- 1Department of Orofacial Sciences, University of California, San Francisco, San Francisco, CA, United States
- 2General Practice Residency, Department of Oral and Maxillofacial Surgery, University of California, San Francisco, San Francisco, CA, United States
- 3Department of Diagnostic Sciences, Tufts University, Boston, MA, United States
The number of cancer survivors are increasing and so are the oral toxicities from cancer therapy. Most patients receiving treatment for cancer develop some form of oral adverse events including, but not limited to, mucositis, opportunistic infections, dry mouth, and/or osteonecrosis of the jaw. One of the most common complications from head and neck cancer radiation therapy is salivary gland dysfunction (SGD). SGD is an umbrella term that includes the subjective sensation of dry mouth (xerostomia) and hyposalivation (objective reduction of the salivary flow rate). Dry mouth in cancer patients may lead to functional defects (e.g., eating, speaking, and swallowing), increase the risk of dental caries and oral candidiasis, and can have a negative effect on the nutritional and psychological status of the patients. The aim of this mini review was to summarize the current criteria for diagnosis and management of SGD associated with cancer treatment.
Introduction
Cancer survival rates continues to increase and by 2040, it is estimated that there will be 26 million cancer survivors in the United States [1–3]. Cancer patients may develop acute and chronic oral toxicities from cancer therapy including mucositis, xerostomia, salivary gland dysfunction (SGD), neurosensory disorders, trismus, jaw necrosis, and infections to name a few. Cancer regimen-related toxicities often lead to devastating consequences, reduced function, poor clinical outcomes, and higher health care cost [4].
SGD is one of the most frequent side effects from cancer therapy [5]. Dry mouth in cancer patients may be secondary to chemotherapy, head and neck radiotherapy, dehydration, and chronic graft-vs. host disease (cGVHD). Saliva has important functions including antimicrobial activity, gustatory function, protection and lubrication of the oral mucosa and esophagus, and remineralization and maintenance of hard and soft tissues in the oral cavity; all these functions have the potential to be compromised by cancer therapy [6]. The aim of this mini review was to describe the established causes and guidelines for diagnosis and management options of SGD in cancer patients, as well as the potential new therapeutic approaches that are currently in study and development.
Definitions and Diagnostic Tests For Salivary Gland Dysfunction
SGD has been defined as “any alteration in the qualitative or quantitative output of saliva caused by an increase (hyperfunction) or decrease (hypofunction) in salivary output” [7].
Hyposalivation is assessed by measuring stimulated and unstimulated salivary flow and at times, by individual major gland secretion. Hyposalivation is defined as a resting (unstimulated) whole saliva flow rate of ≤ 0.1 mL/min and/or a stimulated whole saliva flow rate of ≤ 0.5 mL/min [8, 9]. Commonly used stimulants to assess stimulated salivary flow rates include sugar free gums, paraffin wax, rubber bands, or citric acid. Hyposalivation may or may not result in xerostomia (the subjective feeling of dry mouth), negative impact on function, including eating, speaking, and swallowing, dysgeusia, and/or a burning sensation of the oral mucosa [10, 11].
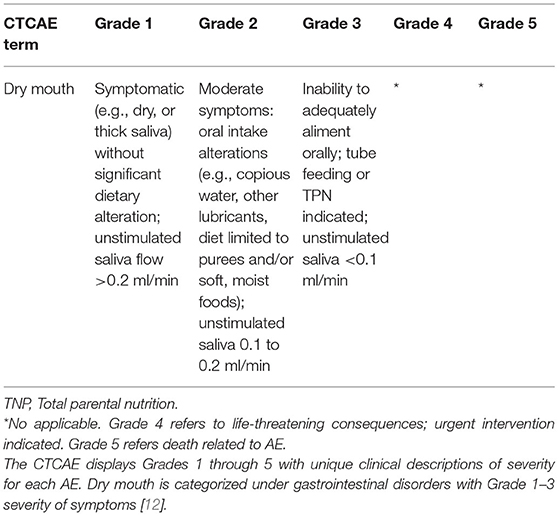
Xerostomia in cancer patients is assessed by patient reported outcomes (PROs) [e.g., Xerostomia Inventory (XI)] or by practitioner reported outcomes [e.g., using the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (PRO-CTCAE)], where in addition to the patient symptoms, the unstimulated saliva is measured (Table 1) [12, 13]. The XI is an 11-item instrument that evaluates and measures the different aspects of xerostomia that are experienced by the patient. A shortened version of XI named Summated Xerostomia Inventory (SXI) comprises five of the original 11 items and was more recently developed to focus on the “experiential aspects of dry mouth” rather than measuring general exocrine gland functions [14].
