- 1Neuro-Ophthalmology Service, Ophthalmology Department, Canberra Hospital, Canberra, ACT, Australia
- 2Medical School, Australian National University, Canberra, ACT, Australia
- 3Medical Imaging Department, Royal Prince Alfred Hospital, Sydney, NSW, Australia
- 4Neurology Department, Royal Prince Alfred Hospital, Sydney, NSW, Australia
- 5Central Clinical School, University of Sydney, Sydney, NSW, Australia
Aim: To characterise the ophthalmic indications for, and ophthalmic efficacy of, transverse sinus stenting in adults with medically refractory idiopathic intracranial hypertension.
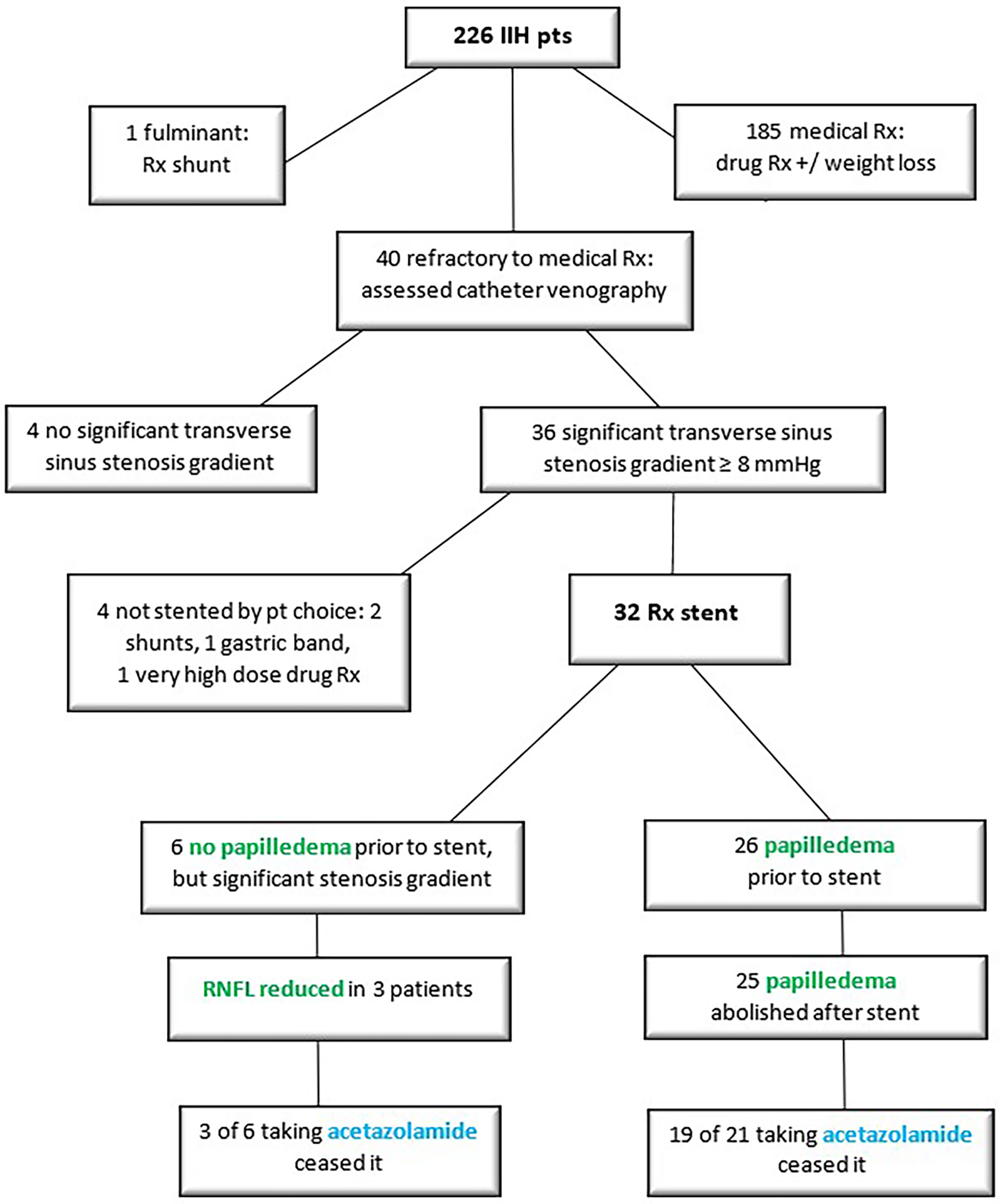
Methods: A retrospective cohort study was undertaken on a single-author database of 226 successive patients with confirmed idiopathic intracranial hypertension (IIH). A total of 32 patients were identified who received a transverse sinus stent for medically refractory disease. This which was defined as visual threat and/or intolerance of maximal medical therapy. Patients with medically refractory disease proceeded to stenting, if found to have a significant transverse sinus stenosis gradient at catheter venography. Visual threat was quantified via the degree of papilledema on optical coherence tomography of the retinal nerve fibre layer, and via the visual field mean deviation. CSF opening pressure at lumbar puncture and cerebral venous sinus pressure measurements from catheter venography were correlated with the ophthalmic data, noting also intolerance of maximal medical therapy. Complications of stenting were fully assessed.
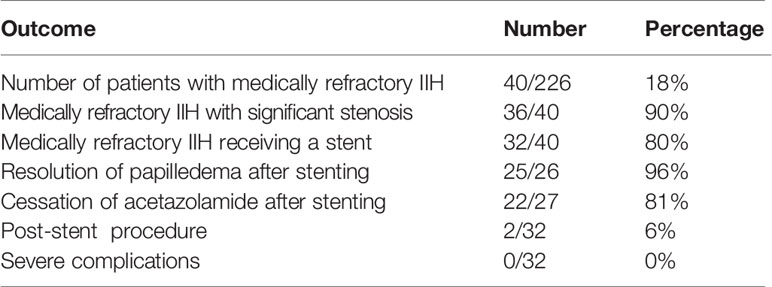
Results: Medically refractory IIH was found in 18% of the total cohort of IIH patients. 90% of those with medically refractory disease had a significant transverse sinus stenosis pressure gradient, and 80% proceeded to stenting. The intervention eliminated papilledema in 96% of stented patients, and allowed 81% to cease acetazolamide. The need for a further procedure was low at 6%, and the safety profile was favourable.
Conclusions: Medically refractory disease in IIH is common (18%), and nearly always associated with a significant transverse sinus stenosis pressure gradient (90%). Endovascular stenting of the stenosis deserves wider uptake as a highly effective, safe, and usually definitive treatment. It safeguards vision by eliminating papilledema (96%), and allows most patients to cease acetazolamide (81%). By analogy with glaucoma, if acetazolamide is the prostaglandin of IIH and CSF diversion the emergency glaucoma filter, stenting is the minimally invasive glaucoma surgery.
Introduction
This paper defines the ophthalmic indications for procedural intervention in an important subset of idiopathic intracranial hypertension (IIH) sufferers. These are adults with medically refractory disease, associated with significant transverse sinus stenosis (stenosis). Such patients have historically progressed to cerebrospinal fluid (CSF) diversion with a shunt (shunting) or optic nerve sheath fenestration (fenestration). Shunting reverses papilledema (1) but is associated with significant morbidity and cost (2), due to infections and the need for repeat procedures (3). Fenestration, while also reversing papilledema (4), is not readily accessed in some regions, and has its own complications (5).
The authors show here that transverse sinus stenosis stenting (stenting) in medically refractory IIH offers effective and safe intervention, when visual threat and/or intolerance of maximal medical therapy co-exist with a significant stenosis pressure gradient as measured at catheter venography. Stenting (Figure 1) reverses papilledema in such patients by alleviating cerebral venous hypertension. The procedure relieves partial venous obstruction, improving CSF drainage, and hence lowering intracranial pressure.
