- 1Oculofacial Plastic Surgery, Miami, FL, United States
- 2Thrive Health, West Vancouver, BC, Canada
- 3School of Medicine, West Virginia University, Morgantown, WV, United States
- 4Tourmaline Bio, Inc., New York, NY, United States
- 5Department of Surgery, Sharp Grossmont Hospital for Neuroscience La Mesa, CA, United States
Elevated serum interleukin-6 (IL-6) levels have been shown to correlate with disease activity in patients with thyroid eye disease (TED), a complex, heterogeneous, autoimmune disease affecting thousands of people worldwide. IL-6 plays a pivotal role in the pathogenesis of TED through three key mechanisms that together may contribute to inflammation, tissue expansion, remodeling, and fibrosis within the orbit. First, IL-6 drives an autoimmune response targeting the thyroid-stimulating hormone receptor (TSHR) by promoting the production of autoantibodies (i.e. TSHR-Ab, TSI), thereby triggering TSHR-dependent immune pathways. Second, IL-6 stimulates the activation and differentiation of orbital fibroblasts, which contributes to the inflammatory process and increase adipogenesis. Finally, IL-6 stimulates T-cell–mediated inflammation, amplifying the immune response within orbital tissues. Although corticosteroids and surgery have served as mainstays of TED treatment, a multimodal approach is often required due to the disease’s heterogeneous presentation and response to current treatment options. TED is a chronic, lifelong condition characterized by periods of exacerbation and remission, with inflammation playing a central role in disease progression and severity. Because inflammation can flare intermittently throughout a patient’s life, there is growing interest in targeting specific components of the immune system to reduce disease activity and severity. This review focuses on the current evidence supporting IL-6 as a key mediator of TED pathogenesis and explores its potential as a diagnostic biomarker and therapeutic target of the disease.
1 Introduction
Interleukin-6 (IL-6) is a multi-functional cytokine that regulates various physiological processes within multiple tissues. IL-6 polymorphisms have been associated with varying risks for, or protection against several different diseases (1, 2). Multiple studies have reported a correlation between higher serum IL-6 levels and disease activity in patients with thyroid eye disease (TED) (3). TED is a complex autoimmune disease caused by local inflammation of the orbital tissues that can lead to visual dysfunction, vision loss, and facial disfigurement that alters the patient’s overall appearance and quality-of-life (QoL). The heterogenous presentation of TED requires a multimodal treatment approach that must also adapt over time to variable inflammatory activity. The therapeutic landscape changed with U.S. Food and Drug Administration (FDA) approval of Teprotumumab in February 2020. Since its introduction, other monoclonal antibodies are being evaluated for safety and efficacy in TED.
Targeted anti-IL-6 monoclonal antibody therapy has been used for TED patients in clinical studies (4). An understanding of IL-6 signaling is required to achieve a balance between targeting the pathological effects of IL-6-mediated inflammation and the unintentional concomitant abolition of its anti-inflammatory and pro-resolution effects. This review focuses on the current evidence suggesting a critical role for IL-6 as a key mediator of TED pathogenesis and discusses the potential utility of IL-6 as a biomarker and therapeutic target for TED.
2 Overview of IL-6
IL-6 is a pleiotropic cytokine produced by various lymphocytes and non-lymphocyte cells including T and B cells, fibroblasts, monocytes, mesangial cells, endothelial cells, keratinocytes, and tumor cells (5). Specifically, IL-6 is known to regulate the proliferation and differentiation of adult hematopoietic stem and progenitor cells in a paracrine manner (6). It induces several complement system proteins and the coagulation cascade, and also functions as an endogenous pyrogen to regulate thermogenesis (7). Notably, IL-6 plays a significant role in inflammation and immune responses by stimulating acute phase proteins (e.g., C-reactive protein [CRP], serum amyloid A, fibrinogen, and hepcidin), promoting T and B cell differentiation and maturation, and activating cells such as fibroblasts (8).
Additionally, IL-6 is a myokine, secreted from skeletal muscle during exercise and acting upon that muscle in paracrine and autocrine fashions. IL-6 also mediates anti-inflammatory and metabolic processes similar to an endocrine hormone. It has metabolic effects that allow for improved insulin signaling, enhanced insulin sensitivity, and increased fatty acid oxidation in skeletal muscles, and may also contribute to exercise-induced vascular remodeling (7). IL-6 is also pivotal for maintaining the integrity of the intestinal epithelium. Conversely, in inflammatory conditions, IL-6 harms this epithelial barrier by increasing intestinal permeability (9). Additionally, several chronic inflammatory conditions and different types of cancer feature aberrant hyperactivation of the IL−6/JAK/STAT3 signaling pathway (10).
IL-6 signals through three primary mechanisms: cis signaling, trans signaling, and trans-presentation (11). Briefly, cis signaling, otherwise termed classical signaling, involves binding of IL-6 to its membrane bound receptor (IL-6R), which activates intracellular pathways that influence local cell behavior (12). Conversely, trans signaling occurs when IL-6 binds to soluble IL-6R (sIL-6R), which can impact distant cells by binding to gp130 receptors (12). While trans-presentation, also known as cluster signaling, involves the presentation of IL-6-IL-6R complexes on the surface of one cell to another, contributing to downstream cell signaling and responses (11).
3 IL-6 and TED
3.1 Overview of TED
TED is a lifelong autoimmune disease with a prevalence of approximately five cases per 100,000 person-years (adjusted for age and sex) within the U.S (13). Although it can affect people of all ages, TED typically manifests during mid-life (e.g., 49 years of age), and occurs more frequently in women than men (14). Several factors have been associated with worsening severity of TED, including age, male sex, poorly controlled thyroid levels, radioactive iodine treatment, and smoking (15, 16). Many patients experience persistent or worsening orbital tissue inflammation for years, even after the initial presentation (e.g., 15.6% rate recurrence within 10 years from the initial episode) (17, 18). This local inflammation can lead to visual dysfunction, chronic pain, and facial disfigurement, resulting in characteristic changes to facial features and significant impacts on daily activities and functionality. Patients with TED ultimately experience a poorer QoL, social withdrawal, and mental health issues (15, 19–21). In two different studies evaluating the mental health of patients with TED, more than one-third of patients (36 - 42%) reported suffering from depression or anxiety, compared to one-fifth of the general U.S. adult population (17, 21).
Although the pathophysiology of TED is complex and not fully understood, the most widely accepted mechanism involves a loss of self-tolerance to the thyroid-stimulating hormone (TSH, or thyrotropin) receptor (TSHR) and insulin-like growth factor-1 receptor (IGF-1R) (22). These receptors are expressed together on fibroblasts in orbital and periocular tissues and interact with each other (23). This autoimmune response triggers an inflammatory cascade within the orbital, facial, and periocular tissues, leading to hypertrophy and fibrosis of the extraocular muscles, adipogenesis, eyelid retraction, and inflammation around the eyes (23, 24). Due to the presence of TSHR in orbital and periocular tissues as well as the thyroid, TED often occurs simultaneously with systemic Graves’ disease (GD), an autoimmune disorder affecting the thyroid gland that can lead to hyperthyroidism and abnormal thyroid hormone levels (25). However, in some cases, TED may present with normal euthyroid status or primary hypothyroidism (such as in Hashimoto’s thyroiditis) (26).
With persisting inflammation or flares of worsening inflammation, patients often experience symptoms such as proptosis, eyelid retraction, orbital pain, periocular redness and edema, and diplopia (26). As the tissues expand within the fixed, bony orbit, they can compress the optic nerve, leading to dysthyroid optic neuropathy and potential permanent vision loss (27, 28). Optic neuropathy in TED may also result from stretching of the optic nerve, which becomes more vulnerable to damage through circumferential straining (29). Inflammation, fibrosis, and hypertrophy of the extraocular fibers can lead to restrictive strabismus and diplopia significantly impairing patient functionality (23). Additionally as the globe moves anteriorly and the eyelids retract away from the eye, patients may suffer from ocular surface disease due to increased exposure of the ocular surface, altered blink dynamics, lagophthalmos, and evaporative dry eye disease (30).
3.2 Genetic associations in TED
IL-6 polymorphisms have been associated with several diseases; though the directions of effect are variable (i.e., conferring increasing risk versus protective effect on a disease) (1, 2). Numerous studies have explored the genetic associations of IL-6 in GD and ocular diseases, including TED (31, 32). To date, four studies have specifically examined IL-6 polymorphisms in TED (31, 33–35). The IL-6-174 G/C polymorphism (rs1800795) is of particular interest because it increases transcriptional activity of IL-6 leading to higher downstream basal IL-6 levels (36). A meta-analysis of IL-6-174 G/C polymorphisms in ocular diseases also found a significant association in TED (31). However, when this polymorphism was assessed in 108 Polish-Caucasian patients with GD and clinically active TED, no association was found with serum IL-6 levels, thyroid autoantibodies, or the development and severity of TED (35).
3.3 The role of IL-6 in TED pathogenesis
Production of IL-6 is regulated by several cell types involved in TED, including fibrocytes, orbital fibroblasts (OFs), adipocytes, and orbital macrophages, and is also the result of OF-lymphocyte crosstalk (37–41). Studies suggest that IL-6 drives TED pathogenesis through three key mechanisms that together may contribute to inflammation, tissue expansion, remodeling, and fibrosis within the orbit. First, IL-6 drives an autoimmune response targeting the TSHR by promoting the production of autoantibodies (i.e. TSHR-Ab, TSI), thereby triggering TSHR-dependent immune pathways. Second, IL-6 stimulates the activation and differentiation of OFs, which contributes to the inflammatory process and increases adipogenesis. Finally, IL-6 stimulates T-cell–mediated inflammation, amplifying the immune response within orbital tissues (Figure 1) (23).
