- 1Section of Periodontics, School of Dentistry, University of California, Los Angeles, Los Angeles, CA, United States
- 2School of Dentistry, Federal University of Pelotas, Pelotas, Brazil
Objectives: To perform a comprehensive and integrative review of the available literature on the potential changes in the microbiome of healthy and individuals with diabetes under periodontal health and disease.
Materials and Methods: The review was conducted by two independent reviewers. Indexed electronic databases (PubMed/Medline, Cochrane Library, Web of Science and Scopus) were searched, including articles published in English and dated from 5 years ago until December 2021. A manual search also was performed to identify co-related articles. Following the removal of duplicates and eligibility criteria, the articles were included in tables for analysis and described in the manuscript.
Results: According to this review, diabetes mellitus was associated with significant changes in the subgingival and salivary microbiome, either in its association with periodontitis or in cases of periodontal health. In addition to affecting microbial diversity in terms of taxonomy, metagenomic studies have shown that this endocrine disorder may also be directly related to increased pathogenicity in the oral microbiome.
Conclusion: Although the reviewed studies demonstrate important differences in the subgingival and salivary microbiome composition because of diabetes mellitus, further studies are needed to clarify the real effects of hyperglycemia on oral microbial profiles and support new diagnostic approaches and innovative treatments.
Introduction
Periodontitis is a chronic multifactorial inflammatory disease associated with dysbiotic plaque biofilms and characterized by the progressive destruction of the tooth-supporting apparatus [1]. Diabetes mellitus is a group of metabolic diseases characterized by abnormal insulin secretion, insulin action, or both situations leading to hyperglycemia [2]. Inflammation plays a central role in periodontitis and diabetes mellitus [3]. Both diseases have a high epidemiological impact worldwide with periodontitis affecting nearly 750 million people [4–6], and an estimated 415 million adults aged 20–79 with diabetes mellitus, including 193 million who are undiagnosed [2, 7–9].
Although there are no phenotypic features unique to periodontitis in patients with diabetes mellitus, diabetes mellitus increases periodontitis risk. In addition, periodontitis affects glycemic control and its complications [10–13]. This “two-way” relationship between periodontitis and diabetes mellitus has stimulated the development of several clinical and experimental studies focusing on identifying the molecular pathways that link these two conditions and understanding how they may potentially affect each other [8, 10, 13–15]. In general, periodontitis effects on diabetes mellitus may be related to bacteremia and/or the presence of inflammatory mediators in the systemic circulation. Consequently, an exaggerated systemic inflammatory response to subgingival bacteria leads to an acute-phase protein burst and, systemically, high levels of pro-inflammatory mediators that facilitate insulin resistance [16–18].
Based on this, it is assumed that the subgingival microbiome plays a fundamental role not only in the periodontitis pathogenesis, as has been reported in several taxonomic identification studies [19–25], but also in the bidirectionality with diabetes mellitus [26, 27]. Herein, we aim to better understand the diabetes mellitus impact on the oral microbiome by reviewing recent studies that analyzed the oral microbiome in individuals with periodontal health, periodontal disease, and diabetes.
Methodological Aspects
This work is a literature review on the oral microbiome and its variations in individuals with diabetes mellitus in periodontal health and disease. The searches were performed by two independent reviewers in the main international databases (PubMed, Medline, Cochrane Library, Web of Science, and Scopus), in addition to a manual search. The search strategy used in all databases included the descriptors and MeSH (medical subject headings) terms “(periodontitis) AND (diabetes) AND (oral microbiome) AND (genetics)” without study design distinction. The analyzed articles were published in English and spanned the past 5 years. A total of 37 studies published between 2016 and 2021 were evaluated after the initial electronic search. After reading all the publications, 26 publications were excluded as they failed to mention the search terms, while 10 were fully evaluated and included in the presented discussion. A second search strategy was carried out, similar to the previous one, but excluding the last descriptor and adding the term “(salivary).” This generated a total of 16 articles, but only 8 matched the search criteria and were included.
Key Principles Of The Oral Microbiome In Healthy Conditions And Periodontitis
The oral microbiome represents an important part of the human microbiota and includes more than a thousand species [28, 29]. As a human being develops from birth to adulthood, the oral microbiome changes, and succession mechanisms are observed [30, 31]. For example, there is a change in the predominance of Escherichia coli, Staphylococcus, and Pseudomonas present before tooth eruption to the predominance of Fusobacterium, Prevotella, and Streptococcus mutans linked to mature oral microbiomes [32–34].
Changes in the oral microflora have also been associated with systemic diseases such as diabetes mellitus as well as oral diseases such as periodontitis [35–37]. In this sense, as an important resource for phylogenetic, taxonomic, genomic, and phenotypic identification of the human oral microbiome [29], the expanded human oral microbiome database (eHOMD) [28] provides access to data on hundreds of cultivable and non-cultivable prokaryotic species [38, 39].
On that note, the microbial biofilm, the primary etiological factor in periodontitis, has been extensively studied and can include 500 species or more among cultivable and non-cultivable strains in a single person, with more than 800 species having been identified in different dental biofilms [40–42]. It is estimated that the total number of species may well-exceed 1,000, although most are non-cultivable [43–45].
