
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol., 27 March 2025
Sec. Molecular and Cellular Oncology
Volume 15 - 2025 | https://doi.org/10.3389/fonc.2025.1556917
Cancer is a disease where cells begin to divide uncontrollably and spread to other parts of the body. Mitotic kinases play a crucial role in the initiation and progression of all human malignancies, making them common therapeutic targets. However, a significant portion of the human kinome has yet to be functionally studied in cancer systems. The NIMA-related kinase family (NEKs), consisting of 11 members distributed across different cellular regions, are important protein kinases that regulate mitotic processes. Emerging research suggests that NEK family members have potential key roles in various malignancies. This review systematically summarizes the expression and regulatory mechanisms of NEK family members in different cancer systems, highlighting that targeting NEKs holds promise as a new therapeutic approach for inhibiting cancer growth and metastasis.
According to data from the World Health Organization, cancer is an important cause of death globally. It was reported that in 2022,there were 19,976,499 new cases of malignant tumors worldwide, an increase of nearly 700,000 compared to 2020 (1). Among these, the highest incidence of malignancies was in lung cancer, followed by breast cancer, colorectal cancer, prostate cancer, stomach cancer and liver cancer. The most challenging obstacles in cancer therapy is the gradual development of therapy resistance by patients, leading to tumor recurrence. Therefore, discovering new targets that can restore treatment sensitivity or provide alternative signal pathways for targeting is a key focus in therapeutic research. Kinases are commonly used targets in oncology (2).The human kinome is vast, comprising 518 kinases, offering a wealth of potential targets for oncology (3).Currently,253 kinase-specific inhibitors are in phases 1-4 of clinical trials (4).Inhibiting kinase activity and preventing the corresponding phosphorylation cascades affect various pathways of tumorigenesis, including cell proliferation,apoptosis, and cell migration. Despite the availability of several kinase-targeted therapies, many kinases within the human kinome remain underexplored, potentially holding new therapeutic targets.
The NIMA-related kinase (NEK) family is a group of serine/threonine protein kinases that play an important role in regulating mitotic processes. In addition to cyclin-dependent kinases (CDKs),Aurora kinases, and Polo-like kinases (PLKs), NEKs are among the most-known kinase families, initially recognized for their role in cell cycle regulation (5).Mammals are composed of 11 NEKs, respectively are NEK1, NEK2, NEK3, NEK4, NEK5, NEK6, NEK7, NEK8, NEK9, NEK10 and NEK11, which are distributed across cilia, centrosomes, the nucleus, cytoplasm, and mitochondria (6).Recent studies have increasingly shown that NEK family members are also involved in mRNA splicing, myogenic differentiation, inflammasome formation, protein transport within the cell, mitochondrial homeostasis, and DNA damage, with their abnormal expression or function closely related to tumor progression. They are associated with the occurrence and development of breast cancer,lung cancer,prostate cancer, pancreatic cancer, thyroid cancer, colorectal cancer, and hepatocellular carcinoma and are associated with poor prognosis for various cancers, making them potential therapeutic targets (7, 8). In recent years, information about the NEK family has been accumulating. Consider the versatility of the NEK family, this article outlines the research of the NEK family in various cancers (Figure 1), as well as the clinical research of the NEK family as a potential biomarker in oncology (Table 1). Meanwhile we introduce the molecular mechanism of the NEK family (Table 2) and the current summary of potential therapeutic inhibitors of the NEK family(Table 3), aiming to provide researchers with a fuller and more comprehensive understanding of the NEK family and to offer a foundation for exploring tumorigenesis mechanisms and designing anti-tumor drugs.
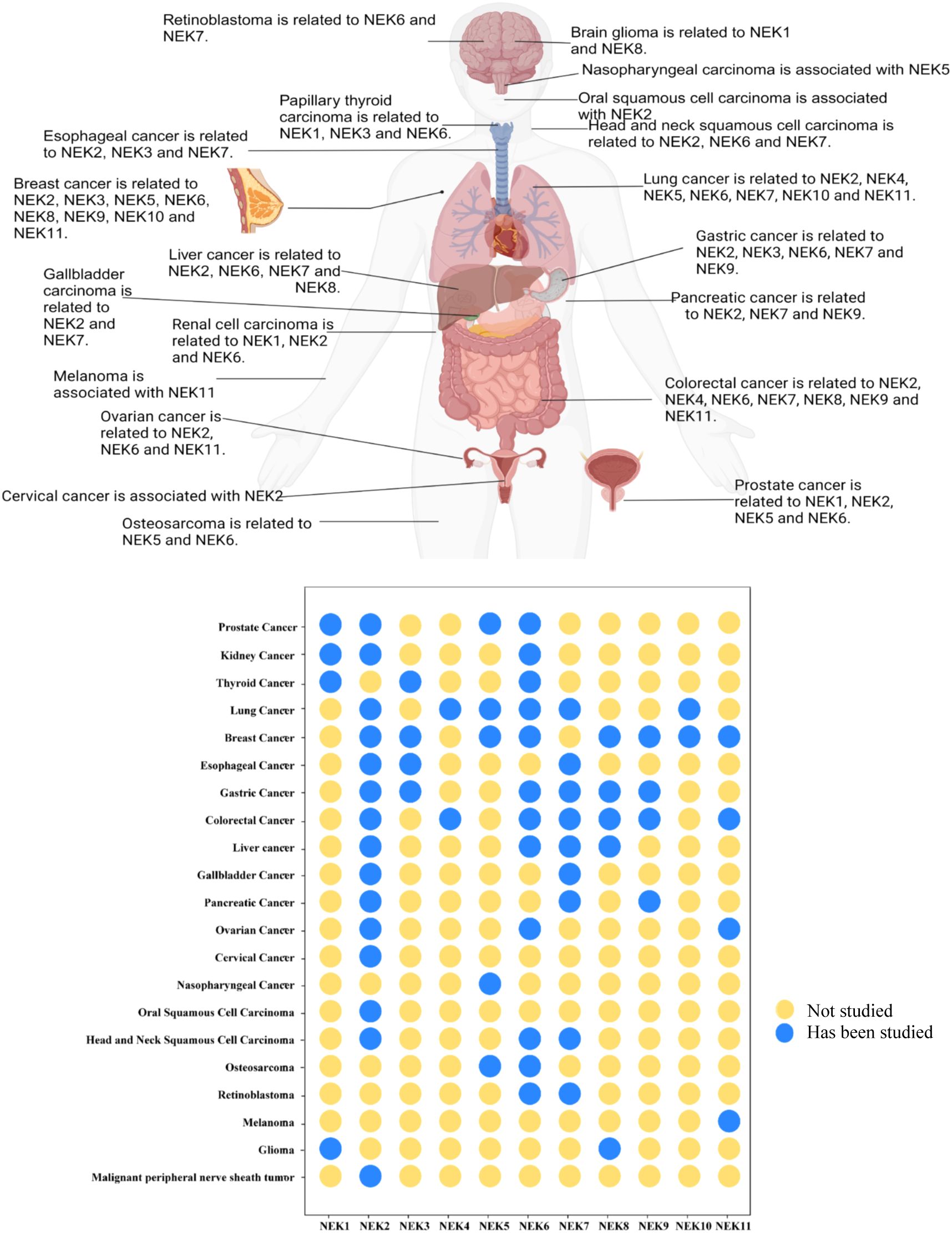
Figure 1. Summary of research progress on the NEK family in the occurrence and development of various cancers based on the literature.(Created in BioRender. Fengying, S. (2025) https://BioRender.com/c45s254).
The kinase domain of NEK family proteins contains two subdomains: a small N-lobe and a large C-lobe (Figure 2A). The structural characteristics of the NEK family determine their diversity in cellular functions. Most NEK family members possess an N-terminal catalytic domain that contains a His-Arg-Asp (HRD) motif, and there is a serine/threonine residue in the activation loop which may be the site of activation modifications. In some NEK family members, this residue undergoes autophosphorylation, while in others, it is phosphorylated by an upstream kinase (9, 10). The C-terminal regions exhibit significant variation in length, sequence, and structure, and their diversified domains allow them to localize in different organelles and participate in specific cellular functions.NEK1 is the longest, possessing a typical N-terminal kinase domain capable of catalyzing the phosphorylation of serine/threonine residues; its C-end contains various functional domains, including multiple coiled-coil regions and domains that regulate protein-protein interactions. The experimental crystal structure details of NEK1 (PDB id: 4APC) are shown in Figure 2B.The C-terminus of NEK2 contains a coiled-coil domain. Such as: there are three splicing variants of NEK2 in mammals:NEK2A and NEK2B differ at their carboxy terminus, while NEK2C lacks the 8 amino acid sequence at the carboxy terminus of NEK2A. NEK2A is uniformly distributed in the nucleus and cytoplasm, NEK2B is mainly found in the cytoplasm, and NEK2C is primarily located in the nuclear region (11). NEK3 is relatively short and contains only a conserved N-terminal kinase domain. NEK5 has a unique DEAD-box domain, which is not present in other NEK family members (5). The sequence identity of the kinase regions of NEK6 and NEK7 exceeds 85%, both lacking complex C-terminal regulatory domains. Thus, their kinase activity mainly relies on interactions with other proteins (such as NEK9) (12). NEK9 has a long C-terminal structure that contains RCC1-like repeat sequences, allowing it to interact with NEK6 and NEK7 to regulate their activity. Generally, autophosphorylation typically occurs within the activation loop of the kinase domain, but the non-catalytic regions at the C-terminus of NEK8 and NEK9 can regulate their localization and activation through autophosphorylation (13, 14).The kinase domain of NEK10 is located in the middle of the protein rather than at the N-terminus.
