
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 12 March 2025
Sec. Hematologic Malignancies
Volume 15 - 2025 | https://doi.org/10.3389/fonc.2025.1550644
 Francesca Gay1,2*
Francesca Gay1,2* Elena Zamagni3,4
Elena Zamagni3,4 Craig Emmitt Cole5,6
Craig Emmitt Cole5,6 Christof Scheid7
Christof Scheid7 Malin Hultcrantz8
Malin Hultcrantz8 Justyna Chorazy9
Justyna Chorazy9 Ike Iheanacho9
Ike Iheanacho9 Anuja Pandey9
Anuja Pandey9 Jacopo Bitetti10
Jacopo Bitetti10 Natalie Boytsov11
Natalie Boytsov11 Molly Purser11
Molly Purser11 Simon McNamara12
Simon McNamara12 Shinsuke Iida13
Shinsuke Iida13Introduction: Anti-CD38-based therapy has become a backbone regimen for the treatment of multiple myeloma (MM), approved in first-, second-, and third-line settings. The effectiveness of anti-CD38-based retreatment after an initial relapse on previous anti-CD38-based therapy is unclear. Here we present the results of a systematic literature review investigating the clinical outcomes of anti-CD38-based retreatment in patients with relapsed/refractory MM.
Methods: Medline/Embase, congress publications, and other sources were searched (to December 8, 2023) for relevant articles in English and screened for eligibility criteria using the Population, Intervention, Comparator, Outcomes, Study Design (PICOS) framework, and data were then extracted for outcomes including progression-free survival (PFS), overall survival (OS), and overall response rate (ORR).
Results: In total, 2938 records were identified from the initial Medline/Embase search and 11 were identified from other sources; 34 were eligible for inclusion, representing 24 studies (6 clinical [n=18–307] and 18 real-world evidence [RWE; n=19–583]). Where reported, median follow-up ranged from 1.9–43.0 months across 6 clinical and 8.7–53.0 months across 10 RWE studies. For clinical trials, anti-CD38-based retreatment resulted in a median PFS of 1.0–2.8 months in all but one trial (19.4 months), a median OS of 10.7–19.1 months (not reached in one trial), and ORRs of 0–75%. RWE studies reported a median PFS of 1.5–8.4 months, a median OS of 8.4–19.0 months (not reached in one study), and ORRs of 24.6–90.0%.
Discussion: Findings from this systematic literature review indicate that clinical outcomes with anti-CD38-based retreatment are variable and offer limited clinical benefit in patients with relapsed/refractory MM, including in those refractory to anti-CD38-based treatment.
Multiple myeloma (MM) is a malignant plasma cell disorder that accounts for 1.8% of all new cancer cases and 2.0% of all cancer-related deaths in the US, with an estimated 5-year survival rate of 61.1% (1). Newly diagnosed MM cases are assessed for transplant eligibility, which considers age, fitness, and comorbidities and helps to assign suitable first-line therapies based on current MM treatment guidelines (2, 3). Novel combinations, particularly quadruplet regimens, have emerged as frontline options in patients who were transplant ineligible and, more recently, transplant eligible, resulting in improved progression-free survival (PFS) and overall survival (OS) (4–6). These regimens are generally composed of a monoclonal antibody targeting CD38 (daratumumab or isatuximab) in combination with a proteasome inhibitor and/or immunomodulatory drug, and a steroid.
With the use of frontline combination regimens, patients become exposed and/or refractory to multiple effective drug classes early on, limiting treatment options in the relapsed/refractory multiple myeloma (RRMM) setting. In particular, the proportion of patients who are anti-CD38-refractory at first relapse is likely to increase, given the clinical efficacy shown with first-line anti-CD38 combination regimens in phase III trials (5–9). Currently, the anti-CD38 agent daratumumab is approved in combination regimens as first-line treatment for both patients who are transplant eligible and ineligible and in combinations or as monotherapy for patients with RRMM, while isatuximab is approved in combination regimens as first-line treatment for patients who are transplant ineligible and as second- or later-line treatment for patients with RRMM (10, 11).
With subsequent therapy options that have distinct mechanisms of action becoming more limited due to previous exposure and/or refractoriness, retreatment with anti-CD38 agents may be more frequently considered (12). Indeed, anti-CD38 retreatment has become relatively common in patients exposed to multiple drug classes, as shown in a real-world study, where 36% of patients exposed to two prior therapies and 48% of patients with triple-refractory MM were retreated with daratumumab (13). At present, the evidence supporting anti-CD38-based retreatment in patients who are anti-CD38 exposed or refractory is unclear and treatment guidelines generally do not recommend retreatment when a patient is considered refractory to the same agent (14, 15).
To understand the impact of anti-CD38 retreatment on patient outcomes, we conducted a systematic literature review (SLR) that aimed to identify, summarize, and draw insights from data on the clinical outcomes of anti-CD38-based retreatment in patients with RRMM.
This SLR was conducted in adherence with Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines (16, 17). Medline, Medline In-Process, and Embase electronic databases were searched for articles published in English from database inception to December 8, 2023. The proceedings from the following five pre-selected annual conferences were also searched (from 2016–2023): The American Association for Cancer Research, the American Society of Clinical Oncology, the American Society of Hematology, the European Hematology Association, and the European Society for Medical Oncology. The methodology for the Medline and Embase search is detailed in Supplementary Table S1. To supplement these searches, ClinicalTrials.gov was reviewed for ongoing clinical trials and trials with data not reported elsewhere, as were the bibliographies of relevant, recently published SLRs for any additional articles of relevance.
The Population, Intervention, Comparator, Outcomes, Study Design (PICOS) framework was used to apply the SLR eligibility criteria (18), and all PICOS inclusion criteria were required to be met for studies to be included in the analysis (Supplementary Table S2). Studies had to include adults with RRMM who were previously treated and retreated with an anti-CD38-based therapy (daratumumab or isatuximab). For studies with mixed patient populations, ≥80% of patients must have been anti-CD38-retreated or data for the patients who were retreated with anti-CD38s had to be reported separately as a subgroup; studies must have reported results for ≥10 patients who were anti-CD38-retreated overall. Studies must have reported at least one of the outcomes of interest with anti-CD38-based retreatment including PFS, OS, time to progression, overall/objective response rate (ORR), complete response (CR) rate, very good partial response (VGPR) rate, and partial response (PR) rate. The main outcome of interest was PFS. Eligible study designs included randomized controlled trials (RCTs), single-arm trials, non-randomized trials, and observational/real-world evidence (RWE) studies. Case reports, qualitative studies, pharmacodynamic/pharmacokinetic studies, genetic studies, cellular or molecular studies, network meta-analyses, and economic evaluations were excluded. Whilst reference lists of relevant SLRs were used for trial identification, they were excluded as discrete studies. Full-text peer-reviewed original research articles, clinical trial records, and conference abstracts were included. Narrative reviews, editorials, protocols, guidelines, letters not reporting original research, errata, notes, or comments were excluded.