Total bacterial levels in the subgingival environment vary according to periodontal conditions, with ~103 in healthy shallow sites and 108 in deep periodontal pockets, including putative pathogens, such as anaerobic gram-negative bacteria, spirochetes, fungi, and even viruses [46]. Gram-negative bacterial species such as Porphyromonas gingivalis, Treponema denticola, and Tanerellaforsythia have been systematically associated with the onset and progression of periodontitis in deeper anaerobic areas, while others dominated the gingival plaque composition in health [46].
Given this diversity, the microbial “complexes” concept emerged, showing that there is a shift in biofilm colonization from health to disease, as well as in the development and progression of periodontitis [43]. Over the last few decades, hypotheses such as the “non-specific plaque hypothesis,” [47] the “specific plaque hypothesis,” [48] the “ecological plaque hypothesis,” [49] and the “fundamental pathogen hypothesis” [50] helped to inform knowledge for future microbiological research on understanding the complex nature of the onset and progression of microbial diseases in the oral environment. Currently, OMICs approaches (e.g., genomics, transcriptomics, proteomics, and metabolomics) have increased the understanding of the oral microbial interactions, and it is now possible to identify all microbial species that colonize the mouth [43, 44].
Subgingival Microbiome Affected By Diabetes Mellitus
Despite several studies focusing on the bidirectional relationship between diabetes mellitus and oral conditions such as periodontitis, the available literature still has limitations regarding the number and quality of studies addressing the impact of diabetes mellitus on the oral microbiome [51, 52].
Since glucose levels in the gingival crevicular fluid are similar to those in serum, the high availability of glucose may favor increased levels of saccharolytic commensals [53, 54], boosting the growth of fermenting species, including Streptococcus anginosus, Filifactor alocis, and generating a selective environmental pressure of glucose availability [55, 56].
In recent years, the development of next-generation sequencing technologies, as well as the Human Microbiome Project based on informative marker genes, community gene inventories (metagenomics), and functional analyses (metatranscriptomics), has contributed to the study of the human microbiome, including the oral microbiome and related systemic diseases [19, 22, 53, 54, 57].
Although some studies derived from high-throughput metagenomic sequencing of the oral microbiome have not been conclusive about the differences caused by diabetes mellitus [58–60], other studies with 16S rRNA gene deep sequencing have suggested that periodontally healthy individuals but, with diabetes mellitus, are at risk for periodontitis due to a decrease in the relative abundance and prevalence of species compatible with health (such as Atopobium and Corynebacterium) and an increase in the pathogenic content of the hyperglycemic microbiota (including Porphyromonas, Prevotella, Campylobacter, and Fusobacterium) [61, 62].
Despite its importance, 16S rRNA-based research is largely limited to taxonomic composition and only allows computational prediction analysis [63] of the microbial genomic potential of the studied communities [54, 64]. To date, metagenomic studies in the diabetes mellitus field have mainly focused on the gut metagenome [65–67]. However, using metagenomic shotgun sequencing to understand the susceptibility to periodontitis in the oral microbiome of individuals with diabetes, a recent study has identified distinct differences in the subgingival microbiome of patients with type 2 diabetes mellitus compared to non-diabetics. While the red complex species genes were less prevalent in the periodontitis state in diabetes mellitus compared to non-diabetics, in the healthy periodontal state, the subgingival microbiome in patients with diabetes mellitus contained more genes from orange complex species [27].
In another study, complete metagenomic shotgun sequencing of the subgingival microbiome showed that periodontitis was associated with a significantly higher relative abundance of oral taxon 439 of the bacterium Anaerolineaceae in patients with type 2 diabetes mellitus [68].
Applying 16s rRNA sequencing, Matsha et al. examined the bacterial composition in subgingival plaque samples from 128 patients with periodontitis and showed that Fusobacteria and Actinobacteria were significantly more abundant in subjects with type 2 diabetes mellitus, where the former increased the odds of diabetes mellitus by 14% and the latter increased the odds by 10%, both in subjects with gingival bleeding. However, according to the authors, it is not clear whether these differences were the consequence of hyperglycemia or the presence of periodontitis [52].
