- Department of Oral and Maxillofacial Diagnostic Sciences, Division of Oral Medicine, University of Florida College of Dentistry, Gainesville, FL, United States
Medication-Related Osteonecrosis of the Jaw (MRONJ) was first reported in 2003. Despite the progress in the understanding of this oral complication in cancer patients for the past 18 years, there is still discussion about the best way to define MRONJ, prevent the complication, how to diagnose, and the options of treatment available. The initial reports associated MRONJ to bisphosphonates and denosumab, medications that work as bone-modifying agents. Later, other agents such as the antiangiogenics, have also been reported to cause the oral complication, either alone or in combination with antiresorptives. Initially, these medications were prescribed to patients with osteoporosis and cancers patients with bone metastasis. Today, because of the effect of the medications in the bone remodeling system, patients with several other diseases such as giant cell tumors, rheumatoid arthritis, Paget's disease of bone, fibrous dysplasia, osteogenesis imperfecta, are managed with these medications, significantly increasing the population of individuals at risk for developing MRONJ. This mini review focused on the cancer patient. It updates the dental clinician on the recent scientific literature about MRONJ and provides information on how to diagnose and manage patients being treated with these medications, suggests protocols to prevent the development of MRONJ, and present ways to manage those patients who develop the oral complication.
Introduction
The history of MRONJ started about 18 years ago when it was first reported [1–3]. After many suggestions to name the complication, including ONJ, BON, BIONJ, ostechemonecrosis, BONJ, and many others, the final universal agreement came with a proposal from the clinical guidelines article from the American Academy of Oral and Maxillofacial Surgeons (AAOMS) in 2014 that named it “medication-related osteonecrosis of the jaws, or MRONJ” [4] due to its association with medications. This is the terminology we will use throughout this mini review. Despite the progress in the understanding of this oral complication in patients with cancer, there is still discussion about the best way to define, diagnose, and stage MRONJ, the mechanisms that lead to the development of the oral complication, management alternatives with medical or surgical interventions, and best prevention measures. We will discuss current knowledge about patient management, with the goal of assisting the dental provider when treating patients, taking drugs reported to be associated with MRONJ and those patients who develop the complication.
Methods
We conducted a brief PubMed review of the recent literature, addressing MRONJ in patients with cancer. Using the key words bisphosphonates and osteonecrosis, the initial search revealed 3,635 publications from 2003 through 2021. From this search, relevant articles written in English were reviewed, and pertinent information was collected. When available, clinical trials were used as the main source of information. Otherwise, important research and personal expert experience developed during the past 18 years will be used. A recent joint clinical practice guidelines manuscript published by the Multinational Association of Supportive Care in Cancer, the International Society of Oral Oncology and the American Society of Clinical Oncology (MASCC/ISOO/ASCO) has revealed a lack of robust clinical trials, making difficult to produce guidelines based solely on scientific evidence [5].
Mini-Review Results
Definition, Diagnosis, and Clinical Staging
MRONJ is an oral manifestation characterized by exposed necrotic jawbone of patients who are using one of the medications that have been associated with the complication. To diagnose this condition, the clinician should confirm the presence of the three following criteria [4, 5]:
• Current or previous treatment with bone-modifying agents, such as a bisphosphonate, denosumab, or an antiangiogenic.
• The presence of an exposed necrotic bone or a bone that can be probed through an intraoral or extraoral fistula in the maxillofacial region and that has persisted for longer than 8 weeks.
• No history of radiation therapy to the jaws or obvious metastatic disease to the jaws.