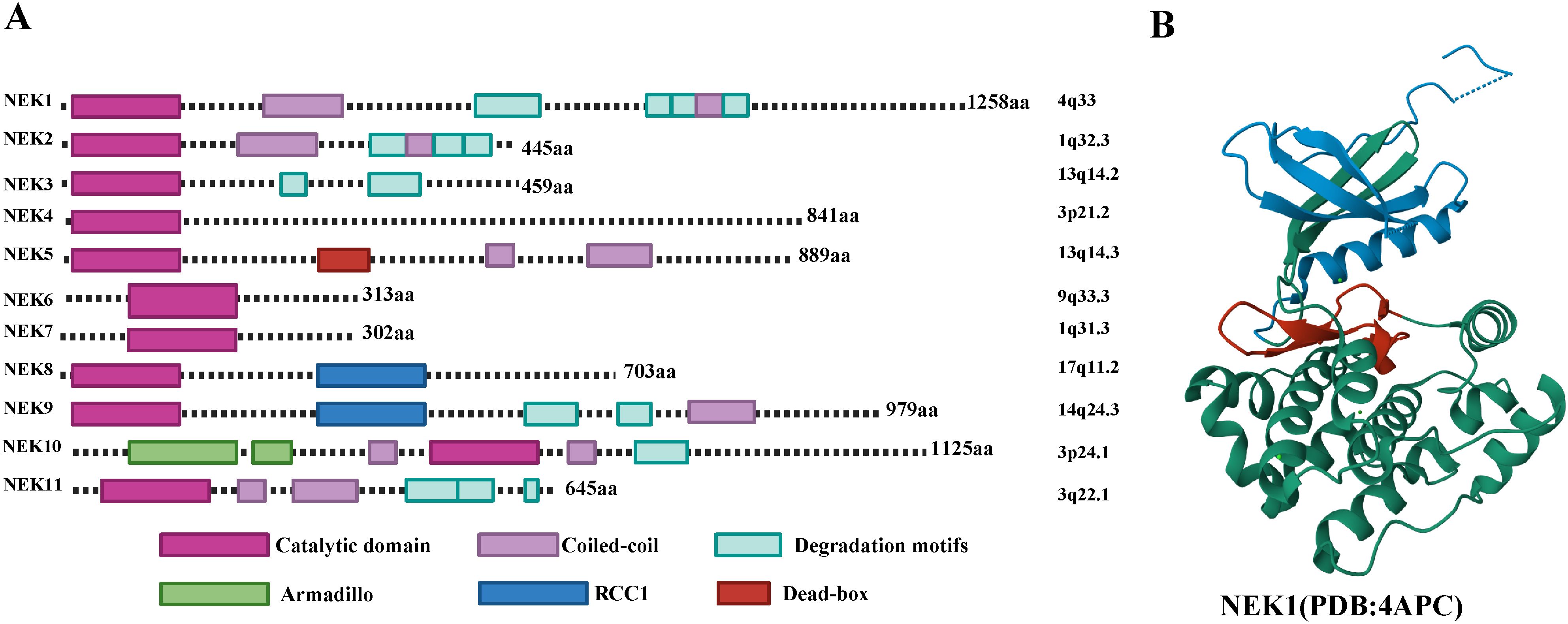
Figure 2. (A) Structural characteristics of the human NEK family. (B) Experimental crystal structure details of NEK1. Blue represents the N-terminal lobe, green represents the C-terminal lobe, and red represents the activation loop.
Cell cycle dysregulation is a major cause of tumorigenesis, and the human NEK family is extensively involved in various stages of the cell cycle. In particular, it controls cell mitosis (7). During mitosis,NEK2,NEK6,NEK7,and NEK9 cooperate to regulate the formation of the bipolar spindle, chromatin condensation, nuclear envelope breakdown, and cytokinesis.NEK3 is associated with cell proliferation and signal transduction, while NEK1,NEK4,NEK5,NEK7,NEK8, NEK10,and NEK11 are all related to DNA damage response.NEK1,NEK8,and NEK4 play roles in cilia in post-mitotic cells (7).Therefore, overexpression of NEKs leads to chromosomal instability and aneuploidy in cancer cells. Moreover, Overexpression of NEKs activates several carcinogenic pathways, resulting in cancer development by acquiring proliferation, invasion, anti-apoptosis, and resistance capabilities.
In the NEK family,NEK1 is the gene most studied in relation to the occurrence and development of prostate cancer. In advanced prostate cancer, the level of phosphorylated NEK1 is significantly elevated. The Tousled Like kinases (TLK) is a phosphorylation inducer (15).TLK1 can activate NEK1 by phosphorylating the keto amino acid at position 141 (T141) of NEK1.Analysis of prostate cancer tissue samples reveals that the level of p-NEK1-T141 increases with higher Gleason scores (the grading system for prostate cancer),suggesting that this phosphorylated state may serve as a biomarker for disease progression (16).Early-stage prostate cancer typically relies on androgen signaling for growth, making androgen deprivation therapy (ADT) a common treatment strategy. VDAC1 is a channel protein on the outer mitochondrial membrane, and studies have found that TLK1 can form a complex with NEK1 to regulate the phosphorylation state of VDAC1,maintaining mitochondrial integrity and preventing apoptosis (17).NEK1 can also phosphorylate multiple residues on the key effector protein Yes-associated protein 1 (YAP1) in the Hippo pathway, enhancing the stability and nuclear retention of YAP1.Phosphorylated YAP1 can bind to the transcription factor transcriptional enhancer associated domain protein(TEAD)and the androgen receptor (AR),regulating gene expression and enhancing the growth and anti-apoptotic capacity of cancer cells, thereby promoting prostate cancer cell resistance to ADT (18).Many patients develop androgen-independent prostate cancer (AIPC) after treatment, which is no longer sensitive to conventional endocrine therapy, leading to treatment difficulties and poor prognosis. Research has shown that TLK1 and NEK1 are highly expressed in AIPC models, and inhibition of TLK1 or NEK1 expression disrupts DNA damage repair signaling pathways, making cells more sensitive to DNA damage. Inhibitors targeting the TLK1/NEK1 axis, such as Thioridazine, can effectively inhibit the growth of AIPC by modulating the activity of TLK1 without affecting its expression levels (19).The combination of Thioridazine with existing ADT can further enhance treatment efficacy, indicating its potential value as a combined therapy strategy.
Additionally, the high expression of NEK1 leads to the development of other tumors. For example, Yanbing Shen and colleagues (20) found that high expression of NEK1 is associated with poor prognosis of thyroid cancer. Talita Diniz Melo-Hanchuk and others further discovered that NEK1 is specifically overexpressed in follicular thyroid carcinoma, with higher levels found in patients with multifocal tumors and lymph node metastasis (6).Moreover,NEK1 expression is elevated in human glioma tissues and human glioma cell lines, correlating with lower survival rates in patients. Jun Zhu and colleagues found (21) that overexpression of NEK1 in glioma cells may be an important TMZ resistance factor. Silencing the NEK1 gene using siRNA significantly inhibited tumor cell growth and induced apoptosis, while also reduce resistance to temozolomide (21). Yumay Chen and colleagues (22) found increased expression of NEK1 in renal cancer. Compared to normal renal tubular epithelial cells, the expression of NEK1 was significantly increased in renal carcinoma tissues and cultured renal carcinoma cell lines. Overexpression of NEK1 results in cancer cells exhibiting greater resistance to DNA-damaging treatments, while downregulation of the NEK1 gene significantly enhances cancer cell sensitivity to these treatments and induces apoptosis. Guang Chen and others have confirmed through fundamental experiment that (23),the VHL protein is an important tumor suppressor and is often involved in the degradation of hypoxia-inducible factor (HIF) protein family. VHL may be involved in the regulation of NEK1. In renal cancer cells with VHL function loss, the stability of HIF-2α increases, and HIF-2α can directly bind to the hypoxia-responsive element (HRE) in the NEK1 gene promoter, thereby enhancing NEK1 expression. This suggests that NEK1 may be a new hypoxia-inducible gene (24).
NEK2 is the most extensively studied gene among various types of cancer.NEK2 expression is low in the G1 phase of the cell cycle, increases in the S and G2 phases with enhanced activity, peaks in the late G2/M phase, and decreases as the cell enters mitosis.Additionally,NEK2 is responsible for initiating centrosome separation during the G2/M phase of the cell cycle (25).Overexpression of NEK2 leads to chromosomal instability and aneuploidy in cancer cells (26).NEK2 is expressed at higher levels in many human cancers compared to normal tissues, including prostate cancer (27),renal cancer (28, 29),non-small cell lung cancer (30, 31),breast cancer (32, 33),gastric cancer (34, 35),esophageal cancer (36, 37),colorectal cancer (38–40),hepatocellular carcinoma (41, 42),pancreatic cancer (43),cholangiocarcinoma (44),ovarian cancer (45, 46),cervical cancer (47),oral squamous cell carcinoma (48),and malignant peripheral nerve sheath tumors (49).Thus, elevated NEK2 expression appears to be a common feature in most human tumors. Studies report that NEK2 expression influences cancer cell proliferation (50, 51).NEK2’s pro-proliferative role may be related to the Wnt/β-catenin pathway. NEK2 can phosphorylate β-catenin, promoting its nuclear translocation (52–54), which the expression of Wnt target genes is activated and cell proliferation is affected.Furthermore,NEK2 can inhibit apoptosis and promote cell survival (31). The mechanism by which NEK2 inhibition leads to apoptosis in tumor cells is still unclear. Hayward and colleagues reported for the first time that overexpression of NEK2 precedes cancer cell metastasis (55).NEK2 is believed to participate in invasion and motility, and promote cancer metastasis (56, 57). NEK2’s pro-metastatic role may also be related to the Wnt/β-catenin pathway. Research shows NEK2 overexpression induces nuclear accumulation of β-catenin in lung cancer cells (26). Additionally, NEK2 can enhance tumor cell invasion and metastasis by regulating the epithelial-mesenchymal transition (EMT) process (58). Drug sensitivity and resistance are important issues in cancer treatment. Some researchers have reported that NEK2 is involved in resistance to 5-fluorouracil,tamoxifen, trastuzumab, paclitaxel, and doxorubicin (59–61).NEK2 and Plk4 may promote the proliferation of cancer cells with genomic alterations, thereby affecting the uptake, metabolism, and cellular response to therapeutic drugs, leading to drug resistance (60).Meanwhile, NEK2 gene silencing combined with paclitaxel or doxorubicin can enhance the sensitivity of TNBC cells to chemotherapy (59).
