- 1Department of Colorectal and Anus Surgery, Yantai Affiliated Hospital of Binzhou Medical University, Yantai, China
- 2Department of Hepatobiliary Surgery, Yantai Affiliated Hospital of Binzhou Medical University, Yantai, China
- 3Department of Gastrointestinal Surgery, Hernia and Abdominal Wall Surgery I, Yantai Affiliated Hospital of Binzhou Medical University, Yantai, China
Colorectal cancer (CRC) poses a significant global health burden, with gut microbiota emerging as a crucial modulator of CRC pathogenesis and therapeutic outcomes. This review synthesizes current evidence on the influence of gut microbiota on tumor immune surveillance and responses to immunotherapies and chemotherapy in CRC. We highlight the role of specific microbial taxa in promoting or inhibiting tumor growth and the potential of microbiota-based biomarkers for predicting treatment efficacy. The review also discusses the implications of microbiota modulation strategies, including diet, probiotics, and fecal microbiota transplantation, for personalized CRC management. By critically evaluating the literature, we aim to provide a comprehensive understanding of the gut microbiota’s dual role in CRC and to inform future research directions in this field.
1 Introduction
Colorectal cancer (CRC) remains a leading cause of cancer-related mortality worldwide, accounting for approximately 10% of all cancer deaths, according to the Global Cancer Observatory (GLOBOCAN 2020) (1). The American Cancer Society estimated that there were about 151,030 new cases of CRC and approximately 52,580 deaths in the United States in 2022 alone (2). Risk factors contributing to the rise of CRC include genetic predispositions, dietary habits, sedentary lifestyles, and the increasing role of alterations in gut microbiota (3, 4). Notably, advancements in our understanding of tumor biology have illuminated the role of the gut microbiome in immune system modulation and its implications for tumor surveillance and therapeutic responses in CRC (5).
The gut microbiota, which is composed of trillions of microorganisms, including bacteria, viruses, fungi, and archaea, plays essential roles in metabolism, vitamin synthesis, and maintaining immune homeostasis (6). Recent studies have underscored the impact of dysbiosis—imbalance in the composition of gut microbiota—on CRC pathogenesis and progression (7). Research highlights differences in microbial composition between CRC patients and healthy individuals, suggesting that specific gut microbial patterns might serve as both biomarkers for early detection and targets for novel therapeutic strategies (8).
The relationship between gut microbiota and CRC extends beyond mere correlation. Experimental models of CRC have demonstrated that certain bacterial species, such as Fusobacterium nucleatum, can promote tumor growth through pro-inflammatory mechanisms and immune evasion, while beneficial species, such as Lactobacillus, might exert protective effects (9, 10). These observations raise critical questions regarding the mechanisms by which gut microbiota modulate both tumor immunity and responsiveness to therapies, particularly immune checkpoint inhibitors and chemotherapy.
Recent investigations suggest a substantial impact of gut microbiota on immune checkpoint blockade efficacy in CRC. For instance, researchers have shown that the presence of specific microbial taxa correlates with enhanced response rates to programmed death factor-1(PD-1) inhibitors (11). Conversely, other studies indicate that certain gut microbial profiles could render tumors resistant to immunotherapy, calling for a more nuanced understanding of microbiota-related mechanisms, which could facilitate the personalized treatment of CRC (12).
However, heterogeneity in study results has led to debates regarding the specific microbial species involved, the methodologies employed in microbiome profiling, and factors such as host genetics and diet that may contribute to these differences (13). For example, while Bacteroides fragilis has been implicated in tumorigenesis in some populations, it has shown protective effects in others, emphasizing the complexity of host-microbe interactions (13). Additionally, the usage of various sequencing techniques and bioinformatics tools adds layers of variability that challenge the reproducibility of findings across studies.
In this review, we will systematically evaluate existing literature focused on gut microbiota’s role in CRC, emphasizing its influence on tumor immune surveillance and therapeutic responses. By critically analyzing different studies and their outcomes, we aim to provide a comprehensive overview of the current understanding, along with highlighting gaps and inconsistencies in the research that warrant further investigation. The goal is to refine our perspective on gut microbiota’s dual role as both a biological entity influencing tumorigenesis and a potential therapeutic target, fostering future research endeavors in CRC prevention and treatment.
2 The role of gut microbiota in CRC
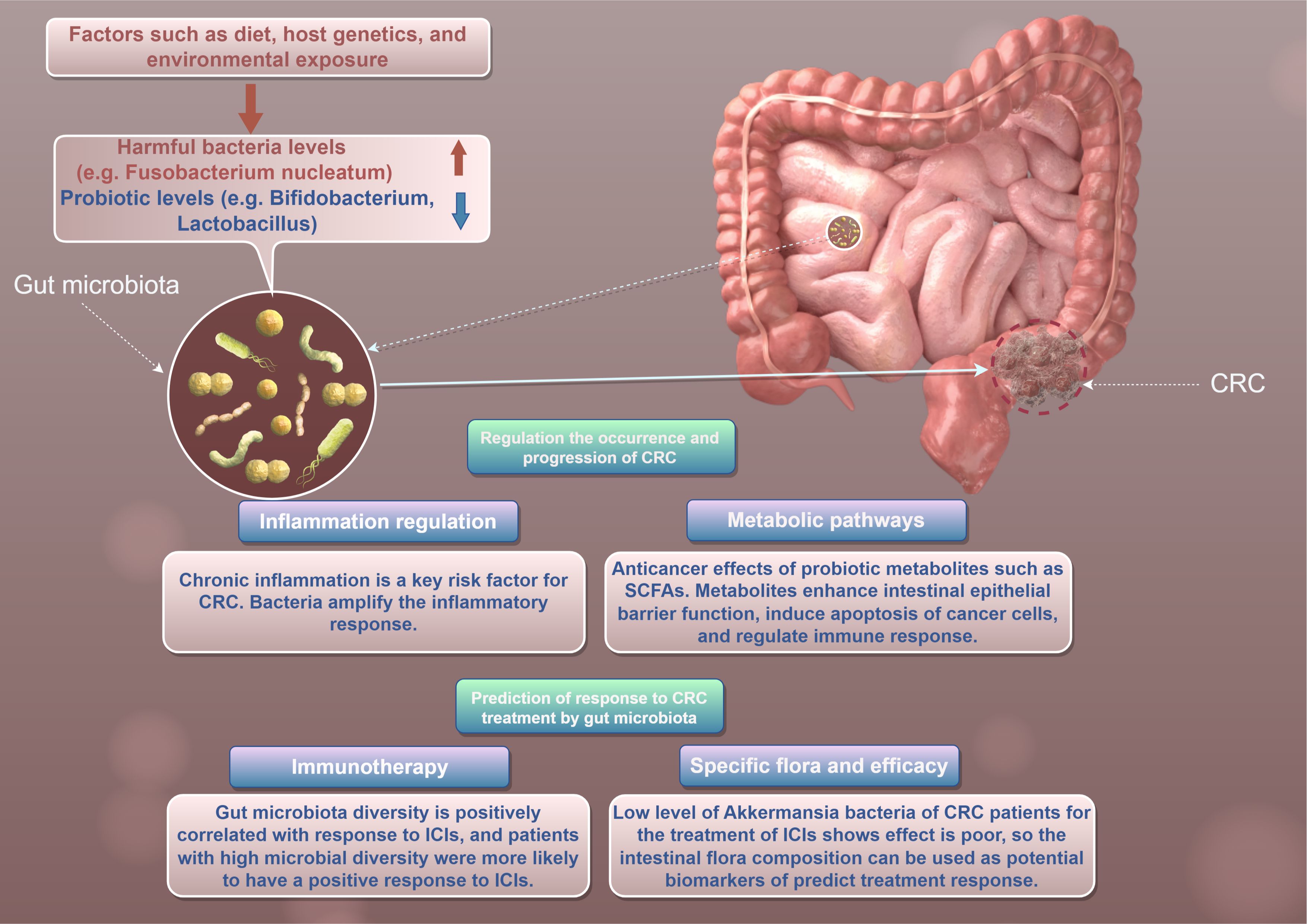
CRC is one of the leading causes of cancer-related morbidity and mortality worldwide (14). Emerging evidence suggests that gut microbiota plays a crucial role in the pathogenesis and progression of CRC (15) (Figure 1). The human gut is home to trillions of microorganisms, collectively referred to as the gut microbiota, which are involved in various physiological processes (6). Recent studies indicate that alterations in gut microbial communities may influence tumor immune surveillance and therapeutic responses in CRC patients (16).
2.1 Gut microbiota composition in CRC
The composition of gut microbiota can vary significantly between healthy individuals and CRC patients (17). Several studies have reported distinct microbial signatures associated with CRC. For instance, Zhu et al. (9) demonstrated that CRC patients exhibited increased levels of invasive bacteria, such as Fusobacterium nucleatum, which have been linked to tumor progression. This bacterium can enhance tumorigenesis through mechanisms such as the promotion of inflammatory responses and modulation of immune cell activities. Conversely, commensal bacteria such as Bifidobacterium may inhibit CRC progression through anti-inflammatory metabolites and immune modulation (18).
Notably, the relationship between gut microbiota and CRC is complex and influenced by various factors, including diet, host genetics, and environmental exposures (19) (Figure 1). Environmental factors, such as diet and microbial exposure in the living environment, play a crucial role in shaping the gut microbiota and its impact on CRC. Different dietary patterns can significantly alter the composition of intestinal microbiota, thereby affecting the occurrence of tumors and therapeutic outcomes.
Dietary patterns, such as high-fat diets, have been shown to increase the abundance of pro-inflammatory bacteria and reduce the diversity of the gut microbiota, which may promote tumor development. In contrast, high-fiber diets can enrich beneficial bacteria, such as Bifidobacterium and Lactobacillus, which are associated with a lower risk of CRC (20). These beneficial bacteria can enhance the production of short-chain fatty acids (SCFAs), which have anti-inflammatory and anti-tumor effects (21).
Moreover, microbial exposure in the living environment can also influence the gut microbiota. For example, exposure to certain bacteria or viruses may alter the gut microbiota composition, leading to an increased risk of CRC (22). Understanding these environmental influences can help enrich the research dimension and provide new insights into the prevention and treatment of CRC.
In a meta-analysis by Wirbel et al., the authors highlighted significant disparities in microbial profiles across different studies, emphasizing the need for standardized methodologies in gut microbiota research (23). Factors contributing to these inconsistencies include population diversity, geographic variations, and methodological differences in sampling and analysis. Future research should focus on exploring the complex interplay between environmental factors, gut microbiota, and CRC to develop more effective prevention and treatment strategies.
