
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 24 March 2025
Sec. Cancer Immunity and Immunotherapy
Volume 15 - 2025 | https://doi.org/10.3389/fonc.2025.1541760
This article is part of the Research TopicCancer Immunity and RadiotherapyView all 8 articles
Recurrent and metastatic advanced cervical cancer carries a poor prognosis, and there is currently no standardized treatment regimen. Immunotherapy, as an emerging and effective treatment strategy, has attracted significant attention. Socazolimab, a programmed death ligand 1 monoclonal antibody developed in China, is primarily used to treat patients with recurrent or metastatic cervical cancer who have failed or are intolerant to first-line platinum-based chemotherapy, regardless of programmed death ligand 1 expression status. We report the case of a Chinese patient with advanced cervical cancer and multiple metastases, who showed significant tumor regression after receiving Socazolimab treatment. Currently, during ongoing immunotherapy, the tumor continues to demonstrate persistent remission, with no new lesions observed. This case suggests that Socazolimab may offer a promising therapeutic option for patients with metastatic cervical cancer. In this report, the safety profile of Socazolimab was favorable, with no severe or uncontrollable adverse events. This case provides compelling evidence of the antitumor activity and manageable toxicity of Socazolimab in metastatic cervical cancer patients, offering valuable insights for further research and clinical application of the drug.
Cervical cancer is the fourth most common cancer among women worldwide and is also one of the top three cancers affecting women under the age of 45 (1). Standard treatment options for early-stage and locally invasive cervical cancer include radical hysterectomy, radical trachelectomy, and pelvic lymphadenectomy, combined with concurrent chemoradiotherapy (2). For patients with recurrent and metastatic advanced cervical cancer, chemotherapy-based systemic treatment is typically the cornerstone of therapy (3). However, due to the poor prognosis in advanced-stage patients and the significant adverse effects associated with existing therapies, there is an urgent need to identify new treatment options. In recent years, immune checkpoint inhibitors (ICIs) have rapidly emerged as a leading form of cancer immunotherapy and have demonstrated significant clinical efficacy (4, 5). In particular, ICIs targeting programmed death-1 (PD-1), programmed death-ligand 1(PD-L1), and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) have become some of the most promising therapeutic approaches in oncology (6–8).
Socazolimab is a monoclonal antibody targeting PD-L1 that has been included in the breakthrough therapy drug review process by the China National Medical Products Administration (NMPA). It is primarily used to treat patients with recurrent or metastatic cervical cancer who have failed or are intolerant to first-line platinum-based chemotherapy, regardless of PD-L1 expression status (9). In 2024, the Chinese Society of Clinical Oncology (CSCO) included Socazolimab (PD-L1) in the ‘Cervical Cancer Diagnosis and Treatment Guidelines 2024,’ recommending it as a ‘systemic therapy for recurrent or metastatic cervical cancer (Level 2B).’ This article presents the case of a patient with recurrent and metastatic cervical cancer who was treated with Socazolimab.
In April 2008, a 46-year-old married and parous Chinese woman with no significant medical history presented with unexplained vaginal bleeding. After undergoing relevant examinations at a local hospital, she was diagnosed with cervical cancer. In May, she underwent radical total hysterectomy, bilateral salpingo-oophorectomy, and pelvic lymphadenectomy. Postoperative pathology revealed squamous cell carcinoma of the cervix with minimal local invasion (stage I-II), and no lymph node or distant organ metastases were observed. The patient did not receive postoperative chemoradiotherapy and did not have regular follow-up examinations.
In August 2019, the patient sought medical attention due to lower limb edema. Chest and abdominal CT scans revealed metastasis to the left iliac vascular region, as well as lymph node involvement in the left iliac vessels and mesorectum. In September 2019, the patient started a treatment regimen consisting of six cycles of paclitaxel plus carboplatin chemotherapy, combined with apatinib. After the fourth cycle, evaluation showed partial remission (PR). However, following the fifth and sixth cycles, a follow-up CT scan revealed no significant reduction in the tumor, and the disease was classified as stable (SD). From March to April 2020, the patient underwent two cycles of albumin-bound paclitaxel, cisplatin, and bevacizumab combination chemotherapy and targeted therapy. A CT scan in April 2020 showed no changes in the tumor.In July 2020, the patient received radiotherapy with 6MV X-ray radiation and Volumetric Modulated Arc Therapy (VMAT). The prescribed doses were as follows: PTV-High 95% volume: 47.5Gy/25 fractions (F); PTV-para 95% volume: 45Gy/25F; PGTV (pelvic wall recurrence site) 95% volume: 61.25Gy/25F. The treatment was completed successfully, and follow-up examinations showed complete remission (CR) of the recurrent lesions (Figure 1).