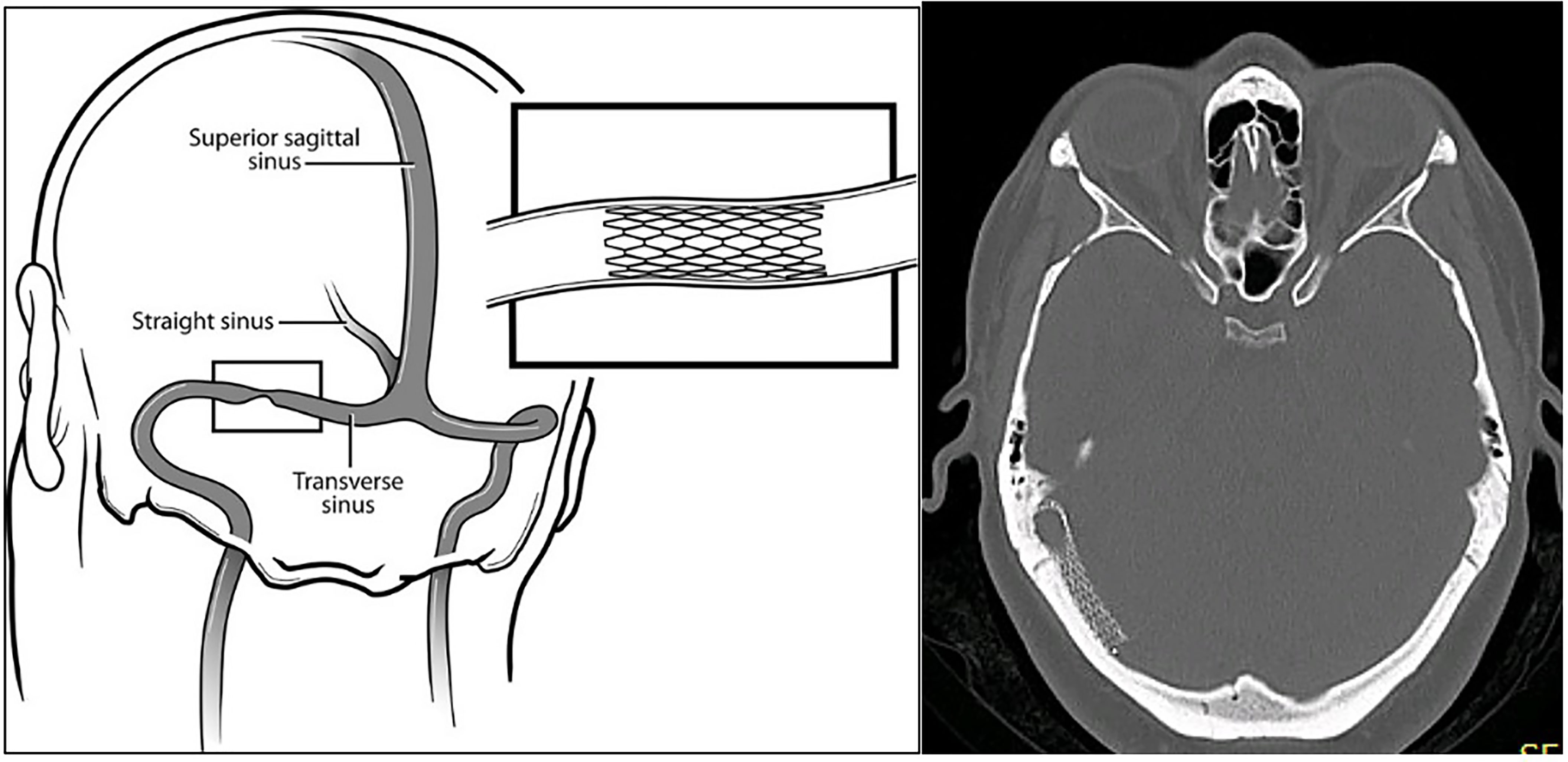
Figure 1 An endovascular stent can be placed to relieve a significant transverse sinus stenosis pressure gradient. (Paul H. Dressel, BFA. Copyright E.I. Levy, published with permission.) The axial CT brain (bone window) with right transverse sinus stent in situ clearly shows the stent fenestrations.
We present comprehensive ophthalmic data to complement the metrics of catheter venography. Systematic ophthalmic assessment and documentation is key in managing IIH - both in identifying those patients who will benefit from a stent, and in assessing the efficacy of the intervention. Our paper advances the IIH literature through its focus on the ophthalmic aspects of stenting (6).
Methods
Ophthalmic Data
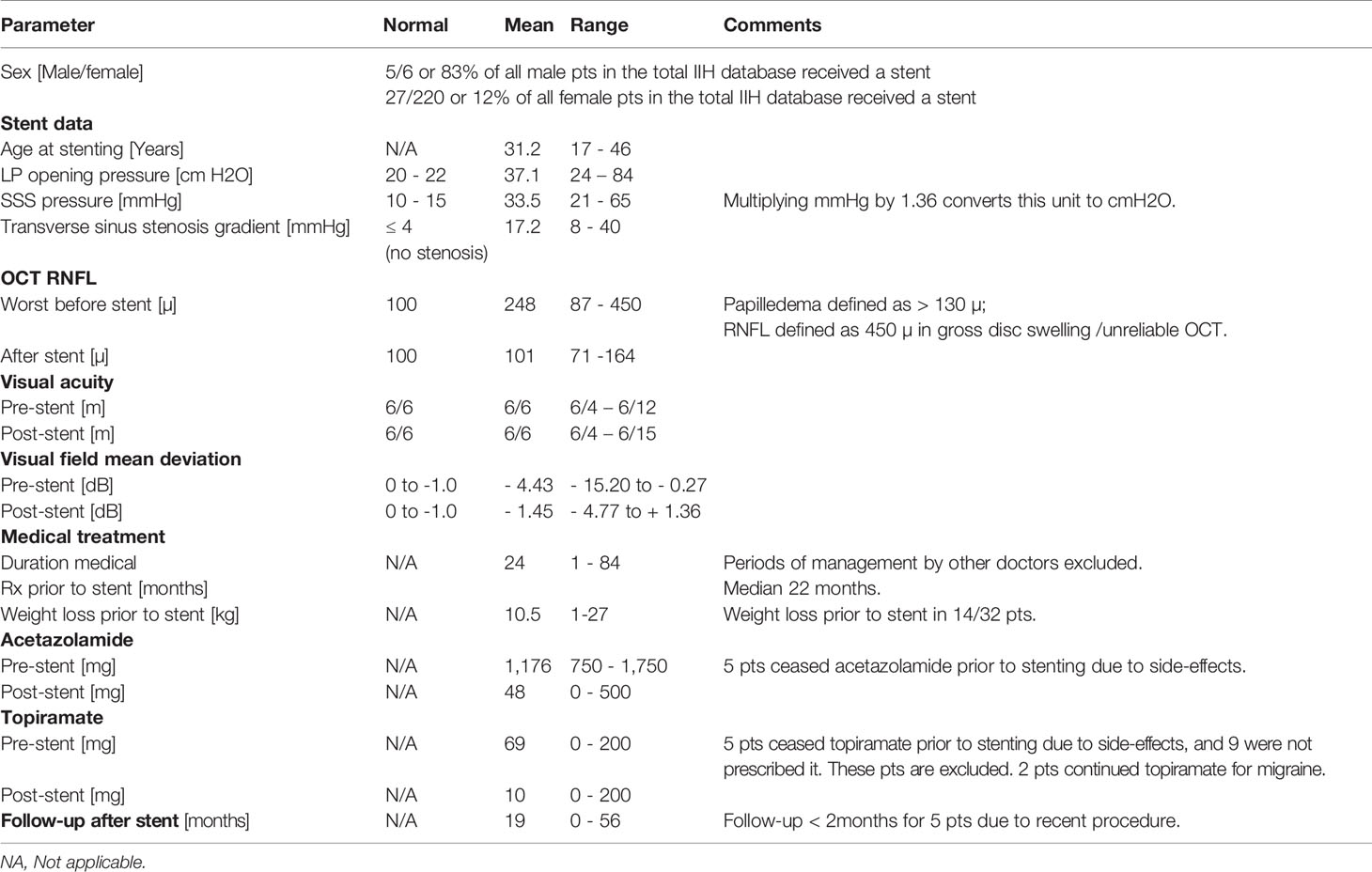
The database of all 226 successive IIH patients seen from 2012 to 2021 at Canberra Hospital was reviewed by its sole author, the senior ophthalmologist listed here as first author. Included patients met the criteria for a diagnosis of IIH (7). Adult patients who had received a stent at Royal Prince Alfred Hospital were then identified. The ophthalmic data of the stented patients was tabulated before and after the procedure, noting best corrected visual acuity (BCVA), visual field mean deviation (MD), average retinal nerve fibre layer thickness (RNFL) on ocular coherence tomography (OCT) (8), IIH medication, and time from diagnosis to stenting (Supplementary Table 1).
Central acuity was measured on a LogMAR vision chart, but recorded as the Snellen equivalent. Automated perimetry was undertaken on a Humphrey Field Analyser, using a 30-2 protocol. Perimetry was supervised by an experienced orthoptist, and repeated if the reliability indices were unsatisfactory. The MD was taken as a proxy for the extent of field loss. The worst visual field (field) in either eye prior to stenting was selected as a marker of disease severity/visual threat.
RNFL was used to assess the degree of disc swelling. Papilledema was defined as an RNFL ≥ 130 µ (normal 100 µ) in at least 1 eye. At this level, papilledema becomes clinically appreciable, and allowance is made for an RNFL somewhat higher than 100 µ in younger patients. The worst RNFL prior to stenting was selected as a marker of disease severity/visual threat. The RNFL was sometimes less than the normal of 100 µ, if optic atrophy had already developed.
Due to segmentation errors, in patients with severe papilledema the RNFL cannot be measured accurately (9), and the machine-generated RNFL average thickness must be disregarded (Figure 2, green arrows). Such RNFLs were recorded as 450 µ rather than ‘unreliable’ (Supplementary Table 1). This value was derived by comparing the physical width of the ‘rolled out’ tomogram of the RNFL in a normal patient to that in a patient with gross papilloedema (Figure 2, red and blue arrows).