Unlike NEK1, NEK3 is primarily overexpressed in papillary thyroid carcinoma with high cellularity. Quantitative analysis of NEK3 expression can distinguish between benign and malignant thyroid tissues, with a sensitivity of 78%, specificity of 80%, and accuracy of 79%.This suggests that NEK3 expression is closely related to the malignancy and invasiveness of thyroid cancer (6).According to the literature, NEK3 expression is upregulated in esophageal adenocarcinoma and its precursor lesion, Barrett’s esophagus (62).NEK3 is overexpressed in gastric cancer and is significantly associated with TNM staging, lymph node metastasis, and poor prognosis (63).In a breast cancer cell models, NEK3 phosphorylation site Thr165 is a key regulator of cancer cell migration and local adhesion remodeling (64).The phosphorylation of NEK3 Thr-165 depends on the activation of the MEK1-ERK1/2 signaling pathway in response to Prolactin (PRL) stimulation in vivo.U0126 can inhibit PRL induced activation of the MEK1-ERK1/2 signaling pathway, thereby significantly reducing the phosphorylation level of NEK3 at the Thr-165 site (64). PRL, which plays a critical role not only in mammary gland development and milk production but also as a pro-carcinogenic factor in breast cancer progression and metastasis, has been shown by SL Miller and colleagues to promote breast cancer cell motility by activating NEK3.This activation leads to changes in the phosphorylation state of cytoskeletal proteins, such as Cofilin, triggering cytoskeletal reorganization and enhancing cell movement (65).
Currently, research on NEK4 in cancer is limited, but it has been reported to be involved in mediating cancer cell death pathways. Although apoptosis can inhibit tumor development and metastasis, cancer cells often acquire the ability to overcome these barriers. The tumor necrosis factor-related apoptosis inducing ligan(TRAIL), a member of the tumor necrosis factor(TNF)family,can trigger apoptosis. Since TRAIL receptors are primarily expressed on cancer cells, TRAIL can selectively mediate tumor cell death. TRAIL has been considered a potential cancer therapeutic agent (66, 67).Survivin is an anti-apoptotic protein which highly expressed in most cancers and leads to chemotherapy resistance, increased tumor recurrence and shortened survival. In vitro studies have found that the loss of NEK4 increases the sensitivity of colorectal and lung cancer cells to down-regulate Survivin-mediated TRAIL-induced apoptosis (68).Lung cancer is a highly metastatic malignant tumor, and epithelial-mesenchymal transition (EMT) is a key process for tumor cells to acquire the ability to invade and metastasize. Both in vivo and in vitro studies have confirmed that NEK4 kinase promotes EMT in lung cancer cells, enhancing their migration and invasion abilities, thereby promoting lung cancer metastasis (69).Suppressing NEK4 increased E-cadherin and Zo1 (epithelial markers), decreased Zeb1 and Smads (mesenchymal markers), and reduced metastasis in mice. This suggests NEK4 enhances EMT by downregulating epithelial markers and upregulating mesenchymal transcription factors, likely through the transforming growth factor-β (TGF-β)/Smad pathway (69).Quercetin is a flavonoid and has diverse biological effects. Actually it is well defied that quercetin can act on the chemo-sensitization and radio-sensitization in various cancer cells (70, 71).Quercetin inhibits protein kinases involved in deregulated cell growth in cancer cells, and NEK4 is a known target of quercetin (72).
Although research on NEK5’s mechanisms in cancer is limited, it can regulate the integrity of cell centrosomes, sequencing data has highlighted its potential as a new target in the cancer field. For instance, microarray analysis has shown that NEK5 expression is upregulated in highly metastatic non-small cell lung cancer (NSCLC) cells compared to low-metastatic cell lines (73).In 2017,RNA sequencing of prostate cancer tissue samples identified NEK5 as a novel RNA candidate biomarker for the disease (74).In osteosarcoma, information from the PHAROS database suggested that NEK5 could be a potential target (75).While these studies link NEK5 expression or signaling with various solid tumor subtypes, all of the data is based on bioinformatics analysis, without further experimental validation. The exact mechanism by which NEK5 drives tumorigenesis remains unclear. In breast cancer, clinical evidence has shown that high NEK5 expression is significantly associated with tumor progression and poor prognosis. Inhibition of NEK5 expression was found to effectively inhibit the proliferation, migration and invasion of tumor cells both in vitro and in vivo. Some mechanism studies suggest that NEK5 upregulates Cyclin A2 expression while downregulating Cyclin D1, Cyclin D3, and Cyclin E1 (76).Another study confirmed that NEK5 activation promotes EMT in breast cancer, thereby enhancing cell migration and invasion (77).In nasopharyngeal carcinoma,NEK5-mediated miRNA regulation has been shown to inhibit hypoxia-induced glycolysis, migration, and invasion. NEK5 is a direct target of miR-381-3p,and interfering with NEK5 can mitigate the pro-glycolytic and pro-metastatic effects of miR-381-3p downregulation (78).
The loss of NEK6 function can lead to mitotic abnormalities, metaphase cell accumulation, chromosome segregation errors, and subsequent apoptosis, involving in the progression of the mitotic cell cycle (79). Recent years, research has found NEK6 is linked to the development of many tumors, with significantly elevated expression levels in solid tumors (79). Bioinformatics analysis suggests that NEK6 is highly expressed in breast cancer and is associated with reduced survival rates in breast cancer patients (80). In breast cancer, both in vivo and in vitro studies have shown NEK6 overexpression promotes proliferation and migration in cancer cells (81). NEK6 expression in hepatocellular carcinoma (HCC) tissue is significantly higher than in normal liver tissue. High NEK6 expression is associated with tumor grading, staging, and poor prognosis (82, 83).NEK6 levels in liver cancer cell lines such as Huh7, Hep3B,and HepG2 are also elevated. Overexpression or inhibition of NEK6 can significantly impact the proliferation of liver cancer cells. Biao Zhang et al. pointed out that NEK6 regulates cyclin B transcription by activating CDC2, promoting the progression of the G2/M phase of the cell cycle. Thereby promoting tumor proliferation (84).In liver cancer,NEK6 inhibits TGF β - mediated transcriptional activity by interacting with Smad4, thereby affecting cell growth and differentiation. Specifically, overexpression of NEK6 inhibits TGF β - induced cell growth arrest and promotes hepatoma carcinoma cell proliferation. Research has found that NEK6 can promote hepatoma carcinoma cell proliferation by regulating the expression of cell cycle related proteins such as Cyclin B and CDC2. NEK6 is also associated with the invasion and metastasis ability of hepatoma carcinoma, and it may affect the migration and invasion ability of hepatoma carcinoma cells by regulating the reorganization of the cytoskeleton and related signaling pathways (85). Additionally, high NEK6 expression in gastric cancer and head and neck squamous cell carcinoma is positively correlated with distant metastasis, lymph node metastasis, TNM staging, as well as poor prognosis. Downregulation of NEK6 expression can inhibit cancer cell invasion and migration (86, 87).Kasap and colleagues reported (88) that NEK6 expression in adenomatous polyps and colorectal adenocarcinoma tissue is significantly higher than in normal colorectal mucosa. Another study found that NEK6 expression in ulcerative colitis and colorectal cancer tissue is significantly elevated compared to normal colorectal mucosa, with the top levels observed in colorectal cancer tissue (89). This suggests that NEK6 plays a role in the early stages of intestinal inflammation, especially in patients with extensive colonic involvement and long-term ulcerative colitis. NEK6 is also involved in prostate cancer, where its knockdown increases intracellular redox balance, enhances DNA damage response, induces apoptosis, and increases sensitivity to chemotherapy drugs. Further studies have revealed that NEK6 enhances androgen receptor signaling, maintaining cell growth in a castration-resistant environment, thus promoting castration resistance. NEK6 is likely a key target for castration-resistant prostate cancer. In ovarian cancer, NEK6 promotes chemotherapy resistance by affecting the interaction between FOXO3 and C-MYC (90).Another study found that NEK6,together with HIF-1α,co-regulates the cytoskeleton, affecting chemotherapy resistance in serous ovarian cancer (91).Quercetin can dose dependently reduce the expression of NEK6 in HeLa cells and has tumor suppressive effects. S206A is a potential NEK6 inhibitor. These two drugs can be used as cancer treatments.
Circular RNAs (circRNAs) and microRNAs (miRNAs) are closely related to the occurrence and progression of various types of cancer through their abnormal expression. Numerous studies have shown that NEK6 influences the development of osteosarcoma (92), colorectal cancer (93), kidney cancer (94), and retinoblastoma (95) by interacting with miR-26a-5p, miR-323a-3p, miR-141-3p, and miR-506-3p. Circ_NEK6 is typically transcribed from the exons of the NEK6 gene. Overexpression of NEK6 circRNA can promote the growth and invasion of thyroid cancer cells (96), while also increasing the resistance of differentiated thyroid cancer cells to iodine-131 (¹³¹I) therapy (97).
