
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oral. Health , 22 September 2022
Sec. Oral Health Promotion
Volume 3 - 2022 | https://doi.org/10.3389/froh.2022.1003679
This article is part of the Research Topic Precision Dentistry and eHealth in Oral Healthcare View all 5 articles
Personalized Oral Healthcare has recently become the new trend word in medicine and dentistry. In this context, saliva diagnostics using various biomarkers seem to be the gateway to personalized dental diagnostics and therapy. But the terminology is not (yet) uniformly defined, furthermore it is unclear to what extent which salivary markers play a relevant role in the therapeutic decision making. In this Scoping Review, an electronic search was conducted in PubMed and Web of Science databases using medical subject headings (MESH terms) “saliva”, “biomarker”, “personality/persons”, and “dentistry”. Only human studies were included, in which repeated salivary measurements were performed to analyze monitoring effects with at least ten patients per group. PRISMA-ScR and Tricco guidelines were followed: (i) to examine what salivary biomarkers have been explored in terms of personalized oral healthcare and precision dentistry, (ii) to investigate the clinical relevance for oral health and its correlation to systemic health, and (iii) to summarize an outlook for future developments based on these results. Out of 899 studies, a total of 57 were included for data extraction in this Scoping Review, mainly focusing on periodontal therapy and patient monitoring. Salivary biomarkers have shown the potential to change the field of dentistry in all dental disciplines as a key for personalized workflows. The increasing interest in dental research is obvious, demonstrated by the growing number of publications in recent years. At this time, however, the predominant discipline is periodontology, which allows biomarker-based monitoring of the disease prevention and progression. The studies included showed heterogeneous methods using manifolds biomarkers. Therefore, no uniformly accepted concept can be presented today. Further clinical research with well-defined outcomes including standardized procedures is necessary.
The maintenance of oral health can be achieved by preventive measures and the early detection and timely treatment of incipient oral infections, namely caries and periodontitis. Both non-communicable diseases are based on a disbalance of microbial biofilms in the oral cavity (1). Preventive measures to combat these pathogenic biofilms on a daily basis were summarized recently in different Consensus Reports (2, 3). Evidently, the most powerful individual tools for prevention and maintenance were based on self-performed oral hygiene and use of cleaning agents containing fluoride. Aside, it seems crucial to control and detect individuals' deviations of the oral health status at an early stage to hinder disease outbreak. While gold standard clinical examinations and radiographs at the dental office may miss developing disorders on a cellular base, other diagnostic tools and modern technical possibilities gain more importance (4, 5). Different biofluids, such as periphery blood, gingival crevicular fluid (GCF), and saliva were evaluated for their diagnostic or prognostic qualification for disease detection (6). The ease and non-invasiveness in saliva collection positions this biofluid as potential alternative to blood testing. Interestingly, the search for saliva diagnostics in different databases demonstrates already a high quantity of clinical investigations using saliva as target vehicle for different biomarkers in oral diseases. Saliva bathes all healthy and diseased oral surfaces in the oral cavity (7, 8). It was shown, that the oral microbiome can be quantified to a certain extent in saliva (9, 10), and even more interesting, host immune response to pathogens can be analyzed nearly in real-time (11). Furthermore, modern technologies allow the detection and quantification of specific substances in low concentrations (4, 12). Today, improvements and further developments in point-of-care devices evolve from technical progress in the era of Covid 19 (13, 14). With that much data and even more technical possibilities, the questions arise, what to actually search for.
Single salivary measurements usually present a condition at a specific timepoint. These measurements can be used to define special patient groups or signature profiles of different diseases and to detect outlier (10). In contrast, repeated salivary measurements provide information on trends, progression of disease, and on response to different treatment strategies (11, 15). Furthermore, it allows the monitoring of patients during maintenance and offers thereby the possibility to retreat timely—if needed. The outcome might help to detect personalized salivary pattern in reaction to different trigger and in future to compute custom-fit therapies for precision dentistry. This might also pave the way for real-time measurements outside the dental office and its implementation in daily life.
Based on these thoughts, the high number of clinical trials using saliva, and on the lack of structure in saliva literature, the three objectives of this Scoping Review are:
• What salivary biomarkers have been investigated in clinical trials in terms of personalized oral healthcare and precision dentistry mapped for disciplines and/or indications (key elements: choice of biomarkers, number of participants, dental disciplines)?
• What has been the clinical relevance for oral health and its correlation to systemic health?
• What can be summarized for a future outlook?
The conduct of this review follows the Arksey and O'Malley framework, modified by Levac (16, 17), omitting the consultation step. Reporting follows the PRISMA-ScR statement (18), shown in Supplementary_Data 1; in addition, the preliminary review protocol including PCC-question is available as Supplementary_Data 2.
Dentistry-related clinical trials, written in English, which investigated salivary analysis at different timepoints, and participants at least 18 years, were included. Different timepoints were defined as a minimum of two separate repeated salivary measurements at different periods. Clinical trials investigating plain general medicine subjects, without reference to the oral health status, were excluded. Clinical trials with less than 10 participants per group, and animal studies, as well as review articles were excluded.
