
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 11 March 2025
Sec. Genitourinary Oncology
Volume 15 - 2025 | https://doi.org/10.3389/fonc.2025.1566848
This article is part of the Research TopicHarnessing Big Data for Precision Medicine: Revolutionizing Diagnosis and Treatment StrategiesView all 39 articles
 Huadong Xie1,2†
Huadong Xie1,2† Yuanbi Huang2†
Yuanbi Huang2† Chengjie Ban3,4†
Chengjie Ban3,4† Wei Wei5
Wei Wei5 Han Tang3,4
Han Tang3,4 Qingming Huang3,4
Qingming Huang3,4 Zhengwei Su3,4
Zhengwei Su3,4 Zhi Cheng1,4
Zhi Cheng1,4 Tianling Liao4
Tianling Liao4 Kangji Liao6,4
Kangji Liao6,4 Liquan Zhou1*
Liquan Zhou1* Xianlin Yi1,3,6,7*
Xianlin Yi1,3,6,7*Objective: We evaluated the prognostic significance of the Lactate Dehydrogenase-to-Serum Albumin Ratio (LAR), Fibrinogen-to-Albumin Ratio (FAR), and Platelet-to-Lymphocyte Ratio (PLR) in patients with high-grade urothelial carcinoma (HGUC) of the bladder who underwent radical cystectomy (RC). These markers have been reported to be associated with the prognosis of various cancers.
Methods: A retrospective analysis was conducted on HGUC patients who underwent RC at Guangxi Medical University Cancer Hospital between January 2013 and June 2021. Optimal cutoff values for LAR, FAR, and PLR were established. Kaplan-Meier survival analysis was used to evaluate survival outcomes, while univariate and multivariable Cox regression analyses identified independent prognostic factors. A nomogram was developed to predict survival, with validation through time-dependent receiver operating characteristic (ROC) curves, calibration plots, and decision curve analysis (DCA).
Results: A total of 180 patients were included, with a follow-up period ranging from 2 to 127 months (49.28 ± 37.87 months). The optimal cutoff values for LAR, PLR, and FAR were 4.46, 139.68, and 0.13, respectively. Multivariable Cox regression identified tumor stage, LAR, PLR, and FAR as independent prognostic factors. Specifically, Stage III (HR = 25.44, 95% CI: 5.20–124.35, p < 0.001) and Stage IV (HR = 11.28, 95% CI: 3.18–40.05, p < 0.001) were independent risk factors for poor survival. A low PLR (HR = 0.45, 95% CI: 0.27–0.76, p = 0.003), low FAR (HR = 0.51, 95% CI: 0.29–0.89, p = 0.018), and low LAR (HR = 0.39, 95% CI: 0.23–0.67, p < 0.001) were independently associated with improved survival. The nomogram demonstrated high accuracy in predicting 1-, 3-, and 5-year overall survival (OS), with area under the curve (AUC) values of 0.866, 0.84, and 0.831, respectively. Further validation confirmed the model’s stability and clinical applicability.
Conclusion: LAR, PLR, and FAR are promising prognostic factors for HGUC of the bladder following RC, showing substantial potential for prognostic evaluation.
Bladder cancer represents a significant global public health challenge and is the second most common malignancy of the urinary system. According to 2022 data, bladder cancer ranks ninth in global incidence and thirteenth in cancer-related mortality (1). Among bladder cancers, urothelial carcinoma is the most prevalent histological subtype, which can be classified into low-grade and high-grade urothelial carcinoma based on pathological grading (2). HGUC is associated with a higher recurrence rate and increased mortality compared to low-grade urothelial carcinoma (3, 4). Despite significant advances in bladder cancer treatment, including surgery, chemotherapy, and immunotherapy, RC remains a common treatment option for patients with HGUC that is clinically staged as high T without distant metastasis (5, 6). However, HGUC is highly malignant, strongly correlating with tumor progression and cancer death rates (3). Therefore, there is an urgent need for effective prognostic factor to predict clinical outcomes more accurately and to guide personalized treatment strategies.
In recent years, there has been increasing interest in hematological indices associated with systemic inflammation and immune status. Studies have shown that indicators such as the LAR, PLR, and FAR are closely linked to the prognosis of various cancers (7–9). Inflammation and immune responses can promote tumor proliferation, invasion, and other mechanisms, accelerating cancer progression (10). Although some studies suggest an association between serum inflammatory markers and bladder cancer prognosis, their clinical significance and prognostic value in HGUC of the bladder have yet to be fully validated. Thus, further reliable evidence is required to explore the clinical applicability of these factors (11–13).
This study aims to explore the potential of hematological indicators, including LAR, PLR, and FAR, as prognostic tools for HGUC of the bladder by analyzing their correlation with OS following RC. The ultimate goal is to construct a nomogram model that can provide more accurate predictions of OS for bladder cancer patients following RC.
This retrospective study analyzed the clinical data of 180 patients with HGUC of the bladder who underwent RC at the Department of Urology, Guangxi Medical University Cancer Hospital, between January 2013 and June 2021. Tumor histological type, grade, and stage were determined according to the 2016 WHO classification and the 8th edition of the American Joint Committee on Cancer (AJCC) staging manual (2, 6). The inclusion criteria were: (I) histologically or clinically diagnosed HGUC≥T1; (II) received RC; (III) complete routine preoperative blood test results within one week prior to surgery; (IV) aged 18 years or older; (V) complete clinical data and follow-up information. The exclusion criteria were: (I) concurrent malignancies; (II) comorbidities affecting survival, such as multi-organ failure, liver disease, autoimmune diseases, hematological disorders, and infectious diseases; (III) history of preoperative antitumor therapy, including radiotherapy, chemotherapy, or immunotherapy; (IV) incomplete clinical or pathological data; (V) loss to follow-up or incomplete follow-up data.