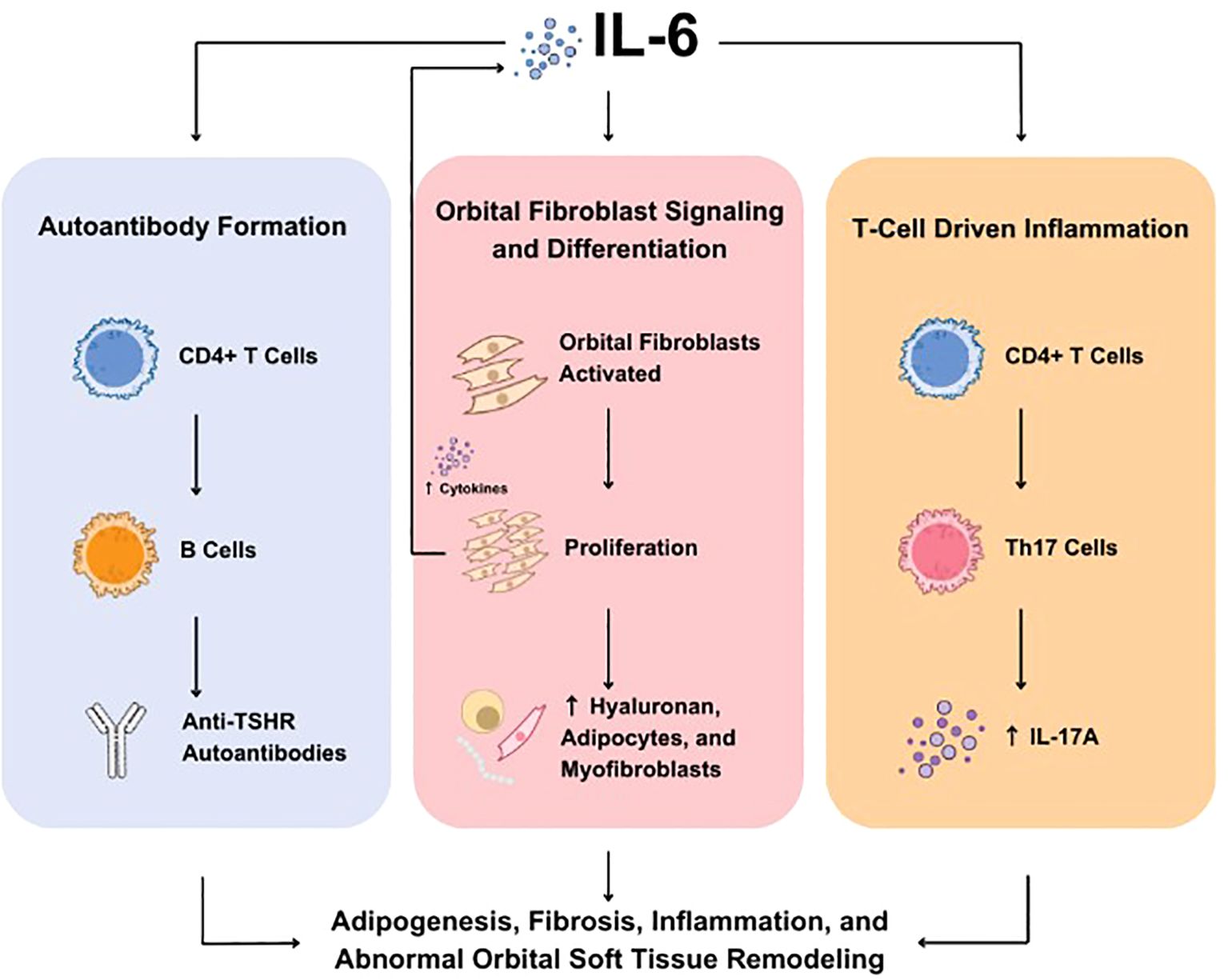
Figure 1. Role of IL-6 in TED. IL-6 is involved in three key molecular mechanisms underlying TED including autoantibody formation (left), OF signaling and differentiation (center), and T-cell driven inflammation (right). IL, interleukin; OF, orbital fibroblasts; TED, thyroid eye disease; Th, T helper; TSHR, thyroid-stimulating hormone receptor.
3.4 Autoantibody formation
The pathophysiology of TED appears to involve autoantibody activation of TSH and IGF-1 receptors, both of which are overexpressed in orbital tissue and on OFs of patients with TED. Serum levels of TSHR antibodies (TSHR-Abs) correlate positively with TED severity (42, 43). Specifically, thyroid-stimulating immunoglobulin (TSI) is predictive of TED and correlates with both the activity and severity of the disease (43). TSHR-Abs promote cellular activation, inflammation, early adipocyte differentiation and stimulation of hyaluronan and group-specific antigen (GAG) production which all lead to orbital tissue changes and remodeling (42). In severe cases, these processes can lead to vision loss (44–46).
IL-6 plays a supporting role in autoantibody formation, as well as in the maturation of memory B cells and plasma cells (47). IL-6 also promotes the generation of T follicular helper (Tfh) cells, which are necessary for germinal center formation, antibody affinity maturation, and the development of high-affinity antibodies (potentially including TSHR-Ab and TSI) (48, 49). Data on Tfh cells in TED are limited, but studies show that Tfh cells in peripheral blood mononuclear cells from TED patients are significantly elevated (10.83%) compared to non-TED controls (0.36%) (50). While no correlation was found between Tfh cell levels and the Clinical Activity Score (CAS), a positive correlation was observed with TSI levels (50). Additionally, Tfh cells were found to be increased locally in orbital tissue in patients with dysthyroid optic neuropathy compared to those with inactive TED (51). Overall, autoantibodies play a central role in TED pathogenesis and disease presentation.
3.5 Orbital fibroblast signaling and differentiation
OFs are key to the pathogenesis of TED and contribute to soft tissue inflammation and hypertrophy (24, 52). In early active TED, the extraocular muscles, as well as adipose and connective tissues, become infiltrated by mononuclear cells (including CD4+ and CD8+ T cells, monocytes, macrophages, B cells, plasma cells, and fibrocytes), which activate OFs through direct cellular interactions or inflammatory mediators, including IL-6 (53).
Furthermore, OFs may be primed to be hyper-responsive to IL-6. Following activation by IL-1β, OFs produce significantly higher levels of IL-6 than dermal fibroblasts (54). Basal levels of IL-6R are also notably higher in OFs compared to dermal fibroblasts (54). This site-specific over-expression of these molecules may offer potential therapeutic targets for TED. Additionally, the IL-6 pathway can influence OFs in TED by modulating several effector functions and molecules that play important roles in the disease. For instance, the proinflammatory phenotype of OFs in TED is enhanced following stimulation with IL-6 and sIL-6R, leading to upregulation of nuclear factor kappa B, chemokines (such as monocyte chemoattractant protein-1 [MPC-1]), molecules involved in antigen presentation (e.g., major histocompatibility complex-I [MHC-I]), and co-stimulatory molecules (e.g., CD40 and intercellular adhesion molecule-1 [ICAM-1]) (39).
TSHR stimulation on OFs has also been implicated in the pathogenesis of TED (55). IL-6 has been shown to upregulate TSHR gene expression in OFs. Cultured OFs isolated from patients with severe TED displayed TSH-dependent cAMP production and increased TSHR mRNA following IL-6 stimulation, whereas OFs from individuals without GD or TED did not (45). However, another study found that stimulating CD34+ and CD34- fibroblasts with IL-6 or IL-6 plus sIL-6R did not significantly increase TSHR expression (39, 45). Although IL-6 may influence autoantigen levels in OFs, further research is needed to clarify this relationship.
IL-6 has also been implicated in fibrotic diseases, including ocular fibrosis (56). IL-6, in combination with sIL-6R, has been shown to upregulate expression of extracellular matrix proteins—such as fibronectin, collagen I, alpha-smooth muscle actin (α-SMA), and tissue inhibitor of metalloprotease 1 (TIMP-1)—in OFs (56). These in vitro effects were attenuated by the addition of tocilizumab, a recombinant humanized monoclonal anti-IL-6R antibody (39). While it’s exact role in TED fibrosis remains unclear, these findings suggest potential involvement of IL-6.
Although limited, some evidence suggests that IL-6 may play a role in driving adipogenesis in OFs derived from TED patients (45). Stimulation of adipogenesis is associated with increased TSHR expression and TSH-dependent cyclic adenosine monophosphate (cAMP) production. The addition of IL-6 during adipocyte differentiation resulted in enhanced expression of both, indicating a potential influence on adipogenesis (45). However, further studies are needed to confirm these findings.
OFs also produce IL-6 in response to various stimuli in TED. Several studies suggest that IL-6 is produced by CD34+ OFs in a TSHR-dependent manner, with TSH and M22 (an activating anti-TSHR mAb) driving IL-6 production (37). Several studies suggest that IL-6 is produced by CD34+ OFs in a TSHR-dependent manner. This production is driven by TSH and M22, an activating anti-TSHR monoclonal antibody (45).
IL-6 production by OFs can also occur through direct cellular costimulation and cross talk. OFs express CD40, a costimulatory molecule involved in cellular activation (38). The interaction between CD40 on OFs and membrane-bound CD40 ligand (CD154) results in the production of inflammatory mediators, including IL-6 (38, 57). This effect was observed in OFs from patients with stable TED but not in control OFs (38). IL-6 is also produced through cross talk between OFs and B and T cells (40, 41). Whether this is dependent on direct costimulation or indirect cross talk remains unclear.
Additionally, OF production of IL-6 is influenced by soluble mediators, including prostaglandin E2 (PGE2) and interleukin-1 beta (IL-1β) (54, 58). PGE2, produced by OFs, stimulates a dose-dependent production of IL-6 (58). Similarly, IL-1β stimulation results in time- and concentration-dependent IL-6 production by OFs (54). Both studies reported greater IL-6 production in PGE2- and IL-1β-stimulated OFs compared to dermal fibroblasts.
In summary, OFs in TED are hyperresponsive to IL-6, which promotes inflammatory and fibrotic responses that likely contribute to the pathogenesis of TED. These findings suggest that IL-6 and its signaling pathways can serve as potential therapeutic targets in the treatment of TED.
3.6 T-cell-driven inflammation and fibrosis
In conjunction with TGF-β, IL-6 is essential for the differentiation of naïve CD4+ T cells into the T helper-17 (Th17) subset, which promotes inflammatory responses and modulates fibrosis, in part through the secretion of the cytokine IL-17A (59, 60).