An electronic search was performed in the database PubMed and Web of Science for relevant papers using “saliva” as the source of analysis, “biomarker” as concept, and “(personalized) dentistry” or “precision dentistry” as context. Search combinations in PubMed included: ("saliva"[MeSH Terms] OR "saliva"[All Fields] OR "salivas"[All Fields] OR "saliva s"[All Fields] OR "salivary"[All Fields]) AND ("biomarker s"[All Fields] OR "biomarkers"[MeSH Terms] OR "biomarkers"[All Fields] OR "biomarker"[All Fields]) AND ((("person s"[All Fields] OR "personable"[All Fields] OR "personableness"[All Fields] OR "personal"[All Fields] OR "personalisation"[All Fields] OR "personalise"[All Fields] OR "personalised"[All Fields] OR "personalising"[All Fields] OR "personality"[MeSH Terms] OR "personality"[All Fields] OR "personalities"[All Fields] OR "personality s"[All Fields] OR "personalization"[All Fields] OR "personalize"[All Fields] OR "personalized"[All Fields] OR "personalizes"[All Fields] OR "personalizing"[All Fields] OR "personally"[All Fields] OR "personals"[All Fields] OR "persons"[MeSH Terms] OR "persons"[All Fields] OR "person"[All Fields]) AND ("dentistry"[MeSH Terms] OR "dentistry"[All Fields] OR "dentistry s"[All Fields])) OR (("precise"[All Fields] OR "precised"[All Fields] OR "precisely"[All Fields] OR "preciseness"[All Fields] OR "precises"[All Fields] OR "precision"[All Fields] OR "precisions"[All Fields]) AND ("dentistry"[MeSH Terms] OR "dentistry"[All Fields] OR "dentistry s"[All Fields]))). All publications were included until the 30th of June 2022. The search in Web of Science was conducted with the terms “(ALL = (saliva*biomarker* ((person* and dentistry*) or (precis* and dentistry*)))).
Title and abstract screening were performed by three authors (PNP, JH, AZ) using a data chart, which was discussed during a first exploratory screening (Supplementary_Data 3). The following data items were extracted during the full-text search: first author, year of publication, country of origin (= where the study was conducted), dental discipline (e.g., periodontology, implantology, prosthodontics), topic (research question), applied salivary biomarker, monitoring intervals, total number of patients, outcome metrics/conclusion of the study, study design (Table 1). All included studies were categorized to dental disciplines, including a total number of patients per group. Major biomarkers of included studies were specified in frequency of occurrence and in their combination.
The Scoping Research was completed on 2022-06-30 and results are current as of this date. Of the 914 titles retrieved by the search, 899 abstracts were further screened, and successively, 60 full-texts identified. A total of 3 full-texts were excluded from the final analysis. Finally, 57 full-texts were included for data extraction (Figure 1).
Included studies were judged to be of sufficient quality considering the specific study design. Detailed information of each study is tabularized for general data in Table 1 (author, year of publication, country of origin, then categorized in study design, dental disciplines including topics investigated and target biomarkers, monitoring intervals, total number of patients, and outcome metrics).
The publication dates range from 2000 to 2022 with continuously increasing numbers in recent years. Study types were categorized in observational clinical studies (n = 50), RCTs (n = 4), case-control studies (n = 2), and pilot study (n = 1). A total of 4,125 patients were investigated with patient monitoring intervals from 2 h up to 3 years, depending on the respective trial design and focused research question. Dental disciplines involved were periodontology (n = 42), general dentistry (n = 7), orthodontics (n = 3), prosthodontics (n = 2), oral surgery (n = 1), implantology (n = 1), and temporo-mandibular disorders (n = 1). As explanation, the term “general dentistry” has been used for diagnostic or therapy protocols fundamental to protecting and maintaining a good standard of oral health, but not related to any dental specialty.
Depending on these classified dental disciplines, a variety of topics with diverse salivary biomarkers using different research techniques and monitoring intervals were reported. Here, main focus of salivary biomarkers investigated was on Matrix-Metalloproteinases (MMP) plus Interleukins (IL). Figure 2 shows a map of disciplines by number of studies and patients included in this Scoping Review, and Figure 3 displays the major salivary biomarkers and their distribution within the studies.
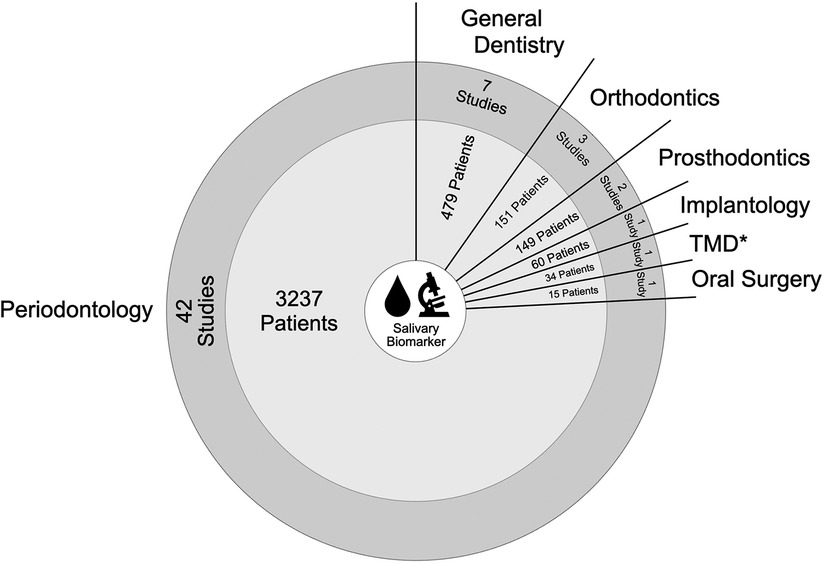
Figure 2. Map of data items associated with number of studies related to dental disciplines including patients involved (* TMD = temporo-mandibular disorder).