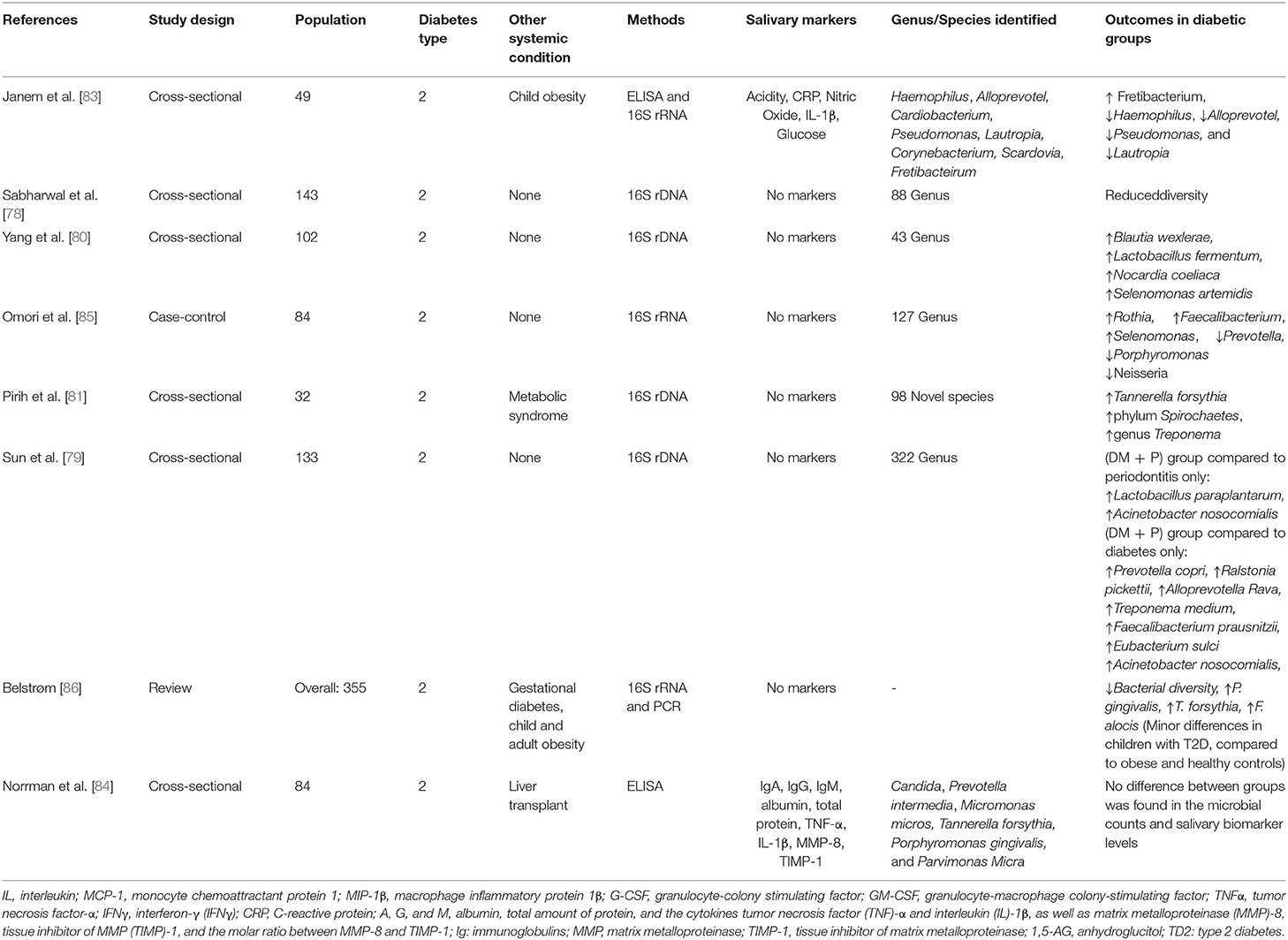
Corroborating with this, a consensus report from the American Academy of Periodontology and the European Federation of Periodontology [69] stated that there was no compelling evidence that diabetes mellitus significantly impacted the oral microbiota. This conclusion was based on several human studies that reported inconsistent and contradictory findings on whether diabetes mellitus altered the bacterial composition in the oral cavity [51]. However, some authors report evidence that type 2 diabetes mellitus reduces the diversity and richness of the subgingival microbiome, and this decrease is even associated with inadequate glycemic controls [56, 59, 70]. A summary of studies examined for this review is shown in Table 1.
Despite being extremely relevant, considerable knowledge gaps remain regarding the composition of the subgingival microbial under diabetic conditions, especially using modern methodologies and omics approaches. However, the few studies available in the literature, including the present review, should be considered to support future investigations and larger studies.
Salivary Microbiome Affected By Diabetes Mellitus
Saliva is a complex fluid composed of the secretions from the minor and major salivary glands, mucosal transudations, serum, among others [74]. In the last two decades, saliva has become the focus of a great number of studies, being adopted in diagnostics of oral and systemic diseases [75] because samples can be easily and non-invasively collected [76]. Saliva contains numerous active biomolecules, its microbiome is from various niches in the oral cavity, and appears to be representative of the overall oral microbiome [77]. An analysis of the salivary microbiome is required to better understand how it relates to both diabetes mellitus and periodontitis status.
Oral microbial diversity appears to decrease in patients with type 2 diabetes and increase with the progression of periodontitis compared with periodontally healthy controls [78]. This could be explained by the following two different mechanisms. First, elevated glucose levels in the saliva of subjects with type 2 diabetes mellitus and pre-diabetes could impact the oral environment, enhancing the growth of certain bacterial species at the expense of others. Second, mouth dehydration, usually associated with type 2 diabetes mellitus, could result in microbial diversity reduction [59, 78–80].
Corroborating these data, Pirih et al. [81] assessed individuals with metabolic syndrome and type 2 diabetes mellitus in comparison to healthy patients. The data showed that the salivary microbiome in health was more diverse than the metabolic syndrome group. In addition, the metabolic syndrome periodontitis group displayed a higher abundance of Tannerella forsythia. In a different study, higher levels of T. forsythia, P. gingivalis, and F. alocis were reported in patients with gestational diabetes [82].
