- Department of Oral Diagnostic Sciences, Faculty of Dentistry, King Abdulaziz University, Jeddah, Saudi Arabia
The oral cavity can present early manifestations of several systemic diseases. Since the emergence of the COVID-19 pandemic, there have been many published studies reporting the direct effect of the virus on orofacial structures. In the present study, oral signs and symptoms of 22 hospital-admitted COVID-19 patients were examined and compared to a matching control group. Loss of taste and smell was the most prevalent symptom (65%), followed by oral dryness (45%) and halitosis (30%). The most common oral lesions were candidal infections (68%). Other less common manifestations were oral ulcerations (36%) followed by the appearance of white patches (27.3%). There was a statistically significant association between candidal infection and age in the study group, where the p-value was 0.008. In the present study, 80% of those who had candida infections were aged 60 years or above. There was no significant association with comorbidities such as diabetes mellitus and hypertension.
Introduction
Severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2], known as the COVID-19 pandemic, is a highly contagious infection caused by a novel single-chain RNA virus with an incubation period of 1–14 days (1–4). The disease can vary from mild flu-like to severe respiratory distress, with the first symptoms, namely, weakness, fever, headache, sore throat, cough, runny nose, abdominal pain, vomiting, and diarrhea, manifesting within 4–5 days (5). Dermatological findings were reported in the form of petechia-like and vesiculobullous lesions, erythematous lesions, and urticarial rash (2, 6). Orofacial manifestations include olfactory dysfunction and anosmia as initial symptoms (7). Oral epithelial cells offer viral entry via the angiotensin-converting enzyme 2 receptor, which facilitate viral replication and can result in tissue inflammation (4). A variable spectrum of oral mucosal manifestations has been reported in the literature, including ulcers, erosions, vesicles, pustules, fissured or depapillated tongue, maculopapular lesions, plaque (8–13), pigmentation, halitosis, white lesions, hemorrhagic crusts, necrosis, erythema, and spontaneous bleeding (14–19). The most common sites of involvement were the tongue (4, 14) followed by the labial mucosa and the palate. Suggested diagnoses of the lesions were aphthous stomatitis, herpetiform lesions, candidiasis, and vasculitis.
Since the outbreak of this pandemic, there have been more than 603,711,760 confirmed cases globally, including 6,484,136 deaths according to the World Health Organization (WHO) (5). The first COVID-19-positive case in the Kingdom of Saudi Arabia was detected on 2 March 2020 (20–23). The number of cases then rapidly escalated. There were 813,986 confirmed cases as of 8 September 2022, with 801,254 recoveries. The fatality rate was 2% with 9,309 deaths (24).
Hospitalization protocols for moderate to severe cases in Saudi Arabia depend on respiratory symptoms and the presence of other comorbidities such as diabetes, cancer, immunosuppression, and obesity (25). Descriptive studies of clinical characteristics of patients with COVID-19 in Saudi Arabia demonstrated significant differences in symptoms as a direct effect of age and comorbidities (23). To date, no published data has yet investigated oral manifestations of hospital-admitted COVID-19 patients in Saudi Arabia. Oral manifestations may help guide early diagnosis of COVID-19, and the objective of this study is to identify the oral signs and symptoms associated with admitted COVID-19 patients and their prevalence in comparison to age, gender, and comorbidity-matched control group.
Methods
Study design
A case-control two-center study was conducted among hospitalized COVID-19 patients in King Fahad General Hospital and East Jeddah General Hospital, Jeddah, Saudi Arabia from 1 September to 30 October 2021. The ethical approval was obtained from the research and studies department, Ministry of Health, Saudi Arabia (KACST, KSA: H-02-J-002).
Study subjects
Inclusion criteria:
Patients of any age admitted for COVID-19.
Exclusion criteria:
• Non-hospitalized COVID-19 patients.
• Hospitalized patients under ventilation at the time of examinations for whom oral examinations were not feasible.
