- 1Department of Cornea and Anterior Segment Services, Shantilal Shanghvi Eye Institute, Mumbai, Maharashtra, India
- 2Department of Vitreo-retina Services, Shantilal Shanghvi Eye Institute, Mumbai, Maharashtra, India
- 3Shantilal Shanghvi Cornea Institute, LV Prasad Eye Institute, Hyderabad, Telangana, India
Infectious scleritis is a severe and potentially vision-threatening inflammation of the sclera caused by microbial invasion. Unlike autoimmune scleritis, infectious scleritis is less common with a prevalence of 5%–10% of all cases of scleritis. The initial clinical presentation may often resemble autoimmune scleritis, thus resulting in a delayed diagnosis and poorer outcomes. A high level of suspicion is required in such cases and risk factors such as with a history of trauma or surgery and worsening or refractory to immunosuppressive medications as these may well be infectious. While in established cases, the classical clinical features enable us to diagnose as infectious etiology without much difficulty, the management remains a challenge. Majority of these cases require an aggressive approach with a combination of antimicrobial therapy and surgical treatment. Poor prognostic factors include delayed diagnosis, fungal etiology, and the presence of keratitis or endophthalmitis. In this review, the authors have summarized the prevalence, clinical and microbiological profile, and management strategies of infectious scleritis and their outcomes.
1 Introduction
Scleritis is a painful state of ocular inflammation with a wide spectrum of clinical presentation and etiological factors (1, 2). It is most often associated with autoimmune or connective tissue disease but may be idiopathic or have an infectious etiology, each of which presage varying severity and prognosis of the disease (3). Infection is an undesirable and rare cause of scleritis, involving deep inflammation of the scleral tissue, posing a significant threat to the globe integrity and vision.
Clinical differentiation between infectious scleritis (IS) and autoimmune scleritis is challenging during the early stages, thereby delaying the correct diagnosis and delaying the institution of appropriate medical therapy (4). Majority of these eyes are treated with immunosuppressive therapy alone, resulting in exacerbation of the infectious process and poorer final outcomes. However, a favorable prognosis can be obtained when early diagnosis and appropriate therapy is implemented. This underscores the critical importance of distinguishing between infectious and autoimmune causes early on (5).
2 Epidemiology
The prevalence of IS is largely determined by the geographical location. Infectious scleritis is a rare clinical entity and accounts for just 5%–10% of all cases of scleritis, with bacterial infection being the most common etiology (53%–87%) (3, 6). Although various organisms have been identified as the cause for IS, Pseudomonas aeruginosa remains the most common pathogen. Yu et al. reported this microbe to be the commonest etiological agent in about 87% of cases, whether reported from the United States, South Korea, Australia, or Singapore (3, 6–8). It is to be noted that fungal infection is far less common, seen in only about 11% of the cases in western cohorts (5). IS was also noted to be predominant in the middle-aged to elderly patients (mean age ranging from 40 to 70 years), with a female preponderance (57%–70%). Infectious scleritis represents a higher percentage of scleritis cases in developing countries, such as India where it accounts for 18%–30% (9, 10). Unlike the western literature, fungus has been reported to be the most common cause of IS in the Indian subcontinent (23.5 to 38%), followed by Pseudomonas spp. (range: 13.7%–24%) (3, 11). In the developing regions, IS most commonly affects younger individuals (mean age ranging from 40 to 50 years). Here men (49%–81%) were more commonly affected than women. Jain et al. have attributed the higher incidence of fungal infection in India to agricultural and environmental factors (3). Aspergillus flavus is the most common fungal isolate in the eyes with fungal scleritis. Moreover, systemic diseases such as tuberculosis is endemic to the Indian subcontinent, so it is not surprising to note that a few publications have reported that almost 75% cases of infectious scleritis are tuberculosis-related scleritis (3, 5).
3 Classification