Excessive salivation is rare, and may occur in cancer patients due to dysphagia, odynophagia, malignancy, or local oral irritation. Non-pharmacologic treatment varies from functional dysphagia therapy to neurosensory approaches. Pharmacologic treatment aims to reduce salivary flow and includes several agents such as glycopyrrolate, scopolamine, atropine, benztropine, and botulinum toxin injection into the salivary glands [15]. Mucolytic agents such as guaifenesin and n-acetylcysteine may decrease the viscosity and volume of mucous secretions and improve comfort [16].
SGD Secondary to Cancer Treatment
Radiotherapy- Induced SGD
Dry mouth is one of the most common and dismal effects of radiation therapy (RT) to the head and neck cancer patients. Salivary gland tissue, in particular acinar cells of the serous glands (parotid), is sensitive to RT with permanent salivary gland damage in patients receiving cumulative doses > 30 Gy [17]. RT can also cause dry mouth due to indirect damage to epithelial and connective tissues of the gland including the blood vessels and nerves [4]. When salivary glands are within the field of radiation, irreversible salivary glands damage occurs in 63–93% of the patients [18]. In head and neck cancer patients undergoing RT, the dysfunction is dose dependent; when 40–50 Gy are administered up to 75% of the parotid gland function may be impaired [19]. The effects of RT on SGD are long term and usually irreversible [18].
Preventive strategies of salivary gland hypofunction and dry mouth secondary to RT have focused on the preservation of salivary gland function, primarily the parotid glands, by new advances in radiation techniques, including the appearance and optimizing of 3D treatment planning, conformal radiation techniques, and intensity-modified radiotherapy (IMRT) [20, 21]. Recent studies also showed that the prevention of hyposalivation secondary to RT may be addressed via use of cytoprotective agents (eg. amifostine), the application of lubricating or stimulatory agents, surgical transfer of submandibular glands, and acupuncture during and following cancer treatment [11, 22–24].
Treatment of salivary gland hypofunction secondary to RT includes systemic parasympathomimetic agents with muscarinic action (pilocarpine HCl and cevimeline) [25]. Pilocarpine is recommended to be administered at a dose of 5 mg 3 times a day for at least 3 months [26]. Cevimeline is also recommended for a minimum of 3 months to achieve clinical results, 30 mg 3 times daily [27]. Common side effects for both medications include increased excess of sweating, dyspepsia, nausea, and diarrhea [28]. Topical agents include over-the-counter saliva substitutes and mucosal lubricants, as well as non-pharmacological approaches to mechanically stimulate salivary flow, such as sugar-free lozenges and gums [29]. Acupuncture and hyperbaric oxygen (HBO) therapy have been also used as a possible intervention for the treatment of radiation-induced xerostomia in patients with a residual functional capacity of the salivary glands with controversial results [11, 30]. Low-level laser therapy (LLLT) has proven effects in promoting biomodulation in the cellular metabolism and has been effectively used as a salivary stimulants in patients with reduce salivary flow rate due to chemotherapy and radiotherapy [31]. More recently, salivary gland transfer and gene therapy, using human aquaporin-1 gene transfer, are strategies that appear potentially useful for preventing salivary gland radiation damage [32, 33]. The regenerative medicine options include adipose tissue–derived mesenchymal stem cell and adult salivary gland–derived stem cells [32].
Graft vs. Host Disease- Induced SGD
Chronic graft vs.-host-disease (cGVHD) is a complication that may occur in 30–70% of patients undergoing allogeneic hematopoietic stem cell transplant (HSCT) [34]. Oral cGVHD is characterized by lichenoid changes, ulcers, erythema, and salivary gland hypofunction [35] Salivary gland involvement is characterized by destruction of secretory acini and ducts, resulting in decreased production of saliva and defense proteins [36]. HSCT patients typically experience dry mouth after receiving conditioning regimens, and this may persist through the period when salivary gland cGVHD develops, making the onset and diagnosis less evident. cGVHD of the salivary glands results in both quantitative and qualitative changes in salivary production, composition, and output [37]. Sialagogues such as pilocarpine and cevimeline have shown to improve symptoms in approximately two thirds of patients and recommended dosing is the same as described for RT-induced hyposalivation [38].
