
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oral. Health , 29 March 2021
Sec. Oral Cancers
Volume 2 - 2021 | https://doi.org/10.3389/froh.2021.638213
This article is part of the Research Topic Prognostic Biomarkers for Oral Cancer View all 5 articles
Overexpression of Cleft Lip and Palate Transmembrane 1-Like (Clptm1L) confers cancer cell survival through the endoplasmic reticulum (ER) stress survival signaling pathway, while TMEM207 impairs the tumor suppressor function of WW domain containing oxidoreductase (WWOX), which sensitizes cancer cells to ER stress-induced apoptosis. In the present study, we examined whether these two ER stress-related proteins, Clptm1L and TMEM207, could be prognostic markers in oral squamous cell carcinoma (OSCC). Immunohistochemical staining using specific antibodies to Clptm1L or TMEM207 revealed that 31 of 89 tissue specimens exhibited concomitant expression of Clptm1L and TMEM207 at the cancer invasion front. A Kaplan–Meier plot of the patient survival curve followed by a log-rank test revealed that the coexpression of Clptm1L and TMEM207 was significantly associated with poor outcome in patients with OSCC (P = 0.00252). Coexpression of Clptm1L and TMEM207 was closely related to lymph node metastasis (P=0.000574). Both univariate and multivariate analyses demonstrated that coexpression of Clptm1L and TMEM207 predicted the poor prognosis of the patients with OSCC. The present study indicated that the double positive Clptm1L and TMEM207 immunoreactivity was closely related to lymph node metastasis with prognostic value in patients with OSCC.
The incidence of oral squamous cell carcinoma (OSCC) has increased in the recent years. Despite progress in chemotherapy and radiotherapy, the prognosis of advanced OSCC remains unfavorable [1]. This is contrary to the improved prognosis of patients with other cancers, such as breast cancer, lymphoma, lung cancer, and gastrointestinal cancers, which has been greatly improved by recent molecular targeted therapies. To develop new molecular targeting therapies with regard to OSCC, there is a need to clarify the pathobiological mechanism, especially the crucial point of signaling cascade in oral squamous carcinogenesis.
The endoplasmic reticulum (ER) is a critical compartment for folding of the secretory, plasma membrane, and various organelle proteins [2]. Under various conditions, because of ER stress, protein folding is impaired, leading to the accumulation of misfolded proteins in the ER [3]. The accumulation of these misfolded proteins is harmful to the cells, and prolonged ER stress typically leads to apoptosis [4]. Therefore, cancer cells are more dependent on stress support survival pathways [5], which could be a good target in molecular therapy.
Cleft Lip and Palate Transmembrane 1-Like (Clptm1L) was first isolated by screening cisplatin resistance-related genes [6]. Subsequently, several studies have demonstrated that Clptm1L is involved in lung carcinogenesis [7–10]. Notably, Clptm1L interacts with GRP78, which mediates ER stress-induced Akt signaling for ER stress survival in pancreatic adenocarcinoma cells [11].
TMEM207 is localized to the ER and binds to the tumor suppressor molecule, WW domain containing oxidoreductase (WWOX), through its PPxY domain [12]. As a result, TMEM207 inhibits the various tumor suppressor function of WWOX, leading to the induction of ER stress-related apoptosis in cancer cells [13, 14]. Notably, the tumor suppressor function of WWOX is dependent on the expression level of GRP78 in ovarian cancer cells [15].
In this study, we aimed to determine the prognostic value of concomitant expression of Clptm1L and TMEM207 in OSCC. Here, we show the robust correlation of Clptm1L and TMEM207 expression with lymph node metastasis and poor prognosis in patients with OSCC.
For this retrospective study, we collected data of all surgically treated patients primarily diagnosed with OSCC. Archived pathological tissue specimens from 89 OSCC cases were used in this study. The present study was conducted in accordance with the ethical standards of the Helsinki Declaration in 1975. The use of tissue samples and review of the clinical records were performed according to protocols approved by the Institutional Review Board of the Gifu University Graduate School of Medicine (specific approval number: 28-524).