The surgical approaches include open and laparoscopic radical cystectomy, both accompanied by urinary diversion. Radical cystectomy is employed for patients with locally resectable muscle-invasive bladder cancer (stages T2-T4a) or high-risk T1, particularly in cases of multifocality or recurrence. For selected patients with advanced-stage disease, palliative cystectomy is performed to alleviate symptoms and improve quality of life.
Clinical data, including patient age, sex, surgical method, pathological results, TNM stage, grade, muscularis infiltration, lymph node metastasis, and distant metastasis, were collected. Preoperative blood test data were also collected, and several indicators were calculated using the following formulas: NLR = neutrophil count/lymphocyte count; LAR = lactate dehydrogenase/serum albumin; PLR = platelet count/lymphocyte count; SII = (neutrophil count × platelet count)/lymphocyte count; MLR = monocyte count/lymphocyte count; FAR = fibrinogen/albumin; PIV = (neutrophil count × platelet count × monocyte count)/lymphocyte count. The optimal cutoff values for NLR, PLR, SII, MLR, FAR, LAR, and PIV were determined using Kaplan-Meier survival analysis with the survminer R package, and patients were divided into high and low groups. Kaplan-Meier survival analysis and log-rank tests were performed using the Survival R package.
Patients were advised to undergo chest and abdominal CT scans every 3–6 months during the first two years post-surgery, followed by annual scans thereafter. Follow-up was conducted via telephone or outpatient visits to monitor patients’ general condition. The follow-up period ended in June 2024. OS was defined as the time from surgery to death.
Data were analyzed using R software (version 4.4.1), IBM SPSS Statistics (version 25), and Zstats 1.0 (www.zstats.net). Continuous variables were expressed as mean ± standard deviation (SD), and categorical variables were presented as percentages. Fisher’s exact test or the chi-square test was used for categorical variables, and t-tests or nonparametric tests were applied to continuous variables based on their distribution. In univariate Cox regression analysis, variables with a p-value < 0.05 were included in the multivariable Cox analysis, and stepwise regression was used to determine the final variables for constructing the nomogram. The accuracy of the nomogram was evaluated using ROC curves and calibration plots. DCA was used to assess the clinical utility of the nomogram, with curves above the “None” and “All” lines indicating a net benefit, suggesting the model’s applicability in clinical practice. Statistical significance was defined as P < 0.05.
This study was approved by the Ethics Committee of Guangxi Medical University Cancer Hospital (No. KY2020069), and all procedures were performed in accordance with the Declaration of Helsinki. The requirement for written informed consent was waived due to the retrospective nature of the study.
A total of 180 patients who underwent RC were included in this study (Figure 1), with follow-up periods ranging from 2 to 127 months (49.28 ± 37.87 months). Among these, 161 were male (89.44%) and 19 were female (10.56%). The mean age was 66.32 ± 11.50 years. The patients were categorized into two groups based on survival status: 94 patients were alive (52.22%) and 86 patients had died (47.78%). Regarding clinical and pathological features, 82 patients (45.56%) were in stage T1, while 98 patients (54.44%) had tumors staged >T1. N0 status was observed in 147 patients (81.67%), while 33 patients (18.33%) had ≥N0 status. M0 status was found in 164 patients (91.11%), and 16 patients (8.89%) had M1 status. Muscle invasion was present in 102 patients (56.67%), while 78 patients (43.33%) had non-muscle-invasive bladder cancer. The optimal cutoff values for NLR, PLR, SII, MLR, FAR, LAR, and PIV were 2.16, 139.68, 648.05, 0.29, 0.13, 4.46, and 333.27, respectively. Based on these cutoff values, patients were classified into high and low groups. Kaplan-Meier analysis indicated that higher preoperative NLR, PLR, SII, MLR, FAR, LAR, and PIV were associated with poorer OS (log-rank P < 0.001, Figure 2). Detailed baseline clinical characteristics of the study population are presented in Table 1.
