
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Oncol., 03 March 2025
Sec. Cancer Molecular Targets and Therapeutics
Volume 15 - 2025 | https://doi.org/10.3389/fonc.2025.1565872
 Heidi C. Ko1*
Heidi C. Ko1* Kyle C. Strickland1,2
Kyle C. Strickland1,2 Dana Jaggessarsingh3
Dana Jaggessarsingh3 Alicia Dillard1
Alicia Dillard1 Michelle Green1
Michelle Green1 Erin Newburn1
Erin Newburn1 Tiffany Sellaro1
Tiffany Sellaro1 Jonathan L. Klein3
Jonathan L. Klein3 Brian Caveney1
Brian Caveney1 Marcia Eisenberg1
Marcia Eisenberg1 Eric A. Severson1
Eric A. Severson1 Shakti Ramkissoon1,4
Shakti Ramkissoon1,4 Rebecca A. Previs1,5
Rebecca A. Previs1,5 Anis Toumeh6
Anis Toumeh6The growing importance of HER2 expression as a biomarker across multiple cancers is largely driven by advances in HER2-directed antibody-drug conjugates. The recent approval of trastuzumab deruxtecan (T-DXd) as a tumor-agnostic therapy has revolutionized treatment strategies for HER2-overexpressed tumors beyond breast, gastric, and colorectal cancers (CRC). This mini-review explores the evolving role of assessing HER2 overexpression in pan-solid tumors, following the recent approval of T-DXd as a tumor-agnostic therapy. It examines how HER2 scoring criteria for pan-tumor indications rely on immunohistochemistry (IHC) assessment, which may be prone to subjective interpretation and interobserver variability, and how these criteria differ from those used in breast, gastric, and CRC tumors. We also address the potential for NGS approaches to identify ERBB2 copy number gain (CNG) and the utility of artificial intelligence (AI) algorithms to enhance the consistency and accuracy of HER2 score interpretation for T-DXd treatment eligibility in solid tumors.
Human epidermal growth factor receptor 2 (HER2) is a member of the epidermal growth factor receptor (EGFR) family of transmembrane glycoproteins with tyrosine kinase activity which are essential in regulating epithelial cell proliferation, differentiation, and survival (1). Abnormal activation of HER2 activity can occur through amplification or mutation of the ERBB2 gene, leading to dysregulated cell growth and tumorigenesis. ERBB2 amplification is a more commonly observed alteration event which is associated with overexpression of HER2 protein, triggering oncogenesis and uncontrolled cell growth through PI3K/AKT/mTOR and MAPK signaling pathways in many cancers (2).
HER2 overexpression is clinically assessed using an immunohistochemistry (IHC) staining assay, with scores ranging from very low or absent (0) to very high (3+). HER2 IHC score of 0 represents a staining pattern where there are fewer than 10 percent of tumor cells with no staining or incomplete membrane staining that is faint or barely perceptible. A score of 1+ is defined as faint or barely perceptible incomplete membrane staining in more than 10 percent of tumor cells. Tumors with weak-moderate complete membrane staining in more than 10 percent of tumor cells are scored as 2+. Lastly, tumors with complete, intense membrane staining involving more than 10 percent of tumor cells are scored as 3+. In cases of equivocal IHC score of 2+ in breast, gastric, and colorectal cancers, reflex in-situ hybridization (ISH) is performed to confirm ERBB2 amplification (3–5).
HER2 overexpression plays a critical role in oncogenesis of breast tumors, arising in almost half of in-situ carcinomas and 20% of invasive breast cancers (1). Beyond breast cancer, ERBB2 amplification and HER2 overexpression are observed across other various tumor types such as gastric, bladder, colorectal, bile duct, and non-small cell lung cancers (2, 6). Overexpression of HER2 suggests an aggressive tumor phenotype and a historically unfavorable prognosis in many cancers (1, 2). However, the development of HER2-targeted therapies has significantly improved the prognosis and survival of patients with HER2-overexpressed cancers, particularly in breast cancer (2). Historically, mechanisms of HER2-targeted therapies included HER2 monoclonal antibodies (mAb) and tyrosine kinase inhibitors (TKIs). In more recent years, anti-HER2 antibody-drug conjugate (ADCs) have emerged as a new standard-of-care treatment for patients with HER2-overexpressed or mutated cancers (2).
ADCs are usually comprised of a tumor antigen-selective mAb covalently attached to a highly potent cytotoxic chemotherapy via a chemical linker (7). The construct of an ADC enables precise delivery of the cytotoxic chemotherapy to the tumor, leading to potent killing of the cancer cells while minimizing the off-target systemic drug toxicities to healthy tissues (7–9).