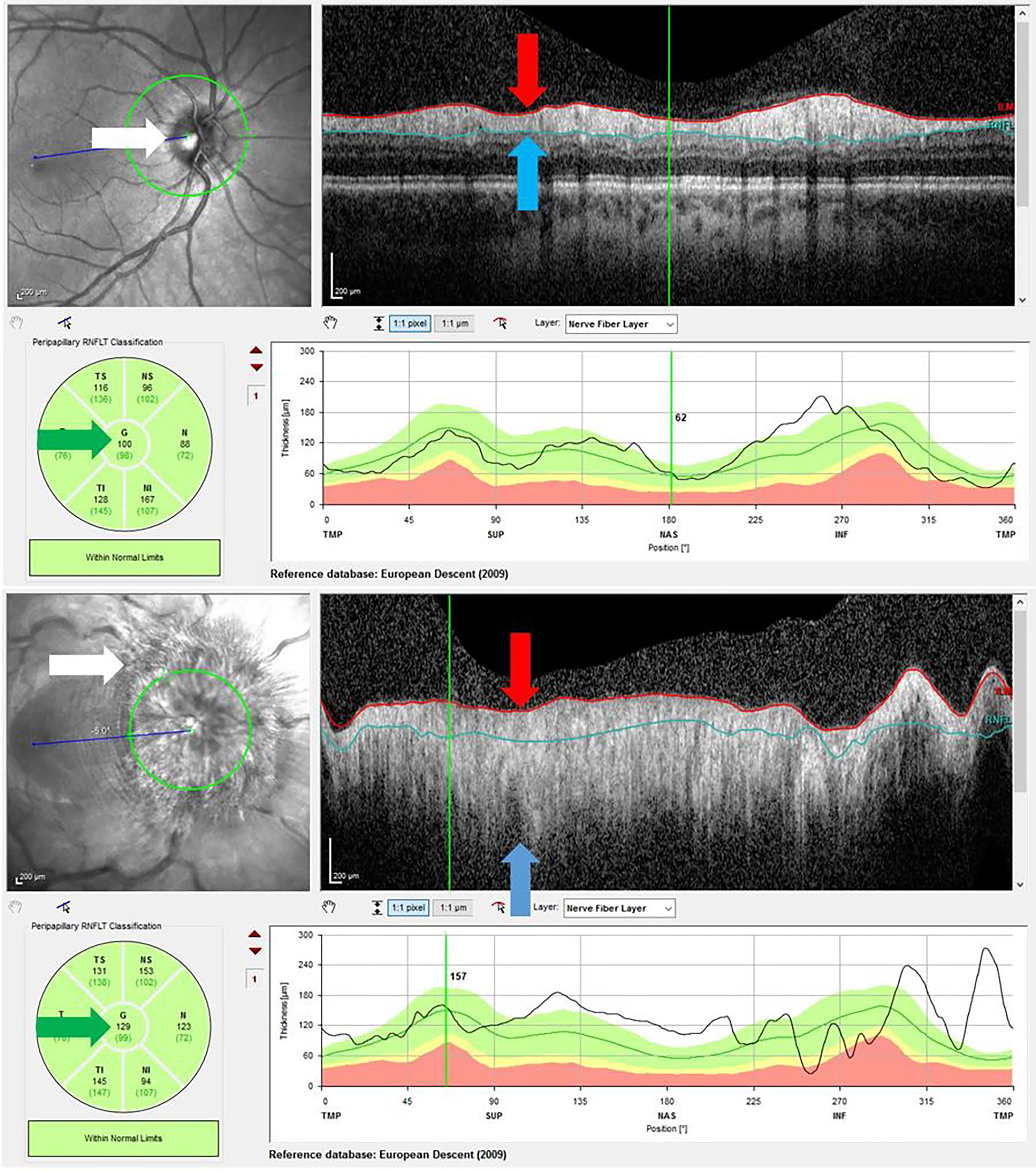
Figure 2 Above – OCT RNFL of normal optic disc. The disc margin is sharp (white arrow), and the RNFL fully contained between the red and blue limit lines (red and blue arrows). The average thickness is 100 μ (green arrow, centre number). Below – OCT RNFL in gross papilledema. The disc margin is obliterated (white arrow), and the RNFL extends far beyond the red and blue limit lines (red and blue arrows). Hence the average thickness measurement of 129 μ (green arrow, centre number) is invalid.
Medication
Note was made of the IIH medication each patient was taking before and after stenting. The highest dose prescribed at any point during treatment was used as a marker of disease severity /visual threat. Inability to continue with medication due to side effects was noted.
Determination of Medically Refractory IIH
Medically refractory IIH was diagnosed if there was visual threat, as assessed on field loss and/or papilledema, and/or if there was intolerance of maximal medical therapy (Table 1). Patients found to have medically refractory disease went on to catheter venography, to ascertain if there was a transverse sinus stenosis pressure gradient that merited stenting.
One patient deemed to have fulminant disease was not considered for stenting. There was such profound papilledema and advanced field loss at presentation that same-day procedural intervention was sought in the form of shunting.
Lumbar Puncture
All patients receiving a stent had previously undergone lumbar puncture (LP) measurement of CSF opening pressure with CSF analysis, to confirm the diagnosis of IIH. Where multiple measurements were available, the highest CSF pressure was selected as a marker of disease severity.
Manometry Data From Catheter Venography
All patients receiving a stent had initially been found to have bilateral variations in transverse sinus anatomy on CT or MR venography (Figure 3). Findings were either bilateral stenosis, or unilateral stenosis with contralateral hypoplasia. Such findings led to assessment with catheter venography of the dominant transverse sinus by a senior neuro-interventionalist. This investigation was performed with the expectation that if a significant stenosis gradient was detected, then it would be treated with stenting in preference to shunting or fenestration.
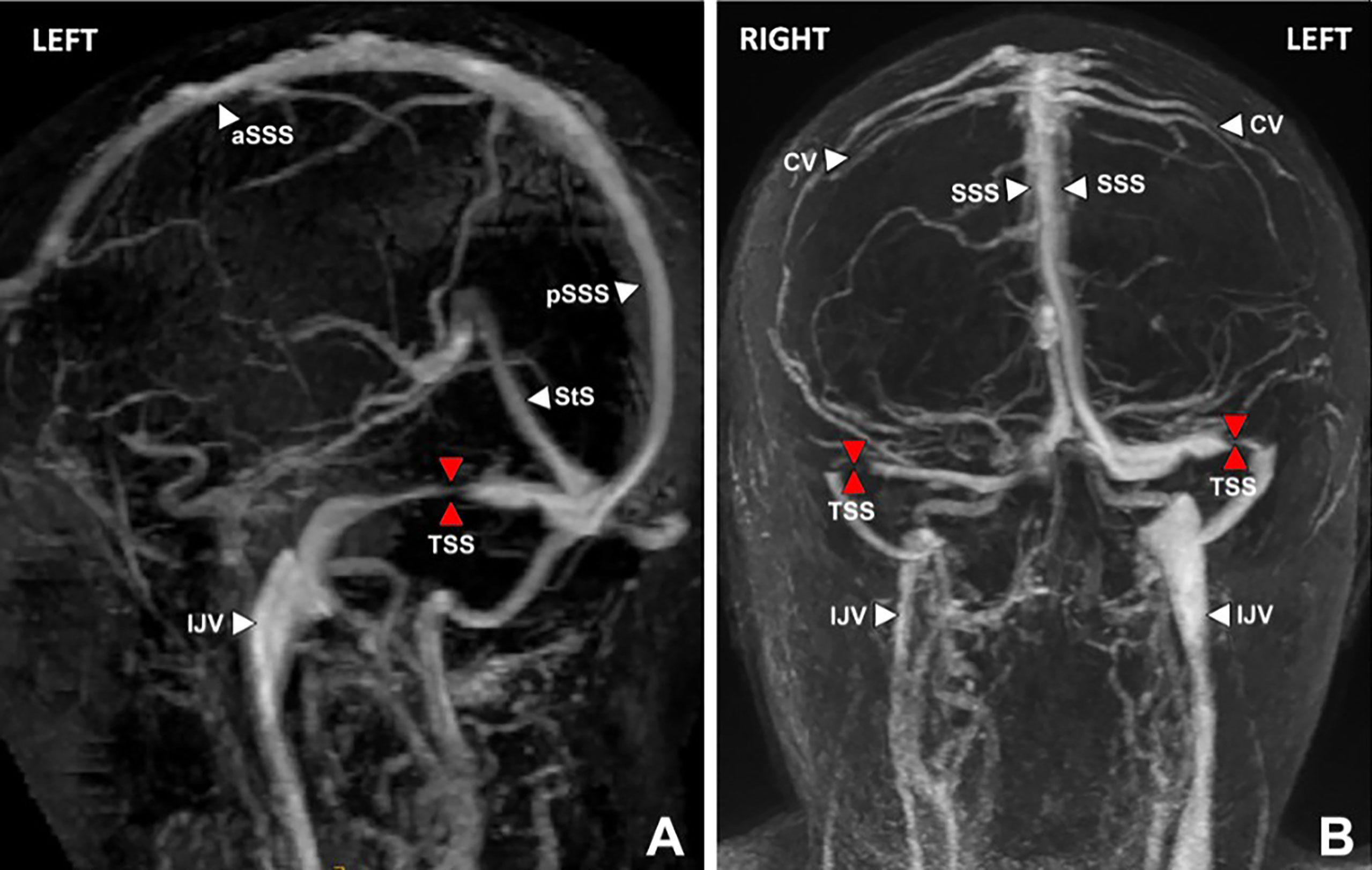
Figure 3 MR venogram brain, lateral oblique (A) and posterior views (B) showing severe stenosis (red arrows) of the dominant left and non-dominant/hypoplastic right transverse sinuses (TSS). Venous hypertension does not develop unless the dominant transverse sinus is abnormal, or both co-dominant transverse sinuses are affected. Key: aSSS, anterior superior saggital sinus; pSSS, posterior superior saggital sinus; StS, straight sinus; TSS, transverse sinus stenosis; IJV, internal jugular vein; CV, cortical vein.
