- 1State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Guangdong Provincial Key Laboratory of Ophthalmology and Vision Science, Guangdong Provincial Clinical Research Center for Ocular Diseases, Sun Yat-sen University, Guangzhou, Guangdong, China
- 2Department of Visual Science, Guangzhou Xinhua University, Guangzhou, China
Purpose: To evaluate the myopia control effect of different concentrations atropine in children and adolescent.
Methods: Meta-analyses and systematic reviews available in the Pubmed, Embase, and Cochrane Library databases from the databases’ inception to August 2023 were searched to evaluate the efficacy and tolerability of different concentrations’ atropine in controlling myopia progression. Overall effects were performed using random-effects model. AMSTAR 2 tool was used to assess the quality of included studies. Prespecified outcomes were weight mean difference (WMD) with 95% credible interval (95% CI) of annual spherical equivalent refraction (SER) changes and annual axial length (AL) changes.
Results: 19 systematic reviews/meta-analyses of different atropine concentrations were included in the analysis. 14 studies reported SER changes, and 17 reported AL changes. In terms of the studies’ overall methodological quality level (measured using AMSTAR 2), 1 study was rated high, 7 moderate, 7 low, and 4 critically low. The 0.01% atropine was found to have statistically significance (annual SER change WMD 0.27 [95% CI 0.21 - 0.34] D/year; annual AL change WMD -0.09 [95% CI -0.1 to -0.07]) mm/year), 0.05% atropine was preferred considering efficacy and tolerability (annual SER change WMD 0.54 [95% CI 0.49 - 0.58] D/year; annual AL change WMD -0.21 [95% CI -0.12 to -0.02]) mm/year).
Conclusions: Different atropine concentrations alleviated children and adolescent myopia progression. However, higher-quality evidence and further investigation are needed to clarify the dose–response relationship, and practical guidelines must be developed to determine myopia control efficacy.
1 Introduction
Myopia has increasing prevalence and has raised wide public concern in the last few decades, especially in Asia (1–3). It is estimated that 49.8% of the world population will suffer from myopia by 2050, and that 9.8% will have high myopia (4). School-age children and adolescents are the main group suffering from myopia, probably due to high educational pressure and limited outdoor times (5). Unawareness of appropriate myopia control approaches may aggravate ametropia and cause it to develop into high myopia, which increases the risk of retinopathy, glaucoma, cataracts, and other oculopathies (6, 7). Irreversible visual acuity damage caused by high myopia leads to loss of productivity, bringing substantial economic burden (8). Thus, the increase in myopia cases must be given great attention, and effective and safe measures must be devised and applied to slow myopia progression.
Atropine, a muscarinic acetylcholine receptor antagonist that pharmacologically retards myopia progression, has been widely studied of late. Several trials have reported the outcomes of the use of various concentrations of atropine eye drops in children and adolescents with myopia (9–11). Systematic reviews and meta-analyses have also been conducted to compare the effectiveness of the concentrations (12, 13). However, the latest clinical trial (i.e., Repka et al. (14)) about low-dose atropine turned out to be invalid in slowing myopia progression, which contradicts the results of previous studies and meta-analyses (12, 14, 15). Available studies focused on the myopia control effect of different subgroups (high dose, moderate dose, and low dose), and incomplete reported concentrations were included in the analysis. Clinical practice requires a comprehensive assessment of different concentrations of atropine used to slow myopia progression.
The examined systematic reviews and meta-analyses showed variable quality and results due to the use of different inclusion criteria and subgroup categories; hence, it is necessary to over review the quality and results of previous studies. The present umbrella review aimed to summarize the different atropine concentrations used to slow myopia progression as reported in published systematic reviews and meta-analyses, assess their tolerability and efficacy in slowing myopia progression, and determine practicable concentrations.
2 Methods
We performed this umbrella review to investigate the efficacy and tolerability of different concentrations of atropine in slowing the myopia progression in adolescents and children. Systematic reviews with and without meta-analyses of randomized control trials (RCTs) and observational studies were included in this review. Study protocol was registered prospectively with PROSPERO (CRD42023466785). The results were reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) protocol statement (Supplementary Table S1). Full details of Methods for conducting this review are presented in Supplementary Materials.
2.1 Data sources and search strategy
The present umbrella review included systematic reviews and meta-analyses of the use of atropine for myopia control in children and adolescents. Pubmed, Embase, and Cochrane Library were searched for relevant studies. Two reviewers independently searched the relevant systematic reviews and meta-analyses conducted and uploaded from the databases’ inception to August 25, 2023. The mesh terms “atropine,” “mydriatics,” “cycloplegia,” “myopia,” “systematic review,” and “meta-analysis” were used for the searches, and the articles’ references were screened to identify additional studies. The detailed search strategies used are shown in the Supplementary Material (Supplementary Table S3).
2.2 Inclusion and exclusion criteria
The inclusion criteria were as follows: (a) study type: systematic review or meta-analysis based on RCTs or observational studies on the topic of myopia control; (b) study participants: aged under 18 years with myopia; (c) intervention and control: the intervention group should have received atropine drops with the concentration varying from 0.01% to 1%, and the control group should have received saline or placebo, or should have been blank control (studies comparing the other myopia control approaches and atropine plus approaches were also included); and (d) report of outcomes: the main outcomes included the changes in spherical equivalent refraction (SER) or axial length (AL) expressed as weight mean difference (WMD) with 95% credible interval (95% CI).
The exclusion criteria were as follows: (a) articles reporting data regarding atropine used in animals; (b) study protocols, conference abstracts, and network meta-analyses, or articles without full texts; (c) articles reporting unavailable data; (d) studies cited incorrect data or references.
2.3 Review and data extraction
The identified studies in the three databases were imported into Endnote X9, and duplicate studies were eliminated. Two independent reviewers (BZ.C. and Y.N.) screened the abstracts and titles to identify and collect eligible studies. The articles’ authors and publication dates, the countries of origin of the included RCTs or observational studies, the study durations and covered years, the numbers of included studies and patients, treatment and control information, outcomes, conclusions, and data from related RCTs or observational studies were extracted. We also searched and screened the original RCTs or observational studies of the systematic reviews and meta-analyses to make sure the data and references were matched correctly.
