- The First Affiliated Hospital of Chongqing Medical University, Chongqing Key Laboratory of Ophthalmology, Chongqing Eye Institute, and Chongqing Branch of National Clinical Research Center for Ocular Diseases, Chongqing, China
Behcet’s disease (BD) is one of the most vision-threatening clinical entities of uveitis. Although the etiopathogenesis of BD remains obscure, accumulating evidence has demonstrated that both genetic and environmental factors may contribute to the development of BD. Genome-wide association studies (GWAS) and candidate association studies have identified several genetic variants strongly associated with BD, including variants in human leukocyte antigen (HLA) -A02, -A03, -A24, -A26, -A31, -B15, -B27, -B35, -B49, -B51, -B57, -B58, -C0704, CIITA, ERAP1, MICA, IL1A-IL1B, IL10, IL12, IL23R, IL-23R/IL-12RB2, IL1RL1-IL18R1, STAT4, TFCP2L1, TRAF5, TNFAIP3, CCR1/CCR3, RIPK2, ADO-ZNF365-EGR2, KLRC4, LACC1, MEFV, IRF8, FUT2, CEBPB-PTPN1, ZMIZ1, RPS6KA4, IL10RA, SIPA1-FIBP-FOSL1, VAMP1, JRKL/CTCN5, IFNGR1 and miRNA-146a. Epigenetic modifications are also reported to play essential roles in the development of BD, including DNA methylation and histone modification. We review here the recent advances in the genetic and epigenetic factors associated with the BD pathogenesis.
Introduction
Uveitis is a group of inflammatory ocular diseases affecting the uveal tract, retina, and retinal blood vessels. It constitutes a number of visual impairments worldwide. Behcet’s disease (BD) is one of the most vision-threatening entities of uveitis. BD patients account for 16.5% of the total uveitis patients according to a Chinese study (1). BD is an autoinflammatory disorder that affects multiple systems, characterized by intraocular inflammation, arthritis, oral and genital ulcers and skin damages (2). While young adults aged 20-40 years are the commonest age group affected by BD, it still can be seen in children and older patients (3). Young male patients are reported to be affected more frequently and more severely than female patients (4). BD affects millions of people worldwide but mainly occurs along the ‘silk road’, with a higher incidence in Turkey, China, Japan and Iran, and a lower incidence in Europe and North America (5).
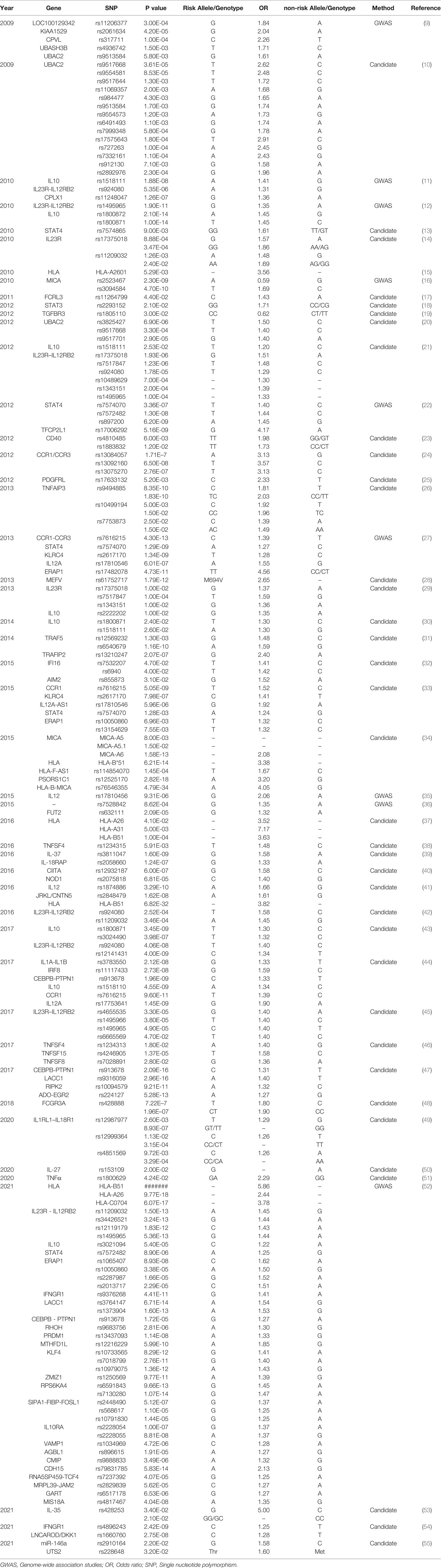
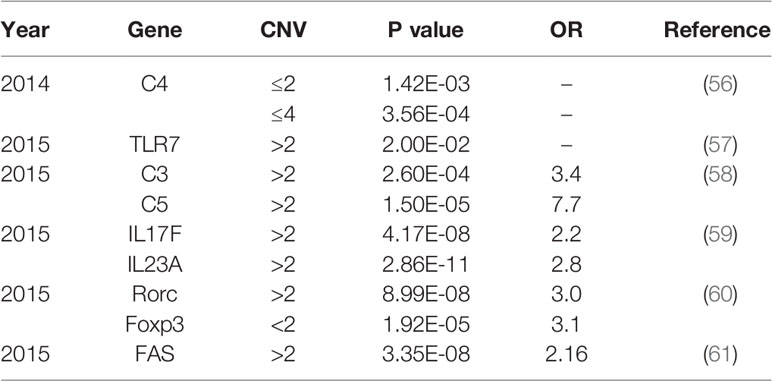
Approximately 70% of BD patients suffer from ocular involvement and the most common ocular finding is uveitis (6). BD patients with uveitis usually display recurrent and refractory ocular involvement, leading to severe visual impairments even blindness. However, the pathogenesis of BD is not entirely understood. Currently, genetic and environmental factors are considered to be responsible for the development of BD. A previous study comprising 21,940,795 individuals in 12 million families in Korea demonstrated that the familial incidence and risk of BD increased a lot among first degree relatives, especially in individuals with an affected twin (165-fold) (7). As a growing number of studies focus on the genetic predisposition for BD, multiple genetic variants associated with BD have been identified (8). Particularly, susceptibility gene for BD can be divided into human leucocyte antigen (HLA) and non-HLA. Identified genetic variants for BD include single nucleotide polymorphisms (SNPs) (Table 1) and copy number variations (CNVs) (Table 2), with variants ranging from single base to large fragment changes. Epigenetic modifications are also reported to be important in the development of BD, including DNA methylation and histone modification (62). The present review aims to summarize the latest genetic and epigenetic progress on BD.
Variants in Human Leucocyte Antigen and Its Related Genes
HLA is located on the chromosome 6p21.3 and functions to encode various important proteins of immune responses. HLA antigen plays a critical role in self/nonself-discrimination by presenting antigens to T cells and eliminating pathogenic microorganisms such as bacteria and viruses (63). Multiple HLA alleles have been identified to be associated with various autoimmune diseases, especially BD in different populations. HLA-A02, -A24, -A26, -A31, -B27, -B51, -B57 were considered to be BD-risk alleles, while HLA-A03, -B15, -B35, -B49, -B58 were thought to be BD-protective (15, 37, 64, 65). HLA-B51 has been recognized as the most significant susceptibility gene for BD in a large number of GWAS and candidate association studies, and it was carried in about 60% of BD patients (22, 41, 66, 67). Moreover, HLA-B51 carriers were more prone to developing ocular manifestations of BD, which was observed towards the east along the Silk Road in Eurasia rather than west-Eurasian (68). However, HLA-B51 was only responsible for 19% of the genetic susceptibility to BD (69). Recently, we discovered HLA-C0704 to be associated with BD in a GWAS study involving a Chinese Han population, including eight independent SNPs at the genome-wide level (52).
Meanwhile, multiple HLA-related gene variants also play essential roles in BD, including variants in the class II major histocompatibility complex transactivator (CIITA), endoplasmic Reticulum Aminopeptidase 1 (ERAP1) and the major histocompatibility complex class I chain related gene A (MICA) (64). CIITA is an important transcriptional coactivator that modulates expression of MHC class II genes, IL-4 and IL-10 (70). In a Chinese Han population, the CIITA SNP rs12932187 G allele and GG genotype were identified to be involved in BD (40). The expression of CIITA was increased while that of IL-10 was decreased in peripheral blood monocytes (PBMC) stimulated by Lipopolysaccharide (LPS) of GG carriers (40).
ERAP1 is an important enzyme that trims peptides for binding onto MHC class I molecules. SNP rs17482078 in ERAP1 was found as a risk factor for BD in a Turkish GWAS and the finding was replicated in Iranian (27, 33). Moreover, SNP rs10050860, rs1065407, rs2287987 and rs2013717 in ERAP1 were also associated with BD in a Chinese GWAS study (52).
MICA is a highly polymorphic non-classic HLA gene that modulates immune responses by binding to its receptors on natural killer (NK) cells, CD8 T cells, and γδT cells (63, 71, 72). The DNA sequence of MICA is highly polymorphic. A tri-nucleotide microsatellite polymorphism (GCT/AGC) n was identified in the MICA transmembrane region designated as An (A4, A5, A5.1, A6, A9) allele (63). Among them, MICA-A6 conferred risk to BD while the rest alleles were thought to be protective, especially in the Middle East and East Asia (71, 73). MICA*009 and MICA* 019 were identified as risk alleles for BD involving a Spanish population (71, 74). However, a recent study in a Han Chinese population showed that no significant difference of MICA*009 polymorphisms were observed between the BD patients and controls, which indicated genetic heterogeneity of MICA gene polymorphisms in different populations (75). Additionally, MICA*049 was firstly reported to have an association with BD in this study, and this association was suggested to be independent from that with HLA-B51. The MICA*009 allele was probably mixed with the MICA*049 allele previously, since the only difference between the MICA*00901(a subtype of MICA*009) and the MICA*049 existed at codon 335 in exon 6 which was not studied. The MICA*009 was distinguished from the MICA*049 by T-ARMS-PCR in this study (75). Consistent with previous studies, the MICA*A6 was strongly associated with BD Chinese population.