2.2 Mechanisms of gut microbiota in CRC pathogenesis
Gut microbiota’s influence on CRC involves multiple mechanisms, including the modulation of inflammation, metabolic pathways, and tumor immune surveillance (24). One of the key pathways through which gut microbiota contribute to CRC development is by promoting chronic inflammation (24). Chronic inflammation is a well-known risk factor for CRC, with cytokines and other pro-inflammatory mediators being implicated in tumorigenesis (25) (Figure 1). For example, Fusobacterium nucleatum can amplify the inflammatory response and activate oncogenic signaling pathways, such as the Wnt/β-catenin pathway, leading to increased cell proliferation and survival (26).
Moreover, certain microbial metabolites, particularly SCFAs produced by beneficial gut bacteria, have been shown to exert protective effects against CRC (27). SCFAs, such as butyrate, can enhance intestinal epithelial barrier function, promote apoptosis in cancerous cells, and regulate immune responses (28). According to a study by Kang et al, the oral administration of butyrate significantly reduced tumor incidence in mouse models of CRC, supporting its potential as a therapeutic strategy (29).
Recent studies have further elaborated on the key signaling pathways and molecular targets through which gut microbiota affect tumor immunity and therapeutic response (30, 31). For instance, Fusobacterium nucleatum has been shown to modulate the tumor microenvironment by inducing the expression of pro-inflammatory cytokines such as IL-6, IL-17, and tumor necrosis factor α, which promote angiogenesis and tumor cell metastasis (30). Additionally, Bacteroides fragilis, another bacterium associated with CRC, produces a toxin (BFT) that disrupts cell junctions of E-cadherin and β-catenin, leading to increased intestinal permeability and inflammation (32). BFT exerts its neoplastic effects by causing DNA damage and accumulating mutations, leading to cell proliferation and transcription of genes involved in tumor progression (c-myc) (33, 34).
The Wnt/β-catenin signaling pathway is a key oncogenic pathway that is often activated in CRC. Fusobacterium nucleatum can activate this pathway, leading to increased cell proliferation and survival (35, 36). Furthermore, the presence of certain bacteria, such as Fusobacterium nucleatum, has been correlated with worse survival rates among CRC patients, highlighting its potential role as a biomarker and prognostic factor (37).
2.3 Gut microbiota as predictors of therapeutic response
Therapeutic outcomes in CRC can also be influenced by gut microbiota composition (38). The advent of immunotherapy has raised interest in understanding how gut microbiota affects patients’ responses to treatments such as immune checkpoint inhibitors(ICIs) (39). Several studies have reported that patients with a diverse gut microbiota are more likely to respond positively to ICIs, with favorable outcomes correlated to higher microbial diversity (40, 41) (Figure 1). Conversely, patients with specific microbial signatures may have poorer responses to treatment (42). For example, a recent study indicated that low levels of the genus Akkermansia were associated with decreased efficacy of anti-PD-1 therapy in CRC patients (43). These findings suggest that gut microbiota profiling could potentially serve as a predictive biomarker for therapeutic responses, guiding personalized treatment strategies in CRC.
Despite the growing body of evidence supporting the role of gut microbiota in CRC, there are several gaps in our understanding that warrant further investigation (44). The mechanistic pathways through which specific gut microbes influence tumorigenesis and immune responses remain inadequately explored. Future studies should aim to delineate these pathways, potentially identifying novel therapeutic targets. Additionally, large-scale, multi-center studies are needed to establish a comprehensive understanding of gut microbiota’s role in CRC across diverse populations. Standardizing methodologies for microbiome profiling and integrating metagenomic, metabolomic, and immune profiling approaches will enrich our insights into the interactions between gut microbes and host biology (45).
In summary, gut microbiota plays a multifaceted role in CRC pathogenesis, impacting tumor immune surveillance and therapeutic responses (46). The evidence suggests that both the composition and activity of gut microbes can markedly influence tumor development and progression, as well as the efficacy of treatment modalities (47). Understanding these complexities opens new avenues for personalized medicine in CRC, as targeting gut microbiota or modulating their composition could form part of future therapeutic strategies. Continued research in this field is essential for translating these findings into clinical practice, ultimately aiming to improve outcomes for CRC patients.
3 Gut microbiota and tumor immune surveillance
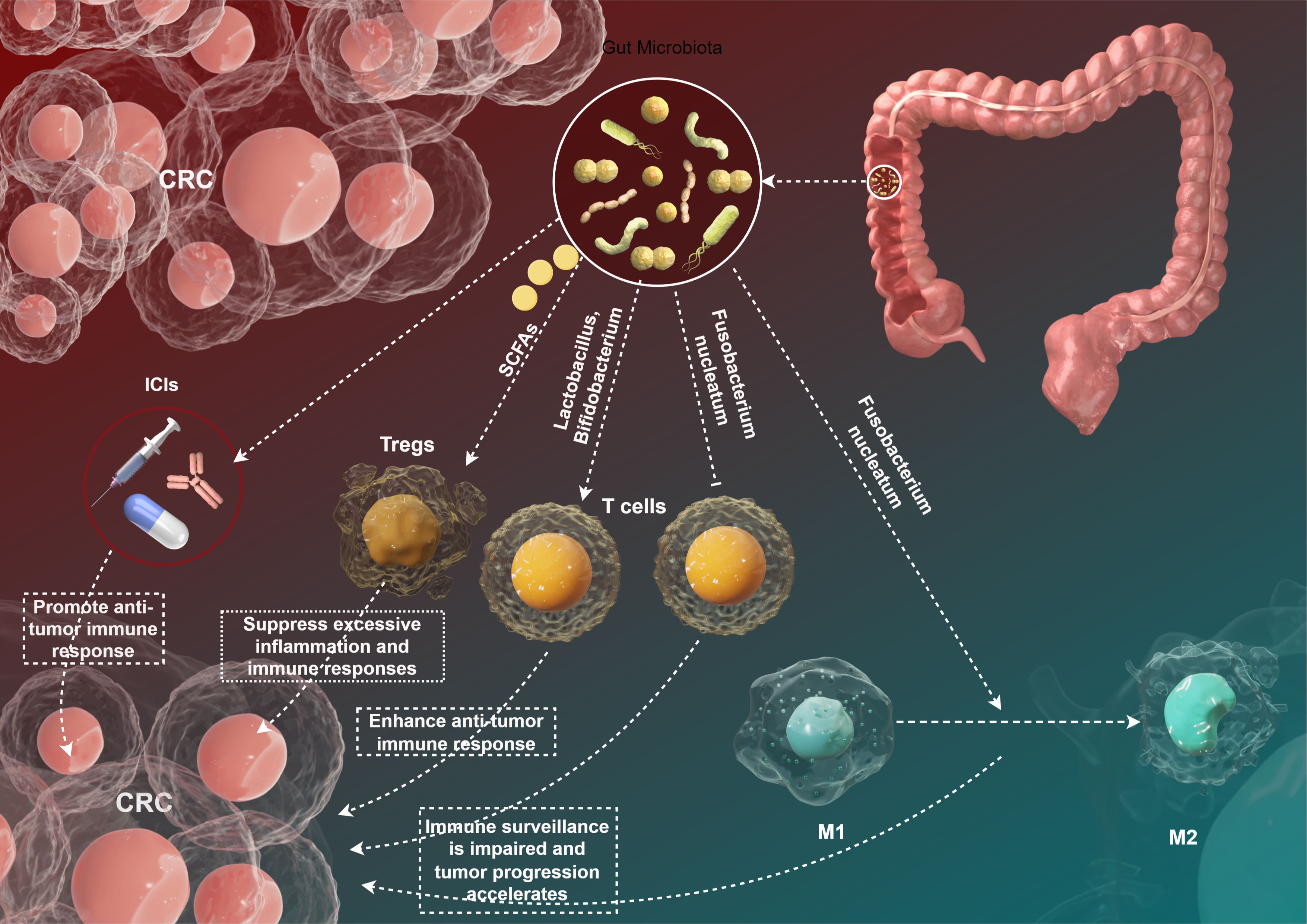
The intricate relationship between gut microbiota and the immune system has garnered significant attention in recent years, particularly regarding its implications for tumor immune surveillance in CRC. The gut microbiota contributes to the maintenance of immune homeostasis and the modulation of inflammatory responses, thus influencing the development and progression of malignancies (Figure 2). In this section, we will explore the current understanding of how gut microbiota interacts with tumor immune surveillance mechanisms, emphasizing various microbial species, their functional roles, and the diverse outcomes on tumor immunity.
3.1 Microbial composition and immune modulation
The composition of gut microbiota varies significantly among individuals, influenced by factors such as diet, lifestyle, and genetic background (6). Specific microbial communities have been shown to have distinct effects on host immunity. For example, a study by Luo et al. identified that a diverse gut microbiota composition is associated with enhanced anti-tumor immunity in CRC mouse models (48). The presence of beneficial bacteria, such as Lactobacillus and Bifidobacterium, was correlated with the activation of T cells and the production of pro-inflammatory cytokines that are crucial for anti-tumor responses (49) (Figure 2). Conversely, dysbiosis—characterized by an imbalance in microbial populations—has been linked to impaired immune surveillance and increased cancer risk (50). The pathogenic bacterium Fusobacterium nucleatum has been implicated in promoting CRC through multiple mechanisms, including the alteration of immune cell function. Fusobacterium nucleatum can inhibit the activity of cytotoxic T lymphocytes and promote the polarization of macrophages toward an M2 phenotype, which is associated with immunosuppression and tumor progression (51) (Figure 2).
3.2 Impact of gut microbiota on immune cell dynamics
Gut microbiota interact with various immune cell types, including dendritic cells (DCs), T cells, and regulatory T cells (Tregs), ultimately influencing the tumor microenvironment (52) (Figure 2). SCFAs, particularly butyrate, have been shown to enhance the differentiation and function of Tregs, promoting an anti-inflammatory environment (53). Arpaia et al. (54) demonstrated that butyrate plays a crucial role in Tregs differentiation, which can limit excessive inflammation and immune reactions against tumor cells. However, the role of Tregs in CRC is context-dependent; while they can prevent autoimmunity, their presence within tumors can also facilitate immune evasion by inhibiting effector T cell responses (54). Moreover, microbial metabolites can influence the maturation of dendritic cells, which are pivotal for T cell activation (55). Sivan et al. (56) reported that specific gut microbiota profiles could enhance the efficacy of immune checkpoint inhibitors by priming DCs to enhance anti-tumor immune responses. This finding underscores the potential for microbiota manipulation as an adjuvant therapy in immunotherapeutic approaches (56).