2.4 Quality and evidence assessment
The methodological quality of the included systematic reviews and meta-analyses was evaluated by independent reviewers using A Measure Tool to Assess Systematic Reviews version 2 (AMSTAR 2). The critical areas were assessed by seven items (16). Each item was classified as “No,” “Partial Yes,” or “Yes” based on its conformance to the criteria. The overall level of methodological quality was categorized as “high,” “moderate,” “low,” or “critically low.” The disparities that arose throughout the evaluation were resolved through discussion by the third reviewer (ZT.Z.).
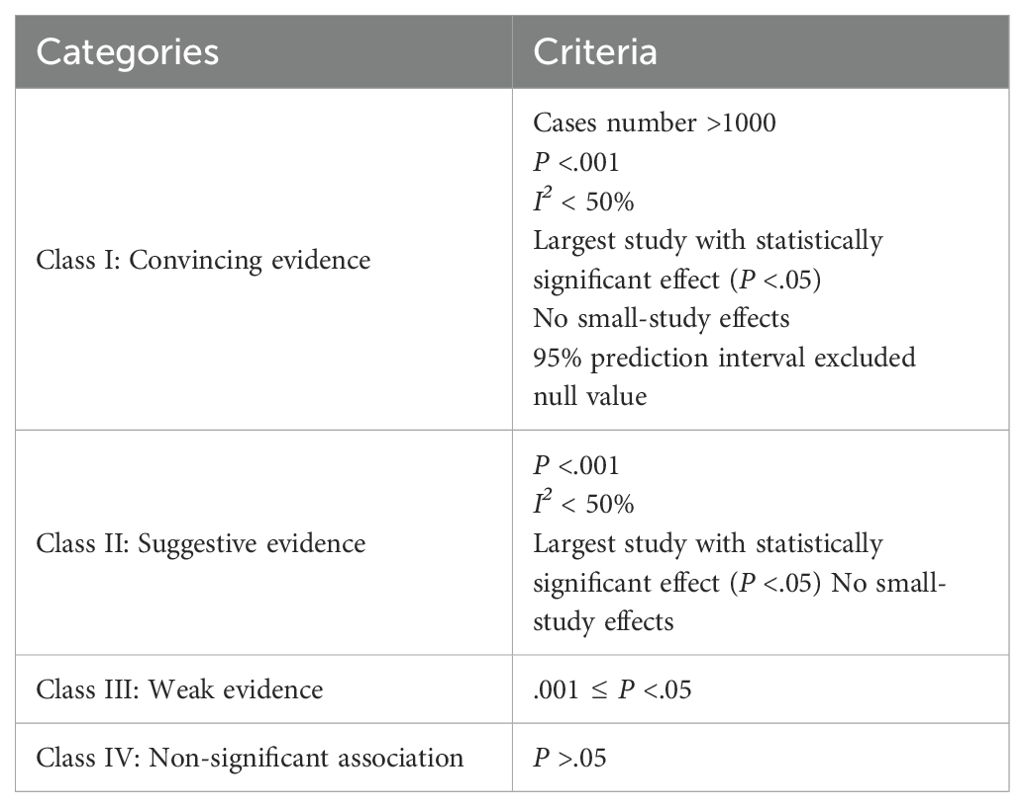
Evidence of outcomes was classified into four categories using evidence classification criteria according to previous umbrella reviews (17–20): class I (convincing evidence), class II (suggestive evidence), class III (weak evidence), and class IV (non-significant) (Table 1).
2.5 Statistical analysis
A considerable number of systematic reviews and meta-analyses published within a short time frame and concentrating on the same field may contain numerous duplicate RCTs, which may introduce bias into the overall results. To evaluate the possible effect caused by including the same RCTs, we calculated the amount of overlap using the corrected covered area (CCA). The primary RCTs served as the rows in the matrix, and the included systematic reviews and meta-analyses served as the columns, as in the study by Mariam et al. (21) The total number of RCTs included in systematic reviews and meta-analyses, RCTs, and included systematic reviews and meta-analyses were denoted by “N” (repetition permitted), “r,” and “c,” respectively; then, CCA = (N − r)/[(r × c) − r]. Minor overlap was indicated by a 0% to 5% CCA value, moderate overlap by 6% to 10%, high overlap by 11% to 15%, and very high overlap by a value > 15%.
Original data from the RCTs or observational studies included in the systematic reviews and meta-analyses were extracted and reanalyzed using Revman 5.4. We calculated the annual changes in SER and AL in each study or collected the annual change data to make them comparable for the different study durations of the RCTs or observational studies. The annual mean differences were calculated by dividing the total change in mean differences by duration (years).
Using the data from the individual RCTs, we retooled various outcome indicators with incongruent systematic review and meta-analysis effect sizes. The random-effects model was used to estimate the overall effect size.
3 Results
Through the research strategy, 201 potentially relevant articles were obtained, 28 of which were excluded as they were duplicates. Following a title- and abstract-based screening, 126 articles were eliminated due to irrelevant content. The remaining 47 articles were then retrieved for full-text evaluation. 28 articles were eliminated. Finally, 19 articles (13, 15, 22–38) were included in the present umbrella review. The flowchart (Figure 1) shows the literature screening procedure.
