
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 17 March 2025
Sec. Breast Cancer
Volume 15 - 2025 | https://doi.org/10.3389/fonc.2025.1543938
 Genlan Zhang1,2
Genlan Zhang1,2 Cui Gao1,2
Cui Gao1,2 Zining Guo3
Zining Guo3 Wenrui Zhao1,2
Wenrui Zhao1,2 Xufang Xu1,2
Xufang Xu1,2 Huaneng Wen4
Huaneng Wen4 Yaoxuan Li1
Yaoxuan Li1 Run Lin1
Run Lin1 Nenggui Xu3
Nenggui Xu3 Shaoyang Cui1*
Shaoyang Cui1*Background: Although acupuncture is recommended for managing breast cancer-related hot flashes, the level of evidence is limited. With the updating of randomized controlled trials, it is necessary to reassess its efficacy.
Objective: To assess the effectiveness of acupuncture in the treatment of hot flashes in patients with breast cancer.
Methods: Up to March 2024, we retrieved data from nine databases and used Stata software (version 14.0, version 17.0) and RevMan software (version 5.3) to conduct a meta-analysis. The Cochrane Collaboration’s risk of bias assessment tool was used for methodological assessment of the risk of bias, and the GRADEpro GDT online assessment tool was used for evidence evaluation.
Results: In total, 11 randomized controlled trials (RCTs) involved 963 participants were included in the meta-analysis. The result of risk of bias revealed that the included RCTs exhibited a high risk of bias, primarily attributable to deficiencies in randomization and blinding methods. The results of primary meta-analysis indicated that acupuncture can improved the hot flash symptom scale score (SMD, -0.54; 95% CI, -0.83 to -0.24; P < 0.05). However, acupuncture does not reduce the frequency of hot flashes(SMD, -0.20; 95% CI, -0.75 to 0.36; P = 0.48). Further subgroup analyses, including the type of control group and the duration of needle retention, etc. showed different results, highlighting the necessity for further research. Sensitivity analysis confirmed the reliability of these finding. In addition, due to various issues, the level of evidence is low.
Conclusions: Although acupuncture treatment for hot flashes in breast cancer shows potential, the evidence for the efficacy of acupuncture is still lacking due to various factors such as bias risk and significant differences between studies, and more high-quality RCTs are needed to confirm the efficacy of acupuncture.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42024531542.
The challenge posed by breast cancer to global public health remains severe, with an estimated 2.3 million new cases and 685,000 deaths each year (1). 80% of breast cancer patients experience hot flashes, which are characterized by a sudden onset of temporary fever with intense sweating (2, 3). Typically, mild hot flashes subside within minutes, while severe hot flashes persist for several hours. It is worth noting that persistent high intensity hot flashes will not only cause fatigue and sleep disorders, which will reduce the quality of life of patients, but may also affect patients’ adherence to anticancer treatment regimens, resulting in failure to achieve optimal therapeutic outcomes, and thus how to effectively manage hot flashes in breast cancer has become a major challenge worldwide (4). Currently, medication is the primary symptom management measure for hot flashes, and hormone replacement therapy (HRT) is the most widely used. Although HRT has achieved some positive results in improving hot flashes, there are side effects such as headache, nausea, palpitations and other uncomfortable symptoms, and even increase the risk of recurrence of hormone-receptor-positive breast cancer (5). In addition to hormone replacement therapy, non-hormonal drugs also have certain clinical applications, including antidepressants, such as venlafaxine and paroxetine; anticonvulsants, such as gabapentin and pregabalin; as well as other medications, such as clonidine and oxybutynin, etc. However, these medications also have a series of side effects, such as venlafaxine, which may cause dry mouth, decreased appetite, nausea, and constipation; oxybutynin may lead to difficulty in urination and cognitive impairment (6). Given the limitations of the aforementioned drug therapies, neurokinin receptor antagonists are receiving increasing attention due to research on novel regulatory pathways for reproductive function. Neurokinin-3 receptor antagonists, represented by fexofoliratan, which was approved by the U.S. Food and Drug Administration (FDA) in 2023, have been proven to be more effective than traditional non-hormonal drugs in reducing the frequency of moderate to severe hot flashes (5). Elinzanetant is an antagonist that can simultaneously target NK1 and NK3 receptors after fexofoliratan, and it has also shown encouraging results in phase III clinical trials, significantly reducing the frequency and severity of moderate to severe vasomotor symptoms, improving sleep disturbances, and enhancing overall quality of life (7–9). Although emerging neurokinin receptor antagonists have shown some potential, the lack of sufficient clinical trials to support the quality of evidence means that further research is still needed to prove their exact efficacy and safety. Therefore, there is an urgent need for a complementary alternative therapy with higher safety and effectiveness to participate in the management of hot flashes in breast cancer patients.
In recent years, complementary and alternative therapies have gained significant momentum in the field of oncology. According to data from the National Health Interview Survey (NHIS) in the United States, more than one-third of cancer survivors have used complementary and alternative therapies within a year. Notably, breast cancer patients are the most frequent users (10, 11). Acupuncture, as one of the important interventions in complementary and alternative therapies, has garnered attention for its effectiveness in alleviating hot flashes in breast cancer patients, thanks to its high safety and ease of operation (10, 12, 13). The latest American Society of Clinical Oncology (ASCO) research indicates that acupuncture can improve the condition of breast cancer patients experiencing hot flashes in the United States, South Korea, and China (14). Moreover, China’s evidence-based guidelines on hot flashes have already recommended the use of acupuncture for treating hot flashes in breast cancer patients, albeit as a weak recommendation (evidence level C) (15). The Society for Integrative Oncology (SIO) in the United States also suggests that acupuncture can be considered to reduce the frequency of hot flashes in breast cancer patients, based on patient preference (16). It is noteworthy that the Oncology Nursing Society guidelines only recommend considering the use of acupuncture for hot flashes in breast cancer within the context of clinical trials (17). Given the differing perspectives on acupuncture for hot flashes in breast cancer patients and the recent emergence of evidence on this topic, it is necessary to conduct a comprehensive systematic review and meta-analysis to evaluate and summarize the evidence on the effectiveness of acupuncture in improving hot flashes in breast cancer patients, as well as to identify gaps and future trends in this field.
This review was conducted in accordance with the Preferred Reporting Items for Systematic Evaluation and Meta-Analyses guidelines. The review protocol is registered in PROSPERO (no. CRD42024531542).
Two reviewers (GZ and CG) independently conducted a comprehensive search of the studies in nine electronic databases (PubMed, Web of Science, Sinomed, Embase, Cochrane Library, EBSCO, China National Knowledge Infrastructure, China Science and Technology Journal Database [VIP], and Wanfang Database) from database inception to March 2024. The searches were conducted using medical subject heading (MeSH) terms and free words, with MeSH terms including “Breast Neoplasm (MeSH),” “Acupuncture (MeSH),” “hot flashes (MeSH),” “Breast Tumors,” “breast cancer,” “acupuncture therapy,” “acupuncture, ear,” “hot flush,” “hectic fever,” and “randomized controlled trial.” The reference lists of the retrieved articles were manually searched and previously published review articles were scrutinized. The search process for the Embase database is detailed in the following flowchart (Table 1).
The inclusion criteria were as follows: (a) Patients with pathologically diagnosed breast cancer who experienced hot flashes (b) Intervention: The experimental group was limited to acupuncture as the sole intervention measure (unrestricted factors included the needle material, treatment point selection, manipulation techniques, and needle retention time), including electroacupuncture, fire needle, and ear acupuncture. The control group received sham acupuncture, conventional medication, no treatment, or were placed on a waiting list (c) Outcome: This study focused on hot flash symptoms in patients with breast cancer, examining the frequency of hot flashes (times/24 h, times/day, times/night), hot flash symptom scores (hot flash score, HFS), hot flash composite score, hot flash-related daily interference scale, Kupperman’s index (KI), visual analog scale, and other recognized effective scales. (d) Study type: Randomized controlled trial, with no restrictions on language.
The exclusion criteria were as follows: (a) The experimental group used acupuncture therapy in combination with other therapies. Additionally, studies were excluded if they did not mention skin penetration during acupuncture, such as laser acupuncture, and if the control group used another acupuncture method. (b) Studies that did not focus on improving hot flash symptoms in postoperative patients with breast cancer as a main research indicator. (c) Animal experiments. (d) Duplicate publications. (e) If only the abstract was available and the full text was not obtainable.
The retrieved literature was imported into NoteExpress. After deduplication, two investigators (GZ and XX) independently read the titles and abstracts of the literature according to the inclusion and exclusion criteria, performed the initial screening according to the inclusion criteria, and subsequently downloaded and read the full texts of the selected literature to make the final inclusion decision. For studies with incomplete original data, attempts were made to contact the original authors via email or telephone to acquire the necessary information.
Two investigators (GZ and CG) independently extracted data from the included original studies and created a database using Microsoft Excel. Information such as the source of the literature, stage of the tumor, intervention measures and duration, measurement indicators, results (mean and standard deviation), and methodological aspects including concealed randomization, intention-to-treat analysis, and blinding were extracted. Any discrepancies related to study inclusion or data extraction were resolved by a third researcher (ZG).
The two reviewers (GZ and CG) independently applied the Cochrane Risk of Bias Tool version 1 to assess the risk of bias in randomized trials. This tool encompasses six domains: (a) sequence generation and allocation concealment; (b) blinding of participants and outcome assessment; (c) blinding of outcome assessors (detection bias); (d) incomplete outcome data; (e) selective outcome reporting; (f) other biases. Each bias risk assessment was categorized as high, low, or unclear (18). In the event of discrepancies, a third researcher (ZG) made a final decision.