Single Nucleotide Polymorphisms in Interleukin Family Genes
Interleukin-10 (IL-10) is a widely expressed cytokine that contributes to preventing inflammatory and autoimmune pathologies. Multiple cells of innate and adaptive immune system exert important functions in BD, including CD4+T cells (76, 77), CD8+ T cells (78), dendritic cells (79), macrophages (80), NK cells (81), neutrophils (82) and B cells (83, 84), and almost all of them could express IL-10 (85). IL-10 was identified as risk locus for many inflammatory diseases, including bacterial sepsis (86), type I diabetes (87), mixed connective tissue disease (MCTD) (88), ankylosing spondylitis (AS) (89) and especially BD (9, 11, 12, 90).
Multiple SNPs of IL-10 were confirmed to be strongly associated with BD. Residing in the intron 3 of IL-10, the SNP rs1554286 was highly associated with BD in Japanese populations (12). A meta-analysis of IL-10 with additional studies in Turkish and Korean cohorts identified that IL-10 locus rs1800872 (P=2.1×10−14) and rs1800871 (P=1.0×10−14) had genome-wide significant associations with BD (12). Association between rs1518111 polymorphism of IL-10 and risk of BD was identified by multiple studies in various populations (11, 27, 30, 34). A recent GWAS study of Chinese BD uveitis involving 978 patients and, 4388 controls also confirmed the risk factor role of the IL-10 variant (rs3021094) (52). In addition, two novel loci of IL10RA (rs2228054 and rs2228055) showed genome-wide significant association with BD uveitis in this study.
Recently, some studies demonstrated how GWAS-identified IL-10 loci contributed to BD progression. IL-10 locus rs1518111 was associated with reduced IL-10 expression, which was a risk factor for BD (11, 12). A previous study proposed that IL-10 loci polymorphism was associated with impaired M2 macrophage (anti-inflammatory) function while promoting M1 macrophage (proinflammatory)-mediated inflammation in BD (91). Our study has identified rs3024490 and rs1800871 of IL-10 as susceptibility loci for BD in Chinese patients. PBMCs of rs3024490/TT genotype carriers showed decreased IL-10 production as compared with GG carriers (43). Interestingly, we further found that the risk allele T of rs3024490, which was located in the enhancer elements of IL-10, may be involved in BD pathogenesis by affecting the binding of TBX1 to IL-10. Since decreased TBX1 expression was shown in BD patients, the binding of TBX1 to IL10 decreased and the expression of IL10 was downregulated. Therefore, the risk of developing BD increased (92). However, whether the genetic changes of TBX1 could directly influence BD remains unknown.
IL-12 is a proinflammatory cytokine which contributes to Th1 activation, NK-cell cytotoxicity and IFNγ production. IL-12 is composed of p40 subunit and p35 subunit. IL-23 is a cytokine that consists of p19 subunit and the common p40 subunit with IL-12. IL-23 functions to influence inflammatory macrophage and memory T cell via binding to IL-23 receptor (IL-23R). IL-12 and IL-23 were reported to be involved in autoimmune inflammations (93). A multi-center GWAS study carried out in Western Europeans, Middle Eastern and Turkish, reported the association between IL-12A/rs17810546 with BD (35). Such a finding was replicated in Iranian BD cohorts (33). The locus rs1874886 of IL-12 was found to be a BD susceptibility factor in Spaniards (41). Activation of IL-23/IL-17 pathway is important in many autoinflammatory diseases, including BD (77). IL-12Rβ1 encodes the common subunit of IL23R and IL-12R, while other subunits are encoded by IL23R and IL12RB2 respectively. Stimulated by IL-23 and IL-12, IL-23R and the IL-12RB play important roles in naïve CD4+T cell differentiation and promote the expression of IL-1β, IL-6, IL-17A and TNFα (94). Multiple SNPs (rs12119179, rs4655535, rs924080, rs11209032, rs1343151, rs1495965, rs1495966, rs17375018, rs7517847, rs34426521, 10489629 and rs1966176) located in IL-23R/IL-12RB2 genes were involved in BD in the populations of Turkey (11), Japanese (12), Han Chinese (14, 42, 43), Iranian (21), Korean (45), Spain (29), and Western Algeria (95). However, little is known concerning the specific molecular mechanisms how these gene variants affect the pathogenesis of BD. The specific roles of these identified SNPs are warranted to be explored in further studies.
IL1RL1–IL18R1 region contains a list of genes involved in immune responses, including IL1RL2, IL1RL1, IL18R1, and IL18RAP. These genes contribute to T cells differentiation and cytokine production, such as TNF-α, IL-17A and IL-23 (96–98). Recent studies showed that IL1RL1–IL18R1 region was involved in various immune-mediated diseases, such as asthma, atopic dermatitis and BD (99, 100). Genetic ablation of IL18R1 or blockade of IL-18 receptor signaling could protect mice from autoimmune disease (98, 101).Three SNPs (rs12999364, rs12987977, and rs4851569) located in the IL1RL1–IL18R1 region were associated with ocular manifestations in Chinese BD patients. Among them, rs12987977 showed the strongest association with BD (P value=8.93e10−7, OR=0.39). Further investigations demonstrated that rs12987977/GG genotype resulted in decreased production of IFN-γ or TNF-α, indicating its anti-inflammatory role (49).
Single Nucleotide Polymorphisms in MicroRNA
MicroRNA (MiRNA), a member of noncoding RNAs, functions in regulating gene expression by inducing cleavage, degradation, or block translation of messenger RNA. Over the last decade, multiple studies showed that miRNAs participate in the post-transcriptional gene regulation of BD and could be used as diagnostic biomarkers (102, 103). In an Iranian population, a significantly increased expression of miR-155 was observed in the PBMCs of BD patients as compared with controls (104). Jadideslam et al. confirmed that in BD patients from Iran the production of miR-21 and miR-146b decreased significantly, while the production of miR-326 increased significantly. MiR-326 expression level was proposed to be a biomarker to predict severe ocular involvement in BD patients (105). In addition, higher proportions of the G/G genotype and G allele of miR-146a rs2910164 variant were found in BD patients as compared with the healthy (55). Other altered expression of miR-25, miR-106b, miR-326 and miR-93, miR-182, miR-638, and miR-4488 has also been described in BD (106–109). However, the specific mechanism how the variants in MiRNA confer risk to BD remains unknown.
Single Nucleotide Polymorphisms in Other Genes
The Janus kinase (JAK)/signal transducers and activators of transcription (STAT) pathway exerts important functions in multiple immune-mediated diseases. Many clinical trials made progress in treating autoimmune diseases using JAK inhibitors, including rheumatoid arthritis (110), psoriatic arthritis (111), ulcerative colitis (112) and psoriasis (113). Several case reports also indicated that targeting JAK/STAT pathway could alleviate the progression of uveitis (114–116). Tofacitinib, a JAK1/3 inhibitor targeting T cell signaling, was shown to be effective for BD patients (117). Our previous study demonstrated that multiple SNPs of JAK1 contributed to the genetic susceptibility of BD with ocular involvement, including rs2780815, rs310241, rs3790532 (118). STAT4 belongs to the STAT family that regulate s gene transcription in response to type I interferon (IFN-I) and various cytokines of IL family (119). STAT4 has been implicated in T-helper cell differentiation, natural killer (NK) cell activation and IFNγ production and contributes to multiple inflammation and autoimmune diseases (119, 120). Recent studies have identified many susceptibility loci of STAT4 in a variety of diseases including rheumatoid arthritis (RA) (121, 122), systemic lupus erythematosus (SLE) (121, 122) and BD (13). A GWAS study initially reported susceptibility locus of rs7574070 STAT4 for BD in Turkish populations, which was replicated in Japanese, Chinese and Iranian populations (22, 27, 33). We found that SNPs rs7572482 and rs897200 in STAT4 were also shown to be associated with BD in a Han Chinese GWAS (22). Among them, the rs897200 risk genotype AA was associated with higher expression of STAT4 and severer clinical symptoms including. This GWAS in Han Chinese population totally identified 10 non-HLA SNPs which showed genome-wide significant associations with BD, including rs17006292 in TFCP2L1 for the first time (22).
TRAF5, a polymorphic gene, is a closely related member of tumor necrosis factor-receptor-associated factors (TRAF) family. It was identified to be associated with various autoimmune diseases including inflammatory bowel disease, diabetes and rheumatoid arthritis (31, 123). In, 2013, a case-control study in Han Chinese descent found that SNP rs12569232, rs10863888 and rs6540679 in TRAF5 conferred susceptibility to BD. Additionally, further investigations showed that TRAF5 gene polymorphisms contributed to regulating TRAF5 production as well as downstream inflammatory cytokines including TNF-α and IL-6 (31). Recently, some SNPs were shown to regulate the expression of long non-coding RNAs (lincRNAs) and then influence disease progression. The SNP rs12569232–residing in the non-coding site of the intergenic region between linc00467 and TRAF5 may affect the pathogenesis of BD through increasing the expression of linc00467. Moreover, the study suggested that overexpression of linc00467 enhanced cell viability of CD4+T cells, which was essential to immune response in BD patients (124).
