
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 25 March 2025
Sec. Genitourinary Oncology
Volume 15 - 2025 | https://doi.org/10.3389/fonc.2025.1568774
This article is part of the Research Topic Urothelial Neoplasms: An Integrated Approach to Prevention, Diagnostics, and Personalized Therapy View all articles
Background and objective: To explore the risk factors and construct a prediction model of the lymphovascular invasion (LVI) in patients with upper tract urothelial carcinoma (UTUC).
Methods: Clinical data of 143 UTUC patients treated in our hospital during Jan. 2010 and Dec. 2022 were retrospectively analyzed. The patients were divided into LVI positive group and LVI negative group according to the postoperative lymphovascular conditions. Kaplan-Meier method was used to evaluate the overall survival (OS) and cancer-specific survival (CSS) of the two groups, and the survival curve was drawn. The correlation between LVI and inclusion indexes was analyzed using univariate and ultivariate. A prediction model was established and receiver operating characteristic (ROC) curve was drawn to analyze the diagnostic value.
Results: The median survival time of LVI positive patients was 78 months (95%CI 44.47-111.53), lower than the 90months (95%CI 72.77-107.23) for LVI negative patients, and the 5-year OS of LVI positive patients was 53.0%, lower than that of LVI negative patients (79.6%). The difference was statistically significant (P=0.005). The 5-year CSS of LVI positive patients was 57.0%, lower than that of LVI negative patients (85.7%, P=0.009). The results of univariate analysis showed that there were statistically significant differences between the two groups (P < 0.05) in exfoliation cytology (P=0.044), hydronephrosis (P=0.015), preoperative fibrinogen level (P=0.003), lymph node status (P=0.014), pathological stage (P=0.001) and grade (P=0.047). Multivariate Logistic regression analysis showed that hydronephrosis (P=0.022), pathological stage (P < 0.001), lymph node status (P=0.025) and fibrinogen level (P=0.019) were independent factors influencing the occurrence of lymphovascular invasion, and the combination of four indexes above was better than any single index. the ROC curve showed that the area under the curve (AUC) of postoperative LVI was the largest when combined with the four predictors, and the AUC was 0.833 (95%CI 0.759-0.907). When the Youden index was 0.594, the sensitivity was 81.1%, and the specificity was 78.3%.
Conclusion: Lymphovascular invasion is related to hydronephrosis, pathological stage, lymph node condition and fibrinogen level. Patients with preoperative hydronephrosis, high pathological stage, lymph node metastasis and high fibrinogen level were at higher risk of lymphovascular invasion.
Urothelial carcinoma can be categorized into upper urinary tract and lower urinary tract divisions. Urothelial carcinoma originating from the pyelocaliceal cavities and ureter is referred to as upper tract urothelial carcinoma (UTUC). UTUC is a relatively uncommon form, accounting for only 5%-10% of all urothelial carcinomas. However, the prognosis for patients with UTUC is poorer compared to those with bladder urothelial carcinoma, with approximately 60% of UTUC patients being diagnosed at an advanced stage (1). Radical nephroureterectomy (RNU) along with bladder cuff resection has long been established as the gold standard treatment for high-risk UTUC, regardless of tumor location (1).
However, the high recurrence rate and poor prognosis following RNU remain significant challenges in the treatment of UTUC (2). Therefore, it is imperative to investigate the prognostic factors and risk of recurrence in UTUC patients. Retrospective studies on UTUC have reported a detection rate of approximately 20% for lymphovascular invasion (LVI) (3), which plays a crucial role in systemic dissemination (4) and negatively impacts patient prognosis. Research has demonstrated that LVI or tumor cell invasion into blood vessels or the lymphatic system may serve as important factors contributing to the unfavorable outcomes observed in solid tumors, such as gastric cancer (5). The growth of UTUC relies on neovascularization for nutritional support, with abundant neovascularization providing a pathway for tumor cells to enter circulation through blood or lymphatic vessels and subsequently metastasize to distant sites. Manoharan M et al. conducted a study involving 357 patients and discovered that LVI was significantly associated with T stage, lymph node metastasis, and tumor grade. Patients with LVI exhibited a markedly higher recurrence rate compared to those without LVI (P < 0.001), as well as a significantly lower long-term survival rate (P < 0.001) (6). Studies conducted by Margulis et al. (7) and Mellouli et al. (8) have also identified LVI as a predictor of postoperative recurrence and poor prognosis in UTUC patients. However, there is currently no clear consensus regarding which factors are more likely associated with LVI. Identifying these factors would be clinically significant as it could help identify patients at higher risk of disease recurrence, enabling early follow-up or adjuvant therapy interventions.