NEK7 is one of the smallest proteins in the mammalian NEK family. For the past few years, many research have reported which the upregulation of NEK7 is linked to the development of several cancers, including head and neck squamous cell carcinoma (98), gallbladder cancer (99), retinoblastoma (100), liver cancer (101, 102), pancreatic cancer (103), lung cancer (104), and esophageal cancer (62), indicating its potential as a biomarker. In gastric cancer, NEK7 is overexpressed and associated with poor prognosis. Cell experiments have shown that NEK7 promotes cell proliferation, while the lack of NEK7 leads to inhibited gastric cancer proliferation and G1/S phase arrest.NEK7 not only directly regulates cancer cell proliferation but may also promote the progression of gastric cancer by interacting with other cells, influencing immune infiltration and changes in immune cell subpopulations (105). In recent years, more and more studies have highlighted NEK7 as an important regulatory factor, playing a key role in the inflammatory response by promoting the assembly and activation of the NLRP3 inflammasome, leading to the release of inflammatory cytokines such as IL-1β and IL-18. The activation of NLRP3 inflammasome mediated by NEK7 is closely related to a variety of inflammatory diseases, including autoimmune, metabolic, and infectious diseases (106).Preliminary results from NEK7 inhibitors have shown promising anti-tumor and anti-inflammatory effects. Quercetin induces pyroptosis in colon cancer cells by activating the NEK7-mediated NLRP3 inflammasome-GSDMD signaling pathway, suggesting quercetin’s potential in colon cancer treatment and providing a foundation for future research (107).
Few studies have reported a connection between NEK8 and tumor development. Most NEK8-related research focuses on its association with cystic kidney disease. However, in recent years, there have been reports indicating that NEK8 plays a pro-cancer role in breast cancer (108), gastric cancer (109), liver cancer (110), glioma (111) and colon cancer (112, 113). The exact mechanism by which NEK8 promotes cancer is not fully understood. Some studies suggest that NEK8 may potentially exert its pro-cancer effect in human gastric cancer through the HIF-1 signaling pathway (109). Von Hippel-Lindau protein(pVHL) interacts with NEK8 and promotes its degradation. Since hypoxia stabilizes HIF-α by preventing its degradation by pVHL, it can be inferred that in a hypoxic tumor environment, HIF-α levels would increase. As NEK8 is regulated by HIF-α (114), this would likely lead to an increase in NEK8 expression. Additionally, the Kaplan - Meier survival analysis (109) that higher expression levels of NEK8 are associated with poor survival in gastric cancer patients. Considering that tumor hypoxia is often related to poor prognosis, it is reasonable to suspect that tumor hypoxia may contribute to increased NEK8 expression, which in turn may promote tumor progression. Knocking down NEK8 can inhibit cell proliferation (110), induce cell cycle arrest, suppress cell migration and invasion, and also reduce the characteristics of tumor stem cells. The WNT/β-catenin pathway plays a crucial role in various cellular processes, including cell growth, differentiation, tumorigenesis, chemoresistance, and cell cycle progression. β-catenin translocation into the nucleus triggers a range of transcription factors, regulating downstream signaling cascades. Eunji Kang and colleagues (108) reported that in breast cancer, NEK8 overexpression promotes β-catenin nuclear translocation, enhancing its activity within the nucleus. Additionally, NEK8 overexpression may induce the phosphorylation of proteins related to cancer progression. Furthermore, co-immunoprecipitation results showed that NEK8 directly interacts with β-catenin, suggesting that NEK8 is responsible for the phosphorylation of β-catenin at Ser552 and Ser675. This phosphorylation leads to the stabilization and nuclear accumulation of β-catenin.
The clinical research on NEK9 in breast cancer is controversial. Zhu and colleagues (115) found that NEK9 expression was elevated in tissue samples from breast cancer patients and was positively correlated with the occurrence and development of breast cancer, serving as an independent risk factor for poor prognosis. However, Xu and colleagues (116) reached the opposite conclusion: they discovered that NEK9 expression was downregulated in breast cancer and that low NEK9 expression was associated with larger, poorly differentiated tumors, lymph node metastasis, distant metastasis, and HER2-positive tumors. Patients with low NEK9 expression had significantly lower overall survival (OS) and disease-free survival (DFS) compared to those with high NEK9 expression. Gao and colleagues (80), on the other hand, found that compared to normal breast tissue, NEK9 was highly expressed in breast cancer tissues, and its high expression was positively correlated with disease-free survival in breast cancer patients. Currently, the role of NEK9 in breast cancer lacks experimental validation. NEK9 has a pro-cancer role in gastric cancer. In gastrointestinal tumors, NEK9 expression is elevated in metastatic gastric cancer lesions compared to non-cancerous tissue and primary cancer lesions, and its increased levels are associated with reduced overall survival in gastric cancer patients (117). Further research revealed that cancer-associated fibroblast-secreted SLIT2 could specifically activate NEK9, significantly promoting gastric cancer cell migration and invasion (117). Additionally, NEK9 acts as an effector of the IL-6/STAT3 signaling pathway. The IL-6/STAT3 pathway can upregulate NEK9 expression, thereby targeting the phosphorylation of ARHGEF2, promote the ability of gastric cancer cell migration and metastasis (118).Dabrafenib can inhibit NEK9, and in cancer cell lines with NRAS - and KRAS - mutations, dabrafenib has a significant inhibitory effect on cancer cell growth by inhibiting NEK9 and CDK16. This suggests that dabrafenib or its analogues may be developed as inhibitors against NEK9 for the treatment of related cancers (119).In colorectal cancer, analysis of clinical data shows that high expression of NEK9 and EG5 is associated with poor prognosis in patients with pathological stage t3 colon cancer. Intervening in the NEK9-EG5 axis could regulate the proliferation, migration, and invasion abilities of colorectal cancer cells (120).Pancreatic cancer is a highly malignant cancer type with a very poor prognosis, and patients have a very low 5-year survival rate. The prolactin receptor (PRLR) belongs to the cell membrane receptor family and primarily mediates the biological effects of prolactin. PRLR exists in two main isoforms: the long isoform (PRLR-LF) and the short isoform (PRLR-SF). PRLR-SF, as an significant metabolic suppressor in pancreatic cancer, can interact with NEK9. The loss of PRLR-SF can activate NEK9, thereby inhibiting the phosphorylation of YAP, a key tumor suppressor in the Hippo pathway. This inhibition leads to enhanced nuclear translocation and transcriptional activity of YAP, which promotes the proliferation and growth of pancreatic cancer cells. Inhibiting the NEK9-Hippo axis activities could be a crucial breakthrough in the treatment of pancreatic cancer.
There is little research on the function of NEK10, except for its involvement in cell cycle regulation. Wen-Liang Gao and colleagues (121) conducted bioinformatics analysis and found that NEK10 is expressed at higher levels in normal breast tissue compared to breast cancer tissue, and that Low expression of NEK10 can lead to poor prognosis in breast cancer patients, Indicating its potential tumor suppressive function. However, this study lacks experimental validation. In contrast, NEK10 acts as an oncogene in lung cancer. NEK10-mediated tyrosine phosphorylation enhances the stability of β-catenin, reducing its chances of ubiquitination and degradation. When NEK10 is overexpressed, lung cancer cells exhibit stronger proliferation and migration capabilities. NEK10, as a potential tumor-promoting factor, could serve as a new therapeutic target for lung adenocarcinoma (122).
NEK11 exhibits both oncogenic and tumor-suppressive functions across different cancer types. NEK11 is necessary for HCT116 cells to produce G2/M phase blockade against ionizing radiation (IR) or chemotherapy drug irinotecan. When NEK11 is depleted by RNAi mediation, cells are unable to enter the G2/M phase arrest normally after DNA damage, but continue to enter the cell cycle, which may cause cells to divide with damaged DNA, leading to genomic instability (123).A research team conducting whole-genome sequencing on melanoma families discovered multiple mutations in NEK11 among melanoma patients, which could lead to its dysfunction. Functional experiments and cell models revealed that cells with NEK11 mutations show increased proliferation and anti-apoptotic properties, suggesting NEK11’s potential role in melanoma development. The link between NEK11 and colorectal cancer has also been identified (124). Studies have shown that the loss of NEK11 in colorectal cancer cells prevents effective DNA repair after damage, leading to cell death. Targeting NEK11 may enhance the efficacy of DNA-damaging agents in treatment (123).
Research has also indicated high NEK11 expression in breast cancer, which is associated with better prognosis (80). Ovarian cancer, a highly lethal gynecological tumor, often develops resistance to chemotherapy, leading to treatment failure. In a 2014 study by Liu et al. (45), the relationship between NEK11 and ovarian cancer was validated. The study concluded that NEK11 expression is significantly lower in chemoresistant ovarian cancer tissues compared to chemosensitive tissues, and NEK11 downregulation is associated with poor prognosis and high recurrence rates. Overexpression of NEK11 can significantly enhance the sensitivity of ovarian cancer cells to chemotherapy, inducing apoptosis. Bioinformatics analysis revealed that NEK11 mRNA is significantly downregulated in ovarian cancer tissues and cisplatin resistant cells (125).NEK11 may serve as a novel biomarker for chemoresistance in ovarian cancer and as a potential therapeutic target, offering new strategies to overcome chemotherapy resistance in ovarian cancer.
The abnormal expression of NEK family genes is involved in the initiation, maintenance, progression, and metastasis of various cancers. These findings indicate that NEKs could be a potential therapeutic target for many cancer treatments. The structural diversity of the NEK family makes them promising drug targets in cancer research. Inhibitors designed to target specific domains, such as kinase domains or protein-binding domains, may offer high selectivity, effectively interfering with cancer cell proliferation and migration. More research is needed to develop targeted interventions for NEKs, which could become an important strategy for future cancer therapies.