The control group was selected from the dental clinics of the same hospitals and matched to the study group for age, gender, and comorbidities. Patients were interviewed and underwent intra-oral examination by two oral medicine consultants (SA and SJ) to identify any associated oral manifestations. Informed consent was obtained from each patient or his/her guardian. Patients' characteristics including age, gender, comorbidities, concomitant medications, time to onset of COVID-19, COVID-19 vaccination, and number of vaccination doses were recorded. All patients underwent two stages of screening recorded on a data collection sheet.
The patients were first asked questions about the presence and severity of the following symptoms after the onset of COVID-19:
1. Change in ability to smell or taste.
2. Orofacial pain.
3. Halitosis.
4. Oral dryness.
5. Any oral ulceration or vesicles/bullae.
The severity of symptoms was recorded using the validated visual analog scale (VAS) (26). Second, oral mucosal examinations using a disposable tongue depressor and a flashlight were performed by the two main investigators to record all possible intraoral lesions with location specifications. Scraping using gauze was done for any white lesions. Intraoral examinations were performed similarly to the control group.
Data analysis
Statistical Package for Social Sciences computer software (SPSS for Mac, Version 28.0; IBM, 2019) was used to analyze the data. Descriptive statistics were calculated on all variables of interest to describe study variables: arithmetic means (x¯), standard deviations (SD), frequencies, and percentages. The chi-squared test (χ2) or Fisher's exact test was used to evaluate the association between two qualitative variables or to detect differences between two or more proportions where appropriate. The level of significance was p = 0.05. All reported p-values were generated from two-sided statistical tests.
Results
Patients
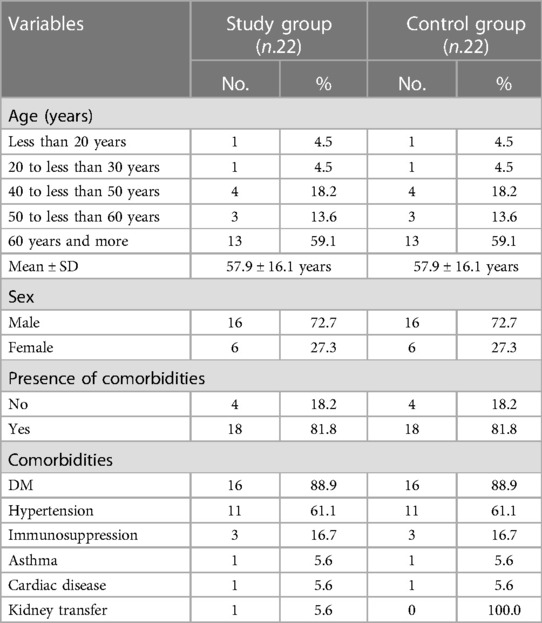
There were 22 patients included in the study: 16 (72.7%) were men and six were women (27.3%) with a mean age of 57.9 ± 16.1 years. There were 18 patients (82%) with comorbidities, 16 (88.9%) were diabetic, and 11 (61.1%) were hypertensive. The control group demonstrated the same sex distribution with matching age and comorbidities (Table 1).
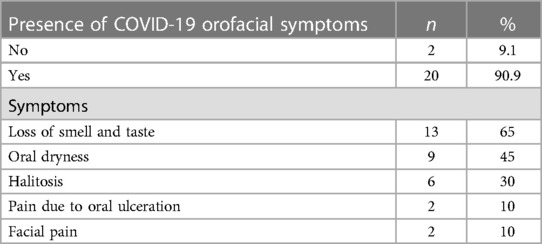
The mean period to COVID-19-confirmed diagnosis was 11.0 ± 6.3 days. In all, 20 patients (90.1%) in the study group reported one or more orofacial symptoms. Approximately two-thirds (65%) of them complained of a loss of smell and taste perception, whereas less than half (45%) presented with oral dryness (Table 2). We found that 27.3% of the study group did not receive the COVID-19 vaccine compared to 72.8% who received either one or two doses (same percentage for both). Figure 1 shows the onset of symptoms after a confirmed COVID-19 diagnosis.