143 patients with UTUC who underwent radical surgery at a high-volume center in China between January 2010 and December 2022 were included in this study. Exclusion criteria comprised patients with other primary or secondary malignancies, those with incomplete clinicopathological data or missing follow-up information, and individuals diagnosed with distant metastases prior to surgery. Their age ranged from 49 to 83 years. There were 78 males (54.5%) and 65 females (45.5%). The tumors were located in the renal pelvis in 43 cases (30.1%), in the ureter in 80 cases (55.9%), and in both the renal pelvis and ureter in 20 cases (14.0%). We retrospectively collected the clinicopathologic data from the medical records, including age, sex, body mass index (BMI), occurrence of hypertension, diabetes and hydronephrosis, lymph node invasion, surgical margin, tumor location and side, T stage, tumor grade, as well as laboratory data including exfoliative cytology, fibrinogen levels, absolute neutrophil count, absolute lymphocyte count, absolute platelet count, and albumin levels. Elderly patients were defined according to the World Health Organization's definition of 65 years old or older. The tumor stage was determined based on the American Joint Committee on Cancer (AJCC) staging system. The tumor grade was defined according to the 2004 World Health Organization (WHO) classification system. LVI was defined as wall invasion, destruction, or intraluminal tumor thrombus of the small vein, arteriole, or lymphatic vessel of the tumor (9).
Firstly, all patients were divided into lymphovascular positive and negative according to the postoperative lymphovascular invasion. The median follow-up duration for all patients was 67 months. The study end was overall survival (OS) and cancer-specific survival (CSS) after RNU. The survival of two groups were compared and the clinicopathological characteristics was evaluated. Additionally, the univariate and multivariate binary logistic regression analyses were performed to evaluate the risk factors of lymphvascular positive patients. Lastly, a novel prediction model was established and its diagnostic value was assessed by constructing a receiver operating characteristic (ROC) curve. The study was approved by the institutional review board from The Luoyang Central Hospital Affiliated to Zhengzhou University. Written informed consent was obtained from the involving patient for the publication of this study. In order to confirm the original diagnosis, we invited an experienced urological pathologist to check all the pathological specimens again. Hydronephrosis was defined as abnormal dilation of the renal pelvis and calyces due to obstruction of the urinary system. Preoperative evaluation comprised color Doppler ultrasonography, CT, and/or MRI to confirm the presence of hydronephrosis and to evaluate potential systemic metastasis.
The Kaplan-Meier method was used to estimate the overall survival (OS) and cancer specific survival (CSS) of the patients, and the survival curve was drawn. The log-rank test was used to compare the OS and CSS. The measurement data that did not conform to the normal distribution were expressed as the median (lower quartile, upper quartile), and non-parametric test was used for comparison between groups. The measurement data conforming to normal distribution were described by mean ± standard deviation, and T-test was used for inter-group comparison. The statistical data were expressed as rate (%) and χ2 test was used for comparison between groups. Univariate analysis was employed to examine the risk factors associated with LVI in UTUC patients, while a binary logistic regression model was utilized for multivariate correlation analysis. The results of the multiple factor analysis were used to construct a novel prediction model, and its predictive value was evaluated using receiver-operating characteristics (ROC) curve. Statistical significance was defined as P < 0.05. A two-sided p-value of <0.05 was considered significant. All the statistical analyses were conducted using SPSS version 26.0 (IBM Corporation, Armonk, NY, USA).
A total of 143 patients with UTUC were included in this study, with 37 cases (25.9%) exhibiting LVI positive and 106 cases (74.1%) showing LVI negative status. A total of 68 patients exhibited positive lymph node status post-surgery. Among them, 18 patients underwent open or intraoperative procedures, while 125 patients received laparoscopic surgery. The median follow-up duration for all patients was 67 months, during which a total of 55 patients succumbed to mortality, including 14 LVI positive patients and 41 LVI negative patients. Among them, tumor specific deaths accounted for the demise of 42 patients, comprising 11 individuals with LVI positive and 31 individuals with LVI negative. UTUC patients with LVI positive exhibited a shorter median survival time at 78 months (95% CI:44.47-111.53) compared to those without LVI involvement (90 months;95%CI:72.77-107.23). The five-year overall survival rate for individuals with LVI positive was lower at 53%, in contrast to the higher rate observed among those without LVI involvement(79.6%). This difference demonstrated statistical significance (P=0.005; Figure 1A). Similarly, the five-year cancer-specific survival rate stood at 57% for individuals with LVI positive while it reached 85.7% for those with LVI negative. The statistical significance remained evident(P=0.009; Figure 1B).