Pathogenic Th17 cells are upregulated in circulation and are found to be a dominant T helper cell type within the orbit of patients with TED (41, 61, 62). Both circulating and orbital Th17 cells positively correlate with the CAS, and orbital Th17 cells negatively correlate with visual acuity (41, 62). In cases of active, severe TED with dysthyroid optic neuropathy, Th17 cells adopt a Th17.1 phenotype (or Th1-like Th17), where they coexpress the master transcription factors T-Bet and RORγt and produce both IFN-γ and IL-17A (both Th1 and Th17, respectively (63). Th17.1 cells appear to be more pathogenic than Th17 cells, displaying enhanced proliferation, increased production of proinflammatory cytokines, and reduced suppression by regulatory T cells (64). Interestingly, it is hypothesized that inhibiting the IL-6 pathway may prevent Th17.1 polarization, providing a mechanistic explanation for reports of tocilizumab use in steroid-resistant, severe TED (65).
The Th17 cell population is thought to influence OF inflammatory status, fibrosis, and adipogenesis in TED (60). Coculture of Th17 cells or IL-17A with OFs results in robust production of IL-6 along with IL-8 and monocyte chemoattractant protein-1 (MCP-1) (41). IL-17A impacts fibrosis in OFs by enhancing TGF-β-induced extracellular matrix production—such as collagen I, fibronectin, matrix metalloprotease-2 (MMP-2), and TIMP-1—in TED-derived OFs (41, 61). The same study reported that IL-17A synergizes with TGF-β to drive enhanced myofibroblast differentiation in CD90+ OFs. The role of IL-17A in adipogenesis remains unclear, as studies suggest both inhibitory and stimulatory effects on adipogenesis in OFs (41, 66).
Similar to the role of IL-6 in T follicular helper cell development for autoantibody production, IL-6 is required for Th17 differentiation and the downstream effector functions that drive inflammation and fibrosis in TED. It remains to be determined how IL-6 blockade might impact these mechanisms in TED.
4 Treatments for TED
The goal of TED treatment is essentially to prevent the worsening of symptom severity and reversal of sequelae of inflammation. Therefore, early intervention with durable treatment options that reduce inflammation is of the utmost critical importance. However, treatment of TED is challenging due to the potential need for multimodal and long-lasting therapeutic strategies (15). Corticosteroids and surgery have been the mainstay of treatment of TED for decades, but these approaches do not fully address the heterogeneity of disease presentation. Furthermore, although teprotumumab has been approved by the FDA, its access is limited outside the U.S.
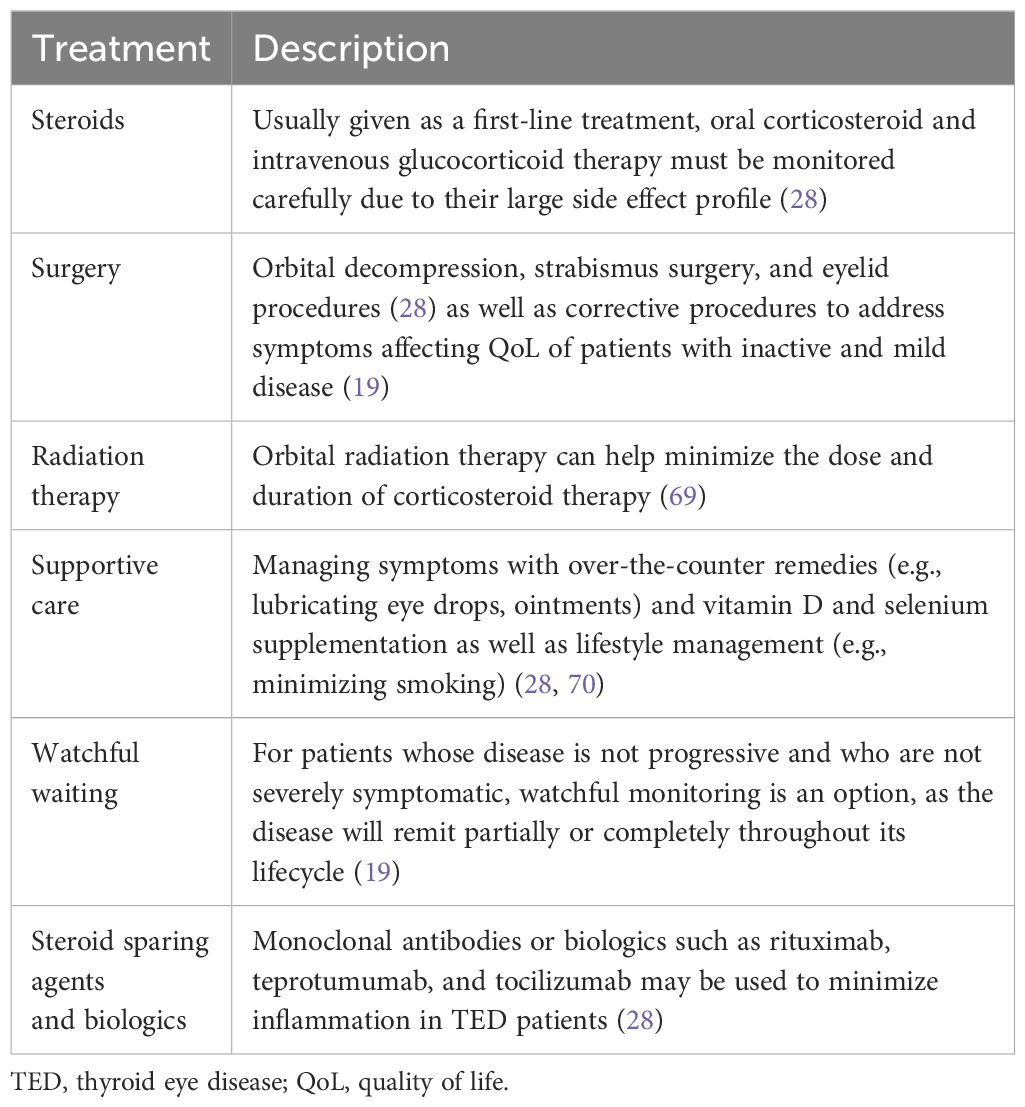
Patients with mild symptoms that do not significantly affect their QoL are often provided with supportive care (including vitamin D and selenium supplementation, artificial tears without preservatives, and optimizing thyroid function). For patients whose symptoms affect their everyday functioning including their ability to work, read, drive, or interfere with their professional and personal interactions, corticosteroids or teprotumumab are typically used (67, 68). Other options include orbital irradiation, steroid-sparing agents, and biologics (Table 1) (28, 70, 71). Notably, many existing non-surgical treatments aim to reduce inflammation but do not address all symptoms of TED (i.e., eyelid retraction, diplopia). Despite good results with current treatment options, TED patients still may have residual proptosis, eyelid retraction, or diplopia, thus requiring surgical intervention. Nonetheless, these symptoms can also recur or worsen with time, necessitating multiple rounds of treatment protocols or corrective surgeries. Ultimately, TED regression and recurrence continue to be a challenge (19, 72, 73).
4.1 Immune-targeting and inflammation-targeting treatments
Given TED is a progressively worsening disease generally characterized by an initial inflammatory phase, there is growing interest in therapeutically targeting specific components within the immune system to reduce the magnitude of disease. Targeted biologics, including monoclonal antibodies, have been an established therapeutic strategy in other autoimmune and inflammatory conditions and are now being further explored as steroid-sparing agents for the treatment of TED. Currently in use or under investigation are antibodies targeting CD20, IL-6, IL-6R, IL-11R, IGF-1R, and the neonatal Fc receptor (FcRn). The monoclonal antibodies teprotumumab (anti-IGF−1R; FDA-approved), rituximab (anti-CD20; off-label), and tocilizumab (anti-IL-6R; off-label) have been evaluated for TED treatment in clinical trials (Table 2) (28). Interestingly, a meta-analysis of 12 trials (five randomized controlled trials [RCTs] and seven observational studies) comparing rituximab, teprotumumab, or tocilizumab in 448 patients with active and moderate-to-severe TED, found that tocilizumab treatment resulted in a greater treatment response (CAS reduction, efficacy response rate, disease inactivation rate), reduction in proptosis, and the lowest association for adverse events (based on odds ratio of the three treatments, while diplopia improved the most with teprotumumab treatment (75).
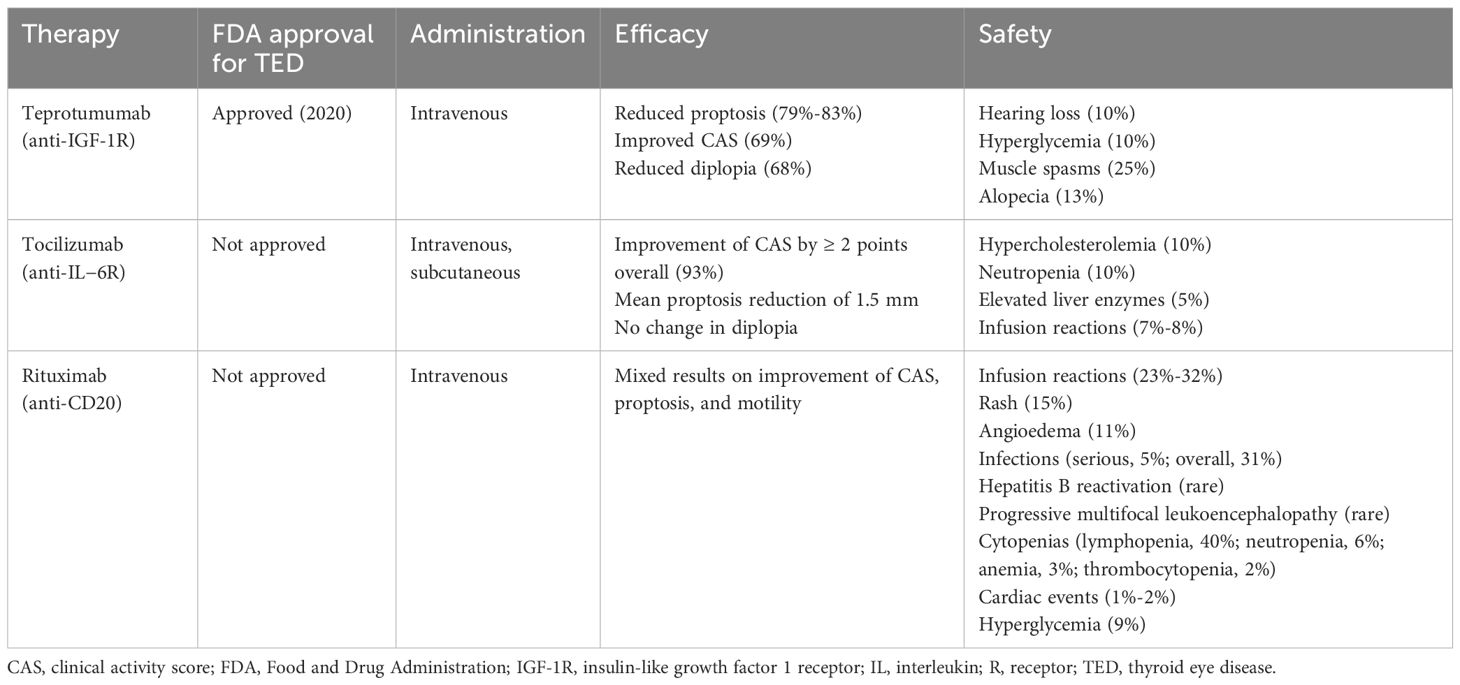
Table 2. Summary and comparison of characteristics of biologic treatments for TED (74)..