In addition to a precise definition and diagnosis, one should stage the complication before management is proposed (Figure 1). Staging of MRONJ is of importance in the decision-making process on how to manage each of the patients. The staging classification proposed in 2014 by the AAOMS guidelines article established the following staging criteria (updated based on new evidence):
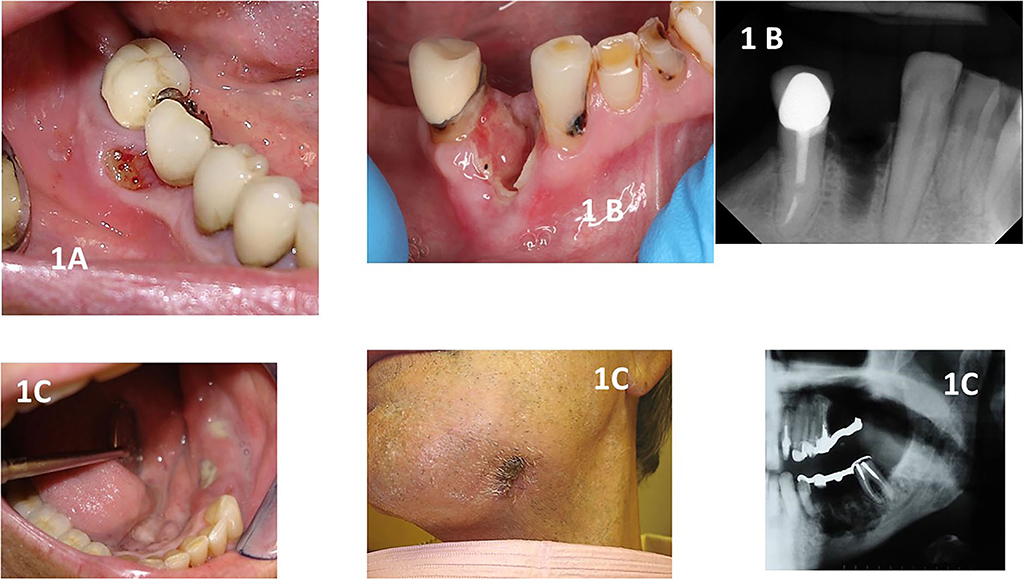
Figure 1. Shows clinical and radiographical images of different stages of MRONJ. (A): stage 1 showing a patient with breast cancer taking zoledronic acid with a small asymptomatic area of exposed necrotic bone that was affecting the use of a removable prosthodontic appliance. (B): shows a patient taking denosumab who had a recent dental extraction. The site was not healing, and exposed alveolar bone could be seen. The patient was in pain and did not respond to antibiotics. The radiographical image shows the non-healing alveolus. (C): shows a patient with multiple myeloma with Stage 3 MRONJ. The patient was in pain and presented to the clinic with swelling of the alveolar mucosa and several areas of infected and exposed bone with pus drainage. The patient complained of paresthesia in the area and had a strong mal odor. One can observe an extraoral fistula on the left submandibular area. The radiograph shows the extensive area of bone destruction, placing the patient at risk for a pathological fracture. Note that the fixed prosthodontic appliance was removed, revealing the exposed bone seen in this figure.
At Risk
No apparent necrotic bone in patients who have been or who are being treated with oral or intravenous antiresorptives and or antiangiogenics.
Stage Zero
No clinical evidence of a necrotic bone but nonspecific clinical findings, radiographic changes, and symptoms. This staging terminology is controversial and may lead to under or overdiagnosis MRONJ. Clinicians should be aware of this possibility when diagnosing patients at risk for MRONJ [6–8].
Stage 1
Exposed and necrotic bones or fistulas that probe to be bones in patients who are asymptomatic and have no evidence of infection.
Stage 2
Exposed and necrotic bones or fistulas that probe to be bones associated with infection as evidenced by pain and erythema in the region of exposed bones with or without purulent drainage.
Stage 3
Exposed and necrotic bones or a fistula that probes to be a bone in patients with pain, infection, and ≥ 1 of the following: exposed and necrotic bones extending beyond the region of alveolar bone (i.e., inferior border and ramus in mandible, maxillary sinus, and zygoma in maxilla), resulting in pathologic fracture, extraoral fistula, oral antral or oral nasal communication, or osteolysis extending to the inferior border of the mandible or sinus floor.