3.3 Gut microbiota and immune checkpoint inhibitors
The advent of ICIs has revolutionized the treatment landscape for various cancers, including CRC (57). The efficacy of ICIs is, in part, influenced by the composition of gut microbiota (39). A pivotal study by Wang et al. demonstrated that patients with a diverse gut microbiota had improved therapeutic responses to PD-1 blockade compared to those exhibiting dysbiosis (58) (Figure 2). The authors found that the presence of specific microbial taxa, including Akkermansia muciniphila and Faecalibacterium prausnitzii, correlated with better clinical outcomes (58). The mechanisms underlying this phenomenon are thought to involve enhanced anti-tumor immunity facilitated by gut microbiota. Microbes can influence the systemic immune environment, potentially increasing the infiltration of effector T cells into tumors (59). Additionally, the production of SCFAs may contribute to enhanced responsiveness to ICIs by modulating local and systemic immune responses (60). However, the relationship between gut microbiota and ICI efficacy remains complex and necessitates deeper investigation. Some studies have reported conflicting results, indicating that certain microbial profiles may be associated with reduced effectiveness of ICIs (38). For example, Routy et al. (61) found that specific Enterobacteriaceae members might be linked to poor responses to anti-PD-1 therapy in advanced melanoma, highlighting the need for personalized approaches in microbiota-targeted therapies.
3.4 Microbial metabolites as mediators of cancer host immune response
In addition to their direct effects on immune cells, gut microbiota also influence cancer host immune responses through the production of various metabolites. These microbial metabolites act as mediators that can modulate immune cell function, inflammation, and tumor microenvironment, thereby affecting antitumor immunity (62). SCFAs, such as butyrate, acetate, and propionate, are produced by the fermentation of dietary fibers by commensal bacteria. SCFAs have been shown to exert anti-inflammatory effects and enhance immune responses against tumors. For instance, butyrate can promote the differentiation of Tregs, which play a crucial role in maintaining immune homeostasis and preventing excessive inflammation (63). Additionally, SCFAs can enhance the production of cytokines such as IL-10 and transforming growth factor-beta (TGF-β), which are essential for immune regulation (60).
Bile acids, which are derived from cholesterol metabolism, can also be modified by gut microbiota. Microbial bile acid metabolites have been shown to influence immune cell function and tumor progression. For example, certain bile acid metabolites can activate the aryl hydrocarbon receptor (AhR), which is involved in the regulation of immune responses and has been implicated in cancer development (64). Tryptophan metabolism by gut microbiota produces various metabolites, including indole-3-acetic acid and indole-3-propionic aci, which have been shown to modulate immune responses. These metabolites can influence the activity of immune cells such as dendritic cells and T cells, thereby affecting antitumor immunity (65). Other microbial metabolites, such as succinate and formate, have also been implicated in modulating immune responses. For example, succinate has been shown to enhance the production of pro-inflammatory cytokines such as IL-1β and tumor necrosis factor-alpha (TNF-α), which can promote antitumor immunity (66).
3.5 The impact of gut microbiota on humoral immunity in antitumor responses
In addition to its role in modulating cellular immunity, gut microbiota also significantly influences humoral immunity, which plays a crucial role in antitumor responses (67). Humoral immunity involves the production of antibodies by B cells, which can recognize and neutralize tumor antigens, thereby inhibiting tumor growth and metastasis. The gut microbiota can affect humoral immunity through multiple mechanisms, including the regulation of B cell development, antibody production, and the modulation of immune cell interactions (67). Gut microbiota plays a vital role in the development and maturation of B cells. Studies have shown that specific microbial communities can promote the differentiation of B cells into plasma cells, which are responsible for antibody production. For instance, segmented filamentous bacteria have been shown to induce the production of IgA antibodies in the gut, which can help maintain gut homeostasis and prevent the overgrowth of pathogenic bacteria (68). Similarly, certain bacteria, such as Bifidobacterium and Lactobacillus, can stimulate the production of IgG antibodies, which are crucial for neutralizing tumor antigens and enhancing antitumor immunity (69).
Gut microbiota can also modulate the interactions between immune cells, including B cells, T cells, and DCs, thereby influencing humoral immunity. For example, SCFAs produced by gut microbiota can enhance the activation and proliferation of B cells, leading to increased antibody production (70, 71). Additionally, SCFAs can modulate the function of DCs, which play a crucial role in antigen presentation and the activation of T cells. By enhancing the function of DCs, gut microbiota can indirectly influence humoral immunity by promoting the activation of T cells, which can help regulate B cell responses (72). The influence of gut microbiota on humoral immunity has significant implications for antitumor responses. Studies have shown that specific microbial communities can enhance the production of antibodies that target tumor antigens, thereby improving antitumor immunity (56, 73). For instance, a study demonstrated that the administration of probiotics could increase the production of IgG antibodies against tumor antigens in CRC patients, leading to enhanced antitumor effects (74). Similarly, another study found that the presence of certain bacteria, such as Lactobacillus rhamnosus, could enhance the production of antibodies that neutralize tumor antigens, thereby inhibiting tumor growth and metastasis (67).
4 Gut microbiota and therapeutic response in CRC
The role of gut microbiota in modulating therapeutic responses in CRC has emerged as a significant area of research (75). Beyond its established function in homeostasis and immunity, the gut microbiome is increasingly recognized for its influence on treatment efficacy, particularly in the context of chemotherapy, targeted therapy, and immunotherapy (47) (Table 1).
4.1 Influence on chemotherapy response
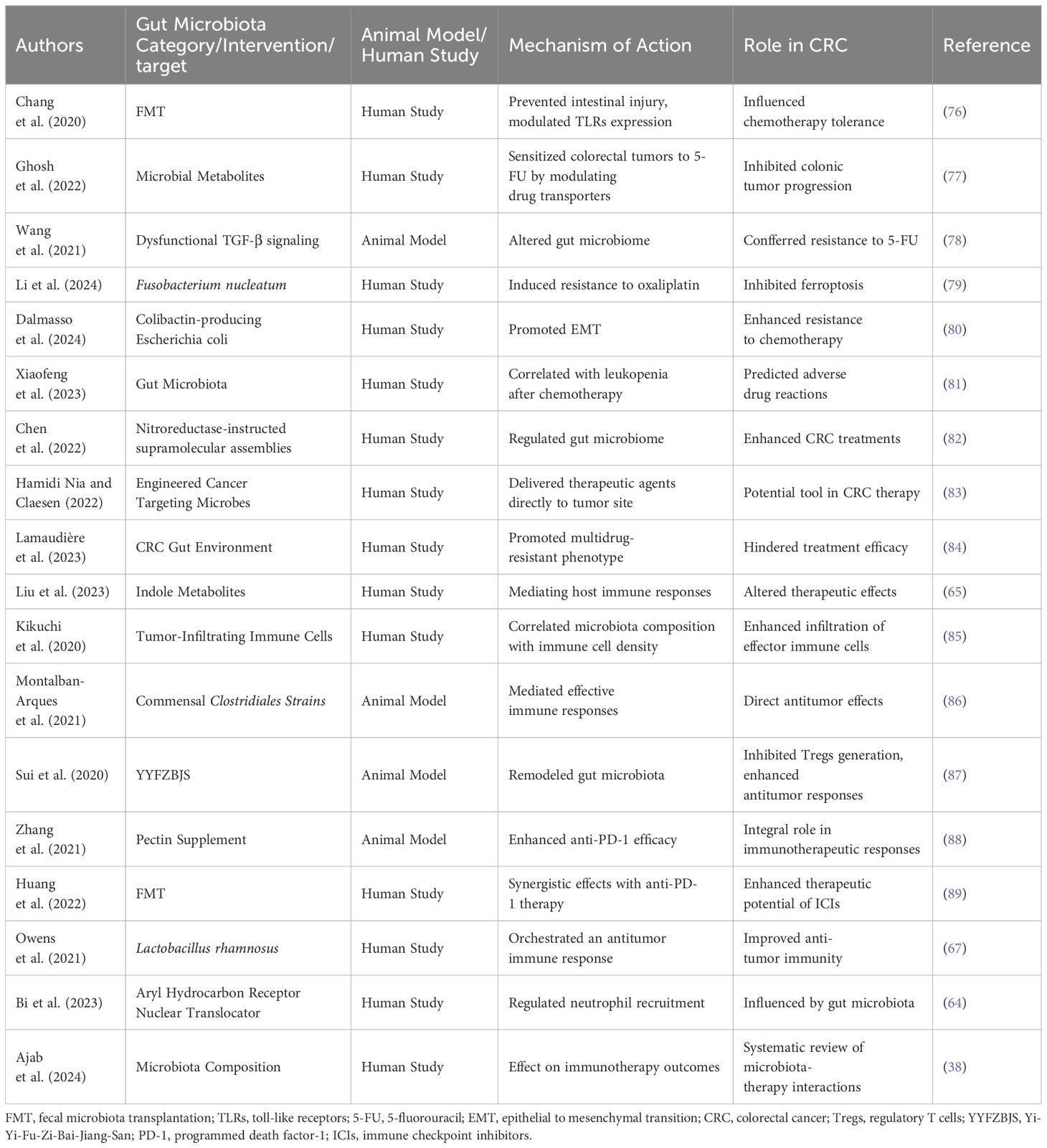
The impact of gut microbiota on chemotherapy outcomes in CRC has garnered increasing attention in recent years. Emerging evidence suggests that the composition and functional capacity of the gut microbiome can significantly alter the effectiveness and tolerability of common chemotherapeutic agents, such as 5-fluorouracil (5-FU) and oxaliplatin. Chang et al. (76) demonstrated that FMT not only prevented intestinal injury associated with chemotherapy but also reduced the systemic toxic effects of 5-FU and oxaliplatin through the modulation of Toll-like receptor (TLR) expression. This indicates that a balanced gut microbiome may enhance the tolerance of chemotherapy, revealing a potential approach for improving patient outcomes.
However, while some studies report beneficial associations between specific microbial taxa and enhanced chemotherapy response, others present contrasting findings. For example, Ghosh et al. (77) reported that certain microbial metabolites can sensitize colorectal tumors to 5-FU by modulating drug transporters via the FOXO3-FOXM1 axis, thereby inhibiting colonic tumor progression. In contrast, Wang et al. (78) found that mice with dysfunctional TGF-β signaling developed an altered gut microbiome that conferred resistance to 5-FU, highlighting the complexity of interactions between host immune responses, microbiome composition, and therapeutic efficacy. This discrepancy suggests that variations in microbiome composition might yield divergent effects on chemotherapy sensitivity, underscoring the need for personalized therapeutic strategies.
Moreover, certain bacterial species have been implicated in chemotherapy resistance mechanisms. Fusobacterium nucleatum, for instance, has been shown to induce resistance to oxaliplatin by inhibiting ferroptosis through the E-cadherin/β-catenin/GPX4 pathway (79). Similarly, another study identified colibactin-producing Escherichia coli as promoting resistance to chemotherapy by inducing epithelial-to-mesenchymal transition (EMT) during tumor progression (80). These findings highlight the potential for certain gut bacteria to not only correlate with adverse chemotherapy outcomes but also actively mediate resistance, complicating the therapeutic landscape for CRC.
In addition to these species, modifications in the gut microbiome post-chemotherapy have been shown to correlate with clinical outcomes. Xiaofeng et al. (81) observed a significant correlation between shifts in the gut microbiota and instances of leukopenia following chemotherapy in CRC patients, suggesting that microbiome alterations may predict adverse drug reactions. Such insights stress the importance of monitoring microbiome composition not only as a potential predictive biomarker but also as a target for therapeutic intervention.