Similar results were found by Saeb et al. [59] where a reduction of biological and phylogenetic diversity in the oral microbiota was apparent in type 2 diabetes mellitus and pre-diabetes in comparison with normoglycemic individuals. Janem et al. [83] found a tendency for lower diversity scores in type 2 diabetes mellitus compared to obese groups. Some variation was noted at the genus level where Haemophilus, Alloprevotella, Pseudomonas, and Lautropia were reduced in diabetes mellitus, while Fretibacterium was increased. Norrman et al. [84] did not find differences between the groups in number of periodontal pathogens; however, it is important to note that the patients from this study included liver transplant recipients with type 2 diabetes mellitus, using several systemic medications, such as analgesics, immunosuppressors, cyclosporine, and corticosteroid.
Omori et al. [85] showed no significant differences in the alpha diversity of the salivary microbiota between elderly patients with type 2 diabetes mellitus and control groups. At the genus level, however, an increased abundance of Rothia, Faecalibacterium, and Selenomonas was observed, as well as a decreased abundance of Prevotella, Porphyromonas, and Neisseria in the diabetic groups compared to the control group.
Similarly, another study showed that the proportions of Lactobacillus paraplantarum and Acinetobacter nosocomialis were relatively higher in the periodontitis with the type 2 diabetes mellitus group compared to patients with periodontitis only. In addition, higher proportions of Prevotella copri, Ralstonia pickettii, Alloprevotella rava, Treponema medium, Faecalibacterium prausnitzii, Eubacterium sulci, and Acinetobacter nosocomialis were found compared to the diabetic group [79]. Interestingly, the proportions of Streptobacillus moniliformis, Streptococcus mutans, and Prevotella jejuni were significantly higher in the group of patients with type 2 diabetes mellitus that received treatment with Metformin [79]. These data suggest that, after effective glycemic control, the salivary microbial composition of periodontitis patients with type 2 diabetes mellitus resembled that of healthy individuals.
Another study compared the salivary microbiome of healthy patients, patients with type 2 diabetes mellitus without treatment, and patients with diabetes treated with metformin or a combination of insulin and other drugs [80]. This study found that Blautia wexlerae, Lactobacillus fermentum, Nocardia coeliaca, and Selenomonas artemidis exhibited a relatively higher abundance in the patients without treatment compared to healthy and the diabetic treatment groups. However, diabetic patients without treatment showed increased severity of periodontitis [80], which could account for the differences in microbial composition.
The differences between the microbiome found in the studies could be justified based on several factors such as: 1) different methods used to assess the microbiome, 2) the association with other systemic diseases, 3) type 2 diabetes mellitus controlled by medication, 4) poorly controlled type 2 diabetes mellitus, 5) the age of the patients, 6) periodontitis classification, 7) sample size, and/or 8) geographic location. Although there is still no consensus on the microbiome in patients with type 2 diabetes mellitus and periodontitis, it is clear that the combined effects of diabetes mellitus and periodontitis on the changes in the salivary microbial composition were significantly greater than that of diabetes mellitus alone, suggesting that periodontitis-related parameters are the main factors influencing the salivary microbial composition [86]. A summary of studies examined for this review is shown in Table 2.
Well-designed longitudinal studies are needed to uncover if salivary microbiome changes precede clinical signs of disease, which would enable the use of salivary microbiome signatures for each disease and its diagnosis and risk assessments [78].
Conclusion
Diabetes mellitus leads to taxonomic differences of microbial species, but little is known about the direct effects of this metabolic disorder on the subgingival and salivary microbiota. Given the uncertainty as to whether the changes reported are due exclusively to hyperglycemia or predominantly, from periodontal inflammation, more studies need to be carried out not only to help answer these questions but mainly to support the development of new periodontal treatments in patients with uncontrolled diabetes.
Author Contributions
DS, SM, and MC conducted bibliographic research and manuscript writing. BB, RL, and FP contributed to the writing and guided the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil) for supporting this manuscript.
References
1. Papapanou PN, Sanz M, Buduneli N, Dietrich T, Feres M, Fine DH, et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol. (2018) 89 Suppl 1:S173–82. doi: 10.1002/JPER.17-0721
2. Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. (2018) 138:271–81. doi: 10.1016/j.diabres.2018.02.023
3. Tsalamandris S, Antonopoulos AS, Oikonomou E, Papamikroulis GA, Vogiatzi G, Papaioannou S, et al. The role of inflammation in diabetes: Current concepts and future perspectives. Eur Cardiol. (2019) 14:50–9. doi: 10.15420/ecr.2018.33.1
4. Brennan DS, Spencer AJ, Roberts-Thomson KF. Quality of life and disability weights associated with periodontal disease. J Dent Res. (2007) 86:713–7. doi: 10.1177/154405910708600805
5. Kassebaum NJ, Bernabe E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. Global burden of severe periodontitis in 1990-2010: a systematic review and meta-regression. J Dent Res. (2014) 93:1045–53. doi: 10.1177/0022034514552491
6. Wu CZ, Yuan YH, Liu HH, Li SS, Zhang BW, Chen W, et al. Epidemiologic relationship between periodontitis and type 2 diabetes mellitus. BMC Oral Health. (2020) 20:204. doi: 10.1186/s12903-020-01180-w
7. Eke PI, Thornton-Evans GO, Wei L, Borgnakke WS, Dye BA, Genco RJ. Periodontitis in US Adults: National Health and Nutrition Examination Survey 2009-2014. J Am Dent Assoc. (2018) 149:576–88.e6. doi: 10.1016/j.adaj.2018.04.023
8. Sanz M, Ceriello A, Buysschaert M, Chapple I, Demmer RT, Graziani F, et al. Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology. J Clin Periodontol. (2018) 45:138–49. doi: 10.1111/jcpe.12808
9. Atlas D. International Diabetes Federation. IDF Diabetes Atlas, 7th edn Brussels. Belgium: International Diabetes Federation (2015).