4.2 IL-6 pathway inhibition overview
The pleotropic and proinflammatory effects of IL-6 are well recognized. Targeting the IL-6 pathway and related-immune processes early in this progressive disease may reduce the duration of symptoms, limit the magnitude of the disease, address the physical changes that can potentially persist, and even allow for long-term disease inactivation. Since 2014, numerous reports have been published surrounding therapeutic targeting of IL-6 signaling in over 200 patients with TED; these have been previously reviewed elsewhere (76). Overall, these studies reported reductions in CAS, proptosis, and thyroid autoantibodies (76). However, reductions in diplopia after treatment with IL-6 antibodies are inconsistent and contain several study limitations and research gaps discussed later in this manuscript. Even though a more comprehensive review has been published previously, it is important to briefly review what is currently known about IL-6 and the emerging data coming down the pipeline (76).
4.3 Targeting IL-6R: tocilizumab and sarilumab
Tocilizumab is a recombinant humanized monoclonal anti-IL-6R antibody with an excellent safety profile even in vulnerable populations like the elderly (77, 78). The majority of published studies targeting the IL-6 pathway in TED involve intravenous use of tocilizumab. Duarte et al. conducted a systematic review which suggested tocilizumab to be mostly effective in reducing inflammatory signs during active TED, with an improvement of ≥ 3 points CAS, and an overall relapse rate of 8.2% (76). In numerous studies tocilizumab also resulted in marked reductions in proptosis (76). A meta-analysis of 12 studies (N=219) including patients with active, steroid-resistant TED who were treated with intravenous tocilizumab reported similar findings: CAS reduction of 4.6 points, proptosis reduction of 2.04 mm, diplopia response rate of 48%, and a reactivation rate of 1% (4).
There is currently only one RCT with published data: a Phase 3 study that enrolled 32 patients with moderate-to-severe corticosteroid-resistant TED (79). Patients received tocilizumab (8 mg/kg at weeks 0, 4, 9, and 12) or placebo. At 16 and 40 weeks from baseline, respectively, 14/15 (93%) and 13/15 (87%) tocilizumab-treated patients had improved CAS (≥2 points) compared with 10/17 (59%) placebo-treated patients (for both timepoints) (79). Although there was a significant reduction in proptosis at week 16, this result did not remain at week 40; at both time points the median change was –1.5mm (79). No patients withdrew from the study due to an adverse event (79). Only two tocilizumab-treated patients experienced a serious adverse event; though it is unclear whether this was due to the study drug (79). One patient who was treated with hydrazides for latent tuberculosis, experienced a moderate transaminase elevation at week 8 that normalized after discontinuing hydrazides, while another patient experienced acute pyelonephritis at week 30 (79). Studies have also reported reductions in antithyroid autoantibodies following treatment with tocilizumab in TED. In one of the first meta-analyses evaluating changes in thyroid autoantibodies following treatment with tocilizumab, TSHR-Ab levels were reported to be reduced from baseline following tocilizumab treatment in TED in five studies (10.62-IU mean reduction in TSHR-Ab) (4). A cohort study of nine patients with active TED who received tocilizumab following intolerance to or disease progression following systemic corticosteroid treatment found similar outcomes (74). After an average of 4.2 tocilizumab infusions, patients’ CAS, TED Scale score, and TSI levels were reduced to 0.4 ± 0.5 (reduction of 6.3 points), 1.2 ± 1.1 (reduction of 9.0 points), and 201% (reduction of 240%), respectively (80). After an average follow-up of 24 months post treatment, no patients experienced recurrence of active disease (80). The findings from these studies are noteworthy given the role of IL-6 in B cell and antibody-mediated immune responses, as well as the role of TSI in TED.
In addition to steroid-resistant patients, tocilizumab use has also been described in patients with moderate-to-severe, longstanding TED resistant to treatment with: corticosteroids, methotrexate, radiation, and surgery. Patients (N=9) were followed for an average of 24.6 weeks. Positive clinical responses were observed in all patients including improvements in proptosis, eyelid edema, diplopia, extraocular movement, visual acuity, color vision, and TSI levels (74). Investigators documented an overall decrease in CAS indicating improved overall patient burden (74). These studies support the potential clinical utility of the IL-6 pathway inhibition for the treatment of recalcitrant TED.
While the studies reporting efficacious results for tocilizumab in TED have provided a basis for hypotheses surrounding IL-6 pathway inhibition, these studies contain several pitfalls that may reduce their strength and limit their interpretation. To date, there is limited data from randomized, placebo-controlled clinical trials. In fact, the lack of controlled trials even prevented a Cochrane database review of tocilizumab in TED as no studies met the standard for review and inclusion (81). Another limitation is the lack of a treatment-naïve population; most studies have evaluated tocilizumab in a steroid-failure population. Therefore, the effect of targeting the IL-6 pathway early in the course of active disease in a treatment-naïve population is unknown. Further research, particularly in the form of RCTs, is required to fully understand tocilizumab’s impact, appropriate dosage, and administration in patients with TED.
Sarilumab is another anti-IL-6R Ab with reported use in TED, but has not yet been studied in an RCT setting (82). Sarilumab is a fully human monoclonal anti-IL-6R Ab that blocks both cis and trans IL-6 signaling as well as IL-6 trans presentation (or cluster signaling) (83, 84). It was first approved by the FDA in 2017 for the treatment of adults with moderate-to-severe active rheumatoid arthritis (RA) who have had an inadequate response or intolerance to one or more disease-modifying antirheumatic drugs. Sarilumab is also approved for RA in the European Union and Japan (85). An observational case series abstract describes the use of sarilumab (200 mg, subcutaneous, every 15 days for a mean of 11 months) in active, moderate-to-severe TED in five patients. In this case series, CAS was reduced by an average of 3 points following treatment with sarilumab; all patients achieved TED inactivity and reduced disease severity, along with reductions in antithyroid antibody levels (82).
4.4 Targeting IL-6: sirukumab, siltuximab, and olamkicept
Sirukumab, siltuximab, and olamkicept are human monoclonal anti-IL-6 Abs that block classic and trans IL-6 signaling (Table 3) (84, 86, 87). Siltuximab received FDA approval in 2014 for patients with multicentric Castleman’s disease who are HIV negative and HHV-8 negative (88). While sirukumab demonstrated efficacy for the treatment of RA in a Phase 3 trial (89). Further, olamkicept is a soluble gp130-Fc-fusion-protein currently in development that inhibits trans IL-6 signaling, affecting IL-6-driven chronic inflammation (90). Currently, there are no RCTs investigating the use of these monoclonal antibodies in TED.
Based on data from two small, single-arm, single-dose studies comparing the immunological effects of siltuximab (which targets IL-6) and tocilizumab (which targets IL-6R), several differences were observed. Firstly, inhibition of IL-6R but not IL-6, led to persistent suppression of IL-6–induced phosphorylated-signal transducer and activator of transcription 3 (p-STAT3) and declines in inducible T-cell costimulator expression on T follicular helper cells (91). While IL-6 inhibition reversed T effector resistance to Treg-mediated suppression and enhanced T cell production of regulatory cytokines (91). In addition, IL-6 and IL-6R inhibition had opposing effects on T-cell receptor-induced p-STAT3 signaling (91). However, a recent meta-analysis of six different RCTs revealed comparable efficacy and safety between IL-6 and IL-6R inhibitors, thus suggesting that there may not be a meaningful clinical difference between targeting IL-6 versus IL-6R (92).
The clinical implications of these observations are currently unknown. Although the protective and reparative functions of IL-6 in TED are currently poorly understood and underappreciated, it will be important to understand how these functions may be impacted by inhibition of IL-6R (trans- and classical-signaling) versus IL-6. A theoretical advantage of targeting IL-6 versus IL-6R, is that IL-6 is found at low levels in circulation compared with IL-6R, which may impact drug load, half-life, and effectiveness (93).
Controlled clinical studies are underway that may shed light on these considerations. There are three Phase 2/3 RCTs are currently recruiting (as of October 2024) to evaluate anti-IL-6 (pacibekitug) or -IL-6R antibodies (satralizumab) as potential treatments for TED (Table 4).
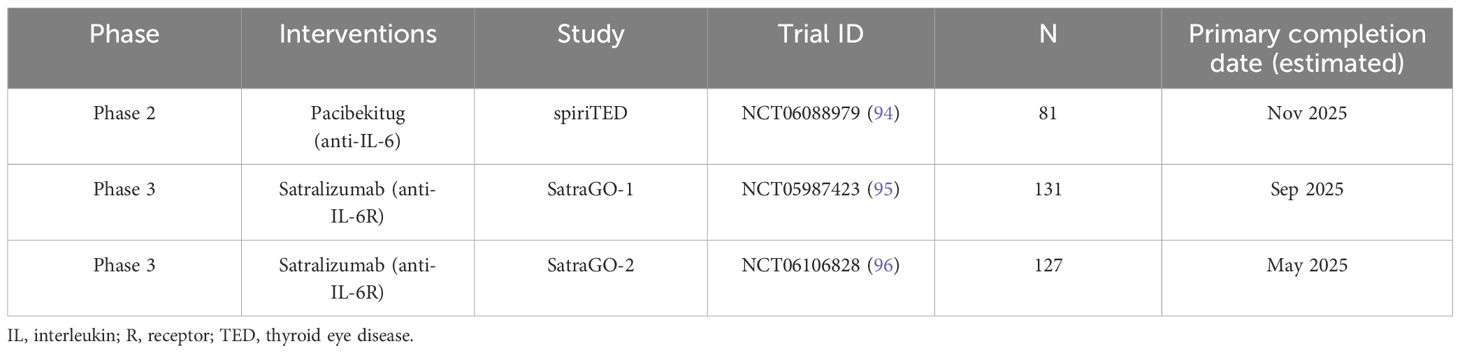
Table 4. Ongoing and upcoming Phase 2 or 3 trials evaluating treatments for TED that target IL-6 or IL−6R.