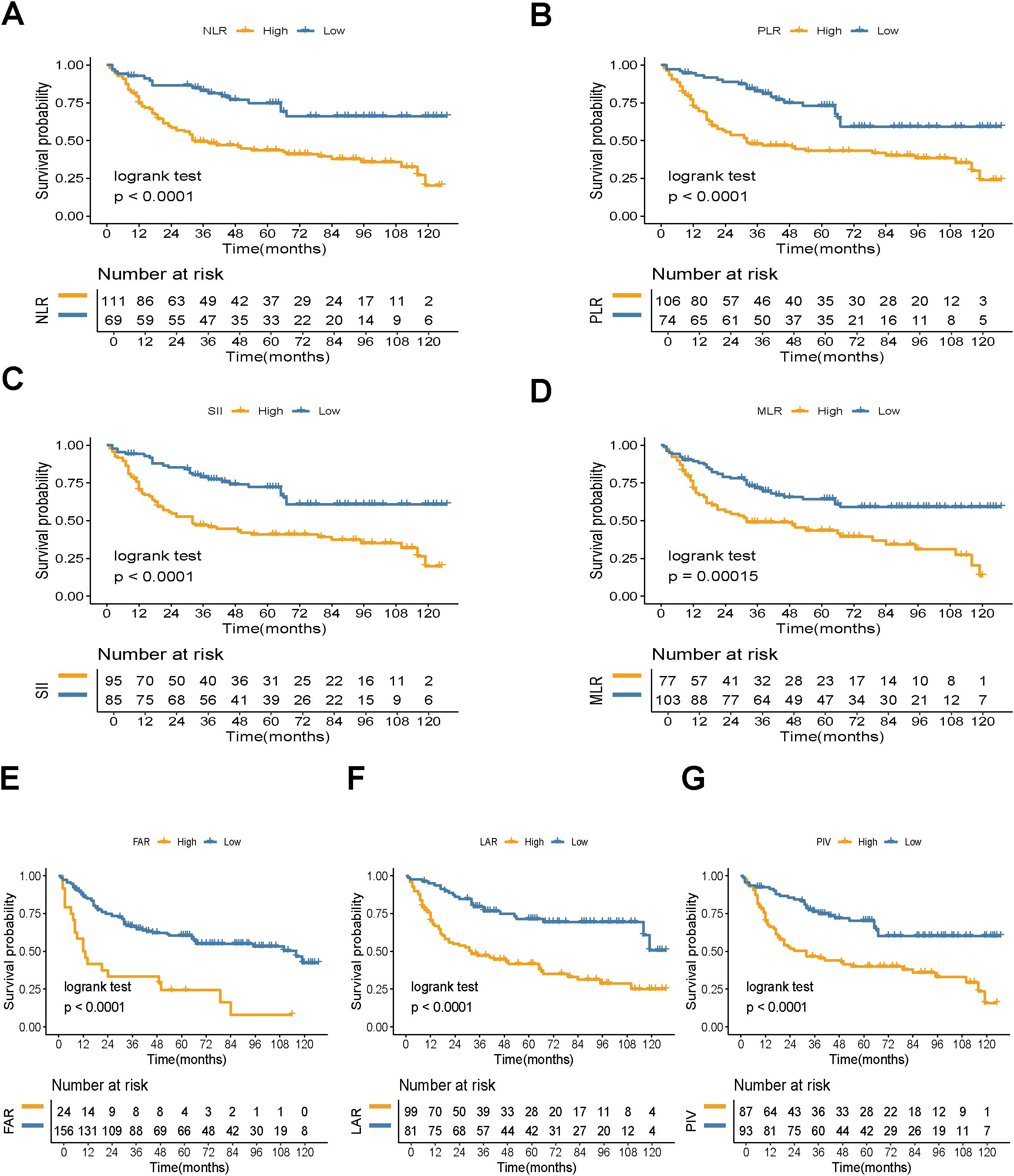
Figure 2. Kaplan-Meier estimate of OS. Kaplan-Meier survival curves for OS based on (A) NLR, (B) PLR, (C) SII, (D) MLR, (E) FAR, (F) LAR, and (G) PIV levels (log-rank test, P < 0.05). NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; SII, systemic immune-inflammation index; MLR, monocyte-to-lymphocyte ratio; FAR, fibrinogen-to-albumin ratio; LAR, lactate dehydrogenase-to-albumin ratio; PIV, pan-immune-inflammation value. OS, overall survival.
Univariate Cox regression analysis identified age, tumor TNM stage, muscle invasion, lymph node metastasis, distant metastasis, NLR, PLR, SII, MLR, FAR, LAR, and PIV as significant factors associated with overall survival (p < 0.05) (Table 2). Variables with p < 0.05 in the univariate analysis were subsequently included in the multivariable Cox regression model. The results indicated that tumor stage, PLR, FAR, and LAR were independent prognostic factors for survival following RC. Stage III (HR = 25.44, 95% CI: 5.20–124.35, p < 0.001) and Stage IV (HR = 11.28, 95% CI: 3.18–40.05, p < 0.001) were independent risk factors for poor survival. Low PLR was associated with improved survival (HR = 0.45, 95% CI: 0.27–0.76, p = 0.003), low FAR was associated with better survival (HR = 0.51, 95% CI: 0.29–0.89, p = 0.018), and low LAR was an independent predictor of better survival (HR = 0.39, 95% CI: 0.23–0.67, p < 0.001).
Based on the results of the multivariable Cox regression analysis, we constructed a nomogram to predict the 1-year, 3-year, and 5-year survival probabilities for patients with HGUC of the bladder following RC. The nomogram includes independent risk factors such as tumor stage, PLR, FAR, and LAR (Figure 3).