An important aspect of the staging system is presented by the Italian consortium on MRONJ, stating that imaging examination is necessary to precisely diagnose and stage the oral complication [9]. Areas of osteosclerosis and bone changes can assist the clinician in determining the real extension of the complication and help in the planning of management [10, 11].
Prevalence of MRONJ
How prevalent is MRONJ among patients being treated with one of the drugs associated with the oral complication? The prevalence is small. For the dental provider, it is important to know that patients with cancer have a higher risk of developing the complication than patients who use the medications for osteoporosis. A prospective controlled study compared zoledronic acid (ZA) and denosumab use in 5,723 patients with cancer. The overall risk of MRONJ was 1.6%. In patients being treated with zoledronic acid, 1.3% developed MRONJ, whereas 1.8% of patients treated with denosumab developed MRONJ [12]. A review from 2008 to 2015 suggested that the frequency of MRONJ is about 1% (range, 2–6.7%) [13]. The recommendation for the clinician is to consider that any patients exposed to the medications are at risk to develop the complication.
What Are the Drugs That Have Been Reported to Be Associated With MRONJ?
The first group of drugs associated with MRONJ were the bisphosphonates Pamidronate (Aredia®) and zoledronic acid (Zometa®). These drugs inhibit osteoclasts and are used to treat patients with cancer with malignancies that metastasized to bones, such as multiple myeloma, breast, prostate, and lung [1, 2, 14]. Following, with the development of denosumab (XGeva®), a humanized monoclonal antibody with similar action over osteoclasts, new cases of MRONJ were reported. Currently, several other drug groups have been associated with MRONJ, including the antiangiogenics, targeted therapy, and biologic immunomodulators [15–17]. The use of these drugs places individuals at risk for the development of MRONJ. The clinician must certify if a patient is being treated with one of the drugs when reviewing medical history so a prevention protocol can be used during patient care. It has been proposed that patients with cancer being treated with a combination of bisphosphonates and antiangiogenics may be at increased risk for MRONJ [18].
Common Signs and Symptoms Associated With MRONJ
The most common signs and symptoms associated with MRONJ observed in patients who have developed the complication include pain, infection with purulent secretion, general jaw discomfort, paresthesia, mal odor, a non-healing extraction site, or a sore associated with an ill-fitting denture [4, 5]. Of major importance is the presence of exposed necrotic bone or bone that could be probed through a fistula, according to the currently accepted definition of MRONJ [4].
It has been postulated that clinicians who manage patients considered at risk for MRONJ would benefit from having a diagnostic test that would indicate increased risk for MRONJ prior to doing invasive dental care. A study has hypothesized that bone remodeling markers may be indicators of the risk of development of MRONJ [19]. However, there is controversy in the literature whether or not such bone remodeling markers may, indeed, indicate risk [20]. A more recent study using different markers of bone changes in patients taking an antiresorptive medications [21] has evaluated 12 different biomarkers in patients with and without MRONJ. They suggested that tartrate-resistant acid phosphatase isoform 5b (TRACP 5b) levels were significantly lower, and the mean Dickkopf-related protein 1 (DKK1) levels were significantly higher than the corresponding values for the control group (without MRONJ). This indicates the need to carefully follow patients with these abnormal biomarkers before and after dental extractions. However, one must always consider the availability of such tests and the cost of running them.
What Is the Mechanism That Leads to MRONJ?
A large body of research has been published in order to establish the mechanism that leads to the formation of MRONJ. The current evidence is that MRONJ is a multifactorial complication resulting from the effect of antiresorptive drugs-inhibiting osteoclasts and altering the bone remodeling system [22], the presence of dental infection both in the periodontium and the periapical areas [23, 24], chronic inflammation and acidic environment [25], dental trauma from dental extractions or invasive surgery [26], diabetes and other chronic disease [27], the use of corticosteroids, and altered local immunity [28]. This continues to be researched with the goal of determining the precise mechanism that results in MRONJ. However, there is no doubt that the antiresorptives and the antiangiogenics play a very important role. There is also evidence that the combined use of bisphosphonates and antiangiogenics in certain types of cancer increases the risk for the complication [29].