Two weeks prior to catheter venography, patients had either ceased or halved their acetazolamide, to solve the therapeutic dilemma of recording an equivocal stenosis gradient whilst on high-dose medical treatment – while the plasma half-life of acetazolamide is 4-8 hours, its pharmacologic effect may last longer (10). Topiramate was not adjusted, given that sudden withdrawal is not recommended, albeit in the context of its use in epilepsy (11).
LP was also avoided prior to venography for at least 2 weeks, as the abrupt lowering of CSF pressure which follows LP may persist even some weeks later. (An LP was sometimes performed immediately after venography, providing immediate treatment if there was concern about the visual status.)
The catheter venography studies were reviewed for the absolute pressure in the superior sagittal sinus (SSS) using the right atrial pressure as a reference point. The relative gradient across the stenosis in the dominant transverse sinus was then measured. A significant stenosis gradient was defined as a minimum of 8 mmHg, for a normal drop in pressure across the transverse sinus of ≤ 4 mmHg. (Note that venography measurements are obtained in mmHg, while lumbar puncture results use cmH2O. Multiplying mmHg by 1.36 converts this unit to cmH2O.)
Immediately after stenting of a significant stenosis, dramatic alteration is seen in the venous anatomy (12), with reduction of the pressure gradient to the normal range (Figure 4).
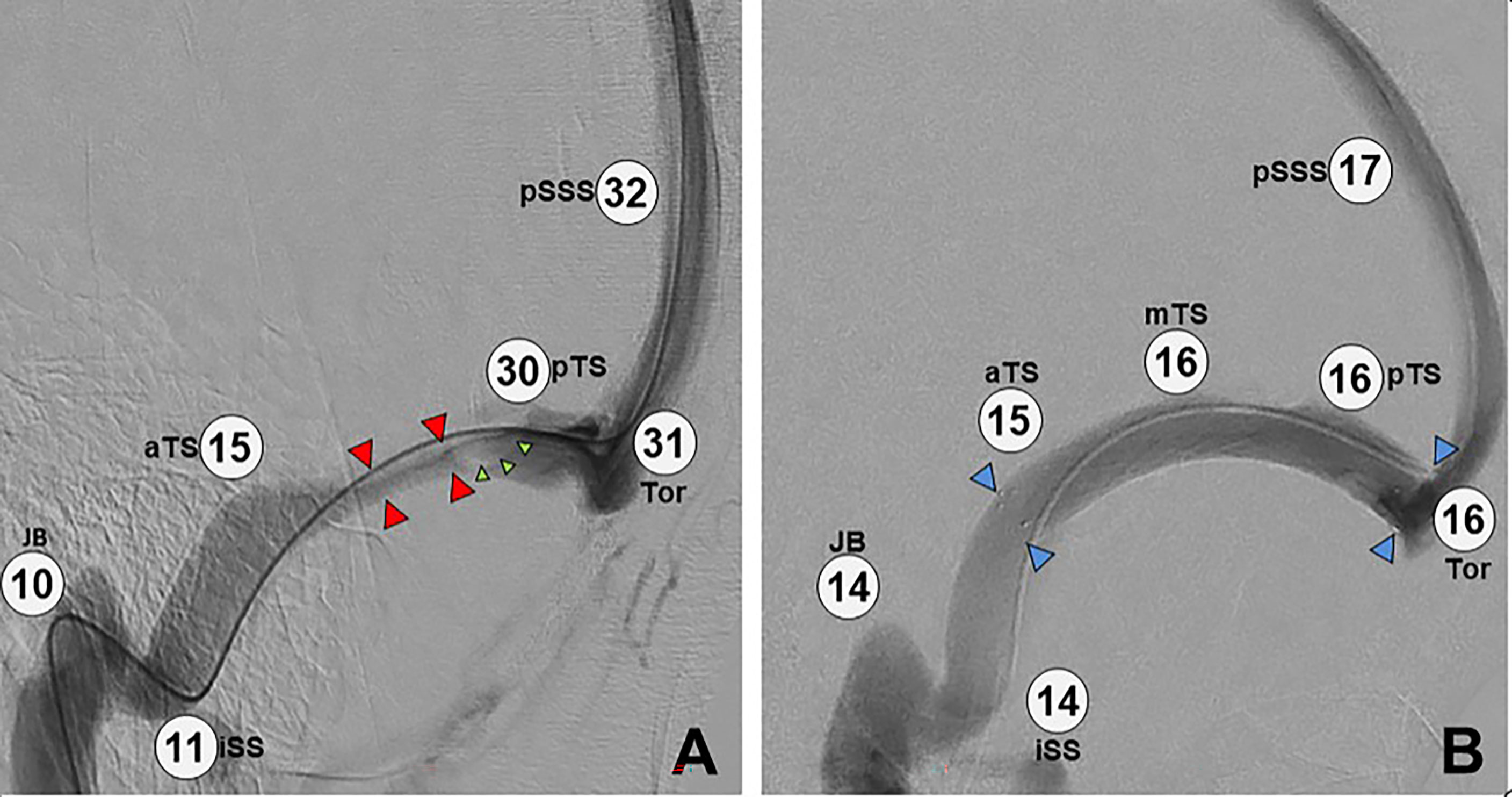
Figure 4 Image intensifier views before and after placement of a transverse sinus stent. (A) Catheter cerebral venography and manometry demonstrating elevated overall pressure (pSSS 32 mmHg) and a long segment of extrinsic stenosis in the left transverse sinus (red arrows), plus a large arachnoid granulation (green arrows). Note the large pressure gradient across the stenosis (30 mmHg – 15 mmHg = 15 mmHg). (B) Post-stenting study in the same patient, demonstrating overall lower pressure (pSSS 17 mmHg), a patent stent (blue arrows) with resolved stenosis, and no significant pressure gradient (16 mmHg – 15 mmHg = 1 mmHg). Key: pSSS, posterior superior saggital sinus; pTS, posterior transverse sinus; Tor, torcula; aTS, anterior transverse sinus; JB, jugular bulb; iSS, inferior saggital sinus; mTS, middle transverse sinus.
Data Quality
The database of 226 consecutive patients diagnosed with IIH in Canberra Hospital’s Ophthalmology Service was compiled by the sole treating senior ophthalmologist and first author, using a standardised template. The entire database was scrutinised to ensure that no patient had disc drusen alone, an alternative cause of papilledema such as cerebral venous sinus thrombosis, or an equivocal diagnosis of IIH in retrospect.
The data regarding stented patients was comprehensive (Supplementary Table 1), with only 29 of 768 ophthalmic and neuro-radiologic data points unavailable or unreliable. All stenting was carried out at one centre by senior neuro-interventionalists. All patients in the IIH database who were stented at Royal Prince Alfred Hospital were included. The sample size of stented patients was reasonable at 32, given that a 2012 meta-analysis of 8 case series and 7 case reports yielded a sample size of 143 (13).
Potential causes of error in data acquisition included patients feeling unwell and so providing an inaccurate field; inaccurate LP measurements due to obesity and positioning issues; and segmentation errors in the RNFL for very swollen discs, as discussed above.
Results
Medically refractory IIH was found in 18% of the total cohort of IIH patients. Of those, 90% with medically refractory disease had a significant transverse sinus stenosis pressure gradient, and 80% proceeded to stenting. The intervention eliminated papilledema in 96% of stented patients, and allowed 81% to cease acetazolamide. The need for a further procedure was low at 6%, and the safety profile was favourable (Table 2, Figure 5).
Additional Results are recorded in Table 3.
Complications of Stenting
A low complication rate was observed in the stented group (Table 4). The only significant complication was the development of mural change within the stent in 2 patients. A differential diagnosis of thrombosis was considered, but anticoagulation was ceased when the appearance was static on serial imaging. A final diagnosis of ingress of an arachnoid granulation through the mesh of the stent was made in both cases.
While headache immediately after stenting due to dural stretch was nearly universal, it was self-limiting within a few weeks.
The complication rate here compared favourably with a 2021 multicentre database study (14), which identified 6 major complications from a total of 811 stents and 1,466 catheter venograms for IIH. These authors found 1 fatality after stenting, equating to a mortality of 0.1%. To place this in context, mortality of CSF shunting in children for hydrocephalus was around 0.5% in a study of 5,955 patients in the US between 1998 and 2000 (15). The high failure and complication rates associated with CSF shunting are referenced above.