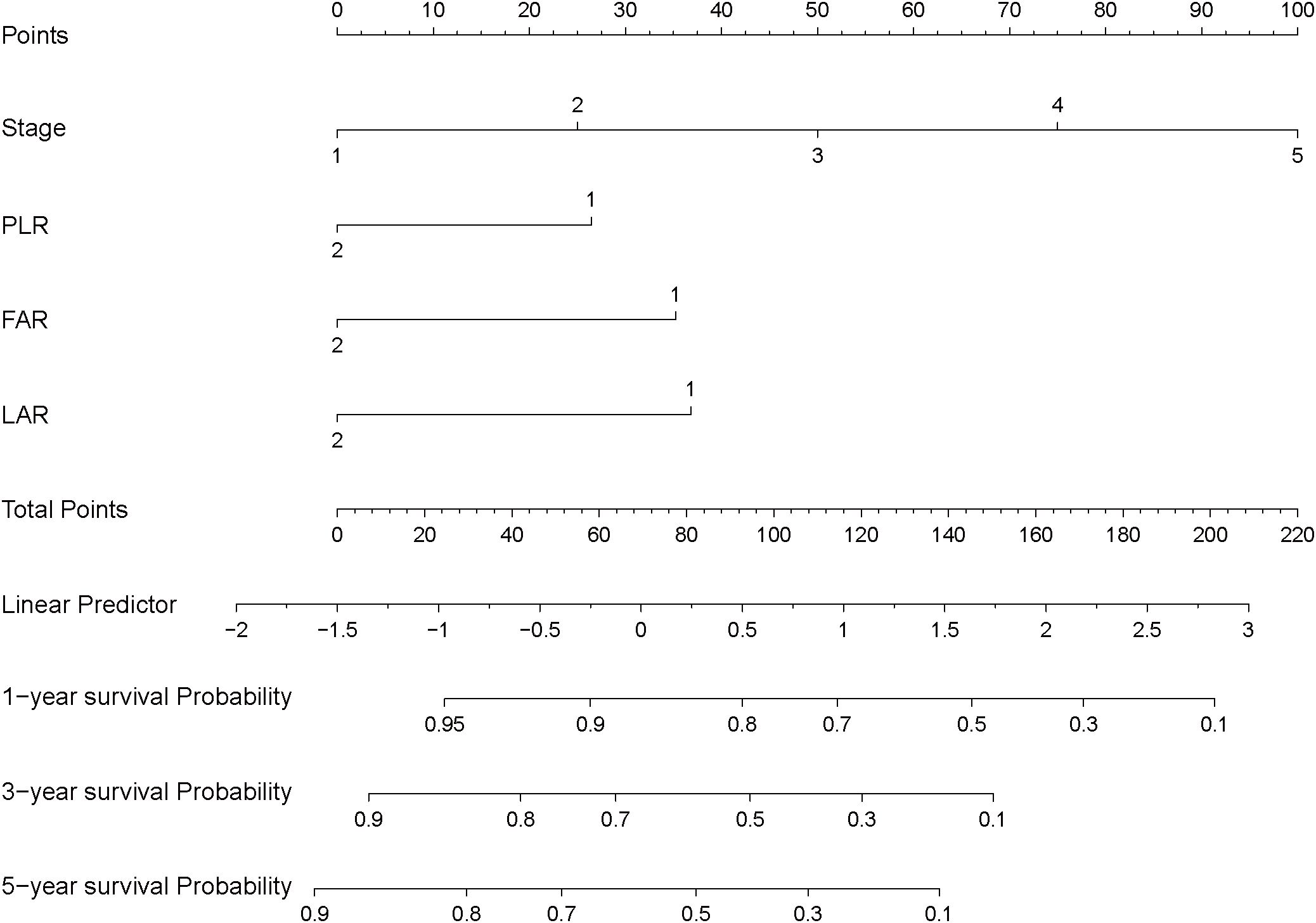
Figure 3. Nomogram for predicting postoperative survival in high-grade urothelial carcinoma of the bladder.
We performed time-dependent ROC curve analysis (Figure 4) to evaluate the predictive performance of the nomogram for OS in patients with HGUC following RC. The AUC for 1-year survival was 0.866, indicating excellent predictive accuracy. For 3-year and 5-year survival predictions, the AUCs were 0.84 and 0.831, respectively, demonstrating that the model maintains stable and reliable predictive performance over longer follow-up periods. These results validate the reliability and stability of the nomogram in clinical settings, underscoring its practical value.
The calibration plot showed that the nomogram provided accurate and consistent predictions for the 1-year, 3-year, and 5-year overall survival probabilities in patients with HGUC after RC. The 1-year survival prediction closely aligned with the observed outcomes, with the curve near the 45-degree reference line and a narrow confidence interval, reflecting high prediction accuracy (Figure 5A). The 3-year survival prediction was generally consistent, although there was slight overestimation in the low-probability range (Figure 5B). The 5-year survival prediction performed well in the medium-probability range but showed some variation in extreme values (Figure 5C).
DCA demonstrated that the postoperative nomogram provided significant clinical value in predicting outcomes at 1-, 3-, and 5-year following RC. The 1-year nomogram (Figure 6A) showed net benefits in the threshold probability range of 0.05 to 0.30, the 3-year nomogram (Figure 6B) in the range of 0.05 to 0.40, and the 5-year nomogram (Figure 6C) in the range of 0.05 to 0.45, all outperforming the “treat all” and “treat none” strategies. These findings suggest that the postoperative nomogram can effectively support clinical decision-making.