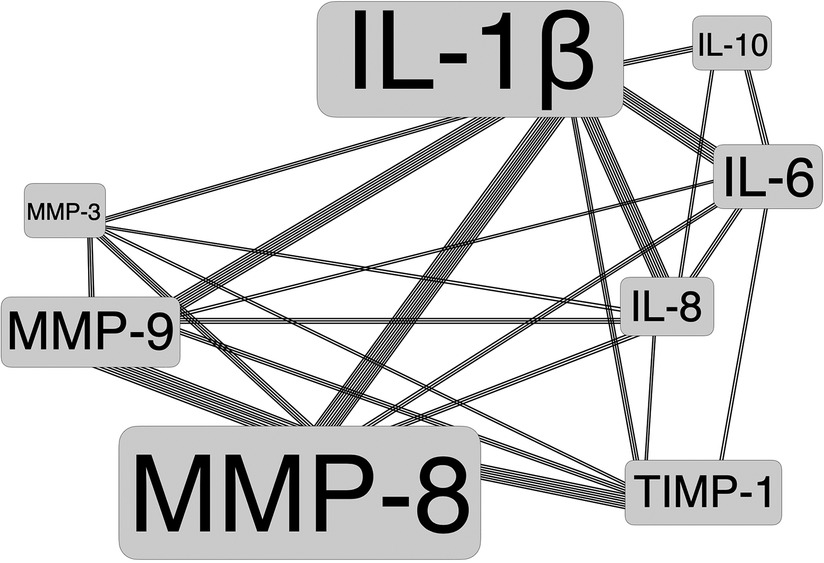
Figure 3. Major salivary biomarkers with frequency of occurrence (as illustrated by font size) and their combinations among studies.
Due to the pronounced heterogeneity of the included studies, a direct comparison among the identified publications was not deemed possible. Therefore, this Scoping Review of the included full-texts followed a descriptive analysis. Supplementary_Data 3 summarizes the detailed information of the included studies.
The aims of this Scoping Review were: (i) to compile studies on salivary biomarkers in terms of personalized oral healthcare and precision dentistry, (ii) to investigate the clinical relevance for oral health, and (iii) to summarize an outlook for future developments based on these results. “Scoping Reviews are also executed in a systematic, replicable manner, but usually intended to identify the types of available evidence in a given field and to discover knowledge gaps, to clarify concepts and definitions, and to examine how research is conducted, eventually, inform research, educational and clinical policy and priorities” (75, 76). A Scoping Review aims to “map the literature on a particular topic or research area and provide an opportunity to identify key concepts, gaps in the research; and types and sources of evidence to inform practice, policymaking, and research” (77). Therefore, the specific format Scoping Review was chosen to screen this relatively young topic and to summarize the current state of research in the context of a broad overview. Although a preliminary review protocol is available as Supplementary_Data 2, it should be noted that this Scoping Review was not registered on an online platform.
In general, precision dentistry provides diagnostic or therapeutic protocols that are as individualized as the disease with specific signs and symptoms. This approach is based on the identification of clinical information enabling the understanding of the patients' unique genomic constitution and how that makes them vulnerable to certain diseases. Each patient is to be treated with comprehensive consideration of individual circumstances using multidisciplinary channels, beyond the solely functional aspect of disease diagnosis. This also includes the continuous adjustment of therapy to keep pace with the progress of dental and medical knowledge (78, 79). It is still a very recent development: the move from purely evidence-based to personalized dentistry – and the meaning (and importance) for the dental sub-disciplines is entirely different.
Several research groups all over the world concentrate on personalized oral healthcare and precision dentistry today. The topic is of great interest in the field of dentistry, although there is (still) an imbalance in the distribution of the dental disciplines involved. The majority of all included publications were assigned to the field of periodontology, representing a proportion of 74% related to the number of studies, or 79% related to the number of patients, respectively. In restorative and reconstructive dentistry, research on salivary biomarkers seems to play a minor role at the present time. A possible explanation could be that periodontal diagnostics and treatment is very standardized following generally accepted principles (80). Therefore, it is much easier to implement personalized workflows in periodontology compared to other disciplines. For example, in reconstructive dentistry, it is per se a highly personalized discipline and uniform standard operating procedures (SOP) are hard to implement, except for situations that are directly comparable, such as complete edentulous patients (81).
The main causes of tooth loss are caries and periodontitis (82). Caries can usually be prevented very well by the patients' self-discipline with oral hygiene devices and the use of fluoride-containing toothpaste combined with mouth rinses. For periodontitis, however, clinical study outcomes could help to understand closer associations of genetic factors in periodontitis patients (83). It is therefore not surprising that with the further development of laboratory methods in recent years, salivary biomarkers have increasingly become the focus of periodontal research (84).
The abundance of matrix metalloproteinases (MMP) and interleukins (IL) as salivary biomarkers was particularly striking in this Scoping Review. It was also observed that a variety of different combinations of MMP and IL were examined. Most commonly, IL-1β, MMP-8, IL-6, and MMP-9 were determined in saliva, followed by IL-8, TIMP-1, IL-10 and MMP-3. Altogether, more than 92 different biomarkers were analyzed in saliva. This leads to the conclusion that currently no consensus exists on which biomarkers should be used for what specific scientific target(s) and with which intention.
Therefore, no clear recommendations can be given related to specific salivary biomarkers associated for personalized oral healthcare principles at this time. MMP and IL seem to be the most promising biomarkers, in particular in periodontology.