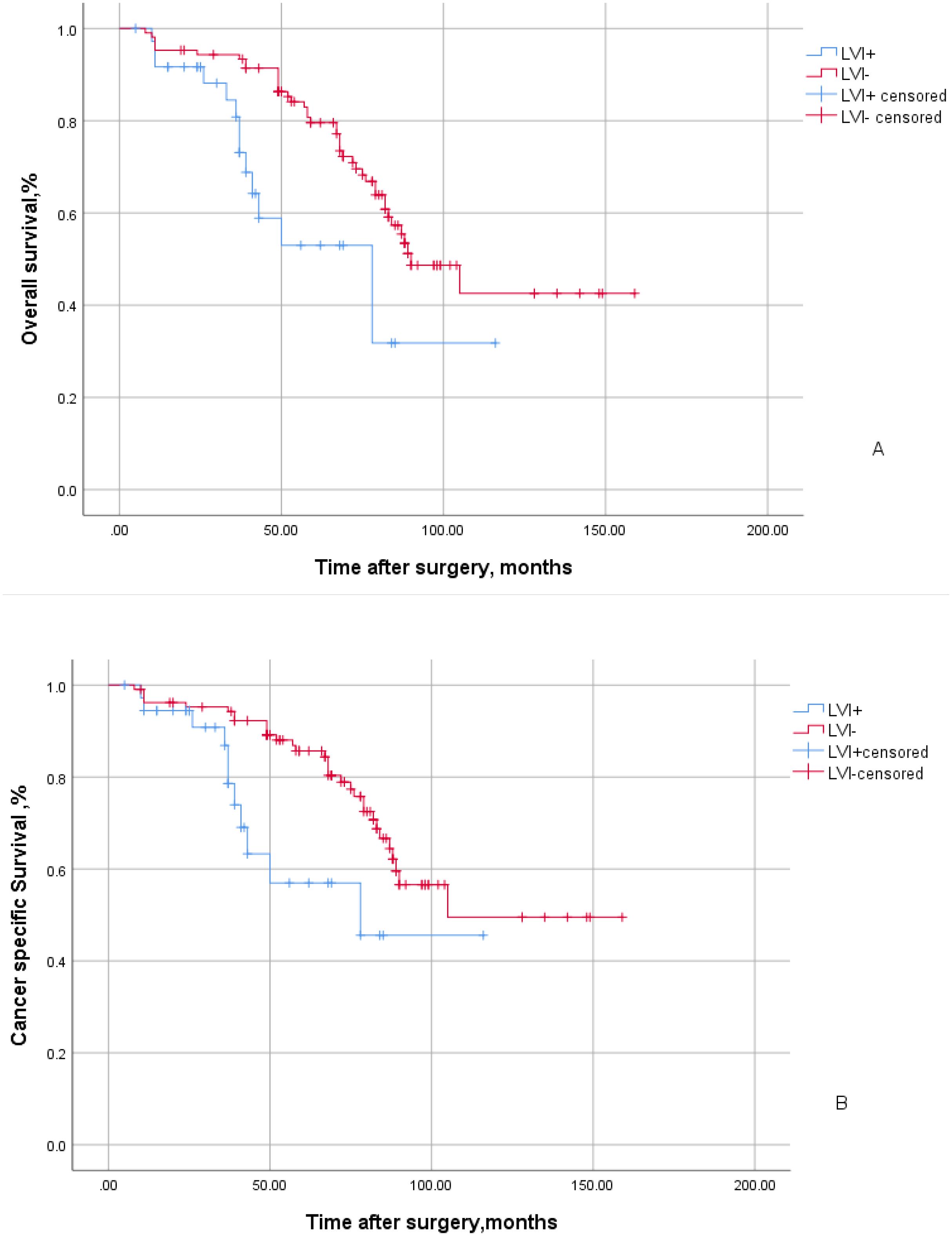
Figure 1. Kaplan-Meier curves of overall survival (A) and cancer specific survival (B) stratified by LVI in 143 patients with UTUC. The blue line indicates LVI positive status and the red line indicates LVI negative status.
Among the 143 patients, 78 (54.5%) had comorbid hypertension, 47 (32.9%) had comorbid diabetes, and 72 (50.3%) had comorbid hydronephrosis. To further elucidate LVI positive risk factors, we conducted single factor analysis which revealed no statistical significance (P > 0.05) in age, sex, hypertension, diabetes, BMI, albumin levels, surgical margin, tumor location and side between the two groups. However, a presence of significant differences were observed in positive exfoliative cytology, preoperative hydronephrosis, fibrinogen levels, positive lymph nodes, and pathological stage and grade (P < 0.05) (Table 1).