The effects of salivary gland hypofunction in these patients include rampant decay and recurrent oral candidiasis, especially if there is ongoing topical corticosteroid therapy for management of mucosal cGVHD, which suppresses mucosal immunity. An additional feature of oral cGVHD is the development of recurrent superficial mucoceles, suggesting that the underlying inflammation may be secondary to generalized mucosal involvement or because of direct salivary gland tissue targeting [39, 40].
Chemotherapy- Induced SGD
Chemotherapy induced dry mouth is prevalent in 10–80% of patients undergoing treatment, regardless of the type of cancer [41]. The onset of oral symptoms generally begins in the 7th to the 10th day after the administration of chemotherapy and resolves after completion of chemotherapy, unlike radiotherapy-induced SGD, which persists for years after radiation therapy is completed. Symptoms include, but are not limited to, dry oral mucosa with consequential oral pseudomembranous candidiasis, halitosis, oral dysesthesia, hypogeusia, and difficulties in chewing, swallowing, and speaking [41].
Changes in salivary gland function can be caused by several chemotherapeutic agents including doxorubicin, cyclophosphamide, fluorouracil, methotrexate, and vinblastine [42]. Some cancer patients may also be on anticholinergic medications for therapy-induced nausea or diarrhea (e.g., for irinotecan-related early diarrhea) which may contribute to xerostomia [7].
Clinical presentation of chemotherapy induced SGD is variable, with some patients presenting with dry mucous membranes of varying severity, while others complaining of excessive salivation with drooling because of dysphagia or odynophagia. As opposed to RT-induced dry mouth, chemotherapy-related dry mouth is typically reversible. Therefore, management is palliative in nature and aims to relieve the temporary symptoms that manifest during treatment. This includes lifestyle modifications, use of saliva substitutes and mucosal lubricants, and short-term use of non-pharmacologic stimuli (gustatory stimuli, sugarless gum) [43, 44]. Acupuncture, tomotherapy, and 1% malic acid spray are currently under investigation, without published results yet.
Immunotherapy–Induced SGD
Immune checkpoint inhibitors (ICIs) have proven to be an effective treatment option for patients with many forms of advanced cancers by preventing cancer cell evasion mechanisms through the suppression of major immune regulatory pathways, such as PD-1/PD-L1 and CTLA-4 [45]. The introduction of ICI therapy has been accompanied by a constellation of adverse events, likely secondary to T-cell–mediated immune reactions, resulting in increases in proinflammatory cytokines or enhancement of complement and autoantibody-mediated immune injury. Immune-related adverse events (irAEs) may virtually affect every organ system [46, 47], including the salivary glands. The development of ICI-induced sicca syndrome usually develops within the first 3–4 months of treatment [48]. Clinical features include severe hyposalivation with xerostomia, ocular dryness, and in some cases parotid gland swelling. Management depends on the severity of the irAE and is with over-the-counter topical agents, sialagogues, and systemic treatment with prednisone 1–2 mg/kg or hydroxychloroquine for severe cases [49]. ICI treatment interruptions may also help [50].
SGD Complications and Management
Dental Caries
Patient who develops dry mouth secondary to cancer therapy are at risk of developing dental caries. A literature review conducted by the Oral Care Study Group of the Multinational Association of Supportive Care in Cancer and the International Society for Oral Oncology (MASCC/ISOO) which included 37 head and neck cancer trials, showed that the prevalence of dental caries in head and neck cancer patients treated with radiation was 24% (4 studies) and 21% respectively (9 studies) for those receiving chemoradiation [51]. Patients with oral chronic GVHD also present with a > 50% increased number of cervical and interproximal dental caries [52]. The management and prevention of dental caries for cancer patients include regular dental care, maintenance of meticulous oral hygiene, daily sodium fluoride application for 3–4 min on teeth using a toothbrush or custom trays (patient should be instructed to avoid rinsing or eating for the next 30 min following the application) and dietary modification, minimizing consumption of cariogenic and acidic foods [53].