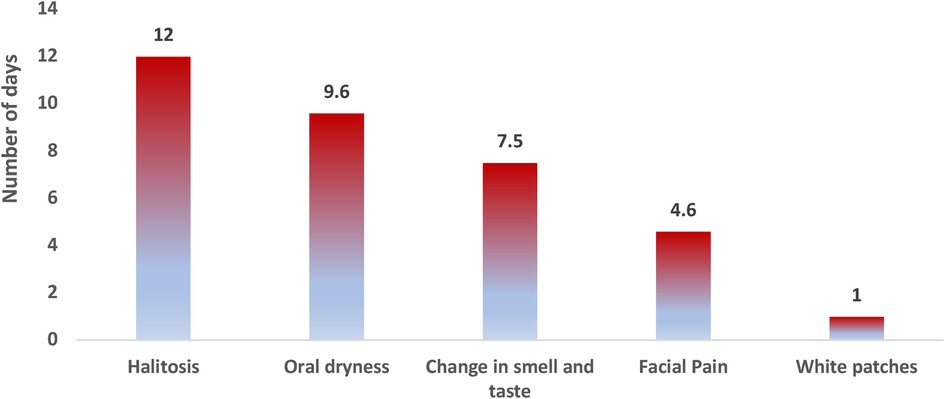
Figure 1. Average time in days for symptom perception to the onset of COVID-19: reported onset of halitosis was 12.0 ± 12.7 days, oral dryness was 9.6 ± 5.2 days, change of smell and taste was 7.5 ± 5.3 days, facial pain was 4.6 ± 2.0 days, and appearance of white patches was 1 ± 0.0 day after the onset of COVID-19 symptoms.
Regarding the severity of symptoms, changes in smell and taste perception came first with a mean degree of severity of 8.9 ± 2.0 points out of 10, followed by halitosis and facial pain with the same mean score of 4.6 ± 2.3 points for both.
Oral lesions
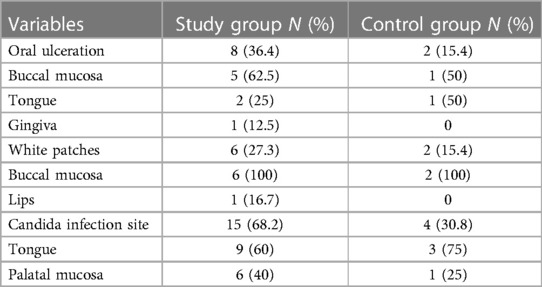
Approximately one-third (36.4%) of the study group had oral ulcerations while they were in the hospital. Petechiae in the palatal mucosa was presented in one patient. More than a quarter (27.3%) of the study group had white patches. More than two-thirds (68.2%) of them had candida. Table 3 details the site distribution for each lesion.
Oral mucosal lesions were reported among more than half (59.1%) of the control group; 30.8% of these lesions were candida infection followed by tongue depapillation, white patches, ulcers, and petechiae (23.1%, 15.4%, 15.4%, and 15.4% respectively (Table 3).
Comparison between the presence of candida infection in the study group and control group denotes a statistically significant difference between them with a p-value of 0.001, where 68.2% of the study group had candida infection compared to only 18.2% of the control group. No statistically significant differences were noted between the two groups in terms of the presence of other oral lesions.
There was no statistically significant association between the oral lesions and comorbidities of the studied group or the control group except for ulcers and hypertension in the control group (p = 0.013). There was a statistically significant association between candidal infection and age in the study group, where the p-value was 0.008. In the present study, 80% of those who had candida infections were aged 60 years or above. There was no statistically significant association noted between candidal infection and sex and comorbidities in the study group or the control group.
There was a statistically significant association noted between the appearance of white patches and immunosuppression as one of the comorbidities of the study group (p-value = 0.049). However, there was no association detected among the control group.
Candidal infection was associated with the number of days to COVID-19 diagnosis, where the p-value was 0.006. The time to diagnosis of COVID-19 was associated with the risk of candida infection; half (53.3%) of those who had oral candidal infection were diagnosed with COVID-19 at least one week previously. However, orofacial manifestations were not associated with the COVID-19 vaccine in the study group.