The identification of HER2 as a therapeutic target in the 1980’s paved the way for development of HER2-targeted ADCs as treatments for HER2-altered cancers (10). All the currently available HER2-targeted ADCs use trastuzumab as the mAb, which was the first fully humanized mouse antibody to be approved by the FDA in 1998 (11). Trastzumab emtansine (T-DM1) is a HER2-directed ADC that combines trastuzumab with a cytotoxic microtubule inhibitor, called emtansine (DM1) via an uncleavable linker (12). In the phase III EMILIA trial, treatment with T-DM1 led to significant improvements in objective response rates (ORR), progression-free survival (PFS), and overall survival (OS) in patients with HER2-overexpressed metastatic breast cancer who previously failed trastuzumab and chemotherapy as compared with capecitabine plus lapatinib (13).
Trastuzumab deruxtecan (T-DXd) is a newer generation HER2-directed ADC which has shown superior therapeutic efficacy to T-DM1 in clinical trials (14). T-DXd is an ADC that consists of trastuzumab and a topoisomerase I inhibitor, DX-8951. T-DXd has unique properties that increase its potency over T-DM1. First, T-DXd contains a higher cytotoxic drug-to-antibody ratio of 8:1 as compared with the 3.5:1 in T-DM1 (12, 15). Second, T-DXd has higher membrane permeability that allows for improved stability in the plasma until it is internalized into the target cells. Third, the linker that attaches the chemotherapy payload to trastuzumab is cleavable, enabling more efficient processing and release of the cytotoxic drug into the cell (12). Finally, once T-DXd is internalized and processed by the target cells, it has a unique ability to enter the surrounding cells and induce a bystander killing effect to the off-target cancer cells, maximizing its therapeutic efficacy even in tumors with heterogeneous expressions of HER2 (12, 14). These characteristics of T-DXd enable potent delivery of the chemotherapy to the cancer cells, resulting in cell cycle arrest and apoptosis (12). The unique properties of T-DXd have broadened its therapeutic efficacy in tumors with lower HER2 expression, classified as HER2-low (IHC 1+ or 2+/ISH-negative) and ultralow (IHC 0 with membrane staining) breast cancer (16, 17). These tumors were previously considered HER2-negative and did not have therapeutic responses to anti-HER2 mAbs and T-DM1 (18, 19).
Early clinical success of T-DXd in solid tumors was established through breast cancer-specific studies, notably DESTINY-Breast01 and 02 trials. Treatment with T-DXd resulted in an ORR of 60.9% (20) and led to an improvement in PFS by 64% (21) in patients with unresectable or metastatic HER2-overexpressed, defined as IHC 3+ or 2+/ISH-positive, breast cancer after progression on prior treatments including anti-HER2 therapies. In 2022, T-DXd further demonstrated therapeutic efficacy in patients with intermediate to low levels of HER2 expression, defined as HER2-low tumors with IHC 1+ or 2+/ISH-negative scores (16). These practice-changing data were reflected in the DESTINY-Breast04 trial, where the risk of disease progression was 50% lower and the risk of death was 36% lower in patients treated with T-DXd compared to chemotherapy, regardless of hormonal receptor status (16). Ultimately, these results led to the U.S Food and Drug Administration (FDA) approval of T-DXd for the treatment of advanced HER2-low breast cancer. More recently, the DESTINY-Breast06 phase III clinical trial demonstrated clinically meaningful activity of T-DXd in chemotherapy-naive patients with even lower levels of HER2 expression, defined as HER2 ultralow (IHC 0 with membrane staining) breast cancer with an improvement in PFS of 22% as compared to chemotherapy (22). Following the positive results benefiting a broader patient population with lower levels of HER2 expression, the U.S FDA has approved T-DXd for the treatment of chemotherapy-naïve patients with unresectable or metastatic HER2-low (IHC 1+ or 2+/ISH-) or HER2-ultralow (IHC 0 with membrane staining) breast cancer after at least one line of endocrine therapy (23–25).
The phase II DESTINY-Gastric01 was the first study that showed the therapeutic efficacy of T-DXd in patients with HER2-overexpressed advanced gastric cancer who have received at least two prior lines of therapy including trastuzumab. HER2 overexpression was defined as IHC 3+ or IHC 2+/ISH+ for ERBB2 amplification (26). Treatment with T-DXd led to an ORR of 51% and a median OS of 12.5 months as compared to an ORR of 14% and a median OS of 8.4 months with chemotherapy (26). Based on these results, the U.S FDA approved T-DXd for the treatment of unresectable and/or metastatic HER2-overexpressed gastric or gastroesophageal junction (GEJ) adenocarcinoma after progression on a prior trastuzumab-based regimen. Additional studies are ongoing to further evaluate the efficacy and safety of T-DXd, either alone or in combination with other therapies, for the treatment of HER2-overexpressed advanced gastric or GEJ adenocarcinoma. An ongoing phase III DESTINY-Gastric04 trial is evaluating T-DXd versus traditional chemotherapy-based regimen in patient with HER2-overexpressed gastric or GEJ adenocarcinoma after progression on trastuzumab-based therapy (27).