5 Potential utility of IL-6 as a biomarker for TED
There has been a strong interest to identify molecular biomarkers that provide clinical utility in diagnosis, management, and treatment of TED, since there are currently no acceptable options (3, 97, 98). Studies evaluating IL-6, sIL-6R, and surrogates of IL-6 pathway activation in those with TED suggest the potential utility of IL−6 pathway activation as a biomarker.
IL-6 levels have been evaluated in both serum and tears of those with TED. Numerous studies have reported significant increases of IL-6 levels within tears isolated from those with TED compared with controls. Tear-derived IL-6 was found to positively correlate with CAS, disease activity, and lacrimal gland enlargement; however differences were not observed between TED and GD (99–103). IL-6 levels in tears were also found to decrease with CAS reductions following treatment of active, moderate-to-severe TED with intravenous methylprednisolone (103). Additionally, a few studies have evaluated levels of IL-6 in the serum of patients with TED. In a study comparing serum levels in patients who had GD with (n=47) or without (n=29) ophthalmopathy, IL-6 levels were significantly elevated in those displaying ocular disease (104); however data correlating serum IL-6 levels with inflammation and activity are inconsistent (104, 105).
IL-6R can be generated by alternative splicing or cleavage from the cell membrane and enter circulation as sIL-6R, where it can bind to circulating IL-6 (106). One study reported significant increases in serum sIL-6R in patients with GD who had active inflammatory TED compared to controls without TED and those with low inflammatory (inactive) TED (105). However localized sIL-6R was not found to be significantly upregulated in TED (99).
Given that IL-6 can be difficult to reliably measure, surrogates of IL-6 pathway activation may serve as alternative biomarkers (107). Currently, studies evaluating surrogates of IL-6 pathway activation (e.g., CRP, neutrophil-to-lymphocyte ratios [NLR]) in TED are limited. CRP production within the liver is induced by IL-6 (108). When compared to healthy controls, CRP was found to be increased in tear-derived exosomes from patients with TED (109). Additionally, NLR is an emerging indicator of systemic inflammation (110) and IL-6 signaling in various diseases (111–113). NLR was found to correlate with CAS, proptosis, and imaging parameters in 87 consecutive patients with TED (114). The same study reported that elevated baseline NLR correlated with reduced clinical outcomes after a median follow-up of 25 months (114). A meta-analysis including 734 subjects also reported significant differences in NLR between TED and control groups (97). Although promising, additional studies are needed to better understand which molecular players involved in IL-6 signaling may serve as appropriate biomarkers for TED.
6 Research gaps and future directions
Several knowledge gaps remain as it relates to the epidemiology and pathogenesis, as well as diagnosis and treatment of TED. Currently, the heterogeneous manifestations of TED among different patient demographics are poorly understood and may be due to genetics or other factors (15, 115). Given it’s pivotal role in TED pathogenesis, further investigations into the mechanisms underlying IL-6 signaling are critical. For example, when coupled with TGF-β, IL-6 drives differentiation of Th17 cells; these cells are then maintained and amplified by IL-23 (116). Interestingly, polymorphisms within IL-23R are strongly associated with TED; though these findings were not replicated in a subsequent study (34, 117). Additional studies exploring variants within IL-23R may provide further insight into the role of Th17 pathway in TED. Beyond IL-23R, larger genetic studies may also aid identification of potential causal relationships between IL-6 polymorphisms and TED.
Additionally, availability and information surrounding clinical biomarkers for TED are extremely limited. Few studies have investigated serum IL-6 levels in patients with TED; conflicting results have also been observed across publications. Studies of other IL-6 pathway markers such as CRP are also limited. This lack of IL-6 pathway biomarkers has hindered clinicians’ ability to properly predict patient responses to IL-6 pathway inhibitors including long-standing options (>10 years) such as tocilizumab. Future studies of potential biomarkers including epigenetic factors, may help improve diagnostic and treatment approaches for TED (3, 118).
Available treatment options such as teprotumumab have surprisingly blurred the line between active and chronic TED. Although data from current publications vary, it is clear that the chronic changes to extraocular muscles and fat once thought to be chronic and permanent can be addressed by treatment interventions such as teprotumumab later in the disease process (26, 52, 72). These new findings also pose challenges of treating a chronic disease already requiring a complex and multimodality approach in the acute onset of presentation. The risks and benefits of each treatment option will need to be evaluated after multiple courses over varying lengths of time. With recent developments in therapies targeting specific components of molecular pathways, it will be important to understand how to optimize the effectiveness of potential therapeutics while mitigating potential short- and long-term side effects due to unintended downstream immunological consequences (71).
In line with the above, there is also a need for well-controlled studies evaluating IL-6 pathway inhibitors for the treatment of TED since data from only one RCT has been published (79). Future studies should aim to assess the tolerability, effectiveness, and QoL impacts of IL-6 pathway inhibitors in patients who are treatment-naïve and those who have received multiple prior modalities of treatment. This will allow for a more comprehensive understanding of the effectiveness and tolerability of IL-6 pathway inhibitors across a broad range of patient types. It will also be important to understand the potential differences in treatment efficacy and tolerability when blocking different components of IL-6R signaling (classical versus trans signaling).
7 Conclusion
The IL-6 pathway is increasingly recognized as a potential therapeutic target in TED. IL-6 is significantly upregulated systemically and locally within the orbits of those with TED. However, additional studies examining the genetic associations of IL-6 as well as IL-6 as a biomarker in TED are needed. Given what is generally known about the pleiotropic functions of IL-6 and specifically reported within TED, IL-6 could play roles in autoantibody formation, OF activation and differentiation, and T cell-mediated mechanisms of disease. Clinical studies evaluating treatments that target the IL-6 pathway have reported reductions in proptosis, inflammation (CAS), and thyroid autoantibodies. However, many of these studies were retrospective and in patients who were also steroid-resistant. The utility of IL-6 pathway inhibition for the treatment of TED remains understudied. To date, only one RCT has been completed that evaluates IL-6 pathway blockade in TED; however, several RCTs are currently underway. Development of additional novel therapies will provide healthcare providers with a range of options to tailor treatment to each patient’s specific needs and circumstances, especially over the chronic course of this heterogeneous disease process.
Author contributions
JM: Writing – original draft, Writing – review & editing. JN: Writing – original draft, Writing – review & editing. BH: Writing – original draft, Writing – review & editing. CA: Writing – original draft, Writing – review & editing. JW: Writing – original draft, Writing – review & editing. AL: Writing – original draft, Writing – review & editing. CT: Writing – original draft, Writing – review & editing. KE: Writing – original draft, Writing – review & editing. KC: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was not received for the research and/or publication of this article.
Acknowledgments
Writing support was provided by Talisa Silzer, PhD, of Sixsense Strategy Group Inc., a Herspiegel company, and Raya Mahbuba, MSc, from Toronto, Ontario, Canada, and was funded by Tourmaline Bio, Inc.
