
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol., 04 March 2025
Sec. Breast Cancer
Volume 15 - 2025 | https://doi.org/10.3389/fonc.2025.1548036
High-grade adenosquamous carcinoma (HGASC) is a rare and aggressive subtype of metaplastic breast cancer (MpBC). This article reports a case of HGASC (pT2N0M0 Stage IIA) in a 43-year-old female and reviews the relevant literature, with a specific focus on distinguishing HGASC from other MpBC subtypes, particularly low-grade adenosquamous carcinoma (LGASC). The patient underwent a skin-sparing mastectomy with abdominal rectus myocutaneous flap reconstruction. Histopathology confirmed HGASC with metaplastic features. Postoperative adjuvant chemotherapy with capecitabine was administered. The case highlights the unique clinical, imaging, and pathological characteristics of HGASC, its therapeutic challenges, and the need for individualized treatment strategies. A five-month follow-up showed no signs of recurrence or metastasis.
Metaplastic breast cancer (MpBC) is a rare and aggressive malignant tumor, accounting for approximately 0.2%–1% of all breast cancers (1). In 2019, the World Health Organization (WHO) revised the histological classification of MpBC based on the stromal and epithelial components of the tumor. The current subtypes include low-grade adenosquamous carcinoma, fibromatosis-like metaplastic carcinoma, squamous cell carcinoma, spindle cell carcinoma, metaplastic carcinoma with heterologous mesenchymal differentiation (such as chondroid, rhabdomyoid, or osseous), and mixed-type metaplastic carcinoma (2). Among these, low-grade adenosquamous carcinoma (LGASC) is indolent and high-grade adenosquamous carcinoma (HGASC) of the breast is an extremely rare subtype, characterized by the simultaneous presence of adenocarcinoma and squamous cell carcinoma components with high invasiveness, accounting for approximately 0.05%–0.1% of all breast cancers (3). Clinically, HGASC is characterized by large tumor size, rapid growth, high invasiveness, and low axillary lymph node metastasis rate (4). This case report aims to shed light on the clinical presentation, imaging, pathology, and treatment of HGASC, emphasizing its distinction from other MpBC subtypes.
A 43-year-old female presented with a two-month history of a palpable mass in the right breast. Approximately two months ago, the patient noticed localized pain in the right breast and palpated a hard mass. There was no tenderness upon palpation, no fever, no local skin redness, no nipple discharge, or nipple retraction. Mammography at a local hospital showed a Breast Imaging - Reporting and Data System (BI-RADS) 4B lesion in the right breast. On July 3, 2024, the patient visited our hospital for further evaluation. A breast biopsy was performed, revealing irregular small glands and small nest-like clusters of cells in the fibrous and sclerotic stroma. The cells showed atypia, and scattered mitotic figures were observed. Metaplastic carcinoma with sclerotic changes could not be ruled out. The biopsy was localized, and excision followed by comprehensive evaluation was recommended. The immunohistochemical results were: Estrogen Receptor (ER)(-), Progesterone Receptor (PR)(-), Human Epidermal Growth Factor Receptor-2 (HER-2)(-), Antigen Ki - 67 (Ki-67) (+10-20%).
Upon admission, the patient had a palpable right breast mass with associated pain, but there was no nipple discharge or fluid leakage. The patient reported premenstrual breast pain, which was linked to emotional stress. The patient had a history of hypertension for 7 years, well-controlled with medication. On physical examination, the breasts were symmetrical, with no nipple inversion or signs of peau d’orange or dimpling. A hard, tender mass of approximately 2×2 cm in size was palpated at the 12 o’clock position of the right breast. The mass had an irregular surface and poor mobility. There was no nipple discharge or arm edema. No significant mass was palpated in the left breast, and no axillary lymphadenopathy was observed bilaterally.
Auxiliary Examinations: Enhanced breast MRI (Figure 1) showed a lobulated mass in the right breast with a size of approximately 22×27×21 mm. T1-weighted imaging (T1WI) showed low signal intensity, while T2-weighted imaging (T2WI) demonstrated slightly increased signal intensity, with cystic changes in the central region. Diffusion-weighted imaging (DWI) revealed a thick-walled mass with high signal intensity, irregular shape, and low signal intensity in the central area. The mass was irregularly enhanced during the early phase of contrast injection, with spiculated margins. The central necrotic area showed no enhancement. The dynamic contrast-enhanced curve(DCE-Curve) showed a washout pattern, consistent with squamous metaplasia but not definitive.