All microbial agents such as bacteria, fungi, viruses, and parasites are reported to cause infectious scleritis, with coinfections caused by two or more clinical pathogens being far less common (4). The infection may have an exogenous or endogenous source. The exogenous route is far more common—due to external ocular wounds following trauma or surgery or spread from an adjacent ocular infection, such as microbial keratitis (Figure 1). These infections tend to be more acute and suppurative, presenting with a greater severity of tissue damage. Endogenous IS is an uncommon clinical entity and often resembles or is misdiagnosed as noninfectious diffuse, nodular, or necrotizing scleritis. Scleritis secondary to systemic infections such as tuberculosis, herpes virus, and syphilis also falls under this category.
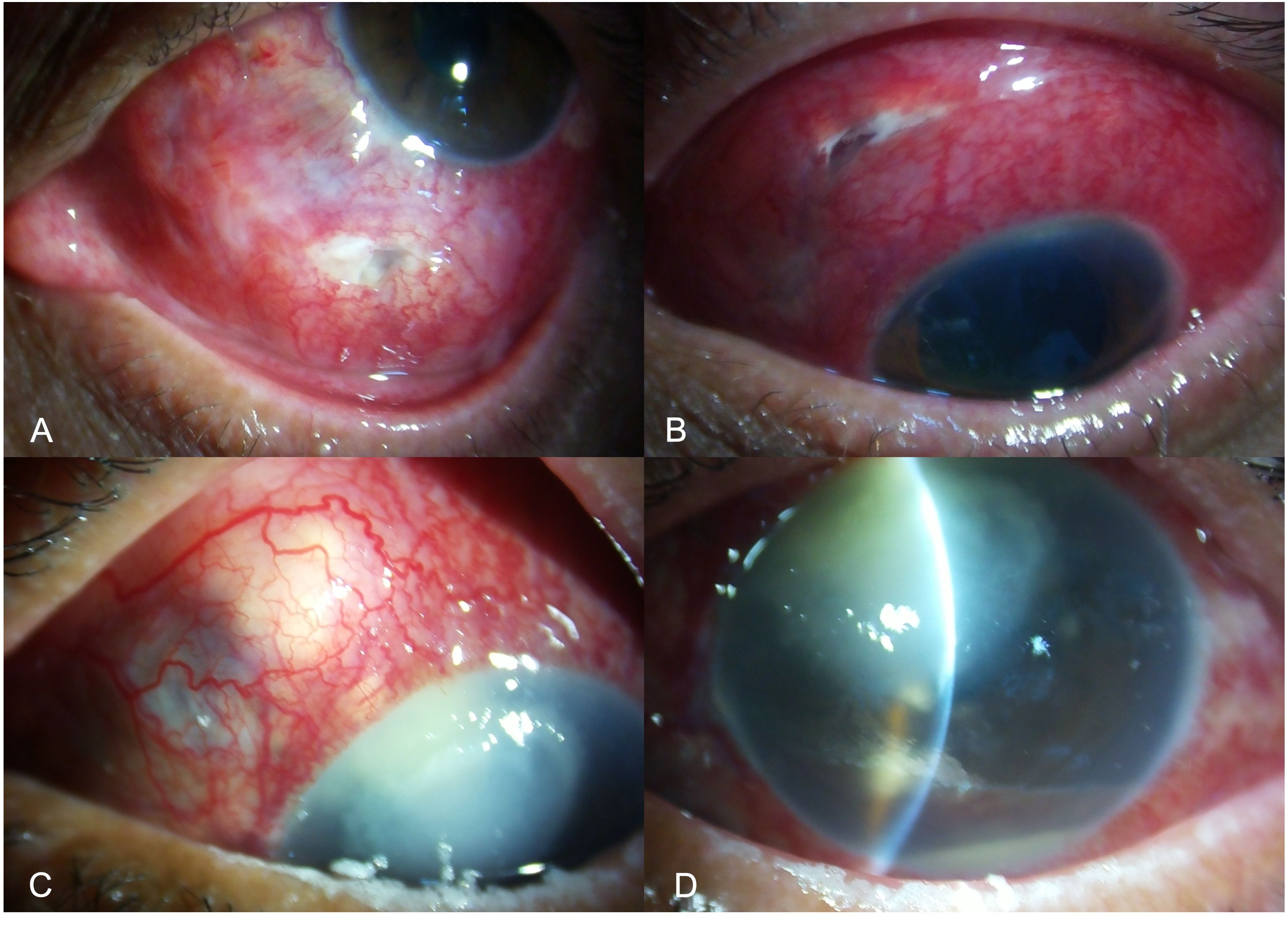
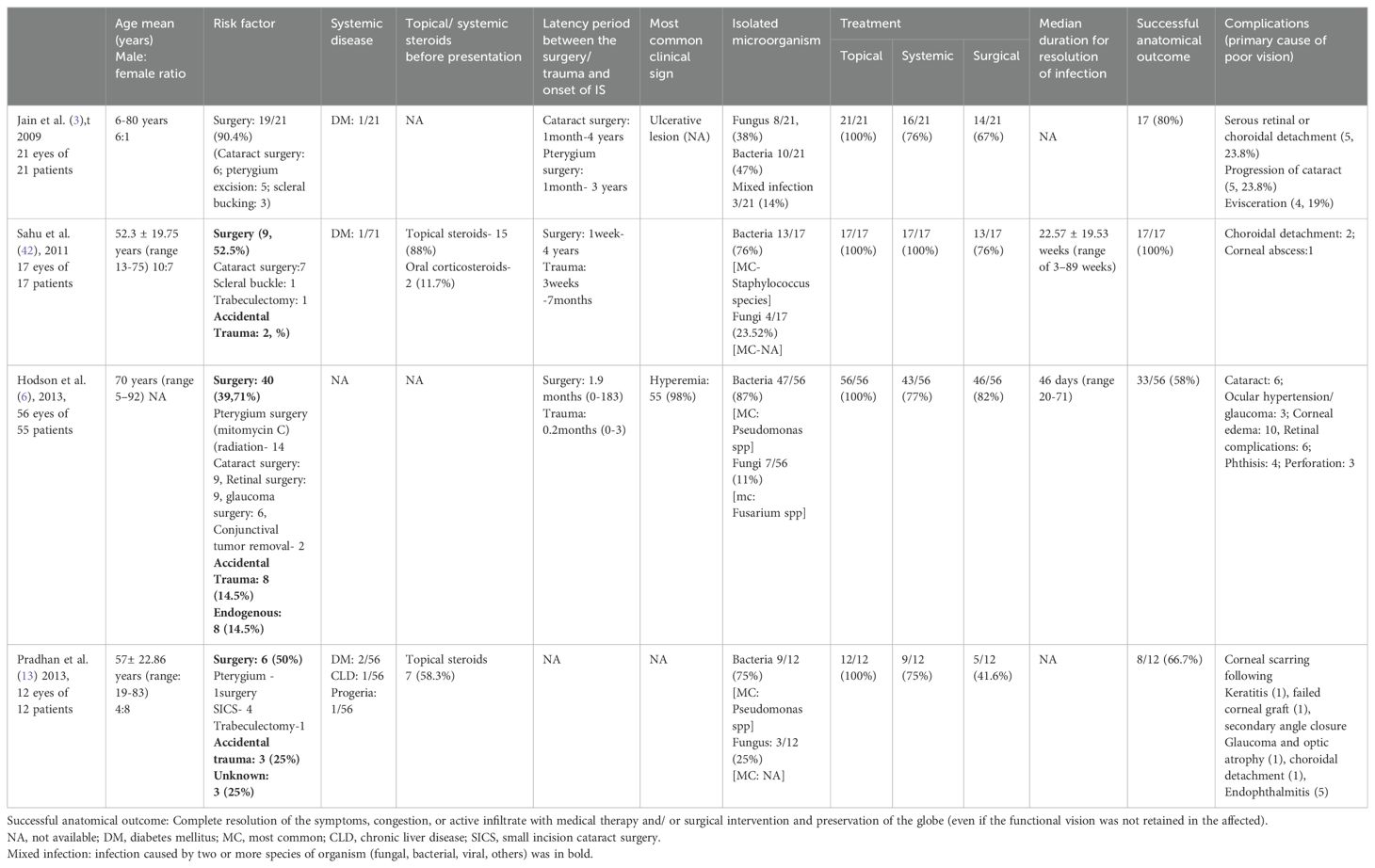
Figure 1. Slit-lamp image of the left eye of a patient presenting with infectious scleritis, a week after penetrating trauma with a wooden stick. The wound involves the inferior scleral tissue with infiltrate and conjunctival necrosis along the wound (A). Slit-lamp image of the left eye in a patient with scleral infection and thinning involving the scleral tunnel following small incision cataract surgery. The nasal edge of the incision shows retraction of the conjunctival tissue with chemosis and infiltrate (B). Slit-lamp image a patient with infective scleral nodule in the super-nasal quadrant (C), due to spread of infection from the adjacent corneal infection following accidental injury with a twig (D). Microbiological examination of the samples collected from the scleral nodule and corneal scraping showed fungal filaments.
IS may also be classified as a primary or secondary infection (4). Primary IS is a term reserved for those eyes where infection is seen as a direct consequence of scleral surgery or accidental ocular trauma. Predisposing factors to a primary infection include systemic infection, systemic/topical steroid use (4), and a previous diagnosis of autoimmune scleritis. Secondary IS is a term reserved for cases where the infection spreads from an adjacent infectious foci like keratitis or endophthalmitis. The risk factors for secondary IS are like those of primary IS, except for history of contact lenses use, presence of debilitating systemic diseases, and corneal tissue devitalization. Regardless of the classification, there are two pathological mechanisms contributing to the development of IS, i.e., direct microbial invasion causing tissue destruction or an immune response triggered by the infectious agent, as seen in eyes with tuberculous scleritis (5).
Posterior infectious scleritis is a rare but potentially destructive disease characterized by infection and inflammation of the posterior sclera. The emergence of posterior scleritis is accompanied by relatively few or, in some cases, no visible clinical signs at all, making diagnosis difficult. Retained intraocular foreign body (IOFB) has been previously reported to cause posterior infectious scleritis (4).
4 Risk factors
4.1 Surgery
Previous ocular surgery is a well-established risk factor for IS, with pterygium surgery being the most common surgical procedure (3, 5, 6). Excessive cauterization and bare sclera technique after pterygium excision increase the susceptibility of IS (Figures 2A, B). Incorrect surgical technique and surgery-induced trauma cause the destruction of the conjunctival and episcleral tissue, including their vascular supply, thus decreasing local tissue immune resistance and exposing the sclera to microbial pathogens. Tissue destruction is propagated by the production of collagenase and subsequent enzymatic degradation in response to the prolonged exposure of the bare sclera to the tear film and atmosphere. Additionally, the use of adjunctive beta irradiation or antimetabolites, such as mitomycin-C and thiotepa, prevents re-epithelization and leaves the sclera vulnerable to microbial invasion (12).
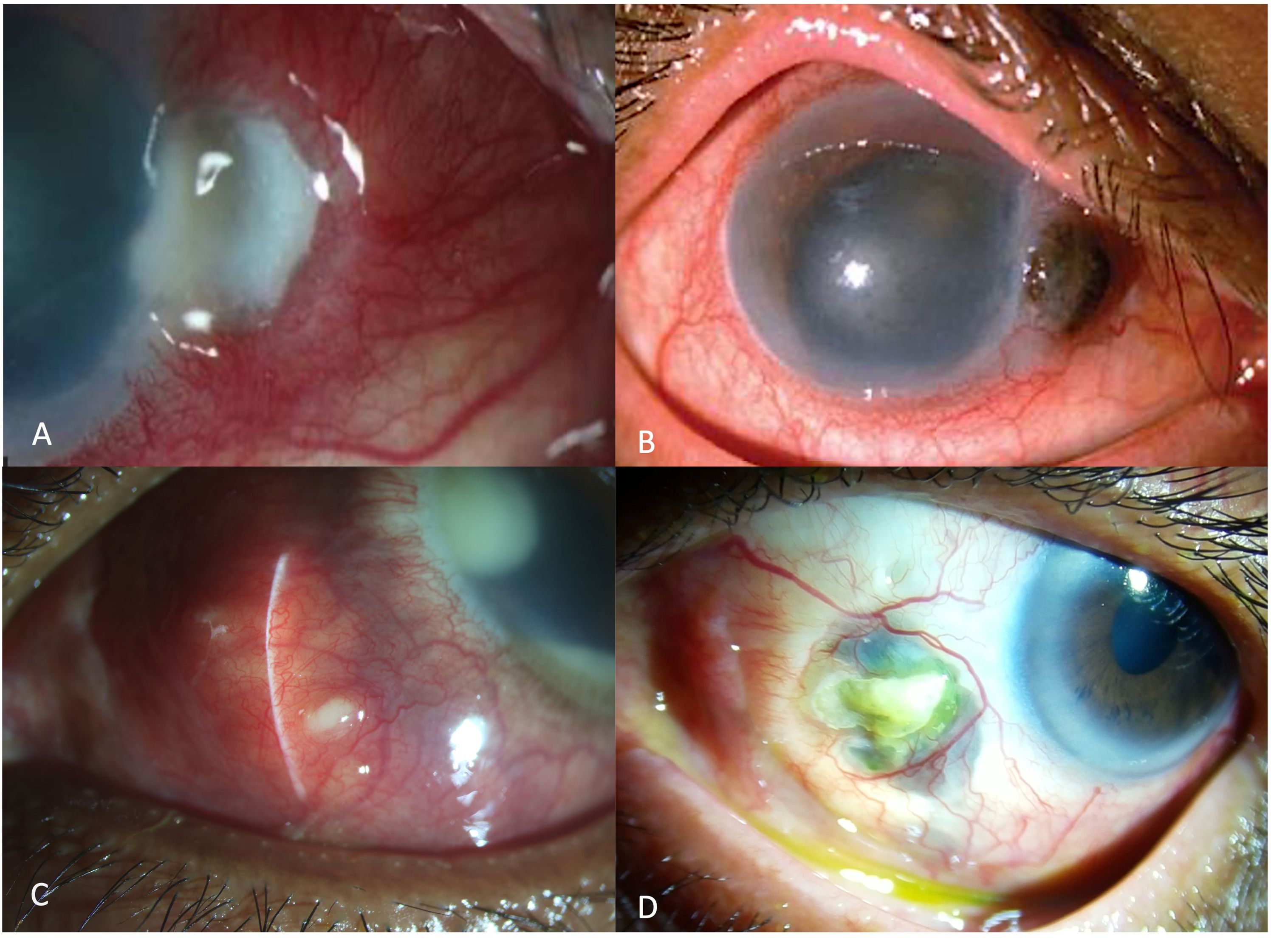
Figure 2. Slit-lamp image of the left eye showing scleral necrosis after pterygium excision, characterized by yellow infiltrate overlying the bare sclera with adjacent corneal stromal lysis (A). Slit lamp image of the right eye of a patient presenting with corneo-scleral perforation after pterygium excision, due to longstanding infectious scleritis after pterygium surgery, recalcitrant to medical therapy (B). Slit-lamp image of the right eye showing an infectious lesion, with a pus-point around the belt-buckle with contagious spread of infection involving the cornea, 3months after a vitreo-retinal surgery for retinal detachment (C). Slit-lamp image of the left eye showing scleral necrosis, infiltrate with thinning and underlying uveal show around the area of the sclerotomy port following retinal surgery (D).