Suggestions of Management Protocols
Patients at Risk for MRONJ and Prevention Protocols
Patients who will be prescribed medications associated with MRONJ should have their oral health stabilized as soon as possible, preferably prior to the start of the drug therapy. A complete evaluation of teeth, periodontium and radiographic examination should be done. The dentist should perform dental extractions of hopeless teeth, scaling and root planning, dental restorations, and implement good oral hygiene. There is evidence that this may prevent or decrease the risk of MRONJ development [5, 6, 30, 31]. A periodic follow-up could be planned, depending on the oral health of each of the patients. If the drug therapy has started, dental procedures can be planned together with the patient's physician. Patients with complex medical conditions may not be exposed to invasive surgical procedures, and a more conservative dental care should be done. One must always consider the best alternative for the overall health of the patient. Although MRONJ is a severe complication, the risk is relatively small. Patients with active dental and periodontal infections may benefit from local treatment to control the infection associated with antibiotic therapy and topical antiseptic rinses [5, 32].
Patients With MRONJ
Dental professionals with expertise in managing MRONJ should be the ones to treat patients with this complication [5]. It is recommended that the decision on how to treat the patient should be done by a multiprofessional team. MRONJ must be staged, the overall medical health status evaluated, and the prognosis of the cancer should be considered. In general, experts propose two modalities of therapy: medical and surgical treatments. However, controversy exists in the literature about which is the best approach [17, 33].
Medical treatment is a more conservative approach. Minor local debridement of areas of exposed necrotic bone can be performed, sharp edges of bone can be eliminated, and active infection managed with antibiotics and topical antibacterial rinses. Some have proposed the use of a combination of pentoxifylline and tocopherol (vitamin E) such as it is done with patients with osteoradionecrosis [34]. However, clinical and radiographic results may take months or years to happen [35, 36]. Patients who cannot use pentoxifylline, cilostasol can be an alternative [37]. Conservative therapy can also be of value to treat patients ineligible to surgery [38].
Surgical treatment as the first choice for the treatment of MRONJ has been proposed regardless of staging [39]. The surgical approach must aim to the removal of all necrotic bones and closure by primary intention [40]. Several studies have shown improvement and resolution of MRONJ with surgical management [41, 42]. Surgical lasers have been used with reasonable success [43, 44].
Discussion
MRONJ is a relatively new oral complication in patients with cancer being treated with medications used in cancer care. We provided current information that may be used by the clinician when managing patients at risk or with MRONJ. However, professional expertise in the diagnosis, staging, and management of patients with MRONJ is of importance for the success of the treatment [5].
It is suggested that a team of medical and dental providers must make all decisions about the best way to manage patients at risk for MRONJ. The idea of the patient discontinuing drug therapy is always present in the clinician's mind. However, the evidence available about a drug holiday prior to invasive dental procedures is not robust [45]. Discontinuation of antiresorptives may lead to more serious complications, such as skeletal-related fractures.
We revised information on the important aspects of taking complete dental and medical histories, detecting signs and symptoms that lead to the suspicion of MRONJ diagnosis and a patient's characteristics that help to determine risk of MRONJ development. We provided basic guidance for the clinician on current proposed prevention and management protocols.
Research Gaps and Future Research Needs
There still exist research gaps that are being investigated by several authors. These gaps provide future research ideas in the field of MRONJ. For example, we still do not know the exact mechanism that leads to the formation of MRONJ. There are several suggestions, but the only thing that can be stated is that it is a multifactorial process [28]. However, what starts MRONJ and how the many mechanisms interact have not been established. There is a lack of information about the mechanisms involved in MRONJ with new, non-antiresorptive drugs that are being reported to be associated with the complication [16]. None of the proposed treatment protocols have been demonstrated to have complete success, but only partial resolution of MRONJ cases [17, 46]. There is a need to better understand the role that osteoimmunity plays [28] and whether or not a genetic predisposition exists among populations [47].