All tissue specimens were obtained surgically, fixed in 10% buffered formalin, and embedded in paraffin. Tissue sections representing the deepest invasion site of each case were immunohistochemically stained. The rabbit conventional antibody to Clptm1L (dilution 1:100, cat no. NBP1-84378) was obtained from Novus Biologicals (Littleton, CO, USA). Generation and characterization of a murine monoclonal antibody recognizing the synthetic peptide VNYNDQHPNGW (a.a. 40–50 of TMEM207) described previously [12], was employed in this study. Anti-TMEM207 antibody was used at 5 μg/ml for immunohistochemical staining. Control antibodies were used as previously described [12, 15]. The tissue sections deparaffinized, incubated with normal goat serum, and incubated with antibodies for overnight at 4°C. After washing, the tissues were immunostained with antibodies using the ImmPRESS™ polymerized reporter enzyme staining system (Vector Laboratories, Inc., Burlingame, CA, USA) as previously reported [15].
We also performed double immunohistochemical staining of twenty cases using a biotin-free polymer detection kit (MACH 2, Biocare Medical, Walnut Creek, CA, USA) according to the manufacturer's protocol.
We evaluated the immunohistochemical staining results as a percentage of immunoreactivity in OSCC cells. The fraction of positive cells stained at the cancer invasion front was scored after examining three high-power fields (400×) per tissue section for each case. The staining was considered negative if <10% of invasive cancer cells exhibited immunoreactivity, and positive if over 10% did. One experienced pathologist read the sections without knowing the pathological grade and clinical data.
Curves for overall survival (OS) were drawn using the Kaplan–Meier method, and differences in survival rates were compared using the log-rank test. The relationship between clinicopathological parameters was examined using the chi-square test. Univariate and multivariate analyses of the Cox proportional hazard model were also used to determine the significant prognostic factors of OS. A P < 0.05 was considered statistically significant.
Clinicopathological features of OSCC cases are shown in Table 1. Representative immunohistochemical staining is shown in Figure 1. Non-cancerous oral stratified squamous epithelium exhibited little Clptm1L or TMEM207 immunoreactivity. In contrast, 50 and 40 of 89 OSCC tissue specimens exhibited Clptm1L or TMEM207 immunoreactivity, respectively. Notably, Clptm1L and TMEM207 expressions were significantly related to each other (Table 2, P = 0.0002). We considered 31 tissue specimens as double positive for Clptm1L and TMEM207. Concomitant expression of Clptm1L and TMEM207 expression was confirmed by double immunohistochemical staining (Figure 2).
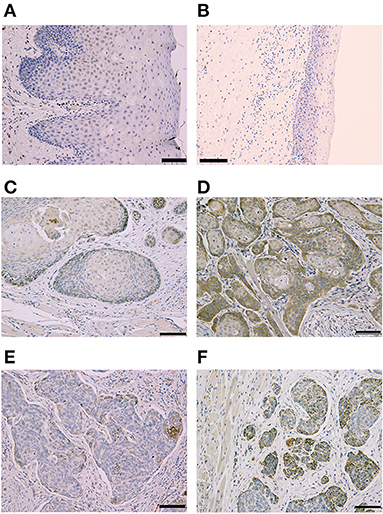
Figure 1. Representative Immunohistochemical staining according to Cleft Lip and Palate Transmembrane 1-Like (Clptm1L) and TMEM207 immunoreactivity at the invasion front of oral squamous cell carcinoma (OSCC). Weak Clptm1L or TMEM207 immunoreactivity was found in non-tumorous oral epithelial cells (A,B), respectively. In contrast, Clptm1L or TMEM207 immunoreactivity was found in many invasive OSCC cells. Representative positive Clptm1L immunoreactivity in tissue specimens from patients with favorable and poor prognosis is shown in (C,D), respectively. TMEM207 immunoreactivity in tissue specimens from patients with favorable and poor prognosis is shown in (E,F), respectively. Note the robust Clptm1L and TMEM207 immunoreactivity in (D,F).