Jain et al. have reported cataract surgery to be the predisposing factor in 30% eyes, followed by pterygium surgery (24%) (3). The reported interval between the surgery and diagnosis of IS ranges from 1 week to 4 years following cataract surgery and 1 month to 3 years following pterygium surgery. The higher prevalence of IS following cataract surgery in developing countries may be attributed to the fact that a higher number of manual incision cataract surgeries are performed using a self-sealing but larger sclero-corneal tunnel compared to clear corneal phacoemulsification performed in the developed countries (13).
Other less common predisposing surgical procedures include vitreo-retinal surgeries such as pars plana vitrectomy (PPV) and scleral buckling (Figures 2C, D). The reported incidence in developing countries is around 6%–14% (3, 9). Reddy et al., in a retrospective study from a tertiary eye care center in India, reported that 75% of the cases with post-surgical IS had a history of vitreo-retinal surgery, especially scleral buckle surgery (10). Majority of these eyes developed infection related to the implanted scleral buckle and presented with severe chronic pain and decreased visual acuity at varying lengths of time in the postoperative period. Smiddy et al. reported that Staphylococcus species caused most of the culture-positive buckle infections. They reported that the organism formed a biofilm around the buckle, thus promoting bacterial sequestration and colonization and inhibiting the penetration of antibiotics (14). These cases are infrequently encountered in the recent decade as there has been a paradigm shift in retinal surgeries from scleral buckle to pars plana vitrectomy.
A newer and rare form of post-surgical infection was noted with the cosmetic surgeries like “eye-whitening” procedures, including regional conjunctivectomy with mitomycin C application, commonly performed in Southern Korea and I-BRITE procedure which is popular in the Unites States, with reports of scleral infection emerging in both. Less frequently, strabismus surgery and glaucoma filtration surgeries have also been known to cause IF (15).
4.2 Trauma
Direct implantation or seeding of the microorganisms in the scleral tissue following accidental penetrating trauma, especially with organic material, is a common cause of IS. Foreign bodies that lodge in or pass through the sclera may also harbor the organisms (5). Nocardia and Aspergillus are soil inhabitants that are associated with the majority of the post-traumatic IS cases (10, 16). Trauma to a distant site in the body may also cause IS through hematogenous spread or self-inoculation. Trauma and incorrect surgical technique cause destruction of the conjunctival and episcleral tissue and its vasculature, predisposing the sclera to be invaded by microorganisms. Additionally, the microbial invasion incites an inflammatory microangiopathy response in the vascular channels (4).
4.3 Iatrogenic
The use of topical and systemic steroids weakens the immune barrier predisposing the eye to IS (9, 17). Though the scleral tissue is radioresistant, IS has been seen in eyes with uveal melanoma that received radiotherapy. Sub-tenon triamcinolone acetonide injections have been known to cause early-onset (bacterial) and late-onset (fungal) infectious scleritis (18). Fungal infections by Aspergillus have been commonly associated with intravenous drug abuse (19).
4.4 Others
IS may present as a secondary spread of infection from adjacent structures, such as keratitis and endophthalmitis, through contiguous spread. Systemic immunosuppression is also a risk factor for IS. Diseases such as human immune-deficiency virus (HIV) infection and diabetes mellitus are commonly associated with IS (4, 20).
5 Clinical presentation
The clinical findings in the case of infectious scleritis, including the mode of onset, presentation, natural history, and the disease course, depend on the precipitating cause of the infection. Based on the above-mentioned features, Murthy et al. have elaborated the differentiating features between infectious and non-infectious scleritis (4).
5.1 Symptoms and signs
There are specific clinical symptoms that should raise a strong suspicion of scleritis, which include ocular pain, redness, watering, decrease in visual acuity headache, and photophobia. Clinical signs suggestive of infective etiology include scleral necrosis, calcified plaques, conjunctival ulceration and sloughing, hypopyon and anterior chamber reaction, corneal infiltrates, yellowish-white uni- or multifocal scleral abscesses or pus points, ulcerative scleral lesion, mucopurulent discharge, and scleral thinning (Figure 3) (10, 21). Exposure of the scleral buckle with surrounding necrosis and discharge, choroidal thickening, exudative retinal detachment, and widening of the sub-tenon’s space are suggestive of buckle-related IS.
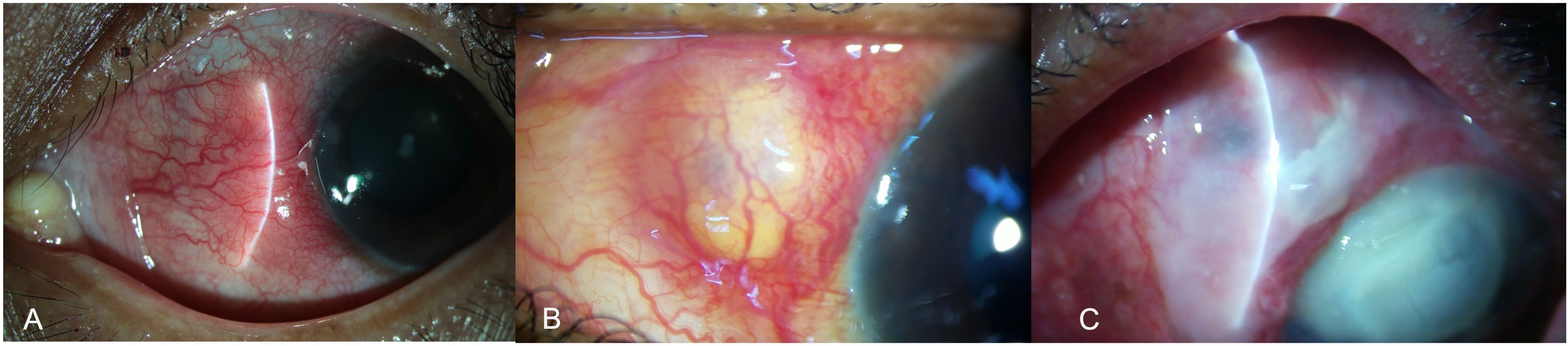
Figure 3. Slit-lamp images showing the various clinical features of infectious scleritis. In the early stages the patients may present with a subconjunctival nodule, with localized conjunctival congestion (A). As the infection worsens the nodule increase in size and appears yellowish (B). Eventually, as the infection spreads, scleral lysis with ulceration and necrosis of the overlying conjunctival is seen with the spread of infection to the adjacent structures like cornea and the anterior chamber (C).
Necrotizing scleritis is most frequently associated with IS (93%), with a yellowish or reddish hue of the lesion due to underlying suppuration (5). Multiple nodules may appear over the course of the disease, which may coalesce and lead to scleral necrosis and thinning. These nodules are mostly seen at around 4 to 5 mm from the limbus in a circumferential pattern. Eventually, a black arc-shaped band appears, extending from the initial lesion, across all the satellite lesions due to scleral thinning and uveal show. This is suggestive of intrascleral dissemination (5). Once these lesions heal, a fibrous band appears overlying the area of scleral thinning, unlike in eyes with immune-mediated scleritis, which heal only with scleral thinning. Poor visual acuity at presentation (<20/200) is associated with poor prognosis and worse visual outcomes, emphasizing the need for early diagnosis and treatment (13).
Patients with posterior infectious scleritis have been reported to present with blurred vision, deep-seated eye pain, particularly during extraocular movements that may radiate around the orbit, diplopia with reduced extraocular movements, and, in a few instances, proptosis, episcleral congestion chemosis, and lid edema. Choroidal mass, serous retinal detachments, usually at the posterior pole, retinal and/or disc edema, retinal and/or choroidal folds, choroidal detachment (CD), and vitritis are usually found on fundus examination. In a small number of cases, anterior uveitis may also be present (4, 22).
5.2 Mode of onset
The natural history, clinical course, and presenting features are indicative of the underlying cause in majority of the eyes with IS (4, 10). In eyes with exogenous IS, history of recent ocular surgery, trauma with organic matter, or presence of concurrent keratitis may help in diagnosing IS. Another characteristic feature of IS is delayed presentation, occurring months to several years after the ocular surgery or trauma (5). Latency is defined as the time interval between the inciting event and the onset of the symptoms. Although this time period is extremely variable, nonsurgical post-traumatic cases tend to have a shorter latency period (0–4 weeks) compared to post-surgical cases (1 week to 36 years). This can be explained by a greater ocular surface damage and a higher load of the infectious agent following trauma (4, 23, 24).
Delayed presentation is a characteristic feature of both IS and surgically induced necrotizing scleritis (SINS). Hence, post-surgical cases of IS should be differentiated from eyes with SINS, with the latter having a longer latency period (up to 36 years and up to 51 years, respectively) (25). The microorganisms may remain dormant inside the sclera tissue, without inciting an inflammatory response, making the diagnosis of IS extremely challenging (5). Secondary infection in eyes with SINS, in the absence of systemic vasculitis, has been reported previously. The underlying mechanism was possibly explained by Lin et al., where the authors postulated that after the onset of SINS, the microorganisms invade the scleral tissue, causing late-onset IS (26). Jain et al. proposed that tear film and conjunctival epithelial alterations may expose underlying necrotic scleral collagen to microorganisms and allow localization, adherence, colonization, and invasion (3).
5.3 The course of the disease
The course of IS is primarily determined by the virulence of the causative organism (7). Fastidious microorganisms like non-tubercular Mycobacteria, Nocardia sp., or fungi usually present as an indolent infection (3, 27). In contrast, microorganisms such as Pseudomonas sp. and Staphylococcus sp. or viral infections have a shorter and more aggressive clinical course (28, 29). Immune-mediated scleritis has a more protracted course determined by the underlying systemic disease (4). Once the systemic disease is in remission, the scleritis component also gradually subsides. The time to resolution after starting the treatment is known to be longer in eyes with IS compared to those with an immune-mediated disease.