All publication titles and abstracts were initially screened for eligibility using the Nested Knowledge platform, an internet-based program that incorporates artificial intelligence screening capabilities and facilitates collaboration among reviewers during the study selection process (19). The first screening was performed by a human reviewer, while the second screening was performed by artificial intelligence for 85% of abstracts and by a human reviewer for 15% of abstracts. Any conflicts were resolved by a third independent human reviewer.
Full-text screening was carried out by two independent human reviewers. Data were extracted by a single investigator, each data point was then validated by a second senior investigator, and any conflicts were resolved through discussion with a third investigator. Key data extracted included study characteristics (e.g., design, location, size, population, objectives, inclusion/exclusion criteria), patient characteristics (e.g., age, sex, disease stage, prior lines of therapy, high-risk cytogenetics, and refractoriness), treatment characteristics (e.g., dosing regimen, route of administration, duration of treatment, concomitant medications), and analysis outcomes. The extracted evidence was assessed using narrative synthesis. A quality assessment of studies included in the SLR was also performed using the Mixed Methods Appraisal Tool (MMAT) (20).
Due to the nature of this analysis, neither ethics committee nor institutional review board approval was needed as no patient participation or consent was required and no personally identifiable information was used, stored, or disclosed.
Of the 2938 records identified from the initial Medline/Embase searches, 130 were duplicates and excluded, 2621 were excluded during title/abstract eligibility screening, and 164 were excluded during full-text screening. To the 23 remaining records, 11 were added from other sources (nine from conference proceedings, one from ClinicalTrials.gov, and one from SLR bibliography searches). The final 34 records collectively represented data from 24 studies, which were included in the SLR (Supplementary Figure S1).
In total, six clinical trials (two RCTs and four single-arm trials) and 18 RWE studies were included in the analysis; 16 studies had sufficient information to perform the MMAT assessment. All of these studies were of sufficient quality to address our research questions, and included appropriate sampling strategies, patient populations, outcomes, and statistical analyses. Randomization was appropriately performed in the RCTs, both of which had complete outcomes data. There were no substantive concerns about the quality of the studies or data.
Characteristics of the studies are summarized in Tables 1, 2. Of the RWE studies, one was a prospective study (EMMY (21)) and 17 were retrospective observational studies. Across clinical trials and RWE studies, there was considerable variation in their characteristics, including number of patients in the overall study population (ranging from 18–307 in clinical trials and 19–583 in RWE studies), treatments used, and the median follow-up periods (ranging from 1.9–53 months). Similarly, there was substantial variation in key patient characteristics, with median prior lines of therapy ranging from 3–7, 11–81% of patients having high-risk cytogenetics, and large variation in refractoriness (3–100%, double-refractory; 11–92%, triple-refractory; 7–67%, penta-refractory; 28–100%, daratumumab-refractory, where reported). Additional key patient characteristics are presented in Supplementary Table S3.
Median PFS data were reported for all six clinical trials (22–27), with treatment groups ranging from 6–65 patients (Figure 1A). Overall, the median PFS was <3 months in all studies except the TRIMM-2 trial. TRIMM-2 evaluated the novel therapy talquetamab in combination with daratumumab, and reported a median PFS of 19.4 months (95% confidence interval [CI] not reported [NR]) in 65 patients after a median follow-up of 11.5 months (22). The ICARIA-MM study (median follow-up 35.3 months for overall study population) reported a median PFS of 2.2 months (95% CI: 0.1–7.6) for nine patients with RRMM who received an isatuximab regimen followed by daratumumab as the first line of subsequent therapy (27). In contrast, the median PFS was 4.2 months (95% CI: 2.8–4.8) for 82 patients who received an isatuximab regimen followed by a non-daratumumab therapy (27).
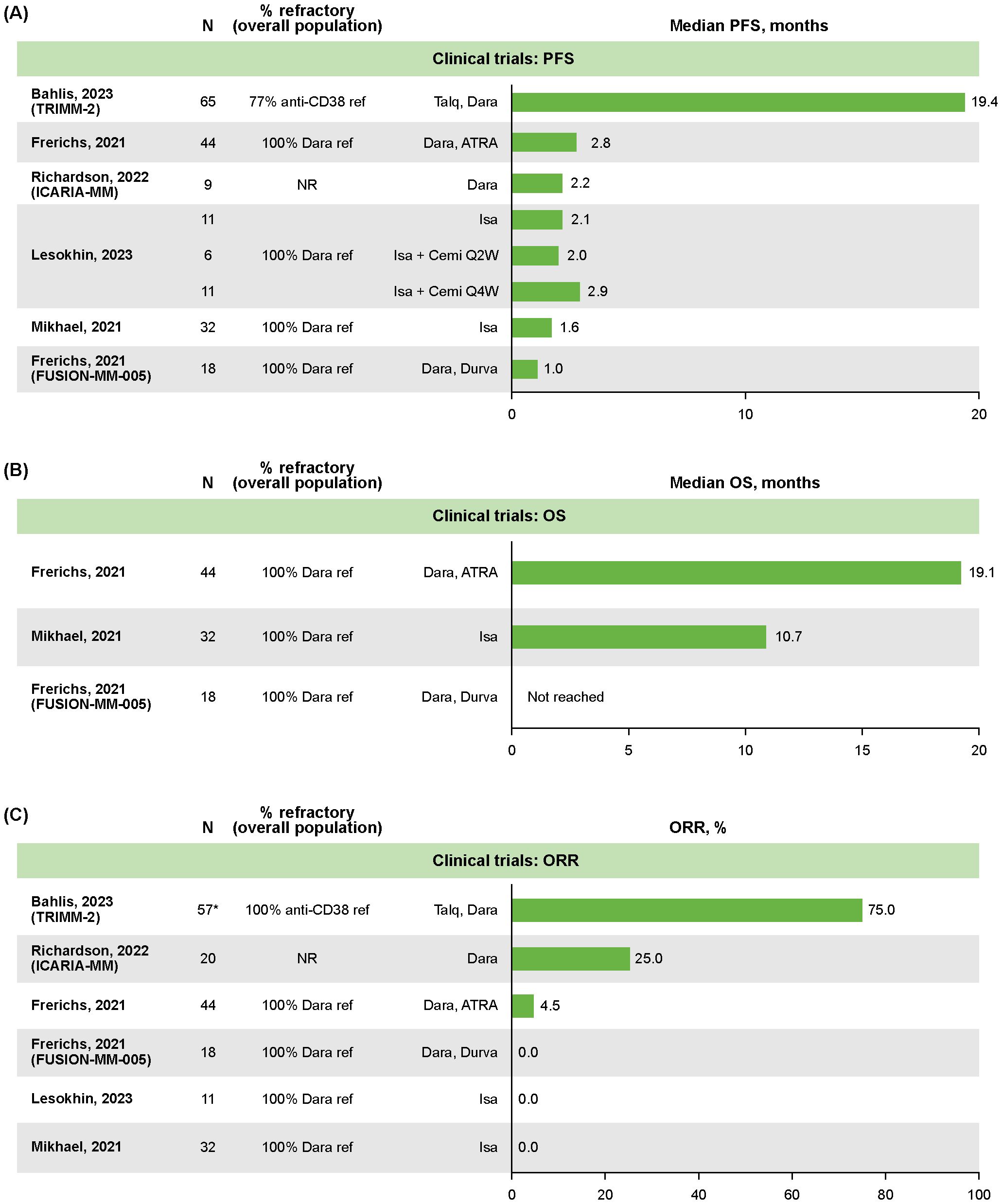
Figure 1. Clinical trial outcomes in patients with RRMM following retreatment with anti CD38-based therapy: (A) median PFS, (B) median OS, (C) ORR. *The N for TRIMM-2 was extrapolated from 88% of 65 patients who were previously exposed to an anti-CD38 drug. ATRA, all-trans retinoic acid; Cemi, cemiplimab; Dara, daratumumab; Durva, durvalumab; Isa, isatuximab; NR, not reported; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; QxW, every x weeks; ref, refractory; RRMM, relapsed/refractory multiple myeloma; Talq, talquetamab.