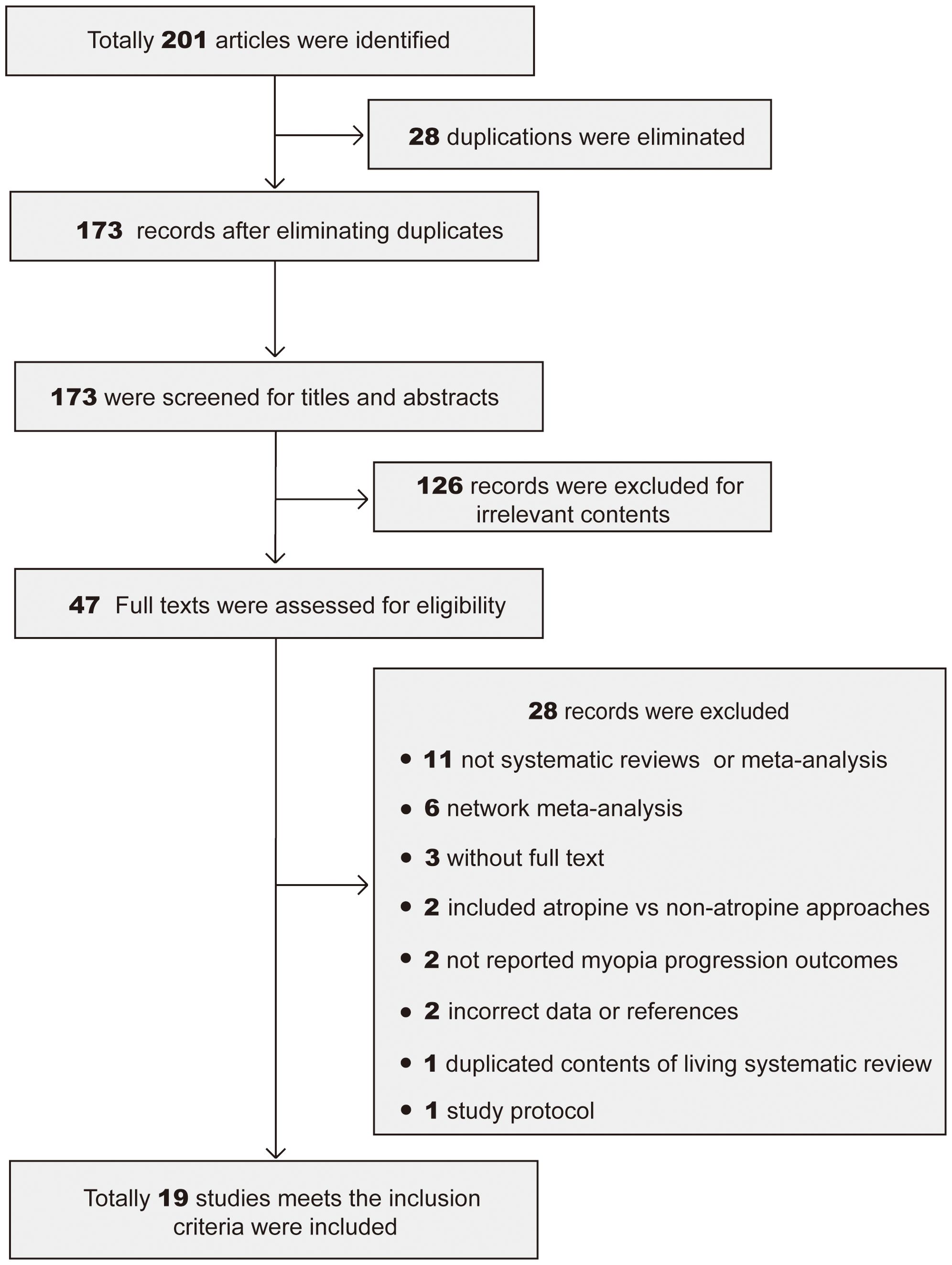
Figure 1. PRISMA flowchart of the study selection process. Totally 201 meta-analyses and systematic reviews were found in 3 databases. 28 duplications were eliminated, 126 articles were eliminated for irrelevant to the topic, 28 articles were eliminated due to improper or incomplete contents. Finally 19 studies were included for analyze.
3.1 Characteristics of the included studies
The characteristics of the 19 systematic reviews and meta-analyses included in the present umbrella review are summarized in Supplementary Table S2. There were 23 RCTs across the 19 systematic reviews and meta-analyses, and their corresponding relationships are shown in Supplementary Table S4. The overall CCA value was 14.14%, indicating a high level of overlap. The CCA of the studies that reported AL changes was 14.93%, indicating high overlap, while the studies that reported SER changes presented very high overlap (CCA = 18.1%). The included systematic reviews and meta-analyses were published between 2011 and 2023, and most of the original RCTs or observational studies in them focused on East Asia, India, Singapore, and the United States.
3.2 Quality assessment
Using AMSTAR 2 tool, we assessed the systematic reviews and meta-analyses, 1 as having high quality, 7 as having moderate quality, 7 as having low quality, and 4 as having critically low quality. Item 13 (adequate addressing of the risk of bias in studies; 13/19 of SRs/MAs, 68.42%) and Item 15 (assessment of presence and likely impact of publication bias; 4/19 of SRs/MAs, 21.05%) were the most frequently absent critical items. Supplementary Table S5 provides the evaluation results of the AMSTAR 2 assessment for each study.
3.3 Myopia control effect
Available studies reported 9 atropine concentrations (0.01%, 0.02%, 0.025%, 0.05%, 0.1%, 0.125%, 0.25%, 0.5%, and 1%). SER change was reported by 73.68% (14/19) of the studies, and AL change was reported by 89.47% (17/19) of the studies. The Overall effect of annual SER change and annual AL change were shown in Figures 2A, 3A. Evidence class and the number of patients in compared subgroups were shown in Figures 2B, 3B.