10. Borgnakke WS, Ylostalo PV, Taylor GW, Genco RJ. Effect of periodontal disease on diabetes: systematic review of epidemiologic observational evidence. J Periodontol. (2013) 84(4 Suppl):S135–52. doi: 10.1902/jop.2013.1340013
11. Cardoso EM, Reis C, Manzanares-Céspedes MC. Chronic periodontitis, inflammatory cytokines, and interrelationship with other chronic diseases. Postgraduate Med. (2018) 130:98–104. doi: 10.1080/00325481.2018.1396876
12. Genco RJ, Borgnakke WS. Diabetes as a potential risk for periodontitis: association studies. Periodontology. (2000) 83:40–5. doi: 10.1111/prd.12270
13. Taylor GW. Bidirectional interrelationships between diabetes and periodontal diseases: an epidemiologic perspective. Ann Periodontol. (2001) 6:99–112. doi: 10.1902/annals.2001.6.1.99
14. Pontes Andersen CC, Flyvbjerg A, Buschard K, Holmstrup P. Relationship between periodontitis and diabetes: lessons from rodent studies. J Periodontol. (2007) 78:1264–75. doi: 10.1902/jop.2007.060491
15. Zheng J, Chen S, Albiero ML, Vieira GHA, Wang J, Feng JQ, et al. Diabetes activates periodontal ligament fibroblasts via NF-kappaB in vivo. J Dent Res. (2018) 97:580–8. doi: 10.1177/0022034518755697
16. Acharya AB, Thakur S, Muddapur MV, Kulkarni RD. Systemic cytokines in type 2 diabetes mellitus and chronic periodontitis. Curr Diabetes Rev. (2018) 14:182–8. doi: 10.2174/1573399812666161220144011
17. Baeza M, Morales A, Cisterna C, Cavalla F, Jara G, Isamitt Y, et al. Effect of periodontal treatment in patients with periodontitis and diabetes: systematic review and meta-analysis. J Appl Oral Sci. (2020) 28:e20190248. doi: 10.1590/1678-7757-2019-0248
18. Preshaw PM, Alba AL, Herrera D, Jepsen S, Konstantinidis A, Makrilakis K, et al. Periodontitis and diabetes: a two-way relationship. Diabetologia. (2012) 55:21–31. doi: 10.1007/s00125-011-2342-y
19. Abusleme L, Dupuy AK, Dutzan N, Silva N, Burleson JA, Strausbaugh LD, et al. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J. (2013) 7:1016–25. doi: 10.1038/ismej.2012.174
20. Colombo AP, Boches SK, Cotton SL, Goodson JM, Kent R, Haffajee AD, et al. Comparisons of subgingival microbial profiles of refractory periodontitis, severe periodontitis, and periodontal health using the human oral microbe identification microarray. J Periodontol. (2009) 80:1421–32. doi: 10.1902/jop.2009.090185
21. Fragkioudakis I, Riggio MP, Apatzidou DA. Understanding the microbial components of periodontal diseases and periodontal treatment-induced microbiological shifts. J Med Microbiol. (2021) 70:e1247. doi: 10.1099/jmm.0.001247
22. Griffen AL, Beall CJ, Campbell JH, Firestone ND, Kumar PS, Yang ZK, et al. Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. ISME J. (2012) 6:1176–85. doi: 10.1038/ismej.2011.191
23. Lamont RJ, Koo H, Hajishengallis G. The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol. (2018) 16:745–59. doi: 10.1038/s41579-018-0089-x
24. Lundmark A, Hu YOO, Huss M, Johannsen G, Andersson AF, Yucel-Lindberg T. Identification of salivary microbiota and its association with host inflammatory mediators in periodontitis. Front Cell Infect Microbiol. (2019) 9:216. doi: 10.3389/fcimb.2019.00216
25. Mombelli A. Microbial colonization of the periodontal pocket and its significance for periodontal therapy. Periodontol. (2000) 76:85–96. doi: 10.1111/prd.12147
26. Khumaedi AI, Purnamasari D, Wijaya IP, Soeroso Y. The relationship of diabetes, periodontitis and cardiovascular disease. Diabetes Metab Syndr. (2019) 13:1675–8. doi: 10.1016/j.dsx.2019.03.023
27. Shi B, Lux R, Klokkevold P, Chang M, Barnard E, Haake S, et al. The subgingival microbiome associated with periodontitis in type 2 diabetes mellitus. Isme J. (2020) 14:519–30. doi: 10.1038/s41396-019-0544-3
28. Chen T, Yu W-H, Izard J, Baranova OV, Lakshmanan A, Dewhirst FE. The Human Oral Microbiome Database: a web accessible resource for investigating oral microbe taxonomic and genomic information. Database. (2010) 2010:baq013. doi: 10.1093/database/baq013
29. Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu WH, et al. The human oral microbiome. J Bacteriol. (2010) 192:5002–17. doi: 10.1128/JB.00542-10