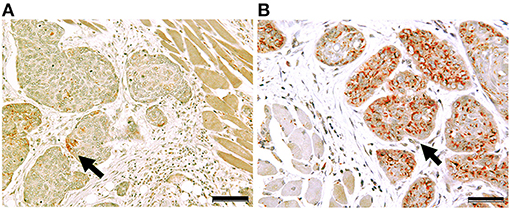
Figure 2. Concomitant expression of Cleft Lip and Palate Transmembrane 1-Like 1 (Clptm1L) and TMEM207 in OSCC cells. Double immunohistochemical staining showed that the Clptm1L, Cleft Lip, and Palate Transmembrane 1-Like (Clptm1L) immunoreactivity (brown color) and TMEM207 immunoreactivity (red color) were co-localized in OSCC cells during cancer invasion in a case of poor outcome (B). In contrast, little Clptm1L or TMEM207 immunoreactivity was found in a case with a favorable outcome (A). Arrows indicate positive staining. Bar indicates 50 μm.
Double Clptm1L and TMEM207 immunoreactivity was significantly related to pT (T1/2 vs. T3/T4) (P = 0.0148), pN stage (P = 0.000574), and smoking habits (P = 0.0032) of patients with OSCC (Table 3). A Kaplan–Meier plot of the patient survival curve followed by log-rank test revealed that the concomitant expression of Clptm1L and TMEM207 was significantly related to poor outcome in patients with OSCC (P = 0.00252) (Figure 3). In the univariate analysis, nodal metastasis (P = 0.0005), UICC stage (I + II vs. III + IV) (P = 0.0089), and double positive for Clptm1L and TMEM207 (P = 0.0046) were significantly related to poor outcome of the patients (Table 4). Multivariate Cox proportional hazards regression analysis indicated that double positive for Clptm1L and TMEM207 expression yielded a hazard ratio of death of 2.466 (95% confidence limit, 1.085–6.386, P = 0.032) in the OSCC series.
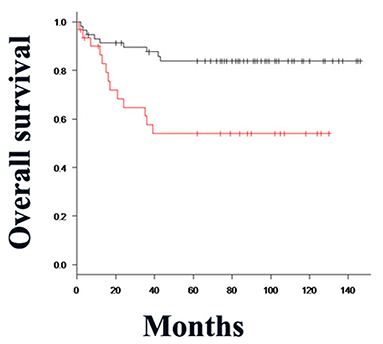
Figure 3. Kaplan–Meier method and differences in the survival rates were compared using the log-rank test for univariate survival analysis. The overall survival rate of patients in the Clptm1L and TMEM207 co-expressed group (red line) was significantly lower than that of patients in the Clptm1L or TMEM20 negative group (black line) (P = 0.00252).
In the present study, we found that Clptm1L and TMEM207 were significantly related to smoking habits in patients with OSCC. Several studies have reported that cigarette smoke induces ER stress in normal and various malignant tumor cells [16]. It is likely that overexpression of Clptm1L and TMEM207 may promote oral squamous cell carcinogenesis by conferring resistance to cigarette smoke-associated ER stress. Alternatively, cigarette smoking-associated ER stress might induce Clptm1L and TMEM207 expression in OSCC cells. Further studies are needed to unravel the relationship between cigarette smoke and Clptm1L and/or TMEM207 expression in OSCC cells.
Using the Kaplan–Meier (log rank) test, the P-value for the difference between concomitant expression of Clptm1L-TMEM207 and others = 0.00252 for OS time Notably, Multivariate Cox's regression analysis unraveled that concomitant expression of Clptm1L-TMEM207 was an independent worse prognosis value (P = 0.032) (Table 4).