Oral Candidiasis
Patients with salivary gland hypofunction may develop a secondary Candida infection in 39–62% of cases while receiving cancer treatment [54, 55] Although Candida albicans has been the most common Candida species detected in cancer patients, the prevalence of non-albicans Candida species (NACS) varies from 37% to 51% and is associated with increased drug resistance [56].Treatment of oral candidiasis involves either topical or systemic antifungal drugs; topical agents are considered preferable to systemic agents due to lower risk of side-effects and drug interactions [55]. The Infectious Diseases Society of America (IDSA) guidelines recommend the use of clotrimazole troches or nystatin suspension (easy to use for patient with hyposalivation) as first-line option for the management of mild candidiasis [57]. A common systemic agent as fluconazole is best used for short courses to prevent the development of resistance. Fluconazole was found to be effective in the prevention of clinical oral fungal infection and in the management of moderate to severe fungal colonization in patients receiving cancer therapy [57]. For fluconazole-refractory cases, the IDSA guidelines recommend the use of itraconazole or posaconazole, with voriconazole and amphotericin B and the echinocandins (caspofungin, anidulafungin, and micafungin) [55].
Malnutrition
In cancer patients an average loss of 8 to 10% of body weight is common, even with early nutritional support [58]. SGD can contribute to difficulties in maintaining adequate nutrition during and after chemotherapy and RT. Cancer therapy may also cause dysphagia, odynophagia, loss of taste, dysgeusia and dehydration, all of which may result in malnutrition [59]. Patients on enteral nutrition had significantly less average weight loss during therapy (3.1 vs. 7.0 kg), required significantly fewer hospitalizations for dehydration and malnutrition, and had fewer interruptions in their cancer treatment (0 % vs. 18%) compared to patients on normal oral food intake [60, 61].
Psychological Disorders
Individuals suffering from cancer are at high risk of experiencing major depressive episodes throughout treatment, although this risk appears to be especially prominent within the first year of diagnosis. The estimated prevalence of anxiety and depression among cancer survivors is 17.9% and 11.6%, respectively [62].
As part of the treatment regimen the most commonly prescribed medications include tricyclic antidepressants (TCAs) and selective serotonin reuptake inhibitors (SSRIs). While both classes of medications have been linked to hyposalivation, in a study done on parotid salivary flow rates, TCAs exhibited a 58% reduction in flow compared to 32% with SSRIs [63]. A meta-analysis performed by Capetta et al. further looked at selective norepinephrine reuptake inhibitors (SNRIs) and found that they significantly increased the risk of developing dry mouth when compared to SSRIs [64]. These data, when combined with the increased risk that head and neck cancer patients already face from SGD, indicate the need for careful prescription of antidepressants for this patient population [65].
Conclusions
SGD is a common and debilitating complication that affects almost two-thirds of patients undergoing cancer treatment. Early diagnosis and management may result in decreased morbidity associated with SGD and improve well-being. Dental specialists are integral part of the cancer treatment team, and provide comprehensive education, supportive care, and the therapy of SGD related to cancer treatment. The current management approach of SGD is mostly palliative and aims to increase the amount of saliva and minimize the risk of secondary effects such as dental caries, dysgeusia and fungal infections.
Patients with SGD from cancer therapy should be followed by their dentist regularly for regular checkups and prescribed sodium fluoride 1.1% gel or toothpaste to reduce the risk of rampant decays.
In summary, SGD therapy is an important component of care prior to and during cancer therapy and amongst survivors. A multi-disciplinary approach is fundamental in the successful management of this complication in oncology and bone marrow transplant patients.
Author Contributions
AVM is participated in conceptualization of the work, data collection and summary, and wrote the mini review. GP participated in data collection and preparation of the manuscript. VS provided feedback on the manuscript. CS participated as an advisor and in the manuscript preparation. AV supervised the work, participated in the analysis of the data, and manuscript preparation. All authors approved the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. Cancer Facts & Figure 2021: American Cancer Society (2021) (Available online at: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2021/cancer-facts-and-figures-2021.pdf.
3. Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. (2018) 391:1023–75. doi: 10.1016/S0140-6736(17)33326-3
4. Epstein JB, Thariat J, Bensadoun RJ, Barasch A, Murphy BA, Kolnick L, et al. Oral complications of cancer and cancer therapy: from cancer treatment to survivorship. CA Cancer J Clin. (2012) 62:400–22. doi: 10.3322/caac.21157
5. Mercadante S, Aielli F, Adile C, Ferrera P, Valle A, Fusco F, et al. Prevalence of oral mucositis, dry mouth, and dysphagia in advanced cancer patients. Support Care Cancer. (2015) 23:3249–55. doi: 10.1007/s00520-015-2720-y
6. Dawes C, Pedersen AM, Villa A, Ekström J, Proctor GB, Vissink A, et al. The functions of human saliva: a review sponsored by the world workshop on oral medicine VI. Arch Oral Biol. (2015) 60:863–74. doi: 10.1016/j.archoralbio.2015.03.004
7. Wolff A, Joshi RK, Ekström J, Aframian D, Pedersen AM, Proctor G, et al. A Guide to medications inducing salivary gland dysfunction, xerostomia, and subjective sialorrhea: a systematic review sponsored by the world workshop on oral medicine VI. Drugs R D. (2017) 17:1–28. doi: 10.1007/s40268-016-0153-9
8. Iorgulescu G. Saliva between normal and pathological. important factors in determining systemic and oral health. J Med Life. (2009) 2:303–7.
9. Navazesh M, Christensen C, Brightman V. Clinical criteria for the diagnosis of salivary gland hypofunction. J Dent Res. (1992) 71:1363–9. doi: 10.1177/00220345920710070301
10. Ghezzi EM, Lange LA, Ship JA. Determination of variation of stimulated salivary flow rates. J Dent Res. (2000) 79:1874–8. doi: 10.1177/00220345000790111001
11. Jensen SB, Pedersen AM, Vissink A, Andersen E, Brown CG, Davies AN, et al. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: prevalence, severity and impact on quality of life. Support Care Cancer. (2010) 18:1039–60. doi: 10.1007/s00520-010-0827-8
12. Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE™): National Cancer Institute. Available online at: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf
13. Meirovitz A, Murdoch-Kinch CA, Schipper M, Pan C, Eisbruch A. Grading xerostomia by physicians or by patients after intensity-modulated radiotherapy of head-and-neck cancer. Int J Radiat Oncol Biol Phys. (2006) 66:445–53. doi: 10.1016/j.ijrobp.2006.05.002
14. Santiago PHR, Song Y, Hanna K, Nair R. Degrees of xerostomia? a rasch analysis of the xerostomia inventory community. Dent Oral Epidemiol. (2020) 48:63–71. doi: 10.1111/cdoe.12504
15. Paine CC 2nd, Snider JW 3rd. When saliva becomes a problem: the challenges and palliative care for patients with sialorrhea. Ann Palliat Med. (2020) 9:1333–9. doi: 10.21037/apm.2020.02.34
17. Grundmann O, Mitchell GC, Limesand KH. Sensitivity of salivary glands to radiation: from animal models to therapies. J Dent Res. (2009) 88:894–903. doi: 10.1177/0022034509343143
18. Mercadante V, Al Hamad A, Lodi G, Porter S, Fedele S. Interventions for the management of radiotherapy-induced xerostomia and hyposalivation: a systematic review and meta-analysis. Oral Oncol. (2017) 66:64–74. doi: 10.1016/j.oraloncology.2016.12.031
19. Deasy JO, Moiseenko V, Marks L, Chao KS, Nam J, Eisbruch A. Radiotherapy dose-volume effects on salivary gland function. Int J Radiat Oncol Biol Phys. (2010) 76(Suppl. 3):S58–63. doi: 10.1016/j.ijrobp.2009.06.090
20. Brennan MT, Elting LS, Spijkervet FK. Systematic reviews of oral complications from cancer therapies, oral care study group, MASCC/ISOO: methodology and quality of the literature. Support Care Cancer. (2010) 18:979–84. doi: 10.1007/s00520-010-0856-3
21. Wendt TG, Abbasi-Senger N, Salz H, Pinquart I, Koscielny S, Przetak S-M, et al. 3D-conformal-intensity modulated radiotherapy with compensators for head and neck cancer: clinical results of normal tissue sparing. Radiation Oncology. (2006) 1:18. doi: 10.1186/1748-717X-1-18
22. Nuchit S, Lam-Ubol A, Paemuang W, Talungchit S, Chokchaitam O, Mungkung O-O, et al. Alleviation of dry mouth by saliva substitutes improved swallowing ability and clinical nutritional status of post-radiotherapy head and neck cancer patients: a randomized controlled trial. Supportive Care Cancer. (2020) 28:2817–28. doi: 10.1007/s00520-019-05132-1