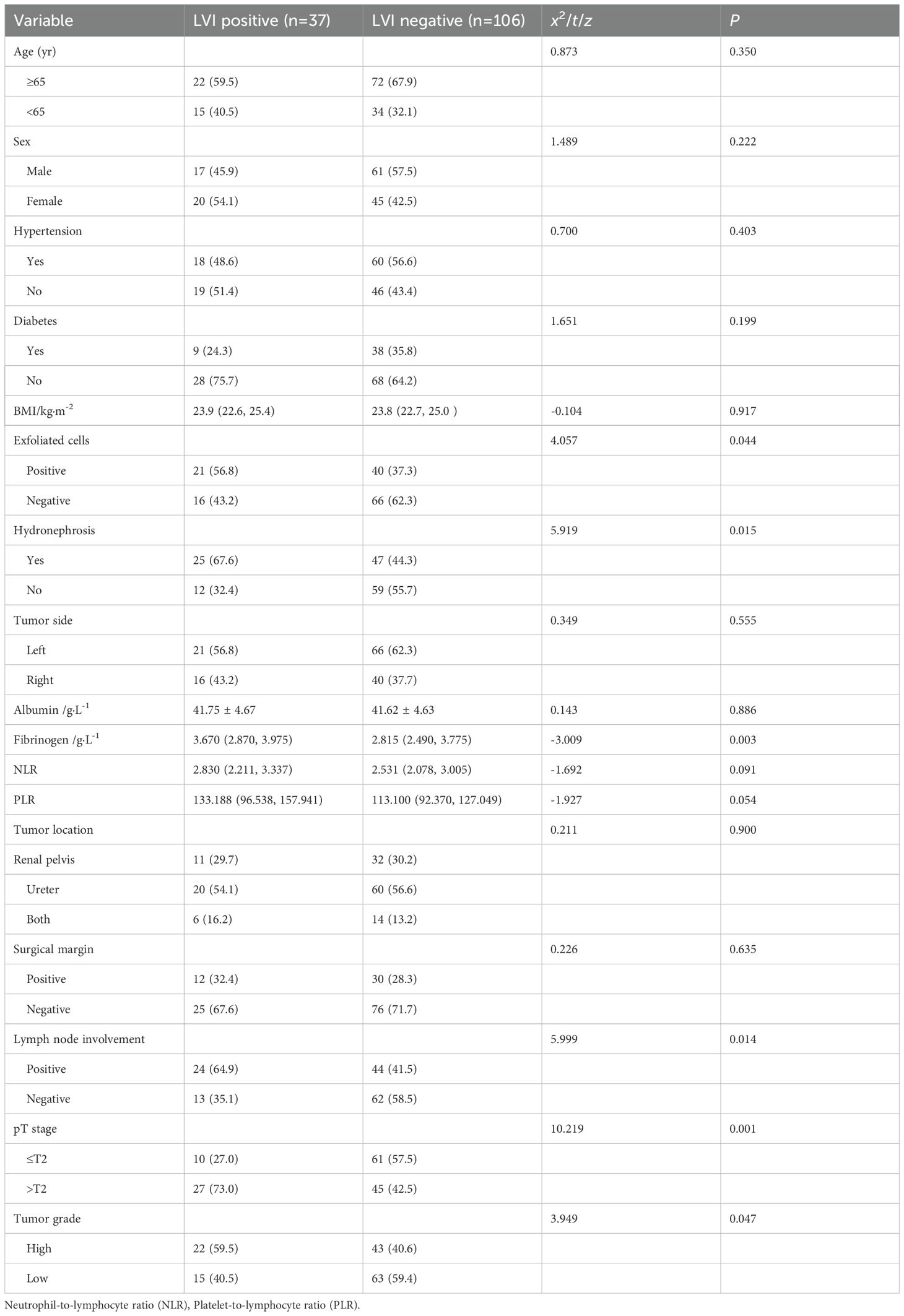
Table 1. The clinicopathological characteristics of UTUC patients with LVI positive and LVI negative groups.
In order to further investigate the risk factors for lymphovascular invasion (LVI), we conducted a multivariable logistic regression analysis, including the following indicators: exfoliated cytology, presence of hydronephrosis and lymph node metastasis, fibrinogen level, tumor grade, and pathological stage. The results revealed significant associations between preoperative hydronephrosis, advanced pathological stage, lymph node metastasis, and fibrinogen level with LVI positivity. However, no significant correlations were observed between pathological grade or exfoliative cytology and LVI positivity (Table 2).
A joint predictor was established using four independent risk factors, including hydronephrosis, lymph node metastasis, pathological stage, and fibrinogen level, to predict positive LVI after UTUC. A ROC curve was constructed and the largest area under the curve (AUC) for the joint predictors was found to be 0.833 (95%CI: 0.759-0.907, P < 0.001). The maximum Youden index was 0.594, with a sensitivity of 81.1% and a specificity of 78.3%, indicating that the combination of the four predictors had a high predictive value for positive LVI after UTUC. (Figure 2)
In recent years, UTUC has emerged as a prominent research focus in the field of urology both domestically and internationally. The absence of symptoms and delayed diagnosis often result in muscle invasive or locally advanced tumors (56%) at the time of presentation, leading to a lower survival rate compared to bladder urothelial carcinoma. Patients with invasive UTUC involving the muscle layer, specifically T2 and T3 stages, have a 5 year survival rate below 50%, whereas those with T4 stage exhibit less than 10% survival rate (10). Therefore, it is crucial to identify prognostic factors associated with UTUC for risk stratification and effective disease management.