The final take-home message is that patients with cancer are prescribed drugs that help mitigate the burden of oncological disease. As any drug, some are associated with the development of MRONJ but have other beneficial effects. Individual patients have different kinds of cancer staging and prognosis, and only their oncologists can determine the risk of discontinuing a medication or of doing an invasive procedure in the oral cavity. It is common for patients with cancer to have additional comorbidities that may preclude invasive surgical procedures. The recommendation for the clinicians is to work in collaboration with the oncology team when making decisions to treat dental disease in this population of patients.
Ethics Statement
Written informed consent was obtained from the individual(s) for the publication of any identifiable images or data included in this article.
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Migliorati CA. Bisphosphanates and oral cavity avascular bone necrosis. J Clin Oncol. (2003) 21:4253–4. doi: 10.1200/JCO.2003.99.132
2. Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. (2003) 61:1115–7. doi: 10.1016/S0278-2391(03)00720-1
3. Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg. (2004) 62:527–34. doi: 10.1016/j.joms.2004.02.004
4. Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, Mehrotra B, et al. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw−2014 update. J Oral Maxillofac Surg. (2014) 72:1938–56. doi: 10.1016/j.joms.2014.04.031
5. Yarom N, Shapiro CL, Peterson DE, Van Poznak CH, Bohlke K, Ruggiero SL, et al. Medication-Related Osteonecrosis of the Jaw: MASCC/ISOO/ASCO Clinical Practice Guideline. J Clin Oncol. (2019) 37:2270–90. doi: 10.1200/JCO.19.01186
6. Khan AA, Morrison A, Hanley DA, Felsenberg D, McCauley LK, O'Ryan F, et al. Diagnosis and management of osteonecrosis of the jaw: a systematic review and international consensus. J Bone Miner Res. (2015) 30:3–23. doi: 10.1002/jbmr.2405
7. Khan AA, Morrison A, Kendler DL, Rizzoli R, Hanley DA, Felsenberg D, et al. Case-Based Review of Osteonecrosis of the Jaw (ONJ) and Application of the International Recommendations for Management From the International Task Force on ONJ. J Clin Densitom. (2017) 20:8–24. doi: 10.1016/j.jocd.2016.09.005
8. Fedele S, Bedogni G, Scoletta M, Favia G, Colella G, Agrillo A, et al. Up to a quarter of patients with osteonecrosis of the jaw associated with antiresorptive agents remain undiagnosed. Br J Oral Maxillofac Surg. (2015) 53:13–7. doi: 10.1016/j.bjoms.2014.09.001
9. Bedogni A, Fedele S, Bedogni G, Scoletta M, Favia G, Colella G, et al. Staging of osteonecrosis of the jaw requires computed tomography for accurate definition of the extent of bony disease. Br J Oral Maxillofac Surg. (2014) 52:603–8. doi: 10.1016/j.bjoms.2014.04.009
10. Assili Z, Dolivet G, Salleron J, Griffaton-Tallandier C, Egloff-Juras C, Phulpin B. A Comparison of the Clinical and Radiological Extent of Denosumab [Xgeva(®)] Related Osteonecrosis of the Jaw: A Retrospective Study. J Clin Med. (2021) 10:2390. doi: 10.3390/jcm10112390