Recently, SNP rs9494885 in the tumor necrosis factor alpha-inducible protein 3 (TNFAIP3) was also identified to be genome-wide significantly associated with BD susceptibility in a Chinese Han population (26). However, no differences in TNFAIP3 expression was shown in different genotypes of rs9494885 (26). Therefore, SNP rs9494885 conferred risk to BD probably through an unknown mechanism rather than directly regulating TNFAIP3 expression.
With a high prevalence of BD in Turkey, studies have reported various novel susceptibility SNPs with genome-wide significance for BD in Turkey, including rs7616215 in CCR1, rs2230801, rs10094579 in RIPK2, rs224127, rs1509966 in ADO-ZNF365-EGR2, rs2617170 in KLRC4, rs2121033 in LACC1, rs61752717 in MEFV, rs7203487, rs142105922, rs11117433 in IRF8, rs681343 in FUT2, rs913678 in CEBPB-PTPN1 (27, 28, 44). Many of those were replicated by Iranian, Japanese and Han Chinese (33, 36, 47, 64).
To demonstrate genetic association between the CCR1/CCR3 gene and BD, we carried out a two-stage case control study in Chinese Han population. The combined studies showed that three SNPs (rs13092160, rs13084057, and rs13075270) conferred risk to BD. Among them, rs13092160 reached the GWAS significance threshold (P value<5e10−8) (24). Additionally, our recent GWAS study in Chinese BD confirmed 7 previously reported variants and proposed 22 novel susceptibility loci in the non-HLA region. The genome-wide significant associations of ZMIZ1, IL10RA, RPS6KA4, SIPA1-FIBP-FOSL1 and VAMP1 with BD were identified (52). Another Chinese candidate association study showed that rs9316059 in LACC1 has an association with BD at the genome-wide level of significance (47). Additionally, a Spanish study identified a novel genetic marker for BD, SNP rs2848479 in JRKL/CTCN5, with a genome-wide association (41).
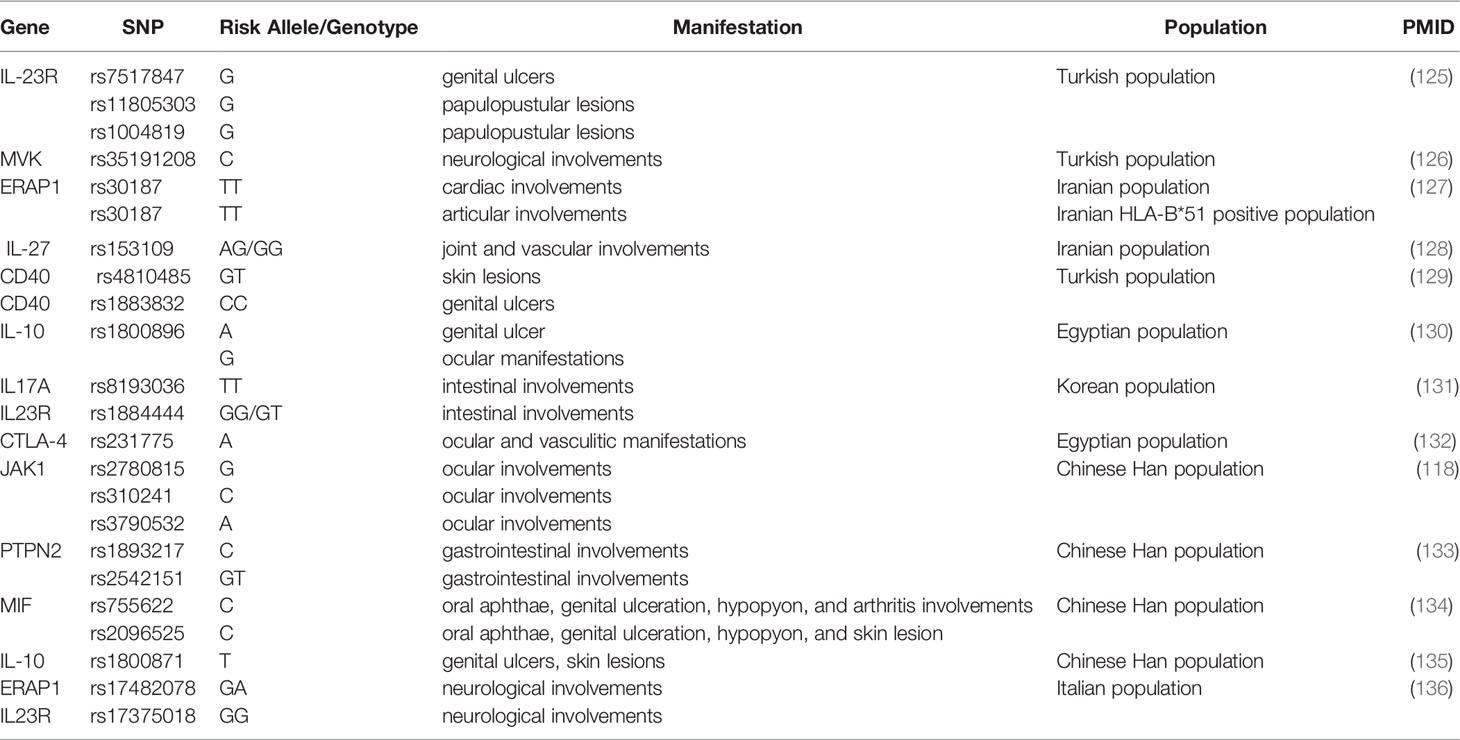
Besides confirming six previously reported susceptibility variants in BD, a study of 9,444 patients and controls from seven different populations identified two novel genome-wide significant loci: rs4896243 in IFNGR1 and rs1660760 within the intergenic region of LNCAROD/DKK1 (54). Another variant in IFNGR1, rs9376268, was also shown to be involved in BD in the Chinese GWAS (52). IFNGR1 is the gene encoding the ligand-binding chain (alpha) of the interferon-gamma receptor, playing an essential role in cell immune response in BD (52). As a growing number of genetic variants have been identified with susceptibility to BD, various studies showed the correlation between genetic variants and specific manifestations of BD (Table 3).
Copy Number Variations
Recently, an increasing number of studies showed that the main cause of structural variation in the genome is copy number variation (CNV). It represents large fragments of DNA sequence variation, involving insertions, duplications, and deletions of sequences (8, 137). CNV significantly contributes to human genetic diversity by influencing the gene expression. Therefore such variations have been found to be associated with autoimmune disorders including BD (56), SLE (138), RA (139) and Grave’s disease (140).
Since complement activation is involved in various immune-mediated diseases, the association of CNV in complements with BD is worthy to study. A previous study detected the copy number of C4 in 905 patients with BD, 205 patients with ankylosing spondylitis (AS) and acute anterior uveitis (AAU), and 1,238 controls by real-time PCR test. A significantly higher ratio of more than 2 copies of C4A patients were observed in BD. However, there was no significance in AS and AAU patients. Functional studies suggested that the high copy number of C4a could moderate C4a expression and promote IL-6 production, which was regarded as a risk factor for BD (56). Additionally, BD patients showed a remarkable high frequency of covering over two copies of C3 and C5 (58).
Toll-like receptors (TLRs), members of pattern-recognition receptors (PRRs), were indicated to be associated with the pathogenesis of various inflammatory or autoimmune human diseases (141, 142). In a Chinese Han population, a research unprecedent declared that the risk of BD could be increased by high copies of the TLR7 gene. TLR7 is located on the X chromosome. The frequencies of >1 copy of TLR7 in male BD patients and >2 copies in female patients were increased, suggested the difference between genders (57).
As a growing number of studies discovered apoptosis-related genes playing critical roles in various autoimmune diseases, recent research investigated whether the CNVs of these genes were associated with BD (61, 143–145). The high copy number of FAS gene, one of the apoptosis-related genes, was identified to be strongly associated with BD (61).In addition, frequencies of more than 2 copies of IL17F and IL23A were significantly increased in Chinese male BD patients as compared with controls (59). Other CNVs associated with BD include Rorc and Foxp3 gene variants (60).
Epigenetic Modifications
Epigenetics usually refer to the heritable changes in gene expression without the DNA sequences alterations (102). It contributes to control gene expression and modulate cell development, differentiation, and activity (146). Epigenetic modifications, containing DNA methylation and histone modification, are considered to be associated with the pathogenesis of BD (62).
DNA methylation is universally considered as the main epigenetic modification of autoimmune diseases. DNA methylation is the covalent addition of methyl groups to the fifth carbon (C5) in the cytosine base, which may regulate physiological and pathological processes (102). A study found a high expression level of IL-6 gene in BD patients with decreased promoter methylation level of the IL-6 mRNA. The change in the methylation level of the IL-6 may be associated with susceptibility to BD (147). Additionally, expression level of the IL-10 gene decreased in BD patients with a high promoter methylation level. Interestingly, the high IL-10 methylation level is significantly associated with severe symptoms in BD patients (148).
An epigenome-wide association study (EWAS) showed, 4332 BD-related differentially methylated CpG sites in Han Chinese. Among them, five methylated sites (cg03546163, cg25114611, cg20228731, cg23261343, and cg14290576) in four genes (FKBP5, FLJ43663, RUNX2, and NFIL3) were significantly hypomethylated. Especially, the study discovered significant overexpression of FKBP5 mRNA with the hypomethylation at cg03546163 and cg25114611 in FKBP5 (149).