Two reviewers (GZ and CG) evaluated the quality of the evidence using the Grading of Recommendations Assessment, Development and Evaluation approach within GRADEpro GDT (http://guidelinedevelopment.org/). The evidence was assessed and classified as high, moderate, low, or very low. The assessment included the risk of bias, inconsistency, indirectness, imprecision, and other considerations. Disagreements were resolved through discussion and consultation with a third reviewer (ZG).
Stata software (versions 14.0 and 17.0) and RevMan 5.3 software were used to conduct the meta-analysis. Continuous variables measured in identical units were analyzed using the mean difference (MD) ± standard deviation (SD) as the effect size, while those measured in different units were analyzed using the standardized mean difference (SMD) ± standard deviation. The 95% confidence interval (CI) was computed, with a P-value < 0.05 denoting statistical significance. Heterogeneity among the studies was then evaluated. The Cochrane I² was used as a criterion for heterogeneity, and the I² was used for reaction heterogeneity statistic. If P ≥ 0.1 and I2 < 50%, the studies were considered to exhibit homogeneity, and a fixed-effects model was applied for the meta-analysis. Conversely, if P < 0.1 and I2 ≥ 50%, heterogeneity was identified, and a random-effects model was adopted. Subgroup analyses were performed to investigate the potential origins of heterogeneity. For studies numbering at least 10, a funnel plot and Egger’s test for asymmetry were used to evaluate publication bias. The quality and certainty of the evidence were tabulated.
The initial search identified 551 articles, of which 307 were removed as duplicates. An additional 257 articles were excluded following a review of their titles and abstracts owing to irrelevance. Fifty articles were deemed suitable for inclusion. After assessing the full texts, 36 articles were excluded. Finally, 14 articles were included in the systematic review. However, two articles did not provide the standard deviation, precluding the extraction of complete data (19, 20). One article, presented only in a graphical format without specific data in the text (21), lacked the necessary information for meta-analysis and was omitted from the analysis after unsuccessful attempts to contact the authors via email. Finally, 11 articles were included in the meta-analysis (Figure 1). In the process of preparing for submission, we meticulously selected an academic article that aligned with the predefined inclusion and exclusion criteria, offering a valuable academic reference for the nuanced interpretation of the study outcomes (14).
The 14 studies involved 963 participants, with 490 in the intervention group and 473 in the control group, and were published between 2006 and 2024. Five studies were conducted (19–23) in the United States, one in Denmark (19), two in Norway (24, 25), one in South Korea (26), one in China (14) and four in Sweden (27–30). Six studies used sham acupuncture as the control (22–25, 28, 31), two used medication as the control (20, 30), two used a blank control (22), one study chose application relaxation as the control group (27) and one study selected delayed acupuncture as the control group (14). The primary outcome measure in four studies (14, 20, 22, 32) was the HFS score; in four studies (24, 25, 27, 30) it was the KI score; and in five studies (23, 24, 27–29) it was the frequency of hot flashes. The duration of treatment and follow-up ranged from two weeks to three years. Table 2 presents the general characteristics of the included studies. The results of the literature quality assessment are presented below. Finally, a total of 11 studies were included in this meta-analysis.
Figures 2, 3 indicate that for random sequence generation, nine studies mentioned the use of computer-generated random tables or sealed envelopes, which were considered to have a low risk of bias, whereas five studies did not describe the relevant content and were considered to be at high risk. Regarding allocation concealment, six studies were considered to have a high risk of bias. For blinding, ten studies were considered to have a high risk of bias in terms of blinding the participants and outcome assessment, and only two studies evaluated the blinding of outcome assessment, which was considered to have a low risk of bias. For incomplete outcome data, five studies did not mention the number of missing participants or reasons and were considered to be at high risk. Overall, the studies included were unsatisfactory in terms of random sequence generation, allocation concealment, participant blinding, and incomplete outcome data.
The primary outcome indicators of this study encompassed two domains: the frequency of hot flashes and the associated scales for assessing hot flash symptoms. In light of the inclusion of studies featuring diverse measurement indicators, these were transformed into a standardized effect size, represented by SMD ± standard deviation. The meta-analysis of hot-flash frequency incorporated five articles, as shown in Figure 4. The Q-test (I2 = 80.10%, P < 0.001) indicated considerable heterogeneity across the selected studies. Consequently, a random-effects model was used for the meta-analysis. The meta-analysis of all five articles yielded results indicating that the acupuncture group did not exhibit a significant reduction in hot flash frequency compared to the control group (SMD, -0.20; 95% CI, -0.75 to 0.36; P = 0.48).
The meta-analysis of scales associated with hot flash symptoms included ten articles, as presented in Figure 5. The Q-test (I2 = 70.03%, P < 0.001) indicated significant heterogeneity among the selected studies. Therefore, a random-effects model was used for meta-analysis. The results indicate that acupuncture can improve hot flash symptoms (SMD, -0.54; 95% CI, -0.83 to -0.24; P < 0.05).
Considering the high heterogeneity in the acupuncture group regarding the frequency of hot flashes and hot flash symptom scores, we conducted a subgroup analysis to explore the sources of this heterogeneity. First, subgroup analysis was performed based on the frequency of hot flashes (Figure 6A). Acupuncture in improving the frequency of hot flashes within 24 h (SMD, -0.09; 95% CI, -0.44 to 0.26; P = 0.26, I2 = 24.86%) showed a reduction in heterogeneity. In terms of the frequency of hot flashes/night (SMD, -0.32; 95% CI, -1.98 to 1.34; P < 0.001, I2 = 93.56%), heterogeneity increased compared to before.

Figure 6. Subgroup analysis of frequency of hot flashes. (A) Subgroup analysis by different frequencies. (B) Subgroup analysis by different control measures. (C) Subgroup analysis by needle retention time. (D) Subgroup analysis by needle insertion depth. (E) Subgroup analysis by age.
A subgroup analysis was conducted based on the type of control group (Figure 6B). The improvement in hot flash symptoms with acupuncture compared to sham acupuncture was not statistically significant (SMD, -0.44; 95% CI, -1.19 to 0.31; I2 = 85.4%), and there was significant heterogeneity among the studies, which may be due to different study designs and patient populations. Further research is needed to determine the effectiveness of acupuncture, and more rigorous designs and methods should be adopted to reduce heterogeneity.
Subgroup analysis conducted based on needle retention time (Figure 6C) revealed slightly decreased heterogeneity for 20 min of needle retention (SMD, -0.09; 95% CI, -0.63 to 0.45; P = 0.1, I2 = 62.07%). For 30 min of needle retention (SMD, -0.67; 95% CI, -1.67 to 0.33; P = 0.02, I2 = 80.69%), heterogeneity increased compared to the previous measurement. No statistically significant difference was found between the two groups (P = 0.32).
Based on the depth of insertion for subgroup analysis (Figure 6D), an insertion depth of 5–20 mm (SMD, 0.08; 95% CI, -0.29 to 0.46; P = 0.56, I² = 0%) had no heterogeneity. In contrast, an insertion depth of 5–30 mm (SMD, -1.16; 95% CI, -1.70 to -0.61). The difference between the two groups was statistically significant (P <0.01). Considering the significant heterogeneity (I² = 85.73%), the results should be cautiously interpreted.
Subgroup analysis conducted based on age (Figure 6E) demonstrated increased heterogeneity for individuals aged <55 years (SMD, -0.26; 95% CI -1.29 to 0.76; P <0.01, I² = 87.57%). For individuals aged >55 years (SMD, -0.09; 95% CI -0.63 to 0.45; P = 0.10, I² = 62.07%), heterogeneity was lower. No statistically significant difference was found between the two age groups (P = 0.77).
A subgroup analysis was conducted based on the scales used to assess hot flash symptoms, as shown in Figure 7A The statistically significant difference in intra-group heterogeneity between the manual acupuncture and the no treatment group (SMD, -1.34; 95% CI, -2.79 to 0.10; P = 0.01, I² = 84.69%) highlighted the effect of acupuncture. There was no heterogeneity observed between the acupuncture group and the sham acupuncture group (SMD, -0.92; 95% CI, -1.26 to -0.59; P = 0.81, I² = 0%), electroacupuncture group and applied relaxation group (SMD, 0.16; 95% CI, -0.33 to 0.64; P = 0.88, I² = 0%), and the hormone therapy group (SMD, -0.27; 95% CI, -0.67 to 0.13; P = 0.50, I² = 0%). The overall effect size (SMD, -0.57; 95% CI, -1.00 to -0.15; P < 0.001) suggests that while there is some evidence to support the effectiveness of acupuncture, the evidence is not consistent. Further research with stricter experimental designs is required to draw definitive conclusions.