ERBB2 amplification or HER2 overexpression is detected in about 2% of all CRC and represents a therapeutic target for treatment eligibility with HER2-targeted therapies (28). Initial HER2-targeted therapies for CRC centered on dual inhibition with HER2-directed mAbs and tyrosine kinase inhibitors in patients with HER2-overexpressed tumors, defined as IHC 3+ or IHC 2+ with ERBB2 amplification on ISH (29). More recently, HER2-directed ADCs have emerged as a therapeutic option for those patients with HER2-overexpressed CRC. Although results from the HERACLES B study exploring the efficacy of T-DM1 in HER2-overexpressed CRC were not significant (30), T-DXd demonstrated clinically meaningful benefits in patients with HER2-overexpressed (IHC 3+) CRC after two prior lines of therapy including trastuzumab and pertuzumab (31). Treatment with T-DXd resulted in an ORR of 45.3% in patients with previously treated HER2-overexpressed CRC (28, 31). Given its clinical efficacy and tolerable safety profile, T-DXd has been approved by the U.S FDA for the treatment of HER2-overexpressed (IHC 3+) CRC.
In NSCLC, T-DXd is approved for use in patients with ERBB2-mutant as well as HER2-overexpressed tumors (IHC 3+) unresectable or metastatic disease. A multi-cohort DESTINY-Lung01 trial showed that treatment with T-DXd resulted in a promising antitumor activity with a confirmed ORR of 55% in patients with previously treated ERBB2-mutant NSCLC (32). A separate cohort of DESTINY-Lung01 demonstrated results of ORR 34% in patients with HER2-overexpressed NSCLC (33).
In an effort to expand T-DXd use across all solid tumors, the recent phase II DESTINY-PanTumor02 trial evaluated its efficacy in HER2-overexpressed (IHC 3+/2+ with ISH positivity), unresectable locally advanced or metastatic solid tumors upon disease progression on first line therapy (34). Eligible patients had solid tumors ranging from biliary tract, bladder, cervical, endometrial, ovarian, pancreatic to other solid cancers that were not breast, colorectal, gastric, or NSCLC. At the primary analysis, the study investigators found that treatment with T-DXd achieved clinically significant results with ORR of 37.1% in all patients and 61.3% in those whose tumors had HER2 IHC 3+ expression. The median duration of response was 11.3 months in all patients and 22.1 months in those with HER2 IHC 3+ tumors (34). Given these encouraging results, the U.S. FDA recently granted an accelerated approval to T-DXd for patients with unresectable or metastatic relapsed HER2-overexpressed (IHC 3+) solid tumors (35). This approval represents the first tumor-agnostic approval for an antibody-drug conjugate and introduces IHC testing as a tumor-agnostic biomarker assessment for solid tumors.
Although HER2 expression has evolved into a tumor-agnostic biomarker with therapeutic implications across all solid tumors, there are practical considerations around this newly defined tumor-agnostic biomarker.
Several pathological and laboratory considerations have emerged since the DESTINY-PanTumor02 trial and subsequent FDA tumor-agnostic approval for T-DXd. First and foremost, HER2 overexpression criteria (IHC 3+), set in DESTINY-PanTumor02, differs significantly from traditional tumor types such as breast, gastric/GEJ, and colorectal cancers (Figure 1) (34, 36). Traditional HER2 testing protocols, developed for breast and gastric cancers and adopted for colorectal cancer, allow for HER2 assessment via IHC followed by reflex to in situ hybridization (ISH) for equivocal (IHC 2+) cases (36). In contrast, recent FDA approval for T-DXd as a tumor-agnostic indication is based on a HER2 IHC 3+ score and does not require ISH confirmation of equivocal cases by IHC (Figure 1). Therefore, while HER2 tumor-agnostic indication expands treatment options for solid tumors, it is critical for laboratories to maintain tumor-specific testing protocols and resulting to ensure appropriate therapeutic decisions.
Second, most tumor-agnostic approvals are dependent on a binary assessment of either the presence or absence of a biomarker, such as TRK inhibitor eligibility for those patients with NTRK fusion-positive solid tumors (37). However, treatment eligibility for T-DXd in solid tumors relies on a more subjective approach of assessing tumor HER2 expression via IHC assay, subject to interobserver variability among pathologists. In addition, HER2 staining intensity may be influenced by various laboratory factors, including sample storage conditions, tissue section thickness, buffer solutions, incubation durations, variations among commercially available antibodies, control tissue selection, and quality control practices. Figure 2 showcases real-world stained images of HER2 IHC and ISH in solid tumors. While the inter-observer agreement is generally better at differentiating IHC 0 and 1+ from 3+ cases, high interobserver variability can occur in discerning 2+ from 3+ scores, which can ultimately influence the eligibility for tumor-agnostic therapy (38, 39).