5.4 Response to immunosuppressive treatment
Topical and systemic immunosuppressants reduce the inflammatory reaction, causing significant symptomatic relief (30, 31). Patients with IS may notice a marked reduction in pain and redness, particularly with topical steroids. This delays the diagnosis, causing the organism to proliferate. Therefore, in any patient with a scleral lesion that initially responds to immunosuppressants, especially topical steroids, but then worsens with subsequent features as described in this review, the clinician should be alerted to the possibility of an infectious cause (32).
5.5 Atypical clinical presentation
Atypical IS is characterized by painless presentation, unaffected visual acuity, lack of corneal involvement, absence of anterior chamber reaction, and normal intraocular pressure (5). IS may commonly masquerade as surgically induced necrotizing scleritis (SINS), ocular tumors, orbital cellulitis, carotico-cavernous fistula, cavernous sinus thrombosis, and autoimmune scleritis (6). Isolated case reports support atypical clinical presentations like aqueous fistula in pneumococcal scleritis, a case of pyomyositis presenting with an inverse hypopyon, and spontaneous IS in immunocompromised patients (33–35). Atypical IS presents with extreme heterogeneity, and a high level of clinical suspicion and thorough diagnostic evaluation before labeling any scleral lesion as IS.
6 Etiology and microbe-specific clinical features
6.1 Bacteria
Bacterial scleritis significantly contributes to cases of IS, particularly in developed countries (6, 23). These pathogens cause tissue destruction through proteases enzyme produced by the microbes and the activated neutrophils and activation of tissue complement pathways (36).
6.1.1 Gram-negative bacteria
Bacteria are the most common cause of IS in developed countries. Among them, Pseudomonas sp., particularly Pseudomonas aeruginosa, is the most common causative organism contributing to almost 51%–85% cases of IS in the west, most often associated with necrotizing scleritis, corneal infection with subsequent scleral infection, and IS post-pterygium surgery (20, 26, 28). The infection is often severe and associated with poor prognosis due to excessive tissue destruction and collagenolysis caused by the enzymes produced by the activated inflammatory cells and the pathogen itself. Stenotrophomonas maltophilia is another less common pathogen known to cause IS following pterygium surgery and secondary to corneal infection (37, 38). The organism has an indolent course, is less fulminant, and is collagenase-negative, causing lesser tissue destruction compared to the previous pathogen. However, the challenge lies in the treatment as the organism is multi-drug resistant. Lin et al. reported a case of S. maltophilia-associated IS that occurred 18 years after a pterygium surgery. The authors also highlighted the importance of long-term follow-up and therapy after a case of IS that recurred and presented with intrascleral dissemination 5 months after complete resolution (38). Esteben and Jeng highlighted the importance of ultrasound biomicroscopy in a case of IS that revealed a dome-shaped mass overlying an area of partial-thickness scleral laceration after minor ocular trauma.
Other gram-negative organisms known to cause IS include Serratia marcescens, Enterobacter cloacae, Escherichia strain, Proteus sp., and others (6, 10, 12, 24).
6.1.2 Gram-positive bacteria
Staphylococcus aureus is frequently associated with post-pterygium and vitreo-retinal surgeries. A staphylococcal infection may also spread from a distant site in the body (24, 39, 40). Arora et al. reported a case of S. aureus IS which presented as pyomyositis with inverse hypopyon and localized scleral thinning in a patient with a large thigh abscess (39). When the infection shows poor or no response to the typical b-lactam class of antibiotics, a possibility of multi-drug-resistant methicillin-resistant S. aureus (MRSA) scleritis should be considered (29). This was highlighted by Lee et al. who reported a case of MRSA 6 months after a pterygium surgery. An initial misdiagnosis of Pseudomonas-associated IS was reconsidered as the lesion showed no response to systemic amikacin and ceftazidime as well as topical ciprofloxacin (29).
Streptococcus pneumoniae infection represents a potentially aggressive form of the disease, commonly seen in children and adults with concomitant bone, joint, cardiovascular, gastrointestinal, or genitourinary infection. S. pneumonia-related IS is an aggressive form of disease requiring a combination of topical, intravenous, and oral medications (33, 41).
Other gram-positive organisms known to cause IS include Enterococcus faecalis, Propionibacterium acnes, Corynebacterium diphtheriae, Listeria monocytogenes, Actinomyces spp., and others (10, 24, 42).
6.1.3 Nocardia scleritis
Nocardia spp. are gram-positive, weakly acid-fast organisms which morphologically resemble fungi due to their filamentous branching structures (3). A disease caused by Nocardia has a long course, with non-specific clinical signs and difficult microbiological analysis. It is important to consider nocardiosis in cases of unilateral anterior scleritis or sclerokeratitis, combined with a trauma history involving organic materials or a recent ophthalmic surgery (11). Diagnosis is often delayed due to unfamiliarity with the pathogen and poorly understood treatment alternatives, particularly in drug-resistant cases, resulting in potential sight-threatening complications. The commonly reported predisposing factors include cataract surgery, scleral buckling surgery, penetrating keratoplasty, sub-tenon triamcinolone acetonide injection for cystoid macular edema, trauma, and contact lens wear (11, 16).
Nocardia is a saprophytic bacterium genus found mostly in soil, water, dust, and decaying waste. India, being an agriculturally oriented emerging country, gives more opportunity for this organism to be transferred from the soil to the eye (3, 11). Nocardia asteroids is the most often reported organism, followed by Nocardia nova. The classical clinical presentation is chronic diffuse scleritis with nodules and/or necrotic abscesses. Studies from India have reported the incidence of Nocardia keratitis ranging from 14% to 25% (3, 10, 11, 13). Sahu et al. reported three cases of Nocardia scleritis and published an extensive review of the published literature highlighting the importance of surgical debridement in addition to topical and systemic medications to achieve complete resolution (11).
6.2 Fungal scleritis
The reported incidence of fungal keratitis in developing and tropical countries ranges from 5% to 38% (3, 10, 24). The high prevalence of fungal keratitis has been attributed to geographical locations having a hot and humid climate and the abundance of fungal spores in the environment. The infection can spread endogenously in immunocompromised people or through intravenous drug use, or it can also be transmitted exogenously by trauma or ocular surgery (43). The risk of infection increases in immunocompromised patients, in those with chronic use of contact lenses, in intravenous drug users, and in those with chronic use of topical steroids.
Filamentous fungi are the most common cause of fungal keratitis, of which Aspergillus flavus is the most common fungal isolate reported, especially from India (6, 44). Rare forms of fungi known to cause infectious scleritis have been reported in the past. Pseudallescheria boydii, a saprophytic fungus commonly isolated from agricultural soil and contaminated water, has been observed to cause significant posterior sclera involvement with multiple abscesses (45). Paecilomyces lilacinus is an environmental mold found in soil and plants that causes human infection only in rare cases (46). Chung et al. reported on a case of P. lilacinus that manifested as several scleral abscesses with fibrinoid anterior chamber response that developed to invade the cornea despite topical and oral antifungals (46). Phthisis bulbi was seen despite aggressive therapy with surgical debridement and intracameral injection of amphotericin B for 4 months. Scedosporium prolificans, an emerging opportunistic fungus, has been reported to cause late-onset infectious scleritis following pterygium surgery with poor response to voriconazole (47).
Fungal scleritis, unlike the bacterial and viral infections, is associated with poor disease course and outcomes (42, 44). Longer latency periods and smaller necrotic areas at the initial presentation have been commonly observed, suggestive of a chronic indolent course (5). Fungal scleritis is often associated with poor visual outcomes due to delayed diagnosis, unavailability of fungicidal agents, poor penetration of the anti-fungal drugs in the avascular scleral tissue, and the ability of the organism to quiescent in the avascular tissue for prolonged periods without inciting an inflammatory response, thus delaying the clinical diagnosis. Table 1 highlights the outcomes of fungal keratitis as reported previously.