Considering the patient’s age and preferences, she underwent a skin-sparing mastectomy with abdominal rectus myocutaneous flap reconstruction. During surgery, sentinel lymph node biopsy was performed, and intraoperative frozen section pathology showed no evidence of metastatic carcinoma. The breast tissue was excised, extending to the surface of the pectoralis major fascia. The excised specimen measured approximately 12×10 cm, with the remaining breast duct removed. Frozen section pathology confirmed negative margins with no evidence of metastatic carcinoma.
Postoperative Pathological Results: The right breast and partial skin tissue excision specimen showed invasive carcinoma with extensive necrosis. The cancer cells were arranged in nest-like patterns, with some spindle-shaped cells and others showing epithelial-like morphology and eosinophilic cytoplasm. The diagnosis was metaplastic carcinoma (high-grade adenosquamous carcinoma) (pT2N0M0 Stage IIA), measuring 2.3×1.8×1.7 cm, with evidence of nerve invasion but no vascular invasion. The skin, base, and surgical margins showed no cancer involvement. Immunohistochemistry results indicated: Ki-67 (+30-40%), ER(-), PR (+<1%, weak staining), HER-2 (1+), Androgen Receptor (AR)(-) (Figure 2).
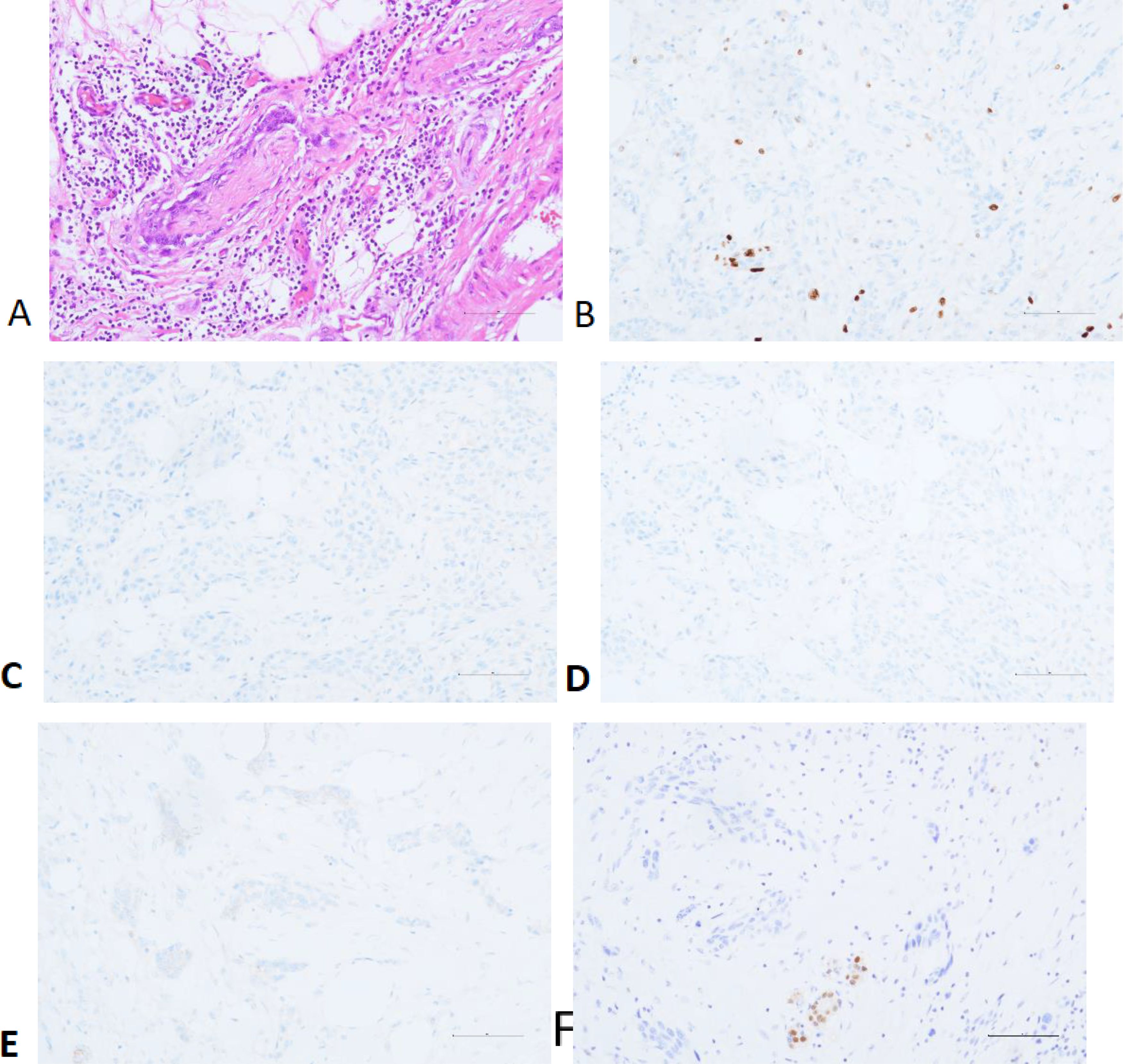
Figure 2. Perineural Invasion (A) and Immunohistochemistry: Ki-67 (B), ER (C), PR (D), HER-2 (E) and AR (F).