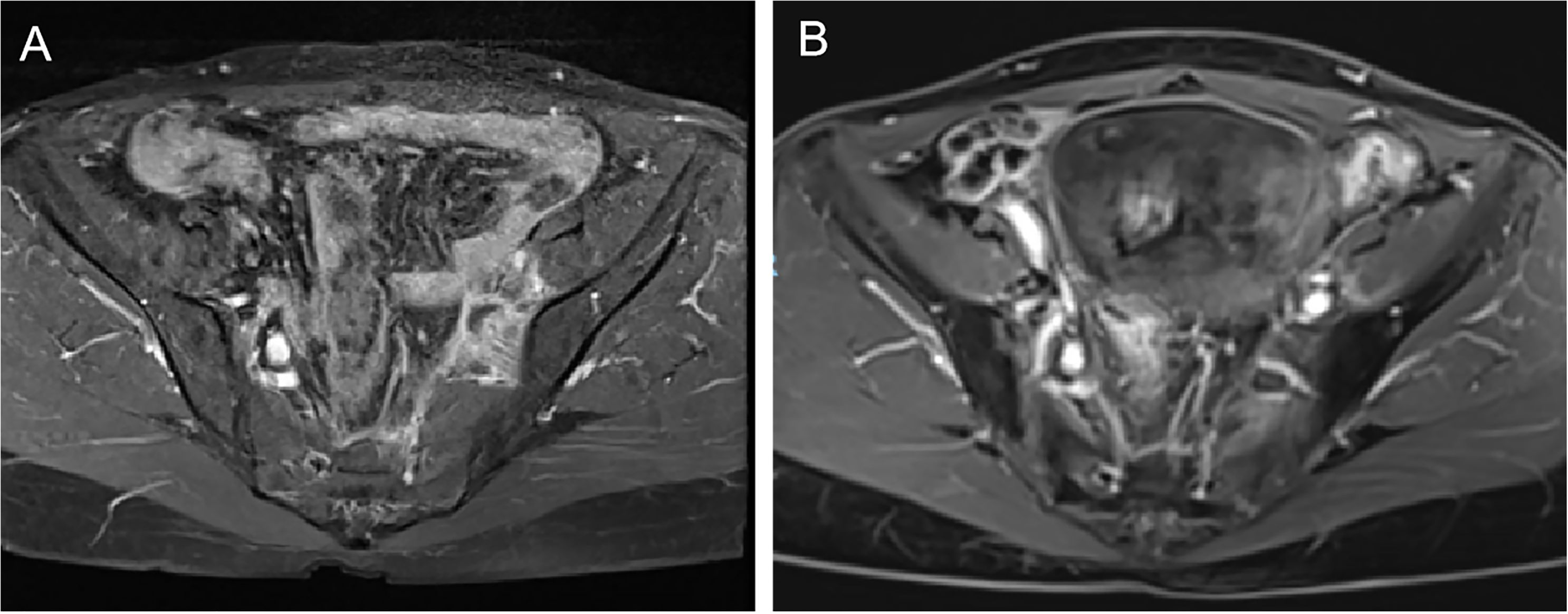
Figure 1. (A) pre-radiotherapy imaging results for the left iliac vascular region. (B) post-radiotherapy imaging results for the left iliac vascular region.
In August 2023, the patient developed an unexplained dry cough and subsequently underwent PET-CT and contrast-enhanced CT at the hospital. Imaging revealed new metastatic lesions in the right upper lung lobe, as well as multiple lymph node metastases in the mediastinum, right lung hilum, and other regions. Severe external compression and critical stenosis of the right main bronchus were also observed. At that time, the patient declined treatment due to personal reasons. However, in May 2024, she returned to the hospital with worsening symptoms. Chest contrast-enhanced CT showed further progression of the metastatic lesion in the right upper lung lobe, along with enlargement of the mediastinal and right hilar lymph nodes, which had merged with the tumor. Additionally, a newly enlarged right supraclavicular lymph node was observed, suspected to be metastatic. The enlarged mediastinal and right hilar lymph nodes were also found to be invading the superior vena cava.