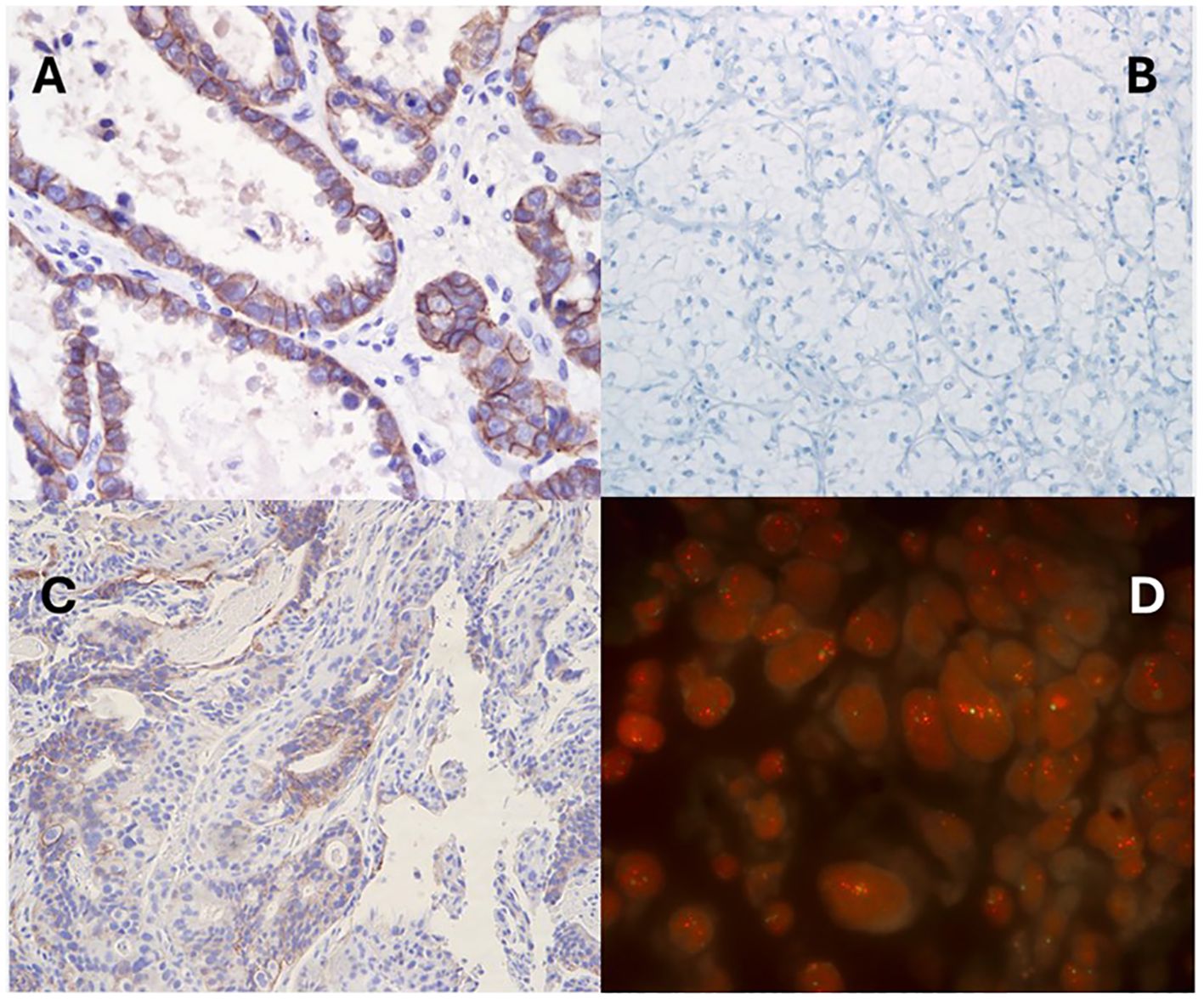
Figure 2. Representative results of HER2 immunohistochemistry and fluorescence in situ hybridization in solid tumors. (A) ovarian clear cell carcinoma with IHC 3+, (B) clear cell renal cell carcinoma with IHC 0, (C) gastroesophageal adenocarcinoma with IHC 2+ with FISH positivity (D).
Third, specific criteria for HER2 expression and patient selection for T-DXd eligibility should be carefully reviewed. DESTINY-PanTumor02 trial allowed enrollment of patients with HER2 IHC 2+/ISH+ and IHC 3+ tumors. However, patients with biliary tract cancers with IHC 2+/ISH+ did not have meaningful treatment responses to T-DXd while patients with HER2 IHC 2+/ISH+ gynecologic cancers had high response rates (34). Based on the consistent results across tumors with IHC 3+ score, the FDA approval recognizes the HER2 overexpression as IHC 3+ for treatment eligibility with T-DXd as a tumor-agnostic therapy. However, additional studies might be warranted in certain tumor types such as endometrial cancer to determine if expanded populations of patients with lower HER2 expression might benefit from T-DXd. In addition, while T-DXd is approved for pan-solid tumor indications, certain tumor types such as sarcomas and renal cell carcinoma were not represented in the DESTINY-PanTumor02 trial (34). As a result, careful patient and tumor selection is essential to minimize the risk of harm and toxicity in those unlikely to benefit from T-DXd.