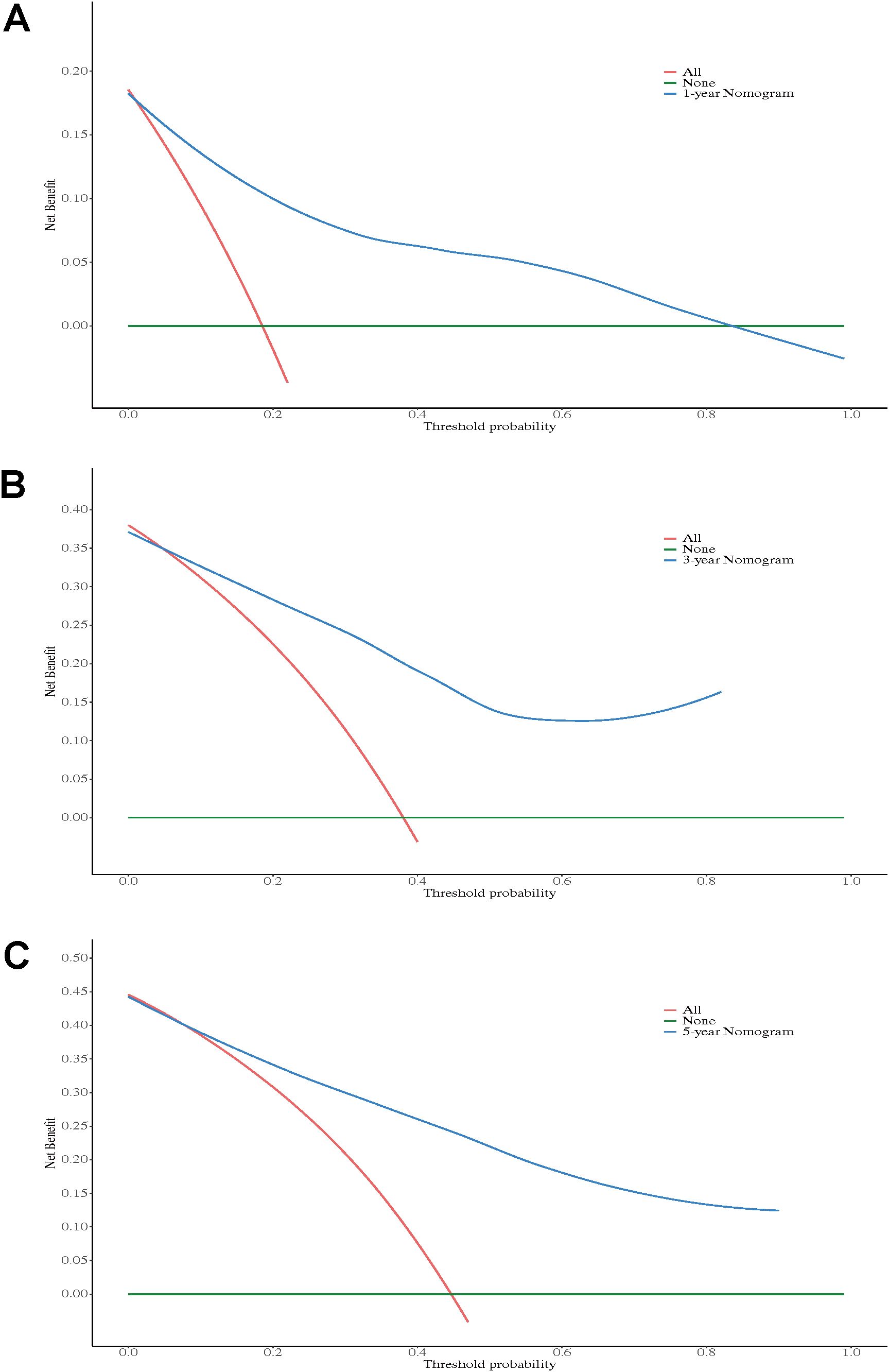
Figure 6. Decision curve analysis of the nomogram for 1-year OS (A), 3-year OS (B), and 5-year OS (C).
Overall, the nomogram demonstrates high practical value in predicting overall survival after RC.
HGUC of the bladder is a highly aggressive malignancy, characterized by high recurrence rates and poor prognosis (2, 14). It remains one of the most challenging urological cancers to treat. This study aimed to explore the relationship between preoperative blood biomarkers and OS in patients with HGUC undergoing RC, with the goal of evaluating the prognostic potential of these markers. Our findings suggest that tumor stage, LAR, FAR, and PLR are independent prognostic factors for patient survival. Based on these variables, we developed and validated a nomogram, providing a significant prognostic tool for clinical assessment in patients after RC for HGUC.
Inflammatory responses are crucial in tumor initiation, progression, and metastasis, and are closely associated with a variety of cancers (15). Bladder urothelial carcinoma is strongly linked to systemic inflammation, with chronic inflammatory states being carcinogenic. Inflammatory macrophages and lymphocytes increase the production of reactive oxygen species (ROS) and reactive nitrogen species (RNS), which promote bladder cancer progression (16). Additionally, studies have shown that bladder tumors are correlated with inflammatory markers in the blood, such as CRP, CD3+, and CD8+ cells, all of which are significantly associated with survival outcomes (13). The role of immune-inflammatory responses in the progression of bladder cancer has been extensively investigated, and research on blood biomarkers associated with bladder cancer progression has gradually increased. Felice Crocetto et al. have proposed that current blood-based immune-inflammatory biomarkers are of significant prognostic value in the progression of bladder cancer (17, 18). Our results further suggest that preoperative LAR, FAR, and PLR are strongly associated with prognosis in bladder cancer patients, underscoring the pivotal role of inflammation in the prognosis of bladder cancer.