The patient recovered well postoperatively and, after discussion, underwent six cycles of adjuvant capecitabine therapy. Regular follow-up examinations were conducted, and at the five-month follow-up, there was no evidence of recurrence or metastasis.
Metaplastic breast carcinoma (MpBC) comprises various histological subtypes, among which high-grade adenosquamous carcinoma (HGASC) represents a distinct entity with unique characteristics. Several studies have demonstrated significant differences between HGASC and other MpBC subtypes, particularly low-grade adenosquamous carcinoma (LGASC), across multiple key aspects (5).
As a rare malignant breast tumor, the relationship between the clinicopathological features, treatment approaches, and clinical outcomes of MpBC remains insufficiently studied (6). In clinical practice, HGASC typically presents on MRI as an irregular mass with spiculated margins, indistinct boundaries, central necrosis, heterogeneous enhancement, and a rapid washout pattern on dynamic contrast-enhanced imaging. A multicenter imaging study analyzing MRI scans of 150 MpBC patients found that these typical features were observed in up to 85% of HGASC cases. In contrast, LGASC often appears as a smaller, well-defined lesion with relatively mild imaging features. Due to its well-demarcated borders and lower enhancement intensity, LGASC is frequently misdiagnosed as a benign sclerotic lesion, typically displaying a more homogeneous enhancement pattern (7).
Clinically, HGASC exhibits greater aggressiveness. A Study has shown (8) that HGASC tumors grow rapidly, and patients often present with larger tumor sizes at diagnosis. Some cases may exhibit breast pain, peau d’orange changes, and a higher likelihood of axillary lymph node and distant metastasis. Conversely, LGASC exhibits slower growth, smaller tumor sizes, and a lower risk of metastasis, with most patients presenting only with a painless breast mass (9).
From a pathological perspective, HGASC is characterized by poor differentiation, marked cellular atypia, and a high mitotic rate, which correlates with its aggressive behavior and poorer prognosis (10). In contrast, LGASC demonstrates better differentiation, lower cellular atypia, and reduced mitotic activity (9).
Immunohistochemically, Ki-67 serves as a proliferation-associated antigen, with high Ki-67 expression levels often linked to increased tumor cell proliferative activity (11). A Study has suggested that although HGASC exhibits high aggressiveness, intratumoral heterogeneity may exist, with tumor cells in different regions displaying varied biological characteristics, including Ki-67 expression levels. This heterogeneity may impact treatment response and prognosis (12). Consequently, although HGASC generally exhibits high Ki-67 expression, its prognosis may not necessarily be as poor as other high-grade tumors (13). In contrast, LGASC typically exhibits lower Ki-67 expression levels, generally ranging from 5% to 15% (14).
Regarding treatment, most MpBC subtypes are classified as undifferentiated triple-negative breast cancer (TNBC), which is insensitive to endocrine therapy and targeted therapy, resulting in a poorer prognosis (15). Currently, surgery remains one of the primary treatment options for highly aggressive MpBC subtypes such as HGASC (16). Studies have found no significant difference in survival rates between mastectomy and breast-conserving surgery, though the latter is associated with a higher local recurrence rate (17). Therefore, in clinical practice, HGASC patients often require wider surgical excision. For tumors with larger sizes or surrounding tissue invasion, radical surgery is often necessary to ensure complete tumor resection (18). In contrast, LGASC, as an indolent subtype of metaplastic breast carcinoma, rarely metastasizes. Consequently, breast-conserving surgery with negative margins is the preferred approach, while adjuvant radiotherapy, endocrine therapy, or chemotherapy is generally unnecessary (14).