The patient underwent a cervical lymph node (right supraclavicular lymph node) biopsy in May 2024, and pathology confirmed metastatic squamous cell carcinoma. Immunohistochemical(IHC) analysis revealed the following results: p16 (diffusely strong +), MSH2 (+), MSH6 (+), PMS2 (slightly weak +), MLH1 (+), Her-2 (0), PD-L1 (BP6099, TC score: 80%), PD-L1 Neg (–), p63 (+), CK5/6 (+), p40 (+), CD5 (–), CD117 (–). In situ hybridization for EBER was negative (Figure 2). The patient was diagnosed with multiple metastases (lung, mediastinum, and cervical lymph nodes) following pelvic recurrence of cervical cancer after chemoradiotherapy.
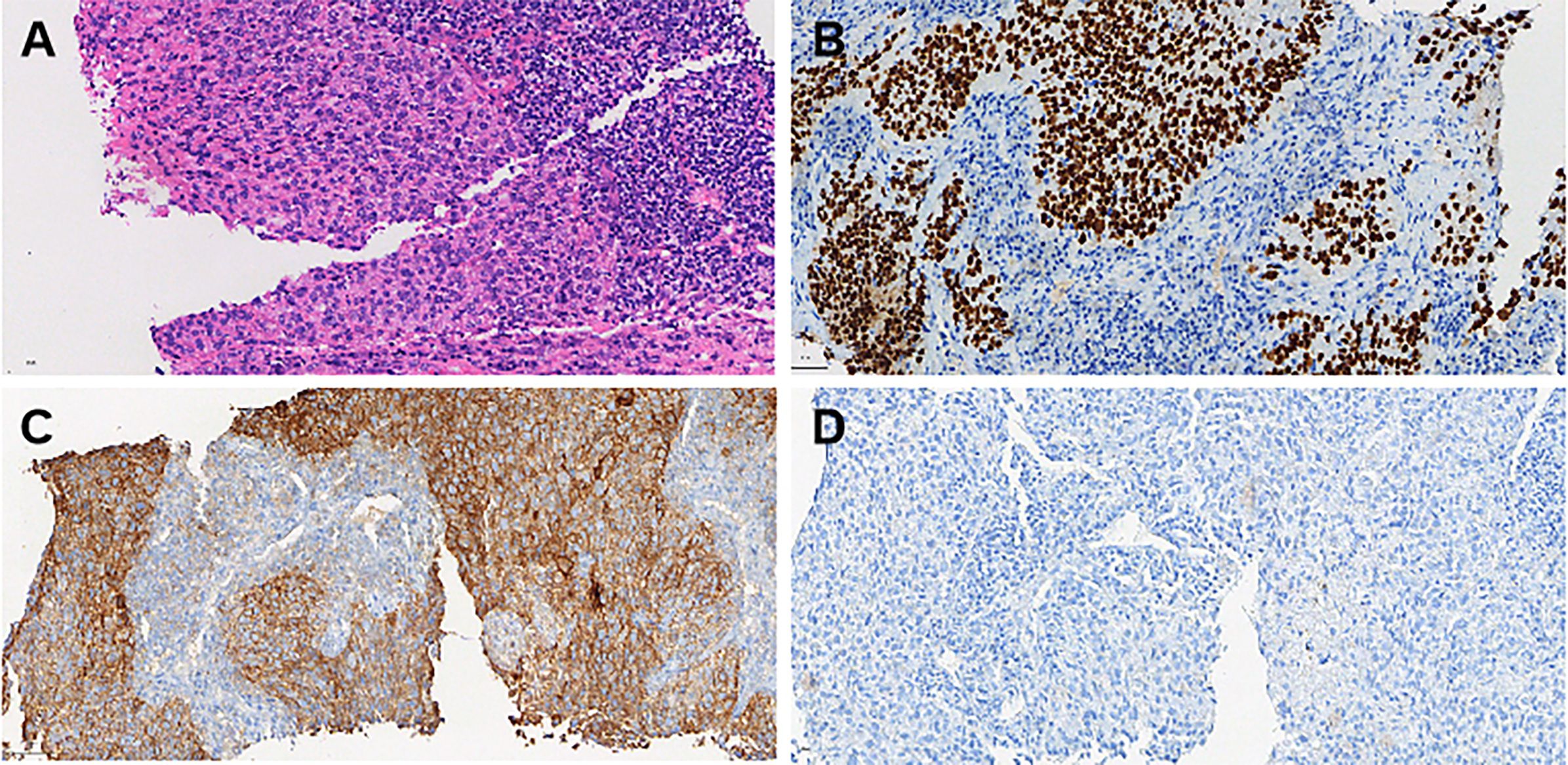
Figure 2. Histopathological and immunohistochemical findings from the cervical lymph node biopsy (May 2024). (A) Hematoxylin and eosin (H&E) staining: The metastatic squamous cell carcinoma (SCC) tissue exhibits pleomorphic tumor cells with hyperchromatic nuclei and irregular architecture. The tumor is arranged in solid nests with prominent keratinization. Scale bar = 50 μm (magnification: 40×). (B) p40 immunohistochemical staining: Strong and diffuse nuclear positivity for p40 confirms the squamous cell carcinoma phenotype of the metastatic lesion. Scale bar = 50 μm (magnification: 40×). (C) PD-L1 (22C3) immunohistochemical staining: Tumor cells demonstrate strong membranous PD-L1 expression with a tumor proportion score (TC) of 80%, indicating potential responsiveness to immune