Histone modifications, including acetylation, methylation, SUMOylation, and ubiquitination, could influence the chromatin structure and gene expression (62). Sirtuin 1 (Sirt1), a histone deacetylase, which could inhibit NF-κB transcription and T-cell proliferation, promote TNF-α induced apoptosis and affect immune tolerance through regulating histone acetylation, was considered as a risk factor for BD with uveitis (150). However, future studies are needed for the full comprehension how epigenetic modifications confer susceptibility to BD.
Conclusion
Uveitis is a sight-threatening inflammatory ocular disease, among which BD is one of the most common and complex entities. Although the pathogenesis of BD is not entirely understood, specific HLA types and gene variants in non-HLA regions have been identified to contribute to the susceptibility of BD. Specifically, SNPs as well as CNVs play critical roles in the gene variants. In addition, epigenetic modifications including DNA methylation and histone modification were also implicated in the development of BD. However, the identified gene variants still account for a small part of the pathogenesis of BD. A large number of BD-related gene variants have yet to be explored.
Author Contributions
YG, ZZ, and PY conceived the structure of manuscript. YG drafted initial manuscript. YG and ZZ made the tables. PY and ZZ revised this manuscript. All authors contributed to the article and approved the submitted version.
Funding
The work was supported by the Chongqing Key Laboratory of Ophthalmology (CSTC, 2008CA5003), Chongqing Outstanding Scientists Project (2019), Chongqing Chief Medical Scientist Project (2018), Chongqing Science & Technology Platform and Base Construction Program (cstc2014pt-sy10002).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Yang P, Zhang Z, Zhou H, Li B, Huang X, Gao Y, et al. Clinical Patterns and Characteristics of Uveitis in a Tertiary Center for Uveitis in China. Curr Eye Res (2005) 30(11):943–8. doi: 10.1080/02713680500263606
2. Sakane T, Takeno M, Suzuki N, Inaba G. Behcet’s Disease. N Engl J Med (1999) 341(17):1284–91. doi: 10.1056/NEJM199910213411707
3. Kone-Paut I. Behcet’s Disease in Children, an Overview. Pediatr Rheumatol Online J (2016) 14(1):10. doi: 10.1186/s12969-016-0070-z
4. Mat MC, Sevim A, Fresko I, Tuzun Y. Behcet’s Disease as a Systemic Disease. Clin Dermatol (2014) 32(3):435–42. doi: 10.1016/j.clindermatol.2013.11.012
5. Mahr A, Maldini C. Epidemiology of Behcet’s Disease. Rev Med Interne (2014) 35(2):81–9. doi: 10.1016/j.revmed.2013.12.005
6. Yang P. Atlas of Uveitis. PR of China: Springer Nature Singapore Pte Ltd. and People’s Medical Publishing House (2021).
7. Ahn HS, Kim HJ, Kazmi SZ, Kang T, Jun JB, Kang MJ, et al. Familial Risk of Behcet’s Disease Among First-Degree Relatives: A Population-Based Aggregation Study in Korea. Rheumatol (Oxford) (2021) 60(6):2697–705. doi: 10.1093/rheumatology/keaa682
8. Hou S, Li N, Liao X, Kijlstra A, Yang P. Uveitis Genetics. Exp Eye Res (2020) 190:107853. doi: 10.1016/j.exer.2019.107853
9. Fei Y, Webb R, Cobb BL, Direskeneli H, Saruhan-Direskeneli G, Sawalha AH. Identification of Novel Genetic Susceptibility Loci for Behcet’s Disease Using a Genome-Wide Association Study. Arthritis Res Ther (2009) 11(3):R66. doi: 10.1186/ar2695
10. Sawalha AH, Hughes T, Nadig A, Yilmaz V, Aksu K, Keser G, et al. A Putative Functional Variant Within the UBAC2 Gene is Associated With Increased Risk of Behcet’s Disease. Arthritis Rheumatol (2011) 63(11):3607–12. doi: 10.1002/art.30604
11. Remmers EF, Cosan F, Kirino Y, Ombrello MJ, Abaci N, Satorius C, et al. Genome-Wide Association Study Identifies Variants in the MHC Class I, IL10, and IL23R-IL12RB2 Regions Associated With Behcet’s Disease. Nat Genet (2010) 42(8):698–702. doi: 10.1038/ng.625
12. Mizuki N, Meguro A, Ota M, Ohno S, Shiota T, Kawagoe T, et al. Genome-Wide Association Studies Identify IL23R-IL12RB2 and IL10 as Behcet’s Disease Susceptibility Loci. Nat Genet (2010) 42(8):703–6. doi: 10.1038/ng.624
13. Hu K, Yang P, Jiang Z, Hou S, Du L, Li F. STAT4 Polymorphism in a Chinese Han Population With Vogt-Koyanagi-Harada Syndrome and Behcet’s Disease. Hum Immunol (2010) 71(7):723–6. doi: 10.1016/j.humimm.2010.04.007
14. Jiang Z, Yang P, Hou S, Du L, Xie L, Zhou H, et al. IL-23R Gene Confers Susceptibility to Behcet’s Disease in a Chinese Han Population. Ann Rheum Dis (2010) 69(7):1325–8. doi: 10.1136/ard.2009.119420
15. Kaburaki T, Takamoto M, Numaga J, Kawashima H, Araie M, Ohnogi Y, et al. Genetic Association of HLA-A*2601 With Ocular Behcet’s Disease in Japanese Patients. Clin Exp Rheumatol (2010) 28(4 Suppl 60):S39–44.
16. Meguro A, Inoko H, Ota M, Katsuyama Y, Oka A, Okada E, et al. Genetics of Behcet Disease Inside and Outside the MHC. Ann Rheum Dis (2010) 69(4):747–54. doi: 10.1136/ard.2009.108571
17. Li K, Zhao M, Hou S, Du L, Kijlstra A, Yang P. Association Between Polymorphisms of FCRL3, a non-HLA Gene, and Behcet’s Disease in a Chinese Population With Ophthalmic Manifestations. Mol Vis (2008) 14:2136–42.
18. Hu K, Hou S, Jiang Z, Kijlstra A, Yang P. JAK2 and STAT3 Polymorphisms in a Han Chinese Population With Behcet’s Disease. Invest Ophthalmol Vis Sci (2012) 53(1):538–41. doi: 10.1167/iovs.11-8440
19. Chen Y, Yang P, Li F, Hou S, Jiang Z, Shu Q, et al. Association Analysis of TGFBR3 Gene With Vogt-Koyanagi-Harada Disease and Behcet’s Disease in the Chinese Han Population. Curr Eye Res (2012) 37(4):312–7. doi: 10.3109/02713683.2011.635398
20. Hou S, Shu Q, Jiang Z, Chen Y, Li F, Chen F, et al. Replication Study Confirms the Association Between UBAC2 and Behcet’s Disease in Two Independent Chinese Sets of Patients and Controls. Arthritis Res Ther (2012) 14(2):R70. doi: 10.1186/ar3789
21. Xavier JM, Shahram F, Davatchi F, Rosa A, Crespo J, Abdollahi BS, et al. Association Study of IL10 and IL23R-IL12RB2 in Iranian Patients With Behcet’s Disease. Arthritis Rheumatol (2012) 64(8):2761–72. doi: 10.1002/art.34437
22. Hou S, Yang Z, Du L, Jiang Z, Shu Q, Chen Y, et al. Identification of a Susceptibility Locus in STAT4 for Behcet’s Disease in Han Chinese in a Genome-Wide Association Study. Arthritis Rheumatol (2012) 64(12):4104–13. doi: 10.1002/art.37708
23. Chen F, Hou S, Jiang Z, Chen Y, Kijlstra A, Rosenbaum JT, et al. CD40 Gene Polymorphisms Confer Risk of Behcet’s Disease But Not of Vogt-Koyanagi-Harada Syndrome in a Han Chinese Population. Rheumatol (Oxford). (2012) 51(1):47–51. doi: 10.1093/rheumatology/ker345
24. Hou S, Xiao X, Li F, Jiang Z, Kijlstra A, Yang P. Two-Stage Association Study in Chinese Han Identifies Two Independent Associations in CCR1/CCR3 Locus as Candidate for Behcet’s Disease Susceptibility. Hum Genet (2012) 131(12):1841–50. doi: 10.1007/s00439-012-1200-4
25. Hou S, Xiao X, Zhou Y, Zhu X, Li F, Kijlstra A, et al. Genetic Variant on PDGFRL Associated With Behcet Disease in Chinese Han Populations. Hum Mutat (2013) 34(1):74–8. doi: 10.1002/humu.22208
26. Li H, Liu Q, Hou S, Du L, Zhou Q, Zhou Y, et al. TNFAIP3 Gene Polymorphisms Confer Risk for Behcet’s Disease in a Chinese Han Population. Hum Genet (2013) 132(3):293–300. doi: 10.1007/s00439-012-1250-7