LAR, the ratio of lactate dehydrogenase (LDH) to serum albumin, has emerged as a reliable prognostic marker. LDH is a key enzyme in aerobic glycolysis, converting pyruvate to lactate and creating a mildly hypoxic environment that contributes to tumor hypoxia, angiogenesis, and poor prognosis (19). Elevated LDH levels are associated with poor prognosis in bladder cancer (20). Serum albumin, an important biomarker for assessing nutritional status, is frequently low in advanced malignancies. Previous studies have shown that low serum albumin levels correlate with poor cancer prognosis (21). When combined, LAR, incorporating both LDH and albumin, provides a more reliable indicator than either marker alone. Several studies have demonstrated the prognostic value of preoperative LAR in cancers such as nasopharyngeal carcinoma, breast cancer, and colorectal cancer (7, 22, 23). Our study further found that a preoperative LAR >4.46(HR = 0.39, 95% CI: 0.23–0.67, p < 0.001) is associated with poor OS in HGUC patients following RC.
PLR, a marker reflecting the ratio of platelets to lymphocytes, is also indicative of inflammation and immune response, and has demonstrated prognostic value in various cancers (24–26). Platelets play a critical role in tumor growth, metastasis, angiogenesis, and immune-inflammatory responses (27), while lymphocytes are integral to tumor immune surveillance (28). Furthermore, lymphocytes are crucial for immune defense, promoting the apoptosis of cytotoxic cells and inhibiting the proliferation and migration of tumor cells. A reduction in lymphocytes can impair anti-tumor immunity (29). We observed that lower PLR(HR = 0.45, 95% CI: 0.27–0.76, p = 0.003) values were associated with better prognosis, consistent with findings in other cancer types. An elevated PLR likely reflects an immunosuppressive state, holding significant prognostic value in bladder cancer.
FAR, the ratio of fibrinogen to albumin, has gained attention as a biomarker that reflects both systemic inflammation and nutritional status. However, its mechanisms in HGUC prognosis remain incompletely understood. Previous studies have shown that fibrinogen induces the expression of intercellular adhesion molecule-1 (ICAM-1), promoting tumor cell migration, angiogenesis, and metastasis in various cancers, including gallbladder cancer (30). Elevated plasma fibrinogen levels have also been closely linked to poor prognosis in non-muscle-invasive bladder cancer (31). Decreased serum albumin levels not only impair immune function but may also promote pro-inflammatory factors associated with cancer progression, increasing cancer risk (32). Low serum albumin has been identified as an independent predictor of poor long-term prognosis in bladder cancer, with an increased risk of postoperative complications and mortality.
A meta-analysis has demonstrated that higher FAR is significantly associated with OS and disease-free survival DFS in breast cancer patients. Similarly, a single-center study involving patients who underwent transurethral resection of bladder tumors found that elevated preoperative FAR levels serve as a predictive indicator for advanced bladder cancer (18). In line with this, our study identified high FAR as a risk factor for poor OS in HGUC patients undergoing RC, further supporting FAR as a potential prognostic marker in RC for HGUC patients. Preoperative lower FAR (HR = 0.51, 95% CI: 0.29–0.89, p = 0.018) may also serve as an important reference factor for clinicians when assessing patient prognosis.
Through univariate and multivariable Cox regression analyses, we confirmed that tumor stage, PLR, FAR, and LAR are independent prognostic factors. Tumor stage, particularly Stage III and IV, was identified as an independent risk factor for poor survival. Lower values of PLR, FAR, and LAR were associated with improved survival outcomes. In the multivariable analysis, the T stage did not achieve statistical significance, which may be attributable to other confounding factors or limitations in sample size. However, the P value for the Stage in the multivariable analysis was statistically significant. We propose that, although the T stage plays a crucial role in treatment decision-making for bladder cancer, incorporating the Stage based on the TNM classification into the analysis may provide a more comprehensive prognostic assessment. Based on these results, we developed a nomogram model that could serve as a personalized guide for treatment decisions.
Our study has several limitations. First, due to its retrospective design, selection bias may have been introduced, leading to an uneven distribution of certain characteristics (e.g., age, stage, and gender) within the study sample. Additionally, postoperative adjuvant therapy, as a potential confounding factor, may have impacted the accuracy of the findings. Second, the relatively small sample size and the use of data from a single center may have resulted in a slight overestimation of the predictive probabilities, and also limit the generalizability of the results. Therefore, future studies should involve larger, multicenter, prospective cohorts to validate these findings and enhance their external validity. Finally, this study did not investigate the specific molecular mechanisms by which LAR, FAR, and PLR influence prognosis in HGUC of the bladder. Future research should focus on elucidating the molecular roles of these factors in bladder cancer, which could offer more precise clinical guidance.
This study identifies LAR, FAR and PLR as independent prognostic factors for patients with HGUC of the bladder undergoing RC. The nomogram developed in this study demonstrates high predictive accuracy and considerable clinical utility. However, further validation through large-scale, multicenter studies is needed.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by The Ethics Committee of the Guangxi Medical University Cancer Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because This study was approved by the Ethics Committee of Guangxi Medical University Cancer Hospital (No. KY2020069), and all procedures were performed in accordance with the Declaration of Helsinki. The requirement for written informed consent was waived due to the retrospective nature of the study.