The exact molecular mechanism, which results in close relation of concomitant Clptm1L-TMEM207 expression and nodal metastasis remains unclear. Interestingly, overexpression of GRP78 increases lymph node metastasis in various cancers [17–19]. GRP78 might be a key molecule associated with high lymph node metastasis rate in Clptm1L and TMEM207 co-expressed OSCC.
Oliveira and Ribeiro-Silva classified immunohistochemical biomarkers with prognostic significance as cell cycle, apoptosis, angiogenesis, cell adhesion, and matrix degradation-related molecules in OSCC [20]. In contrast, the present study revealed that two ER stress-related proteins, Clptm1L and TMEM207, could also be a good biomarker to predict poor outcomes in patients with OSCC. We believe that molecules which help OSCC cells evade ER stress could also be a target of molecular therapy in patients with OSCC.
In conclusion, the present study demonstrated that the expression of two ER stress-related proteins, Clptm1L and TMEM207 immunoreactivity was closely related to lymph node metastasis with prognostic value in patients with OSCC.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Institutional Review Board of the Gifu University Graduate School of Medicine (specific approval number: 28-524). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
KHan and KHat carried out the scoring, performed the statistical analysis, and interpreted the results. CS and YK designed the project and wrote the manuscript. TS contributed to the manuscript text. TT designed and supervised the project. All authors critically revised the manuscript.
This study was supported by grant from the Ministry of Education of Japan (Grant no. 20K07406).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank our colleague, Ms. Masako Azuma for providing tissue specimens. We thank Dr. Hideharu Tanaka for his advise on statistical analysis.
Clptm1L, Cleft Lip and Palate Transmembrane 1-Like; ER, endoplasmic reticulum; WWWOX, WW domain containing oxidoreductase; OSCC, oral squamous cell carcinoma; OS, overall survival; TON, tongue; LG, lower gum; BM, buccal mucosa; UG, upper gum; FOM, floor of mouth; HP, hard palate.
2. Sitia R, and Braakman I. Quality control in the endoplasmic reticulum protein factory. Nature. (2003) 426:891–4. doi: 10.1038/nature02262
3. Xu C, Bailly-maitre B, and Reed JC. Endoplasmic reticulum stress : cell life and death decisions Find the latest version : review series Endoplasmic reticulum stress : cell life and death decisions. J Clin Invest. (2005) 115:2656–64. doi: 10.1172/JCI26373
4. Szegezdi E, Logue SE, Gorman AM, and Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. (2006) 7:880–5. doi: 10.1038/sj.embor.7400779
5. Jäättelä M. Escaping cell death: survival proteins in cancer. Exp Cell Res. (1999) 248:30–43. doi: 10.1006/excr.1999.4455
6. Yamamoto K, Okamoto A, Isonishi S, Ochiai K, and Ohtake Y. A novel gene, CRR9, which was up-regulated in CDDP-resistant ovarian tumor cell line, was associated with apoptosis. Biochem Biophys Res Commun. (2001) 280:1148–54. doi: 10.1006/bbrc.2001.4250
7. James MA, Wen W, Wang Y, Byers LA, Heymach JV, Coombes KR, et al. Functional characterization of CLPTM1L as a lung cancer risk candidate gene in the 5p15. Locus. (2012) 7:1–9. doi: 10.1371/journal.pone.0036116
8. James MA, Vikis HG, Tate E, Rymaszewski AL, and You M. CRR9/CLPTM1L regulates cell survival signaling and is required for ras transformation and lung tumorigenesis. Cancer Res. (2014) 74:1116–27. doi: 10.1158/0008-5472.CAN-13-1617