30. Cephas KD, Kim J, Mathai RA, Barry KA, Dowd SE, Meline BS, et al. Comparative analysis of salivary bacterial microbiome diversity in edentulous infants and their mothers or primary care givers using pyrosequencing. PLoS ONE. (2011) 6:e23503. doi: 10.1371/journal.pone.0023503
31. Lif Holgerson P, Ohman C, Ronnlund A, Johansson I. Maturation of oral microbiota in children with or without dental caries. PLoS ONE. (2015) 10:e0128534. doi: 10.1371/journal.pone.0128534
32. Cavalcanti IM, Del Bel Cury AA, Jenkinson HF, Nobbs AH. Interactions between Streptococcus oralis, Actinomyces oris, and Candida albicans in the development of multispecies oral microbial biofilms on salivary pellicle. Mol Oral Microbiol. (2017) 32:60–73. doi: 10.1111/omi.12154
33. Jakubovics NS. Intermicrobial interactions as a driver for community composition and stratification of oral biofilms. J Mol Biol. (2015) 427:3662–75. doi: 10.1016/j.jmb.2015.09.022
34. Wan AK, Seow WK, Purdie DM, Bird PS, Walsh LJ, Tudehope DI. A longitudinal study of Streptococcus mutans colonization in infants after tooth eruption. J Dent Res. (2003) 82:504–8. doi: 10.1177/154405910308200703
35. Anukam KC, Agbakoba NR. A comparative study of the oral microbiome compositions of healthy postmenopausal, premenopausal, and prepubertal Nigerian females, using 16s rrna metagenomics methods. Niger J Clin Pract. (2017) 20:1250–8. doi: 10.4103/njcp.njcp_32_17
36. Lazarevic V, Whiteson K, Huse S, Hernandez D, Farinelli L, Osteras M, et al. Metagenomic study of the oral microbiota by Illumina high-throughput sequencing. J Microbiol Methods. (2009) 79:266–71. doi: 10.1016/j.mimet.2009.09.012
37. Verma D, Garg PK, Dubey AK. Insights into the human oral microbiome. Arch Microbiol. (2018) 200:525–40. doi: 10.1007/s00203-018-1505-3
38. Escapa IF, Chen T, Huang Y, Gajare P, Dewhirst FE, Lemon KP. New insights into human nostril microbiome from the expanded human oral microbiome database (eHOMD): A resource for the microbiome of the human aerodigestive tract. mSystems. (2018) 3:6. doi: 10.1128/mSystems.00187-18
39. I FE, Huang Y, Chen T, Lin M, Kokaras A, Dewhirst FE, et al. Construction of habitat-specific training sets to achieve species-level assignment in 16S rRNA gene datasets. Microbiome. (2020) 8:65. doi: 10.1186/s40168-020-00841-w
40. Diaz PI. Microbial diversity and interactions in subgingival biofilm communities. Front Oral Biol. (2012) 15:17–40. doi: 10.1159/000329669
41. Mahajan A, Singh B, Kashyap D, Kumar A, Mahajan P. Interspecies communication and periodontal disease. ScientificWorldJournal. (2013) 2013:765434. doi: 10.1155/2013/765434
42. Rosan B, Lamont RJ. Dental plaque formation. Microbes and infection. (2000) 2:1599–607. doi: 10.1016/S1286-4579(00)01316-2
43. Brown JL, Johnston W, Delaney C, Short B, Butcher MC, Young T, et al. Polymicrobial oral biofilm models: simplifying the complex. J Med Microbiol. (2019) 68:1573–84. doi: 10.1099/jmm.0.001063
44. Jenkinson HF. Beyond the oral microbiome. Environ Microbiol. (2011) 13:3077–87. doi: 10.1111/j.1462-2920.2011.02573.x
45. Kinane DF, Stathopoulou PG, Papapanou PN. Authors' reply: Predictive diagnostic tests in periodontal diseases. Nat Rev Dis Primers. (2017) 3:1. doi: 10.1038/nrdp.2017.70
46. Socransky SS, Haffajee AD. The bacterial etiology of destructive periodontal disease: Current concepts. J Periodontol. (1992) 63 Suppl 4S:322–31. doi: 10.1902/jop.1992.63.4s.322
47. Theilade E. The non-specific theory in microbial etiology of inflammatory periodontal diseases. J Clin Periodontol. (1986) 13:905–11. doi: 10.1111/j.1600-051X.1986.tb01425.x
49. Marsh PD. Dental plaque as a biofilm and a microbial community - implications for health and disease. BMC Oral Health. (2006) 6 Suppl 1:S14. doi: 10.1186/1472-6831-6-S1-S14
50. Hajishengallis G, Darveau RP, Curtis MA. The keystone-pathogen hypothesis. Nat Rev Microbiol. (2012) 10:717–25. doi: 10.1038/nrmicro2873
51. Graves DT, Ding Z, Yang Y. The impact of diabetes on periodontal diseases. Periodontol. (2000) 82:214–24. doi: 10.1111/prd.12318
52. Matsha TE, Prince Y, Davids S, Chikte U, Erasmus RT, Kengne AP, et al. Oral microbiome signatures in diabetes mellitus and periodontal disease. J Dent Res. (2020) 99:658–65. doi: 10.1177/0022034520913818
53. Yost S, Duran-Pinedo AE, Teles R, Krishnan K, Frias-Lopez J. Functional signatures of oral dysbiosis during periodontitis progression revealed by microbial metatranscriptome analysis. Genome Med. (2015) 7:1–19. doi: 10.1186/s13073-015-0153-3