Collectively, the interplay between gut microbiota and chemotherapy in CRC illustrates a complex relationship that can influence both efficacy and toxicity of treatment. This necessitates a comprehensive understanding of microbiome dynamics in the context of CRC therapy, paving the way for strategies that incorporate microbiota modulation to enhance therapeutic outcomes and mitigate detrimental effects. Further research is imperative to elucidate these relationships and their underlying mechanisms, ultimately contributing to the development of more effective, personalized cancer treatments.
4.2 Role in targeted therapies
The interplay between gut microbiota and therapeutic responses in CRC is gaining recognition, particularly in the context of targeted therapies. Recent studies suggest that the gut microbiota can modulate the effectiveness of various targeted treatments, including immunotherapies. Chen et al. (82) demonstrated that nitroreductase-instructed supramolecular assemblies can enhance the efficacy of CRC treatments by regulating the gut microbiome, highlighting the potential of microbiota modulation to increase therapeutic success in resistant tumors. Conversely, there is a variability in responses based on individual microbial communities, which can differ significantly between patients, affecting treatment outcomes.
Moreover, engineered microbes have emerged as highly promising tools in CRC therapy. Hamidi Nia and Claesen (83) described the use of cancer-targeting microbes and encapsulation devices to deliver therapeutic agents directly to the tumor site by navigating through the gut microbiome. However, the variability in the gut microbiome composition among patients complicates the development of standardized microbial therapies, raising concerns about the reproducibility of results across diverse populations.
Furthermore, Lamaudière et al. (84) have indicated that the CRC gut environment can promote a multidrug-resistant phenotype of ESKAPE pathogens, which may hinder treatment efficacy. Additionally, the role of gut-derived metabolites, particularly indole metabolites from tryptophan metabolism, has been implicated in mediating host immune responses and altering therapeutic effects, emphasizing the complexity of host-microbiota interactions in treatment (65).
In summary, while evidence supports a significant role of microbiota in CRC targeted therapies, inter-individual variability and microbial community dynamics remain critical hurdles in translating these findings into clinical practice. Further investigations are needed to elucidate consistent mechanisms and optimize therapies based on microbiota profiling, ultimately enhancing patient outcomes in CRC management.
4.3 Immunotherapy and gut microbiota
The gut microbiota has emerged as a critical factor influencing immune responses and therapeutic outcomes in CRC immunotherapy. A growing body of research illustrates how various microbial populations can either promote or inhibit anticancer immune responses, thereby shaping the efficacy of treatments such as ICIs. For example, Kikuchi et al. (85) characterized tumor-infiltrating immune cells in CRC and revealed a correlation between microbiota composition and the density of specific immune populations, such as T cells, in the tumor microenvironment. This suggests that a favorable gut microbiota may enhance the infiltration of effector immune cells, thereby potentially improving therapeutic outcomes.
Moreover, certain bacterial strains have shown promise in augmenting anti-tumor immunity. Montalban-Arques et al. (86) demonstrated that commensal Clostridiales strains could mediate effective immune responses against solid tumors, indicating that specific gut microbiota can have direct antitumor effects. In contrast, other studies highlight the potential for microbial dysbiosis to impair effective immune surveillance. Sui et al. (87) reported that treatment with Yi-Yi-Fu-Zi-Bai-Jiang-San (YYFZBJS) in Apc(Min/+) mice resulted in gut microbiota remodeling, which, in turn, inhibited Tregs generation, thus enhancing anti-tumor responses. This duality in microbiota influence underscores the complexity of interactions between gut communities, immune regulation, and therapeutic efficacy.
The potential of gut microbiota to predict immunotherapy outcomes has also been emphasized in recent studies. Zhang et al. (88) found that pectin supplementation significantly enhanced the efficacy of anti-PD-1 therapy in tumor-bearing mice with humanized gut microbiota derived from CRC patients, highlighting the integral role of specific dietary components and gut microbiota in immunotherapeutic responses. Additionally, Huang et al. (89) reported that FMT combined with anti-PD-1 therapy exhibited synergistic effects, suggesting that modulation of gut microbiota can play a pivotal role in enhancing the therapeutic potential of ICIs.
However, some discrepancies exist within the literature regarding the role of specific microbial taxa in CRC immunotherapy. For instance, while some studies have linked the abundance of certain bacteria, such as Lactobacillus rhamnosus, with improved anti-tumor immunity (67), others suggest the involvement of different microbial populations in negatively impacting immune responses, thus complicating the landscape of microbiota-therapy interactions (38, 64). Furthermore, the role of the AhR nuclear translocator in regulating neutrophil recruitment in CRC was shown to be influenced by gut microbiota, but the interplay remains complex and context-dependent (64).
In conclusion, the gut microbiota significantly influences the immune landscape in CRC and the outcomes of immunotherapy. While progress has been made in understanding these interactions, variability in findings and the complexity of microbiota-host dynamics necessitate further research. More extensive clinical studies that incorporate multi-omics approaches and exhaustive microbiota profiling may provide deeper insights into harnessing gut microbiota for enhanced immunotherapeutic strategies in CRC. Through such synergistic efforts, personalized microbiome-based interventions may one day become a standard adjunct to immunotherapy, ultimately improving patient outcomes in CRC management.
5 Microbiota modulation strategies in CRC: current advances
The translation of microbiota modulation strategies from experimental models to clinical practice remains a critical challenge in CRC management (44). While emerging evidence highlights the therapeutic potential of probiotics, prebiotics, dietary interventions, and FMT, key translational gaps—such as standardized protocols, safety monitoring, and personalized approaches—must be addressed to optimize clinical utility (Table 2). Below, we critically evaluate these strategies with a focus on their practical implementation in CRC care.
5.1 Probiotics
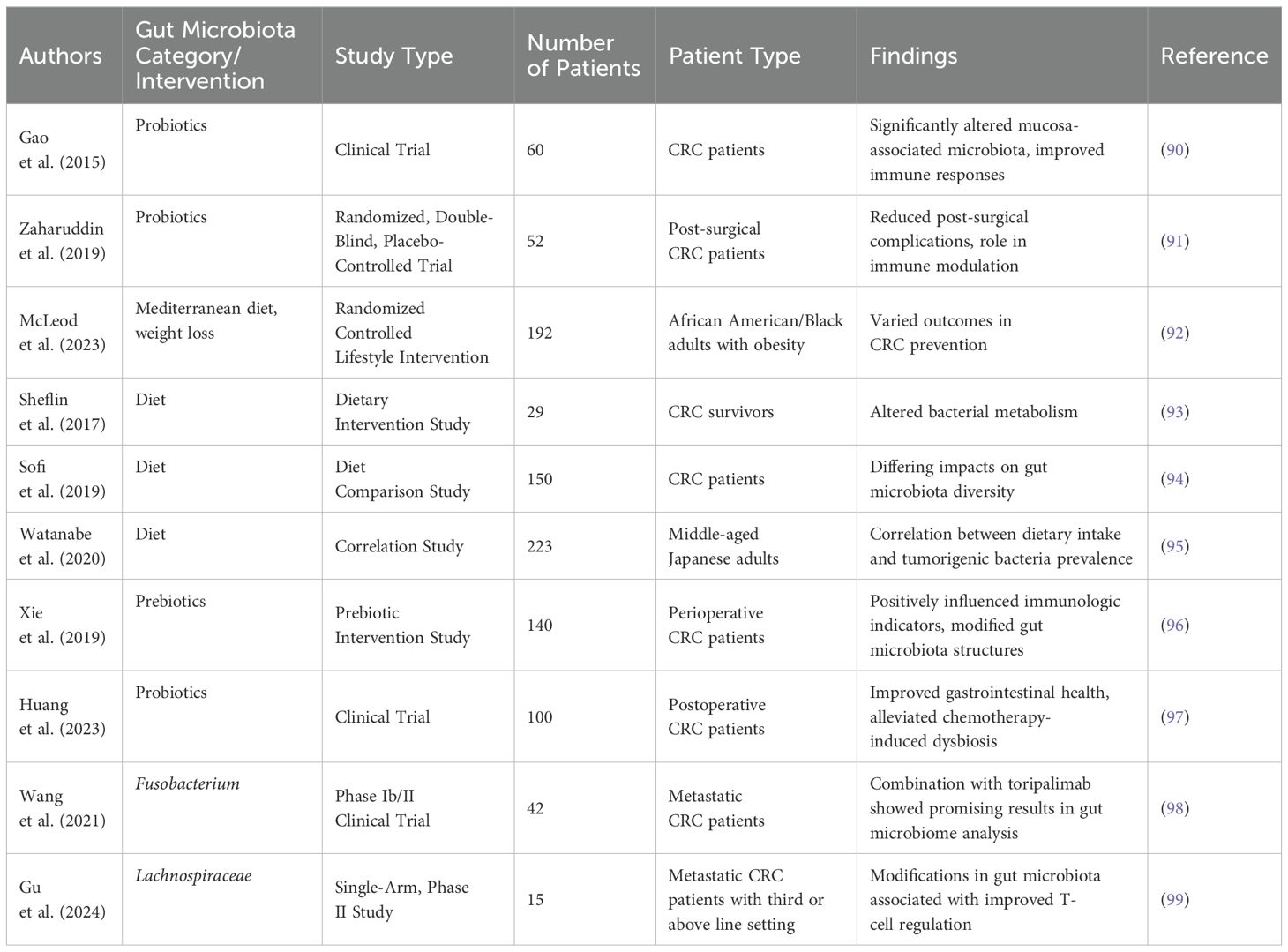
Probiotics, primarily Lactobacillus and Bifidobacterium strains, have demonstrated immunomodulatory and anti-tumor effects in preclinical models. However, clinical translation requires careful consideration of strain specificity, dosing, and timing. Gao et al. (90) demonstrated that the administration of probiotics in CRC patients significantly altered mucosa-associated microbiota, resulting in improved immune responses potentially beneficial for cancer therapy. A randomized, double-blind, placebo-controlled trial by Zaharuddin et al. (91) found that probiotics could reduce post-surgical complications in CRC patients, suggesting a role in post-operative recovery and immune modulation. Conversely, the impact of probiotics does not always lead to uniform results. For example, the study by McLeod et al. focused on a Mediterranean diet supplemented with specific probiotics demonstrating varied outcomes in CRC prevention (92). This disparity could be attributed to the strains of probiotics used and individual patient microbiota composition, emphasizing the need for personalized probiotic interventions. Hence, while probiotics are promising, their efficacy may be influenced by host-specific factors and the complexity of the intestinal ecosystem.