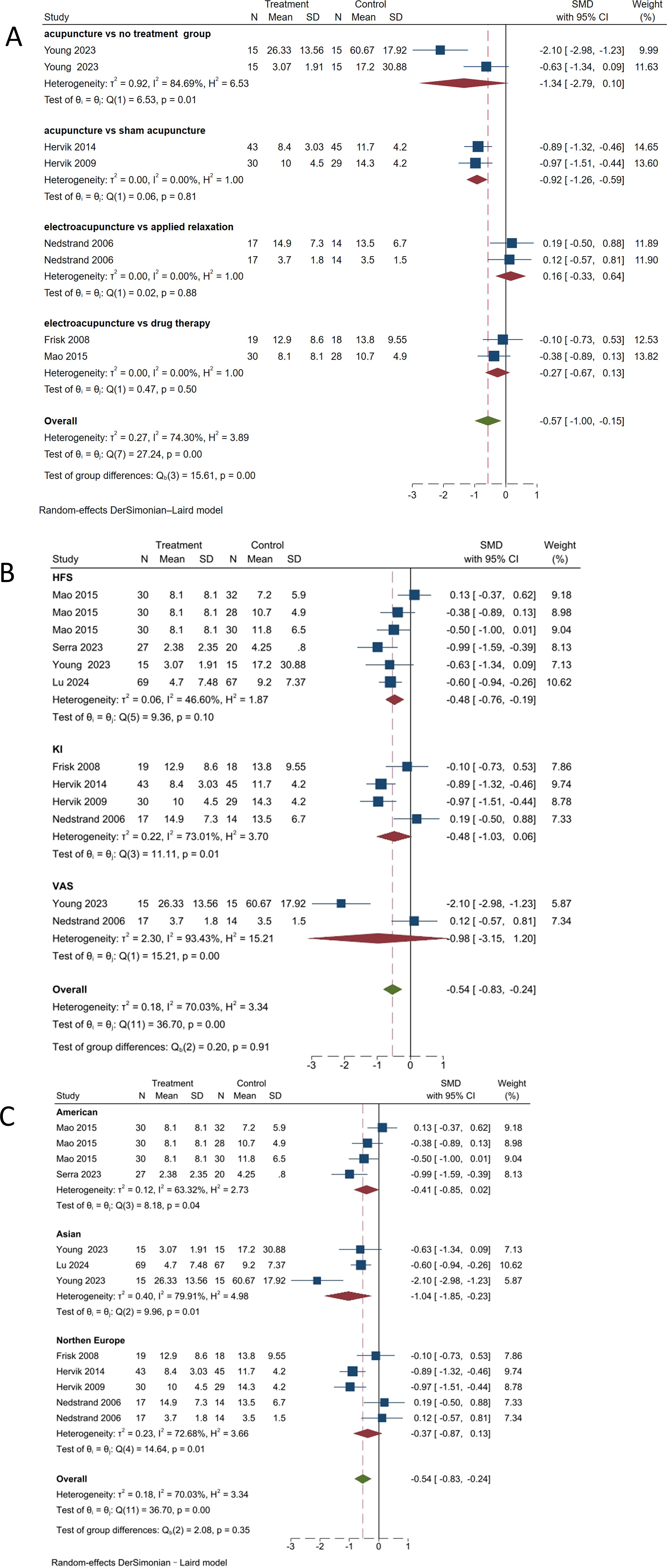
Figure 7. Subgroup analysis of hot flash-related symptom scale. (A) Subgroup analysis by different control types. (B) Subgroup analysis by different scales. (C) Subgroup analysis by different regions.
Given the high possibility of heterogeneity sources caused by different scales, as shown in Figure 7B, using the HFS score as the outcome indicator showed a reduction in heterogeneity (SMD, -0.48; 95% CI, -0.76 to -0.19; P = 0.10, I² = 46.6%). The subgroup analysis with KI score as the outcome indicator showed an increase in heterogeneity (SMD, -0.48; 95% CI, -1.03 to 0.06; P = 0.01, I² = 73.01%). The subgroup analysis with visual analog scale score as the measure of outcome showed an increase in heterogeneity (SMD, -0.98; 95% CI, -3.15 to 1.20; P < 0.001, I² = 93.43%). There were statistically significant differences between the KI and visual analog scale groups; however, there was no significant difference in intergroup heterogeneity (P = 0.91). Therefore, it is necessary to unify outcome indicators as much as possible.
Considering that current studies have primarily focused on Northern Europe and the United States, with fewer studies from Asia, there is a high possibility of regional differences. As shown in Figure 7C, in the United States (SMD, -0.41; 95% CI, -0.85 to 0.02; P = 0.04, I2 = 63.32%), heterogeneity has decreased. In Asia (SMD, -1.04; 95% CI, -1.85 to -0.23; P = 0.01, I2 = 79.91%), heterogeneity has increased compared to before. In Northern Europe (SMD, -0.37; 95% CI, -0.87 to 0.13; P = 0.01, I2 = 72.68%), heterogeneity has increased compared to previously. The differences between the treatment and control groups within each group and in the overall analysis were statistically significant (P < 0.05). There was significant heterogeneity among studies from different regions, indicating that the differences between studies may be influenced by various factors such as regional culture and population characteristics, which should be considered in future studies.
Subgroup analysis performed based on needle retention time (Figure 8A) showed significant heterogeneity for the 20-min retention group (SMD, -1.34; 95% CI, -2.79 to 0.10; P = 0.01, I2 = 84.69%). For the 30-min retention group (SMD, -0.37; 95% CI, -0.65 to -0.09; P = 0.01, I2 = 59.71%), the heterogeneity was somewhat reduced. No statistically significant difference was observed between the two groups (P = 0.20), indicating that needle retention time has little effect on the therapeutic outcome. However, considering the high heterogeneity (I2 = 70.39%), further analysis focusing on needle retention time should be conducted in the future.
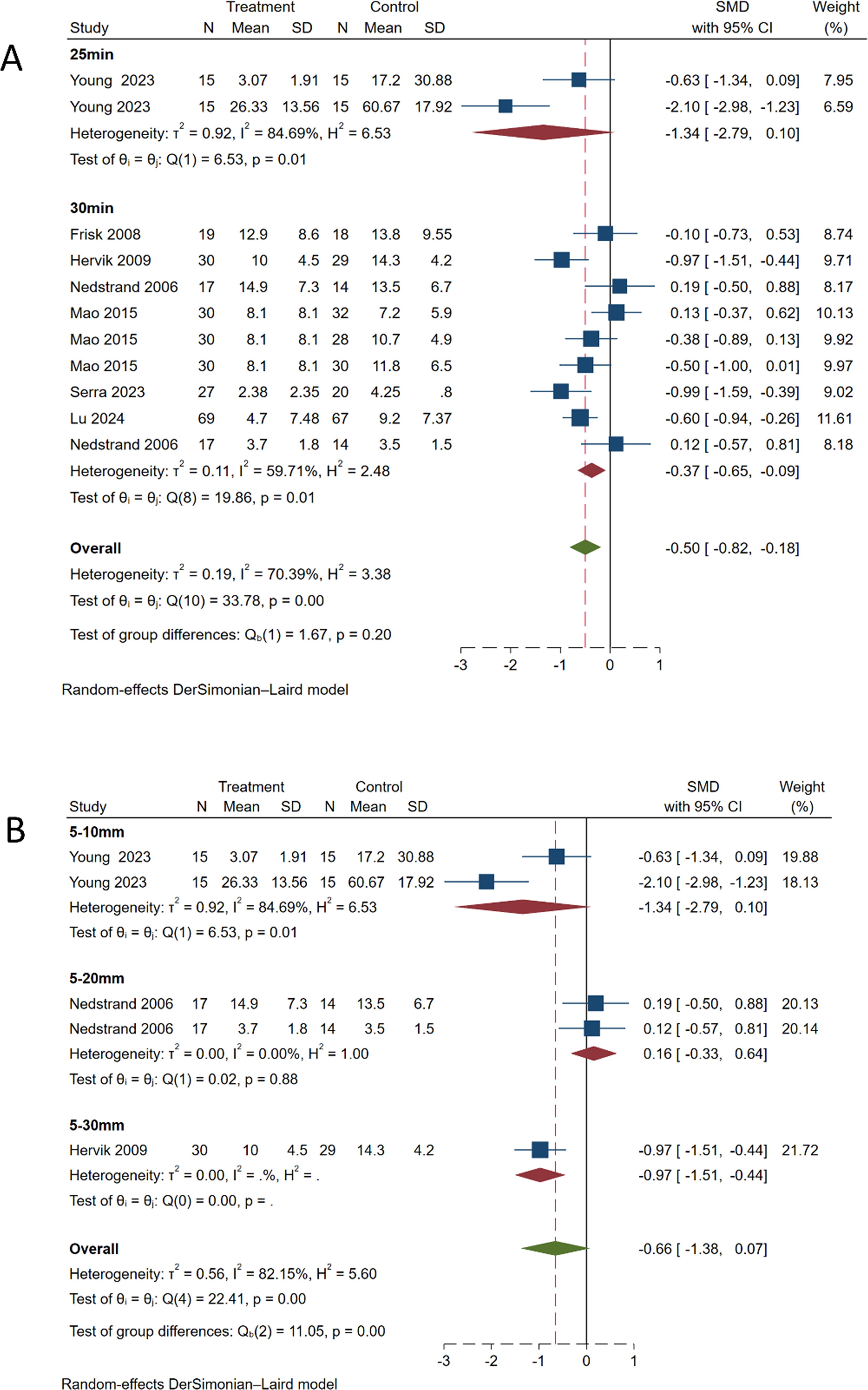
Figure 8. Subgroup analysis of hot flash symptom scale. (A) Subgroup analysis by needle retention time. (B) Subgroup analysis by needle insertion depth.
Subgroup analysis conducted based on the depth of needle insertion (Figure 8B) revealed significant heterogeneity for the 5–10 mm depth group (SMD, -1.34; 95% CI, -2.79 to 0.10; P = 0.01, I2 = 84.69%). For the 5–20 mm depth group (SMD, 0.16; 95% CI, -0.33 to 0.64; P = 0.88, I2 = 0%), no significant heterogeneity was observed. For the 5–30 mm depth group (SMD, -0.97; 95% CI, -1.51 to -0.44). The overall meta-analysis pooled effect (SMD, -0.66; 95% CI, -1.38 to 0.07; P < 0.01, I2 = 82.15%) indicated significant heterogeneity among different groups.