OS was reported in three clinical trials, with median not reached in one study of daratumumab plus durvalumab (N=18) (24), and the remaining two reporting medians of 10.7 months (95% CI: 8.0–19.0; N=32; median follow-up 4.7 months; isatuximab monotherapy [N=32]) (26) and 19.1 months (95% CI: 15.0–23.1; N=44; median follow-up 43 months; daratumumab plus all-trans retinoic acid [N=44]; Figure 1B) (24).
ORR data were reported for all six clinical trials (22–27), with treatment groups ranging from 11–57 patients. The ORR was 0% in three trials, 4.5% (n=NR/44) in a study of daratumumab plus all-trans retinoic acid in patients who were daratumumab refractory (NCT02751255), 25% (n=5/20) in ICARIA-MM, and 75% (n=NR/NR) in TRIMM-2 (Figure 1C) (22, 23, 27).
Median PFS data were reported in nine RWE studies (21, 28–35), ranging from 1.5 months (95% CI: NR; N=8; median follow-up NR) for patients with five lines of anti-CD38 exposure to 8.4 months (95% CI: 2.8–not estimable; N=22; median follow-up 14.2 months) in patients exposed but not refractory to daratumumab who received isatuximab plus pomalidomide and dexamethasone in the IMAGE study (Figure 2A) (29, 34). Overall, median PFS tended to be shorter in studies with >95% of patients who were anti-CD38-refractory, ranging from 3.3 months (95% CI: 0.0–6.9; N=12; median follow-up NR) to 5.0 months (95% CI: 1.5–8.4; N=35; median follow-up NR) (32, 33, 35, 36). In the IMAGE study of patients with RRMM who received isatuximab plus pomalidomide and dexamethasone, those who were refractory to daratumumab (median follow-up 14.2 months) had a median PFS of 3.0 months (95% CI: 2.4–4.8; N=56), while those who were daratumumab naïve had a median PFS of 16.6 months (95% CI: 13.2–not reached; N=215; Supplementary Figure S2) (29).
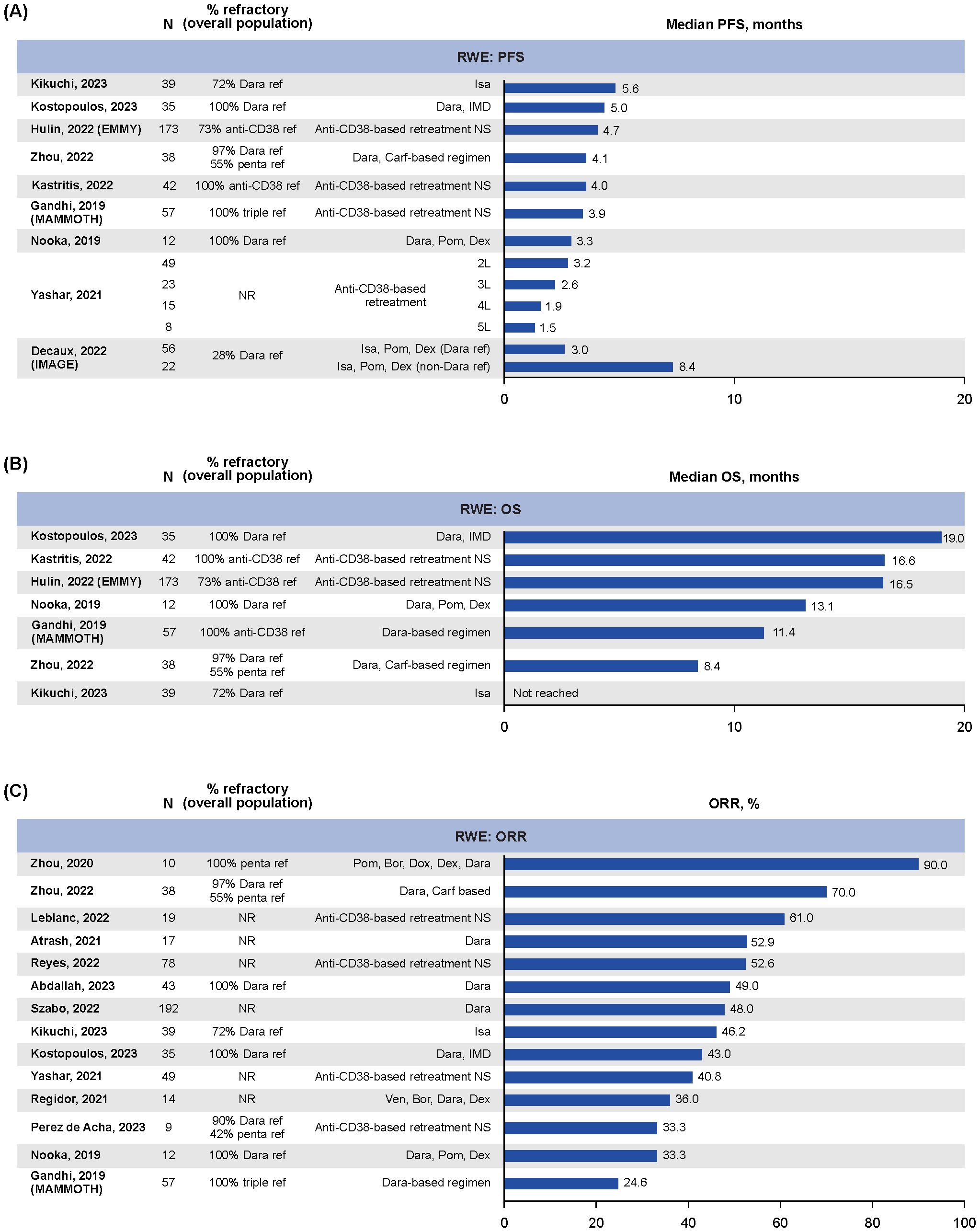
Figure 2. RWE for outcomes in patients with RRMM following retreatment with anti-CD38-based therapy: (A) median PFS, (B) median OS, (C) ORR. 2/3/4/5L, second/third/fourth/fifth line of therapy; Carf, carfilzomib; Dara, daratumumab; Dex, dexamethasone; Dox, doxorubicin; IMD, immunomodulatory drug; Isa, isatuximab; NR, not reported; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; Pom, pomalidomide; ref, refractory; RRMM, relapsed/refractory multiple myeloma; Ven, venetoclax; RWE, real-world evidence.