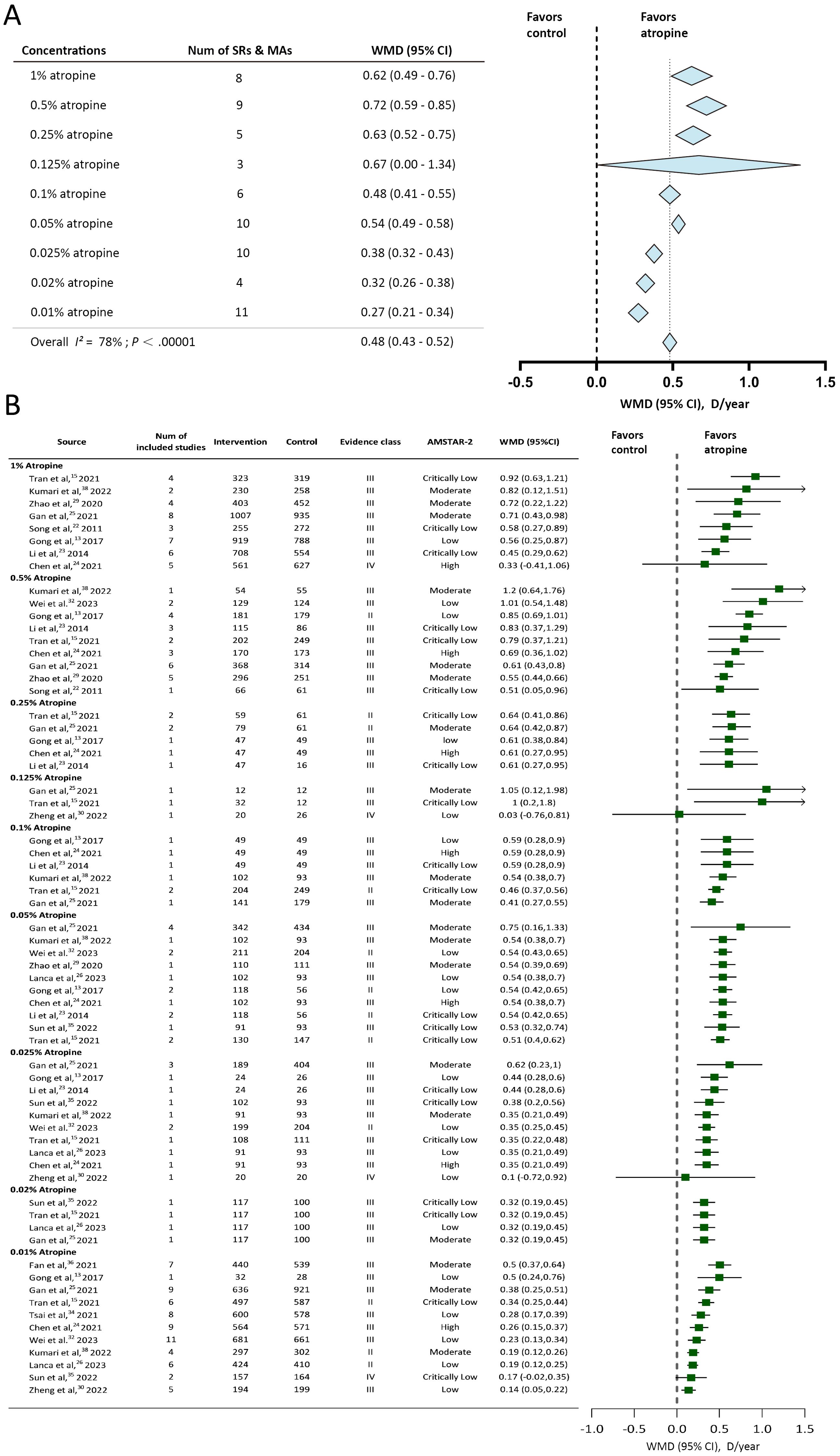
Figure 2. Forest plot of annual Spherical Equivalent Refraction (SER) change for myopic individuals with different concentrations of atropine vs control. (A) Shows the summary effect of each concentration, length of diamonds covers the 95% credible interval (CI) of summary effect sizes. (B) Shows effect sizes of the included systematic reviews or meta-analyses. WMD, mean difference; Num, number; D, diopter; SRs, systematic reviews; MAs, meta-analyses; AMSTAR-2, A Measurement Tool for the Assessment of Multiple Systematic Reviews version 2.
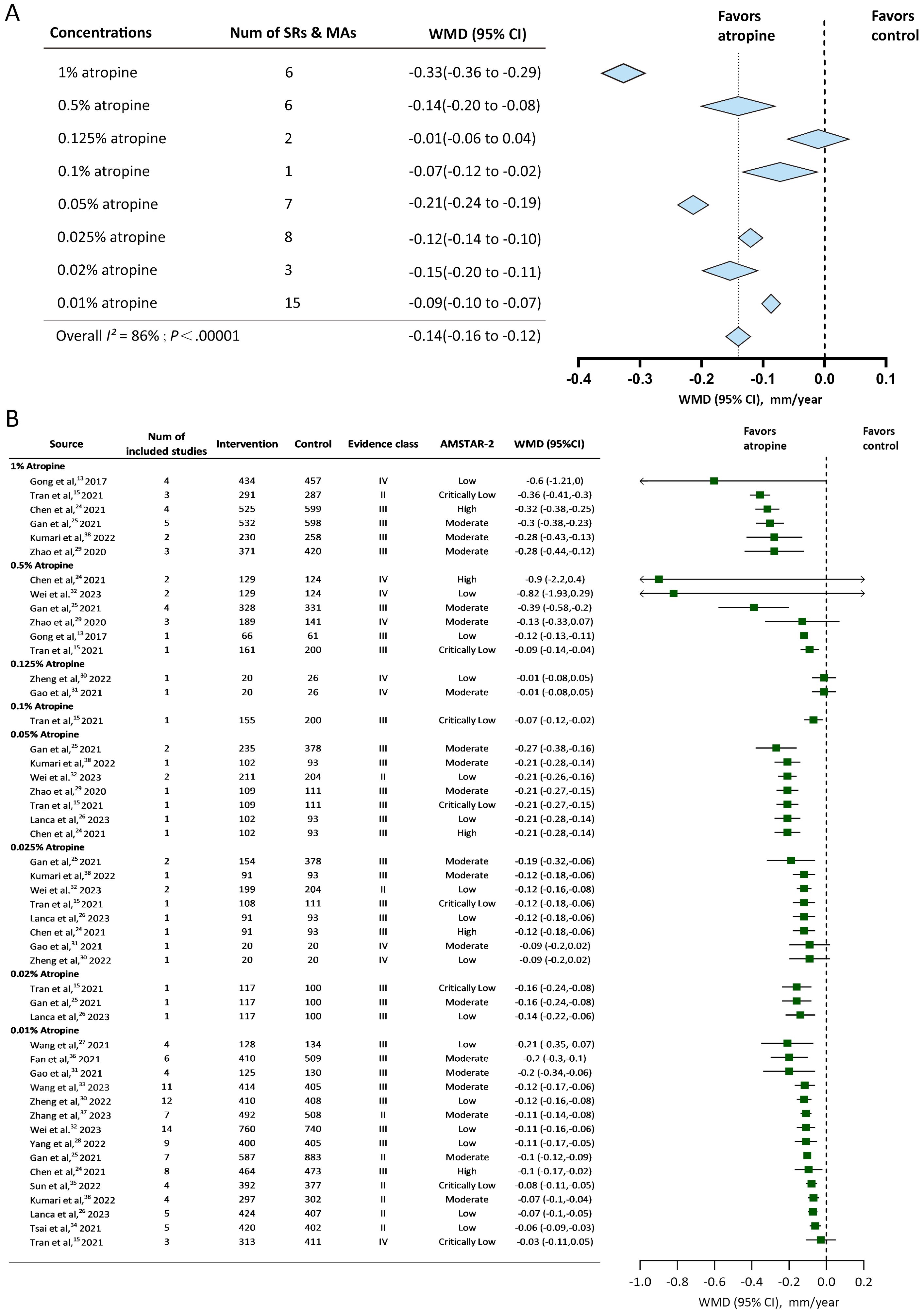
Figure 3. Forest plot of annual Axial Length (AL) change for myopic individuals with different concentrations of atropine vs control. (A) Shows the summary effect of each concentration, length of diamonds covers the 95% credible interval (CI) of summary effect sizes. (B) Shows effect sizes of the included systematic reviews or meta-analyses. WMD, mean difference; Num, number; mm, millimeter; SRs, systematic reviews; MAs, meta-analyses; AMSTAR-2, A Measurement Tool for the Assessment of Multiple Systematic Reviews version 2.
3.3.1 Spherical equivalent refraction change
The included studies reported 9 concentrations of atropine and estimated the myopia control effect using SER change. All of the concentrations showed slower SER progression compared to the control group. The overall effect of 9 concentrations of atropine is 0.48D/year (95% CI -0.43 to 0.52). 1%, 0.5%, 0.25%, 0.125% and 0.05% showed slower SER progression than the overall effect. 0.1% showed an identical annual SER change but a larger 95% credible interval compared to the overall effect. The annual SER change was less in 0.01%, 0.02% and 0.025% atropine than the overall effect. 0.5% atropine showed the maximum annual SER change among 9 concentrations (0.72D/year (95%CI 0.59 to 0.85)). Despite the 0.01% showed the minimum annual SER change among these 9 concentrations, it still showed the significant myopia control effect with an annual SER change of 0.27D/year (95% CI 0.21 to 0.34).
3.3.2 Axial length change
8 concentrations of atropine reported the axial length change to estimate the myopia control effect. All of them except 0.125% showed slowing axial elongation compared to the control group. The overall effect of the annual axial length change was -0.14mm/year (95%CI -0.16 to -0.12). 1%, 0.05%, and 0.02% atropine showed slower axial elongation compared to the overall effect. 0.01%, 0.025%, 0.1% showed less annual axial length change than the overall effect. 0.5% atropine had the same WMD with the overall effect but a larger 95% credible interval. 1% atropine had the best slowing axial elongation effect -0.33mm/year (95%CI -0.36 to -0.29). 0.125% had an annual axial change of -0.01mm/year (95%CI -0.06 to 0.04), which was considered statistically insignificant (P = 0.67) in slowing myopia progression.