The selection of appropriate doses and timing of probiotic administration is crucial. Studies have shown that high doses of probiotics (e.g., 109-1010 CFU/day) may be necessary to achieve significant clinical effects (90). However, long-term safety monitoring is essential, as excessive probiotic use may lead to potential risks such as infection in immunocompromised patients. Future research should focus on defining standardized protocols for probiotic use, including optimal doses, timing, and duration of treatment, to maximize therapeutic benefits while minimizing potential risks.
5.2 Dietary interventions
Dietary interventions have emerged as significant determinants of gut microbiota composition, thereby influencing CRC risk. The study by Sheflin et al. (93) highlights how supplementation with rice bran and navy beans altered bacterial metabolism in CRC survivors, suggesting that these dietary components could beneficially reshape the gut microbiome. Similarly, Sofi et al. (94) reported differing impacts on gut microbiota diversity in patients following meat-based versus pesco-vegetarian diets. This suggests a robust interaction between diet, microbiota and CRC risk. In a contrasting perspective, Watanabe et al. (95) identified a correlation between specific dietary intakes and the prevalence of tumorigenic bacteria, highlighting that not all dietary changes yield positive microbiota outcomes. This inconsistency calls for rigorous investigation into specific dietary components and their mechanisms of action. As dietary interventions continue gaining traction, large-scale, long-term studies are warranted to establish causal relationships and optimize dietary recommendations for CRC patients.
Personalized dietary interventions should be considered based on individual patient characteristics, such as genetic background, gut microbiota composition, and comorbidities (100). Long-term safety monitoring is also necessary to assess potential adverse effects of dietary changes, such as nutritional deficiencies or gastrointestinal discomfort (101). Future research should focus on developing standardized dietary guidelines for CRC patients, incorporating specific dietary components and their optimal amounts to achieve the best clinical outcomes.
5.3 Prebiotics
Prebiotics, defined as non-digestible food components that promote the growth of beneficial gut microorganisms, have also shown significant promise in CRC management. Xie et al. (96) reported that prebiotic supplementation positively influenced immunologic indicators and modified gut microbiota structures in perioperative CRC patients. Their study indicates prebiotics’ ability to promote favorable immune responses that might enhance cancer treatment outcomes. Further supporting prebiotics, Huang et al. (97) found that postoperative probiotic administration, combined with prebiotics, significantly improved gastrointestinal health and alleviated chemotherapy-induced dysbiosis. Future research should focus on elucidating the mechanistic pathways by which prebiotics modulate gut microbiota and their interactions with immunologic responses in CRC.
The selection of appropriate prebiotic types and doses is essential. Common prebiotics include inulin, fructooligosaccharides, and galactooligosaccharides, each with different effects on gut microbiota. Studies have shown that daily doses of 5-10 grams of prebiotics may be effective in modulating gut microbiota (102). However, long-term safety monitoring is necessary to assess potential adverse effects, such as gastrointestinal bloating or diarrhea. Future research should focus on defining standardized protocols for prebiotic use, including optimal types, doses, and duration of treatment, to maximize therapeutic benefits while minimizing potential risks.
5.4 Combination therapies
Combination therapies involving conventional treatments and microbiota modulation strategies have also been investigated. The recent study combining regorafenib therapy with toripalimab showed promising results when analyzing the gut microbiome, underscoring the importance of microbiota in modulating therapeutic responses (98). Similarly, the open-label phase II clinical trial examining Quxie Capsules in conjunction with conventional therapies identified modifications in gut microbiota associated with improved T-cell regulation in metastatic CRC (99). However, the varying outcomes from these trials stress the complexity of the interactions within the microbiome and its multifaceted impact on cancer therapies. Moreover, Taylor et al. (103) questioned the consistency of microbial alterations across different treatment regimens, emphasizing the need for further stratified studies involving controlled variables.
The modulation of gut microbiota as a strategy in CRC management is a rapidly evolving field, showing great promise in enhancing therapeutic responses and immune surveillance. While probiotics, dietary interventions, and prebiotics each present individual merits, the complexity and variability of microbiota responses warrant a nuanced understanding of patient-specific factors. Future studies should aim to bridge the gaps in current evidence, focusing on standardized methodologies to define the optimal use of microbiota modulation. Personalized approaches, considering individual microbiomic compositions, diet, and treatment histories, will be pivotal to harnessing the full potential of microbiota modulation in CRC management.
6 The dilemma we face for the future
The gut microbiota’s intricate relationship with CRC presents a complex dilemma for future research and clinical practice. Our understanding of the microbiota’s role in tumor immune surveillance and therapeutic response is still evolving, with numerous challenges and questions remaining unanswered (44).
One of the primary challenges is the heterogeneity of the gut microbiota across populations. Studies such as those by Mignini et al. and Herlo et al. have highlighted the variability in microbiota composition between CRC patients and healthy individuals, suggesting potential differences in immune responses and treatment outcomes (22, 104). The heterogeneity introduces a dilemma in developing standardized microbiota-based therapies, as the microbial signatures associated with CRC can differ significantly among individuals and even among different tumor sites within the same patient (22). Another dilemma arises from the dynamic nature of the gut microbiota. As noted by Hu et al., the gut microbiota is influenced by various environmental and genetic factors, leading to changes in bacterial diversity and function (44). This dynamism complicates the prediction of treatment response and the development of personalized therapies based on microbiota profiles.
The interaction between the gut microbiota and systemic therapies, including chemotherapy and immunotherapy, is another area of dilemma. While some studies suggest that certain microbiota members can enhance the efficacy of these treatments, others indicate potential resistance induced by specific bacteria. For instance, the work by Mager et al. demonstrated that specific bacteria could enhance the response to checkpoint inhibitor immunotherapy, while Gao et al. found that Fusobacterium nucleatum could also enhance programmed cell death ligand 1 (PD-L1) blockade efficacy in CRC (105, 106). These findings suggest a dual role for the microbiota in therapy, which poses a dilemma in harnessing its benefits without exacerbating resistance.
The potential for microbiota-based biomarkers to predict therapeutic outcomes is a promising yet challenging area. As reviewed by Herlo et al., various bacterial species have been implicated as potential biomarkers for CRC diagnosis and prognosis, but the variability in microbiota composition and the lack of standardized testing protocols present significant hurdles (22). The dilemma here is to identify which microbial signatures are most reliable and how to integrate them into clinical practice without causing undue harm or delaying treatment.
Finally, the ethical and practical considerations of microbiota modulation are a dilemma for future research. FMT has shown promise in altering the gut microbiota to improve treatment outcomes, as suggested by studies like those by Routy et al. and Baruch et al. (107, 108). However, the long-term effects of FMT are not fully understood, and there are concerns about the potential transfer of pathogenic microbes and the ethical implications of using human fecal matter as a treatment.
In conclusion, the future of gut microbiota research in CRC is fraught with dilemmas. The heterogeneity of the microbiota, its dynamic nature, the complex interactions with systemic therapies, the challenge of developing reliable biomarkers, and the ethical considerations of microbiota modulation all require careful consideration. As we move forward, it is crucial to address these dilemmas through multidisciplinary collaboration, rigorous clinical trials, and a deeper understanding of the microbiota’s role in CRC pathophysiology.
7 Discussion
Numerous studies have identified distinct microbial signatures associated with CRC, emphasizing a shift towards pathogenic bacteria and a decrease in beneficial commensals within the gut microbiome of CRC patients (19, 46). Specifically, the overrepresentation of Fusobacterium nucleatum has been implicated in promoting tumorigenesis through inflammatory mediators, disrupting immune homeostasis, and modulating tumor microenvironment (109). Conversely, beneficial genera such as Lactobacillus and Bifidobacterium have been associated with protective effects against CRC due to their roles in reinforcing gut barrier function and modulating immune responses (49, 110).
The complex interactions between the gut microbiota and the tumor microenvironment, including tumor cells, immune cells, and stromal cells, play a crucial role in CRC development and progression. The gut microbiota can influence tumor growth and metastasis by modulating various components of the tumor microenvironment. For instance, certain bacterial species, such as Fusobacterium nucleatum, can promote tumor growth by inducing chronic inflammation and activating oncogenic signaling pathways, such as the Wnt/β-catenin pathway, which leads to increased cell proliferation and survival (26, 35). Additionally, microbial metabolites, such as SCFAs, can modulate the tumor microenvironment by enhancing anti-inflammatory responses and promoting the differentiation of Tregs, which may suppress antitumor immunity (60, 63).
Furthermore, the gut microbiota can influence the tumor stroma by altering the expression of extracellular matrix components and growth factors, thereby affecting tumor cell adhesion, migration, and invasion. For example, BFT that disrupts cell junctions of E-cadherin and β-catenin, leading to increased intestinal permeability and inflammation, which can promote tumor progression (32, 34). These interactions highlight the potential for targeting the gut microbiota to modulate the tumor microenvironment and develop new therapeutic strategies for CRC.
Emerging evidence supports the notion that gut microbiota influences tumor immune surveillance (111, 112). Bacterial metabolites, such as SCFAs produced by fermentation of dietary fibers, have been linked to enhanced anti-tumor immune responses through modulation of T cell differentiation and activation (60, 66). For instance, butyrate has been shown to promote Tregs differentiation, potentially skewing the immune response towards an immunosuppressive phenotype (63). However, the relationship between gut microbiota and immune responses in CRC remains complex and context-dependent. Recent findings indicate that certain bacteria can elicit pro-inflammatory responses that may aid tumor progression while simultaneously overcoming immune checkpoints (16, 113). These dual roles present a conundrum in deciphering whether specific microbial components can be harnessed for therapeutic gain without exacerbating tumor growth. Further mechanistic studies are required to unravel these interactions and ascertain how varying microbiota compositions can selectively augment or diminish immune responses in CRC.
The therapeutic potential of modulating the gut microbiota in CRC treatment is a rapidly evolving area of research (114). Microbiome-targeted therapies, including probiotics, prebiotics, and FMT, have emerged as promising adjuncts to conventional treatments, such as chemotherapy and immunotherapy (115). Some studies suggest that specific microbial profiles could predict patient responses to immune checkpoint inhibitors, with Bacteroides and Prevotella being associated with favorable outcomes (116, 117). However, the clinical application of these findings is hindered by several factors. First, the heterogeneous responses observed in different patient cohorts indicate that microbiome modulation is far from a one-size-fits-all approach. Additionally, the potential side effects of manipulating the microbiome raise concerns about the safety and feasibility of such interventions in clinical settings (118). Moreover, the ethical implications of microbiome research must be considered, particularly in terms of informing patients about the experimental nature of these therapies and the variability of individual responses (119).
The intricate relationship between gut microbiota and CRC underscores its pivotal role in tumor immune surveillance and therapeutic response. While numerous studies highlight the potential of microbiome modulation to enhance immunotherapeutic effects, disparities in findings necessitate cautious interpretation. Future investigations should emphasize large-scale, multi-center studies to elucidate specific microbial signatures and their functional mechanisms, ultimately guiding personalized treatment strategies that integrate microbiota profiling to optimize patient outcomes in CRC management.