9. Puskás LG, Mán I, Szebeni G, Tiszlavicz L, Tsai S, and James MA. Novel Anti-CRR9/CLPTM1L antibodies with antitumorigenic activity inhibit cell surface accumulation, PI3K interaction, and survival signaling. Mol Cancer Ther. (2016) 15:985–97. doi: 10.1158/1535-7163.MCT-15-0717
10. Wang Y, Broderick P, Matakidou A, Eisen T, and Houlston RS. Role of 5p15.33 (TERT-CLPTM1L), 6p21.33 and 15q25.1 (CHRNA5-CHRNA3) variation and lung cancer risk in never-smokers. Carcinogenesis. (2010) 31:234–8. doi: 10.1093/carcin/bgp287
11. Clarke WR, Amundadottir L, and James MA. CLPTM1L/CRR9 ectodomain interaction with GRP78 at the cell surface signals for survival and chemoresistance upon ER stress in pancreatic adenocarcinoma cells. Int J Cancer. (2019) 144:1367–78. doi: 10.1002/ijc.32012
12. Takeuchi T, Adachi Y, and Nagayama T. A WWOX-binding molecule, transmembrane protein 207, is related to the invasiveness of gastric signet-ring cell carcinoma. Carcinogenesis. (2012) 33:548–54. doi: 10.1093/carcin/bgs001
13. Saigo C, Kito Y, and Takeuchi T. Cancerous protein network that inhibits the tumor suppressor function of WW domain-containing oxidoreductase (WWOX) by aberrantly expressed molecule. Front Oncol. (2018) 8:350. doi: 10.3389/fonc.2018.00350
14. Janczar S, Nautiyal J, Xiao Y, Curry E, Sun M, Zanini E, et al. WWOX sensitises ovarian cancer cells to paclitaxel via modulation of the ER stress response. Cell Death Dis. (2017) 8:e2955. doi: 10.1038/cddis.2017.346
15. Takeuchi T, Misaki A, Liang SB, Tachibana A, Hayashi N, Sonobe H, et al. Expression of T-cadherin (CDH13, H-cadherin) in human brain and its characteristics as a negative growth regulator of epidermal growth factor in neuroblastoma cells. J Neurochem. (2000) 74:1489–97. doi: 10.1046/j.1471-4159.2000.0741489.x
16. Jorgensen E, Stinson A, Shan L, Yang J, Gietl D, and Albino AP. Cigarette smoke induces endoplasmic reticulum stress and the unfolded protein response in normal and malignant human lung cells. BMC Cancer. (2008) 8:1–30. doi: 10.1186/1471-2407-8-229
17. Zhang J, Jiang Y, Jia Z, Li Q, Gong W, Wang L, et al. Association of elevated GRP78 expression with increased lymph node metastasis and poor prognosis in patients with gastric cancer. Clin Exp Metastasis. (2006) 23:401–10. doi: 10.1007/s10585-006-9051-9
18. Sun Q, Hua J, Wang Q, Xu W, Zhang J, Zhang J, et al. Expressions of GRP78 and Bax associate with differentiation, metastasis, and apoptosis in non-small cell lung cancer. Mol Biol Rep. (2012) 39:6753–61. doi: 10.1007/s11033-012-1500-8
19. Ren P, Chen C, Yue J, Zhang J, and Yu Z. High expression of glucose-regulated protein 78 (GRP78) is associated with metastasis and poor prognosis in patients with esophageal squamous cell carcinoma. Onco Targets Ther. (2017) 10:617–25. doi: 10.2147/OTT.S123494
Keywords: OSCC, prognosis, TMEM207, CLPTM1L, ER-stress, WWOX
Citation: Hano K, Hatano K, Saigo C, Kito Y, Shibata T and Takeuchi T (2021) Combination of Clptm1L and TMEM207 Expression as a Robust Prognostic Marker in Oral Squamous Cell Carcinoma. Front. Oral. Health 2:638213. doi: 10.3389/froh.2021.638213
Received: 05 December 2020; Accepted: 04 March 2021;
Published: 29 March 2021.
Edited by:
Sabrina Wurzba, McGill University, CanadaReviewed by:
Samapika Routray, All India Institute of Medical Sciences Bhubaneswar, IndiaCopyright © 2021 Hano, Hatano, Saigo, Kito, Shibata and Takeuchi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tamotsu Takeuchi, dGFrZXV0aXQwOEBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.