Median OS data for anti-CD38-based retreatment was reported in seven RWE studies (21, 30–33, 35, 36), with treatment groups ranging from 12–173 patients (Figure 2B). Overall, the median OS ranged from 8.4 months (95% CI: 6.7–10.0; N=38; median follow-up NR) in patients who were heavily pretreated (55% penta-refractory) who had received daratumumab, carfilzomib, dexamethasone, thalidomide, cisplatin, doxorubicin, cyclophosphamide, and etoposide (Dara-KDT-P[A]CE) (35) to 19.0 months (95% CI: 13.5–24.5; N=35; median follow-up NR) in patients treated with daratumumab plus an immunomodulatory drug who were refractory to both (32).
ORR data were reported in 14 RWE studies (31–44), with treatment groups ranging from 9 to 192 patients (Figure 2C). The ORRs ranged from 24.6% (35) to 90.0% (28) (Figure 2C) (33, 36, 40, 41). Best overall response was reported in 11 RWE studies (31–38, 40, 43, 44), with treatment groups ranging from 13–192 patients per treatment group, and showed that ORR was predominantly driven by PR/VGPR, with rates of CR or better reported as 0% in six studies and ranging from 2% (n=1/49) (34) to 20.5% (n=8/39) (31) in the remaining five (Supplementary Figure S3A). Of note, the study that reported an ORR of 90% was in a small subgroup of patients who were penta-refractory (n=10) who were treated with pomalidomide, bortezomib, doxorubicin, dexamethasone, and daratumumab (44).
ORR data for patients receiving anti-CD38-based retreatment compared with patients who were anti-CD38 naïve were reported in three RWE studies, with treatment groups ranging from 19–49 patients (Supplementary Figure S3B) (34, 37, 45). For two of these studies, ORRs were lower with retreatment; 49% vs. 65% for daratumumab-based treatment in patients who were daratumumab-refractory vs. naïve (37), and 89.0% vs. 38.5% vs. 33.0% for daratumumab plus pomalidomide and dexamethasone in patients who were daratumumab/pomalidomide naïve vs. daratumumab refractory vs. daratumumab/pomalidomide refractory (45) (Supplementary Figure S3B) (45).
Only one study reported time between prior daratumumab-based therapy to retreatment (37). In this RWE study, the median time to retreatment was 1.25 months (range 0.25–25; n=21) for patients who responded to retreatment and 0.25 months (range 0.25–39; n=22) for patients who did not respond to retreatment (37).
In recent years, novel combination therapies have improved the outcomes for patients with MM. In particular, the use of anti-CD38 combination therapies as first-line treatment for MM has improved responses and survival compared with historical controls (5–8, 46). However, as patients inevitably relapse or become refractory to early lines of therapy, it is important to evaluate the effectiveness of retreatment with approved RRMM therapies (especially anti-CD38-based regimens) to optimize treatment selection and sequencing. To our knowledge, this is the first SLR to examine retreatment with anti-CD38-based regimens.
Overall, these data suggest limited clinical benefit with anti-CD38-based retreatment (especially in later lines of therapy). Median PFS was <3 months across all clinical trials except for TRIMM-2, and <9 months across all RWE studies. For studies with adequate follow-up to report median OS, medians were <20 months across all trials (both clinical and RWE). ORR was <5% in all but two clinical trials (TRIMM-2 and ICARIA-MM) (14, 22). The TRIMM-2 study, which included 65 patients (88% anti-CD38 exposed and 77% refractory), showed a substantially longer PFS (19.4 months) and greater ORR (75%) than other studies (22). However, the benefits of this unlicensed combination are difficult to attribute specifically to anti-CD38-based retreatment rather than simply to the bispecific antibody talquetamab, a novel CD3- and GPRC5D-targeting agent. Indeed, as a monotherapy, talquetamab demonstrated an ORR of 73–74% in the pivotal cohorts of a phase II study of patients with RRMM who were heavily pretreated (23–29% were penta-refractory in the relevant cohorts) (22, 47, 48). Further randomized studies of anti-CD38 combination therapies (including with talquetamab) vs. anti-CD38 monoclonal antibody free regimens in patients with RRMM are needed to clarify the independent efficacy of each drug component.
There was also high variability in ORR for RWE studies (range 25–90% across 14 studies). Variability in outcome measures is likely due to heterogeneity in patient populations (e.g., the presence or absence of high-risk MM features such as extramedullary disease), small sample sizes, and that most studies were conducted in later lines of therapy (>3) where the composition of combination regimens can vary widely (49). It is interesting to note that for the 11 RWE studies that reported best overall response, the ORR appeared to be primarily driven not just by PR but also VGPR. However, rates of CR were low, and further evidence on durability of responses is needed.
The phase II LYNX study investigated retreatment with daratumumab after up to three lines of therapy, but did not meet the cutoff date for inclusion in this SLR (49). In this study, patients who received 1–3 prior therapy lines, one of which contained daratumumab, were randomized to receive daratumumab plus carfilzomib and dexamethasone or carfilzomib plus dexamethasone, and the primary endpoint was rate of VGPR or better. VGPR or better was achieved in 48.8% (95% CI: 35.1–62.6) of patients in the daratumumab plus carfilzomib and dexamethasone arm vs. 46.2% (95% CI: 32.3–60.4) in the carfilzomib plus dexamethasone arm; the secondary endpoint of PFS was also not notably different between the arms (median 10.7 vs. 10.6 months), and the study was terminated early due to futility (49). The results of LYNX are consistent with the data in this SLR, suggesting that anti-CD38 retreatment confers no clear benefit in patients with RRMM.