Subsequent Procedures
Of the 32 stented patients, 2 went on to a second procedure. Although 2 demonstrated restenosis adjacent to the stent, only 1 needed a second stent. Another patient proceeded to a shunt for persistent elevation of SSS pressure, despite abolition of her transverse sinus stenosis pressure gradient by stenting.
Discussion
Escalating papilledema is eventually blinding (16) and must therefore be reversed. This should ideally be done while it is still pre-perimetric but certainly before there has been severe field loss. Cerebral venous hypertension is now understood to be a key element in papilledema (17), as it reduces the pressure gradient between the CSF compartment and the venous sinuses into which CSF ultimately drains. Hence, venous hypertension leads to raised CSF pressure. A negative feedback loop is established, in which rising CSF pressure compresses the cerebral venous sinuses further, perpetuating the venous hypertension.
In more severe IIH, this cerebral venous hypertension is nearly always accompanied by transverse sinus stenosis. Whether the stenosis is intrinsic or secondary to raised intracranial pressure, stenting of a significant stenosis obliterates the stenosis, relieves the venous hypertension, improves CSF drainage, and so reverses papilledema (18). Stenting is now increasingly recognised in managing medically refractory IIH (19–21).
Patient Selection for Stenting
Visual Threat
Selection for stenting first requires identification of medically refractory disease, one element of which is visual threat. Note that central visual acuity is preserved in papilledema until damage to the optic nerve is very advanced, due to relative sparing of the maculopapillar bundle (22). The visual field is thus the most important quantifier of visual threat (23, 24).
Intolerance of Maximal Medical Therapy
In this study, a patient was considered refractory to medication on failing treatment with acetazolamide ≥ 1,000 mg daily, with the average dose a burdensome 1,176 mg daily (750–1,750mg). Use of acetazolamide in higher doses proved unacceptable to most of our patients due to its side-effect profile (25, 26), although the literature does report tolerability of such doses (27). Bloody diarrhoea, and inability to maintain previous employment or studies due to cognitive change, were the most problematic issues.
The maximum dose of topiramate used was 200 mg, although not all patients were prescribed it. The effects of topiramate on contraception (28) and pregnancy (29) were amongst the concerns limiting the use of this medication.
Despite the issues with medication side-effects, a quite lengthy mean of 24 months (1-84 months, median 22 months) of drug therapy was undertaken prior to stenting. This allowed the patient sufficient time to make every effort to lose weight. It also gave time for a thorough assessment of various dosing regimens. Thus, failure of conservative treatment was truly proved before considering invasive work-up and procedural intervention.
As in glaucoma, high-dose acetazolamide is not a sustainable long-term treatment plan. The patient who complies with high-dose medication experiences a significant decline in quality of life, while the patient who does not comply remains at risk of permanent visual loss.
Stenosis Gradient
The definition of a significant stenosis gradient in this study of ≥ 8 mmHg equalled (30) or approximated (31) the criteria used in similar case series. The average stenosis gradient was found to be 17.2 mmHg (8–40 mmHg), a little lower than the 21 mmHg identified in a comparable study (32).
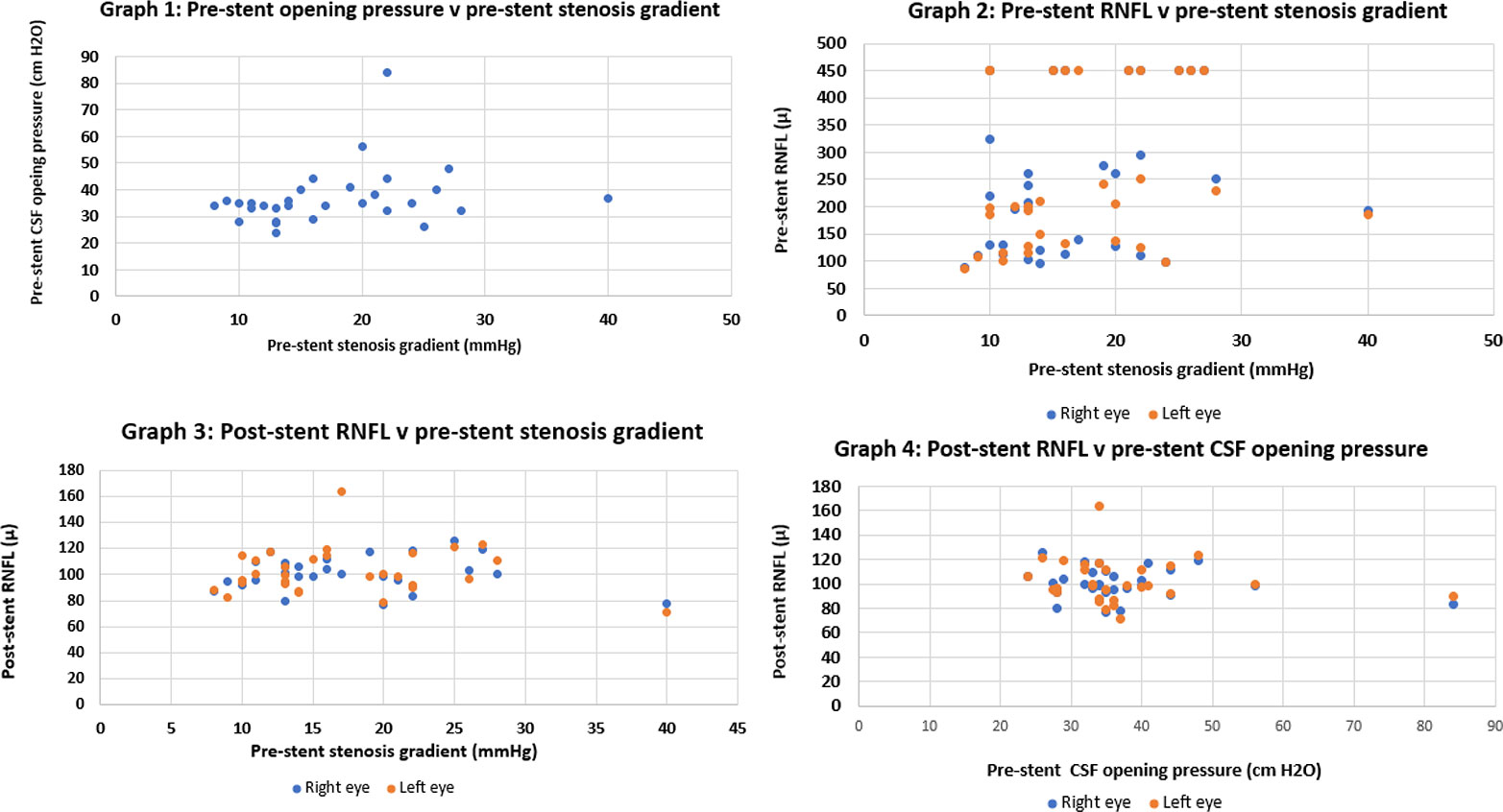
Stenosis gradient did not show any correlation with CSF opening pressure or pre-stent RNFL (Figure 6). Thus, catheter venography should still be considered in a patient whose clinical course suggests medically refractory IIH, even if the LP pressure and/or RNFL are unimpressive.
IIH without papilledema is well recognised (33). In this cohort, 6 patients did not have papilledema as we numerically defined it. They did have abnormal visual fields, medication intolerance or both, as well as a compelling stenosis gradient leading to stenting. In 3 patients, the RNFL declined after stenting. Bearing in mind that the RNFL is the sum of swelling plus atrophy, we surmise that they already had some optic atrophy at the time of stenting.
Assessment Over Time
Sequential assessment of the clinical gestalt over time improves the identification of patients with medically refractory disease. It is important to develop a consistent data set that is updated at each consultation, collating RNFL, fields, fundoscopy, medication, weight, and patient symptoms. This multimodal tracking ensures timely detection of clinical trends that require a change in management.
In quantify to assess the amount of papilledema, objective quantification of RNFL thickness with OCT is much more reliable than subjective Frisen grading of the disc appearance (34). Nevertheless, stereoscopic examination of the disc does provide qualitative information regarding the presence or absence of pallor. Pallor is an indicator of emerging optic atrophy, even if the disc is still swollen.
As in glaucoma, the longitudinal tracking of optic nerve structure (RNFL) and function (visual fields) guides the recommendation to intervene, rather than a rigid numeric rubric. It is perfectly reasonable to trial medical therapy for a grossly swollen pink disc if the visual field shows only mild to moderate blind spot enlargement, and compliance with medication and follow-up is assured. However, if the visual fields show gross blind spot enlargement with significant peripheral loss, or if optic disc pallor is already evident, then early consideration should be given to catheter venography to prevent/minimise permanent structural damage to the optic nerves.
Efficacy of Stenting in Eliminating Papilledema
In this cohort of patients, average RNFL declined by 147 µ (from 248 to 101 µ) after stenting, with resolution of papilledema in 96%. This accorded with a 2018 meta-analysis of 474 stented patients, which demonstrated improvement of papilledema in 94% (35).