An exciting field also seems to be the pre-therapeutic examination of saliva for risk assessment of patients with regard to specific periodontal treatment modalities. This represents a true evolution from purely evidence-based (always applied in the same way) approaches to personalized treatment cascades. It remains very exciting whether new treatment concepts can be derived from this in the future, such as the creation of pre-therapeutic risk profiles of individual patients. Training datasets could be used to compute predictive biomarkers using statistic model predictions and clinical assessments to either differentiate health conditions or to predict treatment outcomes (85). Ideally, clinical measurements, applied threshold values, handling of missing data or data below the detection limit should be described thoroughly and very precisely to allow generalizability.
Only few studies in this Scoping Review examined a possible association of oral and systemic health (n = 6): most common was obesity/nutrition (19, 55, 56), followed by diabetes mellitus (59, 68, 70), and influence of pregnancy (72).
Although salivary biomarker research appears to be extremely promising, it remains to be seen to what extent the MedTech industry will jump on this technology in the future (86). Without an economic driver, it will be difficult to further investigate this costly research topic. Besides periodontology, peri-implantitis might also be an exciting field. This is certainly also in the interest of implant manufacturers, so that financial support for research would be guaranteed. Nevertheless, the results of this Scoping Review revealed only 1 clinical trial focusing on saliva and possible association with peri-implantitis (21). In addition, dentistry could become the door-opener for routine diagnostics in medicine, e.g., assessment of glucose concentration in diabetes patients (84).
Unfortunately, the studies identified demonstrated heterogeneous quality standards, starting with the study design, the number patients included, the salivary biomarkers investigated, and monitoring intervals. Direct comparisons are not possible. It would certainly be helpful if biomarkers could be defined (according to the classification in the different dental disciplines) and then examined under standardized conditions in various clinical studies.
Salivary biomarkers have the potential to change the field of dentistry in all disciplines. The increasing interest in dental research is obvious, demonstrated by the growing number of publications in recent years. At this time, however, the predominant discipline is periodontology. Precision dentistry and personalized workflows are trendy buzzwords, the future research will proof, if the high expectations can be fulfilled. Several research groups investigating diverse salivary biomarkers in a variety of combinations. The limiting factor of Big Data research is the amount of structured data available (87, 88). Therefore, the establishment of an open research data community comprising information of salivary samples could help to foster the further development of personalized oral healthcare and precision dentistry.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Conceptualization: TJ and NPP; Methodology: NPP, JH, ANZ and TJ, Writing-Original Draft Preparation: NPP and TJ, Writing-Review / Editing: JH, ANZ and REJ, Supervision: TJ, Project Administration: NPP and TJ. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/froh.2022.1003679/full#supplementary-material.
1. Marsh PD, Zaura E. Dental biofilm: ecological interactions in health and disease. J Clin Periodontol. (2017) 44(Suppl 18):S12–22. doi: 10.1111/jcpe.12679
2. Jepsen S, Blanco J, Buchalla W, Carvalho JC, Dietrich T, Dorfer C, et al. Prevention and control of dental caries and periodontal diseases at individual and population level: consensus report of group 3 of joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. J Clin Periodontol. (2017) 44(Suppl 18):S85–93. doi: 10.1111/jcpe.12687
3. Sanz M, Beighton D, Curtis MA, Cury JA, Dige I, Dommisch H, et al. Role of microbial biofilms in the maintenance of oral health and in the development of dental caries and periodontal diseases. Consensus report of group 1 of the joint EFP/ORCA workshop on the boundaries between caries and periodontal disease. J Clin Periodontol. (2017) 44(Suppl 18):S5–S11. doi: 10.1111/jcpe.12682
4. Baumgartner D, Johannsen B, Specht M, Lüddecke J, Rombach M, Hin S, et al. Oraldisk: a chair-side compatible molecular platform using whole saliva for monitoring oral health at the dental practice. Biosensors (Basel). (2021) 11:423. doi: 10.3390/bios11110423
5. Mitsakakis K, Stumpf F, Strohmeier O, Klein V, Mark D, Von Stetten F, et al. Chair/bedside diagnosis of oral and respiratory tract infections, and identification of antibiotic resistances for personalised monitoring and treatment. Stud Health Technol Inform. (2016) 224:61–6. doi: 10.3233/978-1-61499-653-8-61