3.4 Tolerability
Fourteen included studies summarized and reported adverse events occurring in the original RCTs or observational studies. Photophobia, accommodation dysfunction, and blurred near vision were the most frequently reported adverse events. Allergy, headache, and systemic flushes were also mentioned in a few cases. For the studies comparing the atropine plus orthokeratology with orthokeratology, contact lens–related events, such as corneal staining, conjunctivitis, and keratitis, were reported in both the intervention and control groups. Gan et al. (25) reported that the odd ratio (OR) of photophobia was 163.57 in high-dose atropine (0.5% and 1%), 8.63 in moderate-dose atropine (0.02% to 0.25%), and 6.04 in low-dose atropine (0.01%). Gong et al. (13) reported that the incidence of photophobia was 43.8% in high-dose atropine, 17.8% in moderate-dose atropine, and 6.3% in low-dose atropine.
4 Discussion
The present umbrella review included systematic reviews and meta-analyses that reported data regarding the use of nine atropine concentrations (from 0.01% to 1%) for myopia control. SER and AL changes were used as outcomes to estimate the myopia control effect. The 0.01%, 0.02%, 0.025%, 0.05%, 0.1%, 0.25%, 0.5%, and 1% concentrations reported significant myopia progression–slowing effects by estimating the SER change. 1%, 0.5%, 0.25%, 0.125% and 0.05% slowed SER progression more than the average overall effect and the 0.01% 0.02% and 0.025% were less than the average level in controlling SER progression. 8 of the concentrations reported axial length change and 7 of them (except 0.125%) showed significant slowdown of axial elongation. 1%, 0.05% and 0.02% showed better efficacy than the overall effect in slowing axial elongation, while 0.01%, 0.025 and 0.1% did not. Combined with the analyzed results of annual SER change and axial length change, 0.05% and 1% showed accordant better myopia control effect than the average efficacy. 0.01% and 0.025% showed accordant less in slowing myopia progression compared to the average efficacy. The residual concentrations (0.02%, 0.1%, 0.25%, 0.5%) showed inconsistent results compared to the average efficacy in annual SER change and axial length change and 0.125% atropine had statistically insignificant effects (P ≥ 0.05). These may be attributed to the insufficient number of participants included and insufficient studies that had reported these concentrations.