Discussion
COVID-19 infection can manifest in different body parts such as the respiratory system, gastrointestinal system, skin, and oral mucosa. In the present study, we identified the oral manifestations of hospital-admitted COVID-19 patients. The most common findings were taste disturbance and candidal infection. Other less common manifestations were oral dryness and oral ulcerations. This is in accordance with the latest update of the living systematic review conducted by dos Santos et al., as xerostomia and taste disturbance were the most common symptoms, while ulcerations were the most common oral lesions followed by herpes-like lesions, candidiasis, and angular cheilitis (27).
One of the first demonstrated symptoms of COVID-19 is taste and smell disturbance. Taste and smell disorders were found to be the most expressed symptoms in Europe but were less common in Asian patients, with an overall prevalence of 38% (27). This disturbance was found to be associated with mild/moderate COVID-19 severity. In the present study, 65% of the study group with severe COVID-19 infection complained of taste and smell disturbance with a mean severity of 8.9 ± 2. The patients reported that loss of smell and taste started 7.5 ± 5.3 days after COVID-19 onset. Giacomelli et al. reported olfactory or taste alteration in 34% of admitted COVID-19 patients with 20.3% of them having it before hospital admission and 13.5% having it during their admission (with a mean time of 6 days from COVID-19) (28).
Oral dryness is another symptom that might be related to the direct effect of the virus on salivary glands (29). Chronic medications such as oral hypoglycemic and antihypertensive medications are additional elements that can affect oral salivation. Of the study group and control group, 89% had diabetes mellitus and 61% had hypertension. Here, 45% of our study group presented with oral dryness with an onset of 9.6 days after COVID-19 diagnosis. None of the control group reported this symptom. However, there was no statistically significant association between the presence of the two comorbidities and oral dryness among the study group. This prevalence is consistent with the meta-analysis conducted by dos Santos et al., which showed a prevalence of 43% (27).
The association between oral ulceration and COVID-19 was first described by Carreras-Presas et al. (30). The impact of COVID-19 on orofacial structures may result from the direct effect of the viral infection on the oral mucosa or as a secondary result of its complications or therapeutic measures. The SARS-CoV-2 virus binding site is to the human angiotensin-converting enzyme-2 (ACE2) receptor. These are well expressed on different oral mucosal tissues such as the tongue and the palate (1, 31–33) (Xu 2020). Another theory suggests that the virus can also infect oral keratinocytes and fibroblasts, which can lead to ulceration and superficial necrosis (3). This might explain the early incidence of oral ulcerations in COVID-19 patients. Ulcerations might be directly related to anemia resulting from the direct hemolytic effect of the COVID-19 virus on red blood cells (34). In our study group, approximately one-third of the subjects had oral ulcerations while they were admitted to the hospital—this value is compatible with the findings from many other documented case reports (8, 30, 35). Only two subjects in the control group had oral ulcerations.
Candidal infection in its pseudomembranous form was the most common oral lesion in our study group (68%); only 18% of the control group showed oral candidiasis. Fungal infection in COVID-19 patients from tertiary centers in Saudi Arabia was also investigated previously (36) and showed that pseudomembranous candidiasis was the most common form of candidiasis in COVID-19 patients and was most likely related to disease comorbidities and hospital admission. COVID-19 is known to increase the risk of secondary opportunistic infections such as candidiasis. In addition, medications used to manage COVID-19, such as azithromycin, will disturb the oral microbiome and increase this risk (3). There was also a statistically significant association noted between the candida infection and the number of days to COVID-19 diagnosis and age of the study group (p-value = 0.006 and 0.008, respectively). It is well established that the risk of candida infection increases in elderly patients. According to the findings of the present study population, candida infection was found mostly in 60-year-old patients. On the other hand, no association was found between comorbidities and candidal infection as about 90% of the sample had diabetes.