23. King M, Joseph S, Albert A, Thomas TV, Nittala MR, Woods WC, et al. Use of amifostine for cytoprotection during radiation therapy: a review. Oncology. (2020) 98:61–80. doi: 10.1159/000502979
24. Peters GJ, van der Vijgh WJ. Protection of normal tissues from the cytotoxic effects of chemotherapy and radiation by amifostine (WR-2721): preclinical aspects. Eur J Cancer. (1995) 31A (Suppl. 1):S1–7. doi: 10.1016/0959-8049(95)00145-9
25. Gil-Montoya JA, Silvestre FJ, Barrios R, Silvestre-Rangil J. Treatment of xerostomia and hyposalivation in the elderly: a systematic review. Med Oral Patol Oral Cir Bucal. (2016) 21:e355–66. doi: 10.4317/medoral.20969
26. Vivino FB, Al-Hashimi I, Khan Z, LeVeque FG, Salisbury PL 3rd, Tran-Johnson TK, et al. Pilocarpine tablets for the treatment of dry mouth and dry eye symptoms in patients with Sjögren syndrome: a randomized, placebo-controlled, fixed-dose, multicenter trial P92-01 study group. Arch Intern Med. (1999) 159:174–81. doi: 10.1001/archinte.159.2.174
27. Fife RS, Chase WF, Dore RK, Wiesenhutter CW, Lockhart PB, Tindall E, et al. Cevimeline for the treatment of xerostomia in patients with Sjögren syndrome: a randomized trial. Arch Intern Med. (2002) 162:1293–300. doi: 10.1001/archinte.162.11.1293
28. Freige C, Ford C. CADTH Rapid Response Reports. In: Pilocarpine for Sjögren's Syndrome-Induced Dry Mouth and Dry Eyes: A Review of Clinical Effectiveness, Cost-Effectiveness, and Guidelines. Ottawa, ON: Canadian Agency for Drugs and Technologies in Health (2020).
29. Sciubba JJ, Goldenberg D. Oral complications of radiotherapy. Lancet Oncol. (2006) 7:175–83. doi: 10.1016/S1470-2045(06)70580-0
30. Garcia MK, Meng Z, Rosenthal DI, Shen Y, Chambers M, Yang P, et al. Effect of true and sham acupuncture on radiation-induced xerostomia among patients with head and neck cancer: a randomized clinical trial. JAMA Netw Open. (2019) 2:e1916910. doi: 10.1001/jamanetworkopen.2019.16910
31. Gonnelli FA, Palma LF, Giordani AJ, Deboni AL, Dias RS, Segreto RA, et al. Low-Level laser for mitigation of low salivary flow rate in head and neck cancer patients undergoing radiochemotherapy: a prospective longitudinal study. Photomed Laser Surg. (2016) 34:326–30. doi: 10.1089/pho.2016.4104
32. Mercadante V, Jensen SB, Smith DK, Bohlke K, Bauman J, Brennan MT, et al. Salivary gland hypofunction and/or xerostomia induced by nonsurgical cancer therapies: ISOO/MASCC/ASCO Guideline. J Clin Oncol. (2021) 39:2825–43. doi: 10.1200/JCO.21.01208
33. Baum BJ, Alevizos I, Chiorini JA, Cotrim AP, Zheng C. Advances in salivary gland gene therapy – oral and systemic implications. Expert Opin Biol Ther. (2015) 15:1443–54. doi: 10.1517/14712598.2015.1064894
34. Lee SJ, Flowers MED. Recognizing and managing chronic graft-versus-host disease. Hematology. (2008) 2008:134–41. doi: 10.1182/asheducation-2008.1.134
35. Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: i. the 2014 diagnosis and staging working group report. Biol Blood Marrow Transplant. (2015) 21:389–401.e1. doi: 10.1016/j.bbmt.2014.12.001
36. Imanguli MM, Atkinson JC, Mitchell SA, Avila DN, Bishop RJ, Cowen EW, et al. Salivary gland involvement in chronic graft-versus-host disease: prevalence, clinical significance, and recommendations for evaluation. Biol Blood Marrow Trans. (2010) 16:1362–9. doi: 10.1016/j.bbmt.2010.03.023
37. Treister N, Duncan C, Cutler C, Lehmann L. How we treat oral chronic graft-versus-host disease. Blood. (2012) 120:3407–18. doi: 10.1182/blood-2012-05-393389
38. Mousavian M, Sroussi H, Villa A, Cutler C, Treister N. Use of prescription sialagogues for management of xerostomia in chronic graft-versus-host-disease. Transplant Cell Ther. (2021) 27:480.e1–5. doi: 10.1016/j.jtct.2021.02.020
39. Tollemar V, Tudzarovski N, Warfvinge G, Yarom N, Remberger M, Heymann R, et al. Histopathological grading of oral mucosal chronic graft-versus-host disease: large cohort analysis. Biol Blood Marrow Trans. (2020) 26:1971–9. doi: 10.1016/j.bbmt.2020.06.031
40. Balasubramaniam R, Alawi F, DeRossi S. Superficial mucoceles in chronic graft-versus-host disease: a case report and review of the literature. Gen Dent. (2009) 57:82–8.