6. Khan ZM, Waheed H, Khurshid Z, Zafar MS, Moin SF, Alam MK. Differentially expressed salivary proteins in dental caries patients. Biomed Res Int. (2021) 2021:5517521. doi: 10.1155/2021/5517521
7. Chojnowska S, Baran T, Wilińska I, Sienicka P, Cabaj-Wiater I, Knaś M. Human saliva as a diagnostic material. Adv Med Sci. (2018) 63:185–91. doi: 10.1016/j.advms.2017.11.002
8. Chambon C, Neyraud E, Sayd T, Bros P, Di Biagio R, Hyvrier F, et al. The salivary proteome reflects some traits of dietary habits in diabetic and non-diabetic older adults. Eur J Nutr. (2021) 60(8):4331–44. doi: 10.1007/s00394-021-02584-2
9. Belibasakis GN, Bostanci N, Marsh PD, Zaura E. Applications of the oral microbiome in personalized dentistry. Arch Oral Biol. (2019) 104:7–12. doi: 10.1016/j.archoralbio.2019.05.023
10. Paqué PN, Herz C, Jenzer JS, Wiedemeier DB, Attin T, Bostanci N, et al. Microbial analysis of Saliva to identify oral diseases using a point-of-care compatible qPCR assay. J Clin Med. (2020) 9(9):2945. doi: 10.3390/jcm9092945
11. Liebsch C, Pitchika V, Pink C, Samietz S, Kastenmüller G, Artati A, et al. The Saliva metabolome in association to oral health Status. J Dent Res. (2019) 98(6):642–51. doi: 10.1177/0022034519842853
12. Katsani KR, Sakellari D. Saliva proteomics updates in biomedicine. J Biol Res (Thessalon). (2019) 26:17. doi: 10.1186/s40709-019-0109-7
13. Azmi I, Faizan MI, Kumar R, Raj Yadav S, Chaudhary N, Kumar Singh D, et al. A saliva-based RNA extraction-free workflow integrated with Cas13a for SARS-CoV-2 detection. Front Cell Infect Microbiol. (2021) 11:632646. doi: 10.3389/fcimb.2021.632646
14. Tapari A, Braliou GG, Papaefthimiou M, Mavriki H, Kontou PI, Nikolopoulos GK, et al. Performance of antigen detection tests for SARS-CoV-2: a systematic review and meta-analysis. Diagnostics (Basel). (2022) 12(6):1388. doi: 10.3390/diagnostics12061388
15. Kinney JS, Morelli T, Braun T, Ramseier CA, Herr AE, Sugai JV, et al. Saliva/pathogen biomarker signatures and periodontal disease progression. J Dent Res. (2011) 90(6):752–8. doi: 10.1177/0022034511399908
16. Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. (2005) 8(1):19–32. doi: 10.1080/1364557032000119616
17. Levac D, Colquhoun H, O’Brien KK. Scoping studies: advancing the methodology. Implement Sci. (2010) 5:69. doi: 10.1186/1748-5908-5-69
18. Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. (2018) 169(7):467–73. doi: 10.7326/M18-0850
19. Al-Hamoudi N, Abduljabbar T, Mirza S, Al-Sowygh ZH, Vohra F, Javed F, et al. Non-surgical periodontal therapy reduces salivary adipocytokines in chronic periodontitis patients with and without obesity. J Investig Clin Dent. (2018) 9(2):e12314. doi: 10.1111/jicd.12314
20. Alajbeg IZ, Vrbanović E, Lapić I, Alajbeg I, Vuletić L. Effect of occlusal splint on oxidative stress markers and psychological aspects of chronic temporomandibular pain: a randomized controlled trial. Sci Rep. (2020) 10(1):10981. doi: 10.1038/s41598-020-67383-x
21. AlJasser R, Zahid M, AlSarhan M, AlOtaibi D, AlOraini S. The effect of conventional versus electronic cigarette use on treatment outcomes of peri-implant disease. BMC Oral Health. (2021) 21(1):480. doi: 10.1186/s12903-021-01784-w
22. Aspiras MB, Barros SP, Moss KL, Barrow DA, Phillips ST, Mendoza L, et al. Clinical and subclinical effects of power brushing following experimental induction of biofilm overgrowth in subjects representing a spectrum of periodontal disease. J Clin Periodontol. (2013) 40(12):1118–25. doi: 10.1111/jcpe.12161
23. Äyräväinen L, Heikkinen AM, Kuuliala A, Ahola K, Koivuniemi R, Laasonen L, et al. Inflammatory biomarkers in saliva and serum of patients with rheumatoid arthritis with respect to periodontal status. Ann Med. (2018) 50(4):333–44. doi: 10.1080/07853890.2018.1468922
24. Bertl K, Schoiber A, Haririan H, Laky M, Steiner I, Rausch WD, et al. Non-surgical periodontal therapy influences salivary melatonin levels. Clin Oral Investig. (2013) 17(4):1219–25. doi: 10.1007/s00784-012-0801-6
25. Bikker FJ, Nascimento GG, Nazmi K, Silbereisen A, Belibasakis GN, Kaman WE, et al. Salivary total protease activity based on a broad-spectrum fluorescence resonance energy transfer approach to monitor induction and resolution of gingival inflammation. Mol Diagn Ther. (2019) 23(5):667–76. doi: 10.1007/s40291-019-00421-1
26. Buczko P, Knaś M, Grycz M, Szarmach I, Zalewska A. Orthodontic treatment modifies the oxidant-antioxidant balance in saliva of clinically healthy subjects. Adv Med Sci. (2017) 62(1):129–35. doi: 10.1016/j.advms.2016.11.004
27. Buduneli N, Kardeşler L, Işik H, Willis CS 3rd, Hawkins SI, Kinane DF, et al. Effects of smoking and gingival inflammation on salivary antioxidant capacity. J Clin Periodontol. (2006) 33(3):159–64. doi: 10.1111/j.1600-051X.2006.00892.x