11. Gaêta-Araujo H, Vanderhaeghen O, Vasconcelos KF, Coucke W, Coropciuc R, Politis C, et al. Osteomyelitis, osteoradionecrosis, or medication-related osteonecrosis of the jaws? Can CBCT enhance radiographic diagnosis? Oral Dis. (2021) 27:312–9. doi: 10.1111/odi.13534
12. Saad F, Brown JE, Van Poznak C, Ibrahim T, Stemmer SM, Stopeck AT, et al. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann Oncol. (2012) 23:1341–7. doi: 10.1093/annonc/mdr435
13. Dodson TB. The Frequency of Medication-related Osteonecrosis of the Jaw and its Associated Risk Factors. Oral Maxillofac Surg Clin North Am. (2015) 27:509–16. doi: 10.1016/j.coms.2015.06.003
14. Migliorati CA, Siegel MA, Elting LS. Bisphosphonate-associated osteonecrosis: a long-term complication of bisphosphonate treatment. Lancet Oncol. (2006) 7:508–14. doi: 10.1016/S1470-2045(06)70726-4
15. Eguia A, Bagán-Debón L, Cardona F. Review and update on drugs related to the development of osteonecrosis of the jaw. Med Oral Patol Oral Cir Bucal. (2020) 25:e71–83. doi: 10.4317/medoral.23191
16. Nicolatou-Galitis O, Kouri M, Papadopoulou E, Vardas E, Galiti D, Epstein JB, et al. Osteonecrosis of the jaw related to non-antiresorptive medications: a systematic review. Support Care Cancer. (2019) 27:383–94. doi: 10.1007/s00520-018-4501-x
17. Schiodt M, Vadhan-Raj S, Chambers MS, Nicolatou-Galitis O, Politis C, Coropciuc R, et al. A multicenter case registry study on medication-related osteonecrosis of the jaw in patients with advanced cancer. Support Care Cancer. (2018) 26:1905–15. doi: 10.1007/s00520-017-4003-2
18. Van Poznak C, Reynolds EL, Estilo CL, Hu M, Schneider BP, Hertz DL, et al. Osteonecrosis of the jaw risk factors in bisphosphonate-treated patients with metastatic cancer. Oral Dis. (2022) 28:193–201. doi: 10.1111/odi.13746
19. Marx RE, Cillo JE Jr, Ulloa JJ. Oral bisphosphonate-induced osteonecrosis: risk factors, prediction of risk using serum CTX testing, prevention, and treatment. J Oral Maxillofac Surg. (2007) 65:2397–410. doi: 10.1016/j.joms.2007.08.003
20. Flichy-Fernández AJ, Alegre-Domingo T, González-Lemonnier S, Balaguer-Martínez J, Peñarrocha-Diago M, Jiménez-Soriano Y, et al. Study of serum CTX in 50 oral surgical patients treated with oral bisphosphonates. Med Oral Patol Oral Cir Bucal. (2012) 17:e367–70. doi: 10.4317/medoral.17583
21. Park JH, Cho S, Kim SJ, Jeong TD, Mun YC, Kim JW. Serum biomarkers for bisphosphonate-related osteonecrosis of the jaw: a prospective clinical study. Osteoporos Int. (2021) 33:367–77. doi: 10.1007/s00198-021-06137-5
22. Aghaloo T, Hazboun R, Tetradis S. Pathophysiology of Osteonecrosis of the Jaws. Oral Maxillofac Surg Clin North Am. (2015) 27:489–96. doi: 10.1016/j.coms.2015.06.001
23. Aghaloo TL, Kang B, Sung EC, Shoff M, Ronconi M, Gotcher JE, et al. Periodontal disease and bisphosphonates induce osteonecrosis of the jaws in the rat. J Bone Miner Res. (2011) 26:1871–82. doi: 10.1002/jbmr.379
24. Aghaloo TL, Cheong S, Bezouglaia O, Kostenuik P, Atti E, Dry SM, et al. RANKL inhibitors induce osteonecrosis of the jaw in mice with periapical disease. J Bone Miner Res. (2014) 29:843–54. doi: 10.1002/jbmr.2097