checkpoint blockade therapy. Scale bar = 50 μm (magnification: 40×). (D) PD-L1 negative control: Negative control staining performed under the same conditions shows the absence of PD-L1 expression in non-tumoral regions, confirming staining specificity. Scale bar = 50 μm (magnification: 40×).
On May 20, 2024, the patient began receiving Socazolimab 200 mg every three weeks as part of her immunotherapy regimen. The treatment proceeded smoothly, with no significant immune-related adverse events observed. After completing the planned course of immunotherapy, a follow-up CT scan in July 2024 (Figure 3) revealed significant improvement in the consolidation of the right upper lung lobe, with the bronchus largely patent. The metastatic lymph nodes in the mediastinum, right supraclavicular fossa, and right lung hilum had also significantly reduced in size, and there was no evidence of invasion into the superior vena cava.
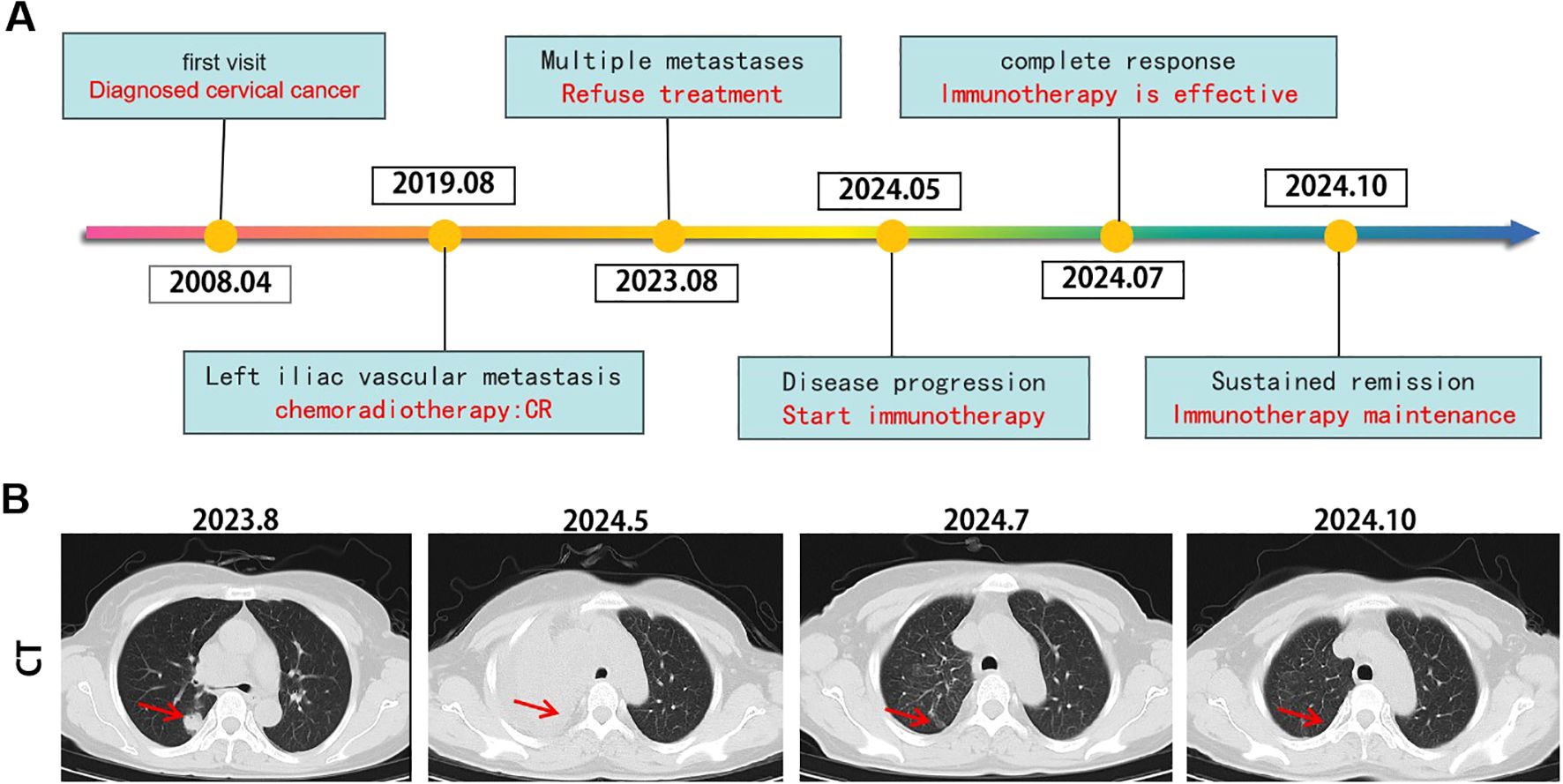
Figure 3. Tumor responses after therapy. (A) Timeline of disease status and corresponding treatment regimens. (B) Time line of treatments of the patient and changes in CT of lung during treatments.
Additionally, the patient’s squamous cell carcinoma (SCC) antigen levels (Figure 4). significantly decreased since the initiation of immunotherapy, and other tumor markers, including carbohydrate antigen 125 (CA125), neuron-specific enolase (NSE), and cytokeratin 19 fragment (CYFRA 21-1), returned to normal levels (Figure 5). Notably, CA125 showed a transient increase during the early stages of immunotherapy. Relevant studies suggest that elevated CA125 is not only associated with malignant tumors but can also serve as an auxiliary diagnostic marker for certain inflammatory diseases, potentially related to the systemic immune response triggered by immunotherapy (10).
Throughout the course of immunotherapy, the patient did not experience any significant immune-related adverse events. A follow-up CT scan and other examinations in October 2024 showed further improvement in the consolidation of the right upper lung lobe. The previously enlarged metastatic lymph nodes in the mediastinum, right supraclavicular region, and right hilum remained stable compared to the July 2024 scan, with no new lesions observed. As of January 20, 2025, the patient continues to receive outpatient immunotherapy.
Immunotherapy, by activating the patient’s immune system and enhancing the anti-tumor immune response, has demonstrated significant therapeutic potential (8). Among the various strategies, blocking the PD-1/PD-L1 pathway is currently the primary therapeutic approach. By disrupting the interaction between PD-1 and PD-L1, it inhibits their suppressive effect on T cells and restores the anti-tumor activity of effector T cells, thereby effectively eliminating tumor cells. This mechanism provides a new avenue for the treatment of various cancers (11–13).