Efforts are ongoing to develop a more precise HER2 diagnostic test to accurately identify patients who could benefit from T-DXd (40, 41). Next-generation sequencing (NGS) has become integral in management of advanced cancers as options for targeted therapies have expanded in oncology. The application of NGS can also facilitate effective tissue stewardship to ensure sufficient material is available to assess all clinically relevant biomarkers in a single test.
Regarding ERBB2/HER2 alterations, NGS has been utilized primarily to identify activating ERBB2 (HER2) mutations, which have therapeutic implications in NSCLC. However, using tissue- or plasma-based NGS testing to detect ERBB2 copy number gain (CNG) as a predictor of response to anti-HER2 therapy is not yet standard practice. The correlation between ERBB2 CNG identified through NGS and HER2 overexpression determined by conventional IHC testing remains less well established. Although ERBB2 CNG is not currently included in the FDA tumor-agnostic approval for HER2 testing, studies have demonstrated high concordance between NGS-detected ERBB2 CNG and HER2 overexpression by traditional IHC/ISH methods, suggesting that NGS could serve as a reliable alternative or surrogate in certain cases (42–44). A recent multi-center phase II basket study has shown that treatment with T-DXd resulted in high ORR (56.5%) with durable responses in patients with advanced ERBB2-amplified solid tumors, detected by cell-free DNA (cfDNA) NGS testing (45). This study suggests that detection of ERBB2 CNG through plasma-based ctDNA NGS assay could potentially serve as an alternative tool for determining T-DXd treatment eligibility in the future, especially when tissue availability is limited. However, NGS does not detect protein overexpression directly, and its results should be interpreted in conjunction with clinical findings and other diagnostic tests.
There have also been developments of artificial intelligence (AI) algorithms to improve the interpretation of HER2 IHC scores to be more consistent and accurate among pathologists (41, 46). Krishnamurthy et al. demonstrated that a fully automated AI tool led to increased interobserver agreement (75% manual to 83.7% AI-assisted review) and scoring accuracy (85.3% manual to 88% AI-assisted review) of HER2 IHC by pathologists (46). Among the cases of HER2 IHC 0 and 1+ cases, AI-assisted review led to even higher interobserver agreement (69.8% manual vs. 87.4% AI-assisted review) and accuracy (81.9% manual vs. 88.8% assisted review) (46). Although T-DXd is currently indicated for pan-solid tumors with HER2 overexpression (IHC 3+), data from breast cancer studies suggest it may also be effective in tumors with lower HER2 expression levels (IHC 1+ or 0), where AI tools can enhance the consistency and accuracy of HER2 interpretation.
The DESTINY-PanTumor02 trial and the subsequent approval of T-DXd have revolutionized the management of HER2-overexpressed solid tumors. It also represents the first tumor-agnostic therapeutic approach that leverages a protein biomarker overexpression and has expanded therapeutic opportunities across multiple cancer types. This evolving landscape of T-DXd eligibility reinforces the need for comprehensive biomarker assessment in solid tumors while balancing the increasing demand for molecular testing and the constraints of limited tissue samples in precision oncology.
HK: Writing – original draft, Writing – review & editing. KS: Writing – original draft, Writing – review & editing. DJ: Writing – original draft, Writing – review & editing. AD: Writing – review & editing. MG: Writing – review & editing. EN: Writing – review & editing. TS: Writing – review & editing. JK: Writing – review & editing. BC: Writing – review & editing. ME: Writing – review & editing. ES: Writing – review & editing. SR: Writing – review & editing. RP: Writing – review & editing, Writing – original draft. AT: Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
HK, KS, DJ, AD, MG, EN, TS, JK, BC, ME, ES, SR, and RP are employees of Labcorp.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Albagoush SA, Zubair M, Limaiem F. Tissue Evaluation for HER2 Tumor Marker. Treasure Island (FL: StatPearls (2024). ineligible companies. Disclosure: Muhammad Zubair declares no relevant financial relationships with ineligible companies. Disclosure: Faten Limaiem declares no relevant financial relationships with ineligible companies.
2. Zhu K, Yang X, Tai H, Zhong X, Luo T, Zheng H, et al. HER2-targeted therapies in cancer: a systematic review. biomark Res. (2024) 12:16. doi: 10.1186/s40364-024-00565-1
3. Wolff AC, Somerfield MR, Dowsett M, Hammond MEH, Hayes DF, McShane LM, et al. Human epidermal growth factor receptor 2 testing in breast cancer: ASCO–college of American pathologists guideline update. J Clin Oncol. (2023) 41:3867–72. doi: 10.1200/JCO.22.02864
4. Abrahao-MaChado LF, Scapulatempo-Neto C. HER2 testing in gastric cancer: An update. World J Gastroenterol. (2016) 22:4619–25. doi: 10.3748/wjg.v22.i19.4619
5. Ahcene Djaballah S, Daniel F, Milani A, Ricagno G, Lonardi S. HER2 in colorectal cancer: the long and winding road from negative predictive factor to positive actionable target. Am Soc Clin Oncol Educ Book. (2022) 2022:219–32.