Conflict of interest
AL, BH, CA, CT, JW, and KE are employees of Tourmaline Bio, Inc. JM is a medical director for Thrive Health, which administers IV infusions of teprotumumab, was a consultant for Horizon/Amgen until September 2023, and has a CDA with Tourmaline Bio, Inc. for planning internal consulting and education. KC is a consultant and principal investigator for Horizon/Amgen, Viridian, Acelyrin, Genentech/Roche, Immunovant, ArgenX, and Tourmaline Bio, Inc. JN has received research funding from Tourmaline Bio, Inc.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Georgakis MK, Malik R, Li X, Gill D, Levin MG, Vy HMT, et al. Genetically downregulated interleukin-6 signaling is associated with a favorable cardiometabolic profile: A phenome-wide association study. Circulation. (2021) 143:1177–80. doi: 10.1161/CIRCULATIONAHA.120.052604
2. Wang X, Yan Z, Ye Q. Interleukin-6 gene polymorphisms and susceptibility to liver diseases: A meta-analysis. Med (Baltimore). (2019) 98:e18408. doi: 10.1097/MD.0000000000018408
3. Ueland HO, Neset MT, Methlie P, Ueland GA, Pakdel F, Rodahl E. Molecular biomarkers in thyroid eye disease: A literature review. Ophthalmic Plast Reconstr Surg. (2023) 39:S19–28. doi: 10.1097/IOP.0000000000002466
4. Sun A, Wang X, Qu J, Wu Y. The efficacy and safety of intravenous tocilizumab to treat Graves’ ophthalmopathy: A systematic review and single-arm meta-analysis. J Clin Endocrinol Metab. (2024) 110(3):e886–96. doi: 10.1210/clinem/dgae711
5. Naka T, Nishimoto N, Kishimoto T. The paradigm of IL-6: from basic science to medicine. Arthritis Res. (2002) 4 Suppl 3:S233–42. doi: 10.1186/ar565
6. Zhao JL, Ma C, O’Connell RM, Mehta A, DiLoreto R, Heath JR, et al. Conversion of danger signals into cytokine signals by hematopoietic stem and progenitor cells for regulation of stress-induced hematopoiesis. Cell Stem Cell. (2014) 14:445–59. doi: 10.1016/j.stem.2014.01.007
7. Villar-Fincheira P, Sanhueza-Olivares F, Norambuena-Soto I, Cancino-Arenas N, Hernandez-Vargas F, Troncoso R, et al. Role of interleukin-6 in vascular health and disease. Front Mol Biosci. (2021) 8:641734. doi: 10.3389/fmolb.2021.641734
8. Tanaka T, Narazaki M, Kishimoto T. Interleukin (IL-6) immunotherapy. Cold Spring Harb Perspect Biol. (2018) 10:1–15. doi: 10.1101/cshperspect.a028456
9. Alhendi A, Naser SA. The dual role of interleukin-6 in Crohn’s disease pathophysiology. Front Immunol. (2023) 14:1295230. doi: 10.3389/fimmu.2023.1295230
10. Johnson DE, O’Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. (2018) 15:234–48. doi: 10.1038/nrclinonc.2018.8
11. Millrine D, Jenkins RH, Hughes STO, Jones SA. Making sense of IL-6 signalling cues in pathophysiology. FEBS Lett. (2022) 596:567–88. doi: 10.1002/1873-3468.14201
12. Lacroix M, Rousseau F, Guilhot F, Malinge P, Magistrelli G, Herren S, et al. Novel insights into interleukin 6 (IL-6) cis- and trans-signaling pathways by differentially manipulating the assembly of the IL-6 signaling complex *. J Biol Chem. (2015) 290:26943–53. doi: 10.1074/jbc.M115.682138
13. Rachmasari KN, Hamadi D, Thapa P, Bradley EA, Stan MN. The epidemiology of thyroid eye disease in olmsted county, minnesota, 2005-2020. Thyroid. (2024) 34(12):1522–30. doi: 10.1089/thy.2024.0304
14. Prummel MF, Bakker A, Wiersinga WM, Baldeschi L, Mourits MP, Kendall-Taylor P, et al. Multi-center study on the characteristics and treatment strategies of patients with Graves’ orbitopathy: the first European Group on Graves’ Orbitopathy experience. Eur J Endocrinol. (2003) 148:491–5. doi: 10.1530/eje.0.1480491
15. Bartalena L, Piantanida E, Gallo D, Lai A, Tanda ML. Epidemiology, natural history, risk factors, and prevention of graves’ Orbitopathy. Front Endocrinol (Lausanne). (2020) 11:615993. doi: 10.3389/fendo.2020.615993
16. Ramesh S, Zhang QE, Sharpe J, Penne R, Haller J, Lum F, et al. Thyroid eye disease and its vision-threatening manifestations in the academy IRIS registry: 2014-2018. Am J Ophthalmol. (2023) 253:74–85. doi: 10.1016/j.ajo.2023.04.013
17. Wang Y, Padnick-Silver L, Francis-Sedlak M, Holt RJ, Foley C, Douglas RS. Inflammatory and noninflammatory thyroid eye disease: comparison of disease signs, symptoms, and quality of life in patients in the United States. Endocr Pract. (2022) 28:842–6. doi: 10.1016/j.eprac.2022.06.003
18. Patel P, Khandji J, Kazim M. Recurrent thyroid eye disease. Ophthalmic Plast Reconstr Surg. (2015) 31:445–8. doi: 10.1097/IOP.0000000000000371
19. Burch HB, Perros P, Bednarczuk T, Cooper DS, Dolman PJ, Leung AM, et al. Management of thyroid eye disease: A consensus statement by the american thyroid association and the european thyroid association. Thyroid. (2022) 32:1439–70. doi: 10.1089/thy.2022.0251
21. Cockerham KP, Padnick-Silver L, Stuertz N, Francis-Sedlak M, Holt RJ. Quality of life in patients with chronic thyroid eye disease in the United States. Ophthalmol Ther. (2021) 10:975–87. doi: 10.1007/s40123-021-00385-8
22. Smith TJ, Hegedus L, Lesser I, Perros P, Dorris K, Kinrade M, et al. How patients experience thyroid eye disease. Front Endocrinol (Lausanne). (2023) 14:1283374. doi: 10.3389/fendo.2023.1283374
23. Bartalena L, Tanda ML. Current concepts regarding Graves’ orbitopathy. J Intern Med. (2022) 292:692–716. doi: 10.1111/joim.v292.5
25. Chin YH, Ng CH, Lee MH, Koh JWH, Kiew J, Yang SP, et al. Prevalence of thyroid eye disease in Graves’ disease: A meta-analysis and systematic review. Clin Endocrinol (Oxf). (2020) 93:363–74. doi: 10.1111/cen.14296
26. Jain AP, Jaru-Ampornpan P, Douglas RS. Thyroid eye disease: Redefining its management-A review. Clin Exp Ophthalmol. (2021) 49:203–11. doi: 10.1111/ceo.13899
27. Łacheta D, Miśkiewicz P, Głuszko A, Nowicka G, Struga M, Kantor I, et al. Immunological aspects of graves’ Ophthalmopathy. BioMed Res Int. (2019) 2019:7453260. doi: 10.1155/2019/7453260
28. Rashad R, Pinto R, Li E, Sohrab M, Distefano AG. Thyroid eye disease. Life (Basel). (2022) 12:1–17. doi: 10.3390/life12122084
29. Agarwal A, Khanam S. Dysthyroid Optic Neuropathy. Treasure Island (FL: StatPearls Publishing LLC (2024).
30. Rana HS, Akella SS, Clabeaux CE, Skurski ZP, Aakalu VK. Ocular surface disease in thyroid eye disease: A narrative review. Ocul Surf. (2022) 24:67–73. doi: 10.1016/j.jtos.2022.02.001
31. Ulhaq ZS, Soraya GV, Budu, Wulandari LR. The role of IL-6-174 G/C polymorphism and intraocular IL-6 levels in the pathogenesis of ocular diseases: a systematic review and meta-analysis. Sci Rep. (2020) 10:17453. doi: 10.1038/s41598-020-74203-9
32. Imani D, Rezaei R, Razi B, Alizadeh S, Mahmoudi M. Association between IL6-174 G/C polymorphism and graves’ Disease: A systematic review and meta-analysis. Acta Med Iran. (2017) 55:665–71.
33. Anvari M, Khalilzadeh O, Esteghamati A, Esfahani SA, Rashidi A, Etemadi A, et al. Genetic susceptibility to Graves’ ophthalmopathy: the role of polymorphisms in proinflammatory cytokine genes. Eye. (2010) 24:1058–63. doi: 10.1038/eye.2009.244
34. Wong KH, Rong SS, Chong KK, Young AL, Pang CP, Chen LJ. Genetic associations of interleukin-related genes with graves’ Ophthalmopathy: a systematic review and meta-analysis. Sci Rep. (2015) 5:16672. doi: 10.1038/srep16672
35. Bednarczuk T, Kuryłowicz A, Hiromatsu Y, Kiljański J, Telichowska A, Nauman J. Association of G-174C polymorphism of the interleukin-6 gene promoter with graves’ Ophthalmopathy. Autoimmunity. (2004) 37:223–6. doi: 10.1080/0891693042000193320
36. Hulkkonen J, Pertovaara M, Antonen J, Pasternack A, Hurme M. Elevated interleukin-6 plasma levels are regulated by the promoter region polymorphism of the IL6 gene in primary Sjögren’s syndrome and correlate with the clinical manifestations of the disease. Rheumatol (Oxford). (2001) 40:656–61. doi: 10.1093/rheumatology/40.6.656
37. Raychaudhuri N, Fernando R, Smith TJ. Thyrotropin regulates IL-6 expression in CD34+ fibrocytes: clear delineation of its cAMP-independent actions. PloS One. (2013) 8:e75100. doi: 10.1371/journal.pone.0075100
38. Hwang CJ, Afifiyan N, Sand D, Naik V, Said J, Pollock SJ, et al. Orbital fibroblasts from patients with thyroid-associated ophthalmopathy overexpress CD40: CD154 hyperinduces IL-6, IL-8, and MCP-1. Invest Ophthalmol Vis Sci. (2009) 50:2262–8. doi: 10.1167/iovs.08-2328
39. Lu Y, Wang Y, Wang Y, Wu Y, Huang Y, Liu X, et al. M1-like macrophages modulate fibrosis and inflammation of orbital fibroblasts in graves’ Orbitopathy: potential relevance to soluble interleukin-6 receptor. Thyroid. (2023) 33:338–50. doi: 10.1089/thy.2022.0254
40. Wang R, Song D, Zhong Y, Li H. Potential role of IGF-1R in the interaction between orbital fibroblasts and B lymphocytes: an implication for B lymphocyte depletion in the active inflammatory phase of thyroid-associated ophthalmopathy. BMC Immunol. (2024) 25:31. doi: 10.1186/s12865-024-00613-3
41. Fang S, Huang Y, Zhong S, Li Y, Zhang Y, Li Y, et al. Regulation of orbital fibrosis and adipogenesis by pathogenic th17 cells in graves orbitopathy. J Clin Endocrinol Metab. (2017) 102:4273–83. doi: 10.1210/jc.2017-01349
43. George A, Diana T, Langericht J, Kahaly GJ. Stimulatory thyrotropin receptor antibodies are a biomarker for graves’ Orbitopathy. Front Endocrinol (Lausanne). (2020) 11:629925. doi: 10.3389/fendo.2020.629925
44. Chiu HI, Wu SB, Tsai CC. The role of fibrogenesis and extracellular matrix proteins in the pathogenesis of graves’ Ophthalmopathy. Int J Mol Sci. (2024) 25:1–16. doi: 10.3390/ijms25063288
45. Jyonouchi SC, Valyasevi RW, Harteneck DA, Dutton CM, Bahn RS. Interleukin-6 stimulates thyrotropin receptor expression in human orbital preadipocyte fibroblasts from patients with Graves’ ophthalmopathy. Thyroid. (2001) 11:929–34. doi: 10.1089/105072501753210984
46. Prabhakar BS, Bahn RS, Smith TJ. Current perspective on the pathogenesis of Graves’ disease and ophthalmopathy. Endocr Rev. (2003) 24:802–35. doi: 10.1210/er.2002-0020
47. Aliyu M, Zohora FT, Anka AU, Ali K, Maleknia S, Saffarioun M, et al. Interleukin-6 cytokine: An overview of the immune regulation, immune dysregulation, and therapeutic approach. Int Immunopharmacol. (2022) 111:109130. doi: 10.1016/j.intimp.2022.109130
48. Bogusławska J, Godlewska M, Gajda E, Piekiełko-Witkowska A. Cellular and molecular basis of thyroid autoimmunity. Eur Thyroid J. (2022) 11. doi: 10.1530/ETJ-21-0024
49. Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. (2014) 41:529–42. doi: 10.1016/j.immuni.2014.10.004
50. Bisnauthsing H, Chan HYE, Chan HYM, So CYS, Chen WX, Ng HK, et al. An abundance of follicular helper T cells in peripheral blood associated with thyroid stimulating immunoglobulin level in the thyroid eye disease. Invest Ophthalmol Visual Sci. (2023) 64:423.