For patients with recurrent or metastatic cervical cancer, particularly those whose disease progresses after first-line chemotherapy and have limited treatment options, immunotherapy holds particular promise. Currently, PD-1/PD-L1 immunotherapy is primarily used as a second-line or subsequent treatment option after the failure of first-line therapy (14). Immunotherapy has shown enhanced efficacy, particularly in patients with high PD-L1 expression (15, 16). In this case report, we observed a favorable response in a patient with recurrent and metastatic cervical cancer who had high PD-L1 expression and received Socazolimab immunotherapy. The patient’s metastatic lesions remained stable and continued to shrink, with no new lesions observed. Immune-related adverse events were manageable. These results suggest the potential of Socazolimab in the treatment of advanced recurrent metastatic cervical cancer.
Socazolimab demonstrates a therapeutic advantage over other drugs due to its dual anti-tumor mechanism: it exerts its anti-tumor effect both through antibody-dependent cellular cytotoxicity (ADCC) and by inhibiting programmed death ligand 1 (PD-L1) (9). More importantly, existing studies (NCT03676959) indicate that Socazolimab is effective in patients with varying levels of PD-L1 expression, providing broader opportunities for immunotherapy in patients with recurrent, metastatic advanced cervical cancer.
In this case, the patient showed sustained lesion remission after receiving Socazolimab treatment. While the significant therapeutic effect observed in this patient may be related to the high expression of PD-L1, the specific mechanism of action of the drug still requires further exploration (17). Although this case demonstrated favorable efficacy, we were unable to assess the effect of Socazolimab in a patient with PD-L1 negative expression.
Overall, Socazolimab immunotherapy has shown sustained tumor regression and manageable toxicity in advanced cervical cancer, representing a promising advancement. However, further research is needed to explore the potential mechanisms of the drug’s action and to develop standardized second-line and subsequent treatment regimens. This will provide more treatment options for patients with advanced cervical cancer and potentially improve survival outcomes.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
The studies involving humans were approved by Shenzhen People’s hospital institutional and research ethics committee. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from primarily isolated as part of your previous study for which ethical approval was obtained. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
ZX: Conceptualization, Data curation, Formal Analysis, Writing – original draft, Writing – review & editing. MW: Conceptualization, Supervision, Validation, Writing – review & editing. LG: Data curation, Methodology, Supervision, Writing – review & editing. GL: Supervision, Writing – review & editing. YCZ: Conceptualization, Data curation, Formal Analysis, Writing – original draft, Writing – review & editing. YYZ: Supervision, Validation, Writing – review & editing. ZL: Funding acquisition, Supervision, Validation, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. The work described in this paper was supported by the Science, Technology & Innovation Commission of Shenzhen Municipality (Nos.JCYJ20240813104127037).
We would like to thank the patient for her participation in this case report and her consent to publish her data. We also acknowledge the support of the Department of Radiotherapy at Shenzhen People’s Hospital for providing the necessary medical resources and infrastructure. This work was supported by the Science, Technology & Innovation Commission of Shenzhen Municipality.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Gennigens C, Jerusalem G, Lapaille L, De Cuypere M, Streel S, Kridelka F, et al. Recurrent or primary metastatic cervical cancer: Current and future treatments. ESMO Open. (2022) 7:100579. doi: 10.1016/j.esmoop.2022.100579