The examined systematic reviews and meta-analyses revealed the effects of some of the atropine concentrations, albeit with some limitations present in recent studies. Several meta-analyses demonstrated the effectiveness of atropine in slowing myopia progression. Chen et al. (24) reported annual SER changes with 0.01%, 0.02% to 0.5%, and 1% atropine. Their findings indicated a statistically significant slowdown in myopia progression with 0.01% atropine (annual SER change WMD 0.26 D/year [95% CI 0.15 - 0.37]) and 0.025% to 0.5% atropine (annual SER change WMD 0.59 D/year [95% CI 0.41 - 0.78]). These results align with ours, suggesting that moderate-dose atropine is more effective than low-dose atropine in slowing myopia progression. The results reported by Gan et al. (25) and Gong et al. (13) also revealed greater myopia progression–slowing effects of high/moderate-dose atropine compared to low-dose atropine. A network meta-analysis was conducted to compare the myopia control effects of different atropine concentrations (12), and 0.05%, 0.5%, and 1% atropine exhibited favorable outcomes. However, each arm included a limited number of studies and might have had unconvincing results. The authors also acknowledged the limitation of insufficiently included studies in their analysis.
Does 0.01% atropine slow myopia progression? Repka et al. (14) reported the latest clinical trial involving the use of 0.01% atropine for myopia control, and the results indicated that 0.01% atropine did not confer benefits for myopia control, contrary to the results of numerous clinical trials and meta-analyses. The clinical trial was conducted rigorously, the refractions of the included participants were measured three times by optometrists, and no previous myopia control was permitted. Furthermore, various races (Black, East Asian, West/South Asian, Hispanic, Latino, and White) were included to prevent the influence of genetic factors. However, most of the clinical trials were conducted in East and South Asia because of the high prevalence of myopia in these regions. Repka et al. (14) ‘s trial was conducted in the United States, and only 13.9% (26/187) of the participants were Asians. The authors also reported that they carried out virtual visits via phone or video due to the coronavirus disease 2019 pandemic. Eye drops were just delivered to families, which made it challenging to ensure compliance with atropine usage. These could probably explain the divergent results regarding the efficacy of 0.01% atropine in controlling myopia. Yam et al. (9) conducted another clinical trial that reported the ineffectiveness of 0.01% atropine compared to placebo in controlling myopia. However, it is notable that the participants were children with SER between + 1.00 D and 0.00 D, which means that the research focused on the myopia prevention effect rather than the myopia control effect. The outcomes of 0.01% atropine may thus be attributed to the differences in the study populations under investigation.
Although the two aforementioned clinical trials reported the myopia control inefficacy of 0.01% atropine, most of the studies in our umbrella review reported a significant myopia progression–slowing effect. The LAMP study (39) reported an annual SER change (mean [SD]) of -0.59 (0.61) D for 0.01% atropine compared to -0.81 (0.53) D for placebo, and an annual AL change of 0.36 (0.29) mm versus 0.41 (0.22) mm. Similarly, Lee et al. (40) reported an annual SER change of -0.31 D (95% CI -0.39 to -0.22) for 0.01% atropine versus -0.53 D (95% CI -0.66 to -0.40) for placebo, and an annual AL change of 0.16 mm (95% CI 0.13 - 0.20) versus 0.25 mm (95% CI 0.20 - 0.30), which are consistent with our results.
The myopia control white paper (41) regarded an annual SER change ≥ 0.25 D/year or an annual AL change ≥ 0.1 mm/year as effective in combined therapies. According to International Myopia Institute (IMI) white papers (42), treatment effects ranging from 70% to 100% were reported with 0.5% and 1% atropine. However, the criteria for the efficacy of monotherapy are void. Due to the lack of practical guidelines for assessing the efficacy of clinical myopia control approaches, the assessment remains challenging. Guidelines for estimating myopia progression are thus needed.
To make the myopia control effect comparable and more accurate, our research standardized the estimated criterion by calculating the annual SER change and annual axial length change. Clinical practice focuses on the efficacy of specific concentrations rather than a rough comparison among concentration subgroups, so the myopia control effect of 9 previously reported concentrations of atropine was respectively reanalyzed in our study, which provides more specific evidence of each concentration in slowing myopia progression. We reanalyzed the myopia control effect based on the available systematic reviews and meta-analyses, providing a higher rank of evidence for the controversial conclusion (for example, the myopia control effect of 0.01% atropine). Furthermore, the quality and the rank of evidence were applied to show the reliability of the analysis.
There are still several limitations in our research. The present umbrella review included comprehensive systematic reviews and meta-analyses and formally assessed the quality of the included studies. More RCTs and observational studies were included and reanalyzed compared to the available meta-analyses to make the results of the review more convincing. However, our research had several limitations. First, there was a small initial number of studies included, and there were inadequate studies in some concentration groups, which may have led to inaccurate results. Second, more than half of the included studies were assessed as having low or critically low quality, increasing the risk of overestimating the effect size. Third, as some concentration groups and included studies did not report adverse events, tolerability was hard to accurately estimate in each concentration.
5 Conclusion
In conclusion, the present umbrella review identified and gave an overview of atropine use and myopia control outcomes. All the reported atropine concentrations showed a myopia progression alleviation effect in children and adolescents. Adverse events occur more in high-dose of atropine than in moderate and low doses of atropine. However, higher-quality multicenter studies with participants spanning a wide range of races are still needed to elucidate the dose–response relationship between atropine and myopia control, and practical guidelines are needed for assessing myopia progression control efficacy.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://github.com/krystian-scott/Re-arranged-Results.