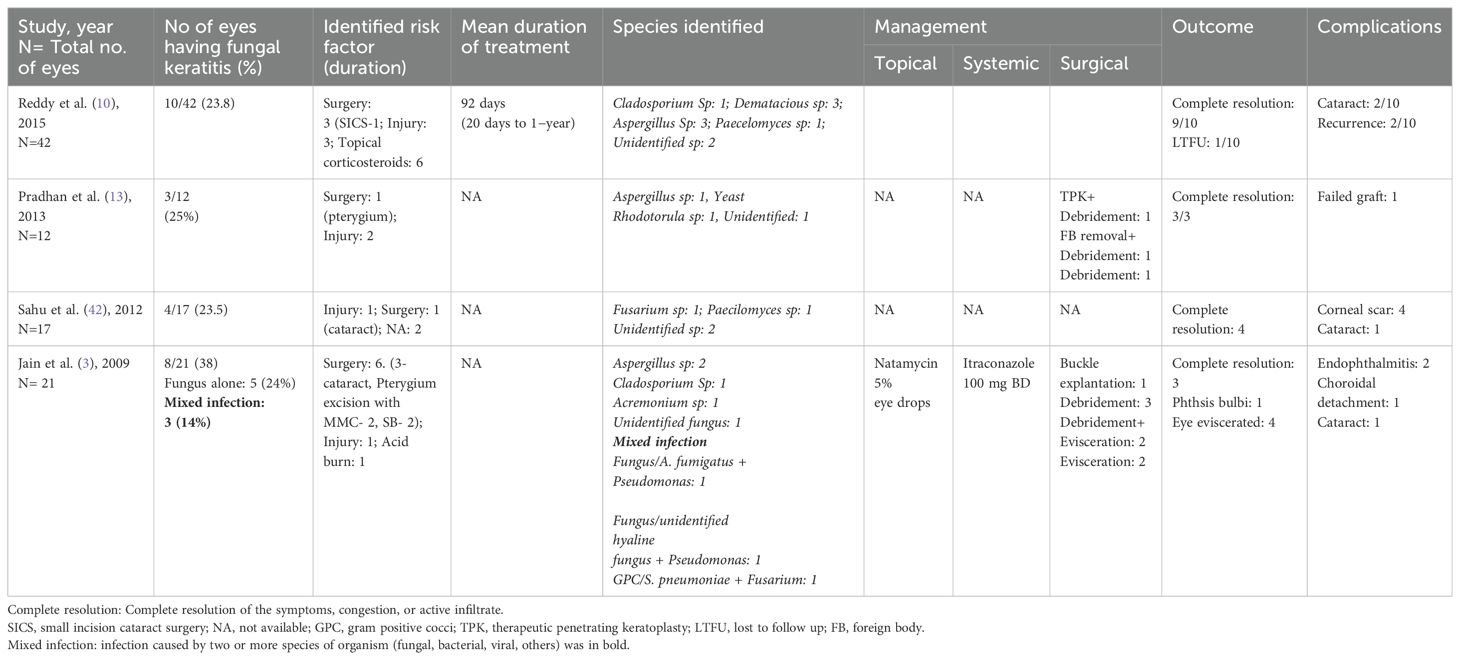
Table 1. Clinical presentation, organisms, management, and outcomes of eyes with fungal scleritis (studies ≥ 10 eyes).
6.3 Viral scleritis
Herpes simplex virus type 1 (HSV-1) and varicella zoster virus (VZV) are most associated with infectious viral scleritis (32, 48). Herpetic scleritis is known to affect middle-aged women, with unilateral clinical findings and moderate to severe ocular pain. As per the current literature, the duration of presentation may be acute (for days) or chronic (3.2 years) (49). Lack of specific signs often result in a diagnostic delay. HSV scleritis commonly presents as unilateral diffuse anterior scleritis and may be associated with keratitis and uveitis. Presence of disproportionate loss of vision, perilimbal devascularization, adjacent corneal thinning, central corneal sparing, significant uveitis, and raised intraocular pressure, in the absence of systemic findings, should raise a suspicion of herpes scleritis. In general, herpes infections are not associated with posterior scleritis, vitreoretinal involvement, or scleromalacia perforans (32, 49).
Zoster scleritis presents as painful, persistent, circumscribed nodules with translucent areas in between and a higher risk of scleral perforation of staphyloma formation (50). A definitive diagnosis by immunohistopathological analysis of scleral biopsies, Giemsa staining of scleral tissue, electron microscopy examination, and cultures is often negative as the infection occurs secondary to viral-induced immune-mediated reaction. Clinical evidence of dendritic or stromal keratitis or positive anti-HSV or anti-VZV titers may help in establishing the diagnosis (4, 50).
Resolution of herpetic scleritis has been seen with oral acyclovir (800 mg) within 3–8 weeks, with the inflammation taking around 5–32 months to resolve (49). The diagnosis of viral scleritis is challenging, but lack of improvement with immunosuppressants and resolution only on starting anti-viral therapy, presence of corneal scars, and iris atrophic patches are clinical indicators of a viral disease.
6.4 Tuberculous scleritis
Mycobacterial scleritis may either result due to direct scleral invasion of the organism, mycobacterium tuberculosis (MTB), or more commonly due to immune-mediated inflammatory microangiopathy (3). Among scleritis, anterior scleritis is the most common pattern of the disease (51). These patients may often present with sclero- keratitis with uveitis. Ocular MTB infection is most often a result of hematogenous dissemination from a distant site (such as lungs) (52). The patient should have a comprehensive investigation using the Mantoux test, sputum analysis, and high-resolution chest CT scan to check for systemic involvement (main focus). For 6–12 months, anti-tubercular therapy consists of 400 mg of ethambutol taken twice daily, 300 mg of isoniazid taken once daily, 600 mg of rifampin taken once daily, and 50 mg of pyridoxine taken once daily.
Mycobacterial infections other than tuberculosis (MOTT) have a low incidence in humans but present in a disseminated form in immunocompromised patients. M. chelonae is among these MOTT species and has been shown to cause scleritis (53), most commonly seen secondary to vitreo-retinal surgeries. Immunocompromised immune status and distant foci of infection like spinal epidural abscess are the predisposing factors. Delayed and insidious presentation area is a hallmark of this infection. Topical amikacin eye drops (2.5%) every hour and oral rifampicin/clarithromycin or doxycycline is the preferred treatment. The in vivo efficacy of the drugs may be poorer than the in vitro sensitivity; thus, combination therapy is advocated, which may include topical ciprofloxacin/moxifloxacin/azithromycin as well. These infections often tend to recur after cessation of therapy; therefore, therapy with at least two drugs and prolonging treatment for 4 weeks to 6 months (in some cases) after the resolution of clinical signs is recommended. Topical corticosteroids are best avoided.
7 Diagnosis
7.1 Scraping
In the presence of adjoining keratitis or ulcerated scleral nodules, samples of infected areas should be taken for microbiology (3, 4). The collected material should be subjected to Gram staining, 10% potassium hydroxide–calcoflur white or trypan blue stain, and 1% acid-fast staining (in cases of suspected mycobacteria and actinomycetes infection) along with inoculation of the material on blood, chocolate, and Sabouraud’s dextrose agar. Inoculating cultures onto Löwenstein–Jensen medium and non-nutrient agar with an Escherichia coli overlay helps identify Mycobacterium and Acanthamoeba species, respectively, if suspected. Utmost care should be taken to avoid contamination of the collected sample with eyelashes, conjunctival tissue, and periocular skin, where the ocular commensals normally reside.
7.2 Tissue biopsy
Scleral or corneo-scleral biopsy (if there is keratitis) is often advised when microbial cultures are negative or reveal specimens with ambiguous identity, or when a poor response is seen with the initial broad-spectrum antimicrobial therapy (24). Biopsy may also be performed with the aim of debulking the infection load or when surgical intervention is anyway warranted with the aim to remove the inciting agent such as scleral buckle, foreign body, or a depot steroid injection. Tissue samples are collected directly from the depth of the lesions (Figures 4A, B).
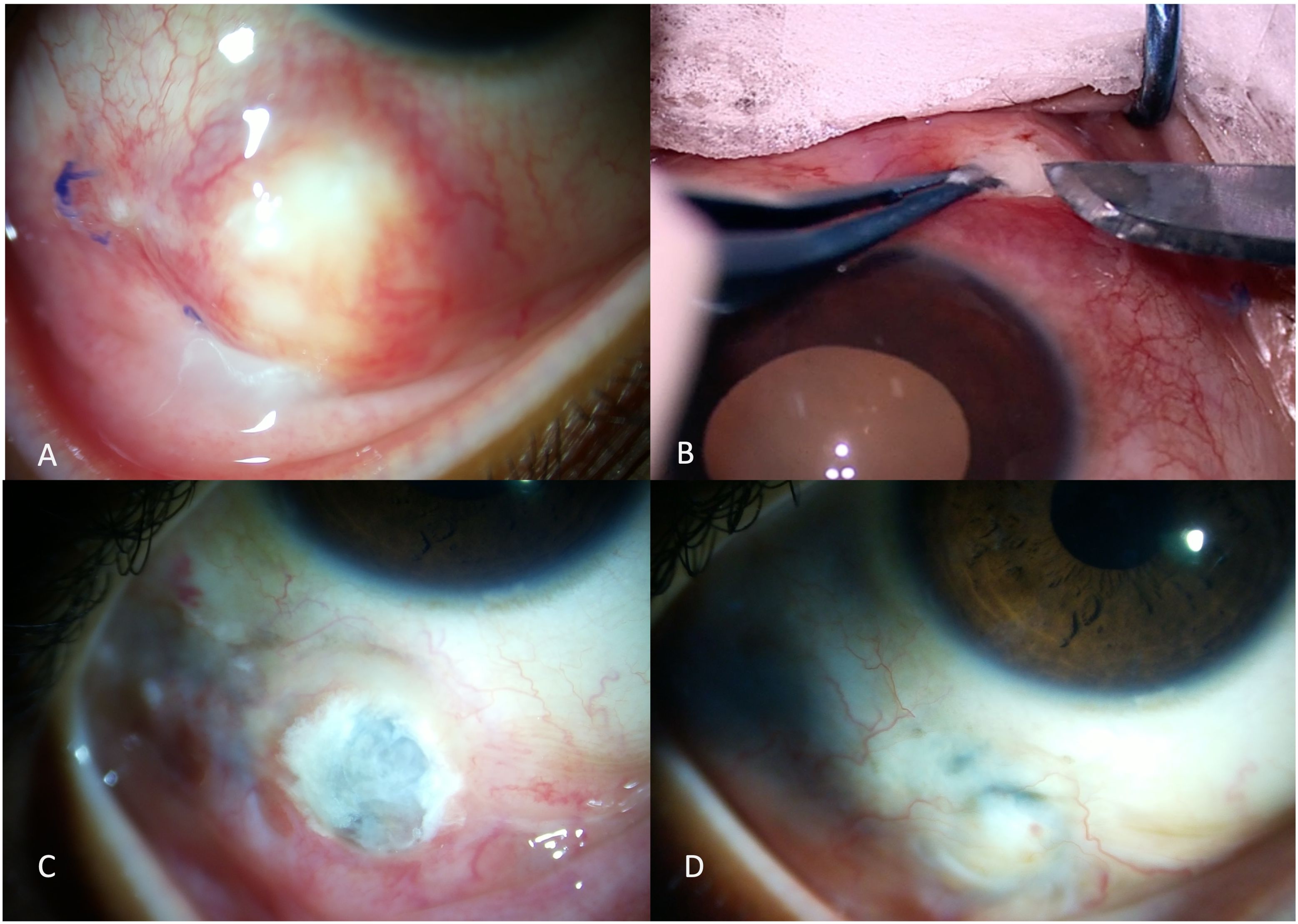
Figure 4. A young girl presented with a scleral nodule associated with redness and discharge for 2 months (A). Surgical debridement with scleral biopsy for microbiological assessment was performed (B). Scleral patch graft was performed for tectonic globe stability in view of scleral thinning (C). The scleral patch graft was conjunctivalized with complete resolution of infection, two months after the intervention (D).