Ten studies related to the associated scales for assessing hot flash symptoms were included, and a meta-regression analysis was performed. Given the high suspicion of heterogeneity caused by different scale types, a meta-regression was conducted with effect size as the dependent variable and scale type as the independent variable, yielding a result of P = 0.572. Considering that the type of control group might be a source of heterogeneity, the meta-regression results showed P = 0.606. Additionally, considering the region as a potential source of heterogeneity, the meta-regression revealed a P-value of 0.208. Meta-regression analysis showed P=0.257, P=0.057, and P=0.828 for age, needle retention time, and needle insertion depth, respectively. These results suggest that they were not sources of heterogeneity. Considering the high heterogeneity in this study, the results of the meta-regression may not be reliable; therefore, a comprehensive evaluation should be conducted in conjunction with subgroup and sensitivity analyses.
Sequential exclusion of each of the 11 included studies was conducted, followed by recalculation of the meta-analysis results following each exclusion. As shown in Figures 9A, B, the recalculated summary estimates remained largely unchanged after the sequential exclusion of individual studies. This suggests that the findings of the meta-analysis within this study are robust and dependable.
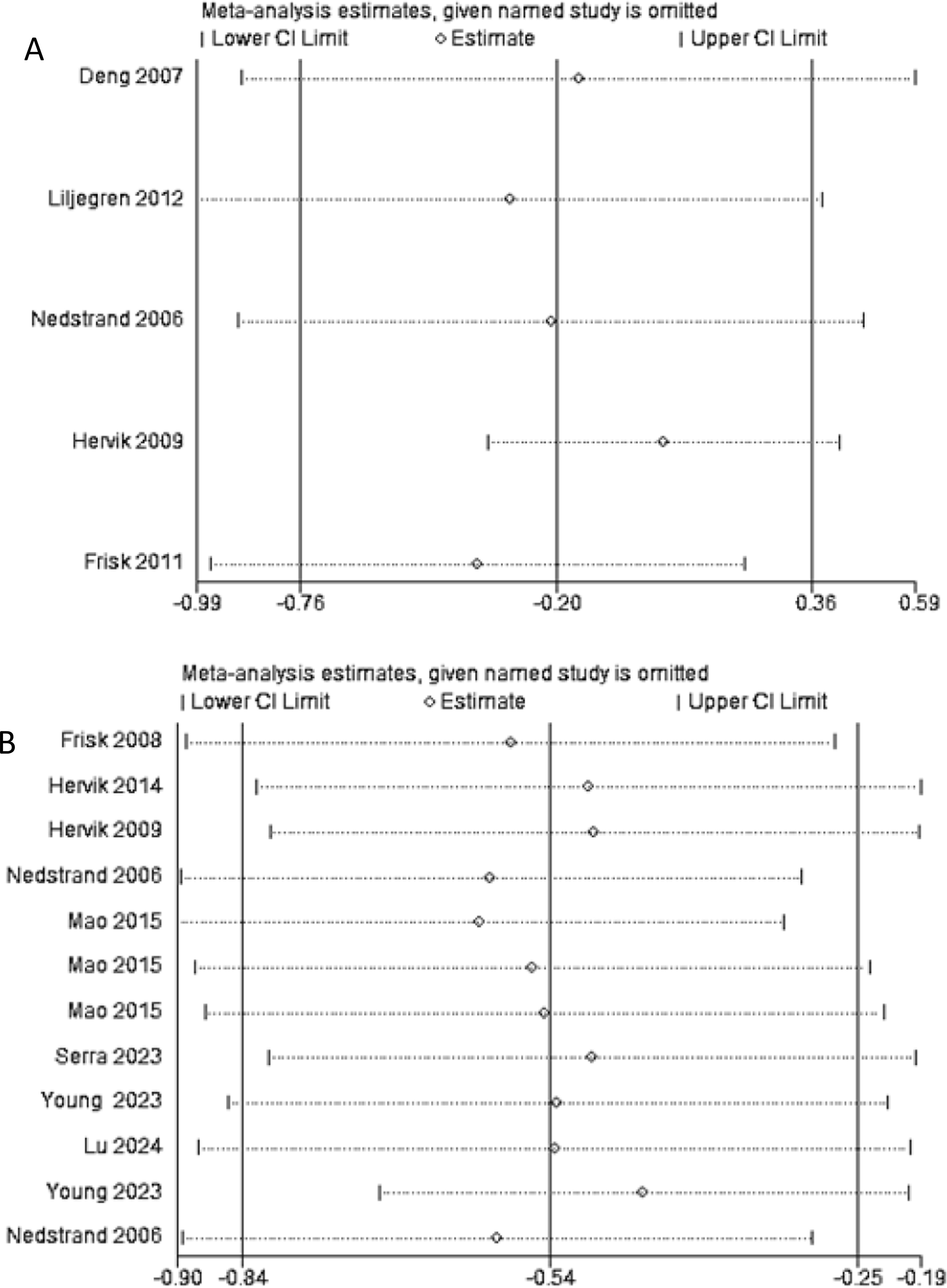
Figure 9. (A) Sensitivity analysis of frequency of hot flashes; (B) Sensitivity analysis of hot flash-related symptom scale.
As shown in Figure 10, the scatter distribution was uniform and essentially symmetrical on both the left and right sides, indicating no significant publication bias. Publication bias was assessed using the Begg and Egger tests. The Begg test result was P = 0.837 and the Egger test result was P = 0.903, both suggesting that there was no publication bias.
Using GERAD pro GDT software to evaluate different outcome indicators, it was found that the evidence provided by Frisk et al. (30), Hervik et al. (25), and Nedstrand, et al. (27) in terms of the KI index was rated as “very low,” and the evidence provided by Hervik et al. (24) on the KI index was rated as “low.” The evidence provided by Deng et al. (23) and Liljegren et al. (28) for the frequency of hot flashes per 24 h was rated as “very low.” In terms of the night-time hot flash frequency, the evidence provided by Frisk et al. (29) was rated as “very low” and the evidence provided by Hervik et al. (24) was rated as “low.” In terms of the hot flash score, two of the articles (14, 22) had “very low” evidence and two had “low” evidence (20, 32). In terms of the visual analog scale, one article (27) had evidence rated as “very low” and another (22) had evidence rated as “very low.” For further details, refer to Table 3.
Adverse events were documented in two studies. One study revealed that the acupuncture group encountered minimal side effects, including slight bleeding or bruising at the insertion site that did not require additional medical treatment. There were a total of 14 grade 1 adverse events recorded among 12 participants (23). Another study indicated that only four women in the acupuncture group had mild and transient side effects, whereas 14 (15%) in the medication group reported symptoms of fatigue, itching, and nausea (19).
Our meta-analysis results show that acupuncture does not have a significant advantage in reducing the frequency of hot flash (SMD, -0.20; 95% CI, -0.75 to 0.36; P = 0.48, I2 = 80.19%); however, in terms of hot flash scores, acupuncture has a certain therapeutic effect compared with sham acupuncture (SMD, -0.92; 95% CI, -1.26 to -0.59; P<0.01, I2 = 0%), which is consistent with the results of a network meta-analysis published in 2020 (33), reflecting that acupuncture has a certain efficacy in improving hot flash symptoms. Nevertheless, a meta-analysis from 2016 demonstrated that acupuncture has no significant advantage in reducing the frequency and severity of hot flashes (P = 0.34 and P = 0.33, respectively), which contradicts our results (34). This discrepancy may be due to our study including more and the latest randomized controlled trials, which also indirectly demonstrates that acupuncture continues to show potential in treating breast cancer-related hot flashes. Therefore, high-quality clinical research is needed to further clarify whether acupuncture should be strongly recommended as a routine adjunctive therapy for breast cancer patients.