51. Sara Maioli MS, Armenti M, Crivicich E, Currò N, Guastella C, Dazzi B, et al. Specific patterns of orbital-resident B and T lymphocytes are related to different clinical presentations of thyroid eye disease. Endocrine Abstracts. (2024) 101. doi: 10.1530/endoabs.101.PS1-01-03
52. Hodgson NM, Rajaii F. Current understanding of the progression and management of thyroid associated orbitopathy: A systematic review. Ophthalmol Ther. (2020) 9:21–33. doi: 10.1007/s40123-019-00226-9
53. Dik WA, Virakul S, van Steensel L. Current perspectives on the role of orbital fibroblasts in the pathogenesis of Graves’ ophthalmopathy. Exp Eye Res. (2016) 142:83–91. doi: 10.1016/j.exer.2015.02.007
54. Chen B, Tsui S, Smith TJ. IL-1 beta induces IL-6 expression in human orbital fibroblasts: identification of an anatomic-site specific phenotypic attribute relevant to thyroid-associated ophthalmopathy. J Immunol. (2005) 175:1310–9. doi: 10.4049/jimmunol.175.2.1310
55. Cui X, Wang F, Liu C. A review of TSHR- and IGF-1R-related pathogenesis and treatment of Graves’ orbitopathy. Front Immunol. (2023) 14:1062045. doi: 10.3389/fimmu.2023.1062045
56. Li Y, Zhao J, Yin Y, Li K, Zhang C, Zheng Y. The role of IL-6 in fibrotic diseases: molecular and cellular mechanisms. Int J Biol Sci. (2022) 18:5405–14. doi: 10.7150/ijbs.75876
57. Sempowski GD, Rozenblit J, Smith TJ, Phipps RP. Human orbital fibroblasts are activated through CD40 to induce proinflammatory cytokine production. Am J Physiol. (1998) 274:C707–14. doi: 10.1152/ajpcell.1998.274.3.C707
58. Raychaudhuri N, Douglas RS, Smith TJ. PGE2 induces IL-6 in orbital fibroblasts through EP2 receptors and increased gene promoter activity: implications to thyroid-associated ophthalmopathy. PloS One. (2010) 5:e15296. doi: 10.1371/journal.pone.0015296
59. Qin H, Wang L, Feng T, Elson CO, Niyongere SA, Lee SJ, et al. TGF-beta promotes Th17 cell development through inhibition of SOCS3. J Immunol. (2009) 183:97–105. doi: 10.4049/jimmunol.0801986
60. Fang S, Lu Y, Huang Y, Zhou H, Fan X. Mechanisms that underly T cell immunity in graves’ Orbitopathy. Front Endocrinol (Lausanne). (2021) 12:648732. doi: 10.3389/fendo.2021.648732
61. Fang S, Huang Y, Wang S, Zhang Y, Luo X, Liu L, et al. IL-17A exacerbates fibrosis by promoting the proinflammatory and profibrotic function of orbital fibroblasts in TAO. J Clin Endocrinol Metab. (2016) 101:2955–65. doi: 10.1210/jc.2016-1882
62. Fang S, Huang Y, Wang N, Zhang S, Zhong S, Li Y, et al. Insights into local orbital immunity: evidence for the involvement of the th17 cell pathway in thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. (2019) 104:1697–711. doi: 10.1210/jc.2018-01626
63. Fang S, Zhang S, Huang Y, Wu Y, Lu Y, Zhong S, et al. Evidence for associations between th1/th17 “Hybrid” Phenotype and altered lipometabolism in very severe graves orbitopathy. J Clin Endocrinol Metab. (2020) 105:1851–67. doi: 10.1210/clinem/dgaa124
64. Basdeo SA, Cluxton D, Sulaimani J, Moran B, Canavan M, Orr C, et al. Ex-th17 (Nonclassical th1) cells are functionally distinct from classical th1 and th17 cells and are not constrained by regulatory T cells. J Immunol. (2017) 198:2249–59. doi: 10.4049/jimmunol.1600737
65. Jiang M, Fu Y, Wang P, Yan Y, Zhao J, Wang Y, et al. Looking beyond th17 cells: A role for th17.1 cells in thyroid-associated ophthalmopathy? Endocrinology. (2023) 164(3):bqad004. doi: 10.1210/endocr/bqad004
66. Huang B, Lang X, Li X. The role of IL-6/JAK2/STAT3 signaling pathway in cancers. Front Oncol. (2022) 12:1023177. doi: 10.3389/fonc.2022.1023177
67. Längericht J, Krämer I, Kahaly GJ. Glucocorticoids in Graves’ orbitopathy: mechanisms of action and clinical application. Ther Adv Endocrinol Metab. (2020) 11:2042018820958335. doi: 10.1177/2042018820958335
68. Smith TJ, Cockerham K, Lelli G, Choudhary C, Taylor S, Barretto N, et al. Utility assessment of moderate to severe thyroid eye disease health states. JAMA Ophthalmol. (2023) 141:159–66. doi: 10.1001/jamaophthalmol.2022.3225
69. Hahn E, Laperriere N, Millar BA, Oestreicher J, McGowan H, Krema H, et al. Orbital radiation therapy for Graves’ ophthalmopathy: measuring clinical efficacy and impact. Pract Radiat Oncol. (2014) 4:233–9. doi: 10.1016/j.prro.2014.02.008
70. Bartalena L, Kahaly GJ, Baldeschi L, Dayan CM, Eckstein A, Marcocci C, et al. The 2021 European Group on Graves’ orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves’ orbitopathy. Eur J Endocrinol. (2021) 185:G43–67. doi: 10.1530/EJE-21-0479
71. Moledina M, Damato EM, Lee V. The changing landscape of thyroid eye disease: current clinical advances and future outlook. Eye (Lond). (2024) 38:1425–37. doi: 10.1038/s41433-024-02967-9
72. Rosenblatt TR, Chiou CA, Yoon MK, Wolkow N, Lee NG, Freitag SK. Proptosis regression after teprotumumab treatment for thyroid eye disease. Ophthalmic Plast Reconstr Surg. (2024) 40:187–91. doi: 10.1097/IOP.0000000000002531
73. Hwang CJ, Rebollo NP, Mechels KB, Perry JD. Reactivation after teprotumumab treatment for active thyroid eye disease. Am J Ophthalmol. (2024) 263:152–9. doi: 10.1016/j.ajo.2023.12.001
74. Silkiss RZ, Paap MK, Roelofs KA, Agi J, Weis E. Treatment of corticosteroid-resistant thyroid eye disease with subcutaneous tocilizumab. Can J Ophthalmol. (2021) 56:66–70. doi: 10.1016/j.jcjo.2020.07.020
75. Hu Y, Chen J, Lin K, Yu X. Efficacy and Safety of intravenous monoclonal antibodies in patients with moderate-to-severe active Graves’ophthalmopathy: a systematic review and meta-analysis. Front Endocrinol (Lausanne). (2023) 14:1160936. doi: 10.3389/fendo.2023.1160936
76. Duarte AF, Xavier NF, Sales Sanz M, Cruz AAV. Efficiency and safety of tocilizumab for the treatment of thyroid eye disease: A systematic review. Ophthalmic Plast Reconstr Surg. (2024) 40:367–73. doi: 10.1097/IOP.0000000000002573
77. Korayem GB, Aljuhani O, Altebainawi AF, Shaya AIA, Alnajjar LI, Alissa A, et al. The safety and effectiveness of tocilizumab in older adult critically ill patients with COVID-19: a multicenter, cohort study. Int J Infect Dis. (2022) 122:252–9. doi: 10.1016/j.ijid.2022.05.038
78. Specker C, Aringer M, Burmester GR, Killy B, Hofmann MW, Kellner H, et al. The safety and effectiveness of tocilizumab in elderly patients with rheumatoid arthritis and in patients with comorbidities associated with age. Clin Exp Rheumatol. (2022) 40:1657–65. doi: 10.55563/clinexprheumatol/f7ff6q
79. Perez-Moreiras JV, Gomez-Reino JJ, Maneiro JR, Perez-Pampin E, Romo Lopez A, Rodríguez Alvarez FM, et al. Efficacy of tocilizumab in patients with moderate-to-severe corticosteroid-resistant graves orbitopathy: A randomized clinical trial. Am J Ophthalmol. (2018) 195:181–90. doi: 10.1016/j.ajo.2018.07.038
80. Smith LD, Moscato EE, Seiff SR. Tocilizumab for the management of thyroid-associated orbitopathy. Ophthalmic Plast Reconstr Surg. (2022) 38:188–92. doi: 10.1097/IOP.0000000000002027
81. Hamed Azzam S, Kang S, Salvi M, Ezra DG. Tocilizumab for thyroid eye disease. Cochrane Database Syst Rev. (2018) 11:CD012984. doi: 10.1002/14651858.cd012984.pub2
82. Moreira Navarrete V, Toyos Sáenz de Miera FJ, Garrido Hermosilla AM, Muñoz Reinoso P, Madrigal Domínguez MJ, Pérez Venegas JJ. AB1315 sarilumab in patients with refractory graves’ orbitopathy. effectiveness and safety in a series of cases in clinical practice. Ann Rheumatic Dis. (2022) 81:1764–5. doi: 10.1136/annrheumdis-2022-eular.4039
83. Huizinga TW, Fleischmann RM, Jasson M, Radin AR, van Adelsberg J, Fiore S, et al. Sarilumab, a fully human monoclonal antibody against IL-6Ralpha in patients with rheumatoid arthritis and an inadequate response to methotrexate: efficacy and safety results from the randomised SARIL-RA-MOBILITY Part A trial. Ann Rheum Dis. (2014) 73:1626–34. doi: 10.1136/annrheumdis-2013-204405
84. Ridker PM, Rane M. Interleukin-6 signaling and anti-interleukin-6 therapeutics in cardiovascular disease. Circ Res. (2021) 128:1728–46. doi: 10.1161/CIRCRESAHA.121.319077
85. Lamb YN, Deeks ED. Sarilumab: A review in moderate to severe rheumatoid arthritis. Drugs. (2018) 78:929–40. doi: 10.1007/s40265-018-0929-z
86. Aletaha D, Bingham CO, Karpouzas GA, Takeuchi T, Thorne C, Bili A, et al. Long-term safety and efficacy of sirukumab for patients with rheumatoid arthritis who previously received sirukumab in randomised controlled trials (SIRROUND-LTE). RMD Open. (2021) 7:1–11. doi: 10.1136/rmdopen-2020-001465
87. Fajgenbaum DC, Kurzrock R. Siltuximab: a targeted therapy for idiopathic multicentric Castleman disease. Immunotherapy. (2016) 8:17–26. doi: 10.2217/imt.15.95
88. Deisseroth A, Ko CW, Nie L, Zirkelbach JF, Zhao L, Bullock J, et al. FDA approval: siltuximab for the treatment of patients with multicentric Castleman disease. Clin Cancer Res. (2015) 21:950–4. doi: 10.1158/1078-0432.CCR-14-1678
89. Takeuchi T, Thorne C, Karpouzas G, Sheng S, Xu W, Rao R, et al. Sirukumab for rheumatoid arthritis: the phase III SIRROUND-D study. Ann Rheum Dis. (2017) 76:2001–8. doi: 10.1136/annrheumdis-2017-211328
90. Schreiber S, Aden K, Bernardes JP, Conrad C, Tran F, Hoper H, et al. Therapeutic interleukin-6 trans-signaling inhibition by olamkicept (sgp130Fc) in patients with active inflammatory bowel disease. Gastroenterology. (2021) 160:2354–66 e11. doi: 10.1053/j.gastro.2021.02.062
91. Speake C, Habib T, Lambert K, Hundhausen C, Lord S, Dufort MJ, et al. IL-6-targeted therapies to block the cytokine or its receptor drive distinct alterations in T cell function. JCI Insight. (2022) 7:1–14. doi: 10.1172/jci.insight.159436
92. Ho Lee Y, Gyu Song G. Comparison of the efficacy and safety of tocilizumab, sarilumab, and olokizumab in patients with active rheumatoid arthritis: a network meta-analysis of randomized controlled trials. Z Rheumatol. (2024) 83:97–106. doi: 10.1007/s00393-022-01315-0
93. Avci AB, Feist E, Burmester GR. Targeting IL-6 or IL-6 receptor in rheumatoid arthritis: what have we learned? BioDrugs. (2024) 38:61–71. doi: 10.1007/s40259-023-00634-1
94. A Study to Investigate Efficacy and Safety of TOUR006 in Participants 18 to 80 Years of Age With Thyroid Eye Disease (spiriTED) ClinicalTrials.gov2024. Available online at: https://clinicaltrials.gov/study/NCT06088979 (Accessed March 20, 2025).