Since RNFL is a proxy for intracranial pressure (36), the impressive decline in RNFL after stenting seen here indicated not only resolution of papilledema, but also resolution of IIH. However, a critical caveat in assessing reduction of papilledema is that a declining RNFL is good news only if the visual field remains stable or improves. If the field is declining along with the RNFL, the patient actually has uncontrolled disease. Since the RNFL is the sum of swelling plus atrophy, the stereoscopic examination of the optic disc for swelling is therefore an important part of interpreting the RNFL and field.
Weight Loss Prior to Stenting
Weight loss is considered a major part of treatment in IIH, as it reduces central venous pressure, and may reverse obesity-related inflammation (37) and metabolic changes (38) implicated in the aetiology of the condition. However, many patients find it impossible to lose and then keep off the recommended minimum 10% of body weight (39). Here only 44% of patients achieved weight loss prior to stenting, with an average loss of 10.5 kg. This amount was not sufficient to achieve clinical remission, even though 72% of patient in another study did achieve remission for this quantum of weight reduction (40).
Those patients not identified in this cohort as losing weight prior to stenting were a heterogenous group, including those of normal weight, those losing no weight or losing it after stenting, those gaining weight, and those keeping incomplete records.
Many things contribute to difficulties with achieving weight loss. Obesity often carries a history of dieting followed by rebound in weight (41), as well as complex psychodynamic issues around body image (42). Bariatric surgery, while superior to community weight loss programs at 2 years (43), is far from readily obtained in the public health system, and too expensive in the private health system for most patients. Even when weight loss is achieved, it does not always produce the desired remission in IIH, as evidenced by the patients in this study who did lose weight prior to procedural intervention. Stenting offers a way out of these multiple impasses.
Visual Outcomes
While the average visual acuity was unaltered by stenting, the average visual field mean deviation improved by 2.88 dB: from -4.43 dB before (-15.20 to -0.27 dB) to -1.45 dB after (-4.77 to +1.36). This was double the 1.43 dB improvement due to acetazolamide alone seen in the 2014 IIH Treatment Trial for patients with mild IIH (44). In our series, 47 of 48 eyes showed improvement in the field mean deviation.
Patient Gender
In this IIH database of 226 patients, 5 of the 6 adult men proved to have medically refractory disease requiring stenting. This is in line with other studies confirming that IIH is more aggressive in men (45), with severe visual loss twice as likely compared to women.
None of the women who went on to stenting presented during pregnancy. Hence there was no need to avoid iodinated contrast and radiation in diagnosis and treatment, as would usually be the case in pregnancy. With a stent in situ, 1 patient went on to 2 uncomplicated pregnancies.
Avoiding Complications During Stenting
Safe stenting starts with co-ordination of care amongst the patient’s ophthalmologist, neurologist, and neuro-radiologic interventionalist, to ensure correct patient selection. Indications for stenting are summarised in Table 5. Appropriate device selection is a fundamental aspect of a safe procedure, but is beyond the scope of this paper. Other important considerations are appropriate training and experience for the interventionalist and for the anaesthetist.
Prevention of Stent Thrombosis
Acute intraluminal stent occlusion due to thrombosis would be potentially life-threatening in a patient who, by definition, has an inadequate contralateral transverse sinus. However, stent thrombosis was not seen in this series, and appears to be extremely rare in patients managed with dual antiplatelet premedication (46). Here all patients were prescribed dual antiplatelets for 1 week prior to stenting, nearly always aspirin 100mg and clopidogrel 75 mg daily. Suppression of platelet function was ascertained immediately prior to the procedure, and a heparin bolus was used on the table. Both anti-platelets were continued for 3 months post-stent, and aspirin was used for another 9 months thereafter. This long duration of antiplatelet medication allows time for endothelium to fully line the stent.
Patients who might struggle to comply with the antiplatelet regime, who will need surgery in the year following stenting, or who are at risk of being lost to follow up in the first year after treatment, should not be offered a stent.
Informed Consent
To facilitate informed consent, catheter venography was carried out as a stand-alone procedure some weeks prior to stenting. It is the authors’ experience that patients are eager for procedural intervention when their vision is threatened, or after many months of unpalatable medication. This makes the need for thorough education about the potential risks of the procedure especially important. Patients considering stenting are counselled that it carries around a 1% risk of serious complication, including stroke and death. More recent literature suggests that the risk is significantly lower than this, as discussed above.
Stenting in Fulminant IIH
Stenting is gaining ground in patients with fulminant disease, defined in one paper as visual field loss to within 5 degrees of fixation, and/or a decrease in visual acuity to ≤ 6/15 in either eye in the presence of papilledema (47). However, the sole fulminant presentation to the first author was not considered for stenting, due to the delay imposed by obtaining catheter venography at another centre and the requirement for a week of antiplatelet therapy prior to stenting. Additionally, it was felt that the commencement of antiplatelet therapy would mean that if a stent proved an inadequate intervention, it would not be possible to perform a lumbar puncture/drain or shunt on an emergency basis.
Stenting Compared to Fenestration and CSF Shunting
There are currently no head-to-head prospective or randomised control trials assessing stenting relative to other procedural interventions. However, meta-analyses comparing them (48–50) do position stenting favourably, due to its high technical and clinical success rates, and low rate of complication.
Since few treatment centres offer all three options of shunting, fenestration, and stenting, it may be difficult to construct trials comparing the three. Nevertheless, as the prevalence of IIH increases (51) along with obesity (52), research opportunities may increase too.
The authors propose that because stenting is a highly effective and safe treatment, it is preferable to shunting. Shunting carries the risks of haemorrhage, infection, epilepsy, and focal neurological deficit. Nor is placement of a shunt technically straightforward in IIH patients, with slit-like ventricles as part of their disease (53). Complications from placing shunt tubing in the abdomen may also be increased by obesity.
The most significant issue with shunts, however, is that they frequently require surgical revision. The average time to revision is a brief 6 months for some 19% of patients (54). Revision rates overall are quoted at 25% within the first year, and up to 85% within the patient’s lifetime (55). Shunt failure may be due to infection, mechanical malfunction, or unsatisfactory rates of CSF drainage, whether excessive or inadequate.
Fenestration, while a proven therapy to protect vision in fulminant and medically refractory IIH (56), is not readily available in some regions. Stent placement by comparison may be more easily accessed. The advent of clot retrieval for large vessel stroke has increased the number of neuro-interventionalists in tertiary hospital imaging departments who can acquire the appropriate training and experience to undertake stenting. The tertiary setting provides neurosurgical back-up, in the very unlikely event of an intracranial complication. Anaesthetic and high dependency services are also available, for the requisite general anaesthesia and post-procedure observation. In summary, stenting avoids the risks associated with shunting, and the limited availability of fenestration.
Limitations of the Study
Headache was deemed too subjective and multifactorial (57) a symptom to be assessed in this paper.
However, headache certainly creates very significant disability for IIH sufferers (58). When responding to a questionnaire about their symptoms, only 18.1% of IIH patients indicated that their lives were most affected by visual problems, with 49.6% most affected by headaches (59).
It has been mentioned above that a declining RNFL must always be interpreted in the context of the visual field to ascertain that the decline represents clinical recovery and not optic atrophy. However, given that significant RNFL thinning can be pre-perimetric (as seen in glaucoma), it is possible that an RNFL decline may have elements of both resolution of swelling and onset of pre-perimetric atrophy. Since stereoscopic assessment of the disc for pallor and swelling is subjective, it would be ideal to have an objective measure of pre-perimetric optic atrophy. Nevertheless, the authors conclude that RNFL decrease in the presence of a normal or stable field is clinically acceptable evidence that vision is protected.
Conclusion
Adults with IIH who fail medical therapy nearly always have a significant transverse sinus stenosis pressure gradient. In such patients, stenting safely and very effectively reverses papilledema. In addition, the majority of patients are able to discontinue medication. Timely stenting protects vision, and improves quality of life. By analogy with glaucoma, if acetazolamide is the prostaglandin of IIH and CSF diversion the emergency glaucoma filter, stenting is the minimally invasive glaucoma surgery (MIGS).
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics Statement
This study involving human participants was reviewed and approved by the ACT Health Human Research Ethics Committee, Low Risk Sub-Committee. Written informed consent for participation was not required for this study, in accordance with the national legislation and the institutional requirements.