41. Pinto VL, Fustinoni SM, Nazário ACP, Facina G, Elias S. Prevalence of xerostomia in women during breast cancer chemotherapy. Rev Bras Enferm. (2020) 73:e20190785. doi: 10.1590/0034-7167-2019-0785
42. Xu Y, Wen N, Sonis ST, Villa A. Oral side effects of immune checkpoint inhibitor therapy (ICIT): an analysis of 4683 patients receiving ICIT for malignancies at Massachusetts general hospital, brigham & women's hospital, and the dana-farber cancer institute, 2011 to 2019. Cancer. (2021) 127:1796–804. doi: 10.1002/cncr.33436
43. Peterson DE, Schubert MM. Oral toxicity. In: Perry MC, editor, The Chemotherapy Source Book. 3rd, Baltimore, MD: Williams & Wilkin (2001).
44. Braga MA, Tarzia O, Bergamaschi CC, Santos FA, Andrade ED, Groppo FC. Comparison of the effects of pilocarpine and cevimeline on salivary flow. Int J Dent Hyg. (2009) 7:126–30. doi: 10.1111/j.1601-5037.2008.00326.x
45. Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nature Reviews Immunol. (2020) 20:651–68. doi: 10.1038/s41577-020-0306-5
46. Postow MA, Sidlow R, Hellmann MD. Immune-Related adverse events associated with immune checkpoint blockade. N Engl J Med. (2018) 378:158–68. doi: 10.1056/NEJMra1703481
47. Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. (2019) 16:563–80. doi: 10.1038/s41571-019-0218-0
48. Warner BM, Baer AN, Lipson EJ, Allen C, Hinrichs C, Rajan A, et al. Sicca syndrome associated with immune checkpoint inhibitor therapy. Oncologist. (2019) 24:1259–69. doi: 10.1634/theoncologist.2018-0823
49. Klein BA, Alves FA, Santana Rodrigues Velho J, Vacharotayangul P, Hanna GJ, Leboeuf NR, et al. Oral manifestations of immune-related adverse events in cancer patients treated with immune checkpoint inhibitors. Oral Dis. (2022) 28:9–22. doi: 10.1111/odi.13964
50. Bustillos H, Indorf A, Alwan L, Thompson J, Jung L. Xerostomia: an immunotherapy-related adverse effect in cancer patients. Supportive Care Cancer. (2022) 30:1681–7. doi: 10.1007/s00520-021-06535-9
51. Deng J, Jackson L, Epstein JB, Migliorati CA, Murphy BA. Dental demineralization and caries in patients with head and neck cancer. Oral Oncol. (2015) 51:824–31. doi: 10.1016/j.oraloncology.2015.06.009
52. Castellarin P, Stevenson K, Biasotto M, Yuan A, Woo S-B, Treister NS. Extensive dental caries in patients with oral chronic graft-versus-host disease. Biol Blood Marrow Transplant. (2012) 18:1573–9. doi: 10.1016/j.bbmt.2012.04.009
53. Lee HJ, Han DH, Kim JH, Wu HG. The effect of comprehensive oral care program on oral health and quality of life in patients undergoing radiotherapy for head and neck cancer: a quasi-experimental case-control study. Medicine (Baltimore). (2021) 100:e25540. doi: 10.1097/MD.0000000000025540
54. Ramirez-Amador V, Silverman S. Jr., Mayer P, Tyler M, Quivey J. Candidal colonization and oral candidiasis in patients undergoing oral and pharyngeal radiation therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. (1997) 84:149–53. doi: 10.1016/S1079-2104(97)90061-5
55. Lalla RV, Latortue MC, Hong CH, Ariyawardana A, D'Amato-Palumbo S, Fischer DJ, et al. A systematic review of oral fungal infections in patients receiving cancer therapy. Supportive Care Cancer. (2010) 18:985–92. doi: 10.1007/s00520-010-0892-z