There was no statistically significant association between the oral lesions and comorbidities of the studied group nor the control group except for hypertension among the control group, which can be attributed to antihypertensive medications that could result in oral mucosal lesions such as lichenoid reaction and ulcerations.
This study is limited by the small sample size. Many COVID-19 hospital-admitted patients were excluded because they were critically ill or intubated and unable to respond to the questions. Moreover, due to the nature of the study, blinding patients was not applicable. However, the results emphasize the importance of oral examinations for critically ill COVID-19 patients to diagnose and manage any related oral complaints aiming to relieve pain and improve quality of life.
Conclusion
Candidal infection and taste disturbance were the most frequent oral lesions in hospital-admitted COVID-19 patients. Other less common manifestations were oral dryness and oral ulcerations. The exact etiopathogenesis of these manifestations is still unknown. The direct impact of the COVID-19 virus on the epithelium is the most likely cause of taste disturbance. However, other manifestations can be the result of co-infections secondary to the compromised medical status of the patients as well as the side effects of medications, such as antibiotics. Intraoral examination for hospitalized patients with COVID-19 is recommended in order to manage any related signs and symptoms.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Research and Studies department, Ministry of Health, Saudi Arabia (KACST, KSA: H-02-J-002). The patients/participants provided their written informed consent to participate in this study.
Author contributions
The authors confirm their contribution to the research paper as follows: study conception and design, data collection—SAlh and SAlj; analysis and interpretation of results—SAlj; draft manuscript preparation—SAlh. All authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to thank the Ministry of Health in Saudi Arabia for their support during the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Anaya-Saavedra G. Oral manifestations accompanying and related to COVID-19: overlooking the obvious. Oral Dis. (2021) 28:2530–2. doi: 10.1111/odi.13857
2. Bardellini E, Bondioni MP, Amadori F, Veneri F, Lougaris V, Meini A, et al. Non-specific oral and cutaneous manifestations of coronavirus disease 2019 in children. Med Oral Patol Oral Cir Bucal. (2021) 26:549–53. doi: 10.4317/medoral.24461
3. Brandão TB, Gueiros LA, Melo TS, Prado-Ribeiro AC, Nesrallah ACFA, Prado G, et al. Oral lesions in patients with SARS-CoV-2 infection: could the oral cavity be a target organ? Oral Surg Oral Med Oral Pathol Oral Radiol. (2021) 131(2):e45–51. doi: 10.1016/j.oooo.2020.07.014
4. Brandini DA, Takamiya AS, Thakkar P, Schaller S, Rahat R, Naqvi AR. COVID-19 and oral diseases: crosstalk, synergy or association? Rev Med Virol. (2021) 3:e2226. doi: 10.1002/rmv.2226
5. Organization WH. Health emergency dashboard WHO (COVID-19) homepage (2021). Available at: https://covid19.who.int/ (Accessed April 26, 2021).