Heterogeneity was a major challenge in our study and we considered those related to the outcome measures, types of controls, regional differences, and other factors. Firstly, the outcome measures for breast cancer-related hot flashes are diverse, and there is currently no consensus on a unified evaluation standard, which is one of the reasons for the significant heterogeneity observed among different studies. Therefore, we call for the establishment of unified outcome measures in the future to more accurately assess the true efficacy of acupuncture. Second, the diversity of control groups is also a potential source of heterogeneity. The control groups in this study include various drug treatments, sham acupuncture, and others. Sham acupuncture is a significant issue in acupuncture clinical trials, with some researchers believing that despite the use of sham acupuncture, the non-specific effects of acupuncture have not been completely excluded, leading to the view that acupuncture is a “powerful placebo.” Research has shown that through functional magnetic resonance imaging (fMRI), it has been found that sham acupuncture can stimulate the anterior cingulate cortex and thalamus, potentially mimicking the effects of real acupuncture (35). Therefore, considering the limitations of sham acupuncture, we believe that future research should further optimize its design and establish a no-treatment control group for comparison to distinguish its specific effects and increase the homogeneity of the studies. Additionally, current clinical research on acupuncture treatment for hot flashes in breast cancer patients lacks comparisons based on the subdivision of drug types. The maximum effect (Emax) pharmacokinetic model for venlafaxine is 13.9%, and for gabapentin, it is 14.8% (36). Therefore, future research can establish different drug control groups to compare the efficacy between specific drug groups and acupuncture groups. Third, the heterogeneity caused by regional differences must be considered. We conducted subgroup analysis by region but did not find the source of heterogeneity as we expected. Currently, most acupuncture research focuses on populations in the United States and Europe, with only a few studies on acupuncture in Asian patients with breast cancer (26, 37). Research shows that black and Hispanic people report a higher incidence of hot flashes and more severe endocrine symptoms (38). A multinational trial by Lu et al. (14) found that a 10-week acupuncture intervention significantly reduced endocrine symptoms and hot flashes in the United States, China, and South Korea. There were differences in efficacy across the centers, with the most significant impact in South Korea and the least in China. Therefore, biological and sociocultural differences among regional populations may lead to heterogeneity. Unfortunately, due to the lack of detailed data in current research, we are unable to conduct further in-depth exploration. Fourth, Huangdi Neijing (The Yellow Emperor’s Classic of Medicine) was the first to mention the theory of constitutional types. Chinese Traditional Chinese Medicine physicians have extended this theory and proposed a distinction between constitutional and syndrome patterns to advance the application of differential diagnosis and treatment based on the constitution, disease, and syndrome, highlighting the importance of personalized medicine. The constitution is a crucial factor in the formation of syndrome patterns and directly influences the clinical manifestations of the disease. Differences in the constitution determine the occurrence, pathogenesis, and prognosis of various diseases (39, 40). Traditional Chinese medicine considers the nature of breast cancer to be rooted in its deficiency, which manifests in excess. This deficiency is mainly characterized by disorders of the Chong and Ren meridians and deficiency of the liver and kidney, whereas excess is predominantly manifested as qi stagnation, phlegm turbidity, and blood stasis. Chinese experts in traditional Chinese medicine generally classify breast cancer into the following syndrome patterns: qi and blood deficiency, Chong and Ren disorders, liver depression and phlegm coagulation, toxin accumulation, spleen deficiency and phlegm dampness, and liver and kidney yin deficiencies. Among the patients with breast cancer, the proportions of patients with yang deficiency, qi stagnation, qi deficiency, yin deficiency, and balanced constitutions were relatively high. Qi stagnation is often related to liver depression and phlegm coagulation; qi deficiency is frequently associated with qi and blood deficiency; and yin deficiency is linked to liver and kidney yin deficiency (41). These correlations indicate that there is a relationship between the TCM constitution and syndrome classification in breast cancer, which influences the occurrence, development, and subsequent treatment of the disease to some extent. Additionally, there is a connection between the clinical and pathological characteristics of breast cancer and TCM constitution. Yin deficiency is related to tissue grading, with a higher grade increasing the likelihood of its occurrence; qi deficiency is associated with pathological types; phlegm dampness is related to lymph node metastasis, with a higher number of metastases increasing its likelihood; and a balanced constitution is related to tissue grading and hormone receptor expression, with a higher grade increasing its likelihood (42, 43). However, current evidence-based medical research on acupuncture treatment for hot flashes in breast cancer rarely includes the constitution or syndrome patterns. Therefore, this may be one of the reasons for the heterogeneity. Overall, Traditional Chinese medicine emphasizes the differential treatment of individualized syndrome patterns from a macro perspective (44). Syndrome differentiation and treatment are core concepts of TCM. We advocate that Chinese scholars conduct evidence-based clinical research, incorporating the constitution and syndrome patterns into the analysis to achieve more accurate personalized treatment from a TCM perspective. We also suggest using modern scientific research methods, such as epidemiological surveys, genomics, and molecular biology, to systematically investigate the relationship between the TCM constitution, syndrome patterns, and heterogeneity. Fifth, we conducted subgroup analyses on needle retention time, needle depth, and patient age, and meta-regression showed that these factors did not account for the source of heterogeneity. At the same time, We also consider that the source of heterogeneity may be related to different breast cancer subtypes and stages. Unfortunately, the studies included in this research did not specify breast cancer subtypes and stages in detail. Additionally, the efficacy of acupuncture is influenced by various factors, including the type of needle, acupoint selection, treatment course and frequency, and technical level of the practitioner, all of which may contribute to the observed heterogeneity (45). Although this study explored the sources of heterogeneity, it could not fully explain them. Through sensitivity analysis, we confirmed the stability of the results, indicating that our conclusions are relatively reliable. The Egger test and funnel plot showed no evidence of publication bias. The quality of evidence was low, with most studies having a high risk of bias, lacking concealment of allocation and blinding, which may have contributed to significant methodological heterogeneity. Therefore, it is necessary to optimize trial design, control variables, and conduct high-quality clinical trials to confirm the efficacy of acupuncture in treating cancer-related hot flashes.
Acupuncture, as an adjuvant therapy for alleviating cancer-related symptoms, is both safe and effective (46). In oncology, acupuncture has been incorporated into clinical practice guidelines for managing symptoms such as breast cancer-related hot flashes, highlighting its potential and prospects in treating cancer-related symptoms (47). However, a significant disparity exists in the quality and level of evidence-based data available for acupuncture in treating hot flashes in patients with breast cancer, highlighting the importance of further exploring its mechanism. As shown in Figure 11, some studies have suggested that the occurrence of hot flashes is related to a decrease in estrogen levels, which is associated with various factors. This decline may cause a series of changes in neural and humoral factors, indirectly leading to hot flash symptoms (48). There are several views on this, the first of which is the hypothalamic-pituitary-ovarian axis theory. The chemotherapy drugs and radiotherapy factors for patients with breast cancer may lead to premature ovarian failure (49), resulting in the disappearance of the feedback inhibition mechanism on the pituitary gland; this imbalance affects the hypothalamic-pituitary-ovarian axis, causing hyperfunction of the hypothalamus and pituitary gland (50). The second is the endocrine-neuroregulatory mechanism. By reducing estrogen levels or blocking its receptor action to inhibit cancer cell growth, the decrease in blood estrogen directly affects the central nervous system. It suppresses the production of neurotransmitters such as 5-hydroxytryptamine and β-endorphin, both of which can regulate the hypothalamic temperature set point and inhibit norepinephrine secretion. When their levels decrease, it directly lowers the temperature set point, leading to dysfunction of the hypothalamic thermoregulatory center. At the same time, it eliminates the negative feedback inhibition on norepinephrine, and the dual action leads to hot flashes (51). Third, reduced estrogen levels decrease the expression of receptors on the surface of immune cells, resulting in insufficient synthesis of immune mediators such as interleukin-2. This immune imbalance results in abnormal levels of inflammatory cytokines, which in turn affect neurotransmitter function, prompting excessive release of norepinephrine and causing hot flashes (52). Fourth, research has indicated that a decrease in estrogen levels stimulates the release of calcitonin gene-related peptide from perivascular calcitonin gene-related peptide fibers, leading to the disruption of vascular homeostasis and vasodilation, which triggers hot flashes (53).
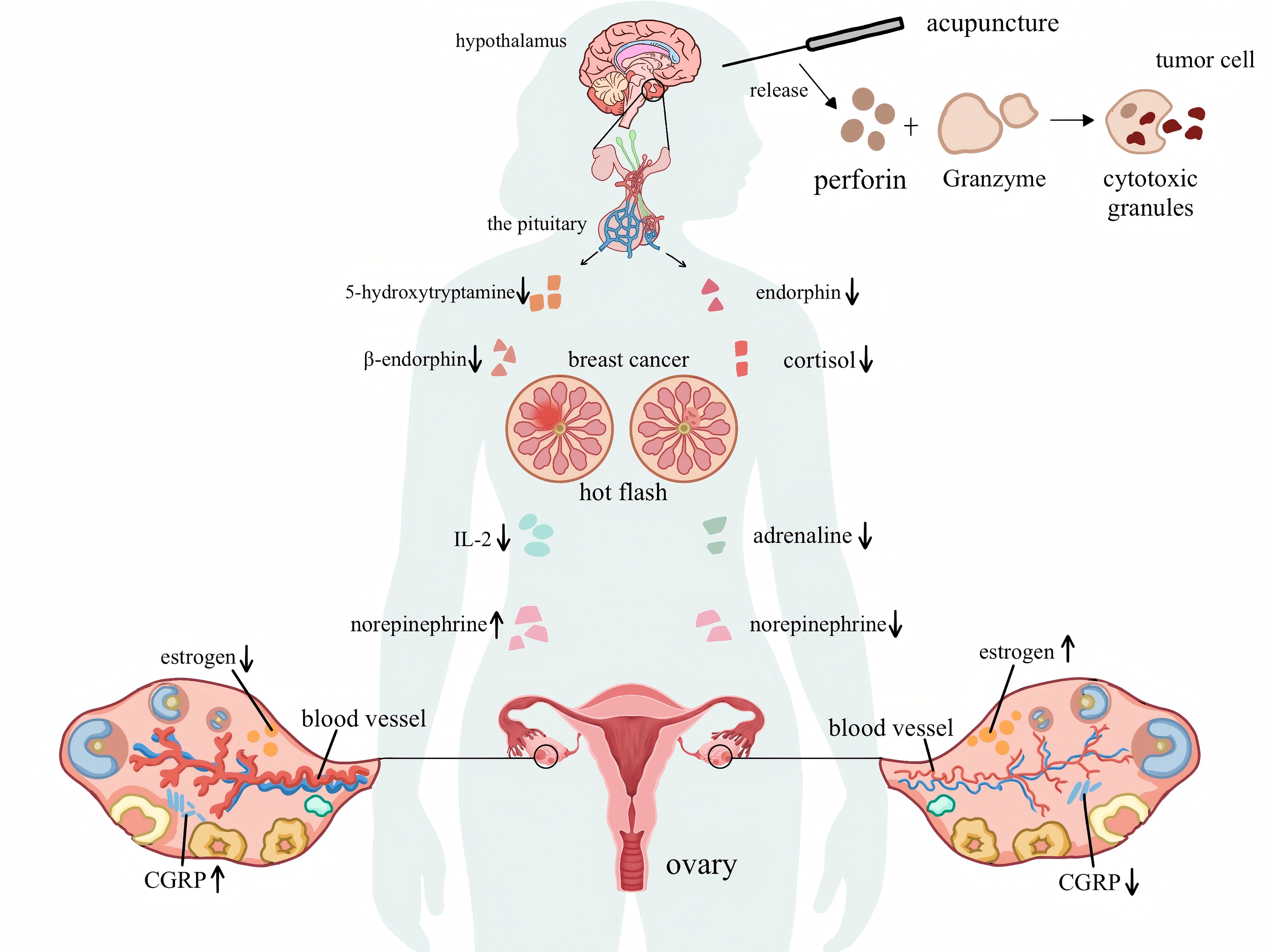
Figure 11. The mechanism diagram of breast cancer hot flashes and the mechanism of acupuncture in improving hot flashes.