One strength of this SLR is that it employed a robust protocol and search strategy, and the screening process ensured that only studies that met PICOS criteria were included. However, as with all studies, there were some limitations that should be considered when interpreting the results. Data availability was limited in some studies; outcomes were reported in few studies and many had small patient populations. There was also considerable variation in the follow-up periods between studies, which likely impacted the outcomes reported. Moreover, the majority of anti-CD38-based retreatment studies were retrospective RWE studies, single-arm trials, or subgroup analyses, which further limited the strength of the analysis. Findings from retrospective studies can be limited by selection bias and confounding factors. Therefore, future prospective studies are needed to validate the results reported in the RWE studies. In addition, the variety of retreatment regimens used across the studies (as well as the heterogeneity in study design, patient populations and characteristics, and outcomes assessed, especially for RWE studies) made it even more challenging to interpret outcomes and response data from the perspective of anti-CD38 retreatment effects, as other agents may have contributed to clinical activity. Considering the variability across patient populations, additional reporting of outcomes by subgroups in future studies would be useful to explore differences in the efficacy of anti-CD38 retreatment based on baseline clinical characteristics. Only one RWE study reported the time to anti-CD38 retreatment, limiting the interpretation of impact of time to treatment (37). Future clinical investigations should prospectively consider the efficacy of retreatment started within six months of anti-CD38 refractoriness vs more than six months, given that the expression level of CD38 is reported to be restored after 6 months, which may offer improved clinical response (40, 50).
Several ongoing trials are evaluating anti-CD38 therapy in combination regimens, including with agents that target B-cell maturation antigen; the bispecific antibodies teclistamab and talquetamab are being assessed with daratumumab for RRMM, while ciltacabtagene autoleucel, a chimeric antigen receptor T-cell treatment, is being assessed with daratumumab for newly-diagnosed MM (51–53). As mentioned in this SLR, the talquetamab plus daratumumab combination from the TRIMM-2 trial has already produced promising results (22), despite the difficulties in clarifying the role of a drug with a new mechanism of action vs the impact of retreatment with anti-CD38 monoclonal antibodies. Combination studies, including studies with anti-CD38 sparing regimens and immunotherapies (54–56), may further inform strategies to overcome variable outcomes in anti-CD38 refractory patients.
In summary, the findings from this SLR indicate that retreatment with current anti-CD38-based regimens offers only a limited clinical benefit in patients with RRMM, with shorter PFS shown in studies with higher rates of patients who are anti-CD38 refractory, and as such anti-CD38-based retreatment remains an investigational option only. The advent of novel and complementary anti-CD38-based combination therapies as well as non-anti-CD38 regimens may help to address this issue, but further clinical studies specifically designed to assess the efficacy and effectiveness of any novel retreatment combinations would be required.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
FG: Investigation, Writing – review & editing. EZ: Investigation, Writing – review & editing. CC: Investigation, Writing – review & editing. CS: Investigation, Writing – review & editing. MH: Investigation, Writing – review & editing. JC: Conceptualization, Methodology, Investigation, Writing – review & editing. II: Conceptualization, Methodology, Data curation, Formal analysis, Investigation, Writing – review & editing. AP: Conceptualization, Methodology, Data curation, Formal analysis, Investigation, Writing – review & editing. JB: Conceptualization, Methodology, Formal analysis, Investigation, Writing – review & editing. NB: Conceptualization, Methodology, Formal analysis, Investigation, Writing – review & editing. MP: Conceptualization, Methodology, Formal analysis, Investigation, Writing – review & editing. SM: Conceptualization, Methodology, Formal analysis, Investigation, Writing – review & editing. SI: Investigation, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was funded by GSK (221354).
For all trials included in the review, the authors would like to thank the participating patients and their families, clinicians, and study investigators. Writing assistance was provided by Alexus Rivas-John, PharmD, and Eithne Maguire, PhD, CMPP, of Fishawack Indicia Ltd, part of Avalere Health, funded by GSK.
FG has served as an advisor for Janssen, Amgen, Bristol Myers Squibb (Celgene), Adaptive Biotechnologies, Roche, AbbVie, GSK, Takeda, Bluebird Bio, Oncopeptides, Pfizer, and Sanofi, and has received honoraria from Janssen, Amgen, Bristol Myers Squibb Celgene, GSK, Takeda, and Sanofi. EZ reports honoraria and participation in advisory boards for Janssen, Bristol Myers Squibb, Sanofi, Pfizer, GSK, Menarini-Stemline, Oncopeptides, and Amgen. CEC has participated in advisory boards/consultation for Pfizer, Sanofi, AstraZeneca, AbbVie, Genentech, Regeneron, GSK, and BMS, and received research funding from GSK and AbbVie. CS reports honoraria and participation in advisory boards from Amgen, Bristol Myers Squibb, GSK, Janssen, Oncopeptides, Pfizer, Sanofi, Stemline, and Takeda and research support from Janssen and Takeda. JC, II, and AP are employees of Evidera. JB, NB, MP, and SM are employees of GSK and hold financial equities in GSK. SI reports research funding from GSK, Janssen, Amgen, Takeda, Ono, Pfizer, AbbVie, Bristol Myers Squibb (and Celgene), Daiichi Sankyo, Otsuka, and Chugai, and honoraria from AstraZeneca, Janssen, Bristol-Myers Squibb (and Celgene), Takeda, Ono, Sanofi, and Pfizer.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This study received funding from GSK. The funder had the following involvement in the study: support of planning, research and publication.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1550644/full#supplementary-material
1. National Cancer Institute: Seer Program. Cancer Stat Facts: Myeloma (2024). Available online at: www.seer.cancer.gov/statfacts/html/mulmy.html (Accessed September 04, 2024).