6. Bemquerer LM, de Arruda JAA, Soares MPD, Mesquita RA, Silva TA. The oral cavity cannot be forgotten in the COVID-19 era: is there a connection between dermatologic and oral manifestations? J Am Acad Dermatol. (2021) 84(3):e143–5. doi: 10.1016/j.jaad.2020.11.034
7. Biadsee A, Biadsee A, Kassem F, Dagan O, Masarwa S, Ormianer Z. Olfactory and oral manifestations of COVID-19: sex-related symptoms-A potential pathway to early diagnosis. Otolaryngol Head Neck Surg. (2020) 163(4):722–8. doi: 10.1177/0194599820934380
8. Cruz Tapia RO, Peraza Labrador AJ, Guimaraes DM, Matos Valdez LH. Oral mucosal lesions in patients with SARS-CoV-2 infection. Report of four cases. Are they a true sign of COVID-19 disease? Spec Care Dentist. (2020) 40(6):555–60. doi: 10.1111/scd.12520
9. de Sousa F, Paradella TC. Considerations on oral manifestations of COVID-19. J Med Virol. (2021) 93(2):667–8. doi: 10.1002/jmv.26451
10. Di Spirito F, Pelella S, Argentino S, Sisalli L, Sbordone L. Oral manifestations and the role of the oral healthcare workers in COVID-19. Oral Dis. (2020) 28:1003–4. doi: 10.1111/odi.13688
11. Díaz Rodríguez M, Jimenez Romera A, Villarroel M. Oral manifestations associated with COVID-19. Oral Dis. (2020) 1:960–2. doi: 10.1111/odi.13555
12. Eghbali Zarch R, Hosseinzadeh P. COVID-19 from the perspective of dentists: a case report and brief review of more than 170 cases. Dermatol Ther. (2021) 34(1):e14717. doi: 10.1111/dth.14717
13. Egido-Moreno S, Valls-Roca-Umbert J, Jané-Salas E, López-López J, Estrugo-Devesa A. COVID-19 and oral lesions, short communication and review. J Clin Exp Dent. (2021) 13(3):e287–94. doi: 10.4317/jced.57981
14. Halepas S, Lee KC, Myers A, Yoon RK, Chung W, Peters SM. Oral manifestations of COVID-2019-related multisystem inflammatory syndrome in children: a review of 47 pediatric patients. J Am Dent Assoc (1939). (2021) 152(3):202–8. doi: 10.1016/j.adaj.2020.11.014
15. Jimenez-Cauhe J, Ortega-Quijano D, Carretero-Barrio I, Suarez-Valle A, Saceda-Corralo D, Moreno-Garcia Del Real C, et al. Erythema multiforme-like eruption in patients with COVID-19 infection: clinical and histological findings. Clin Exp Dermatol. (2020) 45(7):892–5. doi: 10.1111/ced.14281
16. Kitakawa D, Oliveira FE, Neves de Castro P, Carvalho L. Short report—herpes simplex lesion in the lip semimucosa in a COVID-19 patient. Eur Rev Med Pharmacol Sci. (2020) 24(17):9151–3. doi: 10.26355/eurrev_202009_22863
17. La Rosa GRM, Libra M, De Pasquale R, Ferlito S, Pedullà E. Association of viral infections with oral cavity lesions: role of SARS-CoV-2 infection. Front Med (Lausanne). (2020) 7:571214. doi: 10.3389/fmed.2020.571214
18. Maia CMF, Marques NP, de Lucena EHG, de Rezende LF, Martelli DRB, Martelli-Júnior H. Increased number of herpes zoster cases in Brazil related to the COVID-19 pandemic. Int J Infect Dis. (2021) 104:732–3. doi: 10.1016/j.ijid.2021.02.033
19. Maniaci A, Iannella G, Vicini C, Pavone P, Nunnari G, Falsaperla R, et al. A case of COVID-19 with late-onset rash and transient loss of taste and smell in a 15-year-old boy. Am J Case Rep. (2020) 21:e925813. doi: 10.12659/AJCR.925813
20. Alsofayan YM, Althunayyan SM, Khan AA, Hakawi AM, Assiri AM. Clinical characteristics of COVID-19 in Saudi Arabia: a national retrospective study. J Infect Public Health. (2020) 13(7):920–5. doi: 10.1016/j.jiph.2020.05.026
21. Abubakr N, Salem ZA, Kamel AHM. Oral manifestations in mild-to-moderate cases of COVID-19 viral infection in the adult population. Dent Med Probl. (2021) 58(1):7–15. doi: 10.17219/dmp/130814