The mechanism by which acupuncture improves hot flashes remains inconclusive; however, it can be generally categorized into the following aspects: First, by regulating estrogen levels, studies have shown that acupuncture at acupoints such as Guanyuan (CV 4), Neiguan (PC 6), and Zusanli (ST 36) can increase the serum estradiol levels and hypothalamic 5-hydroxytryptamine content in ovariectomized rats (54), reduce the levels of follicle-stimulating and luteinizing hormones, effectively regulate the hypothalamic-pituitary-ovarian axis function, maintain the homeostasis of the thermoregulatory center, and consequently reduce hot flashes. Second, acupuncture may influence neurotransmitter activities by regulating endorphins and norepinephrine in the central nervous system. Acupuncture can reduce the level of substance P and enhance the release of endogenous opioid peptides (55–57). Research has found that electroacupuncture stimulation of the Zusanli (ST36) and ST25 points in mice can activate the sympathetic nerves and adrenal medulla, promoting the release of norepinephrine (58, 59) and improving hot flashes by affecting neurotransmitters. Third, electroacupuncture can regulate vascular relaxation factors by downregulating the expression of calcitonin gene-related peptides (60, 61), thereby reducing the vasodilatory response to alleviate hot flashes. Fourth, research has shown that psychological factors considerably impact the immune system of patients with breast cancer. The mechanism is as follows: chronic stress increases endocrine regulators and cytokines, activating the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system (62); this leads to an increase in the secretion of hormones such as cortisol and adrenaline, which alters the tumor microenvironment and promotes its growth. Meanwhile, chronic stress inhibits the activity of natural killer cells, reducing the body’s ability to kill tumor cells (63). This disruption affects the basic physiological processes of the endocrine and immune systems in patients with breast cancer to regulate tumor growth (64). Simultaneously, acupuncture can enhance the activity and number of natural killer cells, release cytotoxic granules containing perforin and granzymes, improve the body’s ability to kill tumor cells, and alleviate hot flash symptoms by improving the immune status of patients with breast cancer (65–67). As cancer treatment shifts towards personalized treatment, mental health assessments and psychological intervention therapies can be utilized to prevent the occurrence and development of cancer. Further research on the combination of acupuncture and psychological interventions could provide a broader perspective on the relative effectiveness of complementary treatments in maximizing patient benefits.
Acupuncture may also exert its effects by influencing the expression of specific genes. Romero et al. (68) have highlighted that the ADORA1, COMT, TCL1A, and TRPV1 genes can predict breast cancer survivors’ response to acupuncture for hot flashes. Research has also demonstrated that different molecular subtypes of breast cancer have distinct pathological characteristics, exhibit survival differences, and vary in their sensitivity to different treatment regimens (69). Therefore, future research should explore gene expression regulation as a new direction, perform genetic typing of breast cancer, and identify breast cancer survivors who are more sensitive to acupuncture treatment, thereby achieving personalized treatment regimens, optimizing the management of hot flashes, and maximizing the effects of acupuncture.
To explore the mechanism of acupuncture in greater depth and ensure the reliability and validity of research findings, it is necessary to adopt high-quality control groups in acupuncture research. However, the use of high-quality control groups in acupuncture research poses significant challenges. Research suggests that the type of acupuncture control used, whether at non-acupoint locations or non-penetrating needles, may have specific effects (70, 71). Sham or placebo acupuncture can induce tactile stimulation, activate the somatosensory system, and engage the related brain regions, leading to specific physiological responses (72). In the studies included in this review, Liljegren et al. (28) performed sham needling, in which the needle set was placed 1 cm away from the acupuncture point, without piercing the skin, and rotated to make the patient’s skin sensory. The results showed no significant difference between this sham acupuncture and real acupuncture in terms of improving hot flashes. Therefore, reducing the placebo effect of sham acupuncture is crucial for accurately evaluating the actual effects of acupuncture. Overall, future research should conduct large-scale, multicenter, randomized controlled trials to enhance the generalizability and credibility of the results. The study design should be optimized with the selection of appropriate control groups and consideration of the gold standard for double-blind randomized controlled trials. Emphasis should be placed on improving research quality, including the implementation of randomization, blinding, and allocation concealment, to enhance the reliability and comparability of studies. Standardized acupuncture protocols for hot flashes in breast cancer could be formulated, including the combination of specific acupuncture points, the depth of needling, and the duration of needle retention to minimize the influence of the differences in the treatment protocols on the results of the study. To delve deeper into the mechanisms of acupuncture, functional magnetic resonance imaging (fMRI) techniques can be used to observe the effects of acupuncture on specific areas of the brain, such as the hypothalamus and amygdala, and the relationship between these areas and the occurrence of hot flashes, as well as exploring the regulation of gene expression, genotyping breast cancer, and identifying patients with the type of breast cancer that is more sensitive to acupuncture treatment to optimize hot flash management.
Concerning innovation, this study thoroughly explores and summarizes the mechanisms of acupuncture in treating hot flashes in patients with breast cancer, providing a reference and new directions for future research, thereby offering more robust evidence support for the application of acupuncture in this field. Additionally, compared to previous meta-analyses, this study provides an update, expanding the clinical protocols for acupuncture treatment of hot flashes in patients with breast cancer and enhancing the quality of clinical evidence. Regarding limitations, a high degree of heterogeneity exists among these studies, and the sample sizes are relatively small, which restricts our ability to accurately assess the true efficacy of acupuncture in treating breast cancer-related hot flashes. The overall quality of the research evidence is low, and the subjective nature of the outcome measures may have affected result reliability.
This meta-analysis revealed that acupuncture did not significantly reduce the frequency of hot flashes in patients with breast cancer. Nevertheless, an analysis of clinical hot flash rating scales indicated that acupuncture can alleviate hot flash symptoms. These findings suggest that the clinical effectiveness of acupuncture for treating hot flashes in patients with breast cancer is characterized by certain uncertainties and limitations. This uncertainty and limitation may be due to the relatively low quality of clinical research in this field, as well as the lack of standardized diagnostic and treatment protocols. To establish definitive evidence of acupuncture’s efficacy in this context, future research should prioritize conducting high-quality, large-scale, multi-center, randomized controlled trials. Additionally, efforts should be made to enhance the rigor of trial methodologies and develop standardized acupuncture protocols to ensure diagnostic and treatment standardization.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
GZ: Formal analysis, Methodology, Writing – original draft, Writing – review & editing. CG: Formal analysis, Software, Writing – original draft. ZG: Methodology, Writing – review & editing. WZ: Data curation, Writing – original draft. XX: Data curation, Methodology, Writing – original draft. HW: Data curation, Methodology, Writing – original draft. YL: Conceptualization, Writing – original draft. RL: Data curation, Methodology, Writing – original draft. NX: Conceptualization, Funding acquisition, Writing – review & editing. SC: Conceptualization, Funding acquisition, Methodology, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the General Program of the National Natural Science Foundation of China (grant number 82374573) and the Shenzhen “Healthcare Three Project” (grant number SZSM201502044) awarded to NX and SC.
We extend our gratitude to all the investigators and supporters who contributed to this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
CI, confidence interval; HFS, hot flash score; KI, Kupperman’s index; MeSH, medical subject heading; SD, standard deviation; SMD, standardized mean difference.