7.3 Serological evaluation
In every case of endogenous IS, the primary focus of infection should be ascertained by performing appropriate serological testing and blood and urine cultures (26).
7.4 Diagnosis of posterior scleritis
The diagnosis of posterior infectious scleritis is usually made through a combination of clinical examination, imaging studies, and laboratory test. B-scan ultrasonography is invaluable for diagnosing this disease, and it reveals several signs including the thickening and edema of the posterior sclera, a scleral mass lesion suggesting an abscess, diffuse choroidal thickening, and the classic T-sign (22, 54). Ancillary tests, including enhanced-depth imaging optical coherence tomography (EDI-OCT), fundus fluorescein angiography (FFA), indocyanine green angiography (ICGA), computerized tomography (CT), and magnetic resonance imaging (MRI), have a limited role in the setting of infectious posterior scleritis but can be performed in some cases to aid in the differential diagnosis.
8 Management
Management of infectious scleritis (anterior and posterior) is extremely challenging, often complicated by delayed diagnosis and poor drug penetration due to avascular scleral tissue. Early recognition and appropriate treatment is important to prevent vision-threatening complications like scleral perforation, dissemination of infection, enucleation, or evisceration (5). Treatment is primarily based on the predisposing factor and the causative pathogen. Management involves a combination of medical and surgical therapy, with only 18% of patients responding to medical therapy alone and most of them requiring one or more surgical interventions (6). Preventive strategies have improved outcomes in eyes with infectious scleritis (24, 47). Post-surgical infections may be prevented by avoiding the excessive use of cautery intraoperatively and the judicious use of adjunctive therapy, especially in eyes undergoing pterygium and cataract surgery, sparing the episcleral vessels which allow better wound healing and resistance to infection (24). Bare-sclera technique of pterygium surgery has been discouraged as it increases the vulnerability of sclera to secondary infection, particularly in the setting of scleral ischemia which requires prompt intervention either in the form of conjunctival autografting or other forms of scleral reinforcement.
8.1 Medical management
a. While awaiting microbiological reports, broad-spectrum fortified antibiotic therapy covering both Gram-positive and Gram-negative organisms should be initiated (fortified cefazolin 5% and ciprofloxacin 0.3% or tobramycin 0.3% eye drops is the most common combination therapy used), and the medication should be modified depending on the initial smear results and subsequent culture and sensitivity results (10). Various combinations of empirical treatment have been reported, with a combination of topical and systemic therapy known to provide the most favorable outcomes (3, 10, 42). Advanced cases may require intravenous antibiotics for better penetration into the sclera. A therapeutic response is expected within 24 to 48 h of initiating the antibiotics; however, minimal or no response should indicate a change in strategy and alternate therapy should be initiated (4).
b. In cases with suspected bacterial scleritis, an initial apparent worsening may occur due to the bactericidal effect of the drugs and release of endotoxins from the dying bacteria, and this may need addition of topical or oral corticosteroids.
c. Auto-immune scleritis must be suspected in eyes with persistently negative microbiology, despite repeated testing, and when the histopathological study of the tissue biopsy reveals an inflammatory picture. Treatment with oral corticosteroids, immunosuppressive drugs, or biologics should be considered as a last resort with or without antibiotic coverage.
d. In eyes with a history of ocular trauma, particularly with organic matter, antifungal therapy like natamycin or voriconazole may be initiated, although many practitioners would prefer the use of antifungals only after the fungus etiology is confirmed. The use of empirical antifungal therapy as the primary treatment was discouraged by Murthy et al. (4) The authors argued that irrespective of history of trauma, bacterial infection is the most common etiology for IS, and hence antifungals should only be started following a documented worsening or failure on antibacterial therapy or negative smear and cultures. Moreover, response to antifungal therapy is slow and takes several weeks, during which crucial time may be lost if the actual underlying etiology is different.
e. Subconjunctival antibiotics and intravenous antibiotics as an adjuvant to topical and oral antibiotics have shown positive outcomes, especially in postsurgical eyes (55).
f. The technique of suprapalpebral lavage in patients with infectious keratoscleritis was initially described by Hessburg (56). Outcomes of suprapalpebral lavage therapy for the treatment of IS in eyes showing a poor response to topical fortified antibiotics was reported by Meallet in six eyes. Levofloxacin (500 mg/100 ml) and tobramycin (100 mg/L) were used for continuous irrigation of the ocular surface. The author reported several advantages of their technique apart from a continuous drug delivery system, which included continuous irrigation of the necrotic debris and the bacterial load from the ocular surface and being an extremely useful technique in patients having limited financial support and access to healthcare facility. This technique also requires minimal dependency on the healthcare staff as the tube insertion into the superior fornix required 30 min for the procedure, and the antibiotic solution bags required to be changed every 1 to 3 days for tobramycin and levofloxacin antibiotics. However, complications like postoperative hyphema, cataract formation, and systemic absorption of the antibiotics remain a major limitation.
8.2 Role of topical and systemic corticosteroids
g. Topical and systemic corticosteroids must be administered with extreme caution and should be monitored regularly (4, 42). Corticosteroids suppress the host immune response, causing reduced tissue destruction. Steroids should be started to suppress the inflammation only after the infection has been adequately controlled with adequate anti-infectious therapy and when suspected fungal or mixed infection is ruled out with multiple negative biopsies and histopathological tissue evaluation is suggestive of inflammatory microangiopathy.
h. The use of topical or systemic steroids in conjunction with antibacterial therapy has always remained controversial despite a few reports stating successful outcomes (10, 13, 47). When used alongside antibiotics, steroids inhibit the upregulation of host cytokines and chemokines caused by the microorganism, thus reducing the activity of the proteolytic enzymes and polymorphonuclear leucocytes causing tissue destruction. Pradhan et al. reported successful outcomes following the use of topical dexamethasone in combination with antimicrobial therapy in eyes with infectious scleritis associated with severe intraocular inflammation in 58.3% eyes (7/12) (13).
i. Successful outcomes have been reported by Huang et al. with low-dose oral corticosteroids (28). The authors claim that low-dose oral steroids eliminate the direct contact of topical steroids and reduces the risk of exacerbation or late recurrence of scleritis. Ahmad et al. reported successful outcomes in Pseudomonas scleritis with oral steroids. The steroids were initiated after 48 h of antibiotic therapy in microbiologically proven cases, after response was noted and fungal infection was ruled out (55). These patients (61%) achieved better visual outcomes (visual acuity >20/200) compared to those who did not (39%) after the infection resolved. Therefore, in Pseudomonas and other severe bacterial infections, topical and oral corticosteroids are recommended with close observation.
8.3 Surgical management
Pyogenic scleral infection is difficult to treat due to poor penetration of the antimicrobial drugs in the avascular necrotic sclera. Superior outcomes have been seen with combined medical and surgical intervention compared to medical therapy alone (6).
Early surgical debridement offers several benefits like providing sample for microbiology and histopathological evaluation for the early identification of the infectious agent, removing the offending material like the glaucoma drainage device or the scleral buckle and, most importantly, promoting drug penetration by reducing the infective load. Several studies have reported that on wound exploration, the area of scleral involvement is often found to be bigger than what is seen clinically (13, 26, 38, 44). Recovery is known to occur faster, and better prognosis is seen in eyes where early (within 2 to 3 weeks) surgical debridement is performed compared to delayed intervention. Multiple wound debridement, with copious irrigation of the wound with antifungals and antibiotics, helps in preventing seeding and expedites healing (6, 13, 26, 42, 44). Despite these reports, there is a significant discrepancy among practitioners about the effect of surgical debridement on the anatomical and visual outcomes in eyes with IS. Some authors have reported that early surgical debridement shortens the disease course and improves visual outcomes. With early surgical intervention, Tittler et al. demonstrated 100% globe preservation, best corrected visual acuity between 20/120 and 20/400, lesser complications, and shorter hospital stay with early surgical debridement, preferably within 2.5 days (12).
The appearance of “tunnel lesions” was initially described by Raber et al. in an eye with Pseudomonas keratitis, extending to involve deeper scleral structures (57). The authors believed that the presence of tunnel lesions and delayed surgical intervention contributed to the poor outcome. Similar lesions were observed by Lin et al. during early surgical debridement (26). They described a slender lesion extending only into the deeper scleral layers, with the overlying superficial sclera and conjunctival tissue being uninvolved. The authors claim that after surgical debridement, daily wound irrigation with antibiotic solution helps in the early resolution of the infection. The irrigation helps to remove the necrotic tissue, microorganism load, and proteolytic enzymes which accumulate in the deep furrows after debridement. In eyes with severe scleral thinning and underlying uveal show, a corneal or scleral patch graft may be required to reestablish tectonic globe stability (Figures 4C, D). In the presence of scleral thinning but with absent uveal exposure, tenon’s advancement or a multilayered amniotic membrane transplantation may be required. All of these surgical interventions must be performed when the infection is adequately controlled. Various biomaterials that have successfully restored globe integrity include autologous perichondrium grafts of the external ear cartilage, autologous fascia lata grafts (with amniotic membrane for large defects), bovine pericardium grafts, and umbilical amnion grafts, followed by amniotic membrane transplantation (5).