Author contributions
BC: Conceptualization, Formal analysis, Methodology, Software, Supervision, Writing – original draft. YN: Conceptualization, Funding acquisition, Methodology, Writing – review & editing. JC: Data curation, Investigation, Methodology, Writing – review & editing. SX: Writing – review & editing. ZZ: Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by Science and Technology Program of Guangzhou, China (20210210145) and National Natural Science Foundation of Guangdong, China (2023A1515011652).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fopht.2024.1447558/full#supplementary-material
References
1. Morgan IG, French AN, Ashby RS, Guo X, Ding X, He M, et al. The epidemics of myopia: Aetiology and prevention. Prog Retin Eye Res. (2018) 62:134–49. doi: 10.1016/j.preteyeres.2017.09.004
2. Wolffsohn JS, Calossi A, Cho P, Gifford K, Jones L, Jones D, et al. Global trends in myopia management attitudes and strategies in clinical practice - 2019 Update. Cont Lens Anterior Eye. (2020) 43:9–17. doi: 10.1016/j.clae.2019.11.002
3. Jonas JB, Ang M, Cho P, Guggenheim JA, He MG, Jong M, et al. IMI prevention of myopia and its progression. Invest Ophthalmol Vis Sci. (2021) 62:6. doi: 10.1167/iovs.62.5.6
4. Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. (2016) 123:1036–42. doi: 10.1016/j.ophtha.2016.01.006
5. Morgan IG, Rose KA. Myopia and international educational performance. Ophthalmic Physiol Opt. (2013) 33:329–38. doi: 10.1111/opo.12040
6. Baird PN, Saw SM, Lanca C, Guggenheim JA, Smith Iii EL, Zhou X, et al. Myopia. Nat Rev Dis Primers. (2020) 6:99. doi: 10.1038/s41572-020-00231-4
7. Haarman AEG, Enthoven CA, Tideman JWL, Tedja MS, Verhoeven VJM, Klaver CCW. The complications of myopia: A review and meta-analysis. Invest Ophthalmol Vis Sci. (2020) 61:49. doi: 10.1167/iovs.61.4.49
8. Ma Y, Wen Y, Zhong H, Lin S, Liang L, Yang Y, et al. Healthcare utilization and economic burden of myopia in urban China: A nationwide cost-of-illness study. J Glob Health. (2022) 12:11003. doi: 10.7189/jogh.12.11003
9. Yam JC, Zhang XJ, Zhang Y, Yip BHK, Tang F, Wong ES, et al. Effect of low-concentration atropine eyedrops vs placebo on myopia incidence in children: the LAMP2 randomized clinical trial. Jama. (2023) 329:472–81. doi: 10.1001/jama.2022.24162
10. Azuara-Blanco A, Logan N, Strang N, Saunders K, Allen PM, Weir R, et al. Low-dose (0.01%) atropine eye-drops to reduce progression of myopia in children: a multicentre placebo-controlled randomised trial in the UK (CHAMP-UK)-study protocol. Br J Ophthalmol. (2020) 104:950–5. doi: 10.1136/bjophthalmol-2019-314819
11. Wei S, Li SM, An W, Du J, Liang X, Sun Y, et al. Safety and efficacy of low-dose atropine eyedrops for the treatment of myopia progression in chinese children: A randomized clinical trial. JAMA Ophthalmol. (2020) 138:1178–84. doi: 10.1001/jamaophthalmol.2020.3820
12. Ha A, Kim SJ, Shim SR, Kim YK, Jung JH. Efficacy and safety of 8 atropine concentrations for myopia control in children: A network meta-analysis. Ophthalmology. (2022) 129:322–33. doi: 10.1016/j.ophtha.2021.10.016
13. Gong Q, Janowski M, Luo M, Wei H, Chen B, Yang G, et al. Efficacy and adverse effects of atropine in childhood myopia: A meta-analysis. JAMA Ophthalmol. (2017) 135:624–30. doi: 10.1001/jamaophthalmol.2017.1091
14. Repka MX, Weise KK, Chandler DL, Wu R, Melia BM, Manny RE, et al. Low-dose 0.01% Atropine eye drops vs placebo for myopia control: A randomized clinical trial. JAMA Ophthalmol. (2023) 141:756–65. doi: 10.1001/jamaophthalmol.2023.2855
15. Tran HDM, Sankaridurg P, Naduvilath T, Ha TTX, Tran TD, Jong M, et al. A meta-analysis assessing change in pupillary diameter, accommodative amplitude, and efficacy of atropine for myopia control. Asia Pac J Ophthalmol (Phila). (2021) 10:450–60. doi: 10.1097/apo.0000000000000414
16. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. Bmj. (2017) 358:j4008. doi: 10.1136/bmj.j4008
17. Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. Bmj. (2014) 348:g2035. doi: 10.1136/bmj.g2035
18. Sawangjit R, Chuenchom C, Sanverm T, Chaiyakunapruk N, Jiranukool J, Nithipaijit R, et al. Efficacy and safety of herbal medicine on dementia and cognitive function: An umbrella review of systematic reviews and meta-analysis. Phytother Res. (2023) 37:2364–80. doi: 10.1002/ptr.7759
19. Belbasis L, Bellou V, Evangelou E, Ioannidis JP, Tzoulaki I. Environmental risk factors and multiple sclerosis: an umbrella review of systematic reviews and meta-analyses. Lancet Neurol. (2015) 14:263–73. doi: 10.1016/s1474-4422(14)70267-4
20. Dragioti E, Solmi M, Favaro A, Fusar-Poli P, Dazzan P, Thompson T, et al. Association of antidepressant use with adverse health outcomes: A systematic umbrella review. JAMA Psychiatry. (2019) 76:1241–55. doi: 10.1001/jamapsychiatry.2019.2859
21. Kirvalidze M, Abbadi A, Dahlberg L, Sacco LB, Calderón-Larrañaga A, Morin L. Estimating pairwise overlap in umbrella reviews: Considerations for using the corrected covered area (CCA) index methodology. Res Synth Methods. (2023) 14:764–7. doi: 10.1002/jrsm.1658
22. Song YY, Wang H, Wang BS, Qi H, Rong ZX, Chen HZ. Atropine in ameliorating the progression of myopia in children with mild to moderate myopia: a meta-analysis of controlled clinical trials. J Ocul Pharmacol Ther. (2011) 27:361–8. doi: 10.1089/jop.2011.0017