HL: Writing – original draft. CG: Writing – review & editing. JL: Writing – review & editing. MJ: Writing – review & editing. YZ: Writing – review & editing. RC: Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This work was funded by National Natural Science Foundation of China Grants (NO. 82105044, 82303031). Shandong Provincial Natural Science Foundation Youth Project (ZR2021QH106)
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Han B, Zheng R, Zeng H, Wang S, Sun K, Chen R, et al. Cancer incidence and mortality in China, 2022. J Natl Cancer Cent. (2024) 4:47–53. doi: 10.1016/j.jncc.2024.01.006
2. Lapenna S, Giordano A. Cell cycle kinases as therapeutic targets for cancer. Nat Rev Drug Discovery. (2009) 8:547–66. doi: 10.1038/nrd2907
3. Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. (2002) 298:1912–34. doi: 10.1126/science.1075762
4. Meyer C, Bournez C, Carles F, Peyrat G, Aci-Sèche S, Bourg S, et al. Comparative assessment of protein kinase inhibitors in public databases and in PKIDB. Molecules. (2020) 25. doi: 10.3390/molecules25143226
5. Moniz L, Dutt P, Haider N, Stambolic V. Nek family of kinases in cell cycle, checkpoint control and cancer. Cell Div. (2011) 6:18. doi: 10.1186/1747-1028-6-18
6. Melo-Hanchuk TD, Martins MB, Cunha LL, Soares FA, Ward LS, Vassallo J, et al. Expression of the NEK family in normal and cancer tissue: an immunohistochemical study. BMC Cancer. (2020) 20:23. doi: 10.1186/s12885-019-6408-4
7. Panchal NK, Evan Prince S. The NEK family of serine/threonine kinases as a biomarker for cancer. Clin Exp Med. (2023) 23:17–30. doi: 10.1007/s10238-021-00782-0
8. Pavan ICB, Peres de Oliveira A, Dias PRF, Basei FL, Issayama LK, Ferezin C, et al. On broken ne(c)ks and broken DNA: the role of human NEKs in the DNA damage response. Cells. (2021) 10. doi: 10.3390/cells10030507
9. Belham C, Roig J, Caldwell JA, Aoyama Y, Kemp BE, Comb M, et al. A mitotic cascade of NIMA family kinases. Nercc1/Nek9 activates the Nek6 and Nek7 kinases. J Biol Chem. (2003) 278:34897–909. doi: 10.1074/jbc.M303663200
10. Rellos P, Ivins FJ, Baxter JE, Pike A, Nott TJ, Parkinson DM, et al. Structure and regulation of the human Nek2 centrosomal kinase. J Biol Chem. (2007) 282:6833–42. doi: 10.1074/jbc.M609721200
11. Wu W, Baxter JE, Wattam SL, Hayward DG, Fardilha M, Knebel A, et al. Alternative splicing controls nuclear translocation of the cell cycle-regulated Nek2 kinase. J Biol Chem. (2007) 282:26431–40. doi: 10.1074/jbc.M704969200
12. Kandli M, Feige E, Chen A, Kilfin G, Motro B. Isolation and characterization of two evolutionarily conserved murine kinases (Nek6 and nek7) related to the fungal mitotic regulator, NIMA. Genomics. (2000) 68:187–96. doi: 10.1006/geno.2000.6293
13. Bertran MT, Sdelci S, Regué L, Avruch J, Caelles C, Roig J. Nek9 is a Plk1-activated kinase that controls early centrosome separation through Nek6/7 and Eg5. EMBO J. (2011) 30:2634–47. doi: 10.1038/emboj.2011.179
14. Zalli D, Bayliss R, Fry AM. The Nek8 protein kinase, mutated in the human cystic kidney disease nephronophthisis, is both activated and degraded during ciliogenesis. Hum Mol Genet. (2012) 21:1155–71. doi: 10.1093/hmg/ddr544
15. De Benedetti A. The tousled-like kinases as guardians of genome integrity. ISRN Mol Biol. (2012) 2012:1–9. doi: 10.5402/2012/627596
16. Singh V, Aiswal PK, Ghosh I, Koul HK, Yu X, De Benedetti A. The TLK1-Nek1 axis promotes prostate cancer progression. Cancer Lett. (2019) 453:131–41. doi: 10.1016/j.canlet.2019.03.041
17. Singh V, Khalil MI, De Benedetti A. The TLK1/Nek1 axis contributes to mitochondrial integrity and apoptosis prevention via phosphorylation of VDAC1. Cell Cycle. (2020) 19:363–75. doi: 10.1080/15384101.2019.1711317
18. Ghosh I, Khalil MI, Mirza R, King J, Olatunde D, De Benedetti A. NEK1-mediated phosphorylation of YAP1 is key to prostate cancer progression. Biomedicines. (2023) 11. doi: 10.3390/biomedicines11030734
19. Singh V, Aiswal PK, Ghosh I, Koul HK, Yu X, De Benedetti A. Targeting the TLK1/NEK1 DDR axis with Thioridazine suppresses outgrowth of androgen independent prostate tumors. Int J Cancer. (2019) 145:1055–67. doi: 10.1002/ijc.v145.4
20. Shen Y, He W, Wang D, Cao D, Mei H, Luan T, et al. Identification of gene coexpression modules and prognostic genes associated with papillary thyroid cancer. J Oncol 2022. (2022) p:9025198. doi: 10.1155/2022/9025198
21. Zhu J, Cai Y, Liu P, Zhao W. Frequent Nek1 overexpression in human gliomas. Biochem Biophys Res Commun. (2016) 476:522–7. doi: 10.1016/j.bbrc.2016.05.156
22. Chen Y, Chen CF, Polci R, Wei R, Riley DJ, Chen PL. Increased Nek1 expression in renal cell carcinoma cells is associated with decreased sensitivity to DNA-damaging treatment. Oncotarget. (2014) 5:4283–94. doi: 10.18632/oncotarget.v5i12
23. Chen G, Zhou J, Chen J, Zhu J, Liu SC, Ding XF, et al. VHL regulates NEK1 via both HIF-2α pathway and ubiquitin-proteasome pathway in renal cancer cell. Biochem Biophys Res Commun. (2019) 509:797–802. doi: 10.1016/j.bbrc.2019.01.001
24. Li Y, Chen L, Feng L, Zhu M, Shen Q, Fang Y, et al. NEK2 promotes proliferation, migration and tumor growth of gastric cancer cells via regulating KDM5B/H3K4me3. Am J Cancer Res. (2019) 9:2364–78.
25. Fry AM, Mayor T, Meraldi P, Stierhof YD, Tanaka KN, Nigg EA, et al. C-Nap1, a novel centrosomal coiled-coil protein and candidate substrate of the cell cycle-regulated protein kinase Nek2. J Cell Biol. (1998) 141:1563–74. doi: 10.1083/jcb.141.7.1563
26. Xia J, Franqui Machin R, Gu Z, Zhan F. Role of NEK2A in human cancer and its therapeutic potentials. BioMed Res Int 2015. (2015) p:862461. doi: 10.1155/2015/862461
27. Zhang FB, Du Y, Tian Y, Ji ZG, Yang PQ. MiR-1299 functions as a tumor suppressor to inhibit the proliferation and metastasis of prostate cancer by targeting NEK2. Eur Rev Med Pharmacol Sci. (2019) 23:530–8. doi: 10.26355/eurrev_201901_16865
28. Feng X, Jiang Y, Cui Y, Xu Y, Zhang Q, Xia Q, et al. NEK2 is associated with poor prognosis of clear cell renal cell carcinoma and promotes tumor cell growth and metastasis. Gene. (2023) 851:147040. doi: 10.1016/j.gene.2022.147040
29. Zhou J, Lai J, Cheng Y, Qu W. NEK2 serves as a novel biomarker and enhances the tumorigenicity of clear-cellRenal-cell carcinoma by activating WNT/beta-catenin pathway. Evid Based Complement Alternat Med. (2022) 2022:1890823. doi: 10.1155/2022/1890823
30. Bai R, Yuan C, Sun W, Zhang J, Luo Y, Gao Y, et al. NEK2 plays an active role in Tumorigenesis and Tumor Microenvironment in Non-Small Cell Lung Cancer. Int J Biol Sci. (2021) 17:1995–2008. doi: 10.7150/ijbs.59019
31. Zhao D, Han W, Liu X, Cui D, Chen . MicroRNA-128 promotes apoptosis in lung cancer by directly targeting NIMA-related kinase 2. Thorac Cancer. (2017) 8:304–11. doi: 10.1111/tca.2017.8.issue-4
32. Chen Y, Wu N, Liu L, Dong H, Liu X. microRNA-128-3p overexpression inhibits breast cancer stem cell characteristics through suppression of Wnt signalling pathway by down-regulating NEK2. J Cell Mol Med. (2020) 24:7353–69. doi: 10.1111/jcmm.v24.13
33. Xing Z, Zhang M, Wang X, Liu J, Liu G, Feng K, et al. Silencing of Nek2 suppresses the proliferation, migration and invasion and induces apoptosis of breast cancer cells by regulating ERK/MAPK signaling. J Mol Histol. (2021) 52:809–21. doi: 10.1007/s10735-021-09979-9
34. Wan H, Xu L, Zhang H, Wu F, Zeng W, Li T. High expression of NEK2 promotes gastric cancer progression via activating AKT signaling. J Physiol Biochem. (2021) 77:25–34. doi: 10.1007/s13105-020-00776-8
35. Wu J, Luo D, Tou L, Xu H, Jiang C, Wu D, et al. NEK2 affects the ferroptosis sensitivity of gastric cancer cells by regulating the expression of HMOX1 through Keap1/Nrf2. Mol Cell Biochem. (2025) 480(1):425–37. doi: 10.1007/s11010-024-04960-y
36. Gu S, Yasen Y, Wang M, Huang B, Zhou Y, Wang W. NEK2 promotes the migration, invasion, proliferation of ESCC and mediates ESCC immunotherapy. Heliyon. (2024) 10:e29682. doi: 10.1016/j.heliyon.2024.e29682
37. Guo D, Zhou S, Liu R, Yao W, Li S, Zhang X, et al. NEK2 contributes to radioresistance in esophageal squamous cell carcinoma by inducing protective autophagy via regulating TRIM21. Cancer Cell Int. (2024) 24:179. doi: 10.1186/s12935-024-03367-5
38. Lu L, Zhai X, Yuan R. Clinical significance and prognostic value of Nek2 protein expression in colon cancer. Int J Clin Exp Pathol. (2015) 8:15467–73.