Author Contributions
KR compiled all data and drafted the manuscript and figures. GP, TA, and SW carried out the venography, manometry and stenting, and provided the venous pressure data. SW and MH prepared the radiologic images. GP and MH provided critical revision of the manuscript. All authors approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Valuable editorial input from Peter McCluskey is gratefully acknowledged, and sincere thanks go to Marina Kaidaloff for outstanding administrative support.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fopht.2022.885583/full#supplementary-material
References
1. Uretsky S. Surgical Interventions for Idiopathic Intracranial Hypertension. Curr Opin Ophthalmol (2009) 20:451–5. doi: 10.1097/ICU.0b013e3283313c1c
2. Ahmed RM, Zmudzki F, Parker GD, Owler BK, Halmagyi GM. Transverse Sinus Stenting for Pseudotumor Cerebri: A Cost Comparison With CSF Shunting. Am J Neuroradiol (2014) 35:952–8. doi: 10.3174/ajnr.A3806
3. Owler B. CSF Shunt Failure: An Ongoing Epidemic? J Neurol Neurosurg Psychiatry (2009) 80:1185. doi: 10.1136/jnnp.2009.174524
4. Spitze A, Malik A, Al-Zubidi N, Golnik K, Lee A. Optic Nerve Sheath Fenestration vs Cerebrospinal Diversion Procedures: What Is the Preferred Surgical Procedure for the Treatment of Idiopathic Intracranial Hypertension Failing Maximum Medical Therapy? J Neuroophthalmol (2013) 33:183–8. doi: 10.1097/WNO.0b013e318292d06f
5. Gilbert AL, Chwalisz B, Mallery R. Complications of Optic Nerve Sheath Fenestration as a Treatment for Idiopathic Intracranial Hypertension. Semin Ophthalmol (2018) 33:36–41. doi: 10.1080/08820538.2017.1353810
6. Kabanovski A, Kisilevsky E, Yang Y, Margolin E. Dural Venous Sinus Stenting in the Treatment of Idiopathic Intracranial Hypertension: A Systematic Review and Critique of Literature. Survey Ophthal (2022) 67:271–87. doi: 10.1016/j.survophthal.2021.05.002
7. Mollan SP, Davies B, Silver NC, Shaw S, Mallucci CL, Wakerley BR, et al. Idiopathic Intracranial Hypertension: Consensus Guidelines on Management. J Neurol Neurosurg Psychiatry (2018) 89:1088–100. doi: 10.1136/jnnp-2017-317440
8. Fraser C, Lueck CJ. Optical Coherence Tomography: A Window to the Brain? Pract Neurol (2021) 21:313–21. doi: 10.1136/practneurol-2020-002824
9. Aojula A, Mollan SP, Horsburgh J, Yiangou A, Markey KA, Mitchell JL, et al. Segmentation Error in Spectral Domain Optical Coherence Tomography Measures of the Retinal Nerve Fibre Layer Thickness in Idiopathic Intracranial Hypertension. BMC Ophthalmol (2017) 17:article 257. doi: 10.1186/s12886-017-0652-7
10. Van Berkel MA, Elefritz JL. Evaluating Off-Label Uses of Acetazolamide. Am J Health Syst Pharm (2018) 75:524–31. doi: 10.2146/ajhp170279
11. Garnett WR, St Louis EK, Henry TR, Bramley T. Transitional Polytherapy: Tricks of the Trade for Monotherapy to Monotherapy AED Conversions. Curr Neuropharmacol (2009) 7:83–95. doi: 10.2174/157015909788848884
12. King JO, Mitchell PJ, Thomson KR, Tress BM. Manometry Combined With Cervical Puncture in Idiopathic Intracranial Hypertension. Neurology (2002) 58:26–30. doi: 10.1212/wnl.58.1.26
13. Puffer RC, Mustafa W, Lanzino G. Venous Sinus Stenting for Idiopathic Intracranial Hypertension: A Review of the Literature. J Neurointerv Surg (2013) 5:483–6. doi: 10.1136/neurintsurg-2012-010468
14. Townsend R, Jost A, Amans M, Hui F, Bender MT, Satti SR, et al. Major Complications of Dural Venous Sinus Stenting for Idiopathic Intracranial Hypertension: Case Series and Management Considerations. J NeuroInterv Surg (2022) 14:neurintsurg-2021-017361. doi: 10.1136/neurintsurg-2021-017361
15. Smith ER, Butler WE, Barker FG. In-Hospital Mortality Rates After Ventriculoperit1al Shunt Procedures in the United States, 1998 to 2000: Relation to Hospital and Surgeon Volume of Care. J Neurosurg (2004) 100(Suppl Pediatrics):90–7. doi: 10.3171/ped.2004.100.2.0090
16. Best J, Silvestri G, Burton B, Foot B, Acheson J. The Incidence of Blindness Due to Idiopathic Intracranial Hypertension in the UK. Open Ophthalmol J (2013) 28:26–9. doi: 10.2174/1874364101307010026
17. Dinkin M, Oliveira C. Men Are From Mars, Idiopathic Intracranial Hypertension Is From Venous: The Role of Venous Sinus Stenosis and Stenting in Idiopathic Intracranial Hypertension. Semin Neurol (2019) 39:692–703. doi: 10.1055/s-0039-3399506
18. Ahmed RM, Wilkinson M, Parker GD, Thurtell MJ, Macdonald J, McCluskey PJ, et al. Transverse Sinus Stenting for Idiopathic Intracranial Hypertension: A Review of 52 Patients and of Model Predictions. Am J Neuroradiol (2011) 32:1408–14. doi: 10.3174/ajnr.A2575
19. Kulhari A, He M, Fourcand F, Singh A, Zacharatos H, Mehta S, et al. Safety and Clinical Outcomes After Transverse Venous Sinus Stenting for Treatment of Refractory Idiopathic Intracranial Hypertension: Single Center Experience. J Vasc Intervent Neurol (2020) 11:6–12.
20. Labeyrie MA, Fantoni M, Vever U. Intracranial Venous Sinus Stenting for the Treatment of Lateral Sinus Stenoses: An Analysis of 200 Patients. Diagn Interv Imaging (2021) 102:619–27. doi: 10.1016/j.diii.2021.05.008
21. Patsalides A, Oliveira C, Wilcox J, Brown K, Grover K, Gobin YP, et al. Venous Sinus Stenting Lowers the Intracranial Pressure in Patients With Idiopathic Intracranial Hypertension. J Neurointerv Surg (2019) 11:175–8. doi: 10.1136/neurintsurg-2018-014032
22. Trobe JD. Papilledema: The Vexing Issues. J Neuro-Ophthal (2011) 31:175–86. doi: 10.1097/WNO.0b013e31821a8b0b
23. Wall M. The Importance of Visual Field Testing in Idiopathic Intracranial Hypertension. Continuum (Minneap Minn) (2014) 20(4 Neuro-ophthalmology):1067–74. doi: 10.1212/01.CON.0000453302.20110.29
24. McCluskey P, Lam D, Ang T, Todd M, Halmagyi GM. Optic Nerve Sheath Fenestration for Treating Papilledema in the Era of Cerebral Venous Sinus Stenting. Manuscript in preparation.
25. Schmickl CN, Owens RL, Orr JE, Edwards BA, Malhotra A. Side Effects of Acetazolamide: A Systematic Review and Meta-Analysis Assessing Overall Risk and Dose Dependence. BMJ Open Respir Res (2020) 7:e000557. doi: 10.1136/bmjresp-2020-000557
26. Costello F, Skolnik K, Sarna J, Varughese R. Respiratory Complications Associated With Acetazolamide Use in the Management of Idiopathic Intracranial Hypertension. J Neuro-Oph (2019) 39:511–2. doi: 10.1097/WNO.0000000000000813
27. ten Hove MW, Friedman DI, Patel AD, Irrcher I, Wall M, McDermott M, et al. (NORDIC Idiopathic Intracranial Hypertension Study Group) Safety and Tolerability of Acetazolamide in the Idiopathic Intracranial Hypertension Treatment Trial. J Neuroophthalmol (2016) 36:13–9. doi: 10.1097/WNO.0000000000000322
28. Rosenfeld WE, Doose DR, Walker SA, Nayak RK. Effect of Topiramate on the Pharmacokinetics of an Oral Contraceptive Containing Norethindr1 and Ethinyl Estradiol in Patients With Epilepsy. Epilepsia (1997) 38:317–23. doi: 10.1111/j.1528-1157.1997.tb01123.x
29. FDA. Drug Safety Communication: Risk of Oral Clefts in Children Born to Mothers Taking Topamax (Topiramate) (2018) (Accessed 26 Feb 22).