6. Yan Y, Lu L, Liu C, Li W, Liu T, Fu W. HER2/neu over-expression predicts poor outcome in early gastric cancer without lymph node metastasis. Clin Res Hepatol Gastroenterol. (2015) 39:121–6. doi: 10.1016/j.clinre.2014.06.019
7. Joubert N, Beck A, Dumontet C, Denevault-Sabourin C. Antibody-drug conjugates: the last decade. Pharm (Basel). (2020) 13. doi: 10.3390/ph13090245
8. Fu Z, Li S, Han S, Shi C, Zhang Y. Antibody drug conjugate: the “biological missile” for targeted cancer therapy. Signal Transduct Target Ther. (2022) 7:93. doi: 10.1038/s41392-022-00947-7
9. Gogia P, Ashraf H, Bhasin S, Xu Y. Antibody-drug conjugates: A review of approved drugs and their clinical level of evidence. Cancers (Basel). (2023) 15. doi: 10.3390/cancers15153886
10. Rassy E, Rached L, Pistilli B. Antibody drug conjugates targeting HER2: Clinical development in metastatic breast cancer. Breast. (2022) 66:217–26. doi: 10.1016/j.breast.2022.10.016
11. Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of Chemotherapy plus a Monoclonal Antibody against HER2 for Metastatic Breast Cancer That Overexpresses HER2. New Engl J Med. (2001) 344:783–92. doi: 10.1056/NEJM200103153441101
12. Doi T, Shitara K, Naito Y, Shimomura A, Fujiwara Y, Yonemori K, et al. Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody-drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: a phase 1 dose-escalation study. Lancet Oncol. (2017) 18:1512–22. doi: 10.1016/S1470-2045(17)30604-6
13. Diéras V, Miles D, Verma S, Pegram M, Welslau M, Baselga J, et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. (2017) 18:732–42. doi: 10.1016/S1470-2045(17)30312-1
14. Nakada T, Sugihara K, Jikoh T, Abe Y, Agatsuma T. The latest research and development into the antibody-drug conjugate, [fam-] trastuzumab deruxtecan (DS-8201a), for HER2 cancer therapy. Chem Pharm Bull (Tokyo). (2019) 67:173–85. doi: 10.1248/cpb.c18-00744
15. Hunter FW, Barker HR, Lipert B, Rothé F, Gebhart G, Piccart-Gebhart MJ, et al. Mechanisms of resistance to trastuzumab emtansine (T-DM1) in HER2-positive breast cancer. Br J Cancer. (2020) 122:603–12. doi: 10.1038/s41416-019-0635-y
16. Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. (2022) 387:9–20. doi: 10.1056/NEJMoa2203690
17. Bardia A, Hu X, Dent R, Yonemori K, Barrios Carlos H, O’Shaughnessy Joyce A, et al. Trastuzumab deruxtecan after endocrine therapy in metastatic breast cancer. New Engl J Med. (2024) 391:2110–22. doi: 10.1056/NEJMoa2407086
18. Denkert C, Seither F, Schneeweiss A, Link T, Blohmer JU, Just M, et al. Clinical and molecular characteristics of HER2-low-positive breast cancer: pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol. (2021) 22:1151–61. doi: 10.1016/S1470-2045(21)00301-6
19. Fehrenbacher L, Cecchini RS, Geyer CE Jr, Rastogi P, Costantino JP, Atkins JN, et al. NSABP B-47/NRG oncology phase III randomized trial comparing adjuvant chemotherapy with or without trastuzumab in high-risk invasive breast cancer negative for HER2 by FISH and with IHC 1+ or 2. J Clin Oncol. (2020) 38:444–53. doi: 10.1200/JCO.19.01455
20. Modi S, Saura C, Yamashita T, Park YH, Kim S-B, Tamura K, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. New Engl J Med. (2020) 382:610–21. doi: 10.1056/NEJMoa1914510
21. André F, Hee Park Y, Kim S-B, Takano T, Im S-A, Borges G, et al. Trastuzumab deruxtecan versus treatment of physician’s choice in patients with HER2-positive metastatic breast cancer (DESTINY-Breast02): a randomised, open-label, multicentre, phase 3 trial. Lancet. (2023) 401:1773–85.