2. Monteith BE, Sandhu I, Lee AS. Management of multiple myeloma: A review for general practitioners in oncology. Curr Oncol. (2023) 30:4382–401. doi: 10.3390/curroncol30050334
3. Rajkumar SV, Kumar S. Multiple myeloma current treatment algorithms. Blood Cancer J. (2020) 10:94. doi: 10.1038/s41408-020-00359-2
4. Rajkumar SV. Multiple myeloma: 2024 update on diagnosis, risk-stratification, and management. Am J Hematol. (2024) 99:1802–24. doi: 10.1002/ajh.27422
5. Facon T, Kumar SK, Plesner T, Orlowski RZ, Moreau P, Bahlis N, et al. Daratumumab, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone alone in newly diagnosed multiple myeloma (Maia): overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. (2021) 22:1582–96. doi: 10.1016/S1470-2045(21)00466-6
6. Sonneveld P, Dimopoulos MA, Boccadoro M, Quach H, Ho PJ, Beksac M, et al. Daratumumab, bortezomib, lenalidomide, and dexamethasone for multiple myeloma. New Engl J Med. (2024) 390:301–13. doi: 10.1056/NEJMoa2312054
7. Facon T, Dimopoulos MA, Leleu XP, Beksac M, Pour L, Hajek R, et al. Isatuximab, bortezomib, lenalidomide, and dexamethasone for multiple myeloma. New Engl J Med. (2024) 391:1597–1609. doi: 10.1056/NEJMoa2400712
8. Leleu X, Hulin C, Lambert J, Bobin A, Perrot A, Karlin L, et al. Isatuximab, lenalidomide, dexamethasone and bortezomib in transplant-ineligible multiple myeloma: the randomized phase 3 benefit trial. Nat Med. (2024) 30:2235–41. doi: 10.1038/s41591-024-03050-2
9. Gay F, Kortüm KM, Gao Y, Cook E, D’Estrubé T, Hanna M, et al. P-362 a real-world study to assess treatment patterns and outcomes of patients (Pts) with 2l+ Relapsed or refractory multiple myeloma in Italy, Germany, and the United Kingdom. Clin Lymphoma Myeloma Leukemia. (2024) 24:S242. doi: 10.1016/S2152-2650(24)02264-X
10. Jannsen. Darzalex® (Daratumumab). Highlights of Prescribing Information (2024). Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/761036s050lbl.pdf (Accessed September 4, 2024).
11. Sanofi. Sarclisa® (Isatuximab). Highlights of Prescribing Information (2024). Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/761113s014lbl.pdf (Accessed September 26, 2024).
12. Dimopoulos MA, Moreau P, Terpos E, Mateos MV, Zweegman S, Cook G, et al. Multiple myeloma: EHA-ESMO clinical practice guidelines for diagnosis, treatment and follow-up(†). Ann Oncol: Off J Eur Soc Med Oncol. (2021) 32:309–22. doi: 10.1016/j.annonc.2020.11.014
13. Wang PF, Yee CW, Gorsh B, Zichlin ML, Paka P, Bhak RH, et al. Treatment patterns and overall survival of patients with double-class and triple-class refractory multiple myeloma: A US electronic health record database study. Leuk Lymphoma. (2023) 64:398–406. doi: 10.1080/10428194.2022.2140284
14. Leleu X, Martin T, Weisel K, Schjesvold F, Iida S, Malavasi F, et al. Anti-cd38 antibody therapy for patients with relapsed/refractory multiple myeloma: differential mechanisms of action and recent clinical trial outcomes. Ann Hematol. (2022) 101:2123–37. doi: 10.1007/s00277-022-04917-5
15. Varughese P, Smith R, Xue M, Dorrow N, Hogea C, Maiese EM, et al. Real-world treatment patterns and outcomes of triple-class treated patients with multiple myeloma in the United States. Expert Rev Hematol. (2023) 16:65–74. doi: 10.1080/17474086.2023.2154648
16. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The prisma 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clin Res Ed). (2021) 372:n71. doi: 10.1136/bmj.n71
17. Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (Prisma-P) 2015: elaboration and explanation. BMJ (Clin Res Ed). (2015) 350:g7647. doi: 10.1136/bmj.g7647
18. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clin Res Ed). (2009) 339:b2700. doi: 10.1136/bmj.b2700
19. Nested Knowledge. (2024). Available online at: https://about.nested-knowledge.com/ (Accessed November 18, 2024).
20. Hong QN, Gonzalez-Reyes A, Pluye P. Improving the usefulness of a tool for appraising the quality of qualitative, quantitative and mixed methods studies, the mixed methods appraisal tool (Mmat). J Eval Clin Pract. (2018) 24:459–67. doi: 10.1111/jep.12884
21. Hulin C, Perrot A, Macro M, Vekhoff A, Leleu X, Karlin L, et al. Retreatment of patients with anti cd38-based combinations in multiple myeloma in real-life: results from the emmy cohort study. Blood. (2022) 140:7133–5. doi: 10.1182/blood-2022-159424
22. Bahlis Nj WK, Mateos MV, Goldschmidt H, Martin T, Morillo D, Reece D, et al. Talquetamab (Tal) + Daratumumab (Dara) in patients (Pts) with relapsed/refractory multiple myeloma (Rrmm): updated trimm-2 results. Hemasphere. (2023) 7:e90062ca. doi: 10.1097/01.HS9.0000967680.90062.ca
23. Frerichs KA, Minnema MC, Levin MD, Broijl A, Bos GMJ, Kersten MJ, et al. Efficacy and safety of daratumumab combined with all-trans retinoic acid in relapsed/refractory multiple myeloma. Blood Adv. (2021) 5:5128–39. doi: 10.1182/bloodadvances.2021005220
24. Frerichs KA, Verkleij CPM, Dimopoulos MA, Marin Soto JA, Zweegman S, Young MH, et al. Efficacy and safety of durvalumab combined with daratumumab in daratumumab-refractory multiple myeloma patients. Cancers (Basel). (2021) 13:2452. doi: 10.3390/cancers13102452
25. Lesokhin A, LeBlanc R, Dimopoulos MA, Capra M, Carlo-Stella C, Karlin L, et al. Isatuximab in combination with cemiplimab in patients with relapsed/refractory multiple myeloma: A phase 1/2 study. Cancer Med. (2023) 12:10254–66. doi: 10.1002/cam4.5753
26. Mikhael J, Belhadj-Merzoug K, Hulin C, Vincent L, Moreau P, Gasparetto C, et al. A phase 2 study of isatuximab monotherapy in patients with multiple myeloma who are refractory to daratumumab. Blood Cancer J. (2021) 11:89. doi: 10.1038/s41408-021-00478-4
27. Richardson PG, Perrot A, San-Miguel J, Beksac M, Spicka I, Leleu X, et al. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (Icaria-mm): follow-up analysis of a randomised, phase 3 study. Lancet Oncol. (2022) 23:416–27. doi: 10.1016/S1470-2045(22)00019-5
28. Costa LJ, Cornell RF, Callander NS, Chhabra S, Liedtke M, Kansagra A, et al. Efficacy of treatments for patients with triple-class refractory (Tcr) multiple myeloma (Mm): benchmark for new agents utilizing real-world data (Rwd). Blood. (2021) 138:3786. doi: 10.1182/blood-2021-144387