22. Al-Khatib A. Oral manifestations in COVID-19 patients. Oral Dis. (2021) 27(Suppl 3):779–80. doi: 10.1111/odi.13477
23. Al-Omari A, Alhuqbani WN, Zaidi ARZ, Al-Subaie MF, AlHindi AM, Abogosh AK, et al. Clinical characteristics of non-intensive care unit COVID-19 patients in Saudi Arabia: a descriptive cross-sectional study. J Infect Public Health. (2020) 13(11):1639–44. doi: 10.1016/j.jiph.2020.09.003
24. Riad A, Gomaa E, Hockova B, Klugar M. Oral candidiasis of COVID-19 patients: case report and review of evidence. J Cosmet Dermatol. (2021) 20:1580–4. doi: 10.1111/jocd.14066
25. Aziz S, Arabi YM, Alhazzani W, Evans L, Citerio G, Fischkoff K, et al. Managing ICU surge during the COVID-19 crisis: rapid guidelines. Intensive Care Med. (2020) 46(7):1303–25. doi: 10.1007/s00134-020-06092-5
26. Couper MP, Tourangeau R, Conrad FG, Singer E. Evaluating the effectiveness of visual analog scales: a web experiment. Soc Sci Comput Rev. (2006) 24(2):227–45. doi: 10.1177/0894439305281503
27. Amorim dos Santos J, Normando AGC, Carvalho da Silva RL, Acevedo AC, De Luca Canto G, Sugaya N, et al. Oral manifestations in patients with COVID-19: a 6-month update. J Dent Res. (2021) 100(12):1321–9. doi: 10.1177/00220345211029637
28. Giacomelli A, Pezzati L, Conti F, Bernacchia D, Siano M, Oreni L, et al. Self-reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: a cross-sectional study. Clin Infect Dis. (2020) 71(15):889–90. doi: 10.1093/cid/ciaa330
29. Farook FF, Mohamed Nuzaim MN, Taha Ababneh K, Alshammari A, Alkadi L. COVID-19 pandemic: oral health challenges and recommendations. Eur J Dent. (2020) 14(S01):S165–70. doi: 10.1055/s-0040-1718641
30. Martín Carreras-Presas C, Amaro Sánchez J, López-Sánchez AF, Jané-Salas E, Somacarrera Pérez ML. Oral vesiculobullous lesions associated with SARS-CoV-2 infection. Oral Dis. (2021) 27(Suppl 3):710–2. doi: 10.1111/odi.13382
31. Amorim Dos Santos J, Normando AGC, Carvalho da Silva RL, Acevedo AC, De Luca Canto G, Sugaya N, et al. Oral manifestations in patients with COVID-19: a living systematic review. J Dent Res. (2021) 100(2):141–54. doi: 10.1177/0022034520957289
32. Vieira AR. Oral manifestations in coronavirus disease 2019 (COVID-19). Oral Dis. (2021) 27( Suppl 3):770. doi: 10.1111/odi.13463
33. Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. (2020) 12(01):8. doi: 10.1038/s41368-020-0074-x
34. Sarode GS, Sarode SC, Gadbail AR, Gondivkar S, Sharma NK, Patil S. Are oral manifestations related to SARS-CoV-2 mediated hemolysis and anemia? Med Hypotheses. (2021) 146:110413. doi: 10.1016/j.mehy.2020.110413
35. Sinadinos A, Shelswell J. Oral ulceration and blistering in patients with COVID-19. Evid Based Dent. (2020) 21(2):49. doi: 10.1038/s41432-020-0100-z
Keywords: COVID-19, oral manifestations, oral signs and symptoms, oral ulcerations, taste alteration, candidal infection
Citation: Alhamed S and Aljohani S (2023) Oral manifestations in hospital-admitted COVID-19 patients: a case control study. Front. Oral. Health 4:1180017. doi: 10.3389/froh.2023.1180017
Received: 5 March 2023; Accepted: 25 July 2023;
Published: 10 August 2023.
Edited by:
Daniel Manoil, University of Geneva, SwitzerlandReviewed by:
Anas Ahmed Shamala, University of Science & Technology, YemenBenoit Gilbert, Hôpitaux universitaires de Genève (HUG), Switzerland
© 2023 Alhamed and Aljohani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Suad Aljohani c3JhbGpvaGFuaUBrYXUuZWR1LnNh
†These authors have contributed equally to this work
‡ORCID Sana Alhamed orcid.org/0000-0002-2150-3817 Suad Aljohani orcid.org/0000-0002-4169-6046
 Sana Alhamed†,‡
Sana Alhamed†,‡ Suad Aljohani
Suad Aljohani