28. Chang CH, Han ML, Teng NC, Lee CY, Huang WT, Lin CT, et al. Cigarette smoking aggravates the activity of periodontal disease by disrupting redox homeostasis- an observational study. Sci Rep. (2018) 8(1):11055. doi: 10.1038/s41598-018-29163-6
29. Cutando A, López-Valverde A, Gómez-de-Diego R, Arias-Santiago S, de Vicente-Jiménez J. Effect of gingival application of melatonin on alkaline and acid phosphatase, osteopontin and osteocalcin in patients with diabetes and periodontal disease. Med Oral Patol Oral Cir Bucal. (2013) 18(4):e657–63. doi: 10.4317/medoral.18832
30. Dede F, Ozden FO, Avcı B. 8-hydroxy-deoxyguanosine Levels in gingival crevicular fluid and saliva in patients with chronic periodontitis after initial periodontal treatment. J Periodontol. (2013) 84(6):821–8. doi: 10.1902/jop.2012.120195
31. Fine DH, Markowitz K, Fairlie K, Tischio-Bereski D, Ferrandiz J, Godboley D, et al. Macrophage inflammatory protein-1α shows predictive value as a risk marker for subjects and sites vulnerable to bone loss in a longitudinal model of aggressive periodontitis. PLoS One. (2014) 9(6):e98541. doi: 10.1371/journal.pone.0098541
32. Fujimori K, Yoneda T, Tomofuji T, Ekuni D, Azuma T, Maruyama T, et al. Detection of salivary miRNAs that predict chronic periodontitis progression: a cohort study. Int J Environ Res Public Health. (2021) 18(15):8010. doi: 10.3390/ijerph18158010
33. Ghallab N, Shaker O. Salivary-soluble CD44 levels in smokers and non-smokers with chronic periodontitis: a pilot study. J Periodontol. (2010) 81(5):710–7. doi: 10.1902/jop.2010.090630
34. Gutiérrez-Corrales A, Campano-Cuevas E, Castillo-Dalí G, Serrera-Figallo M, Torres-Lagares D, Gutiérrez-Pérez JL. Relationship between salivary biomarkers and postoperative swelling after the extraction of impacted lower third molars. Int J Oral Maxillofac Surg. (2017) 46(2):243–9. doi: 10.1016/j.ijom.2016.10.005
35. Hassan SH, El-Refai MI, Ghallab NA, Kasem RF, Shaker OG. Effect of periodontal surgery on osteoprotegerin levels in gingival crevicular fluid, saliva, and gingival tissues of chronic periodontitis patients. Dis Markers. (2015) 2015:341259. doi: 10.1155/2015/341259
36. Hendek MK, Erdemir EO, Kisa U. Evaluation of salivary procalcitonin levels in different periodontal diseases. J Periodontol. (2015) 86(6):820–6. doi: 10.1902/jop.2015.130751
37. Hodosy J, Celec P. Daytime of sampling, tooth-brushing and ascorbic acid influence salivary thiobarbituric acid reacting substances–a potential clinical marker of gingival status. Dis Markers. (2005) 21(4):203–7. doi: 10.1155/2005/209643
38. Jentsch H, Sievert Y, Göcke R. Lactoferrin and other markers from gingival crevicular fluid and saliva before and after periodontal treatment. J Clin Periodontol. (2004) 31(7):511–4. doi: 10.1111/j.1600-051X.2004.00512.x
39. Jenzsch A, Eick S, Rassoul F, Purschwitz R, Jentsch H. Nutritional intervention in patients with periodontal disease: clinical, immunological and microbiological variables during 12 months. Br J Nutr. (2009) 101(6):879–85. doi: 10.1017/S0007114508047776
40. Justino AB, Teixeira RR, Peixoto LG, Jaramillo OLB, Espindola FS. Effect of saliva collection methods and oral hygiene on salivary biomarkers. Scand J Clin Lab Invest. (2017) 77(6):415–22. doi: 10.1080/00365513.2017.1334261
41. Kamodyová N, Tóthová L, Celec P. Salivary markers of oxidative stress and antioxidant status: influence of external factors. Dis Markers. (2013) 34(5):313–21. doi: 10.1155/2013/341302
42. Kibayashi M, Tanaka M, Nishida N, Kuboniwa M, Kataoka K, Nagata H, et al. Longitudinal study of the association between smoking as a periodontitis risk and salivary biomarkers related to periodontitis. J Periodontol. (2007) 78(5):859–67. doi: 10.1902/jop.2007.060292
43. Kim BS, Han DH, Lee H, Oh B. Association of salivary microbiota with dental caries incidence with dentine involvement after 4 years. J Microbiol Biotechnol. (2018) 28(3):454–64. doi: 10.4014/jmb.1710.10028
44. Kochurova EV, Nikolenko VN. Matrixins in the salivary fluid of patients with tumors of the maxillofacial region during orthopedic rehabilitation with different prosthetic structures. Bull Exp Biol Med. (2017) 163(5):663–6. doi: 10.1007/s10517-017-3874-z
45. Koppolu P, Sirisha S, Mishra A, Deshpande K, Lingam AS, Alotaibi DH, et al. Alkaline phosphatase and acid phosphatase levels in saliva and serum of patients with healthy periodontium, gingivitis, and periodontitis before and after scaling with root planing: a clinico-biochemical study. Saudi J Biol Sci. (2021) 28(1):380–5. doi: 10.1016/j.sjbs.2020.10.016
46. Kuboniwa M, Sakanaka A, Hashino E, Bamba T, Fukusaki E, Amano A. Prediction of periodontal inflammation via metabolic profiling of Saliva. J Dent Res. (2016) 95(12):1381–6. doi: 10.1177/0022034516661142
47. Lee CH, Chen YW, Tu YK, Wu YC, Chang PC. The potential of salivary biomarkers for predicting the sensitivity and monitoring the response to nonsurgical periodontal therapy: a preliminary assessment. J Periodontal Res. (2018) 53(4):545–54. doi: 10.1111/jre.12544
