 33(11):13028–39. doi: 10.1096/fj.201901590R
57. Ferguson B, Bokka NR, Maddipati KR, Ayilavarapu S, Weltman R, Zhu L, et al. Distinct profiles of specialized pro-resolving lipid mediators and corresponding receptor gene expression in periodontal inflammation. Front Immunol. (2020) 11:1307. doi: 10.3389/fimmu.2020.01307
58. Brown EM, Clardy J, Xavier RJ. Gut microbiome lipid metabolism and its impact on host physiology. Cell Host Microbe. (2023) 31(2):173–86. doi: 10.1016/j.chom.2023.01.009
59. Wang K, Liang X, Pang Y, Jiang C. The role of gut Microbiota in host lipid metabolism: an eye on causation and connection. Small Methods. (2020) 4(7):1900604. doi: 10.1002/smtd.201900604
60. Wang RX, Colgan SP. Special pro-resolving mediator (SPM) actions in regulating gastro-intestinal inflammation and gut mucosal immune responses. Mol Aspects Med. (2017) 58:93–101. doi: 10.1016/j.mam.2017.02.002
61. Basil MC, Levy BD. Specialized pro-resolving mediators: endogenous regulators of infection and inflammation. Nat Rev Immunol. (2016) 16(1):51–67. doi: 10.1038/nri.2015.4
62. Lamichhane S, Sen P, Alves MA, Ribeiro HC, Raunioniemi P, Hyötyläinen T, et al. Linking gut microbiome and lipid metabolism: moving beyond associations. Metabolites. (2021) 11(1):55. doi: 10.3390/metabo11010055
63. Costantini L, Molinari R, Farinon B, Merendino N. Impact of Omega-3 fatty acids on the gut Microbiota. Int J Mol Sci. (2017) 18(12):2645. doi: 10.3390/ijms18122645
64. Caesar R, Tremaroli V, Kovatcheva-Datchary P, Cani PD, Bäckhed F. Crosstalk between gut Microbiota and dietary lipids aggravates WAT inflammation through TLR signaling. Cell Metab. (2015) 22(4):658–68. doi: 10.1016/j.cmet.2015.07.026
65. Patterson E, Doherty RMO, Murphy EF, Wall R, Sullivan OO, Nilaweera K, et al. Impact of dietary fatty acids on metabolic activity and host intestinal microbiota composition in C57BL/6J mice. Brit J Nutr. (2014) 111(11):1905–17. doi: 10.1017/S0007114514000117
66. van der Meer-Janssen YPM, van Galen J, Batenburg JJ, Helms JB. Lipids in host–pathogen interactions: pathogens exploit the complexity of the host cell lipidome. Prog Lipid Res. (2010) 49(1):1–26. doi: 10.1016/j.plipres.2009.07.003
67. Miyamoto J, Igarashi M, Watanabe K, Karaki SI, Mukouyama H, Kishino S, et al. Gut microbiota confers host resistance to obesity by metabolizing dietary polyunsaturated fatty acids. Nat Commun. (2019) 10(1):4007. doi: 10.1038/s41467-019-11978-0
68. Kishino S, Takeuchi M, Park SB, Hirata A, Kitamura N, Kunisawa J, et al. Polyunsaturated fatty acid saturation by gut lactic acid bacteria affecting host lipid composition. Proc National Acad Sci. (2013) 110(44):17808–13. doi: 10.1073/pnas.1312937110
69. Franzosa EA, Sirota-Madi A, Avila-Pacheco J, Fornelos N, Haiser HJ, Reinker S, et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat Microbiol. (2019) 4(2):293–305. doi: 10.1038/s41564-018-0306-4
70. Fornelos N, Franzosa EA, Bishai J, Annand JW, Oka A, Lloyd-Price J, et al. Growth effects of N-acylethanolamines on gut bacteria reflect altered bacterial abundances in inflammatory bowel disease. Nat Microbiol. (2020) 5(3):486–97. doi: 10.1038/s41564-019-0655-7
71. Chatterjee R, Chowdhury AR, Mukherjee D, Chakravortty D. Lipid larceny: channelizing host lipids for establishing successful pathogenesis by bacteria. Virulence. (2021) 12(1):195–216. doi: 10.1080/21505594.2020.1869441
72. Saika A, Nagatake T, Kunisawa J. Host- and microbe-dependent dietary lipid metabolism in the control of allergy, inflammation, and immunity. Front Nutr. (2019) 6:36. doi: 10.3389/fnut.2019.00036
73. Huang CB, Ebersole JL. A novel bioactivity of omega-3 polyunsaturated fatty acids and their ester derivatives. Mol Oral Microbiol. (2010) 25(1):75–80. doi: 10.1111/j.2041-1014.2009.00553.x
74. Sun M, Zhou Z, Dong J, Zhang J, Xia Y, Shu R. Antibacterial and antibiofilm activities of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) against periodontopathic bacteria. Microb Pathog. (2016) 99:196–203. doi: 10.1016/j.micpath.2016.08.025
75. Stańdo-Retecka M, Piatek P, Namiecinska M, Bonikowski R, Lewkowicz P, Lewkowicz N. Clinical and microbiological outcomes of subgingival instrumentation supplemented with high-dose omega-3 polyunsaturated fatty acids in periodontal treatment – a randomized clinical trial. BMC Oral Heal. (2023) 23(1):290. doi: 10.1186/s12903-023-03018-7
76. Park SY, Hwang BO, Lim M, Ok SH, Lee SK, Chun KS, et al. Oral–gut microbiome axis in gastrointestinal disease and cancer. Cancers (Basel). (2021) 13(9):2124. doi: 10.3390/cancers13092124
77. Olsen I, Yamazaki K. Can oral bacteria affect the microbiome of the gut? J Oral Microbiol. (2019) 11(1):1586422. doi: 10.1080/20002297.2019.1586422
78. Lee CT, Li R, Zhu L, Tribble GD, Zheng WJ, Ferguson B, et al. Subgingival microbiome and specialized pro-resolving lipid mediator pathway profiles are correlated in periodontal inflammation. Front Immunol. (2021) 12:691216. doi: 10.3389/fimmu.2021.691216
Keywords: specialized pro-resolving mediators, lipid mediator, periodontitis, microbiome, host modulation, omega - 3 - fatty acid, host-pathogen interactions
Citation: Lee CT and Tribble GD (2023) Roles of specialized pro-resolving mediators and omega-3 polyunsaturated fatty acids in periodontal inflammation and impact on oral microbiota. Front. Oral. Health 4:1217088. doi: 10.3389/froh.2023.1217088
Received: 4 May 2023; Accepted: 11 July 2023;
Published: 25 July 2023.
Edited by:
Oleh Andrukhov, University Clinic of Dentistry, Medical University of Vienna, AustriaReviewed by:
Renato Correa Viana Casarin, Universidade Estadual de Campinas, BrazilFrancisco Mesa, University of Granada, Spain
Henrique Ballassini Abdalla, São Leopoldo Mandic School, Brazil
© 2023 Lee and Tribble. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chun-Teh Lee Y2h1bi10ZWgubGVlQHV0aC50bWMuZWR1 Gena D. Tribble Z2VuYS5kLnRyaWJibGVAdXRoLnRtYy5lZHU=
 Chun-Teh Lee
Chun-Teh Lee Gena D. Tribble
Gena D. Tribble