HX: Conceptualization, Data curation, Methodology, Software, Validation, Writing – original draft, Writing – review & editing. YH: Conceptualization, Data curation, Software, Writing – original draft, Writing – review & editing. CB: Conceptualization, Data curation, Software, Writing – original draft, Writing – review & editing. WW: Conceptualization, Data curation, Writing – review & editing. HT: Conceptualization, Data curation, Writing – review & editing. QH: Conceptualization, Data curation, Writing – review & editing. ZS: Conceptualization, Data curation, Writing – review & editing. ZC: Conceptualization, Data curation, Writing – review & editing. TL: Conceptualization, Data curation, Writing – review & editing. KL: Conceptualization, Data curation, Writing – review & editing. LZ: Supervision, Writing – review & editing. XY: Funding acquisition, Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Guangxi Higher Education Institutions Young and Middle-aged Teachers’ Scientific Research Capacity Improvement Program (2023KY0127). The National Natural Science Foundation of China (32260241, 31860289). Joint Project on Regional High-Incidence Diseases Research of Guangxi Natural Science Foundation under Grant No. 2023GXNSFDA026021. Guangxi Medical and Health Appropriate Technology Development and Promotion Application Project (S2022123). The self‑financing research of the Health Department of Guangxi Autonomous Region (Z20200986, Z20210478, Z-B20241441).
No support from any others organization for the submitted work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE. The 2016 WHO classification of tumours of the urinary system and male genital organs-part B: prostate and bladder tumours. Eur Urol. (2016) 70:106–19. doi: 10.1016/j.eururo.2016.02.028
3. Kirkali Z, Chan T, Manoharan M, Algaba F, Busch C, Cheng L, et al. Bladder cancer: Epidemiology, staging and grading, and diagnosis. Urology. (2005) 66:4–34. doi: 10.1016/j.urology.2005.07.062
4. Reisz PA, Laviana AA, Chang SS. Management of high-grade T1 urothelial carcinoma. Curr Urol Rep. (2018) 19:103. doi: 10.1007/s11934-018-0850-8
5. Montorsi F, Bandini M, Briganti A, Dasgupta P, Gallina A, Gallucci M, et al. Re-establishing the role of robot-assisted radical cystectomy after the 2020 EAU muscle-invasive and metastatic bladder cancer guideline panel recommendations. Eur Urol. (2020) 78:489–91. doi: 10.1016/j.eururo.2020.06.035
6. Babjuk M, Burger M, Capoun O, Cohen D, Compérat EM, Dominguez Escrig JL, et al. European association of urology guidelines on non-muscle-invasive bladder cancer (Ta, T1, and carcinoma in situ). Eur Urol. (2022) 81:75–94. doi: 10.1016/j.eururo.2021.08.010
7. Peng RR, Liang ZG, Chen KH, Li L, Qu S, Zhu XD. Nomogram based on lactate dehydrogenase-to-albumin ratio (LAR) and platelet-to-lymphocyte ratio (PLR) for predicting survival in nasopharyngeal carcinoma. J Inflammation Res. (2021) 14:4019–33. doi: 10.2147/JIR.S322475
8. Wang Z, Shen X. Prognostic and clinicopathological significance of fibrinogen-to-albumin ratio (FAR) in patients with breast cancer: a meta-analysis. World J Surg Oncol. (2024) 22:220. doi: 10.1186/s12957-024-03506-2
9. Gavriilidis P, Pawlik TM. Inflammatory indicators such as systemic immune inflammation index (SIII), systemic inflammatory response index (SIRI), neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) as prognostic factors of curative hepatic resections for hepatocellular carcinoma. Hepatobiliary Surg Nutr. (2024) 13:509–11. doi: 10.21037/hbsn-23-631
10. Shadpour P, Zamani M, Aghaalikhani N, Rashtchizadeh N. Inflammatory cytokines in bladder cancer. J Cell Physiol. (2019) 234:14489–99. doi: 10.1002/jcp.28252
11. Nabavizadeh R, Bobrek K, Master VA. Risk stratification for bladder cancer: Biomarkers of inflammation and immune activation. Urol Oncol. (2020) 38:706–12. doi: 10.1016/j.urolonc.2020.04.006
12. Soria F, Shariat SF. Biomarkers for the prediction of oncologic outcomes in non-muscle invasive bladder cancer: state of affairs and new frontiers. Transl Androl Urol. (2018) 7:S753–5. doi: 10.21037/tau.2018.08.10
13. Masson-Lecomte A, Rava M, Real FX, Hartmann A, Allory Y, Malats N. Inflammatory biomarkers and bladder cancer prognosis: a systematic review. Eur Urol. (2014) 66:1078–91. doi: 10.1016/j.eururo.2014.07.033
14. Song Y, Jin D, Ou N, Luo Z, Chen G, Chen J, et al. Gene expression profiles identified novel urine biomarkers for diagnosis and prognosis of high-grade bladder urothelial carcinoma. Front Oncol. (2020) 10:394. doi: 10.3389/fonc.2020.00394