27. Kirino Y, Bertsias G, Ishigatsubo Y, Mizuki N, Tugal-Tutkun I, Seyahi E, et al. Genome-Wide Association Analysis Identifies New Susceptibility Loci for Behcet’s Disease and Epistasis Between HLA-B*51 and ERAP1. Nat Genet (2013) 45(2):202–7. doi: 10.1038/ng.2520
28. Kirino Y, Zhou Q, Ishigatsubo Y, Mizuki N, Tugal-Tutkun I, Seyahi E, et al. Targeted Resequencing Implicates the Familial Mediterranean Fever Gene MEFV and the Toll-Like Receptor 4 Gene TLR4 in Behcet Disease. Proc Natl Acad Sci USA (2013) 110(20):8134–9. doi: 10.1073/pnas.1306352110
29. Montes-Cano MA, Conde-Jaldon M, Garcia-Lozano JR, Ortiz-Fernandez L, Ortego-Centeno N, Castillo-Palma MJ, et al. HLA and Non-HLA Genes in Behcet’s Disease: A Multicentric Study in the Spanish Population. Arthritis Res Ther (2013) 15(5):R145. doi: 10.1186/ar4328
30. Wu Z, Zheng W, Xu J, Sun F, Chen H, Li P, et al. IL10 Polymorphisms Associated With Behcet’s Disease in Chinese Han. Hum Immunol (2014) 75(3):271–6. doi: 10.1016/j.humimm.2013.11.009
31. Xiang Q, Chen L, Hou S, Fang J, Zhou Y, Bai L, et al. TRAF5 and TRAF3IP2 Gene Polymorphisms are Associated With Behcet’s Disease and Vogt-Koyanagi-Harada Syndrome: A Case-Control Study. PloS One (2014) 9(1):e84214. doi: 10.1371/journal.pone.0084214
32. Ortiz-Fernandez L, Garcia-Lozano JR, Montes-Cano MA, Conde-Jaldon M, Ortego-Centeno N, Garcia-Hernandez FJ, et al. Variants of the IFI16 Gene Affecting the Levels of Expression of mRNA are Associated With Susceptibility to Behcet Disease. J Rheumatol (2015) 42(4):695–701. doi: 10.3899/jrheum.140949
33. Sousa I, Shahram F, Francisco D, Davatchi F, Abdollahi BS, Ghaderibarmi F, et al. Brief Report: Association of CCR1, KLRC4, IL12A-AS1, STAT4, and ERAP1 With Behcet’s Disease in Iranians. Arthritis Rheumatol (2015) 67(10):2742–8. doi: 10.1002/art.39240
34. Carapito R, Shahram F, Michel S, Le Gentil M, Radosavljevic M, Meguro A, et al. On the Genetics of the Silk Route: Association Analysis of HLA, IL10, and IL23R-IL12RB2 Regions With Behcet’s Disease in an Iranian Population. Immunogenetics (2015) 67(5-6):289–93. doi: 10.1007/s00251-015-0841-6
35. Kappen JH, Medina-Gomez C, van Hagen PM, Stolk L, Estrada K, Rivadeneira F, et al. Genome-Wide Association Study in an Admixed Case Series Reveals IL12A as a New Candidate in Behcet Disease. PloS One (2015) 10(3):e0119085. doi: 10.1371/journal.pone.0119085
36. Xavier JM, Shahram F, Sousa I, Davatchi F, Matos M, Abdollahi BS, et al. FUT2: Filling the Gap Between Genes and Environment in Behcet’s Disease? Ann Rheum Dis (2015) 74(3):618–24. doi: 10.1136/annrheumdis-2013-204475
37. Al-Okaily F, Al-Rashidi S, Al-Balawi M, Mustafa M, Arfin M, Al-Asmari A. Genetic Association of HLA-A*26, -A*31, and -B*51 With Behcet’s Disease in Saudi Patients. Clin Med Insights Arthritis Musculoskelet Disord (2016) 9:167–73. doi: 10.4137/CMAMD.S39879
38. Lu S, Song S, Hou S, Li H, Yang P. Association of TNFSF4 Polymorphisms With Vogt-Koyanagi-Harada and Behcet’s Disease in Han Chinese. Sci Rep (2016) 6:37257. doi: 10.1038/srep37257
39. Tan H, Deng B, Yu H, Yang Y, Ding L, Zhang Q, et al. Genetic Analysis of Innate Immunity in Behcet’s Disease Identifies an Association With IL-37 and IL-18rap. Sci Rep (2016) 6:35802. doi: 10.1038/srep35802
40. Li L, Yu H, Jiang Y, Deng B, Bai L, Kijlstra A, et al. Genetic Variations of NLR Family Genes in Behcet’s Disease. Sci Rep (2016) 6:20098. doi: 10.1038/srep20098
41. Ortiz-Fernandez L, Carmona FD, Montes-Cano MA, Garcia-Lozano JR, Conde-Jaldon M, Ortego-Centeno N, et al. Genetic Analysis With the Immunochip Platform in Behcet Disease. Identification of Residues Associated in the HLA Class I Region and New Susceptibility Loci. PloS One (2016) 11(8):e0161305. doi: 10.1371/journal.pone.0161305
42. Qin X, Xu J, Wu Z, Sun F, Chen H, Zheng W, et al. Association Study of Rs924080 and Rs11209032 Polymorphisms of IL23R-IL12RB2 in a Northern Chinese Han Population With Behcet’s Disease. Hum Immunol (2016) 77(12):1284–90. doi: 10.1016/j.humimm.2016.09.006
43. Yu H, Zheng M, Zhang L, Li H, Zhu Y, Cheng L, et al. Identification of Susceptibility SNPs in IL10 and IL23R-IL12RB2 for Behcet’s Disease in Han Chinese. J Allergy Clin Immunol (2017) 139(2):621–7. doi: 10.1016/j.jaci.2016.05.024
44. Takeuchi M, Mizuki N, Meguro A, Ombrello MJ, Kirino Y, Satorius C, et al. Dense Genotyping of Immune-Related Loci Implicates Host Responses to Microbial Exposure in Behcet’s Disease Susceptibility. Nat Genet (2017) 49(3):438–43. doi: 10.1038/ng.3786
45. Kang EH, Kim S, Park MY, Choi JY, Choi IA, Kim MJ, et al. Behcet’s Disease Risk Association Fine-Mapped on the IL23R-IL12RB2 Intergenic Region in Koreans. Arthritis Res Ther (2017) 19(1):227. doi: 10.1186/s13075-017-1435-5
46. Jiang Y, Cheng L, Li X, Zhou W, Zhang L. Associations Between TNFSF4, TNFSF8 and TNFSF15 and Behcet’s Disease But Not VKH Syndrome in Han Chinese. Oncotarget. (2017) 8(62):105037–46. doi: 10.18632/oncotarget.22064
47. Wu P, Du L, Hou S, Su G, Yang L, Hu J, et al. Association of LACC1, CEBPB-PTPN1, RIPK2 and ADO-EGR2 With Ocular Behcet’s Disease in a Chinese Han Population. Br J Ophthalmol (2018) 102(9):1308–14. doi: 10.1136/bjophthalmol-2017-311753
48. Zhang D, Qin J, Li L, Su G, Huang G, Cao Q, et al. Analysis of the Association Between Fc Receptor Family Gene Polymorphisms and Ocular Behcet’s Disease in Han Chinese. Sci Rep (2018) 8(1):4850. doi: 10.1038/s41598-018-23222-8
49. Tan X, Zhou Q, Lv M, Tan H, Wang Q, Zhang L, et al. Functional Genetic Polymorphisms in the IL1RL1-IL18R1 Region Confer Risk for Ocular Behcet’s Disease in a Chinese Han Population. Front Genet (2020) 11:645. doi: 10.3389/fgene.2020.00645
50. Gholijani N, Daryabor G, Kalantar K, Yazdani MR, Shenavandeh S, Zahed M, et al. Interleukin-27 Gene Variant Rs153109 is Associated With Enhanced Cytokine Serum Levels and Susceptibility to Behcet’s Disease in the Iranian Population. Eur Cytokine Netw (2020) 31(4):140–6. doi: 10.1684/ecn.2020.0458
51. Padula MC, Leccese P, Lascaro N, Radice RP, Limongi AR, Sorrento GG, et al. Correlation of Tumor Necrosis Factor-Alpha -308G>A Polymorphism With Susceptibility, Clinical Manifestations, and Severity in Behcet Syndrome: Evidences From an Italian Genetic Case-Control Study. DNA Cell Biol (2020) 39(7):1104–10. doi: 10.1089/dna.2020.5361
52. Su G, Zhong Z, Zhou Q, Du L, Ye Z, Li F, et al. Identification of Novel Risk Loci for Behcet’s Disease-Related Uveitis in a Chinese Population in a Genome-Wide Association Study. Arthritis Rheumatol (2022) 74(4):671–81. doi: 10.1002/art.41998
53. Feng M, Zhou S, Liu T, Yu Y, Su Q, Li X, et al. Association Between Interleukin 35 Gene Single Nucleotide Polymorphisms and the Uveitis Immune Status in a Chinese Han Population. Front Immunol (2021) 12:758554. doi: 10.3389/fimmu.2021.758554
54. Ortiz Fernandez L, Coit P, Yilmaz V, Yentur SP, Alibaz-Oner F, Aksu K, et al. Genetic Association of a Gain-Of-Function IFNGR1 Polymorphism and the Intergenic Region LNCAROD/DKK1 With Behcet’s Disease. Arthritis Rheumatol (2021) 73(7):1244–52. doi: 10.1002/art.41637
55. Kamal A, Elgengehy FT, Elawady Z, Fawzy NA, El Sisi O. Role of miR-146a Rs2910164 and UTS2 Rs228648 Genetic Variants in Behcet’s Disease. Immunol Invest. (2021) 19: 1–10. doi: 10.1080/08820139.2021.1883647