22. Curigliano G, Hu X, Dent RA, Yonemori K, Barrios CH, O'Shaughnessy J, et al. Trastuzumab deruxtecan (T-DXd) vs physician’s choice of chemotherapy (TPC) in patients (pts) with hormone receptor-positive (HR+), human epidermal growth factor receptor 2 (HER2)-low or HER2-ultralow metastatic breast cancer (mBC) with prior endocrine therapy (ET): Primary results from DESTINY-Breast06 (DB-06). J Clin Oncol. (2024) 42:LBA1000–LBA1000. doi: 10.1200/JCO.2024.42.17_suppl.LBA1000
23. AstraZeneca. Enhertu granted priority review in the US for patients with HER2-low or HER2-ultralow metastatic breast cancer who have received at least one line of endocrine therapy(2024). Available online at: https://www.astrazeneca.com/media-centre/press-releases/2024/enhertu-granted-priority-review-us-for-patients-her2-low-or-her2-ultralow-metastatic-breast-cancer-who-have-received-at-least-1-line-endocrine-therapy.html (Accessed October 4, 2024).
24. Schettini F, Chic N, Braso-Maristany F, Pare L, Pascual T, Conte B, et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer. (2021) 7:1. doi: 10.1038/s41523-020-00208-2
25. Enhertu approved in the US as first HER2-directed therapy for patients with HER2-low or HER2-ultralow metastatic breast cancer following disease progression after one or more endocrine therapies(2025). Available online at: https://www.astrazeneca.com/media-centre/press-releases/2025/enhertu-approved-in-us-for-breast-cancer-post-et.html (Accessed February 3, 2025).
26. Shitara K, Bang Y-J, Iwasa S, Sugimoto N, Ryu M-H, Sakai D, et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. New Engl J Med. (2020) 382:2419–30. doi: 10.1056/NEJMoa2004413
27. Shitara K, Barlaskar F, Franke F, Kawaguchi Y, Shen L, Kamio T, et al. P-159 Trastuzumab deruxtecan (T-DXd) in patients with HER2-positive gastric cancer (GC) or gastroesophageal junction (GEJ) adenocarcinoma who have progressed on or after a trastuzumab-containing regimen (DESTINY-Gastric04): A randomized phase 3 study. Ann Oncol. (2022) 33:S306–7. doi: 10.1016/j.annonc.2022.04.249
28. Babkoff A, Zick A, Hubert A, Tarantino P, Grinshpun A. Unleashing the power of anti-HER2 therapies in metastatic colorectal cancer: paving the way for a brighter future. ESMO Gastrointestinal Oncol. (2024) 3:100032. doi: 10.1016/j.esmogo.2023.100032
29. Strickler JH, Cercek A, Siena S, André T, Ng K, Van Cutsem E, et al. Tucatinib plus trastuzumab for chemotherapy-refractory, HER2-positive, RAS wild-type unresectable or metastatic colorectal cancer (MOUNTAINEER): a multicentre, open-label, phase 2 study. Lancet Oncol. (2023) 24:496–508. doi: 10.1016/S1470-2045(23)00150-X
30. Sartore-Bianchi A, Lonardi S, Martino C, Fenocchio E, Tosi F, Ghezzi S, et al. Pertuzumab and trastuzumab emtansine in patients with HER2-amplified metastatic colorectal cancer: the phase II HERACLES-B trial. ESMO Open. (2020) 5:e000911. doi: 10.1136/esmoopen-2020-000911
31. Yoshino T, Di Bartolomeo M, Raghav K, Masuishi T, Loupakis F, Kawakami H, et al. Final results of DESTINY-CRC01 investigating trastuzumab deruxtecan in patients with HER2-expressing metastatic colorectal cancer. Nat Commun. (2023) 14:3332. doi: 10.1038/s41467-023-38032-4
32. Li BT, Smit EF, Goto Y, Nakagawa K, Udagawa H, Mazières J, et al. Trastuzumab deruxtecan in HER2-mutant non&x2013;Small-cell lung cancer. New Engl J Med. (2022) 386:241–51. doi: 10.1056/NEJMoa2112431
33. Smit EF, Felip E, Uprety D, Nagasaka M, Nakagawa K, Rodríguez LP-A, et al. Trastuzumab deruxtecan in patients with metastatic non-small-cell lung cancer (DESTINY-Lung01): Primary results of the HER2-overexpressing cohorts from a single-arm, phase 2 trial. Lancet Oncol. (2024) 25:439–54. doi: 10.1016/S1470-2045(24)00064-0
34. Meric-Bernstam F, Makker V, Oaknin A, Oh DY, Banerjee S, González-Martín A, et al. Efficacy and safety of trastuzumab deruxtecan in patients with HER2-expressing solid tumors: primary results from the DESTINY-panTumor02 phase II trial. J Clin Oncol. (2024) 42:47–58. doi: 10.1200/JCO.23.02005
35. Administration, F.a.D. FDA grants accelerated approval to fam-trastuzumab deruxtecan-nxki for unresectable or metastatic HER2-positive solid tumors(2024). Available online at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-fam-trastuzumab-deruxtecan-nxki-unresectable-or-metastatic-her2 (Accessed October 16, 2024).