39. Ren Q, Li B, Liu M, Hu Z, Wang Y. Prognostic value of NEK2 overexpression in digestive system cancers: a meta-analysis and systematic review. Onco Targets Ther. (2018) 11:7169–78. doi: 10.2147/OTT.S169911
40. Takahashi Y, Iwaya T, Sawada G, Kurashige J, Matsumura T, Uchi R, et al. Up-regulation of NEK2 by microRNA-128 methylation is associated with poor prognosis in colorectal cancer. Ann Surg Oncol. (2014) 21:205–12. doi: 10.1245/s10434-013-3264-3
41. Lin S, Zhou S, Jiang S, Liu X, Wang Y, Zheng X, et al. NEK2 regulates stem-like properties and predicts poor prognosis in hepatocellular carcinoma. Oncol Rep. (2016) 36:853–62. doi: 10.3892/or.2016.4896
42. Zhang MX, Xu X, Zhang M, Han P, Deng N, Yu J, T T, et al. Effect of silencing NEK2 on biological behaviors of HepG2 in human hepatoma cells and MAPK signal pathway. Tumour Biol. (2016) 37:2023–35. doi: 10.1007/s13277-015-3993-y
43. Zhang X, Huang X, Xu J, Li E, Lao M, Tang T, et al. NEK2 inhibition triggers anti-pancreatic cancer immunity by targeting PD-L1. Nat Commun. (2021) 12:4536. doi: 10.1038/s41467-021-24769-3
44. Kokuryo T, Senga T, Yokoyama Y, Nagino M, Nimura Y, Hamaguchi M. Nek2 as an effective target for inhibition of tumorigenic growth and peritoneal dissemination of cholangiocarcinoma. Cancer Res. (2007) 67:9637–42. doi: 10.1158/0008-5472.CAN-07-1489
45. Liu X, Gao Y, Lu Y, Zhang J, Li L, Yin F. Upregulation of NEK2 is associated with drug resistance in ovarian cancer. Oncol Rep. (2014) 31:745–54. doi: 10.3892/or.2013.2910
46. Liu XL, Liu H, Han N, Li F, Sun F, Fan D. PCAT1 promotes the proliferative and migratory potentials of ovarian cancer via targeting NEK2. Eur Rev Med Pharmacol Sci. (2019) 23:8239–48. doi: 10.26355/eurrev_201910_19133
47. Xu T, Zeng Y, Shi L, Yang Q, Chen Y, Wu G, et al. Targeting NEK2 impairs oncogenesis and radioresistance via inhibiting the Wnt1/beta-catenin signaling pathway in cervical cancer. J Exp Clin Cancer Res. (2020) 39:183. doi: 10.1186/s13046-020-01659-y
48. Ye TT, Chen W, Pei J, Jia Y, Wu H. Expression and clinical significance of NEK2 and EMT related molecules in oral squamous cell carcinoma. Shanghai Kou Qiang Yi Xue. (2023) 32:640–4. doi: 10.19439/j.sjos.2023.06.014
49. Stricker TP, Henriksen K, Tonsgard J, Montag A, Krausz T, Pytel P. Expression profiling of 519 kinase genes in matched Malignant peripheral nerve sheath tumor/plexiform neurofibroma samples is discriminatory and identifies mitotic regulators BUB1B, PBK and NEK2 as overexpressed with transformation. Mod Pathol. (2013) 26:930–43. doi: 10.1038/modpathol.2012.242
50. Wang S, Li W, Liu N, Zhang F, Liu H, Liu F, et al. Nek2A contributes to tumorigenic growth and possibly functions as potential therapeutic target for human breast cancer. J Cell Biochem. (2012) 113:1904–14. doi: 10.1002/jcb.v113.6
51. Zhong X, Guan X, Dong Q, Yang S, Liu W, Zhang L. Examining Nek2 as a better proliferation marker in non-small cell lung cancer prognosis. Tumour Biol. (2014) 35:7155–62. doi: 10.1007/s13277-014-1935-8
52. Ko MJ, Seo Y, Seo D, Park S, Seo J, Jeon E, et al. RPL17 promotes colorectal cancer proliferation and stemness through ERK and NEK2/beta-catenin signaling pathways. J Cancer. (2022) 13:2570–83. doi: 10.7150/jca.69428
53. Mbom BC, Siemers K, Ostrowski M, Nelson W, Barth A. Nek2 phosphorylates and stabilizes beta-catenin at mitotic centrosomes downstream of Plk1. Mol Biol Cell. (2014) 25:977–91. doi: 10.1091/mbc.e13-06-0349
54. Neal CP, Fry A, Moreman C, McGregor A, Garcea G, Berry D, et al. Overexpression of the Nek2 kinase in colorectal cancer correlates with beta-catenin relocalization and shortened cancer-specific survival. J Surg Oncol. (2014) 110:828–38. doi: 10.1002/jso.23717
55. Hayward DG, Clarke R, Faragher A, Pillai M, Hagan I, Fry A, et al. The centrosomal kinase Nek2 displays elevated levels of protein expression in human breast cancer. Cancer Res. (2004) 64:7370–6. doi: 10.1158/0008-5472.CAN-04-0960
56. Kokuryo T, Yokoyama Y, Yamaguchi J, Tsunoda N, Ebata T, Nagino M. NEK2 is an effective target for cancer therapy with potential to induce regression of multiple human Malignancies. Anticancer Res. (2019) 39:2251–8. doi: 10.21873/anticanres.13341
57. Kokuryo T, Hibino S, Suzuki K, Watanabe K, Yokoyama Y, Nagino M, et al. Nek2 siRNA therapy using a portal venous port-catheter system for liver metastasis in pancreatic cancer. Cancer Sci. (2016) 107:1315–20. doi: 10.1111/cas.2016.107.issue-9
58. Zhang Y, Wang W, Wang Y, Huang X, Zhang Z, Chen B, et al. NEK2 promotes hepatocellular carcinoma migration and invasion through modulation of the epithelial-mesenchymal transition. Oncol Rep. (2018) 39:1023–33. doi: 10.3892/or.2018.6224
59. Lee J, Gollahon L. Nek2-targeted ASO or siRNA pretreatment enhances anticancer drug sensitivity in triple−negative breast cancer cells. Int J Oncol. (2013) 42:839–47. doi: 10.3892/ijo.2013.1788
60. Marina M, Saavedra HI. Nek2 and Plk4: prognostic markers, drivers of breast tumorigenesis and drug resistance. Front Biosci (Landmark Ed). (2014) 19:352–65. doi: 10.2741/4212
61. Wen S, Liu Y, Yang M, Yang K, Huang J, Feng D. Increased NEK2 in hepatocellular carcinoma promotes cancer progression and drug resistance by promoting PP1/Akt and Wnt activation. Oncol Rep. (2016) 36:2193–9. doi: 10.3892/or.2016.5009
62. Chen L, Ballout F, Lu H, Hu T, Zhu S, Chen Z, et al. Differential expression of NEK kinase family members in esophageal adenocarcinoma and barrett’s esophagus. Cancers (Basel). (2023) 15. doi: 10.3390/cancers15194821
63. Cao Y, Song J, Chen J, Xiao J, Ni J, Wu C. Overexpression of NEK3 is associated with poor prognosis in patients with gastric cancer. Med (Baltimore). (2018) 97:e9630. doi: 10.1097/MD.0000000000009630
64. Harrington KM, Clevenger CV. Identification of NEK3 kinase threonine 165 as a novel regulatory phosphorylation site that modulates focal adhesion remodeling necessary for breast cancer cell migration. J Biol Chem. (2016) 291:21388–406. doi: 10.1074/jbc.M116.726190
65. Miller SL, Antico G, Raghunath P, Tomaszewski J, Clevenger C, et al. Nek3 kinase regulates prolactin-mediated cytoskeletal reorganization and motility of breast cancer cells. Oncogene. (2007) 26:4668–78. doi: 10.1038/sj.onc.1210264
66. Ana Filipa Brito MR, Abrantes AM, Pires AS, Teixo RJ, Tralhão JG, Botelho MF. Quercetin in cancer treatment, alone or in combination with conventional therapeutics. Bentham Sci. (2015) 22:685–94. doi: 10.2174/0929867322666150812145435
67. Refaat A, Abd-Rabou A, Reda A. TRAIL combinations: The new ‘trail’ for cancer therapy (Review). Oncol Lett. (2014) 7:1327–32. doi: 10.3892/ol.2014.1922
68. Park SJ, Jo DS, Jo SY, Shin DW, Shim S, Jo YK, et al. Inhibition of never in mitosis A (NIMA)-related kinase-4 reduces survivin expression and sensitizes cancer cells to TRAIL-induced cell death. Oncotarget. (2016) 7:65957–67. doi: 10.18632/oncotarget.11781
69. Ding NH, Zhang L, Xiao Z, Rong Z, Li Z, He J, et al. NEK4 kinase regulates EMT to promote lung cancer metastasis. J Cell Mol Med. (2018) 22:5877–87. doi: 10.1111/jcmm.2018.22.issue-12
70. Russo GL, Russo M, Spagnuolo C, Tedesco I, Bilotto S, Iannitti R, et al. Quercetin: a pleiotropic kinase inhibitor against cancer. Cancer Treat Res. (2014) 159:185–205. doi: 10.1007/978-3-642-38007-5_11
71. Brito AF, Ribeiro M, Abrantes A, Pires A, Teixo R, Tralhão J, et al. Quercetin in cancer treatment, alone or in combination with conventional therapeutics? Curr Med Chem. (2015) 22:3025–39. doi: 10.2174/0929867322666150812145435
72. Siegelin MD, Reuss D, Habel A, Rami A, Von deimling A. Quercetin promotes degradation of survivin and thereby enhances death-receptor–mediated apoptosis in glioma cells. Neuro-Oncology. (2009) 11:122–31. doi: 10.1215/15228517-2008-085