25. Otto S, Hafner S, Mast G, Tischer T, Volkmer E, Schieker M, et al. Bisphosphonate-related osteonecrosis of the jaw: is pH the missing part in the pathogenesis puzzle? J Oral Maxillofac Surg. (2010) 68:1158–61. doi: 10.1016/j.joms.2009.07.079
26. Hasegawa T, Ueda N, Yamada SI, Kato S, Iwata E, Hayashida S, et al. Denosumab-related osteonecrosis of the jaw after tooth extraction and the effects of a short drug holiday in cancer patients: a multicenter retrospective study. Osteoporos Int. (2021) 32:2323–33. doi: 10.1007/s00198-021-05995-3
27. Peer A, Khamaisi M. Diabetes as a risk factor for medication-related osteonecrosis of the jaw. J Dent Res. (2015) 94:252–60. doi: 10.1177/0022034514560768
28. Chang J, Hakam AE, McCauley LK. Current Understanding of the Pathophysiology of Osteonecrosis of the Jaw. Curr Osteoporos Rep. (2018) 16:584–95. doi: 10.1007/s11914-018-0474-4
29. Nicolatou-Galitis O, Migliorati C. Osteonecrosis of the jaw (ONJ) in patients who receive Bone Targeting Agents (BTAs): the power of e-learning. Ecancermedicalscience. (2018) 12:ed77. doi: 10.3332/ecancer.2018.ed77
30. Campisi G, Mauceri R, Bertoldo F, Bettini G, Biasotto M, Colella G, et al. Medication-Related Osteonecrosis of Jaws (MRONJ) Prevention and Diagnosis: Italian Consensus Update 2020. Int J Environ Res Public Health. (2020) 17:5998. doi: 10.3390/ijerph17165998
31. Migliorati CA, Saunders D, Conlon MS, Ingstad HK, Vaagen P, Palazzolo MJ, et al. Assessing the association between bisphosphonate exposure and delayed mucosal healing after tooth extraction. J Am Dent Assoc. (2013) 144:406–14. doi: 10.14219/jada.archive.2013.0134
32. Tartaroti NC, Marques MM, Naclério-Homem MDG, Migliorati CA, Zindel Deboni MC. Antimicrobial photodynamic and photobiomodulation adjuvant therapies for prevention and treatment of medication-related osteonecrosis of the jaws: Case series and long-term follow-up. Photodiagnosis Photodyn Ther. (2020) 29:101651. doi: 10.1016/j.pdpdt.2020.101651
33. Owosho AA, Liang STY, Sax AZ, Wu K, Yom SK, Huryn JM, et al. Medication-related osteonecrosis of the jaw: An update on the memorial sloan kettering cancer center experience and the role of premedication dental evaluation in prevention. Oral Surg Oral Med Oral Pathol Oral Radiol. (2018) 125:440–5. doi: 10.1016/j.oooo.2018.02.003
34. Martos-Fernández M, Saez-Barba M, López-López J, Estrugo-Devesa A, Balibrea-Del-Castillo JM, Bescós-Atín C. Pentoxifylline, tocopherol, and clodronate for the treatment of mandibular osteoradionecrosis: a systematic review. Oral Surg Oral Med Oral Pathol Oral Radiol. (2018) 125:431–9. doi: 10.1016/j.oooo.2018.02.004
35. Owosho AA, Estilo CL, Huryn JM, Yom SK. Pentoxifylline and tocopherol in the management of cancer patients with medication-related osteonecrosis of the jaw: an observational retrospective study of initial case series. Oral Surg Oral Med Oral Pathol Oral Radiol. (2016) 122:455–9. doi: 10.1016/j.oooo.2016.06.019
36. Cavalcante RC, Tomasetti G. Pentoxifylline and tocopherol protocol to treat medication-related osteonecrosis of the jaw: a systematic literature review. J Craniomaxillofac Surg. (2020) 48:1080–6. doi: 10.1016/j.jcms.2020.09.008