In terms of adjuvant therapy, HGASC patients exhibit significantly lower survival rates than other breast cancer subtypes, particularly those with tumor diameters >2 cm, lymph node metastasis, and ER-/PR-negative status, which are associated with higher recurrence risks and shorter disease-free survival (19). Studies suggest that due to its high malignancy, HGASC exhibits relatively low sensitivity to conventional chemotherapy agents such as anthracyclines and taxanes, often requiring higher doses or combination regimens (13). Given its biological similarities to TNBC, clinicians often refer to TNBC treatment protocols, selectively incorporating capecitabine. While no specific molecular targets have been identified, future immunohistochemical analyses indicating high PD-L1 expression or increased tumor mutational burden may support the use of PD-1/PD-L1 inhibitors in combination with chemotherapy to improve treatment outcomes in HGASC (20).
In conclusion, the high aggressiveness and poor chemotherapeutic response of HGASC make it one of the more challenging breast cancer subtypes in terms of prognosis. Further large-scale studies are needed to optimize treatment strategies. Based on current evidence, surgical intervention and adjuvant therapy may provide survival benefits, but the specific efficacy and optimal treatment protocols require further validation and investigation.
Upon being diagnosed with high-grade adenosquamous carcinoma (HGASC), the patient initially experienced distress and concern due to the aggressive nature of the disease and the relatively limited treatment options. The rapid tumor growth caused both physical and emotional distress, significantly impacting the patient’s well-being. Throughout the treatment process, the patient underwent a comprehensive evaluation and a multidisciplinary treatment plan. Following surgical resection, the patient reported a sense of relief upon knowing that the primary tumor had been removed. However, anxiety persisted regarding the potential for recurrence and the limited effectiveness of conventional chemotherapy. During postoperative chemotherapy, the patient experienced side effects such as fatigue and numbness in the fingers. Nevertheless, she remained optimistic, adhered to the treatment regimen, and attended regular follow-up appointments. The patient expressed appreciation for the overall treatment process and gratitude toward the medical team for their efforts in tailoring a personalized treatment plan. Furthermore, she expressed a strong willingness to contribute to future research endeavors, hoping that her experience could help improve treatment strategies for others facing similar diagnoses.
XX: Writing – original draft, Writing – review & editing. JL: Supervision, Writing – review & editing. YF: Resources, Writing – original draft. YunW: Resources, Writing – original draft. YueW: Software, Writing – original draft. DL: Writing – review & editing. SZ: Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by the Major Innovation Project of Science and Technology of the China Academy of Chinese Medical Sciences (grant number: CI2021A01903).
All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Evans DGR, Howell A, Howell Sacha J. Metaplastic breast cancer and BRCA1: first strong evidence of a link. Eur J Hum Genet. (2023) 31(11):1207–8.
2. Nagtegaal ID, Odze RD, Klimstra D, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. (2020) 76:182–8. doi: 10.1111/his.13975
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
3. Alipour S, Seifollahi A, Anbiaee R. Lactating breast abscess: a rare presentation of adenosquamous breast carcinoma. Singapore Med J. (2013) 54(12):e247-9. doi: 10.11622/smedj.2013251
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
4. Hu Q, Chen WX, Zhong SL, Li J, Luo Z, Tang JH, et al. Current progress in the treatment of metaplastic breast carcinoma. Asian Pac J Cancer Prev. (2013) 14:6221–5. doi: 10.7314/APJCP.2013.14.11.6221
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
5. Tan Y, Yang B, Chen Y, Yan X. Outcomes of metaplastic breast cancer versus triple-negative breast cancer: a propensity score matching analysis. World J Surg. (2023) 47:3192–202. doi: 10.1007/s00268-023-07106-1
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
6. Thomas HR, Hu B, Boyraz B, Johnson A, Bossuyt VI, Spring L, et al. Metaplastic breast cancer: a review. Crit Rev Oncol Hematol. (2023) 182:103924. doi: 10.1016/j.critrevonc.2023.103924
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