23. Li SM, Wu SS, Kang MT, Liu Y, Jia SM, Li SY, et al. Atropine slows myopia progression more in Asian than white children by meta-analysis. Optom Vis Sci. (2014) 91:342–50. doi: 10.1097/opx.0000000000000178
24. Chen C, Yao J. Efficacy and adverse effects of atropine for myopia control in children: A meta-analysis of randomised controlled trials. J Ophthalmol. (2021) 2021. doi: 10.1155/2021/4274572
25. Gan J, Li SM, Wu S, Cao K, Ma D, He X, et al. Varying dose of atropine in slowing myopia progression in children over different follow-up periods by meta-analysis. Front Med. (2021) 8:756398. doi: 10.3389/fmed.2021.756398
26. Lanca C, Pang CP, Grzybowski A. Effectiveness of myopia control interventions: A systematic review of 12 randomized control trials published between 2019 and 2021. Front Public Health. (2023) 11:1125000. doi: 10.3389/fpubh.2023.1125000
27. Wang S, Wang J, Wang N. Combined orthokeratology with atropine for children with myopia: A meta-analysis. Ophthalmic Res. (2021) 64:723–31. doi: 10.1159/000510779
28. Yang N, Bai J, Liu L. Low concentration atropine combined with orthokeratology in the treatment of axial elongation in children with myopia: A meta-analysis. Eur J Ophthalmol. (2022) 32:221–8. doi: 10.1177/1120672121998903
29. Zhao C, Cai C, Ding Q, Dai H. Efficacy and safety of atropine to control myopia progression: a systematic review and meta-analysis. BMC Ophthalmol. (2020) 20:478. doi: 10.1186/s12886-020-01746-w
30. Zheng NN, Tan KW. The synergistic efficacy and safety of combined low-concentration atropine and orthokeratology for slowing the progression of myopia: A meta-analysis. Ophthalmic Physiol Opt. (2022) 42:1214–26. doi: 10.1111/opo.13029
31. Gao C, Wan S, Zhang Y, Han J. The efficacy of atropine combined with orthokeratology in slowing axial elongation of myopia children: A meta-analysis. Eye Contact Lens. (2021) 47:98–103. doi: 10.1097/icl.0000000000000746
32. Wei XL, Wu T, Dang KR, Hu KK, Lu XT, Gong M, et al. Efficacy and safety of atropine at different concentrations in prevention of myopia progression in Asian children: a systematic review and Meta-analysis of randomized clinical trials. Int J Ophthalmol. (2023) 16:1326–36. doi: 10.18240/ijo.2023.08.20
33. Wang Z, Wang P, Jiang B, Meng Y, Qie S, Yan Z. The efficacy and safety of 0.01% atropine alone or combined with orthokeratology for children with myopia: A meta-analysis. PLoS One. (2023) 18:e0282286. doi: 10.1371/journal.pone.0282286
34. Tsai HR, Chen TL, Wang JH, Huang HK, Chiu CJ. Is 0.01% atropine an effective and safe treatment for myopic children? a systemic review and meta-analysis. J Clin Med. (2021) 10(17). doi: 10.3390/jcm10173766
35. Sun W, Hasebe S. Efficacy of 0.01% Atropine eye drops in controlling myopia progression and axial elongation in children: A meta-analysis based on randomized controlled trials. Acta Med Okayama. (2022) 76:457–63. doi: 10.18926/amo/63905
36. Fan HB, Tang XP, Zou YC, Yang LY, Tao J, Wang Y, et al. Meta-analysis of 0.01% Atropine for Asian adolescents and children of low and medium myopia in diopter and axial length. Int Eye Science. (2021) 21:854–60. doi: 10.3980/j.issn.1672-5123.2021.5.22. 文.
37. Zhang M, Qiu W, An M, Sun Y, Zhang Z, Yu C. Analysis of the reports of low-concentration atropine in controlling myopia in children. J Fr Ophtalmol. (2023) 46:239–48. doi: 10.1016/j.jfo.2022.09.024
38. Kumari S, Anand R, Tripathi S, Mishra RC, Jha SK. Efficacy of atropine for myopia control in children: A meta-analysis of randomized controlled trials. J Family Med Prim Care. (2022) 11:6668–77. doi: 10.4103/jfmpc.jfmpc_1339_22
39. Yam JC, Jiang Y, Tang SM, Law AKP, Chan JJ, Wong E, et al. Low-concentration atropine for myopia progression (LAMP) study: A randomized, double-blinded, placebo-controlled trial of 0.05%, 0.025%, and 0.01% Atropine eye drops in myopia control. Ophthalmology. (2019) 126:113–24. doi: 10.1016/j.ophtha.2018.05.029
40. Lee SS, Lingham G, Blaszkowska M, Sanfilippo PG, Koay A, FranChina M, et al. Low-concentration atropine eyedrops for myopia control in a multi-racial cohort of Australian children: A randomised clinical trial. Clin Exp Ophthalmol. (2022) 50:1001–12. doi: 10.1111/ceo.14148
41. Lu F, Chinese Optometric Association COS, Ophthalmology and Optometry Committee OA, Chinese Doctor Association, Ophthalmology and Optometry Group OC, Chinese Non-government Medical Institutions Association, et al. Expert consensus on myopia management white paper. Chin J Optom Ophthalmol Vis Sci. (2022) 24:9. doi: 10.3760/cma.j.cn115909-20220812-00321
Keywords: myopia, atropine, concentration, children, axial length, spherical equivalent refraction (SER)
Citation: Chen B, Ni Y, Chen J, Xing S and Zhang Z (2024) Comparison of different concentrations atropine in controlling children and adolescent myopia: an umbrella review of systematic reviews and meta-analyses. Front. Ophthalmol. 4:1447558. doi: 10.3389/fopht.2024.1447558
Received: 11 June 2024; Accepted: 29 September 2024;
Published: 23 October 2024.
Edited by:
Lei Wan, China Medical University, TaiwanReviewed by:
Hongfei Ye, Xinhua Hospital Affiliated to Shanghai Jiaotong University School of Medicine, ChinaJiahe Gan, Tongji University, China
Copyright © 2024 Chen, Ni, Chen, Xing and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhaotian Zhang, emhhbmd6aGFvdGlhbkBnenpvYy5jb20=
 Baizhou Chen
Baizhou Chen Yao Ni
Yao Ni Jinghan Chen2
Jinghan Chen2 Zhaotian Zhang
Zhaotian Zhang