3. Abu-Rustum NR, Yashar CM, Arend R, Barber E, Bradley K, Brooks R, et al. NCCN guidelines® insights: Cervical cancer, version 1.2024. J Natl Compr Canc Netw. (2023) 21:1224–33. doi: 10.6004/jnccn.2023.0062
4. Dyer BA, Zamarin D, Eskandar RN, Mayadev JM. Role of immunotherapy in the management of locally advanced and recurrent/metastatic cervical cancer. J Natl Compr Canc Netw. (2019) 17:91–7. doi: 10.6004/jnccn.2018.7108
5. Ferrall L, Lin KY, Roden RBS, Hung CF, Wu TC. Cervical cancer immunotherapy: Facts and hopes. Clin Cancer Res. (2021) 27:4953–73. doi: 10.1158/1078-0432.CCR-20-2833
6. Li K, Chen J, Hu Y, Wang YZ, Shen Y, Chen G, et al. Neoadjuvant chemotherapy plus camrelizumab for locally advanced cervical cancer (NACI study): A multicentre, single-arm, phase 2 trial. Lancet Oncol. (2024) 25:76–85. doi: 10.1016/S1470-2045(23)00531-4
7. Xie Y, Kong W, Zhao X, Zhang H, Luo D, Chen S. Immune checkpoint inhibitors in cervical cancer: Current status and research progress. Front Oncol. (2022) 12:984896. doi: 10.3389/fonc.2022.984896
8. Naimi A, Mohammed RN, Raji A, Chupradit S, Yumashev AV, Suksatan W, et al. Tumor immunotherapies by immune checkpoint inhibitors (ICIs); the pros and cons. Cell Commun Signal. (2022) 20:44. doi: 10.1186/s12964-022-00854-y
9. An J, Tang J, Li BX, Xiong H, Qiu H, Luo L, et al. Efficacy and safety of the anti–PD-L1 mAb socazolimab for recurrent or metastatic cervical cancer: a phase I dose-escalation and expansion study. Clin Cancer Res. (2022) 28:5098–106. doi: 10.1158/1078-0432.CCR-22-1280
10. Zhao P, Yu Q, Zhang A, He F, Xu S, Chen L. Serum CA-125 for the diagnosis of pulmonary tuberculosis: A systematic review and meta-analysis. BMC Infect Dis. (2021) 21:1091. doi: 10.1186/s12879-021-06772-7
11. Tang Q, Chen Y, Li X, Long S, Shi Y, Yu Y, et al. The role of PD-1/PD-L1 and application of immune-checkpoint inhibitors in human cancers. Front Immunol. (2022) 13:964442. doi: 10.3389/fimmu.2022.964442
12. Zhang H, Liu L, Liu J, Dang P, Hu S, Yuan W, et al. Roles of tumor-associated macrophages in anti-PD-1/PD-L1 immunotherapy for solid cancers. Mol Cancer. (2023) 22:58. doi: 10.1186/s12943-023-01725-x
13. Hudson K, Cross N, Jordan-Mahy N, Leyland R. The extrinsic and intrinsic roles of PD-L1 and its receptor PD-1: Implications for immunotherapy treatment. Front Immunol. (2020) 11:568931. doi: 10.3389/fimmu.2020.568931
14. Kokka F, Bryant A, Olaitan A, Brockbank E, Powell M, Oram D. Hysterectomy with radiotherapy or chemotherapy or both for women with locally advanced cervical cancer. Cochrane Database Syst Rev. (2022) 8:CD010260. doi: 10.1002/14651858.CD010260.pub3
15. Lorusso D, Xiang Y, Hasegawa K, Scambia G, Leiva M, Ramos-Elias P, et al. Pembrolizumab or placebo with chemoradiotherapy followed by pembrolizumab or placebo for newly diagnosed, high-risk, locally advanced cervical cancer (ENGOT-cx11/GOG-3047/KEYNOTE-A18): A randomised, double-blind, phase 3 clinical trial. Lancet. (2024) 403:1341–50. doi: 10.1016/S0140-6736(24)00317-9
16. Chung HC, Ros W, Delord JP, Perets R, Italiano A, Shapira-Frommer R, et al. Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: Results from the phase II KEYNOTE-158 study. J Clin Oncol. (2019) 37:1470–8. doi: 10.1200/JCO.18.01265
Keywords: advanced cervical cancer, immune checkpoint inhibitor, PD-L1, Socazolimab, case report
Citation: Xu Z, Wang M, Gong L, Luo G, Zhang Y, Zhou Y and Li Z (2025) Promising outcomes of the PD-L1 inhibitor Socazolimab in recurrent and metastatic cervical cancer: a case report. Front. Oncol. 15:1541760. doi: 10.3389/fonc.2025.1541760
Received: 08 December 2024; Accepted: 10 March 2025;
Published: 24 March 2025.
Edited by:
Giuseppe Giaccone, Cornell University, United StatesReviewed by:
Amauri Silva Justo Junior, Brazilian Biosciences National Laboratory (LNBio), BrazilCopyright © 2025 Xu, Wang, Gong, Luo, Zhang, Zhou and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yucong Zhang, c3pybXpoYW5nM0AxNjMuY29t; Yayan Zhou, aGVsbGVuemhvdTc3QDE2My5jb20=; Zihuang Li, bGkuemlodWFuZ0Bzemhvc3BpdGFsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.