48. Liu KH, Hwang SJ. Effect of smoking cessation for 1 year on periodontal biomarkers in gingival crevicular fluid. J Periodontal Res. (2016) 51(3):366–75. doi: 10.1111/jre.12316
49. Morelli T, Stella M, Barros SP, Marchesan JT, Moss KL, Kim SJ, et al. Salivary biomarkers in a biofilm overgrowth model. J Periodontol. (2014) 85(12):1770–8. doi: 10.1902/jop.2014.140180
50. Nascimento GG, Baelum V, Sorsa T, Tervahartiala T, Skottrup PD, López R. Salivary levels of MPO, MMP-8 and TIMP-1 are associated with gingival inflammation response patterns during experimental gingivitis. Cytokine. (2019) 115:135–41. doi: 10.1016/j.cyto.2018.12.002
51. Nishida N, Yamamoto Y, Tanaka M, Kataoka K, Kuboniwa M, Nakayama K, et al. Association between involuntary smoking and salivary markers related to periodontitis: a 2-year longitudinal study. J Periodontol. (2008) 79(12):2233–40. doi: 10.1902/jop.2008.080149
52. Novakovic N, Todorovic T, Rakic M, Milinkovic I, Dozic I, Jankovic S, et al. Salivary antioxidants as periodontal biomarkers in evaluation of tissue status and treatment outcome. J Periodontal Res. (2014) 49(1):129–36. doi: 10.1111/jre.12088
53. Oktay S, Bal OO, Kuru L, Yarat A, Noyan U. Is sialic acid a promising marker for periodontal diseases? Niger J Clin Pract. (2020) 23(5):603–9. doi: 10.4103/njcp.njcp_499_19
54. Önder C, Kurgan Ş, Altıngöz SM, Bağış N, Uyanık M, Serdar MA, et al. Impact of non-surgical periodontal therapy on saliva and serum levels of markers of oxidative stress. Clin Oral Investig. (2017) 21(6):1961–9. doi: 10.1007/s00784-016-1984-z
55. Dede FÖ, Doğan ŞB, Ballı U, Avcı B, Durmuşlar MC. The effect of initial periodontal treatment on plasma, gingival crevicular fluid and salivary levels of 8-hydroxy-deoxyguanosine in obesity. Arch Oral Biol. (2016) 62:80–5. doi: 10.1016/j.archoralbio.2015.11.014
56. Park JY, Ko KA, Lee JY, Oh JW, Lim HC, Lee DW, et al. Clinical and immunological efficacy of mangosteen and propolis extracted complex in patients with gingivitis: a multi-centered randomized controlled clinical trial. Nutrients. (2021) 13(8):2604. doi: 10.3390/nu13082604
57. Parwani SR, Chitnis PJ, Parwani RN. Salivary nitric oxide levels in inflammatory periodontal disease - a case-control and interventional study. Int J Dent Hyg. (2012) 10(1):67–73. doi: 10.1111/j.1601-5037.2011.00508.x
58. Prakasam S, Srinivasan M. Evaluation of salivary biomarker profiles following non-surgical management of chronic periodontitis. Oral Dis. (2014) 20(2):171–7. doi: 10.1111/odi.12085
59. Raghav D, Alqahtani F, Albaker FJ, Bhagat TV, Kola Z. Intricate assessment and evaluation of long-term implant success as affected by clinicomicrobial and salivary diagnostics in type II diabetic patients: a longitudinal study. J Contemp Dent Pract. (2017) 18(5):405–9. doi: 10.5005/jp-journals-10024-2055
60. Rabelo MS, Gomes GH, Foz AM, Stadler AF, Cutler CW, Susin C, et al. Short-term effect of non-surgical periodontal treatment on local and systemic cytokine levels: role of hyperglycemia. Cytokine. (2021) 138:155360. doi: 10.1016/j.cyto.2020.155360
61. Ramseier AC, Petitat C, Trepp S, Lang NP, Eick S, Adam R, et al. Clinical parameters and oral fluid biomarkers in gingivitis subjects using an electric toothbrush with irrigator vs a manual toothbrush alone over 8 weeks: a randomised controlled clinical trial. Oral Health Prev Dent. (2021) 19(1):137–47.33615769
62. Rangbulla V, Nirola A, Gupta M, Batra P, Gupta M. Salivary IgA, interleukin-1β and MMP-8 as salivary biomarkers in chronic periodontitis patients. Chin J Dent Res. (2017) 20(1):43–51. doi: 10.3290/j.cjdr.a37741
63. Saloom HF, Papageorgiou SN, Carpenter GH, Cobourne MT. Impact of obesity on orthodontic tooth movement in adolescents: a prospective clinical cohort study. J Dent Res. (2017) 96(5):547–54. doi: 10.1177/0022034516688448
64. Sánchez GA, Miozza VA, Delgado A, Busch L. Salivary IL-1β and PGE2 as biomarkers of periodontal status, before and after periodontal treatment. J Clin Periodontol. (2013) 40(12):1112–7. doi: 10.1111/jcpe.12164
65. Sexton WM, Lin Y, Kryscio RJ, Dawson DR 3rd, Ebersole JL, Miller CS. Salivary biomarkers of periodontal disease in response to treatment. J Clin Periodontol. (2011) 38(5):434–41. doi: 10.1111/j.1600-051X.2011.01706.x
66. Silbereisen A, Alassiri S, Bao K, Grossmann J, Nanni P, Fernandez C, et al. Label-free quantitative proteomics versus antibody-based assays to measure neutrophil-derived enzymes in saliva. Proteomics Clin Appl. (2020) 14(3):e1900050. doi: 10.1002/prca.201900050
67. Syndergaard B, Al-Sabbagh M, Kryscio RJ, Xi J, Ding X, Ebersole JL, et al. Salivary biomarkers associated with gingivitis and response to therapy. J Periodontol. (2014) 85(8):e295–303. doi: 10.1902/jop.2014.130696