56. Hou S, Qi J, Liao D, Zhang Q, Fang J, Zhou Y, et al. Copy Number Variations of Complement Component C4 are Associated With Behcet’s Disease But Not With Ankylosing Spondylitis Associated With Acute Anterior Uveitis. Arthritis Rheumatol (2013) 65(11):2963–70. doi: 10.1002/art.38116
57. Fang J, Chen L, Tang J, Hou S, Liao D, Ye Z, et al. Association Between Copy Number Variations of TLR7 and Ocular Behcet’s Disease in a Chinese Han Population. Invest Ophthalmol Vis Sci (2015) 56(3):1517–23. doi: 10.1167/iovs.14-15030
58. Xu D, Hou S, Zhang J, Jiang Y, Kijlstra A, Yang P. Copy Number Variations and Gene Polymorphisms of Complement Components in Ocular Behcet’s Disease and Vogt-Koyanagi-Harada Syndrome. Sci Rep (2015) 5:12989. doi: 10.1038/srep12989
59. Hou S, Liao D, Zhang J, Fang J, Chen L, Qi J, et al. Genetic Variations of IL17F and IL23A Show Associations With Behcet’s Disease and Vogt-Koyanagi-Harada Syndrome. Ophthalmology. (2015) 122(3):518–23. doi: 10.1016/j.ophtha.2014.09.025
60. Liao D, Hou S, Zhang J, Fang J, Liu Y, Bai L, et al. Copy Number Variants and Genetic Polymorphisms in TBX21, GATA3, Rorc, Foxp3 and Susceptibility to Behcet’s Disease and Vogt-Koyanagi-Harada Syndrome. Sci Rep (2015) 5:9511. doi: 10.1038/srep09511
61. Yu H, Luo L, Wu L, Zheng M, Zhang L, Liu Y, et al. FAS Gene Copy Numbers are Associated With Susceptibility to Behcet Disease and VKH Syndrome in Han Chinese. Hum Mutat (2015) 36(11):1064–9. doi: 10.1002/humu.22829
62. Ma X, Wang X, Zheng G, Tan G, Zhou F, Wei W, et al. Critical Role of Gut Microbiota and Epigenetic Factors in the Pathogenesis of Behcet’s Disease. Front Cell Dev Biol (2021) 9:719235. doi: 10.3389/fcell.2021.719235
63. Mizuki N, Ota M, Kimura M, Ohno S, Ando H, Katsuyama Y, et al. Triplet Repeat Polymorphism in the Transmembrane Region of the MICA Gene: A Strong Association of Six GCT Repetitions With Behcet Disease. Proc Natl Acad Sci USA (1997) 94(4):1298–303. doi: 10.1073/pnas.94.4.1298
64. Deng Y, Zhu W, Zhou X. Immune Regulatory Genes Are Major Genetic Factors to Behcet Disease: Systematic Review. Open Rheumatol J (2018) 12:70–85. doi: 10.2174/1874312901812010070
65. Hughes T, Coit P, Adler A, Yilmaz V, Aksu K, Duzgun N, et al. Identification of Multiple Independent Susceptibility Loci in the HLA Region in Behcet’s Disease. Nat Genet (2013) 45(3):319–24. doi: 10.1038/ng.2551
66. Yazici H, Chamberlain MA, Schreuder I, D’Amaro J, Muftuoglu M. HLA Antigens in Behcet’s Disease: A Reappraisal by a Comparative Study of Turkish and British Patients. Ann Rheum Dis (1980) 39(4):344–8. doi: 10.1136/ard.39.4.344
67. Gul A, Ohno S. HLA-B*51 and Behcet Disease. Ocul Immunol Inflamm (2012) 20(1):37–43. doi: 10.3109/09273948.2011.634978
68. Horie Y, Meguro A, Ohta T, Lee EB, Namba K, Mizuuchi K, et al. HLA-B51 Carriers are Susceptible to Ocular Symptoms of Behcet Disease and the Association Between the Two Becomes Stronger Towards the East Along the Silk Road: A Literature Survey. Ocul Immunol Inflamm (2017) 25(1):37–40. doi: 10.3109/09273948.2015.1136422
69. de Menthon M, Lavalley MP, Maldini C, Guillevin L, Mahr A. HLA-B51/B5 and the Risk of Behcet’s Disease: A Systematic Review and Meta-Analysis of Case-Control Genetic Association Studies. Arthritis Rheumatol (2009) 61(10):1287–96. doi: 10.1002/art.24642
70. Leon Machado JA, Steimle V. The MHC Class II Transactivator CIITA: Not (Quite) the Odd-One-Out Anymore Among NLR Proteins. Int J Mol Sci (2021) 22(3):1074. doi: 10.3390/ijms22031074
71. Zhang J, Liao D, Yang L, Hou S. Association Between Functional MICA-TM and Behcet’s Disease: A Systematic Review and Meta-Analysis. Sci Rep (2016) 6:21033. doi: 10.1038/srep21033
72. Groh V, Steinle A, Bauer S, Spies T. Recognition of Stress-Induced MHC Molecules by Intestinal Epithelial Gammadelta T Cells. Science. (1998) 279(5357):1737–40. doi: 10.1126/science.279.5357.1737
73. Wei F, Zhang YU, Li W. A Meta-Analysis of the Association Between Behcet’s Disease and MICA-A6. BioMed Rep (2016) 4(6):741–5. doi: 10.3892/br.2016.644
74. Fiorentino DF, Zlotnik A, Vieira P, Mosmann TR, Howard M, Moore KW, et al. IL-10 Acts on the Antigen-Presenting Cell to Inhibit Cytokine Production by Th1 Cells. J Immunol (1991) 146(10):3444–51.
75. Zhu W, Deng Y, Wang J, Guo X, Ding W, Chao J, et al. MICA*049, Not MICA*009, is Associated With Behcet’s Disease in a Chinese Population. Sci Rep (2019) 9(1):10856. doi: 10.1038/s41598-019-47289-z
76. Shimizu J, Takai K, Fujiwara N, Arimitsu N, Ueda Y, Wakisaka S, et al. Excessive CD4+ T Cells Co-Expressing Interleukin-17 and Interferon-Gamma in Patients With Behcet’s Disease. Clin Exp Immunol (2012) 168(1):68–74. doi: 10.1111/j.1365-2249.2011.04543.x
77. Zhong Z, Su G, Kijlstra A, Yang P. Activation of the Interleukin-23/Interleukin-17 Signalling Pathway in Autoinflammatory and Autoimmune Uveitis. Prog Retin Eye Res (2021) 80:100866. doi: 10.1016/j.preteyeres.2020.100866
78. Vural S, Kerl K, Ertop Dogan P, Vollmer S, Puchta U, He M, et al. Lesional Activation of Tc 17 Cells in Behcet Disease and Psoriasis Supports HLA Class I-Mediated Autoimmune Responses. Br J Dermatol (2021) 185(6):1209–20. doi: 10.1111/bjd.20643
79. Pay S, Simsek I, Erdem H, Pekel A, Musabak U, Sengul A, et al. Dendritic Cell Subsets and Type I Interferon System in Behcet’s Disease: Does Functional Abnormality in Plasmacytoid Dendritic Cells Contribute to Th1 Polarization? Clin Exp Rheumatol (2007) 25(4 Suppl 45):S34–40.
80. Hirahara L, Takase-Minegishi K, Kirino Y, Iizuka-Iribe Y, Soejima Y, Yoshimi R, et al. The Roles of Monocytes and Macrophages in Behcet’s Disease With Focus on M1 and M2 Polarization. Front Immunol (2022) 13:852297. doi: 10.3389/fimmu.2022.852297
81. Petrushkin H, Hasan MS, Stanford MR, Fortune F, Wallace GR. Behcet’s Disease: Do Natural Killer Cells Play a Significant Role? Front Immunol (2015) 6:134. doi: 10.3389/fimmu.2015.00134
82. Emmi G, Becatti M, Bettiol A, Hatemi G, Prisco D, Fiorillo C. Behcet’s Syndrome as a Model of Thrombo-Inflammation: The Role of Neutrophils. Front Immunol (2019) 10:1085. doi: 10.3389/fimmu.2019.01085
83. Suzuki N, Sakane T, Ueda Y, Tsunematsu T. Abnormal B Cell Function in Patients With Behcet’s Disease. Arthritis Rheumatol (1986) 29(2):212–9. doi: 10.1002/art.1780290209
84. Hetta HF, Mohamed AAA, Zahran AM, AM S, My Sayed M, Ga Saleh M, et al. Possible Role of Regulatory B Cells in Different Behcet’s Disease Phenotypes and Therapies: First Report From Egypt. J Inflammation Res (2021) 14:737–44. doi: 10.2147/JIR.S279912
85. Saraiva M, O’Garra A. The Regulation of IL-10 Production by Immune Cells. Nat Rev Immunol (2010) 10(3):170–81. doi: 10.1038/nri2711
86. Vivas MC, Villamarin-Guerrero HF, Sanchez CA. Interleukin-10 (IL-10) 1082 Promoter Polymorphisms and Plasma IL-10 Levels in Patients With Bacterial Sepsis. Rom J Intern Med (2021) 59(1):50–7. doi: 10.2478/rjim-2020-0033
87. Barrett JC, Clayton DG, Concannon P, Akolkar B, Cooper JD, Erlich HA, et al. Genome-Wide Association Study and Meta-Analysis Find That Over 40 Loci Affect Risk of Type 1 Diabetes. Nat Genet (2009) 41(6):703–7. doi: 10.1038/ng.381
88. Paradowska-Gorycka A, Jurkowska M, Czuszynska Z, Felis-Giemza A, Manczak M, Zdrojewski Z, et al. IL-10, IL-12B and IL-17 Gene Polymorphisms in Patients With Mixed Connective Tissue Disease. Mod Rheumatol (2015) 25(3):487–9. doi: 10.3109/14397595.2014.951143