54. Teles F, Wang Y, Hajishengallis G, Hasturk H, Marchesan JT. Impact of systemic factors in shaping the periodontal microbiome. Periodontol. (2000) 85:126–60. doi: 10.1111/prd.12356
55. Chopra P, Kumar T. Correlation of glucose level among venous, gingival and finger-prick blood samples in diabetic patients. J Ind Soc Periodontol. (2011) 15:288. doi: 10.4103/0972-124X.85678
56. Longo PL, Dabdoub S, Kumar P, Artese HPC, Dib SA, Romito GA, et al. Glycaemic status affects the subgingival microbiome of diabetic patients. J Clin Periodontol. (2018) 45:932–40. doi: 10.1111/jcpe.12908
57. Duran-Pinedo AE, Chen T, Teles R, Starr JR, Wang X, Krishnan K, et al. Community-wide transcriptome of the oral microbiome in subjects with and without periodontitis. ISME J. (2014) 8:1659–72. doi: 10.1038/ismej.2014.23
58. Ogawa T, Honda-Ogawa M, Ikebe K, Notomi Y, Iwamoto Y, Shirobayashi I, et al. Characterizations of oral microbiota in elderly nursing home residents with diabetes. J Oral Sci. (2017) 2017:16-0722. doi: 10.2334/josnusd.16-0722
59. Saeb ATM, Al-Rubeaan KA, Aldosary K, Udaya Raja GK, Mani B, Abouelhoda M, et al. Relative reduction of biological and phylogenetic diversity of the oral microbiota of diabetes and pre-diabetes patients. Microb Pathog. (2019) 128:215–29. doi: 10.1016/j.micpath.2019.01.009
60. Tam J, Hoffmann T, Fischer S, Bornstein S, Gräßler J, Noack B. Obesity alters composition and diversity of the oral microbiota in patients with type 2 diabetes mellitus independently of glycemic control. PLoS ONE. (2018) 13:e0204724. doi: 10.1371/journal.pone.0204724
61. Ganesan SM, Joshi V, Fellows M, Dabdoub SM, Nagaraja HN, O'Donnell B, et al. A tale of two risks: smoking, diabetes and the subgingival microbiome. Isme J. (2017) 11:2075–89. doi: 10.1038/ismej.2017.73
62. Long J, Cai Q, Steinwandel M, Hargreaves MK, Bordenstein SR, Blot WJ, et al. Association of oral microbiome with type 2 diabetes risk. J Periodontal Res. (2017) 52:636–43. doi: 10.1111/jre.12432
63. Langille MG, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol. (2013) 31:814–21. doi: 10.1038/nbt.2676
64. Laudadio I, Fulci V, Palone F, Stronati L, Cucchiara S, Carissimi C. Quantitative assessment of shotgun metagenomics and 16S rDNA amplicon sequencing in the study of human gut microbiome. OMICS. (2018) 22:248–54. doi: 10.1089/omi.2018.0013
65. Brunkwall L, Orho-Melander M. The gut microbiome as a target for prevention and treatment of hyperglycaemia in type 2 diabetes: from current human evidence to future possibilities. Diabetologia. (2017) 60:943–51. doi: 10.1007/s00125-017-4278-3
66. Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. (2012) 490:55–60. doi: 10.1038/nature11450
67. Wang X, Xu X, Xia Y. Further analysis reveals new gut microbiome markers of type 2 diabetes mellitus. Antonie Van Leeuwenhoek. (2017) 110:445–53. doi: 10.1007/s10482-016-0805-3
68. Farina R, Severi M, Carrieri A, Miotto E, Sabbioni S, Trombelli L, et al. Whole metagenomic shotgun sequencing of the subgingival microbiome of diabetics and non-diabetics with different periodontal conditions. Arch Oral Biol. (2019) 104:13–23. doi: 10.1016/j.archoralbio.2019.05.025
69. Chapple IL Genco R working working group 2 of the joint EFPAAPw. Diabetes and periodontal diseases: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Periodontol. (2013) 84(4 Suppl):S106–12. doi: 10.1902/jop.2013.1340011
70. Demmer RT, Breskin A, Rosenbaum M, Zuk A, LeDuc C, Leibel R, et al. The subgingival microbiome, systemic inflammation and insulin resistance: The oral infections, glucose intolerance and insulin resistance study. J Clin Periodontol. (2017) 44:255–65. doi: 10.1111/jcpe.12664
71. Joaquim CR, Miranda TS, Marins LM, Silva HDP, Feres M, Figueiredo LC, et al. The combined and individual impact of diabetes and smoking on key subgingival periodontal pathogens in patients with chronic periodontitis. J Periodontal Res. (2018) 53:315–23. doi: 10.1111/jre.12516
72. Babaev EA, Balmasova IP, Mkrtumyan AM, Kostryukova SN, Vakhitova ES, Il'ina EN, et al. Metagenomic analysis of gingival sulcus microbiota and pathogenesis of periodontitis associated with type 2 diabetes mellitus. Bull Exp Biol Med. (2017) 163:718–21. doi: 10.1007/s10517-017-3888-6