30. Dinkin MJ, Patsalides A. Venous Sinus Stenting in Idiopathic Intracranial Hypertension: Results of a Prospective Trial. J Neuroophthalmol (2017) 37:113–21. doi: 10.1097/WNO.0000000000000426
31. Fields JD, Javedani PP, Falardeau J, Nesbit GM, Dogan A, Helseth EK, et al. Dural Venous Sinus Angioplasty and Stenting for the Treatment of Idiopathic Intracranial Hypertension. J NeuroInterv Surg (2013) 5:62–8. doi: 10.1136/neurintsurg-2011-010156
32. Koovor JM, Lopez GV, Riley K, Tejada J. Transverse Venous Sinus Stenting for Idiopathic Intracranial Hypertension: Safety and Feasibility. Neuroradiol J (2018) 31:513–7. doi: 10.1177/1971400918782320
33. Mollan SP, Chong YJ, Grech O, Sinclair AJ, Wakerley BR. Current Perspectives on Idiopathic Intracranial Hypertension Without Papilloedema. Life (2021) 11:472. doi: 10.3390/life11060472
34. Sinclair AJ, Burdon MA, Nightingale PG, Matthews TD, Jacks A, Lawden M, et al. Rating Papilledema: An Evaluation of the Frisén Classification in Idiopathic Intracranial Hypertension. J Neurol (2012) 259:1406–12. doi: 10.1007/s00415-011-6365-6
35. Nicholson P, Brinjikji W, Radovanovic I, Hilditch CA, Tsang ACO, Krings T, et al. Venous Sinus Stenting for Idiopathic Intracranial Hypertension: A Systematic Review and Meta-Analysis. J NeuroInterv Surg (2019) 11:380–5. doi: 10.1136/neurintsurg-2018-014172
36. Vijay V, Mollan SP, Mitchell JL. Using Optical Coherence Tomography as a Surrogate of Measurements of Intracranial Pressure in Idiopathic Intracranial Hypertension. JAMA Ophthalmol (2020) 138:1–8. doi: 10.1001/jamaophthalmol.2020.4242
37. Sinclair AJ, Ball AK, Burdon MA, Clarke CE, Stewart PM, Curnow SJ, et al. Exploring the Pathogenesis of IIH: An Inflammatory Perspective. J Neuroimmunol (2008) 201-202:212–20. doi: 10.1016/j.jneuroim.2008.06.029
38. Glueck CJ, Aregawi D, Goldenberg N, Golnik KC, Sieve L, Wang P. Idiopathic Intracranial Hypertension, Polycystic-Ovary Syndrome, and Thrombophilia. J Lab Clin Med (2005) 145:72–82. doi: 10.1016/j.lab.2004.09.011
39. Ottridge R, Mollan SP, Botfield H, Frew E, Ives NJ, Matthews T, et al. Randomised Controlled Trial of Bariatric Surgery Versus a Community Weight Loss Programme for the Sustained Treatment of Idiopathic Intracranial Hypertension: The Idiopathic Intracranial Hypertension Weight Trial (IIH:WT) Protocol. BMJ Open (2017) 7:e017426. doi: 10.1136/bmjopen-2017-017426
40. Ang JL, Teo KZ, Fraser CL. Weight Loss in Idiopathic Intracranial Hypertension: A Retrospective Review of Outcomes in the Clinical Setting. J Neuro-Ophth (2021) 41:e458–63. doi: 10.1097/WNO.0000000000001107
41. Mann T. Why do Dieters Regain Weight?, in: Psychological Science Agenda (2018). Available at: http://www.apa.org/science/about/psa/2018/05/calorie-deprivation (Accessed 20/03/22).
42. Weinberger NA, Kersting A, Riedel-Heller SG, Luck-Sikorski C. Body Dissatisfaction in Individuals With Obesity Compared to Normal-Weight Individuals: A Systematic Review and Meta-Analysis. Obes Facts (2016) 9:424–41. doi: 10.1159/000454837
43. Mollan SP, Mitchell JL, Ottridge RS, Aguiar M, Yiangou A, Alimajstorovic Z, et al. Effectiveness of Bariatric Surgery vs CommunityWeight Management Intervention for the Treatment of Idiopathic Intracranial Hypertension - A Randomized Clinical Trial. JAMA Neurol (2021) 78:678–86. doi: 10.1001/jamaneurol.2021.0659
44. NORDIC Idiopathic Intracranial Hypertension Study Group Writing Committee, Wall M, McDermott MP, Kieburtz KD, Corbett JJ, Feldon SE, Friedman DI, et al. Effect of Acetazolamide on Visual Function in Patients With Idiopathic Intracranial Hypertension and Mild Visual Loss: The Idiopathic Intracranial Hypertension Treatment Trial. JAMA (2014) 311:1641–51. doi: 10.1001/jama.2014.3312
45. Bruce BB, Kedar S, Van Stavern GP, Monaghan D, Acierno MD, Braswell RA, et al. Idiopathic Intracranial Hypertension in Men. Neurology (2009) 72:304–9. doi: 10.1212/01.wnl.0000333254.84120.f5
46. Teleb MS, Cziep ME, Lazzaro MA, Gheith A, Asif K, Remler B, et al. Idiopathic Intracranial Hypertension: A Systematic Analysis of Transverse Sinus Stenting. Intervent Neurol (2014) 2:132–43. doi: 10.1159/000357503
47. Zehri AH, Lee KE, Kartchner J, Arnel M, Martin T, Wolfe SQ, et al. Efficacy of Dural Venous Sinus Stenting in Treating Idiopathic Intracranial Hypertension With Acute Vision Loss. Neuroradiol J (2022) 35:86–93. doi: 10.1177/19714009211026923
48. Satti SR, Leishangthem L, Chaudry MI. Meta-Analysis of CSF Diversion Procedures and Dural Venous Sinus Stenting in the Setting of Medically Refractory Idiopathic Intracranial Hypertension. Am J Neuroradiol (2015) 36:1899–904. doi: 10.3174/ajnr.A4377
49. Julayanont P, Karukote A, Ruthirago D, Panikkath D, Panikkath R. Idiopathic Intracranial Hypertension: Ongoing Clinical Challenges and Future Prospects. J Pain Res (2016) 9:87–99. doi: 10.2147/JPR.S60633
50. Scherman D, Dmytriw A, Nguyen G, Nguyen NT, Tchantchaleishvili N, Maingard J, et al. Shunting, Optic Nerve Sheath Fenestration and Dural Venous Stenting for Medically Refractory Idiopathic Intracranial Hypertension: Systematic Review and Meta-Analysis. Ann Eye Sci (2018) 3:6. doi: 10.21037/aes.2018.05.01
51. Lichtenberg I, Blackwood E, Gordon J, Hammond S, Hawke S. The Prevalence of Idiopathic Intracranial Hypertension and Associated Co-Morbidities in Central Western New South Wales. J Neurol Neurosurg Psychiatry (2017) 88:e1. doi: 10.1136/jnnp-2017-316074.10
52. Miah L, Strafford H, Fonferko-Shadrach B, Hollinghurst J, Sawhney IMS, Hadjikoutis S, et al. Incidence, Prevalence and Healthcare Outcomes in Idiopathic Intracranial Hypertension: A Population Study. Neurology (2021) 96:e1251–61. doi: 10.1212/WNL.0000000000011463
53. Galloway L, Karia K, White AM, Byrne ME, Sinclair AJ, Mollan SP, et al. Cerebrospinal Fluid Shunting Protocol for Idiopathic Intracranial Hypertension for an Improved Revision Rate. J Neurosurg (2021) 8:1–6. doi: 10.3171/2021.5.JNS21821
54. Farahmand D, Hilmarsson H, Högfeldt M, Tisell M. Perioperative Risk Factors for Short Term Shunt Revisions in Adult Hydrocephalus Patients. J Neurol Neurosurg Psychiatry (2009) 80:1248–53. doi: 10.1136/jnnp.2007.141416
55. Sunderland GJ, Jenkinson MD, Conroy EJ, Gamble C, Mallucci CL. Neurosurgical CSF Diversion in Idiopathic Intracranial Hypertension: A Narrative Review. Life (2021) 11:393. doi: 10.3390/life11050393
56. Obi EE, Lakhani BK, Burns J, Sampath R. Optic Nerve Sheath Fenestration for Idiopathic Intracranial Hypertension: A 7-Year Review of Visual Outcomes in a Tertiary Centre. Clin Neurol Neurosurg (2015) 137:94–101. doi: 10.1016/j.clineuro.2015.05.020
57. Friedman DI, Quiros PA, Subramanian PS, Mejico LJ, Gao S, McDermott M, et al. Headache in Idiopathic Intracranial Hypertension: Findings From the Idiopathic Intracranial Hypertension Treatment Trial. Headache: J Head Face Pain (2017) 57:1195–205. doi: 10.1111/head.13153
58. Mollan SP, Grech O, Sinclair AJ. Headache Attributed to Idiopathic Intracranial Hypertension and Persistent Post-Idiopathic Intracranial Hypertension Headache: A Narrative Review. Headache: J Head Face Pain (2021) 61:808–16. doi: 10.1111/head.14125
Keywords: medically refractory, IIH, transverse sinus stenosis, stenting for IIH, cerebral venous hypertension, visual threat in IIH
Citation: Reid K, Winters HS, Ang T, Parker GD and Halmagyi GM (2022) Transverse Sinus Stenting Reverses Medically Refractory Idiopathic Intracranial Hypertension. Front. Ophthalmol. 2:885583. doi: 10.3389/fopht.2022.885583
Received: 28 February 2022; Accepted: 06 April 2022;
Published: 21 June 2022.
Edited by:
Marc Joshua Dinkin, NewYork-Presbyterian, United StatesReviewed by:
Prem Subramanian, University of Colorado, United StatesEvangelos Anagnostou, National and Kapodistrian University of Athens, Greece
Copyright © 2022 Reid, Winters, Ang, Parker and Halmagyi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kate Reid, a2F0ZS5yZWlkQGFudS5lZHUuYXU=
†ORCID: Kate Reid, orcid.org/0000-0003-3110-8893
H. Stephen Winters, orcid.org/0000-0001-5996-4857
Timothy Ang, orcid.org/0000-0001-8405-0459
Geoffrey Parker, orcid.org/0000-0001-8225-7835
G. Michael Halmagyi, orcid.org/0000-0002-9743-466X
 Kate Reid
Kate Reid H. Stephen Winters3†
H. Stephen Winters3† G. Michael Halmagyi
G. Michael Halmagyi