68. Tatarakis N, Kinney JS, Inglehart M, Braun TM, Shelburne C, Lang NP, et al. Clinical, microbiological, and salivary biomarker profiles of dental implant patients with type 2 diabetes. Clin Oral Implants Res. (2014) 25(7):803–12. doi: 10.1111/clr.12139
69. Varghese J, Bhat V, Chianeh YR, Kamath V, Al-Haj Husain N, Özcan M. Salivary 8-hydroxyguanosine levels in smokers and non-smokers with chronic periodontitis. Odontology. (2020) 108(4):569–77. doi: 10.1007/s10266-020-00496-x
70. Venza M, Visalli M, Cucinotta M, Cicciù D, Passi P, Teti D. Salivary histamine level as a predictor of periodontal disease in type 2 diabetic and non-diabetic subjects. J Periodontol. (2006) 77(9):1564–71. doi: 10.1902/jop.2006.050373
71. Wu JQ, Jiang JH, Xu L, Liang C, Wang XJ, Bai Y. Magnetic bead-based salivary peptidome profiling for accelerated osteogenic orthodontic treatments. Chin J Dent Res. (2018) 21(1):41–9. doi: 10.3290/j.cjdr.a39917
72. Yarkac FU, Gokturk O, Demir O. Effect of non-surgical periodontal therapy on the degree of gingival inflammation and stress markers related to pregnancy. J Appl Oral Sci. (2018) 26:e20170630. doi: 10.1590/1678-7757-2017-0630
73. Yoshida RA, Gorjão R, Mayer MPA, Corazza PFL, Guare RO, Ferreira A, et al. Inflammatory markers in the saliva of cerebral palsy individuals with gingivitis after periodontal treatment. Braz Oral Res. (2019) 33:e033. doi: 10.1590/1807-3107bor-2019.vol33.0033
74. Yoshie H, Tai H, Kobayashi T, Oda-Gou E, Nomura Y, Numabe Y, et al. Salivary enzyme levels after scaling and interleukin-1 genotypes in Japanese patients with chronic periodontitis. J Periodontol. (2007) 78(3):498–503. doi: 10.1902/jop.2007.060216
75. Colquhoun HL, Levac D, O'Brien KK, Straus S, Tricco AC, Perrier L, et al. Scoping reviews: time for clarity in definition, methods, and reporting. J Clin Epidemiol. (2014) 67(12):1291–4. doi: 10.1016/j.jclinepi.2014.03.013
76. Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. (2018) 18(1):143. doi: 10.1186/s12874-018-0611-x
77. Daudt HM, van Mossel C, Scott SJ. Enhancing the scoping study methodology: a large, inter-professional team's experience with Arksey and O'Malley's Framework. BMC Med Res Methodol. (2013) 13:48. doi: 10.1186/1471-2288-13-48
78. Ashley EA. The precision medicine initiative: a new national effort. JAMA. (2015) 313(21):2119–20. doi: 10.1001/jama.2015.3595
79. Chowkwanyun M, Bayer R, Galea S. “Precision” public health - between novelty and hype. N Engl J Med. (2018) 379(15):1398–400. doi: 10.1056/NEJMp1806634
80. Chiappelli F. Evidence-based dentistry: two decades and beyond. J Evid Based Dent Pract. (2019) 19(1):7–16. doi: 10.1016/j.jebdp.2018.05.001
81. Joda T, Zitzmann NU. Personalized workflows in reconstructive dentistry-current possibilities and future opportunities. Clin Oral Investig. (2022) 26(6):4283–90. doi: 10.1007/s00784-022-04475-0
82. Gerritsen AE, Allen PF, Witter DJ, Bronkhorst EM, Creugers NH. Tooth loss and oral health-related quality of life: a systematic review and meta-analysis. Health Qual Life Outcomes. (2010) 8:126. doi: 10.1186/1477-7525-8-126
83. Shungin D, Haworth S, Divaris K, Agler CS, Kamatani Y, Keun Lee M, et al. Genome-wide analysis of dental caries and periodontitis combining clinical and self-reported data. Nat Commun. (2019) 10(1):2773. doi: 10.1038/s41467-019-10630-1
84. Giannobile WV, Kornman KS, Williams RC. Personalized medicine enters dentistry: what might this mean for clinical practice? J Am Dent Assoc. (2013) 144(8):874–6. doi: 10.14219/jada.archive.2013.0200
85. Paqué PN, Herz C, Wiedemeier DB, Mitsakakis K, Attin T, Bao K, et al. Salivary biomarkers for dental caries detection and personalized monitoring. J Pers Med. (2021) 11(3):235. doi: 10.3390/jpm11030235
86. Joda T, Yeung AWK, Hung K, Zitzmann NU, Bornstein MM. Disruptive innovation in dentistry: what it is and what could be next. J Dent Res. (2021) 100(5):448–53. doi: 10.1177/0022034520978774
87. Joda T, Waltimo T, Probst-Hensch N, Pauli-Magnus C, Zitzmann NU. Health data in dentistry: an attempt to master the digital challenge. Public Health Genomics. (2019) 22(1-2):1–7. doi: 10.1159/000501643
Keywords: saliva, oral infections, gingivitis, periodontitis, diagnostics
Citation: Paqué PN, Hjerppe J, Zuercher AN, Jung RE and Joda T (2022) Salivary biomarkers as key to monitor personalized oral healthcare and precision dentistry: A scoping review. Front. Oral. Health 3:1003679. doi: 10.3389/froh.2022.1003679
Received: 26 July 2022; Accepted: 1 September 2022;
Published: 22 September 2022.
Edited by:
Gustavo G. Nascimento, Aarhus University, DenmarkReviewed by:
Leandro Machado Oliveira, Federal University of Santa Maria, Brazil© 2022 Paqué, Hjerppe, Zuercher, Jung and Joda. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tim Joda dGltLmpvZGFAenptLnV6aC5jaA==
Specialty Section: This article was submitted to Oral Health Promotion, a section of the journal Frontiers in Oral Health
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.