89. Braga M, Lara-Armi FF, Neves JSF, Rocha-Loures MA, Terron-Monich MS, Bahls-Pinto LD, et al. Influence of IL10 (Rs1800896) Polymorphism and TNF-Alpha, IL-10, IL-17A, and IL-17f Serum Levels in Ankylosing Spondylitis. Front Immunol (2021) 12:653611. doi: 10.3389/fimmu.2021.653611
90. Wallace GR, Kondeatis E, Vaughan RW, Verity DH, Chen Y, Fortune F, et al. IL-10 Genotype Analysis in Patients With Behcet’s Disease. Hum Immunol (2007) 68(2):122–7. doi: 10.1016/j.humimm.2006.11.010
91. Nakano H, Kirino Y, Takeno M, Higashitani K, Nagai H, Yoshimi R, et al. GWAS-Identified CCR1 and IL10 Loci Contribute to M1 Macrophage-Predominant Inflammation in Behcet’s Disease. Arthritis Res Ther (2018) 20(1):124. doi: 10.1186/s13075-018-1613-0
92. Tan H, Su G, Tan X, Qin Y, Chen L, Yuan G, et al. SNP-Mediated Binding of TBX1 to the Enhancer Element of IL-10 Reduces the Risk of Behcet’s Disease. Epigenomics. (2021) 13(19):1523–37. doi: 10.2217/epi-2021-0215
93. Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, et al. Divergent Pro- and Antiinflammatory Roles for IL-23 and IL-12 in Joint Autoimmune Inflammation. J Exp Med (2003) 198(12):1951–7. doi: 10.1084/jem.20030896
94. Iwakura Y, Ishigame H. The IL-23/IL-17 Axis in Inflammation. J Clin Invest. (2006) 116(5):1218–22. doi: 10.1172/JCI28508
95. Khaib Dit Naib O, Aribi M, Idder A, Chiali A, Sairi H, Touitou I, et al. Association Analysis of IL10, TNF-Alpha, and IL23R-IL12RB2 SNPs With Behcet’s Disease Risk in Western Algeria. Front Immunol (2013) 4:342. doi: 10.3389/fimmu.2013.00342
96. Blumberg H, Dinh H, Dean C Jr., Trueblood ES, Bailey K, Shows D, et al. IL-1RL2 and its Ligands Contribute to the Cytokine Network in Psoriasis. J Immunol (2010) 185(7):4354–62. doi: 10.4049/jimmunol.1000313
97. Pastorelli L, Garg RR, Hoang SB, Spina L, Mattioli B, Scarpa M, et al. Epithelial-Derived IL-33 and its Receptor ST2 are Dysregulated in Ulcerative Colitis and in Experimental Th1/Th2 Driven Enteritis. Proc Natl Acad Sci USA (2010) 107(17):8017–22. doi: 10.1073/pnas.0912678107
98. Gutcher I, Urich E, Wolter K, Prinz M, Becher B. Interleukin 18-Independent Engagement of Interleukin 18 Receptor-Alpha is Required for Autoimmune Inflammation. Nat Immunol (2006) 7(9):946–53. doi: 10.1038/ni1377
99. Vonk JM, Nieuwenhuis MAE, Dijk FN, Boudier A, Siroux V, Bouzigon E, et al. Novel Genes and Insights in Complete Asthma Remission: A Genome-Wide Association Study on Clinical and Complete Asthma Remission. Clin Exp Allergy (2018) 48(10):1286–96. doi: 10.1111/cea.13181
100. Hirota T, Takahashi A, Kubo M, Tsunoda T, Tomita K, Sakashita M, et al. Genome-Wide Association Study Identifies Eight New Susceptibility Loci for Atopic Dermatitis in the Japanese Population. Nat Genet (2012) 44(11):1222–6. doi: 10.1038/ng.2438
101. Kinoshita K, Yamagata T, Nozaki Y, Sugiyama M, Ikoma S, Funauchi M, et al. Blockade of IL-18 Receptor Signaling Delays the Onset of Autoimmune Disease in MRL-Faslpr Mice. J Immunol (2004) 173(8):5312–8. doi: 10.4049/jimmunol.173.8.5312
102. Alipour S, Nouri M, Sakhinia E, Samadi N, Roshanravan N, Ghavami A, et al. Epigenetic Alterations in Chronic Disease Focusing on Behcet’s Disease: Review. BioMed Pharmacother. (2017) 91:526–33. doi: 10.1016/j.biopha.2017.04.106
103. Akbaba TH, Sag E, Balci-Peynircioglu B, Ozen S. Epigenetics for Clinicians From the Perspective of Pediatric Rheumatic Diseases. Curr Rheumatol Rep (2020) 22(8):46. doi: 10.1007/s11926-020-00912-9
104. Kolahi S, Farajzadeh MJ, Alipour S, Abhari A, Farhadi J, Bahavarnia N, et al. Determination of Mir-155 and Mir-146a Expression Rates and its Association With Expression Level of TNF-Alpha and CTLA4 Genes in Patients With Behcet’s Disease. Immunol Lett (2018) 204:55–9. doi: 10.1016/j.imlet.2018.10.012
105. Jadideslam G, Ansarin K, Sakhinia E, Babaloo Z, Abhari A, Alipour S, et al. Expression Levels of miR-21, miR-146b and miR-326 as Potential Biomarkers in Behcet’s Disease. biomark Med (2019) 13(16):1339–48. doi: 10.2217/bmm-2019-0098
106. Yu H, Liu Y, Bai L, Kijlstra A, Yang P. Predisposition to Behcet’s Disease and VKH Syndrome by Genetic Variants of miR-182. J Mol Med (Berl). (2014) 92(9):961–7. doi: 10.1007/s00109-014-1159-9
107. Woo MY, Yun SJ, Cho O, Kim K, Lee ES, Park S. MicroRNAs Differentially Expressed in Behcet Disease are Involved in Interleukin-6 Production. J Inflammation (Lond). (2016) 13:22. doi: 10.1186/s12950-016-0130-7
108. Karasneh J, Gul A, Ollier WE, Silman AJ, Worthington J. Whole-Genome Screening for Susceptibility Genes in Multicase Families With Behcet’s Disease. Arthritis Rheumatol (2005) 52(6):1836–42. doi: 10.1002/art.21060
109. Ahmadi M, Yousefi M, Abbaspour-Aghdam S, Dolati S, Aghebati-Maleki L, Eghbal-Fard S, et al. Disturbed Th17/Treg Balance, Cytokines, and miRNAs in Peripheral Blood of Patients With Behcet’s Disease. J Cell Physiol (2019) 234(4):3985–94. doi: 10.1002/jcp.27207
110. Aletaha D, Smolen JS. Diagnosis and Management of Rheumatoid Arthritis: A Review. JAMA. (2018) 320(13):1360–72. doi: 10.1001/jama.2018.13103
111. Mease P, Hall S, FitzGerald O, van der Heijde D, Merola JF, Avila-Zapata F, et al. Tofacitinib or Adalimumab Versus Placebo for Psoriatic Arthritis. N Engl J Med (2017) 377(16):1537–50. doi: 10.1056/NEJMoa1615975
112. Sandborn WJ, Ghosh S, Panes J, Vranic I, Su C, Rousell S, et al. Tofacitinib, an Oral Janus Kinase Inhibitor, in Active Ulcerative Colitis. N Engl J Med (2012) 367(7):616–24. doi: 10.1056/NEJMoa1112168
113. Papp KA, Menter MA, Abe M, Elewski B, Feldman SR, Gottlieb AB, et al. Tofacitinib, an Oral Janus Kinase Inhibitor, for the Treatment of Chronic Plaque Psoriasis: Results From Two Randomized, Placebo-Controlled, Phase III Trials. Br J Dermatol (2015) 173(4):949–61. doi: 10.1111/bjd.14018
114. Paley MA, Karacal H, Rao PK, Margolis TP, Miner JJ. Tofacitinib for Refractory Uveitis and Scleritis. Am J Ophthalmol Case Rep (2019) 13:53–5. doi: 10.1016/j.ajoc.2018.12.001
115. Bauermann P, Heiligenhaus A, Heinz C. Effect of Janus Kinase Inhibitor Treatment on Anterior Uveitis and Associated Macular Edema in an Adult Patient With Juvenile Idiopathic Arthritis. Ocul Immunol Inflamm (2019) 27(8):1232–4. doi: 10.1080/09273948.2019.1605453
116. Pyare R, Kaushik V, Dutta Majumder P, Biswas J. Tofacitinib in Recalcitrant Scleritis: First Case Report From India. Indian J Ophthalmol (2020) 68(9):1988–90. doi: 10.4103/ijo.IJO_534_20
117. Liu J, Hou Y, Sun L, Li C, Li L, Zhao Y, et al. A Pilot Study of Tofacitinib for Refractory Behcet’s Syndrome. Ann Rheum Dis (2020) 79(11):1517–20. doi: 10.1136/annrheumdis-2020-217307
118. Hou S, Qi J, Zhang Q, Liao D, Li Q, Hu K, et al. Genetic Variants in the JAK1 Gene Confer Higher Risk of Behcet’s Disease With Ocular Involvement in Han Chinese. Hum Genet (2013) 132(9):1049–58. doi: 10.1007/s00439-013-1312-5
119. Yang C, Mai H, Peng J, Zhou B, Hou J, Jiang D. STAT4: An Immunoregulator Contributing to Diverse Human Diseases. Int J Biol Sci (2020) 16(9):1575–85. doi: 10.7150/ijbs.41852
120. Korman BD, Kastner DL, Gregersen PK, Remmers EF. STAT4: Genetics, Mechanisms, and Implications for Autoimmunity. Curr Allergy Asthma Rep (2008) 8(5):398–403. doi: 10.1007/s11882-008-0077-8