Poor visual outcomes despite surgical debridement were reported by Jain et al. (3) Though resolution of infection was seen in 71% eyes, useful vision (vision better than 20/200) could be attained only in 33% cases. Majority of these eyes had fungal infection.
9 Outcomes
In eyes with IS, outcomes following medical or surgical intervention may be defined broadly into anatomical (globe integrity) and functional (visual acuity) outcomes along with the associated complications. Though majority of the studies have demonstrated poor outcomes in eyes with IS, there is enough evidence supporting that the outcomes depend on multiple factors like early diagnosis, prompt treatment and medical or surgical intervention (47). The clinical profile and treatment outcomes of studies (including >12 eyes) are elaborated in Table 2.
Poor prognostic factors include eyes with fungal scleritis compared to bacterial infection, associated kerato-scleritis or endophthalmitis versus isolated scleritis, presenting visual acuity of 20/200 or worse, and medical therapy alone compared to combined medical and surgical treatment. These findings were consistently reported in several studies (3, 6, 10, 42). Fungal scleritis is associated with poor anatomical and functional outcomes due to delayed diagnosis, limited number of fungicidal agents, poor drug penetration in the avascular sclera, and the ability of the organism to remain dormant within the scleral lamellae for prolonged periods, causing progressive worsening.
Surprisingly, visual outcomes were not affected by the inciting factor (surgery, trauma, endogenous infection) or the infectious organism (6, 27, 58). Various treatment techniques such as topical medications alone, combined topical and systemic medical therapy, supra-palpebral antibiotic lavage, surgical debridement, tectonic patch graft using amniotic membrane, and corneal or scleral tissue and lamellar grafts have yielded variable outcomes. However, better prognosis is seen in eyes treated with combined topical and systemic therapy compared to topical therapy alone (10).
Repeated surgical debridement resulted in a shorter treatment course with superior overall outcomes by majority of the studies. Successful anatomical and visual outcomes were reported with a combination of early surgical debridement and medical therapy (11, 13). However, Hodson et al. reported poor functional outcomes (best corrected Snellen visual acuity less than 20/200) despite a combined medical and surgical treatment (6). In their study, 50% of the eyes did not retain functional vision. Enucleation or evisceration was required in 13% of the eyes and was more common in eyes with involvement of the adjacent structures, i.e., eyes with either associated keratitis or endophthalmitis. Therefore, the discrepancy among researchers regarding the effect of surgical debridement on anatomical and functional outcomes persists. Pradhan et al. reported better anatomical outcomes in eyes that underwent surgical debridement (13). However, unlike other studies, the authors did not find any significant difference in the visual outcomes between medically and surgically treated eyes (27, 28).
10 Conclusion
Infectious scleritis, though rare, is an important and impending vision-threatening cause of scleritis. With the known guarded prognosis, it is essential for eye care professionals to consider IS as a differential diagnosis of scleritis. A high level of suspicion is required when the clinical picture is not established, particularly in patients with a history of trauma or surgery, and in those with presumed immune-mediated scleritis but are refractory to immunosuppressive medications. The inciting factor should not be neglected, irrespective of the time when it occurs as the microorganism is known to remain dormant within the avascular sclera. Corneal opacities, cataract, fibrotic pupillary membranes, and repairable retina detachments are the reported sequalae following IS. The diagnosis and management are extremely challenging, requiring early identification of the causative organism with prompt institution of aggressive medical and surgical treatment for better outcomes. Recurrences are rare, but it is important to follow up regularly for a prolonged period, even after clinical resolution of the infection.
Author contributions
SS: Data curation, Methodology, Writing – original draft, Writing – review & editing. JS: Writing – original draft, Writing – review & editing. SM: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Project administration, Resources, Visualization.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Watson PG, Doyne Memorial Lecture. The nature and the treatment of scleral inflammation. Trans Ophthalmol Soc U K. (1982) 102:257–81.
2. Watson PG, Hayreh SS. Scleritis and episcleritis. Br J Ophthalmol. (1976) 60:163–91. doi: 10.1136/bjo.60.3.163
3. Jain V, Garg P, Sharma S. Microbial scleritis—experience from a developing country. Eye. (2009) 23:255–61. doi: 10.1038/sj.eye.6703099
4. Murthy S, Sabhapandit S, Balamurugan S, Subramaniam P, Sainz-de-la-Maza M, Agarwal M, et al. Scleritis: Differentiating infectious from non-infectious entities. Indian J Ophthalmol. (2020) 68:1818. doi: 10.4103/ijo.IJO_2032_20
5. Yu J, Syed ZA, Rapuano CJ. Infectious scleritis: pathophysiology, diagnosis, and management. Eye Contact Lens. (2021) 47:434–41. doi: 10.1097/ICL.0000000000000813
6. Hodson KL, Galor A, Karp CL, Davis JL, Albini TA, Perez VL, et al. Epidemiology and visual outcomes in patients with infectious scleritis. Cornea. (2013) 32:466–72. doi: 10.1097/ICO.0b013e318259c952
7. Ahn SJ, Oh JY, Kim MK, Lee JH, Wee WR. Clinical features, predisposing factors, and treatment outcomes of scleritis in the Korean population. Korean J Ophthalmol KJO. (2010) 24:331–5. doi: 10.3341/kjo.2010.24.6.331
8. Lane J, Nyugen E, Morrison J, Lim L, Stawell R, Hodgson L, et al. Clinical features of scleritis across the asia-pacific region. Ocul Immunol Inflamm. (2019) 27:920–6. doi: 10.1080/09273948.2018.1484496
9. Sahu SK, Das S, Sahani D, Sharma S. Fungal scleritis masquerading as surgically induced necrotizing scleritis: a case report. J Med Case Rep. (2013) 7:288. doi: 10.1186/1752-1947-7-288
10. Reddy JC, Murthy SI, Reddy AK, Garg P. Risk factors and clinical outcomes of bacterial and fungal scleritis at a tertiary eye care hospital. Middle East Afr J Ophthalmol. (2015) 22:203–11. doi: 10.4103/0974-9233.150634
11. Sahu SK, Sharma S, Das S. Nocardia scleritis-clinical presentation and management: a report of three cases and review of literature. J Ophthalmic Inflammation Infect. (2012) 2:7–11. doi: 10.1007/s12348-011-0043-9
12. Tittler EH, Nguyen P, Rue KS, Vasconcelos-Santos DV, Song JC, Irvine JA, et al. Early surgical debridement in the management of infectious scleritis after pterygium excision. J Ophthalmic Inflammation Infect. (2012) 2:81–7. doi: 10.1007/s12348-012-0062-1
13. Pradhan ZS, Jacob P. Infectious scleritis: clinical spectrum and management outcomes in India. Indian J Ophthalmol. (2013) 61:590–3. doi: 10.4103/0301-4738.121085
14. Smiddy WE, Miller D, Flynn HW. Scleral buckle removal following retinal reattachment surgery: clinical and microbiologic aspects. Ophthalmic Surg. (1993) 24:440–5. doi: 10.3928/1542-8877-19930701-04
15. Moshirfar M, McCaughey MV, Fenzl CR, Santiago-Caban L, Kramer GD, Mamalis N. Delayed manifestation of bilateral scleral thinning after I-BRITE(®) procedure and review of literature for cosmetic eye-whitening procedures. Clin Ophthalmol Auckl NZ. (2015) 9:445–51. doi: 10.2147/OPTH.S78390
16. da Cunha LP, Juncal V, Carvalhaes CG, Leão SC, Chimara E, Freitas D. Nocardial scleritis: A case report and a suggested algorithm for disease management based on a literature review. Am J Ophthalmol Case Rep. (2018) 10:1–5. doi: 10.1016/j.ajoc.2018.01.018
17. Reynolds MG, Alfonso E. Treatment of infectious scleritis and keratoscleritis. Am J Ophthalmol. (1991) 112:543–7. doi: 10.1016/S0002-9394(14)76856-4
18. Seth RK, Gaudio PA. Nocardia asteroides necrotizing scleritis associated with subtenon triamcinolone acetonide injection. Ocul Immunol Inflamm. (2008) 16:139–40. doi: 10.1080/09273940802140804