29. Decaux O, Lafore R, Iaquinta D, Tekle C, Leleu X. Isatuximab plus pomalidomide and dexamethasone in patients with relapsed and/or refractory multiple myeloma in real-life context in France: image subgroup analysis based on prior lines of therapy and refractory status. Blood. (2022) 140:10969–70. doi: 10.1182/blood-2022-162602
30. Kastritis E, Theodorakakou F, Ntanasis-Stathopoulos I, Spiliopoulou V, Solia E, Malandrakis P, et al. Management and outcomes of anti-cd38 refractory myeloma patients: the impact of retreatment and of subsequent therapies. Blood. (2022) 140:7324–5. doi: 10.1182/blood-2022-165792
31. Kikuchi T, Tsukada N, Nomura M, Kasuya Y, Oda Y, Sato K, et al. Real-world clinical outcomes in patients with multiple myeloma treated with isatuximab after daratumumab treatment. Ann Hematol. (2023) 102:1477–83. doi: 10.1007/s00277-023-05218-1
32. Kostopoulos I, Fotiou D, Krevvata M, Gavriatopoulou M, Ntanasis-Stathopoulos I, Roussakis P, et al. Efficacy and immune modulation of daratumumab plus imid combination in patients refractory to both agents. Hemasphere. (2023) 7:e7850799. doi: 10.1097/01.hs9.0000975260.78507.99
33. Nooka AK, Joseph NS, Kaufman JL, Heffner LT, Gupta VA, Gleason C, et al. Clinical efficacy of daratumumab, pomalidomide, and dexamethasone in patients with relapsed or refractory myeloma: utility of re-treatment with daratumumab among refractory patients. Cancer. (2019) 125:2991–3000. doi: 10.1002/cncr.32178
34. Yashar D, Regidor BSS, Jew SK, Bujarski S, Goldwater M-S, Swift R, et al. Retrospective analysis of response rates to anti-cd38 monoclonal antibody containing regimens among multiple myeloma patients with prior exposure to daratumumab or isatuximab. Blood. (2021) 138:3785. doi: 10.1182/blood-2021-154350
35. Zhou X, Ruckdeschel A, Peter J, Bockle D, Hornburger H, Danhof S, et al. Salvage therapy with “Dara-kdt-P(a)Ce” in heavily pretreated, high-risk, proliferative, relapsed/refractory multiple myeloma. Hematol Oncol. (2022) 40:202–11. doi: 10.1002/hon.2949
36. Gandhi UH, Cornell RF, Lakshman A, Gahvari ZJ, McGehee E, Jagosky MH, et al. Outcomes of patients with multiple myeloma refractory to cd38-targeted monoclonal antibody therapy. Leukemia. (2019) 33:2266–75. doi: 10.1038/s41375-019-0435-7
37. Abdallah AO, Mahmoudjafari Z, Ahmed N, Cui W, Shune L, McGuirk J, et al. Clinical efficacy of retreatment of daratumumab-based therapy (D2) in daratumumab-refractory multiple myeloma. Eur J Haematol. (2023) 110:626–32. doi: 10.1111/ejh.13942
38. Atrash S, Thompson-Leduc P, Tai MH, Kaila S, Gray K, Ghelerter I, et al. Treatment patterns and effectiveness of patients with multiple myeloma initiating daratumumab across different lines of therapy: A real-world chart review study. BMC Cancer. (2021) 21:1207. doi: 10.1186/s12885-021-08881-7
39. Leblanc R, Venner CP, Masih-Khan E, Kardjadj M, Chu MP, Jimenez-Zepeda VH, et al. Impact of daratumumab-containing regimens in outcomes of subsequent treatments of relapsed multiple myeloma in real-world practice from the Canadian myeloma research group database. Blood. (2022) 140:7192–4. doi: 10.1182/blood-2022-158679
40. Perez de Acha O, Reiman L, Jayabalan DS, Walker ZJ, Bosma G, Keller AL, et al. Cd38 antibody re-treatment in daratumumab-refractory multiple myeloma after time on other therapies. Blood Adv. (2023) 7:6430–40. doi: 10.1182/bloodadvances.2023010162
41. Regidor B, Goldwater MS, Wang J, Bujarski S, Swift R, Eades B, et al. Low dose venetoclax in combination with bortezomib, daratumumab, and dexamethasone for the treatment of relapsed/refractory multiple myeloma patients-a single-center retrospective study. Ann Hematol. (2021) 100:2061–70. doi: 10.1007/s00277-021-04555-3
42. Reyes KR, Liu Y-C, Huang C-Y, Banerjee R, Martin T, Shah N, et al. Clinical outcomes and salvage therapies in patients with relapsed/refractory multiple myeloma following progression on bcma-targeted car-T therapy. Blood. (2022) 140:617–9. doi: 10.1182/blood-2022-160401
43. Szabo AG, Thorsen J, Iversen KF, Levring MB, Preiss B, Helleberg C, et al. The clinical course and life expectancy of patients with multiple myeloma who discontinue their first daratumumab-containing line of therapy. Am J Hematol. (2022) 97:E117–E20. doi: 10.1002/ajh.26449
44. Zhou X, Steinhardt MJ, Grathwohl D, Meckel K, Nickel K, Leicht HB, et al. Multiagent therapy with pomalidomide, bortezomib, doxorubicin, dexamethasone, and daratumumab (“Pom-pad-dara”) in relapsed/refractory multiple myeloma. Cancer Med. (2020) 9:5819–26. doi: 10.1002/cam4.3209
45. Nooka AK, Joseph N, Boise LH, Gleason C, Kaufman JL, Lonial S. Clinical efficacy of daratumumab, pomalidomide and dexamethasone in relapsed, refractory myeloma patients: utility of retreatment with daratumumab among refractory patients. Blood. (2016) 128:492–. doi: 10.1182/blood.V128.22.492.492
46. Leleu XP, Hulin C, Lambert J, Bobin A, Manier S, Perrot A, et al. Phase 3 randomized study of isatuximab (Isa) plus lenalidomide and dexamethasone (Rd) with bortezomib versus isard in patients with newly diagnosed transplant ineligible multiple myeloma (Ndmm ti). J Clin Oncol. (2024) 42:7501. doi: 10.1200/JCO.2024.42.16_suppl.7501
47. Chari A, Minnema MC, Berdeja JG, Oriol A, van de Donk N, Rodriguez-Otero P, et al. Talquetamab, a T-cell-redirecting gprc5d bispecific antibody for multiple myeloma. New Engl J Med. (2022) 387:2232–44. doi: 10.1056/NEJMoa2204591
48. Schinke CD, Touzeau C, Minnema MC, van de Donk NWCJ, Rodríguez-Otero P, Mateos M-V, et al. Pivotal phase 2 monumental-1 results of talquetamab (Tal), a gprc5dxcd3 bispecific antibody (Bsab), for relapsed/refractory multiple myeloma (Rrmm). J Clin Oncol. (2023) 41:8036. doi: 10.1200/JCO.2023.41.16_suppl.8036
49. ClinicalTrials.gov. Daratumumab Retreatment in Participants with Multiple Myeloma Who Have Been Previously Treated with Daratumumab. Available online at: https://clinicaltrials.gov/study/NCT03871829 (Accessed September 9, 2024).