8 Conclusion
In conclusion, the intricate relationship between gut microbiota and CRC underscores its pivotal role in tumor immune surveillance and therapeutic response. While numerous studies highlight the potential of microbiome modulation to enhance immunotherapeutic effects, disparities in findings necessitate cautious interpretation. Future studies should prioritize the development of standardized methodologies for microbiome profiling and the integration of multi-omics approaches to better understand the complex interactions between gut microbiota and CRC. These advancements will ultimately guide personalized treatment strategies that incorporate microbiota profiling, optimizing patient outcomes in CRC management.
Author contributions
CZ: Data curation, Visualization, Writing – original draft, Writing – review & editing. YW: Data curation, Resources, Writing – review & editing. LC: Investigation, Writing – review & editing. XC: Methodology, Writing – review & editing. CL: Conceptualization, Data curation, Funding acquisition, Resources, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by Shandong Province Medical and Health Science and Technology Development Plan Project (NO. 202204010880).
Acknowledgments
We would like to thank all the authors involved in writing this review and the Yantai Affiliated Hospital of Binzhou Medical College for facilitating the publication of this manuscript. Also, thanks for the convenience of FIGDRAW’s online photo making site.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
3. He M, Huan L, Wang X, Fan Y, Huang J. Nine dietary habits and risk of colorectal cancer: a Mendelian randomization study. BMC Med Genomics. (2024) 17:21. doi: 10.1186/s12920-023-01782-7
4. Valle L. Genetic predisposition to colorectal cancer: where we stand and future perspectives. World J Gastroenterol. (2014) 20:9828–49. doi: 10.3748/wjg.v20.i29.9828
5. Shakhpazyan NK, Mikhaleva LM, Bedzhanyan AL, Gioeva ZV, Mikhalev AI, Midiber KY, et al. Exploring the role of the gut microbiota in modulating colorectal cancer immunity. Cells. (2024) 13:1437. doi: 10.3390/cells13171437
6. Hills RD Jr., Pontefract BA, Mishcon HR, Black CA, Sutton SC, Theberge CR. Gut microbiome: profound implications for diet and disease. Nutrients. (2019) 11:1613–29. doi: 10.3390/nu11071613
7. Chawrylak K, Leśniewska M, Mielniczek K, Sędłak K, Pelc Z, Pawlik TM, et al. Gut microbiota-adversary or ally? Its role and significance in colorectal cancer pathogenesis, progression, and treatment. Cancers (Basel). (2024) 16:2236. doi: 10.3390/cancers16122236
8. Ibeanu GC, Rowaiye AB, Okoli JC, Eze DU. Microbiome differences in colorectal cancer patients and healthy individuals: implications for vaccine antigen discovery. Immunotargets Ther. (2024) 13:749–74. doi: 10.2147/itt.S486731
9. Zhu H, Li M, Bi D, Yang H, Gao Y, Song F, et al. Fusobacterium nucleatum promotes tumor progression in KRAS p.G12D-mutant colorectal cancer by binding to DHX15. Nat Commun. (2024) 15:1688. doi: 10.1038/s41467-024-45572-w
10. Pan H-W, Du L-T, Li W, Yang Y-M, Zhang Y, Wang C-X. Biodiversity and richness shifts of mucosa-associated gut microbiota with progression of colorectal cancer. Res Microbiol. (2020) 171:107–14. doi: 10.1016/j.resmic.2020.01.001
11. Gunjur A, Shao Y, Rozday T, Klein O, Mu A, Haak BW, et al. A gut microbial signature for combination immune checkpoint blockade across cancer types. Nat Med. (2024) 30:797–809. doi: 10.1038/s41591-024-02823-z
12. Shan J, Han D, Shen C, Lei Q, Zhang Y. Mechanism and strategies of immunotherapy resistance in colorectal cancer. Front Immunol. (2022) 13:1016646. doi: 10.3389/fimmu.2022.1016646
13. Tegegne HA, Savidge TC. Leveraging human microbiomes for disease prediction and treatment. Trends Pharmacol Sci. (2024) 30:797–809. doi: 10.1016/j.tips.2024.11.007
14. Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol. (2019) 14:89–103. doi: 10.5114/pg.2018.81072
15. Kim J, Lee HK. Potential role of the gut microbiome in colorectal cancer progression. Front Immunol. (2021) 12:807648. doi: 10.3389/fimmu.2021.807648
16. Hou X, Zheng Z, Wei J, Zhao L. Effects of gut microbiota on immune responses and immunotherapy in colorectal cancer. Front Immunol. (2022) 13:1030745. doi: 10.3389/fimmu.2022.1030745
17. Zhao L, Fang Y, Zhang J, Wei C, Ji H, Zhao J, et al. Changes in intestinal microbiota and their relationship with patient characteristics in colorectal cancer. Clin Med Insights Oncol. (2024) 18:11795549241307632. doi: 10.1177/11795549241307632
18. Elahi Z, Shariati A, Bostanghadiri N, Dadgar-Zankbar L, Razavi S, Norzaee S, et al. Association of Lactobacillus, Firmicutes, Bifidobacterium, Clostridium, and Enterococcus with colorectal cancer in Iranian patients. Heliyon. (2023) 9:e22602. doi: 10.1016/j.heliyon.2023.e22602
19. Rebersek M. Gut microbiome and its role in colorectal cancer. BMC Cancer. (2021) 21:1325. doi: 10.1186/s12885-021-09054-2
20. De Filippo C, Chioccioli S, Meriggi N, Troise AD, Vitali F, Mejia Monroy M, et al. Gut microbiota drives colon cancer risk associated with diet: a comparative analysis of meat-based and pesco-vegetarian diets. Microbiome. (2024) 12:180. doi: 10.1186/s40168-024-01900-2
21. Li S, Duan Y, Luo S, Zhou F, Wu Q, Lu Z. Short-chain fatty acids and cancer. Trends Cancer. (2024). doi: 10.1016/j.trecan.2024.11.003
22. Herlo L-F, Salcudean A, Sirli R, Iurciuc S, Herlo A, Nelson-Twakor A, et al. Gut microbiota signatures in colorectal cancer as a potential diagnostic biomarker in the future: A systematic review. Int J Mol Sci. (2024) 25:7937. doi: 10.3390/ijms25147937
23. Wirbel J, Pyl PT, Kartal E, Zych K, Kashani A, Milanese A, et al. Meta-analysis of fecal metagenomes reveals global microbial signatures that are specific for colorectal cancer. Nat Med. (2019) 25:679–89. doi: 10.1038/s41591-019-0406-6
24. Grellier N, Severino A, ArChilei S, Kim J, Gasbarrini A, Cammarota G, et al. Gut microbiota in inflammation and colorectal cancer: A potential Toolbox for Clinicians. Best Pract Res Clin Gastroenterol. (2024) 72:101942. doi: 10.1016/j.bpg.2024.101942
25. Zhang F, Qiao S. Research progress on the relationship between inflammation and colorectal cancer. Ann Gastroenterol Surg. (2022) 6:204–11. doi: 10.1002/ags3.12517
26. Liu J, Xiao Q, Xiao J, Niu C, Li Y, Zhang X, et al. Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduction Targeted Ther. (2022) 7:3. doi: 10.1038/s41392-021-00762-6
27. Duizer C, de Zoete MR. The role of microbiota-derived metabolites in colorectal cancer. Int J Mol Sci. (2023) 24:8024–49. doi: 10.3390/ijms24098024
28. Canani RB, Costanzo MD, Leone L, Pedata M, Meli R, Calignano A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J Gastroenterol. (2011) 17:1519–28. doi: 10.3748/wjg.v17.i12.1519
29. Kang J, Sun M, Chang Y, Chen H, Zhang J, Liang X, et al. Butyrate ameliorates colorectal cancer through regulating intestinal microecological disorders. Anticancer Drugs. (2023) 34:227–37. doi: 10.1097/cad.0000000000001413
30. Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. (2013) 14:207–15. doi: 10.1016/j.chom.2013.07.007
31. Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe. (2013) 14:195–206. doi: 10.1016/j.chom.2013.07.012
32. Cheng WT, Kantilal HK, Davamani F. The mechanism of bacteroides fragilis toxin contributes to colon cancer formation. Malays J Med Sci. (2020) 27:9–21. doi: 10.21315/mjms2020.27.4.2
33. Butt J, Jenab M, Werner J, Fedirko V, Weiderpass E, Dahm CC, et al. Association of Pre-diagnostic Antibody Responses to Escherichia coli and Bacteroides fragilis Toxin Proteins with Colorectal Cancer in a European Cohort. Gut Microbes. (2021) 13:1–14. doi: 10.1080/19490976.2021.1903825
34. Cariño AMD, Balanag GA, Magat EM, Fellizar A, Ortin TS, Villaflores O, et al. Antibody response to enterotoxigenic Bacteroides fragilis of Filipino colorectal cancer patients. Asian BioMed (Res Rev News). (2023) 17:273–80. doi: 10.2478/abm-2023-0070
35. Mondal T, Chattopadhyay D, Saha Mondal P, Das S, Mondal A, Das A, et al. Fusobacterium nucleatum modulates the Wnt/β-catenin pathway in colorectal cancer development. Int J Biol Macromol. (2025) 299:140196. doi: 10.1016/j.ijbiomac.2025.140196
36. Maurice MM, Angers S. Mechanistic insights into Wnt-β-catenin pathway activation and signal transduction. Nat Rev Mol Cell Biol. (2025). doi: 10.1038/s41580-024-00823-y
37. Sameni F, Elkhichi PA, Dadashi A, Sadeghi M, Goudarzi M, Eshkalak MP, et al. Global prevalence of Fusobacterium nucleatum and Bacteroides fragilis in patients with colorectal cancer: an overview of case reports/case series and meta-analysis of prevalence studies. BMC Gastroenterol. (2025) 25:71. doi: 10.1186/s12876-025-03664-x
38. Ajab SM, Zoughbor SH, Labania LA, Östlundh LM, Orsud HS, Olanda MA, et al. Microbiota composition effect on immunotherapy outcomes in colorectal cancer patients: A systematic review. PloS One. (2024) 19:e0307639. doi: 10.1371/journal.pone.0307639
39. Lu Y, Yuan X, Wang M, He Z, Li H, Wang J, et al. Gut microbiota influence immunotherapy responses: mechanisms and therapeutic strategies. J Hematol Oncol. (2022) 15:47. doi: 10.1186/s13045-022-01273-9