In our study, the rate of lymphovascular invasion (LVI) positivity was 25.9%, which is consistent with the LVI detection rate of 25% reported in previous studies (11, 12). Furthermore, numerous prognostic factors associated with disease recurrence or survival have been identified in prior research, among which LVI plays a pivotal and significant role in tumor dissemination (13). The prognostic value of LVI has been documented in various urological malignancies including penile cancer, prostate cancer, and bladder cancer (14). Recently, there have been relevant studies investigating the correlation between LVI and prognosis in patients with UTUC (15). Cancer growth is frequently associated with an expansion of lymphatic vessels (lymphangiogenesis) within and surrounding the primary tumor (histologically most frequently detected with the D2-40 anti-podoplanin antibody) and with lymphangiogenesis in tumor-draining lymph nodes. A large number of experimental and clinical studies have clearly demonstrated that the density of tumor-associated lymphatic vessels, the detection of lymphatic invasion by tumor cells, and intratumoral expression of the main lymphangiogenic growth factor VEGF-C all correlate with lymph node metastasis and poor patient outcome in many cancer types (16). Elenkov AA et al. (17) and Kamai T et al. (18) have highlighted that LVI serves as an independent prognostic factor for overall survival in UTUC patients following surgery. These findings align with the results of our study, emphasizing the importance of elucidating the risk factors associated with LVI in postoperative patients' prognosis.
Previous studies have demonstrated that LVI is associated with prognostic factors identified in UTUC, such as advanced tumor stage, higher pathological grade, and tumor necrosis (19, 20). A meta-analysis revealed a significant correlation between LVI and poor cancer-specific survival, overall survival, and recurrence-free survival. Furthermore, LVI was significantly associated with advanced tumor stage, higher pathological grade, and lymph node metastasis (21). Similarly, Lee HY et al. (22) reported a significant correlation between LVI and tumor stage and grade. In this study of 143 UTUC patients, there were 37 cases of LVI positive patients with pathological stage > T2; among them, 27 cases (73.0%) had high-grade tumors (59.5%). Among the 106 cases of LVI negative patients with pathological staging > T2, there were a total of 45 cases (42.5%) with high grade tumors (40.6%). Univariate analysis indicated a significant difference between LVI positive and LVI negative patients (P=0.001). Multivariate analysis further confirmed that pathological stage remained significantly correlated with LVI positivity (P < 0.001).
The presence of lymph node metastasis has been identified as a significant indicator of tumor progression in previous studies. Li X et al. found that a total of 73 patients (18.7%) had local recurrence within a median follow-up of 41 months (range 3-80 months), and multivariate analysis showed that lymph node metastasis was an independent predictor of increased local recurrence (23). Sato R et al. (24) discovered that the presence of lymph node metastasis was an independent prognostic factor for poor overall survival in 68 patients with UTUC who underwent radical nephroureterectomy (RNU). Milojevic B et al. (25) demonstrated that lymph node metastasis independently predicted reduced tumor specific survival. The results of both univariate and multivariate analyses revealed a significant association between LVI and lymph node status (P < 0.05), suggesting that lymph node metastasis can serve as a risk factor for postoperative LVI in UTUC patients.
In patients with bladder cancer, Stimson CJ et al. (26) reported that concomitant hydronephrosis before radical cystectomy was an independent predictor of adverse pathological features. Messer JC et al. (27) and Chung PH et al. (28) also reported this relationship in their separate studies of patients with high-grade UTUC. Sakano S et al. (29) found a significant correlation between positive urine cytology and hydronephrosis and LVI through their study. Ito Y et al. (30) found that the higher the degree of hydronephrosis, the higher the tumor pathological stage and the positive LVI were significantly correlated. This was consistent with the conclusions drawn in this paper. The incidence of preoperative hydronephrosis was 25 cases (67.6%) among the 37 patients with lymphovascular invasion positive, while it was 47 cases (44.3%) among the 106 patients with LVI negative patients. Both univariate analysis (P=0.015) and multivariate analysis (P=0.022) demonstrated a statistically significant difference. Qian S et al. (31) propose that hydronephrosis can be attributed to luminal obstruction, intraluminal infiltration, or exogenous compression. Consequently, hydronephrosis can result in outward expansion and longitudinal thinning of the upper urinary tract, thereby facilitating the dissemination of cancer cells to local or distant organs. Another study also demonstrated a higher likelihood of developing high grade tumors and lymphovascular invasion positive following radical nephroureterectomy treatment in patients with positive urine cytology (32). The positive preoperative exfoliative cytology was observed in 21 out of 37 patients (56.8%) with lymphovascular invasion (LVI) and in 40 out of 106 patients (37.3%) without LVI. Univariate analysis (P=0.044) demonstrated that urine exfoliative cytology independently increased the risk of positive LVI. However, multivariate analysis showed no statistically significant difference between urine cytology positivity and LVI occurrence (P = 0.071). Further validation is required in a multi-institutional UTUC database to establish the association between urine cytology and LVI.