73. Sun R, Meng X, Wang W, Liu B, Lv X, Yuan J, et al. Five genes may predict metastasis in non-small cell lung cancer using bioinformatics analysis. Oncol Lett. (2019) 18:1723–32. doi: 10.3892/ol.2019.10498
74. Nikitina AS, Sharova EI, Danilenko SA, Butusova TB, Vasiliev AO, Govorov AV, et al. Novel RNA biomarkers of prostate cancer revealed by RNA-seq analysis of formalin-fixed samples obtained from Russian patients. Oncotarget. (2017) 8:32990–3001. doi: 10.18632/oncotarget.16518
75. Zhi LQ, Yang YX, Yao SX, Qing Z, Ma JB. Identification of novel target for osteosarcoma by network analysis. Med Sci Monit. (2018) 24:5914–24. doi: 10.12659/MSM.909973
76. Panchal NK, Evan Prince S. The NEK family of serine/threonine kinases as a biomarker for cancer. Clin Exp Med. (2022). doi: 10.1007/s10238-021-00782-0
77. Matossian MD, Elliott S, Van hoang T, Burks HE, Wright MK, Alzoubi MS, et al. NEK5 activity regulates the mesenchymal and migratory phenotype in breast cancer cells. Breast Cancer Res Treat. (2021) 189:49–61. doi: 10.1007/s10549-021-06295-4
78. Zhao CH, Bai XF, Hu XH. Knockdown of lncRNA XIST inhibits hypoxia-induced glycolysis, migration and invasion through regulating miR-381-3p/NEK5 axis in nasopharyngeal carcinoma. Eur Rev Med Pharmacol Sci. (2020) 24:2505–17. doi: 10.26355/eurrev_202003_20518
79. Panchal NK, Mohanty S, Prince SE. NIMA-related kinase-6 (NEK6) as an executable target in cancer. Clin Trans Oncol. (2022) 25:66–77. doi: 10.1007/s12094-022-02926-4
80. Gao WL, Niu L, Chen WL, Zhang YQ, Huang WH. Integrative analysis of the expression levels and prognostic values for NEK family members in breast cancer. Front Genet. (2022) 13:798170. doi: 10.3389/fgene.2022.798170
81. He Z, Ni X, Xia L, Shao Z. Overexpression of NIMA-related kinase 6 (NEK6) contributes to Malignant growth and dismal prognosis in Human Breast Cancer. Pathol Res Pract. (2018) 214:1648–54. doi: 10.1016/j.prp.2018.07.030
82. Cao X, Xia Y, Yang J, Jiang J, Chen L, Ni R, et al. Clinical and biological significance of never in mitosis gene A-related kinase 6 (NEK6) expression in hepatic cell cancer. Pathol Oncol Res. (2012) 18:201–7. doi: 10.1007/s12253-011-9429-0
83. Zhang H, Li B. NIMA-related kinase 6 as an effective target inhibits the hepatocarcinogenesis and progression of hepatocellular carcinoma. Heliyon. (2023) 9:e15971. doi: 10.1016/j.heliyon.2023.e15971
84. Zhang B, Zhang H, Wang D, Han S, Wang K, Yao A, et al. Never in mitosis gene A-related kinase 6 promotes cell proliferation of hepatocellular carcinoma via cyclin B modulation. Oncol Lett. (2014) 8:1163–8. doi: 10.3892/ol.2014.2300
85. Zuo J, Ma H, Cai H, Wu Y, Jiang W, Yu L. An inhibitory role of NEK6 in TGFβ/Smad signaling pathway. BMB Rep. (2015) 48:473–8. doi: 10.5483/BMBRep.2015.48.8.225
86. Xu J, He Q, He X, Shao Q, Tao H, Ye Z. Expression of NEK-6 in gastric cancer and its clinical significance. Zhonghua Wei Chang Wai Ke Za Zhi. (2015) 18:1036–40.
87. Yang ZM, Liao B, Yang SS, Su T, Zhang J, Wang WM. Predictive role of NEK6 in prognosis and immune infiltration in head and neck squamous cell carcinoma. Front Endocrinol (Lausanne). (2022) 13:943686. doi: 10.3389/fendo.2022.943686
88. Kasap E, Gerceker E, Boyacıoglu SÖ, Yuceyar H, Yıldırm H, Ayhan S, et al. The potential role of the NEK6, AURKA, AURKB, and PAK1 genes in adenomatous colorectal polyps and colorectal adenocarcinoma. Tumour Biol. (2016) 37:3071–80. doi: 10.1007/s13277-015-4131-6
89. Gerceker E, Boyacıoglu SO, Kasap E, Baykan A, Yuceyar H, Yıldırım H, et al. Never in mitosis gene A-related kinase 6 and aurora kinase A: New gene biomarkers in the conversion from ulcerative colitis to colorectal cancer. Oncol Rep. (2015) 34:1905–14. doi: 10.3892/or.2015.4187
90. Liu J, Wang H, Wan H, Yang J, Gao L, Wang Z, et al. NEK6 dampens FOXO3 nuclear translocation to stabilize C-MYC and promotes subsequent de novo purine synthesis to support ovarian cancer chemoresistance. Cell Death Dis. (2024) 15:661. doi: 10.1038/s41419-024-07045-2
91. Donato MD, Fanelli M, Mariani M, Raspaglio G, Pandya D, He S, et al. Nek6 and Hif-1alpha cooperate with the cytoskeletal gateway of drug resistance to drive outcome in serous ovarian cancer. Am J Cancer Res. (2015) 5:1862–77.
92. Zhu M, Sun Y, Xue H, Wu G, Wang Z, Shi J, et al. NEK6 promotes the progression of osteosarcoma through activating STAT3 signaling pathway by down-regulation of miR-26a-5p. Int J Gen Med. (2023) 16:2831–48. doi: 10.2147/IJGM.S413461
93. Hong Z, Chen Z, Pan J, Shi Z, Wang C, Qiu C. MicroRNA-323a-3p negatively regulates NEK6 in colon adenocarcinoma cells. J Oncol 2022. (2022) p:7007718. doi: 10.1155/2022/7007718
94. Liu Y, Fu W, Yin F, Xia L, Zhang Y, Wang B, et al. miR-141-3p suppresses development of clear cell renal cell carcinoma by regulating NEK6. Anticancer Drugs. (2022) 33:e125–33. doi: 10.1097/CAD.0000000000001158
95. Retraction: MiR-506-3p inhibits cell proliferation, induces cell cycle arrest and apoptosis in retinoblastoma by directly targeting NEK6 by Lina Wu, Zhen Chen, Yiqiao Xing. Cell Biol Int. (2019) 43:1524. doi: 10.1002/cbin.v43.12
96. Chen F, Feng Z, Zhu J, Liu P, Yang C, Huang R. Emerging roles of circRNA_NEK6 targeting miR-370-3p in the proliferation and invasion of thyroid cancer via Wnt signaling pathway. Cancer Biol Ther. (2018) 19:1139–52. doi: 10.1080/15384047.2018.1480888
97. Chen F, Yin S, Feng Z, Liu C, Lv J, Chen Y, et al. Knockdown of circ_NEK6 Decreased (131)I Resistance of Differentiated Thyroid Carcinoma via Regulating miR-370-3p/MYH9 Axis. Technol Cancer Res Treat. (2021) 20:15330338211004950. doi: 10.1177/15330338211004950
98. Saloura V, Cho H, Kiyotani K, Alachkar H, Zuo Z, Nakakido M, et al. WHSC1 promotes oncogenesis through regulation of NIMA-related kinase-7 in squamous cell carcinoma of the head and neck. Mol Cancer Res. (2015) 13:293–304. doi: 10.1158/1541-7786.MCR-14-0292-T
99. Wang R, Song Y, Xu X, Wu Q, Liu C. The expression of Nek7, FoxM1, and Plk1 in gallbladder cancer and their relationships to clinicopathologic features and survival. Clin Transl Oncol. (2013) 15:626–32. doi: 10.1007/s12094-012-0978-9
100. Zhang J, Wang L, Zhang Y. Downregulation of NIMA-related kinase-7 inhibits cell proliferation by inducing cell cycle arrest in human retinoblastoma cells. Exp Ther Med. (2018) 15:1360–6. doi: 10.3892/etm.2017.5558
101. Yan Z, Da Q, Li Z, Lin Q, Yi J, Su Y, et al. Inhibition of NEK7 suppressed hepatocellular carcinoma progression by mediating cancer cell pyroptosis. Front Oncol. (2022) 12:812655. doi: 10.3389/fonc.2022.812655
102. Zhou L, Wang Z, Xu X, Wan Y, Qu K, Fan H, et al. Nek7 is overexpressed in hepatocellular carcinoma and promotes hepatocellular carcinoma cell proliferation in vitro and in vivo. Oncotarget. (2016) 7:18620–30. doi: 10.18632/oncotarget.v7i14
103. Yan Z, Qu J, Li Z, Yi J, Su Y, Lin Q, et al. NEK7 promotes pancreatic cancer progression and its expression is correlated with poor prognosis. Front Oncol. (2021) 11:705797. doi: 10.3389/fonc.2021.705797
104. Yan L, Bai M, Xu J, Li X, Wu C, Zhou Y, et al. Retracted Article: CircRNA PVT1 modulates cell metastasis via the miR-181a-5p/NEK7 axis and cisplatin chemoresistance through miR-181a-5p-mediated autophagy in non-small cell lung cancer. RSC Adv. (2019) 9:42324–34. doi: 10.1039/C9RA08872E
105. Li YK, Zhu X, Zhan Y, Yuan W, Jin WL, et al. NEK7 promotes gastric cancer progression as a cell proliferation regulator. Cancer Cell Int. (2021) 21:438. doi: 10.1186/s12935-021-02148-8
106. Liu G, Chen X, Wang Q, Yuan L. NEK7: a potential therapy target for NLRP3-related diseases. Biosci Trends. (2020) 14:74–82. doi: 10.5582/bst.2020.01029