19. Fincher T, Fulcher SFA. Diagnostic and therapeutic challenge of Aspergillus flavus scleritis. Cornea. (2007) 26:618–20. doi: 10.1097/ICO.0b013e318033de67
20. Sainz de la Maza M, Hemady RK, Foster CS. Infectious scleritis: report of four cases. Doc Ophthalmol Adv Ophthalmol. (1993) 83:33–41. doi: 10.1007/BF01203568
21. Al Barqi M, Behrens A, Alfawaz AM. Clinical features and visual outcomes of scleritis patients presented to tertiary care eye centers in Saudi Arabia. Int J Ophthalmol. (2015) 8:1215–9. doi: 10.3980/j.issn.2222-3959.2015.06.25
22. Lee DH, Cho H, Choi EY, Kim M. Clinical features and long-term treatment outcome of posterior scleritis. Ann Transl Med. (2022) 10:1162. doi: 10.21037/atm-22-721
23. Ho YF, Yeh LK, Tan HY, Chen HC, Chen YF, Lin HC, et al. Infectious scleritis in Taiwan—A 10-year review in a tertiary-care hospital. Cornea. (2014) 33:838–43. doi: 10.1097/ICO.0000000000000174
24. Hsiao CH, Chen JJ, Huang SC, Ma HK, Chen PY, Tsai RJ. Intrascleral dissemination of infectious scleritis following pterygium excision. Br J Ophthalmol. (1998) 82:29–34. doi: 10.1136/bjo.82.1.29
25. Mahmood S, Suresh PS, Carley F, Bruce IN, Tullo AB. Surgically induced necrotising scleritis: report of a case presenting 51 years following strabismus surgery. Eye Lond Engl. (2002) 16:503–4. doi: 10.1038/sj.eye.6700033
26. Lin CP, Shih MH, Tsai MC. Clinical experiences of infectious scleral ulceration: a complication of pterygium operation. Br J Ophthalmol. (1997) 81:980–3. doi: 10.1136/bjo.81.11.980
27. Cunningham MA, Alexander JK, Matoba AY, Jones DB, Wilhemus KR. Management and outcome of microbial anterior scleritis. Cornea. (2011) 30:1020–3. doi: 10.1097/ICO.0b013e31820967bd
28. Huang SC, Lai HC, Lai IC. The treatment of Pseudomonas keratoscleritis after pterygium excision. Cornea. (1999) 18:608–11. doi: 10.1097/00003226-199909000-00016
29. Lee JE, Oum BS, Choi HY, Lee JS. Methicillin-resistant Staphylococcus aureus sclerokeratitis after pterygium excision. Cornea. (2007) 26:744–6. doi: 10.1097/ICO.0b013e31804e45ba
30. McCluskey P, Wakefield D. Current concepts in the management of scleritis. Aust N Z J Ophthalmol. (1988) 16:169–76. doi: 10.1111/j.1442-9071.1988.tb01206.x
31. Wakefield D, Di Girolamo N, Thurau S, Wildner G, McCluskey P. Scleritis: Immunopathogenesis and molecular basis for therapy. Prog Retin Eye Res. (2013) 35:44–62. doi: 10.1016/j.preteyeres.2013.02.004
32. Gonzalez-Gonzalez LA, Molina-Prat N, Doctor P, Tauber J, Sainz de la Maza MT, Foster CS. Clinical features and presentation of infectious scleritis from herpes viruses: a report of 35 cases. Ophthalmology. (2012) 119:1460–4. doi: 10.1016/j.ophtha.2012.01.033
33. Paula JS, Simão MLH, Rocha EM, Romão E, Velasco Cruz AA. Atypical pneumococcal scleritis after pterygium excision: case report and literature review. Cornea. (2006) 25:115–7. doi: 10.1097/01.ico.0000164784.18290.45
34. Shaikh SI, Biswas J, Rishi P. Nodular syphilitic scleritis masquerading as an ocular tumor. J Ophthalmic Inflammation Infect. (2015) 5:8. doi: 10.1186/s12348-015-0040-5
35. Agarwal R, Tripathi M, Kashyap S, Sharma S. Recalcitrant infective scleritis masquerading an autoimmune necrotising scleritis: a primary presentation of biopsy-proven granulomatosis with polyangiitis. BMJ Case Rep. (2021) 14:e239517. doi: 10.1136/bcr-2020-239517
36. Su CY, Tsai JJ, Chang YC, Lin CP. Immunologic and clinical manifestations of infectious scleritis after pterygium excision. Cornea. (2006) 25:663–6. doi: 10.1097/01.ico.0000214228.44109.0f
37. Ramos-Esteban JC, Jeng BH. Posttraumatic Stenotrophomonas maltophilia infectious scleritis. Cornea. (2008) 27:232–5. doi: 10.1097/ICO.0b013e318159a968
38. Lin HC, Ma DHK, Chen YF, Yeh LK, Hsiao CH. Late-onset intrascleral dissemination of Stenotrophomonas maltophilia scleritis after pterygium excision. Cornea. (2011) 30:712–5. doi: 10.1097/ICO.0b013e31820007ed
39. Arora R, Shroff D, Narula R, Chauhan D, Mehta DK. Inverse hypopyon as the presenting feature of infectious scleritis in a case of tropical pyomyositis. Can J Ophthalmol J Can Ophtalmol. (2006) 41:769–71. doi: 10.3129/i06-075
40. Gharaee H, Khalife M, Poor SSH, Abrishami M. Infectious scleritis after subtenon triamcinolone acetonide injection. Ocul Immunol Inflamm. (2011) 19:284–5. doi: 10.3109/09273948.2011.584010
41. Altman AJ, Cohen EJ, Berger ST, Mondino BJ. Scleritis and streptococcus pneumoniae. Cornea. (1991) 10:341–5. doi: 10.1097/00003226-199107000-00010
42. Kumar Sahu S, Das S, Sharma S, Sahu K. Clinico-microbiological profile and treatment outcome of infectious scleritis: experience from a tertiary eye care center of India. Int J Inflamm. (2012) 2012:753560. doi: 10.1155/2012/753560
43. Stenson S, Brookner A, Rosenthal S. Bilateral endogenous necrotizing scleritis due to Aspergillus oryzae. Ann Ophthalmol. (1982) 14:67–72.
44. Jain N, Kate A, Basu S. Deep anterior lamellar limbo-keratoplasty for bilateral limbal stem cell deficiency with corneal scarring in chemical injury sequelae: Two case reports. Int J Surg Case Rep. (2022) 97:107409. doi: 10.1016/j.ijscr.2022.107409
45. Taravella MJ, Johnson DW, Petty JG, Keyser RB, Foster CS, Lundberg BE. Infectious posterior scleritis caused by Pseudallescheria boydii. Clinicopathologic findings. Ophthalmology. (1997) 104:1312–6. doi: 10.1016/S0161-6420(97)30142-0
46. Chung PC, Lin HC, Hwang YS, Tsai YJ, Ngan KW, Huang SCM, et al. Paecilomyces lilacinus scleritis with secondary keratitis. Cornea. (2007) 26:232–4. doi: 10.1097/01.ico.0000248384.16896.7d
47. Ramenaden ER, Raiji VR. Clinical characteristics and visual outcomes in infectious scleritis: a review. Clin Ophthalmol Auckl NZ. (2013) 7:2113–22. doi: 10.2147/OPTH.S37809
48. Livir-Rallatos C, El-Shabrawi Y, Zatirakis P, Pellett PE, Stamey FR, Foster CS. Recurrent nodular scleritis associated with varicella zoster virus. Am J Ophthalmol. (1998) 126:594–7. doi: 10.1016/S0002-9394(98)00120-2
49. Bhat PV, Jakobiec FA, Kurbanyan K, Zhao T, Foster CS. Chronic herpes simplex scleritis: characterization of 9 cases of an underrecognized clinical entity. Am J Ophthalmol. (2009) 148:779–89. doi: 10.1016/j.ajo.2009.06.025
51. Okhravi N, Odufuwa B, McCluskey P, Lightman S. Scleritis. Surv Ophthalmol. (2005) 50:351–63. doi: 10.1016/j.survophthal.2005.04.001
52. Agarwal M, Patnaik G, Agarwal S, Iyer G, Anand AR, Ar G, et al. Tuberculous scleritis and multidrug resistance. Ocul Immunol Inflamm. (2022) 30:915–24. doi: 10.1080/09273948.2020.1853176
53. Metta H, Corti M, Brunzini R. Disseminated infection due to Mycobacterium chelonae with scleritis, spondylodiscitis and spinal epidural abscess. Braz J Infect Dis Off Publ Braz Soc Infect Dis. (2008) 12:260–2. doi: 10.1590/S1413-86702008000300019
54. Biswas J, Mittal S, Ganesh SK, Shetty NS, Gopal L. Posterior scleritis: clinical profile and imaging characteristics. Indian J Ophthalmol. (1998) 46:195–202.
55. Ahmad S, Lopez M, Attala M, Galor A, Stanciu NA, Miller D, et al. Interventions and outcomes in patients with infectious pseudomonas scleritis: A 10-year perspective. Ocul Immunol Inflamm. (2019) 27:499–506. doi: 10.1080/09273948.2017.1372484
56. Meallet MA. Subpalpebral lavage antibiotic treatment for severe infectious scleritis and keratitis. Cornea. (2006) 25:159–63. doi: 10.1097/01.ico.0000177837.31249.19
57. Raber IM, Laibson PR, Kurz GH, Bernardino VB. Pseudomonas corneoscleral ulcers. Am J Ophthalmol. (1981) 92:353–62. doi: 10.1016/0002-9394(81)90524-9
Keywords: infectious scleritis, scleral debridement, pseudomonas scleritis, fungal scleritis, management of infectious scleritis, microbiological profile of infectious scleritis
Citation: Sharma S, Sheth JU and Murthy SI (2025) Infectious scleritis: a review of etiologies, clinical features, and management strategies. Front. Ophthalmol. 5:1493831. doi: 10.3389/fopht.2025.1493831
Received: 09 September 2024; Accepted: 07 January 2025;
Published: 07 February 2025.
Edited by:
Irmak Karaca, Stanford University, United StatesReviewed by:
Timothy Janetos, Northwestern Medicine, United StatesSuzan Güven, Ege University, Türkiye
Copyright © 2025 Sharma, Sheth and Murthy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Somasheila I. Murthy, c29tYXNoZWlsYS5tdXJ0aHlAc3NlaW4uaW5kLmlu
 Supriya Sharma
Supriya Sharma Jay U. Sheth
Jay U. Sheth Somasheila I. Murthy
Somasheila I. Murthy