In conducting research on the risk factors associated with LVI positivity, in addition to typical clinical and pathological factors such as pathological staging classification, other factors including clotting factor inflammation, malnutrition, and systemic influences have been reported to significantly impact tumor occurrence and development (33). This study incorporates the analysis of fibrinogen levels, albumin levels, NLR (neutrophil-to-lymphocyte ratio), and PLR (platelet-to-lymphocyte ratio). Both univariate and multivariate analyses demonstrate that fibrinogen level is a statistically significant factor. Firstly, fibrinogen can accumulate around cancer cells, promoting their proliferation and serving as a source of certain growth factors such as vascular endothelial growth factor and fibroblast growth factor, which facilitate tumor angiogenesis (34). Secondly, fibrinogen and its degradation products can enhance platelet adhesion to cancer cells, thereby promoting cancer metastasis. Consequently, the ascending levels of fibrinogen can serve as an indicator of tumor inhibition, while a persistent increase in fibrinogen levels often suggests the potential for tumor metastasis (35). Additionally, the hematological marker fibrinogen holds clinical value due to its affordability and easy accessibility.
However, The present study has certain limitations. The first limitation lies in the inherent constraints of a single-center retrospective study. Secondly, even after employing rigorous multivariate analysis to control for potential confounding factors, it is important to acknowledge that complete elimination of selection bias may not have been achieved. The rarity and low incidence of UTUC necessitate further expansion of the number of risk factors and sample size, as well as the need for multi-center prospective studies to provide additional confirmation and obtain more precise conclusions.
After conducting research, we found that the hydronephrosis, high pathologic stage, lymph node metastasis, and high level of fibrinogen were identified as independent risk factors for LVI positivity. This study provides clinicians with valuable insights to assess the condition of UTUC patients with LVI and identify those at a higher risk. Early preoperative management and auxiliary treatment based on these findings hold significant clinical significance.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. The studies involving human participants were reviewed and approved by The Luoyang Central Hospital Affiliated to Zhengzhou University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
QL: Writing – original draft. PW: Writing – original draft. YK: Writing – original draft. XL: Writing – review & editing. HZ: Writing – review & editing. JS: Writing – original draft, Writing – review & editing. JY: Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Roupret M, Seisen T, Birtle AJ, Capoun O, Comperat EM, Dominguez-Escrig JL, et al. European association of urology guidelines on upper urinary tract urothelial carcinoma: 2023 update. Eur Urol. (2023) 84:49–64. doi: 10.1016/j.eururo.2023.03.013
2. Margulis V, Shariat SF, Matin SF, Kamat AM, Zigeuner R, Kikuchi E, et al. Outcomes of radical nephroureterectomy: a series from the upper tract urothelial carcinoma collaboration. Cancer. (2009) 115:1224–33. doi: 10.1002/cncr.v115:6
3. Novara G, Matsumoto K, Kassouf W, Walton TJ, Fritsche HM, Bastian PJ, et al. Prognostic role of lymphovascular invasion in patients with urothelial carcinoma of the upper urinary tract: An international validation study. Eur Urol. (2010) 57:1064–71. doi: 10.1016/j.eururo.2009.12.029
4. Alitalo K, Mohla S, Ruoslahti E. Lymphangiogenesis and cancer: meeting report. Cancer Res. (2004) 64:9225–9. doi: 10.1158/0008-5472.CAN-04-2475
5. Bu Z, Zheng Z, Li Z, Zhang L, Wu A, Wu X, et al. Lymphatic vascular invasion is an independent correlated factor for lymph node metastasis and the prognosis of resectable T2 gastric cancer patients. Tumou Biol. (2013) 34:1005–12. doi: 10.1007/s13277-012-0637-3