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Rada G, Capurro D, Pantoja T, Corbalán J, Moreno G, Letelier LM, et al. Non-hormonal interventions for hot flushes in women with a history of breast cancer. Cochrane Database Syst Rev. (2010) 9:CD4923. doi: 10.1002/14651858.CD004923
3. Hong D, Bi L, Zhou J, Tong Y, Zhao Q, Chen J, et al. Incidence of menopausal symptoms in postmenopausal breast cancer patients treated with aromatase inhibitors. Oncotarget. (2017) 8:40558–67. doi: 10.18632/oncotarget.17194
4. Bień A, Rzońca E, Iwanowicz-Palus G, Pańczyk-Szeptuch M. The influence of climacteric symptoms on women’s lives and activities. Int J Environ Res Public Health. (2015) 12:3835–46. doi: 10.3390/ijerph120403835
5. Morga A, Ajmera M, Gao E, Patterson-Lomba O, Zhao A, Mancuso S, et al. Systematic review and network meta-analysis comparing the efficacy of fezolinetant with hormone and nonhormone therapies for treatment of vasomotor symptoms due to menopause. Menopause. (2024) 31:68–76. doi: 10.1097/GME.0000000000002281
6. Franzoi MA, Agostinetto E, Perachino M, Del ML, de Azambuja E, Vaz-Luis I, et al. Evidence-based approaches for the management of side-effects of adjuvant endocrine therapy in patients with breast cancer. Lancet Oncol. (2021) 22:e303–13. doi: 10.1016/S1470-2045(20)30666-5
7. Servayge J, Verduyn AC, Page A, Lagaert L, Tjalma W. Clinical guidelines for managing menopausal symptoms in women with (a history of) breast cancer. Facts Views Vis Obgyn. (2023) 15:297–308. doi: 10.52054/FVVO.15.4.102
8. Sassarini J, Anderson RA. Elinzanetant: a phase iii therapy for postmenopausal patients with vasomotor symptoms. Expert Opin Investig Drugs. (2024) 33:19–26. doi: 10.1080/13543784.2024.2305122
9. Fasero M, Sanchez M, Baquedano L, Gippini I, Fuentes D, Navarro C, et al. Management of menopausal hot flushes. Recommendations from the spanish menopause society. Eur J Obstet Gynecol Reprod Biol X. (2025) 25:100366. doi: 10.1016/j.eurox.2025.100366
10. Romeiser JL, Chen Z, Nanavati K, Williams AA. Correlates and patterns of using complementary health approaches among individuals with recent and longer-term cancer diagnoses: a us national cross-sectional study. J Cancer Surviv. (2024). doi: 10.1007/s11764-024-01665-5
11. John GM, Hershman DL, Falci L, Shi Z, Tsai WY, Greenlee H. Complementary and alternative medicine use among us cancer survivors. J Cancer Surviv. (2016) 10:850–64. doi: 10.1007/s11764-016-0530-y
12. Zia FZ, Olaku O, Bao T, Berger A, Deng G, Fan AY, et al. The national cancer institute’s conference on acupuncture for symptom management in oncology: state of the science, evidence, and research gaps. J Natl Cancer Inst Monogr. (2017) 2017(52). doi: 10.1093/jncimonographs/lgx005
13. Mao JJ, Pillai GG, Andrade CJ, Ligibel JA, Basu P, Cohen L, et al. Integrative oncology: addressing the global challenges of cancer prevention and treatment. CA Cancer J Clin. (2022) 72:144–64. doi: 10.3322/caac.21706
14. Lu W, Giobbie-Hurder A, Tanasijevic A, Kassis SB, Park SH, Jeong YJ, et al. Acupuncture for hot flashes in hormone receptor-positive breast cancer: a pooled analysis of individual patient data from parallel randomized trials. Cancer. (2024) 130(18):3219–28. doi: 10.1002/cncr.35374
15. Wenwen C, Dan W, Jingwen Y, Xiaojun X, Xiaoyan J, Ping W, et al. Clinical practice guidelines for acupuncture and moxibustion in the prevention and treatment of tidal fever in patients with breast cancer. World Chin Med. (2022) 17:3174–79. doi: 10.3969/j.issn.1673-7202.2022.22.009
16. Lyman GH, Greenlee H, Bohlke K, Bao T, DeMichele AM, Deng GE, et al. Integrative therapies during and after breast cancer treatment: asco endorsement of the sio clinical practice guideline. J Clin Oncol. (2018) 36:2647–55. doi: 10.1200/JCO.2018.79.2721
17. Kaplan M, Mahon SM, Lubejko BG, Ginex PK. Hot flashes: clinical summary of the ons guidelines™ for cancer treatment-related hot flashes in women with breast cancer and men with prostate cancer. Clin J Oncol Nurs. (2020) 24:430–33. doi: 10.1188/20.CJON.430-433
18. Higgins J, Green S, Higgins J. Cochrane handbook for systematic reviews of interventions, version 5.1.0. The cochrane collaboration (2013). Available at: www.handbook.cochrane.org.
19. Bokmand S, Flyger H, Bollig G. Acupuncture relieves menopausal discomfort in breast cancer patients: a prospective, double blinded, randomized study. Breast. (2012) 56:25. doi: 10.1016/j.breast.2012.07.015
20. Mao JBMX, Farrar JT. Electroacupuncture versus gabapentin for hot flashes among breast cancer survivors: a randomized placebo-controlled trial. J Clin Oncol. (2015) 33:3615–20. doi: 10.1200/JCO.2015.60.9412
21. Walker ERAK, Levine RA. Acupuncture versus venlafaxine for the management of vasomotor symptoms in patients with hormone receptor-positive breast cancer: a randomized controlled trial. J Clin Oncol. (2010) 28:634–40. doi: 10.1200/JCO.2009.23.5150
22. Serra DFSW, Chadha M. Acupuncture reduces severity of hot flashes in breast cancer: a randomized single-blind trial. Holist Nurs Pract. (2023) 37:330–36. doi: 10.1097/HNP.0000000000000612
23. Deng G, Vickers AJ, Yeung KS, D’Andrea GM, Xiao H, Heerdt AS, et al. Randomized, controlled trial of acupuncture for the treatment of hot flashes in breast cancer patients. J Clin Oncol. (2007) 25:5584–90. doi: 10.1200/JCO.2007.12.0774
24. Jill H, Odd ML. Acupuncture for the treatment of hot flashes in breast cancer patients, a randomized, controlled trial. Breast Cancer Res Treat. (2009) 116(2):311–16. doi: 10.1007/s10549-008-0210-3
25. Hervik J, Mjaland O. Long term follow up of breast cancer patients treated with acupuncture for hot flashes. Springerplus. (2014) 50:S86. doi: 10.1186/2193-1801-3-141
26. Jeong YJ, Park YS, Kwon HJ, Shin IH, Bong JG, Park SH. Acupuncture for the treatment of hot flashes in patients with breast cancer receiving antiestrogen therapy: a pilot study in korean women. J Altern Complement Med. (2013) 19:690–96. doi: 10.1089/acm.2012.0347
27. Nedstrand EWYH, Wijma K. Psychological well-being improves in women with breast cancer after treatment with applied relaxation or electro-acupuncture for vasomotor symptom. J Psychosomatic Obstet Gynaecol. (2006) 27:193–99. doi: 10.1080/01674820600724797
28. Liljegren AGPL, Rotstein S. Reducing vasomotor symptoms with acupuncture in breast cancer patients treated with adjuvant tamoxifen: a randomized controlled trial. Breast Cancer Res Treat. (2012) 135:791–98. doi: 10.1007/s10549-010-1283-3
29. Frisk JKLM, Hammar M. Acupuncture improves health-related quality-of-life (hrqol) and sleep in women with breast cancer and hot flushes. Support Care Cancer. (2011) 20:715–24. doi: 10.1007/s00520-011-1134-8
30. Frisk J, K Llstr MA, Wall N, Hammar M. Long term follow up of acupuncture and hormone therapy on hot flushes and well-being in women with breast cancer - a prospective, randomized multi centre trial. J Int Menopause Soc. (2008) 69(2). doi: 10.1080/13697130801958709
31. Ting B, Ling C, Claire S, Kelly B, Karineh T, Jeff G, et al. Patient-reported outcomes in women with breast cancer enrolled in a dual-center, double-blind, randomized controlled trial assessing the effect of acupuncture in reducing aromatase inhibitor-induced musculoskeletal symptoms. Cancer. (2014) 120(3):381–89. doi: 10.1002/cncr.28352
32. Jeong YCHK, Park SH. Impact of acupuncture on hot flashes in breast cancer patients receiving adjuvant antiestrogen therapy with tamoxifen: a randomized controlled trial. J Integr Complement Med. (2023) 29:241–52. doi: 10.1089/jicm.2022.0636
33. Liu J, Nie G, Li Y, Wen Z, Lu L, Xie L, et al. Nonhormonal hot flash management for breast cancer survivors: a systematic review and network meta-analysis. Evid Based Complement Alternat Med. (2020) 2020:4243175. doi: 10.1155/2020/4243175
34. Chien TJ, Hsu CH, Liu CY, Fang CJ. Effect of acupuncture on hot flush and menopause symptoms in breast cancer- a systematic review and meta-analysis. PloS One. (2017) 12:e180918. doi: 10.1371/journal.pone.0180918
35. Qin W, Tian J, Bai L, Pan X, Yang L, Chen P, et al. Fmri connectivity analysis of acupuncture effects on an amygdala-associated brain network. Mol Pain. (2008) 4:55. doi: 10.1186/1744-8069-4-55
36. Li L, Xu L, Wu J, Dong L, Zhao S, Zheng Q. Comparative efficacy of nonhormonal drugs on menopausal hot flashes. Eur J Clin Pharmacol. (2016) 72:1051–58. doi: 10.1007/s00228-016-2090-5
37. Salehi A, Marzban M, Zadeh AR. Acupuncture for treating hot flashes in breast cancer patients: an updated meta-analysis. Support Care Cancer. (2016) 24:4895–99. doi: 10.1007/s00520-016-3345-5
38. Wheeler SB, Spencer J, Pinheiro LC, Murphy CC, Earp JA, Carey L, et al. Endocrine therapy nonadherence and discontinuation in black and white women. J Natl Cancer Inst. (2019) 111:498–508. doi: 10.1093/jnci/djy136
39. Yu S, Chen B, Xie W, Wang B, Chen A, Ba D, et al. Study on the distribution of tcm syndrome types of concomitant symptoms in patients with breast cancer after endocrine therapy. Guiding J Traditional Chin Med Pharm. (2022) 28:145–49. doi: 10.13862/j.cn43-1446/r.2022.10.035
40. He X, Zhang Z, Ren C, Ma X. Research progress on constitution of traditional chinese medicine. Traditional Chin Med. (2022) 4:686–93. doi: 10.12677/TCM.2022.114098
41. Zheng W, Zhou D, Ji Q, Zhou X, Li J, Zhao J. Correlation between breast cancer incidence and traditional chinese medicine constitution and syndrome differentiation. Chin J Clin Healthcare. (2024) 27:361–65. doi: 10.3969/J.issn.1672-6790.2024.03.017
42. Wang J, Li Y, Li L, Zhang Y, Zheng L, Zhang Y, et al. Genomics study on nine types of tcm constitution. China J Traditional Chin Med Pharm. (2014) 29:3871–73. doi: CNKI:SUN:BXYY.0.2014-12-058
43. Li M. Study on the distribution of tcm constitution types of breast cancer and its correlation with high-risk and prognostic factors[the Master’s Degree]. Shandong: Shandong University of Traditional Chinese Medicine (2020).