50. Nijhof IS, Casneuf T, van Velzen J, van Kessel B, Axel AE, Syed K, et al. Cd38 expression and complement inhibitors affect response and resistance to daratumumab therapy in myeloma. Blood. (2016) 128:959–70. doi: 10.1182/blood-2016-03-703439
51. Clinicaltrials.Gov. A Study of Subcutaneous Daratumumab Regimens in Combination with Bispecific T Cell Redirection Antibodies for the Treatment of Participants with Multiple Myeloma (2024). Available online at: https://Clinicaltrials.Gov/Study/Nct04108195 (Accessed December 20, 2024).
52. Clinicaltrials.Gov. A Study of Teclistamab in Combination with Daratumumab Subcutaneously (Sc) (Tec-Dara) Versus Daratumumab Sc, Pomalidomide, and Dexamethasone (Dpd) or Daratumumab Sc, Bortezomib, and Dexamethasone (Dvd) in Participants with Relapsed or Refractory Multiple Myeloma (Majestec-3) (2024). Available online at: https://Clinicaltrials.Gov/Study/Nct05083169 (Accessed December 20, 2024).
53. Clinicaltrials.Gov. A Study of Daratumumab, Bortezomib, Lenalidomide and Dexamethasone (Dvrd) Followed by Ciltacabtagene Autoleucel Versus Daratumumab, Bortezomib, Lenalidomide and Dexamethasone (Dvrd) Followed by Autologous Stem Cell Transplant (Asct) in Participants with Newly Diagnosed Multiple Myeloma (Cartitude-6) (2024). Available online at: https://Clinicaltrials.Gov/Study/Nct05257083 (Accessed December 20, 2024).
54. Dimopoulos MA, Dytfeld D, Grosicki S, Moreau P, Takezako N, Hori M, et al. Elotuzumab plus pomalidomide and dexamethasone for relapsed/refractory multiple myeloma: final overall survival analysis from the randomized phase II eloquent-3 trial. J Clin Oncol. (2023) 41:568–78. doi: 10.1200/JCO.21.02815
55. Hartley-Brown MA, Mo CC, Nadeem O, Midha S, Laubach JP, Richardson PG. Mezigdomide-a novel cereblon E3 ligase modulator under investigation in relapsed/refractory multiple myeloma. Cancers (Basel). (2024) 16:1166. doi: 10.3390/cancers16061166
56. Rodriguez-Otero P, Ailawadhi S, Arnulf B, Patel K, Cavo M, Nooka AK, et al. Ide-cel or standard regimens in relapsed and refractory multiple myeloma. New Engl J Med. (2023) 388:1002–14. doi: 10.1056/NEJMoa2213614
57. Richardson PG, Perrot A, San-Miguel J, Beksac M, Spicka I, Leleu X, et al. Isatuximab plus pomalidomide/low-dose dexamethasone versus pomalidomide/low-dose dexamethasone in patients with relapsed/refractory multiple myeloma (Icaria-mm): characterization of subsequent antimyeloma therapies. Blood. (2022) 140:608–10. doi: 10.1182/blood-2022-159710
58. Perrot A, Richardson P, San-Miguel J, Beksac M, Spicka I, Leleu X, et al. Updates from icaria-mm, a phase 3 study of isatuximab (Isa) plus pomalidomide and low-dose dexamethasone (Pd) versus pd in relapsed and refractory multiple myeloma (Rrmm). Hemasphere. (2021) 5:49. doi: 10.1097/hs9.0000000000000566
59. Richardson P, Perrot A, San Miguel J, Beksac M, Spicka I, LeLeu X, et al. Updates from icaria-mm, a phase 3 study of isatuximab (Isa) plus pomalidomide and low-dose dexamethasone (Pd) versus pd in relapsed and refractory multiple myeloma (Rrmm). J Clin Oncol. (2021) 39:8017. doi: 10.1200/JCO.2021.39.15_suppl.8017
60. ClinicalTrials.gov. A Study to Determine the Efficacy of the Combination of Daratumumab (Dara) Plus Durvalumab (Durva) (D2) in Subjects with Relapsed and Refractory Multiple Myeloma (Rrmm) (Fusion-Mm-005) . Available online at: https://clinicaltrials.gov/study/NCT03000452 (Accessed September 10, 2024).
61. Fotiou D, Kostopoulos IV, Krevvata M, Gavriatopoulou M, Ntanasis-Stathopoulos I, Roussakis P, et al. Evaluation of efficacy and immune modulation associated with the addition of imids to daratumumab backbone in patients refractory to both drug classes. Blood. (2021) 138:1668. doi: 10.1182/blood-2021-150032
62. Fotiou D, Gavriatopoulou M, Ntanasis-Stathopoulos I, Migkou M, Kanellias N, Malandrakis P, et al. The addition of imids for patients with daratumumab-refractory multiple myeloma can overcome refractoriness to both agents. Blood. (2020) 136:21. doi: 10.1182/blood-2020-136323
63. Kastritis E, Theodorakakou F, Ntanasis-Stathopoulos I, Spiliopoulou V, Solia E, Malandrakis P, et al. Management and outcomes of anti-cd38 refractory patients: the impact of retreatment and of subsequent therapies. Hemasphere. (2023) 7:e975. doi: 10.1097/HS9.0000000000000975
64. Girvan A, Yu J, Emechebe N, Kamalakar R, Luo Y. Real-world treatment patterns and outcomes of daratumumab retreatment in multiple myeloma in the United States. Blood. (2022) 140:5266–7. doi: 10.1182/blood-2022-158270
Keywords: anti-CD38, multiple myeloma, relapsed/refractory, systematic literature review, retreatment
Citation: Gay F, Zamagni E, Cole CE, Scheid C, Hultcrantz M, Chorazy J, Iheanacho I, Pandey A, Bitetti J, Boytsov N, Purser M, McNamara S and Iida S (2025) Clinical outcomes associated with anti-CD38-based retreatment in relapsed/refractory multiple myeloma: a systematic literature review. Front. Oncol. 15:1550644. doi: 10.3389/fonc.2025.1550644
Received: 23 December 2024; Accepted: 07 February 2025;
Published: 12 March 2025.
Edited by:
Mohamed A. Yassin, Qatar University, QatarReviewed by:
Francesca Cottini, The Ohio State University, United StatesCopyright © 2025 Gay, Zamagni, Cole, Scheid, Hultcrantz, Chorazy, Iheanacho, Pandey, Bitetti, Boytsov, Purser, McNamara and Iida. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesca Gay, ZnJhbmNlc2NhLmdheUB1bml0by5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.