36. Zhang H, Finkelman BS, Ettel MG, Velez MJ, Turner BM, Hicks DG. HER2 evaluation for clinical decision making in human solid tumours: pearls and pitfalls. Histopathology. (2024) 85:3–19. doi: 10.1111/his.15170
37. Tateo V, Marchese PV, Mollica V, Massari F, Kurzrock R, Adashek JJ. Agnostic approvals in oncology: getting the right drug to the right patient with the right genomics. Pharm (Basel). (2023) 16. doi: 10.3390/ph16040614
38. McCormick SR, Lillemoe TJ, Beneke J, Schrauth J, Reinartz J. HER2 assessment by immunohistochemical analysis and fluorescence in situ hybridization: comparison of HercepTest and PathVysion commercial assays. Am J Clin Pathol. (2002) 117:935–43. doi: 10.1309/3643-F955-7Q6B-EWWL
39. Lacroix-Triki M, Mathoulin-Pelissier S, Ghnassia JP, Macgrogan G, Vincent-Salomon A, Brouste V, et al. High inter-observer agreement in immunohistochemical evaluation of HER-2/neu expression in breast cancer: a multicentre GEFPICS study. Eur J Cancer. (2006) 42:2946–53. doi: 10.1016/j.ejca.2006.06.020
40. Liu M, Vathiotis I, Robbins CJ, Chan NNN, Moutafi M, Burela S, et al. Quantitative measurement of HER2 expression in non–small cell lung cancer with a high-sensitivity assay. Modern Pathol. (2024) 37:100556. doi: 10.1016/j.modpat.2024.100556
41. Moutafi M, Robbins CJ, Yaghoobi V, Fernandez AI, Martinez-Morilla S, Xirou V, et al. Quantitative measurement of HER2 expression to subclassify ERBB2 unamplified breast cancer. Lab Invest. (2022) 102:1101–8. doi: 10.1038/s41374-022-00804-9
42. Ross DS, Zehir A, Cheng DT, Benayed R, Nafa K, Hechtman JF, et al. Next-generation assessment of human epidermal growth factor receptor 2 (ERBB2) amplification status: clinical validation in the context of a hybrid capture-based, comprehensive solid tumor genomic profiling assay. J Mol Diagn. (2017) 19:244–54. doi: 10.1016/j.jmoldx.2016.09.010
43. Stein SM, Snider J, Ali SM, Miksad RA, Alexander BM, Castellanos E, et al. Real-world association of HER2/ERBB2 concordance with trastuzumab clinical benefit in advanced esophagogastric cancer. Future Oncol. (2021) 17:4101–14. doi: 10.2217/fon-2021-0203
44. Dumbrava EEI, Balaji K, Raghav K, Hess K, Javle M, Blum-Murphy M, et al. Targeting ERBB2 (HER2) amplification identified by next-generation sequencing in patients with advanced or metastatic solid tumors beyond conventional indications. JCO Precis Oncol. (2019) 3. doi: 10.1200/PO.18.00345
45. Yagisawa M, Taniguchi H, Satoh T, Kadowaki S, Sunakawa Y, Nishina T, et al. Trastuzumab deruxtecan in advanced solid tumors with human epidermal growth factor receptor 2 amplification identified by plasma cell-free DNA testing: A multicenter, single-arm, phase II basket trial. J Clin Oncol. (2024) 42:3817–25. doi: 10.1200/JCO.23.02626
46. Krishnamurthy S, Schnitt SJ, Vincent-Salomon A, Canas-Marques R, Colon E, Kantekure K, et al. Fully automated artificial intelligence solution for human epidermal growth factor receptor 2 immunohistochemistry scoring in breast cancer: A multireader study. JCO Precis Oncol. (2024) 8:e2400353. doi: 10.1200/PO.24.00353
Keywords: HER2 overexpression, ERBB2 amplification, pan-tumor testing, antibody-drug conjugate, next-generation sequencing, tissue stewardship, precision oncology, tumor-agnostic biomarker
Citation: Ko HC, Strickland KC, Jaggessarsingh D, Dillard A, Green M, Newburn E, Sellaro T, Klein JL, Caveney B, Eisenberg M, Severson EA, Ramkissoon S, Previs RA and Toumeh A (2025) From tissue-specific to tissue-agnostic: HER2 overexpression and the rise of antibody-drug conjugates. Front. Oncol. 15:1565872. doi: 10.3389/fonc.2025.1565872
Received: 23 January 2025; Accepted: 18 February 2025;
Published: 03 March 2025.
Edited by:
Paul Mathew, Tufts Medical Center, United StatesReviewed by:
Jacob Elkon, Tufts Medical Center, United StatesCopyright © 2025 Ko, Strickland, Jaggessarsingh, Dillard, Green, Newburn, Sellaro, Klein, Caveney, Eisenberg, Severson, Ramkissoon, Previs and Toumeh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Heidi C. Ko, aGVpZGkua29AbGFiY29ycC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.