56. Tarapan S, Matangkasombut O, Trachootham D, Sattabanasuk V, Talungchit S, Paemuang W, et al. OralCandidacolonization in xerostomic postradiotherapy head and neck cancer patients. Oral Dis. (2019) 25:1798–808. doi: 10.1111/odi.13151
57. Pappas PG, Kauffman CA, Andes D, Benjamin DK, Calandra TF, Edwards JE, et al. Clinical practice guidelines for the management candidiasis: 2009 update by the infectious diseases society of America. Clin Infect Dis. (2009) 48:503–35. doi: 10.1086/596757
58. Hardy S, Haas K, Vanston VJ, Angelo M. Prophylactic feeding tubes in head and neck cancers #318. J Palliat Med. (2016) 19:1343–4. doi: 10.1089/jpm.2016.0381
59. Pajak TF, Laramore GE, Marcial VA, Fazekas JT, Cooper J, Rubin P, et al. Elapsed treatment days–a critical item for radiotherapy quality control review in head and neck trials: RTOG report. Int J Radiat Oncol Biol Phys. (1991) 20:13–20. doi: 10.1016/0360-3016(91)90132-N
60. Bossola M. Nutritional interventions in head and neck cancer patients undergoing chemoradiotherapy: a narrative review. Nutrients. (2015) 7:265–76. doi: 10.3390/nu7010265
61. Xiao C, Hanlon A, Zhang Q, Ang K, Rosenthal DI, Nguyen-Tan PF, et al. Symptom clusters in patients with head and neck cancer receiving concurrent chemoradiotherapy. Oral Oncol. (2013) 49:360–6. doi: 10.1016/j.oraloncology.2012.10.004
62. Mitchell AJ, Ferguson DW, Gill J, Paul J, Symonds P. Depression and anxiety in long-term cancer survivors compared with spouses and healthy controls: a systematic review and meta-analysis. Lancet Oncol. (2013) 14:721–32. doi: 10.1016/S1470-2045(13)70244-4
63. Daly C. Oral and dental effects of antidepressants. Aust Prescr. (2016) 39:84. doi: 10.18773/austprescr.2016.035
64. Cappetta K, Beyer C, Johnson JA, Bloch MH. Meta-analysis: risk of dry mouth with second generation antidepressants. Prog Neuropsychopharmacol Biol Psychiatry. (2018) 84(Pt A):282–93. doi: 10.1016/j.pnpbp.2017.12.012
65. Lydiatt WM, Bessette D, Schmid KK, Sayles H, Burke WJ. Prevention of depression with escitalopram in patients undergoing treatment for head and neck cancer: randomized, double-blind, placebo-controlled clinical trial. JAMA Otolaryngol Head Neck Surg. (2013) 139:678–86. doi: 10.1001/jamaoto.2013.3371
Keywords: cancer, dry mouth, xerostomia, hyposalivation, radiotherapy, chemotherapy, management
Citation: Vistoso Monreal A, Polonsky G, Shiboski C, Sankar V and Villa A (2022) Salivary Gland Dysfunction Secondary to Cancer Treatment. Front. Oral. Health 3:907778. doi: 10.3389/froh.2022.907778
Received: 30 March 2022; Accepted: 12 May 2022;
Published: 09 June 2022.
Edited by:
Giulia Ottaviani, University of Milan, ItalyReviewed by:
Sven E. Niklander, Universidad Andres Bello, ChileGrace Bradley, University of Toronto, Canada
Copyright © 2022 Vistoso Monreal, Polonsky, Shiboski, Sankar and Villa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anette Vistoso Monreal, YW5ldHRlLnZpc3Rvc29tb25yZWFsQHVjc2YuZWR1
 Anette Vistoso Monreal
Anette Vistoso Monreal Gregory Polonsky
Gregory Polonsky Caroline Shiboski
Caroline Shiboski Vidya Sankar
Vidya Sankar Alessandro Villa
Alessandro Villa