44. Shen X, Liu J. Precision medicine and traditional chinese medicine. Chin J Integrated Traditional Western Med. (2018) 38:105–08. doi: 10.7661/j.cjim.20171025.278
45. Ma R, Li Y, Liu S, Zhao W. Acupuncture for cancer-related hiccups: protocol for a systematic review and meta-analysis of randomized controlled trials. Med (Baltimore). (2020) 99:e19973. doi: 10.1097/MD.0000000000019973
46. Zhou X, Zhang J, Jiang L, Zhang S, Gu Y, Tang J, et al. Therapeutic efficacy of acupuncture point stimulation for stomach cancer pain: a systematic review and meta-analysis. Front Neurol. (2024) 15:1334657. doi: 10.3389/fneur.2024.1334657
47. Guo Z, Liang Y, Liu W, Huang B, Zheng H, Cui S, et al. Effectiveness and safety of acupuncture for cancer-related hiccups: a systematic review and meta-analysis. Front Neurol. (2024) 15:1480656. doi: 10.3389/fneur.2024.1480656
48. Leon-Ferre RA, Majithia N, Loprinzi CL. Management of hot flashes in women with breast cancer receiving ovarian function suppression. Cancer Treat Rev. (2017) 52:82–90. doi: 10.1016/j.ctrv.2016.11.012
49. Kaifeng Y, Zhen C, Shaowu C, Huiping L, Lan LI, Hong Z, et al. Research progress on the mechanism of chemotherapy-induced premature ovarian failure and the prevention and treatment with traditional chinese medicine. J Traditional Chin Med Univ Hunan. (2022) 42:332–36. doi: 10.3969/j.issn.1674-070X.2022.02.028
50. Yang M, Bai W. Hormonal changes in women during menopause and related diseases. China Med News. (2013) 24):20.
51. Sun Y, Yang Q, Li Y, Wang W, Wang K, Qin L. Monoamine neurotransmitter theories of menopausal hot flashes. Acta Anatomica Sin. (2019) 50:857–60. doi: 10.16098/j.issn.0529-1356.2019.06.029
52. Yu S, Liu H, Miao M. Molecular mechanism and features of menopausal syndrome treated by chinese medicine. J Cihinese Med. (2012) 27:338–40. doi: 10.16368/j.issn.1674-8999.2012.03.013
53. Ying Y, Fen L, Qiao-li Z, Xin-wen Z, Yong-hui R. Effect of the levels of serum gonadal hormone and plasma calcitonin gene-related peptide on the hot flushes symptom of perimenopausal women. Clin Med China. (2008) 24:1283–85. doi: 10.3760/cma.j.issn.1008-6315.2008.12.039
54. Xu T, Shi M, Han K, Zhao F. The effects of serum e2 level and the hypothalamus 5-ht content by acupuncture on perimenopausal model of rats. J Nanjing Univ Traditional Chin Med. (2010) 26:296–98. doi: 10.14148/j.issn.1672-0482.2010.04.007
55. Hsieh YL, Yang SA, Yang CC, Chou LW. Dry needling at myofascial trigger spots of rabbit skeletal muscles modulates the biochemicals associated with pain, inflammation, and hypoxia. Evid Based Complement Alternat Med. (2012) 2012:342165. doi: 10.1155/2012/342165
56. Fredy DM, Harpin D, Mihardja H. The role of acupuncture for myofascial pain syndrome (mps) in interventional pain management. J Complement Integr Med. (2022) 19:213–17. doi: 10.1515/jcim-2021-0525
57. Xiong J, Zhou X, Luo X, Gong X, Jiang L, Luo Q, et al. Acupuncture therapy on myofascial pain syndrome: a systematic review and meta-analysis. Front Neurol. (2024) 15:1374542. doi: 10.3389/fneur.2024.1374542
58. Zhang Z, Cui X, Liu K, Gao X, Zhou Q, Xi H, et al. Adrenal sympathetic nerve mediated the anti-inflammatory effect of electroacupuncture at st25 acupoint in a rat model of sepsis. Anat Rec (Hoboken). (2023) 306:3178–88. doi: 10.1002/ar.25102
59. Liu S, Wang Z, Su Y, Qi L, Yang W, Fu M, et al. A neuroanatomical basis for electroacupuncture to drive the vagal-adrenal axis. Nature. (2021) 598:641–45. doi: 10.1038/s41586-021-04001-4
60. He XF, Wei JJ, Shou SY, Fang JQ, Jiang YL. Effects of electroacupuncture at 2 and 100 hz on rat type 2 diabetic neuropathic pain and hyperalgesia-related protein expression in the dorsal root ganglion. J Zhejiang Univ Sci B. (2017) 18:239–48. doi: 10.1631/jzus.B1600247
61. Spetz HA, Frisk J, Hammar ML. Acupuncture as treatment of hot flashes and the possible role of calcitonin gene-related peptide. Evid Based Complement Alternat Med. (2012) 2012:579321. doi: 10.1155/2012/579321
62. Karaca Z, Grossman A, Kelestimur F. Investigation of the hypothalamo-pituitary-adrenal (hpa) axis: a contemporary synthesis. Rev Endocr Metab Disord. (2021) 22:179–204. doi: 10.1007/s11154-020-09611-3
63. Gao YH, Wang JY, Qiao LN, Chen SP, Tan LH, Xu QL, et al. Nk cells mediate the cumulative analgesic effect of electroacupuncture in a rat model of neuropathic pain. BMC Complement Altern Med. (2014) 14:316. doi: 10.1186/1472-6882-14-316
64. Chen X, Wang M, Yu K, Xu S, Qiu P, Lyu Z, et al. Chronic stress-induced immune dysregulation in breast cancer: implications of psychosocial factors. J Transl Int Med. (2023) 11:226–33. doi: 10.2478/jtim-2021-0050
65. Wang M, Liu W, Ge J, Liu S. The immunomodulatory mechanisms for acupuncture practice. Front Immunol. (2023) 14:1147718. doi: 10.3389/fimmu.2023.1147718
66. O’Brien KL, Finlay DK. Immunometabolism and natural killer cell responses. Nat Rev Immunol. (2019) 19:282–90. doi: 10.1038/s41577-019-0139-2
67. Watanabe M, Kainuma E, Tomiyama C. Repetitive manual acupuncture increases markers of innate immunity in mice subjected to restraint stress. Acupunct Med. (2015) 33:312–18. doi: 10.1136/acupmed-2014-010660
68. Romero S, Li QS, Orlow I, Gonen M, Su HI, Mao JJ. Genetic predictors to acupuncture response for hot flashes: an exploratory study of breast cancer survivors. Menopause. (2020) 27:913–17. doi: 10.1097/GME.0000000000001545
69. Tekpli X, Lien T, Røssevold AH, Nebdal D, Borgen E, Ohnstad HO, et al. An independent poor-prognosis subtype of breast cancer defined by a distinct tumor immune microenvironment. Nat Commun. (2019) 10:5499. doi: 10.1038/s41467-019-13329-5
70. Zhu D, Gao Y, Chang J, Kong J. Placebo acupuncture devices: considerations for acupuncture research. Evid Based Complement Alternat Med. (2013) 2013:628907. doi: 10.1155/2013/628907
71. Mao JJ, Xie SX, Farrar JT, Stricker CT, Bowman MA, Bruner D, et al. A randomised trial of electro-acupuncture for arthralgia related to aromatase inhibitor use. Eur J Cancer. (2014) 50:267–76. doi: 10.1016/j.ejca.2013.09.022
Keywords: acupuncture, hot flashes, breast cancer, systematic review, meta-analysis
Citation: Zhang G, Gao C, Guo Z, Zhao W, Xu X, Wen H, Li Y, Lin R, Xu N and Cui S (2025) How effective is acupuncture in treating hot flashes in breast cancer patients? A systematic review and meta-analysis. Front. Oncol. 15:1543938. doi: 10.3389/fonc.2025.1543938
Received: 12 December 2024; Accepted: 24 February 2025;
Published: 17 March 2025.
Edited by:
Matteo Ghilli, Pisana University Hospital, ItalyReviewed by:
Xiaoqian Hu, The University of Hong Kong, Hong Kong SAR, ChinaCopyright © 2025 Zhang, Gao, Guo, Zhao, Xu, Wen, Li, Lin, Xu and Cui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaoyang Cui, aGVyYjEwN0AxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.