40. Roberto M, Carconi C, Cerreti M, Schipilliti FM, Botticelli A, Mazzuca F, et al. The challenge of ICIs resistance in solid tumours: could microbiota and its diversity be our secret weapon? Front Immunol. (2021) 12:704942. doi: 10.3389/fimmu.2021.704942
41. Zhang H, Hong Y, Wu T, Ben E, Li S, Hu L, et al. Role of gut microbiota in regulating immune checkpoint inhibitor therapy for glioblastoma. Front Immunol. (2024) 15:1401967. doi: 10.3389/fimmu.2024.1401967
42. Zhao L, Cho WC, Nicolls MR. Colorectal cancer-associated microbiome patterns and signatures. Front Genet. (2021) 12:787176. doi: 10.3389/fgene.2021.787176
43. Derosa L, Routy B, Thomas AM, Iebba V, Zalcman G, Friard S, et al. Intestinal Akkermansia muciniphila predicts clinical response to PD-1 blockade in patients with advanced non-small-cell lung cancer. Nat Med. (2022) 28:315–24. doi: 10.1038/s41591-021-01655-5
44. Hu Y, Zhou P, Deng K, Zhou Y, Hu K. Targeting the gut microbiota: a new strategy for colorectal cancer treatment. J Trans Med. (2024) 22:915. doi: 10.1186/s12967-024-05671-0
45. Shtossel O, Koren O, Shai I, Rinott E, Louzoun Y. Gut microbiome-metabolome interactions predict host condition. Microbiome. (2024) 12:24. doi: 10.1186/s40168-023-01737-1
46. Coker OO, Liu C, Wu WKK, Wong SH, Jia W, Sung JJY, et al. Altered gut metabolites and microbiota interactions are implicated in colorectal carcinogenesis and can be non-invasive diagnostic biomarkers. Microbiome. (2022) 10:35. doi: 10.1186/s40168-021-01208-5
47. Zhao L-Y, Mei J-X, Yu G, Lei L, Zhang W-H, Liu K, et al. Role of the gut microbiota in anticancer therapy: from molecular mechanisms to clinical applications. Signal Transduction Targeted Ther. (2023) 8:201. doi: 10.1038/s41392-023-01406-7
48. Luo M, Wang Q, Sun Y, Jiang Y, Wang Q, Gu Y, et al. Fasting-mimicking diet remodels gut microbiota and suppresses colorectal cancer progression. NPJ Biofilms Microbiomes. (2024) 10:53. doi: 10.1038/s41522-024-00520-w
49. Mazziotta C, Tognon M, Martini F, Torreggiani E, Rotondo JC. Probiotics mechanism of action on immune cells and beneficial effects on human health. Cells. (2023) 12:1. doi: 10.3390/cells12010184
50. Chen J, Domingue JC, Sears CL. Microbiota dysbiosis in select human cancers: Evidence of association and causality. Semin Immunol. (2017) 32:25–34. doi: 10.1016/j.smim.2017.08.001
51. Luo M, Li Q, Gu Q, Zhang C. Fusobacterium nucleatum: a novel regulator of antitumor immune checkpoint blockade therapy in colorectal cancer. Am J Cancer Res. (2024) 14:3962–75. doi: 10.62347/myza2640
52. Li C, Jiang P, Wei S, Xu X, Wang J. Regulatory T cells in tumor microenvironment: new mechanisms, potential therapeutic strategies and future prospects. Mol Cancer. (2020) 19:116. doi: 10.1186/s12943-020-01234-1
53. Wang J, Zhu N, Su X, Gao Y, Yang R. Gut-microbiota-derived metabolites maintain gut and systemic immune homeostasis. Cells. (2023) 12:793. doi: 10.3390/cells12050793
54. Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. (2013) 504:451–5. doi: 10.1038/nature12726
55. Calvo-Barreiro L, Zhang L, Abdel-Rahman SA, Naik SP, Gabr M. Gut microbial-derived metabolites as immune modulators of T helper 17 and regulatory T cells. Int J Mol Sci. (2023) 24:1806–19. doi: 10.3390/ijms24021806
56. Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. (2015) 350:1084–9. doi: 10.1126/science.aac4255
57. Weng J, Li S, Zhu Z, Liu Q, Zhang R, Yang Y, et al. Exploring immunotherapy in colorectal cancer. J Hematol Oncol. (2022) 15:95. doi: 10.1186/s13045-022-01294-4
58. Wang Y, Ma R, Liu F, Lee SA, Zhang L. Modulation of gut microbiota: A novel paradigm of enhancing the efficacy of programmed death-1 and programmed death ligand-1 blockade therapy. Front Immunol. (2018) 9:374. doi: 10.3389/fimmu.2018.00374
59. Mohseni AH, Taghinezhad-S S, Casolaro V, Lv Z, Li D. Potential links between the microbiota and T cell immunity determine the tumor cell fate. Cell Death Dis. (2023) 14:154. doi: 10.1038/s41419-023-05560-2
60. Abdelhalim KA. Short-chain fatty acids (SCFAs) from gastrointestinal disorders, metabolism, epigenetics, central nervous system to cancer - A mini-review. Chemico-Biological Interact. (2024) 388:110851. doi: 10.1016/j.cbi.2023.110851
61. Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. (2018) 359:91–7. doi: 10.1126/science.aan3706
62. Zhu X, Li K, Liu G, Wu R, Zhang Y, Wang S, et al. Microbial metabolite butyrate promotes anti-PD-1 antitumor efficacy by modulating T cell receptor signaling of cytotoxic CD8 T cell. Gut Microbes. (2023) 15:2249143. doi: 10.1080/19490976.2023.2249143
63. Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. (2013) 504:446–50. doi: 10.1038/nature12721
64. Bi Y, Yang Q, Li Z, Wang Y, Wang Y, Jia A, et al. Aryl hydrocarbon receptor nuclear translocator limits the recruitment and function of regulatory neutrophils against colorectal cancer by regulating the gut microbiota. J Exp Clin Cancer Res. (2023) 42:53. doi: 10.1186/s13046-023-02627-y
65. Liu Y, Pei Z, Pan T, Wang H, Chen W, Lu W. Indole metabolites and colorectal cancer: Gut microbial tryptophan metabolism, host gut microbiome biomarkers, and potential intervention mechanisms. Microbiol Res. (2023) 272:127392. doi: 10.1016/j.micres.2023.127392
66. Al-Qadami GH, Secombe KR, Subramaniam CB, Wardill HR, Bowen JM. Gut microbiota-derived short-chain fatty acids: impact on cancer treatment response and toxicities. Microorganisms. (2022) 10:2048–60. doi: 10.3390/microorganisms10102048
67. Owens JA, Saeedi BJ, Naudin CR, Hunter-Chang S, Barbian ME, Eboka RU, et al. Lactobacillus rhamnosus GG orchestrates an antitumor immune response. Cell Mol Gastroenterol Hepatol. (2021) 12:1311–27. doi: 10.1016/j.jcmgh.2021.06.001
68. Gao L, Li H, Liu X, Li H, Li P, Lu W, et al. Humoral immune responses primed by the alteration of gut microbiota were associated with galactose-deficient IgA1 production in IgA nephropathy. Front Immunol. (2024) 15:1415026. doi: 10.3389/fimmu.2024.1415026
69. Marco ML, Tachon S. Environmental factors influencing the efficacy of probiotic bacteria. Curr Opin Biotechnol. (2013) 24:207–13. doi: 10.1016/j.copbio.2012.10.002
70. Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. (2014) 157:121–41. doi: 10.1016/j.cell.2014.03.011
71. Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. (2016) 16:341–52. doi: 10.1038/nri.2016.42
72. Ivanov II, Honda K. Intestinal commensal microbes as immune modulators. Cell Host Microbe. (2012) 12:496–508. doi: 10.1016/j.chom.2012.09.009
73. Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. (2018) 359:97–103. doi: 10.1126/science.aan4236
74. Zitvogel L, Daillère R, Roberti MP, Routy B, Kroemer G. Anticancer effects of the microbiome and its products. Nat Rev Microbiol. (2017) 15:465–78. doi: 10.1038/nrmicro.2017.44
75. Lang T, Zhu R, Zhu X, Yan W, Li Y, Zhai Y, et al. Combining gut microbiota modulation and chemotherapy by capecitabine-loaded prebiotic nanoparticle improves colorectal cancer therapy. Nat Commun. (2023) 14:4746. doi: 10.1038/s41467-023-40439-y
76. Chang CW, Lee HC, Li LH, Chiang Chiau JS, Wang TE, Chuang WH, et al. Fecal microbiota transplantation prevents intestinal injury, upregulation of toll-like receptors, and 5-fluorouracil/oxaliplatin-induced toxicity in colorectal cancer. Int J Mol Sci. (2020) 21:386–98. doi: 10.3390/ijms21020386
77. Ghosh S, Singh R, Vanwinkle ZM, Guo H, Vemula PK, Goel A, et al. Microbial metabolite restricts 5-fluorouracil-resistant colonic tumor progression by sensitizing drug transporters via regulation of FOXO3-FOXM1 axis. Theranostics. (2022) 12:5574–95. doi: 10.7150/thno.70754
78. Wang Z, Hopson LM, Singleton SS, Yang X, Jogunoori W, Mazumder R, et al. Mice with dysfunctional TGF-β signaling develop altered intestinal microbiome and colorectal cancer resistant to 5FU. Biochim Biophys Acta Mol Basis Dis. (2021) 1867:166179. doi: 10.1016/j.bbadis.2021.166179
79. Li B, Wei Z, Wang Z, Xu F, Yang J, Lin B, et al. Fusobacterium nucleatum induces oxaliplatin resistance by inhibiting ferroptosis through E-cadherin/β-catenin/GPX4 axis in colorectal cancer. Free Radic Biol Med. (2024) 220:125–38. doi: 10.1016/j.freeradbiomed.2024.04.226
80. Dalmasso G, Cougnoux A, Faïs T, Bonnin V, Mottet-Auselo B, Nguyen HT, et al. Colibactin-producing Escherichia coli enhance resistance to chemotherapeutic drugs by promoting epithelial to mesenchymal transition and cancer stem cell emergence. Gut Microbes. (2024) 16:2310215. doi: 10.1080/19490976.2024.2310215
81. Xiaofeng N, Jian C, Jingjing W, Zhanbo Q, Yifei S, Jing Z, et al. Correlation of gut microbiota with leukopenia after chemotherapy in patients with colorectal cancer. BMC Microbiol. (2023) 23:349. doi: 10.1186/s12866-023-03067-6
82. Chen J, Zhang P, Zhao Y, Zhao J, Wu X, Zhang R, et al. Nitroreductase-instructed supramolecular assemblies for microbiome regulation to enhance colorectal cancer treatments. Sci Adv. (2022) 8:eadd2789. doi: 10.1126/sciadv.add2789
83. Hamidi Nia L, Claesen J. Engineered cancer targeting microbes and encapsulation devices for human gut microbiome applications. Biochemistry. (2022) 61:2841–8. doi: 10.1021/acs.biochem.2c00251
84. Lamaudière MTF, Arasaradnam R, Weedall GD, Morozov IY. The colorectal cancer gut environment regulates activity of the microbiome and promotes the multidrug resistant phenotype of ESKAPE and other pathogens. mSphere. (2023) 8:e0062622. doi: 10.1128/msphere.00626-22