15. Coussens LM, Werb Z. Inflammation and cancer. Nature. (2002) 420:860–7. doi: 10.1038/nature01322
16. Wigner P, Grębowski R, Bijak M, Saluk-Bijak J, Szemraj J. The interplay between oxidative stress, inflammation and angiogenesis in bladder cancer development. Int J Mol Sci. (2021) 22:4483. doi: 10.3390/ijms22094483
17. Ferro M, Caputo VF, Barone B, Imbimbo C, de Cobelli O, Crocetto F. Lymphocyte to monocyte ratio: A new independent prognostic factor in bladder cancer progression? Front Oncol. (2021) 11:754649. doi: 10.3389/fonc.2021.754649
18. Barone B, Napolitano L, Reccia P, De Luca L, Morra S, Turco C, et al. Preoperative fibrinogen-to-albumin ratio as potential predictor of bladder cancer: A monocentric retrospective study. Medicina (Kaunas). (2022) 58:1490. doi: 10.3390/medicina58101490
19. Sharma D, Singh M, Rani R. Role of LDH in tumor glycolysis: Regulation of LDHA by small molecules for cancer therapeutics. Semin Cancer Biol. (2022) 87:184–95. doi: 10.1016/j.semcancer.2022.11.007
20. Gu S, Yang C. Serum lactate dehydrogenase level predicts the prognosis in bladder cancer patients. BMC Urol. (2023) 23:65. doi: 10.1186/s12894-023-01239-0
21. Xiang M, Zhang H, Tian J, Yuan Y, Xu Z, Chen J. Low serum albumin levels and high neutrophil counts are predictive of a poorer prognosis in patients with metastatic breast cancer. Oncol Lett. (2022) 24:432. doi: 10.3892/ol.2022.13552
22. He J, Tong L, Wu P, Wu Y, Shi W, Chen L. Prognostic significance of preoperative lactate dehydrogenase to albumin ratio in breast cancer: A retrospective study. Int J Gen Med. (2023) 16:507–14. doi: 10.2147/IJGM.S396871
23. Hu Y, Zhou Y, Cao Y, Wang H, Yang Y, Jiang R, et al. Nomograms based on lactate dehydrogenase to albumin ratio for predicting survival in colorectal cancer. Int J Med Sci. (2022) 19:1003–12. doi: 10.7150/ijms.71971
24. Gong Z, Xin R, Li L, Lv L, Wu X. Platelet-to-lymphocyte ratio associated with the clinicopathological features and prognostic value of breast cancer: A meta-analysis. Int J Biol Markers. (2022) 37:339–48. doi: 10.1177/03936155221118098
25. Hirahara T, Arigami T, Yanagita S, Matsushita D, Uchikado Y, Kita Y, et al. Combined neutrophil-lymphocyte ratio and platelet-lymphocyte ratio predicts chemotherapy response and prognosis in patients with advanced gastric cancer. BMC Cancer. (2019) 19:672. doi: 10.1186/s12885-019-5903-y
26. Wang ZY, Xu B, Wang LN, Zhu XD, Huang C, Shen YH, et al. Platelet-to-lymphocyte ratio predicts tumor response and survival of patients with hepatocellular carcinoma undergoing immunotherapies. Int Immunopharmacol. (2024) 131:111863. doi: 10.1016/j.intimp.2024.111863
27. Franco AT, Corken A, Ware J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood. (2015) 126:582–8. doi: 10.1182/blood-2014-08-531582
28. Yossef R, Krishna S, Sindiri S, Lowery FJ, Copeland AR, Gartner JJ, et al. Phenotypic signatures of circulating neoantigen-reactive CD8+ T cells in patients with metastatic cancers. Cancer Cell. (2023) 41:2154–2165.e5. doi: 10.1016/j.ccell.2023.11.005
29. Paijens ST, Vledder A, de Bruyn M, Nijman HW. Tumor-infiltrating lymphocytes in the immunotherapy era. Cell Mol Immunol. (2021) 18:842–59. doi: 10.1038/s41423-020-00565-9
30. Jiang C, Li Y, Li Y, Liu L, Wang XA, Wu W, et al. Fibrinogen promotes gallbladder cancer cell metastasis and extravasation by inducing ICAM1 expression. Med Oncol. (2022) 40:10. doi: 10.1007/s12032-022-01874-x
31. Li X, Shu K, Zhou J, Yu Q, Cui S, Liu J, et al. Preoperative plasma fibrinogen and D-dimer as prognostic biomarkers for non-muscle-invasive bladder cancer. Clin Genitourin Cancer. (2020) 18:11–19.e1. doi: 10.1016/j.clgc.2019.10.025
Keywords: lactate dehydrogenase-to-serum albumin ratio, platelet-to-lymphocyte ratio, fibrinogen-to-albumin ratio, high-grade urothelial carcinoma, prognosis
Citation: Xie H, Huang Y, Ban C, Wei W, Tang H, Huang Q, Su Z, Cheng Z, Liao T, Liao K, Zhou L and Yi X (2025) LAR, FAR, and PLR as prognostic factors in high-grade urothelial carcinoma of the bladder after surgery. Front. Oncol. 15:1566848. doi: 10.3389/fonc.2025.1566848
Received: 28 January 2025; Accepted: 25 February 2025;
Published: 11 March 2025.
Edited by:
Lushan Xiao, Southern Medical University, ChinaReviewed by:
Biagio Barone, ASL Napoli 1 Centro, ItalyCopyright © 2025 Xie, Huang, Ban, Wei, Tang, Huang, Su, Cheng, Liao, Liao, Zhou and Yi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liquan Zhou, emxxZHIxOTcyQDE2My5jb20=; Xianlin Yi, eWl4aWFubGluQGd4bXUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.