37. de Carvalho EF, Bertotti M, Migliorati CA, Rocha AC. Cilostazol and Tocopherol in the Management of Medication-Related Osteonecrosis of the Jaw: New Insights From a Case Report. J Oral Maxillofac Surg. (2021) 79:2499–506. doi: 10.1016/j.joms.2021.06.036
38. Albanese M, Zotti F, Capocasale G, Bonetti S, Lonardi F, Nocini PF. Conservative non-surgical management in medication related osteonecrosis of the jaw: a retrospective study. Clin Exp Dent Res. (2020) 6:512–8. doi: 10.1002/cre2.303
39. Ruggiero SL, Kohn N. Disease Stage and Mode of Therapy Are Important Determinants of Treatment Outcomes for Medication-Related Osteonecrosis of the Jaw. J Oral Maxillofac Surg. (2015) 73(12 Suppl):S94–s100. doi: 10.1016/j.joms.2015.09.024
40. Çanakçi FG, Er N, Duygu G, Varol GF. Surgical management of stage-2 medication-related osteonecrosis of the jaw with transplantation of human amniotic membrane: Preliminary results. J Stomatol Oral Maxillofac Surg. (2021) S2468-7855(21)00197-X. doi: 10.1016/j.jormas.2021.09.011
41. El-Rabbany M, Lam DK, Shah PS, Azarpazhooh A. Surgical management of medication-related osteonecrosis of the jaw is associated with improved disease resolution: a retrospective cohort study. J Oral Maxillofac Surg. (2019) 77:1816–22. doi: 10.1016/j.joms.2019.03.040
42. Blus C, Giannelli G, Szmukler-Moncler S, Orru G. Treatment of medication-related osteonecrosis of the jaws (MRONJ) with ultrasonic piezoelectric bone surgery. A case series of 20 treated sites. Oral Maxillofac Surg. (2017) 21:41–8. doi: 10.1007/s10006-016-0597-7
43. Momesso GAC, Lemos CAA, Santiago-Júnior JF, Faverani LP, Pellizzer EP. Laser surgery in management of medication-related osteonecrosis of the jaws: a meta-analysis. Oral Maxillofac Surg. (2020) 24:133–44. doi: 10.1007/s10006-020-00831-0
44. Russmueller G, Seemann R, Weiss K, Stadler V, Speiss M, Perisanidis C, et al. The association of medication-related osteonecrosis of the jaw with Actinomyces spp. infection Sci Rep. (2016) 6:31604. doi: 10.1038/srep31604
45. Hadaya D, Soundia A, Gkouveris I, Bezouglaia O, Dry SM, Pirih FQ, et al. Antiresorptive-Type and Discontinuation-Timing Affect ONJ Burden. J Dent Res. (2021) 100:746–53. doi: 10.1177/0022034520986804
46. Otto S, Pautke C, Van den Wyngaert T, Niepel D, Schiødt M. Medication-related osteonecrosis of the jaw: prevention, diagnosis and management in patients with cancer and bone metastases. Cancer Treat Rev. (2018) 69:177–87. doi: 10.1016/j.ctrv.2018.06.007
Keywords: ONJ, MRONJ, osteonecrosis of the jaw, osteonecrosis, oral complications, cancer therapy
Citation: Migliorati CA (2022) Oral Complications in Cancer Patients–Medication-Related Osteonecrosis of the Jaw (MRONJ). Front. Oral. Health 3:866871. doi: 10.3389/froh.2022.866871
Received: 31 January 2022; Accepted: 18 March 2022;
Published: 26 April 2022.
Edited by:
Nathaniel Simon Treister, Brigham and Women's Hospital and Harvard Medical School, United StatesReviewed by:
Marco Meleti, University of Parma, ItalyCopyright © 2022 Migliorati. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cesar Augusto Migliorati, Yy5taWdsaW9yYXRpQGRlbnRhbC51ZmwuZWR1
 Cesar Augusto Migliorati
Cesar Augusto Migliorati