107. Feng SH, Zhao B, Zhan X, Li R, Yang Q, Wang SM, et al. Quercetin-induced pyroptosis in colon cancer through NEK7-mediated NLRP3 inflammasome-GSDMD signaling pathway activation. Am J Cancer Res. (2024) 14:934–58. doi: 10.62347/MKAN3550
108. Kang E, Kim H, Lee H, Han W. Never in mitosis gene A-related kinase-8 promotes proliferation, migration, invasion, and stemness of breast cancer cells via beta-catenin signalling activation. Sci Rep. (2023) 13:6829. doi: 10.1038/s41598-023-32631-3
109. Ding XF, Chen J, Zhou J, Chen G, Wu YL. Never-in-mitosis A-related kinase 8, a novel target of von-Hippel-Lindau tumor suppressor protein, promotes gastric cancer cell proliferation. Oncol Lett. (2018) 16:5900–6. doi: 10.3892/ol.2018.9328
110. Chan WL, Yuo CY, Yang WK, Hung SY, Chang YS, Chiu CC, et al. Transcribed pseudogene psiPPM1K generates endogenous siRNA to suppress oncogenic cell growth in hepatocellular carcinoma. Nucleic Acids Res. (2013) 41:3734–47. doi: 10.1093/nar/gkt047
111. Xiao M, Du C, Zhang C, Zhang X, Li S, Zhang D, et al. Bioinformatics analysis of the prognostic value of NEK8 and its effects on immune cell infiltration in glioma. J Cell Mol Med. (2021) 25:8748–63. doi: 10.1111/jcmm.v25.18
112. Cao B, Zhang K, Pan C, Dong Y, Lu FN. NEK8 regulates colorectal cancer progression via phosphorylating MYC. Cell Communication Signaling. (2023) 21. doi: 10.1186/s12964-023-01215-z
113. Sun R, Yang Y, Lü W, Yang Y, Li Y, Liu Z, et al. Single-cell transcriptomic analysis of normal and pathological tissues from the same patient uncovers colon cancer progression. Cell Bioscience. (2023) 13. doi: 10.1186/s13578-023-01002-w
114. Ding X-F, Zhou J, Hu Q, Liu S, Chen G. The Tumor Suppressor pVHL Down-regulates Never-in-Mitosis A-related Kinase 8 via Hypoxia-inducible Factors to Maintain Cilia in Human Renal Cancer Cells. J Biol Chem. (2015) 290:1389–94. doi: 10.1074/jbc.M114.589226
115. Zhaoyu Z, Huanyu X, Yajie Z, Ziyu D, Hui L, Feng S. Study on the expression of lncRNA ATB and nek9 in breast cancer patients based on Q-PCR technology and its relationship with the disease. Contrast Media Mol Imaging. (2022) 2022. doi: 10.1155/2022/2634080
116. Xu Z, Shen W, Pan A, Sun F, Zhang J, Gao P, et al. Decreased Nek9 expression correlates with aggressive behaviour and predicts unfavourable prognosis in breast cancer. Pathology. (2020) 52:329–35. doi: 10.1016/j.pathol.2019.11.008
117. Lu G, Du R, Dong J, Sun Y, Zhou F, Feng F, et al. Cancer associated fibroblast derived SLIT2 drives gastric cancer cell metastasis by activating NEK9. Cell Death Dis. (2023) 14. doi: 10.1038/s41419-023-05965-z
118. Lu G, Tian S, Sun Y, Dong J, Wang N, Zeng J, et al. NEK9, a novel effector of IL-6/STAT3, regulates metastasis of gastric cancer by targeting ARHGEF2 phosphorylation. Theranostics. (2021) 11:2460–74. doi: 10.7150/thno.53169
119. Phadke M, Remsing Rix LL, Smalley I, Bryant AT, Luo Y, Lawrence HR, et al. Dabrafenib inhibits the growth of BRAF-WT cancers through CDK16 and NEK9 inhibition. Mol Oncol. (2017) 12:74–88.
120. Kim M, Jeong H, Ju H, Song J, Jang S, Choi J. Overexpression of the NEK9–EG5 axis is a novel metastatic marker in pathologic stage T3 colon cancer. Sci Rep. (2023) 13. doi: 10.1038/s41598-022-26249-0
121. Gao WL, Niu L, Chen WL, Zhang YQ, Huang WI. Integrative analysis of the expression levels and prognostic values for NEK family members in breast cancer. Front. Genet. (2022) 13:798170.
122. Dutt P, Haider N, Mouaaz S, Podmore L, Stambolic V. beta-catenin turnover is regulated by Nek10-mediated tyrosine phosphorylation in A549 lung adenocarcinoma cells. Proc Natl Acad Sci U.S.A. (2024) 121:e2300606121. doi: 10.1073/pnas.2300606121
123. Sabir S, Sahota N, Jones G, Fry A. Loss of nek11 prevents G2/M arrest and promotes cell death in HCT116 colorectal cancer cells exposed to therapeutic DNA damaging agents. PloS One. (2015) 10. doi: 10.1371/journal.pone.0140975
124. Christodoulou E, van Doorn R, Visser M, Teunisse A, Versluis M, van der Velden P, et al. NEK11 as a candidate high-penetrance melanoma susceptibility gene. J Med Genet. (2020) 57:203–10. doi: 10.1136/jmedgenet-2019-106134
125. Liu X, Gao Y, Lu Y, Zhang J, Li L, Yin F. Downregulation of NEK11 is associated with drug resistance in ovarian cancer. Int J Oncol. (2014) 45:1266–74. doi: 10.3892/ijo.2014.2503
126. Zhou J, Lai J, Cheng Y, Qu W. NEK2 serves as a novel biomarker and enhances the tumorigenicity of clear-cellRenal-cell carcinoma by activating WNT/β-catenin pathway. Evid Based Complement Alternat Med. (2022) 2022:1890823. doi: 10.1155/2022/1890823
127. Bai R, Yuan C, Sun W, Zhang J, Luo Y, Gao Y, et al. NEK2 plays an active role in Tumorigenesis and Tumor Microenvironment in Non-Small Cell Lung Cancer: Retraction. Int J Biol Sci. (2022) 18:3943. doi: 10.7150/ijbs.75303
128. Pei J, Zhang J, Yang X, Wu Z, Sun C, Wang Z, et al. NEK5 promotes breast cancer cell proliferation through up-regulation of Cyclin A2. Mol Carcinogenesis. (2019) 58:933–43. doi: 10.1002/mc.22982
129. Donato MD, Fanelli M, Mariani M, Raspaglio G, Pandya D, He S, et al. Nek6 and Hif-1α cooperate with the cytoskeletal gateway of drug resistance to drive outcome in serous ovarian cancer. Am J Cancer Res. (2015) 5:1862–77.
130. Bowers AJ, Boylan JF. Nek8, a NIMA family kinase member, is overexpressed in primary human breast tumors. Gene. (2004) 328:135–42. doi: 10.1016/j.gene.2003.12.002
131. Kang E, Kim H, Lee H, Han W. Never in mitosis gene A-related kinase-8 promotes proliferation, migration, invasion, and stemness of breast cancer cells via β-catenin signalling activation. Sci Rep. (2023) 13:6829. doi: 10.1038/s41598-023-32631-3
132. Wang L, He C, Hu B, Li P. Expression and pathological significance of nek8 in colorectal carcinoma tissues. J Guizhou Med Univ. (2017) 42(3):352–5. doi: 10.19367/j.cnki.1000-2707.2017.03.024
133. Nie H, Huang P, Jiang S, Yang Q, Hu L, Yang X, et al. The short isoform of PRLR suppresses the pentose phosphate pathway and nucleotide synthesis through the NEK9-Hippo axis in pancreatic cancer. Theranostics. (2021) 11:3898–915. doi: 10.7150/thno.51712
134. Khalil MI, Ghosh I, Singh V, Chen J, Zhu H, De Benedetti A, et al. NEK1 phosphorylation of YAP promotes its stabilization and transcriptional output. Cancers. (2020) 12. doi: 10.3390/cancers12123666
135. Mundt F, Rajput S, Li S, Ruggles K, Mooradian A, Mertins P, et al. Mass spectrometry–based proteomics reveals potential roles of NEK9 and MAP2K4 in resistance to PI3K inhibition in triple-negative breast cancers. Cancer Res. (2018) 78:2732–46. doi: 10.1158/0008-5472.CAN-17-1990
136. Reinhardt LS, Morás A, Henn J, Arantes P, Ferro M, Braganhol E, et al. Nek1-inhibitor and temozolomide-loaded microfibers as a co-therapy strategy for glioblastoma treatment. Int J Pharmaceutics. (2022) 617. doi: 10.1016/j.ijpharm.2022.121584
137. Henise JC, Taunton J. Irreversible nek2 kinase inhibitors with cellular activity. J Medicinal Chem. (2011) 54:4133–46. doi: 10.1021/jm200222m
Keywords: NEK family, mitosis, therapeutic target, cancer, proliferation, metastasis
Citation: Li H, Li J, Zhang Y, Cao R, Guo C and Jiao M (2025) The NIMA-related kinase family and cancer. Front. Oncol. 15:1556917. doi: 10.3389/fonc.2025.1556917
Received: 07 January 2025; Accepted: 11 March 2025;
Published: 27 March 2025.
Edited by:
Prem S Subramaniam, Columbia University, United StatesReviewed by:
Keith R. Laderoute, Laderoute Consulting, LLC, United StatesCopyright © 2025 Li, Li, Zhang, Cao, Guo and Jiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Congcong Guo, Z2NjaG9zcGl0YWxAMTYzLmNvbQ==; Mingwen Jiao, am13cWZzaG9zcGl0YWxAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.