6. Manoharan M, Katkoori D, Kishore TA, Jorda M, Luongo T, Soloway MS. Lymphovascular invasion in radical cystectomy specimen: is it an independent prognostic factor in patients without lymph node metastases? World J Urol. (2010) 28:233–7. doi: 10.1007/s00345-009-0448-3
7. Margulis V, Shariat SF, Matin SF, Kamat AM, Zigeuner R, Kikuchi E, et al. Outcomes of radical nephroureterectomy: A series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer. (2009) 115:1224–33. doi: 10.1002/cncr.v115:6
8. Mellouli M, Charfi S, Smaoui W, Kallel R, Khabir A, Bouacida M, et al. Prognostic role of lymphovascular invasion in patients with urothelial carcinoma of the upper urinary tract. Urol J. (2017) 14:5008–12. doi: 10.22037/uj.v14i5.3673
9. Ito T, Murakawa T, Sato H, Tanabe A, Maekawa M, Yoshida Y, et al. Simple preoperative computed tomography image analysis shows good predictive performance for pathological vessel invasion in clinical stage IA non-small cell lung cancer. Interact Cardiovasc Thorac Surg. (2012) 15:633–8. doi: 10.1093/icvts/ivs163
10. Lughezzani G, Burger M, Margulis V, Matin SF, Novara G, Roupret M, et al. Prognostic factors in upper urinary tract urothelial carcinomas: a comprehensive review of the current literature. Eur Urol. (2012) 62:100–4. doi: 10.1016/j.eururo.2012.02.030
11. Kikuchi E, Margulis V, Karakiewicz PI, Roscigno M, Mikami S, Lotan Y, et al. Lymphovascular invasion predicts clinical outcomes in patients with node-negative upper tract urothelial carcinoma. J Clin Oncol. (2009) 27:612–8. doi: 10.1200/JCO.2008.17.2361
12. Liu W, Sun L, Guan F, Wang F, Zhang G. Prognostic value of lymphovascular invasion in upper urinary tract urothelial carcinoma after radical nephroureterectomy: a systematic review and meta-analysis. Dis Markers. (2019) 2019:1–15. doi: 10.1155/2019/7386140
13. Sundar SS, Ganesan TS. Role of lymphangiogenesis in cancer. J Clin Oncol. (2007) 25:4298–307. doi: 10.1200/JCO.2006.07.1092
14. Hurel S, Roupret M, Ouzzane A, Rozet F, Xylinas E, Zerbib M, et al. Impact of lymphovascular invasion on oncological outcomes in patients with upper tract urothelial carcinoma after radical nephroureterectomy. BJU Int. (2023) 111:1199–207. doi: 10.1111/bju.12116
15. Takemoto K, Hayashi T, Hsi RS, Kobatake K, Sekino Y, Kitano H, et al. Histological variants and lymphovascular invasion in upper tract urothelial carcinoma can stratify prognosis after radical nephroureterectomy. Urol Oncol. (2022) 40:539.e9–16. doi: 10.1016/j.urolonc.2022.08.010
16. Dieterich LC, Tacconi C, Ducoli L, Detmar M. Lymphatic vessels in cancer. Physiol Rev. (2022) 102:1837–79. doi: 10.1152/physrev.00039.2021
17. Elenkov AA, Timev A, Dimitrov P, Vasilev V, Krastanov A, Georgiev M, et al. Clinicopathological prognostic factors for upper tract urothelial carcinoma. Cent Eur J Urol. (2016) 69:57–62. doi: 10.5173/ceju.2016.713
18. Kamai T, Shirataki H, Nakanishi K, Furuya N, Kambara T, Abe H, et al. Increased Racl activity and Pakl overexpression are associated with lymphovascular invasion and lymph node metastasis of upper urinary tract cancer. BMC Cancer. (2010) 10:164. doi: 10.1186/1471-2407-10-164
19. Kikuchi E, Margulis V, Karakiewicz PI, Roscigno M, Mikami S, Lotan Y, et al. Lymphovascular invasion predicts clinical outcomes in patients with node-negative upper tract urothélial carcinoma. J Clin Oncol. (2009) 27:612–8. doi: 10.1200/JCO.2008.17.2361
20. Novara G, Matsumoto K, Kassouf W, Walton TJ, Fritsche HM, Bastian PJ, et al. Prognostic role of lymphovascular invasion in patients with urothelial carcinoma of the upper urinary tract: an international validation study. Eur Urol. (2010) 57:1064–71. doi: 10.1016/j.eururo.2009.12.029
21. Zhang L, Wu B, Zha Z, Zhao H, Yuan J, Feng Y. The prognostic value of lymphovascular invasion in patients with upper tract urinary carcinoma after surgery: an updated systematic review and meta-analysis. Front Oncol. (2020) 10:487. doi: 10.3389/fonc.2020.00487
22. Lee HY, Li CC, Huang CN, Ke HL, Li WM, Liang PI, et al. Prognostic significance of lymphovascular invasion in upper urinary tract urothelial carcinoma is influenced by tumor location. Ann Surg Oncol. (2015) 22:1392–400. doi: 10.1245/s10434-014-4103-x