73. Bachtiar BM, Theodorea CF, Tahapary DL, Astrella C, n/a N, Bachtiar EW. A pilot study of red complex and three genera subgingival microbiome in periodontitis subjects with and without diabetes, evaluated by MinION platform. F1000Res. (2021) 10:79. doi: 10.12688/f1000research.28216.3
74. Bandhakavi S, Stone MD, Onsongo G, Van Riper SK, Griffin TJ. A dynamic range compression and three-dimensional peptide fractionation analysis platform expands proteome coverage and the diagnostic potential of whole saliva. J Proteome Res. (2009) 8:5590–600. doi: 10.1021/pr900675w
75. Lamy E, Capela-Silva F, Tvarijonaviciute A. Research on saliva secretion and composition. Biomed Res Int. (2018) 2018:7406312. doi: 10.1155/2018/7406312
76. Hu S, Xie Y, Ramachandran P, Ogorzalek Loo RR, Li Y, Loo JA, et al. Large-scale identification of proteins in human salivary proteome by liquid chromatography/mass spectrometry and two-dimensional gel electrophoresis-mass spectrometry. Proteomics. (2005) 5:1714–28. doi: 10.1002/pmic.200401037
77. Hasan NA, Young BA, Minard-Smith AT, Saeed K, Li H, Heizer EM, et al. Microbial community profiling of human saliva using shotgun metagenomic sequencing. PLoS ONE. (2014) 9:e97699. doi: 10.1371/journal.pone.0097699
78. Sabharwal A, Ganley K, Miecznikowski JC, Haase EM, Barnes V, Scannapieco FA. The salivary microbiome of diabetic and non-diabetic adults with periodontal disease. J Periodontol. (2019) 90:26–34. doi: 10.1002/JPER.18-0167
79. Sun X, Li M, Xia L, Fang Z, Yu S, Gao J, et al. Alteration of salivary microbiome in periodontitis with or without type-2 diabetes mellitus and metformin treatment. Sci Rep. (2020) 10:15363. doi: 10.1038/s41598-020-72035-1
80. Yang Y, Liu S, Wang Y, Wang Z, Ding W, Sun X, et al. Changes of saliva microbiota in the onset and after the treatment of diabetes in patients with periodontitis. Aging (Albany NY). (2020) 12:13090–114. doi: 10.18632/aging.103399
81. Pirih FQ, Monajemzadeh S, Singh N, Sinacola RS, Shin JM, Chen T, et al. Association between metabolic syndrome and periodontitis: The role of lipids, inflammatory cytokines, altered host response, and the microbiome. Periodontol. (2000) 87:50–75. doi: 10.1111/prd.12379
82. Gogeneni H, Buduneli N, Ceyhan-Öztürk B, Gümüş P, Akcali A, Zeller I, et al. Increased infection with key periodontal pathogens during gestational diabetes mellitus. J Clin Periodontol. (2015) 42:506–12. doi: 10.1111/jcpe.12418
83. Janem WF, Scannapieco FA, Sabharwal A, Tsompana M, Berman HA, Haase EM, et al. Salivary inflammatory markers and microbiome in normoglycemic lean and obese children compared to obese children with type 2 diabetes. PLoS ONE. (2017) 12:e0172647. doi: 10.1371/journal.pone.0172647
84. Norrman AE, Tervahartiala T, Sahlberg E, Sorsa T, Ruokonen H, Grönroos L, et al. Salivary biomarkers and oral health in liver transplant recipients, with an emphasis on diabetes. Diagnostics. (2021) 11:40662. doi: 10.3390/diagnostics11040662
85. Omori M, Kato-Kogoe N, Sakaguchi S, Kamiya K, Fukui N, Gu YH, et al. Characterization of salivary microbiota in elderly patients with type 2 diabetes mellitus: a matched case-control study. Clin Oral Investig. (2021) 26:493–504. doi: 10.1007/s00784-021-04027-y
Keywords: periodontitis, diabetes mellitus, oral microbiome, genetics, integrative review
Citation: Silva DNdA, Casarin M, Monajemzadeh S, Bezerra BdB, Lux R and Pirih FQ (2022) The Microbiome in Periodontitis and Diabetes. Front. Oral. Health 3:859209. doi: 10.3389/froh.2022.859209
Received: 21 January 2022; Accepted: 14 March 2022;
Published: 08 April 2022.
Edited by:
Georgios Kotsakis, The University of Texas Health Science Center at San Antonio, United StatesReviewed by:
Sukirth M. Ganesan, The University of Iowa, United StatesNagihan Bostanci, Karolinska Institutet (KI), Sweden
Copyright © 2022 Silva, Casarin, Monajemzadeh, Bezerra, Lux and Pirih. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Flavia Q. Pirih, ZnBpcmloQGRlbnRpc3RyeS51Y2xhLmVkdQ==
 Davi Neto de Araújo Silva
Davi Neto de Araújo Silva Maísa Casarin
Maísa Casarin Sepehr Monajemzadeh
Sepehr Monajemzadeh Beatriz de Brito Bezerra
Beatriz de Brito Bezerra Renate Lux
Renate Lux Flavia Q. Pirih
Flavia Q. Pirih