95. A Study to Evaluate the Efficacy, Safety, Pharmacokinetics, and Pharmacodynamics of Satralizumab in Participants With Thyroid Eye Disease (SatraGO-1) ClinicalTrials.gov2024. Available online at: https://clinicaltrials.gov/study/NCT05987423 (Accessed March 20, 2025).
96. A Study to Evaluate the Efficacy, Safety, Pharmacokinetics, and Pharmacodynamics of Satralizumab in Participants With Thyroid Eye Disease (SatraGO-2) Clinicaltrials.gov2024. Available online at: https://clinicaltrials.gov/study/NCT06106828 (Accessed March 20, 2025).
97. Chuang SH, Chang CH. Inflammatory markers in thyroid eye disease: A meta-analysis. Endocr Res. (2024) 49:193–202. doi: 10.1080/07435800.2024.2362787
98. Tong X, Shen Q. Identification of immune-related regulatory networks and diagnostic biomarkers in thyroid eye disease. Int Ophthalmol. (2024) 44:38. doi: 10.1007/s10792-024-03017-9
99. Kishazi E, Dor M, Eperon S, Oberic A, Turck N, Hamedani M. Differential profiling of lacrimal cytokines in patients suffering from thyroid-associated orbitopathy. Sci Rep. (2018) 8:10792. doi: 10.1038/s41598-018-29113-2
100. Huang D, Luo Q, Yang H, Mao Y. Changes of lacrimal gland and tear inflammatory cytokines in thyroid-associated ophthalmopathy. Invest Ophthalmol Vis Sci. (2014) 55:4935–43. doi: 10.1167/iovs.13-13704
101. Huang D, Xu N, Song Y, Wang P, Yang H. Inflammatory cytokine profiles in the tears of thyroid-associated ophthalmopathy. Graefes Arch Clin Exp Ophthalmol. (2012) 250:619–25. doi: 10.1007/s00417-011-1863-x
102. Ujhelyi B, Gogolak P, Erdei A, Nagy V, Balazs E, Rajnavolgyi E, et al. Graves’ orbitopathy results in profound changes in tear composition: a study of plasminogen activator inhibitor-1 and seven cytokines. Thyroid. (2012) 22:407–14. doi: 10.1089/thy.2011.0248
103. Xu N, Cui Y, Fu D, Sun F. Tear inflammatory cytokines and ocular surface changes in patients with active thyroid eye disease treated with high-dose intravenous glucocorticoids. J Endocrinol Invest. (2020) 43:901–10. doi: 10.1007/s40618-019-01174-8
104. Molnár I, Balázs C. High circulating IL-6 level in Graves’ ophthalmopathy. Autoimmunity. (1997) 25:91–6. doi: 10.3109/08916939708996275
105. Salvi M, Girasole G, Pedrazzoni M, Passeri M, Giuliani N, Minelli R, et al. Increased serum concentrations of interleukin-6 (IL-6) and soluble IL-6 receptor in patients with Graves’ disease. J Clin Endocrinol Metab. (1996) 81:2976–9. doi: 10.1210/jcem.81.8.8768861
106. Chalaris A, Garbers C, Rabe B, Rose-John S, Scheller J. The soluble Interleukin 6 receptor: generation and role in inflammation and cancer. Eur J Cell Biol. (2011) 90:484–94. doi: 10.1016/j.ejcb.2010.10.007
107. McElvaney OJ, Curley GF, Rose-John S, McElvaney NG. Interleukin-6: obstacles to targeting a complex cytokine in critical illness. Lancet Respir Med. (2021) 9:643–54. doi: 10.1016/s2213-2600(21)00103-x
108. Nehring SM, Goyal A, Patel BC. C Reactive Protein. Treasure Island (FL: StatPearls Publishing LLC (2024).
109. Han JS, Kim SE, Jin JQ, Park NR, Lee JY, Kim HL, et al. Tear-derived exosome proteins are increased in patients with thyroid eye disease. Int J Mol Sci. (2021) 22:1–12. doi: 10.3390/ijms22031115
110. Buonacera A, Stancanelli B, Colaci M, Malatino L. Neutrophil to lymphocyte ratio: an emerging marker of the relationships between the immune system and diseases. Int J Mol Sci. (2022) 23:1–10. doi: 10.3390/ijms23073636
111. Pop V, Seicean A, Soritau O, Buiga R, Barsan M, Balacescu L, et al. Interleukin-6 correlated with neutrophil-to-lymphocyte ratio in pancreatic cancer. Ann Oncol. (2019) 30:iv15–iv6. doi: 10.1093/annonc/mdz155.056
112. Du J, Liu J, Zhang X, Chen X, Yu R, Gu D, et al. Pre-treatment neutrophil-to-lymphocyte ratio predicts survival in patients with laryngeal cancer. Oncol Lett. (2018) 15:1664–72. doi: 10.3892/ol.2017.7501
113. Adamstein NH, Cornel JH, Davidson M, Libby P, de Remigis A, Jensen C, et al. Association of interleukin 6 inhibition with ziltivekimab and the neutrophil-lymphocyte ratio: A secondary analysis of the RESCUE clinical trial. JAMA Cardiol. (2023) 8:177–81. doi: 10.1001/jamacardio.2022.4277
114. Zhang X, Han C, Wang H, Sun X, Dou X, He X, et al. The correlation of the neutrophil-lymphocyte ratio to clinical and imaging parameters in patients with thyroid eye disease. Endocr Connect. (2022) 11. doi: 10.1530/EC-22-0260
115. Manji N, Carr-Smith JD, Boelaert K, Allahabadia A, Armitage M, Chatterjee VK, et al. Influences of age, gender, smoking, and family history on autoimmune thyroid disease phenotype. J Clin Endocrinol Metab. (2006) 91:4873–80. doi: 10.1210/jc.2006-1402
116. Bunte K, Beikler T. Th17 cells and the IL-23/IL-17 axis in the pathogenesis of periodontitis and immune-mediated inflammatory diseases. Int J Mol Sci. (2019) 20:1–24. doi: 10.3390/ijms20143394
117. Huber AK, Jacobson EM, Jazdzewski K, Concepcion ES, Tomer Y. Interleukin (IL)-23 receptor is a major susceptibility gene for Graves’ ophthalmopathy: the IL-23/T-helper 17 axis extends to thyroid autoimmunity. J Clin Endocrinol Metab. (2008) 93:1077–81. doi: 10.1210/jc.2007-2190
Keywords: IL-6, IL-6R, thyroid eye disease (TED), Graves’ ophthalmopathy, Graves’ orbitopathy, Graves’ disease, thyroid-associated orbitopathy (TAO)
Citation: Murdock J, Nguyen J, Hurtgen BJ, Andorfer C, Walsh J, Lin A, Tubbs C, Erickson K and Cockerham K (2025) The role of IL-6 in thyroid eye disease: an update on emerging treatments. Front. Ophthalmol. 5:1544436. doi: 10.3389/fopht.2025.1544436
Received: 12 December 2024; Accepted: 24 March 2025;
Published: 14 April 2025.
Edited by:
Cheng-Rong Yu, National Eye Institute (NIH), United StatesReviewed by:
Sathyadeepak Ramesh, The Center for Eye & Facial Plastic Surgery, United StatesRudolf Gesztelyi, University of Debrecen, Hungary
Copyright © 2025 Murdock, Nguyen, Hurtgen, Andorfer, Walsh, Lin, Tubbs, Erickson and Cockerham. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kristine Erickson, a2VyaWNrc29uQHRvdXJtYWxpbmViaW8uY29t
 Jennifer Murdock
Jennifer Murdock John Nguyen
John Nguyen Brady J. Hurtgen
Brady J. Hurtgen Cathy Andorfer4
Cathy Andorfer4 Christopher Tubbs
Christopher Tubbs Kristine Erickson
Kristine Erickson Kimberly Cockerham
Kimberly Cockerham