85. Kikuchi T, Mimura K, Ashizawa M, Okayama H, Endo E, Saito K, et al. Characterization of tumor-infiltrating immune cells in relation to microbiota in colorectal cancers. Cancer Immunol Immunother. (2020) 69:23–32. doi: 10.1007/s00262-019-02433-6
86. Montalban-Arques A, Katkeviciute E, Busenhart P, Bircher A, Wirbel J, Zeller G, et al. Commensal Clostridiales strains mediate effective anti-cancer immune response against solid tumors. Cell Host Microbe. (2021) 29:1573–1588.e7. doi: 10.1016/j.chom.2021.08.001
87. Sui H, Zhang L, Gu K, Chai N, Ji Q, Zhou L, et al. YYFZBJS ameliorates colorectal cancer progression in Apc(Min/+) mice by remodeling gut microbiota and inhibiting regulatory T-cell generation. Cell Commun Signal. (2020) 18:113. doi: 10.1186/s12964-020-00596-9
88. Zhang SL, Mao YQ, Zhang ZY, Li ZM, Kong CY, Chen HL, et al. Pectin supplement significantly enhanced the anti-PD-1 efficacy in tumor-bearing mice humanized with gut microbiota from patients with colorectal cancer. Theranostics. (2021) 11:4155–70. doi: 10.7150/thno.54476
89. Huang J, Zheng X, Kang W, Hao H, Mao Y, Zhang H, et al. Metagenomic and metabolomic analyses reveal synergistic effects of fecal microbiota transplantation and anti-PD-1 therapy on treating colorectal cancer. Front Immunol. (2022) 13:874922. doi: 10.3389/fimmu.2022.874922
90. Gao Z, Guo B, Gao R, Zhu Q, Wu W, Qin H. Probiotics modify human intestinal mucosa-associated microbiota in patients with colorectal cancer. Mol Med Rep. (2015) 12:6119–27. doi: 10.3892/mmr.2015.4124
91. Zaharuddin L, Mokhtar NM, Muhammad Nawawi KN, Raja Ali RA. A randomized double-blind placebo-controlled trial of probiotics in post-surgical colorectal cancer. BMC Gastroenterol. (2019) 19:131. doi: 10.1186/s12876-019-1047-4
92. McLeod A, Wolf P, Chapkin RS, Davidson LA, Ivanov I, Berbaum M, et al. Design of the Building Research in CRC prevention (BRIDGE-CRC) trial: a 6-month, parallel group Mediterranean diet and weight loss randomized controlled lifestyle intervention targeting the bile acid-gut microbiome axis to reduce colorectal cancer risk among African American/Black adults with obesity. Trials. (2023) 24:113. doi: 10.1186/s13063-023-07115-4
93. Sheflin AM, Borresen EC, Kirkwood JS, Boot CM, Whitney AK, Lu S, et al. Dietary supplementation with rice bran or navy bean alters gut bacterial metabolism in colorectal cancer survivors. Mol Nutr Food Res. (2017) 61:132–42. doi: 10.1002/mnfr.201500905
94. Sofi F, Dinu M, Pagliai G, Pierre F, Gueraud F, Bowman J, et al. Fecal microbiome as determinant of the effect of diet on colorectal cancer risk: comparison of meat-based versus pesco-vegetarian diets (the MeaTIc study). Trials. (2019) 20:688. doi: 10.1186/s13063-019-3801-x
95. Watanabe D, Murakami H, Ohno H, Tanisawa K, Konishi K, Tsunematsu Y, et al. Association between dietary intake and the prevalence of tumourigenic bacteria in the gut microbiota of middle-aged Japanese adults. Sci Rep. (2020) 10:15221. doi: 10.1038/s41598-020-72245-7
96. Xie X, He Y, Li H, Yu D, Na L, Sun T, et al. Effects of prebiotics on immunologic indicators and intestinal microbiota structure in perioperative colorectal cancer patients. Nutrition. (2019) 61:132–42. doi: 10.1016/j.nut.2018.10.038
97. Huang F, Li S, Chen W, Han Y, Yao Y, Yang L, et al. Postoperative probiotics administration attenuates gastrointestinal complications and gut microbiota dysbiosis caused by chemotherapy in colorectal cancer patients. Nutrients. (2023) 15:356–67. doi: 10.3390/nu15020356
98. Wang F, He MM, Yao YC, Zhao X, Wang ZQ, Jin Y, et al. Regorafenib plus toripalimab in patients with metastatic colorectal cancer: a phase Ib/II clinical trial and gut microbiome analysis. Cell Rep Med. (2021) 2:100383. doi: 10.1016/j.xcrm.2021.100383
99. Gu Z, Wang L, Zhai Ma J, Zhang T, Yang Y. Conventional therapy combined with quxie capsule modulating gut microbiome in metastatic colorectal cancer patients with the third or above line setting: result from an investigator-initiated, open-label, single-arm, phase II study. Integr Cancer Ther. (2024) 23:15347354241280390. doi: 10.1177/15347354241280390
100. Song M, Chan AT. Diet, gut microbiota, and colorectal cancer prevention: A review of potential mechanisms and promising targets for future research. Curr Colorectal Cancer Rep. (2017) 13:429–39. doi: 10.1007/s11888-017-0389-y
101. Sonnenburg JL, Bäckhed F. Diet-microbiota interactions as moderators of human metabolism. Nature. (2016) 535:56–64. doi: 10.1038/nature18846
102. Davani-Davari D, Negahdaripour M, Karimzadeh I, Seifan M, Mohkam M, Masoumi SJ, et al. Prebiotics: definition, types, sources, mechanisms, and clinical applications. Foods. (2019) 8:92–105. doi: 10.3390/foods8030092
103. Taylor WS, Pearson J, Miller A, Schmeier S, Frizelle FA, Purcell RV. MinION Sequencing of colorectal cancer tumour microbiomes-A comparison with amplicon-based and RNA-Sequencing. PloS One. (2020) 15:e0233170. doi: 10.1371/journal.pone.0233170
104. Mignini I, Piccirilli G, Galasso L, Termite F, Esposto G, Ainora ME, et al. From the colon to the liver: how gut microbiota may influence colorectal cancer metastatic potential. J Clin Med. (2024) 13:420–32. doi: 10.3390/jcm13020420
105. Mager LF, Burkhard R, Pett N, Cooke NCA, Brown K, Ramay H, et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science. (2020) 369:1481–9. doi: 10.1126/science.abc3421
106. Gao Y, Bi D, Xie R, Li M, Guo J, Liu H, et al. Fusobacterium nucleatum enhances the efficacy of PD-L1 blockade in colorectal cancer. Signal Transduct Target Ther. (2021) 6:398. doi: 10.1038/s41392-021-00795-x
107. Routy B, Lenehan JG, Miller WH Jr., Jamal R, Messaoudene M, Daisley BA, et al. Fecal microbiota transplantation plus anti-PD-1 immunotherapy in advanced melanoma: a phase I trial. Nat Med. (2023) 29:2121–32. doi: 10.1038/s41591-023-02453-x
108. Baruch EN, Youngster I, Ben-Betzalel G, Ortenberg R, Lahat A, Katz L, et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. (2021) 371:602–9. doi: 10.1126/science.abb5920
109. Proença MA, Biselli JM, Succi M, Severino FE, Berardinelli GN, Caetano A, et al. Relationship between Fusobacterium nucleatum, inflammatory mediators and microRNAs in colorectal carcinogenesis. World J Gastroenterol. (2018) 24:5351–65. doi: 10.3748/wjg.v24.i47.5351
110. Fong W, Li Q, Yu J. Gut microbiota modulation: a novel strategy for prevention and treatment of colorectal cancer. Oncogene. (2020) 39:4925–43. doi: 10.1038/s41388-020-1341-1
111. Aghamajidi A, Maleki Vareki S. The effect of the gut microbiota on systemic and anti-tumor immunity and response to systemic therapy against cancer. Cancers (Basel). (2022) 14:3563–78. doi: 10.3390/cancers14153563
112. Luu M, Schütz B, Lauth M, Visekruna A. The impact of gut microbiota-derived metabolites on the tumor immune microenvironment. Cancers (Basel). (2023) 15:1588–605. doi: 10.3390/cancers15051588
113. Zhuang YP, Zhou HL, Chen HB, Zheng MY, Liang YW, Gu YT, et al. Gut microbiota interactions with antitumor immunity in colorectal cancer: From understanding to application. BioMed Pharmacother. (2023) 165:115040. doi: 10.1016/j.biopha.2023.115040
114. Sánchez-Alcoholado L, Ramos-Molina B, Otero A, Laborda-Illanes A, Ordóñez R, Medina JA, et al. The role of the gut microbiome in colorectal cancer development and therapy response. Cancers (Basel). (2020) 12:1406–20. doi: 10.3390/cancers12061406
115. Zhang T, Li X, Li J, Sun F, Duan L. Gut microbiome-targeted therapies as adjuvant treatments in inflammatory bowel diseases: a systematic review and network meta-analysis. J Gastroenterol Hepatol. (2024). doi: 10.1111/jgh.16795
116. Bouferraa Y, Fares C, Bou Zerdan M, Boyce Kennedy L. Microbial influences on immune checkpoint inhibitor response in melanoma: the interplay between skin and gut microbiota. Int J Mol Sci. (2023) 24:9702–15. doi: 10.3390/ijms24119702
117. Griffin ME, Hang HC. Microbial mechanisms to improve immune checkpoint blockade responsiveness. Neoplasia. (2022) 31:100818. doi: 10.1016/j.neo.2022.100818
118. Parigi TL, Vieujean S, Paridaens K, Dalgaard K, Peyrin-Biroulet L, Danese S. Efficacy, safety, and concerns on microbiota modulation, antibiotics, probiotics, and fecal microbial transplant for inflammatory bowel disease and other gastrointestinal conditions: results from an international survey. Microorganisms. (2023) 11:2806–19. doi: 10.3390/microorganisms11112806
Keywords: colorectal cancer, gut microbiota, tumor immune surveillance, therapeutic response, probiotics, prebiotics, fecal microbiota transplantation
Citation: Zhang C, Wang Y, Cheng L, Cao X and Liu C (2025) Gut microbiota in colorectal cancer: a review of its influence on tumor immune surveillance and therapeutic response. Front. Oncol. 15:1557959. doi: 10.3389/fonc.2025.1557959
Received: 09 January 2025; Accepted: 14 February 2025;
Published: 05 March 2025.
Edited by:
Liang Qiao, The University of Sydney, AustraliaReviewed by:
Dengke Yin, Anhui University of Chinese Medicine, ChinaKe Xiao, Shandong University, China
Copyright © 2025 Zhang, Wang, Cheng, Cao and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunyuan Liu, YnlmeXB3a0AxMjYuY29t
†These authors have contributed equally to this work
 Chunlei Zhang
Chunlei Zhang Yong Wang2†
Yong Wang2†