7. Jia Y, He C, Liu L, Sun L, Chen Y, Luo Y, et al. A retrospective study of the imaging and pathological features of metaplastic breast carcinoma and review of the literature. Med Sci Monit. (2019) 25:248–58. doi: 10.12659/MSM.912107
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
8. Boria F, Tagliati C, Baldassarre S, Ercolani P, Marconi E, Simonetti BF, et al. Morphological MR features and quantitative ADC evaluation in invasive breast cancer: correlation with prognostic factors. Clin Imaging. (2018) 47:123–30. doi: 10.1016/j.clinimag.2018.02.011
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
9. Leng L, Hua X, Liu W, Jian W. Hormone receptor expression in low-grade adenosquamous carcinoma of the breast progressing to high-grade metaplastic carcinoma: a case report. Med (Baltimore). (2024) 103:e11689. doi: 10.1097/MD.0000000000039131
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
10. Tian Z, Wei B, Tang F, Wei W, Gilcrease MZ, Huo L, et al. Prognostic significance of tumor grading and staging in mammary carcinomas with neuroendocrine differentiation. Hum Pathol. (2011) 42(8):1169–77. doi: 10.1016/j.humpath.2010.11.014
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
11. Gobbi H, Simpson JF, Jensen RA, Olson SJ, Page DL. Metaplastic spindle cell breast tumors arising within papillomas, complex sclerosing lesions, and nipple adenomas. Mod Pathol. (2003) 16:893–901. doi: 10.1097/01.MP.0000085027.75201.B5
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
12. Pia-Foschini M, Reis-Filho JS, Eusebi V, Lakhani SR. Salivary gland-like tumours of the breast: surgical and molecular pathology. J Clin Pathol. (2003) 56:587–93. doi: 10.1136/jcp.56.7.497
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
13. Sae-Kho TM, Bhatt A, Solanki MH, Jeans EB, Corbin KS, Fazzio RT, et al. Imaging features of adenosquamous carcinoma of the breast - a rare variant of metaplastic breast carcinoma. BJR Case Rep. (2022) 7:20210108. doi: 10.1259/bjrcr.20210108
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
14. Tamminen A, Boström P. Low-grade adenosquamous carcinoma of the breast: a single-center retrospective study and a systematic literature review. Cancers (Basel). (2024) 16:4246. doi: 10.3390/cancers16244246
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
15. Corso G, Frassoni S, Girardi A, De Camilli E, Montagna E, Intra M, et al. Metaplastic breast cancer: prognostic and therapeutic considerations. J Surg Oncol. (2021) 123:61–70. doi: 10.1002/jso.v123.1
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
16. Hu J, Dong F, Zhang Y, Shen J, Ming J, Huang T. Triple-negative metaplastic breast cancer: treatment and prognosis by type of surgery. Am J Transl Res. (2021) 13:11689–96.
17. Luini A, Aguilar M, Gatti G, Fasani R, Botteri E, Brito JA, et al. Metaplastic carcinoma of the breast, an unusual disease with worse prognosis: the experience of the European Institute of Oncology and review of the literature. Breast Cancer Res Treat. (2007) 101:349–53. doi: 10.1007/s10549-006-9301-1
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
18. Kahn J, Gillespie A, Tsokos M, Ondos J, Dombi E, Camphausen K, et al. Radiation therapy in management of sporadic and neurofibromatosis type 1-associated Malignant peripheral nerve sheath tumors. Front Oncol. (2014) 4:23. doi: 10.3389/fonc.2014.00324
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
19. Rayson D, Adjei AA, Suman VJ, Wold LE, Ingle JN. Metaplastic breast cancer: prognosis and response to systemic therapy. Ann Oncol. (1999) 10:413–9. doi: 10.1023/A:1008329910362
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
20. Rakha EA, Tan PH, Varga Z, Tse GM, Shaaban AM, Climent F, et al. Prognostic factors in metaplastic carcinoma of the breast: a multi-institutional study. Br J Cancer. (2015) 112:283–9. doi: 10.1038/bjc.2014.592
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
Keywords: high-grade adenosquamous carcinoma, metaplastic breast cancer, case report, breast cancer surgery, adjuvant chemotherapy
Citation: Xing X, Li J, Fan Y, Wang Y, Wang Y, Liao D and Zhang S (2025) A case of high-grade adenosquamous carcinoma of the breast: case report and literature review. Front. Oncol. 15:1548036. doi: 10.3389/fonc.2025.1548036
Received: 19 December 2024; Accepted: 13 February 2025;
Published: 04 March 2025.
Edited by:
Robert Wesolowski, The Ohio State University, United StatesReviewed by:
Claudio Siani, G. Pascale National Cancer Institute Foundation (IRCCS), ItalyCopyright © 2025 Xing, Li, Fan, Wang, Wang, Liao and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daixiang Liao, MjcxNTQ0MDY2QHFxLmNvbQ==; Shiyun Zhang, emhzaHkwMDFAcXEuY29t
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.