23. Li X, Cui M, Gu X, Fang D, Li H, Qin S, et al. Pattern and risk factors of local recurrence after nephroureterectomy for upper tract urothelial carcinoma. World J Surg Oncol. (2020) 18:114. doi: 10.1186/s12957-020-01877-w
24. Sato R, Watanabe K, Matsushita Y, Watanabe H, Motoyama D, Ito T, et al. Prognostic assessments in patients with upper tract urothelial carcinoma undergoing radical nephroureterectomy and systematic regional lymph node dissection. Urologia. (2022) 89:354–7. doi: 10.1177/03915603211034943
25. Milojevic B, Bumbasirevic U, Santric V, Kajmakovic B, Dragicevic D, Radisavcevic D, et al. Prognostic significance of tumor multifocality on outcomes in patients with upper tract urothelial carcinoma after radical nephroureterectomy: A cohort study. Curr Probl Cancer. (2021) 45:100747. doi: 10.1016/j.currproblcancer.2021.100747
26. Stimson CJ, Cookson MS, Barocas DA, Clark PE, Humphrey JE, Patel SG, et al. Preoperative hydronephrosis predicts extravesical and node positive disease in patients undergoing cystectomy for bladder cancer. J Urol. (2010) 183:1732–7. doi: 10.1016/j.juro.2010.01.028
27. Messer JC, Terrell JD, Herman MP, Ng CK, Scherr DS, et al. Multi-institutional validation of the ability of preoperative hydronephrosis to predict advanced pathologic tumor stage in upper-tract urothelial carcinoma. Urol Oncol. (2013) 31:904–8. doi: 10.1016/j.urolonc.2011.07.011
28. Chung PH, Krabbe LM, Darwish OM, Westerman ME, Bagrodia A, Gayed BA, et al. Degree of hydronephrosis predicts adverse pathological features and worse oncologic outcomes in patients with high-grade urothelial carcinoma of the upper urinary tract. Urol Oncol. (2014) 32:981–8. doi: 10.1016/j.urolonc.2014.02.018
29. Sakano S, Inamoto T, Inoue R, Matsumoto H, Nagao K, Yamamoto Y, et al. Positive voided urine cytology predicts worse pathological findings of nephroureterectomy specimens in patients with upper tract urothelial carcinoma: Does selective ureteral cytology have an additional efficacy? Jpn J Clin Oncol. (2015) 45:968−972. doi: 10.1093/jjco/hyv114
30. Ito Y, Kikuchi E, Tanaka N, Miyajima A, Mikami S, et al. Preoperative hydronephrosis grade independently predicts worse pathological outcomes in patients undergoing nephroureterectomy for upper tract urothelial carcinoma. Urol. (2011) 185:1621–6. doi: 10.1016/j.juro.2010.12.035
31. Qian S, et al. Preoperative hydronephrosis predicts adverse pathological features and postoperative survival in patients with high-grade upper tract urothelial carcinoma. Int Braz J Urol. (2021) 47:159–68. doi: 10.1590/s1677-5538.ibju.2020.0021
32. Tanaka N, Kikuchi E, Kanao K, Matsumoto K, Shirotake S, Kobayashi H, et al. The predictive value of positive urine cytology for outcomes following radical nephroureterectomy in patients with primary upper tract urothelial carcinoma: A multi−institutional study. Urol Oncol. (2014) 32:e19−e26. doi: 10.1016/j.urolonc.2013.07.003
33. Ku JH, et al. Preoperative serum albumin as a prognostic factor in patients with upper urinary tract urothelial carcinoma. Int Braz J Urol. (2014) 40:753–62. doi: 10.1590/S1677-5538.IBJU.2014.06.06
34. Calıskan S, Sungur M. Fibrinogen and D-dimer levels in prostate cancer: Preliminary results. Prostate Int. (2017) 5:110–2. doi: 10.1016/j.prnil.2017.05.001
Keywords: upper tract urothelial carcinoma, nephroureterectomy, lymphovascular invasion, risk factors, prediction model
Citation: Li Q, Wei P, Kang Y, Li X, Zhang H, Yang J and Sun J (2025) To explore the risk factors of lymphovascular invasion in patients with upper tract urothelial carcinoma and construct a prediction model. Front. Oncol. 15:1568774. doi: 10.3389/fonc.2025.1568774
Received: 30 January 2025; Accepted: 06 March 2025;
Published: 25 March 2025.
Edited by:
Sanja Stifter-Vretenar, Skejby Sygehus, DenmarkReviewed by:
Žilvinas Venclovas, Lithuanian University of Health Sciences, LithuaniaCopyright © 2025 Li, Wei, Kang, Li, Zhang, Yang and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiantao Sun, amlhbnRhb2h4QDE2My5jb20=; Jinhui Yang, cHJpbmNlcHNAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.