121. Remmers EF, Plenge RM, Lee AT, Graham RR, Hom G, Behrens TW, et al. STAT4 and the Risk of Rheumatoid Arthritis and Systemic Lupus Erythematosus. N Engl J Med (2007) 357(10):977–86. doi: 10.1056/NEJMoa073003
122. Ebrahimiyan H, Mostafaei S, Aslani S, Jamshidi A, Mahmoudi M. Studying the Association Between STAT4 Gene Polymorphism and Susceptibility to Rheumatoid Arthritis Disease: An Updated Meta-Analysis. Iran J Immunol (2019) 16(1):71–83. doi: 10.1016/j.mgene.2018.03.010
123. Potter C, Eyre S, Cope A, Worthington J, Barton A. Investigation of Association Between the TRAF Family Genes and RA Susceptibility. Ann Rheum Dis (2007) 66(10):1322–6. doi: 10.1136/ard.2006.065706
124. Wang Q, Yi S, Du Z, Huang X, Xu J, Cao Q, et al. The Rs12569232 SNP Association With Vogt-Koyanagi-Harada Disease and Behcet’s Disease Is Probably Mediated by Regulation of Linc00467 Expression. Ocul Immunol Inflamm (2021) 29(7-8):1464–70. doi: 10.1080/09273948.2020.1745244
125. Yalcin B, Atakan N, Dogan S. Association of Interleukin-23 Receptor Gene Polymorphism With Behcet Disease. Clin Exp Dermatol (2014) 39(8):881–7. doi: 10.1111/ced.12400
126. Arslan Tas D, Erken E, Yildiz F, Dinkci S, Sakalli H. Mevalonate Kinase Gene Mutations and Their Clinical Correlations in Behcet’s Disease. Int J Rheum Dis (2014) 17(4):435–43. doi: 10.1111/1756-185X.12243
127. Mahmoudi M, Ashraf-Ganjouei A, Javinani A, Shahram F, Meguro A, Mizuki N, et al. Epistatic Interaction of ERAP1 and HLA-B*51 in Iranian Patients With Behcet’s Disease. Sci Rep (2018) 8(1):17612. doi: 10.1038/s41598-018-35700-0
128. Ozguclu S, Duman T, Ates FSO, Kucuksahin O, Colak S, Olmez U. Serum Interleukin-37 Level and Interleukin-37 Gene Polymorphism in Patients With Behcet Disease. Clin Rheumatol (2019) 38(2):495–502. doi: 10.1007/s10067-018-4288-7
129. Inal EE, Rustemoglu A, Inanir A, Ekinci D, Gul U, Yigit S, et al. Associations of Rs4810485 and Rs1883832 Polymorphisms of CD40 Gene With Susceptibility and Clinical Findings of Behcet’s Disease. Rheumatol Int (2015) 35(5):837–43. doi: 10.1007/s00296-014-3171-3
130. Talaat RM, Ashour ME, Bassyouni IH, Raouf AA. Polymorphisms of Interleukin 6 and Interleukin 10 in Egyptian People With Behcet’s Disease. Immunobiol (2014) 219(8):573–82. doi: 10.1016/j.imbio.2014.03.004
131. Kim ES, Kim SW, Moon CM, Park JJ, Kim TI, Kim WH, et al. Interactions Between IL17A, IL23R, and STAT4 Polymorphisms Confer Susceptibility to Intestinal Behcet’s Disease in Korean Population. Life Sci (2012) 90(19-20):740–6. doi: 10.1016/j.lfs.2012.03.017
132. Abdel Galil SM, Hagrass HA. The Role of CTLA-4 Exon-1 49 a/G Polymorphism and Soluble CTLA-4 Protein Level in Egyptian Patients With Behcet’s Disease. BioMed Res Int (2014) 2014:513915. doi: 10.1155/2014/513915
133. Wu Z, Chen H, Sun F, Xu J, Zheng W, Li P, et al. PTPN2 Rs1893217 Single-Nucleotide Polymorphism is Associated With Risk of Behcet’s Disease in a Chinese Han Population. Clin Exp Rheumatol (2014) 32(4 Suppl 84):S20–6.
134. Zheng X, Wang D, Hou S, Zhang C, Lei B, Xiao X, et al. Association of Macrophage Migration Inhibitory Factor Gene Polymorphisms With Behcet’s Disease in a Han Chinese Population. Ophthalmol (2012) 119(12):2514–8. doi: 10.1016/j.ophtha.2012.06.039
135. Hu J, Hou S, Zhu X, Fang J, Zhou Y, Liu Y, et al. Interleukin-10 Gene Polymorphisms are Associated With Behcet’s Disease But Not With Vogt-Koyanagi-Harada Syndrome in the Chinese Han Population. Mol Vis (2015) 21:589–603.
136. Padula MC, Leccese P, Lascaro N, Padula AA, Carbone T, Martelli G, et al. A First Step for the Molecular Characterization of Neurological Involvement of Behcet Syndrome: An Italian Pivotal Study. J Mol Neurosci (2021) 71(6):1284–9. doi: 10.1007/s12031-020-01755-w
137. Mace A, Kutalik Z, Valsesia A. Copy Number Variation. Methods Mol Biol (2018) 1793:231–58. doi: 10.1007/978-1-4939-7868-7_14
138. Boteva L, Morris DL, Cortes-Hernandez J, Martin J, Vyse TJ, Fernando MM. Genetically Determined Partial Complement C4 Deficiency States are Not Independent Risk Factors for SLE in UK and Spanish Populations. Am J Hum Genet (2012) 90(3):445–56. doi: 10.1016/j.ajhg.2012.01.012
139. Rigby WF, Wu YL, Zan M, Zhou B, Rosengren S, Carlson C, et al. Increased Frequency of Complement C4B Deficiency in Rheumatoid Arthritis. Arthritis Rheumatol (2012) 64(5):1338–44. doi: 10.1002/art.33472
140. Liu YH, Wan L, Chang CT, Liao WL, Chen WC, Tsai Y, et al. Association Between Copy Number Variation of Complement Component C4 and Graves’ Disease. J BioMed Sci (2011) 18:71. doi: 10.1186/1423-0127-18-71
141. Chang JH, McCluskey P, Wakefield D. Expression of Toll-Like Receptor 4 and its Associated Lipopolysaccharide Receptor Complex by Resident Antigen-Presenting Cells in the Human Uvea. Invest Ophthalmol Vis Sci (2004) 45(6):1871–8. doi: 10.1167/iovs.03-1113
142. Ortiz-Fernandez L, Garcia-Lozano JR, Montes-Cano MA, Conde-Jaldon M, Leo E, Ortego-Centeno N, et al. Association of Haplotypes of the TLR8 Locus With Susceptibility to Crohn’s and Behcet’s Diseases. Clin Exp Rheumatol (2015) 33(6 Suppl 94):S117–22.
143. Wajant H. The Fas Signaling Pathway: More Than a Paradigm. Science. (2002) 296(5573):1635–6. doi: 10.1126/science.1071553
144. Fan X, Shangguan L, Li M, Li CY, Liu B. Functional Polymorphisms of the FAS/FASLG Genes are Associated With Risk of Alopecia Areata in a Chinese Population: A Case-Control Analysis. Br J Dermatol (2010) 163(2):340–4. doi: 10.1111/j.1365-2133.2010.09808.x
145. Yildir S, Sezgin M, Barlas IO, Turkoz G, Ankarali HC, Sahin G, et al. Relation of the Fas and FasL Gene Polymorphisms With Susceptibility to and Severity of Rheumatoid Arthritis. Rheumatol Int (2013) 33(10):2637–45. doi: 10.1007/s00296-013-2793-1
146. Allis CD, Jenuwein T. The Molecular Hallmarks of Epigenetic Control. Nat Rev Genet (2016) 17(8):487–500. doi: 10.1038/nrg.2016.59
147. Alipour S, Sakhinia E, Khabbazi A, Samadi N, Babaloo Z, Azad M, et al. Methylation Status of Interleukin-6 Gene Promoter in Patients With Behcet’s Disease. Reumatol Clin (Engl Ed) (2020) 16(3):229–34. doi: 10.1016/j.reuma.2018.06.006
148. Alipour S, Nouri M, Khabbazi A, Samadi N, Babaloo Z, Abolhasani S, et al. Hypermethylation of IL-10 Gene Is Responsible for its Low mRNA Expression in Behcet’s Disease. J Cell Biochem (2018) 119(8):6614–22. doi: 10.1002/jcb.26809
149. Yu H, Du L, Yi S, Wang Q, Zhu Y, Qiu Y, et al. Epigenome-Wide Association Study Identifies Behcet’s Disease-Associated Methylation Loci in Han Chinese. Rheumatol (Oxford). (2019) 58(9):1574–84. doi: 10.1093/rheumatology/kez043
Keywords: Behcet’s disease, genetics, single nucleotide polymorphism, copy number variation, epigenetic modification
Citation: Gao Y, Zhong Z and Yang P (2022) Genetics in Behcet’s Disease: An Update Review. Front. Ophthalmol. 2:916887. doi: 10.3389/fopht.2022.916887
Received: 10 April 2022; Accepted: 04 May 2022;
Published: 03 June 2022.
Edited by:
Heping Xu, Queen’s University Belfast, United KingdomReviewed by:
Graham Wallace, University of Birmingham, United KingdomWai Kit Chu, The Chinese University of Hong Kong, China
Santos Castañeda, Hospital de La Princesa, Spain
Copyright © 2022 Gao, Zhong and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peizeng Yang, cGVpemVuZ3ljbXVAMTI2LmNvbQ==
 Yu Gao
Yu Gao Zhenyu Zhong
Zhenyu Zhong Peizeng Yang
Peizeng Yang