
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol., 14 April 2025
Sec. Cancer Imaging and Image-directed Interventions
Volume 15 - 2025 | https://doi.org/10.3389/fonc.2025.1539911
This article is part of the Research TopicMethods and Applications of Tumour Metabolic Imaging in the Preclinical and Clinical SettingView all 7 articles
Dynamic imaging has obtained remarkable achievements among a variety of malignant tumors due to the development of multiple simplified scanning protocols and the emergence of whole-body PET/CT scanners, which promote wider application of dynamic PET/CT. In this paper, we mainly review the acquisition protocols of dynamic imaging, related kinetic parameters, advantages and the application of dynamic PET/CT imaging in malignant tumors, including lung cancer, hepatocellular carcinoma, breast cancer, pancreatic carcinoma, prostate neoplasm, and cancer of head and neck. Dynamic PET/CT imaging is increasingly being applied the diagnosis, staging, efficacy monitoring, and prognosis evaluation of malignant tumors. Although standardized uptake value is the most frequently employed semi-quantitative assessment index for static imaging, it is susceptible to several factors, thus cannot be used to evaluate the tracer kinetic information of the lesion. Dynamic PET/CT imaging can be used to achieve continuous assessment of the metabolic activity of a lesion over a certain time frame through quantitative measurement of kinetic parameters, such as the net uptake rate constant. Compared with conventional static imaging, dynamic scanning can be used for the early estimation of minute metabolic changes in tumors. Besides, dynamic scanning can directly and effectively reflect tracer uptake. Nevertheless, the intricacy of parameter analysis and the lengthy scanning time related to dynamic scanning limits its clinical application. Dynamic imaging has obtained remarkable achievements among a variety of malignant tumors due to the development of multiple simplified scanning protocols and the emergence of whole-body PET/CT scanners, which promote wider application of dynamic PET/CT. In this paper, we mainly review the acquisition protocols of dynamic imaging, related kinetic parameters, advantages and the application of dynamic PET/CT imaging in malignant tumors, including lung cancer, hepatocellular carcinoma, breast cancer, pancreatic carcinoma, prostate neoplasm, and cancer of head and neck.
Positron emission tomography/computed tomography (PET/CT) is a molecular imaging technique widely used to identify and analyze physiological, pathological, biochemical, and metabolic alterations in human tissues through radiolabeled tracers. PET/CT can be used to diagnose and evaluate various diseases. Although standardized uptake value (SUV) is a widely used parameter to measure the amount of tracer uptake in both healthy and diseased tissues, it is influenced by several factors, such as physiological factors (body weight, blood glucose levels, and respiratory movement of the patient), procedural factors (injection dose, imaging time, and region of interest (ROI) outlining), and physical factors (partial volume effect, acquisition mode) (1). The degree of glucose metabolism in tumors correlates with factors such as histological type, tumor grade and size. For instance, the SUVmax of squamous carcinoma, poorly differentiated adenocarcinoma, small cell carcinoma and large cell carcinoma was significantly increased, while the SUVmax of well-differentiated adenocarcinoma, carcinoma in situ and minimally invasive carcinoma was only mildly increased or not significantly increased. Even for lung cancers of the same histologic type, SUVmax was only mildly increased in low-grade and significantly increased in high-grade. In addition, due to the influence of spatial resolution, the increase in SUV of lung cancer <1 cm is not obvious (2, 3). Furthermore, the problem of false positives is still widespread, and active infectious lesions such as pneumonia and tuberculosis can be characterized by a marked increase in SUVmax (3). This is due to the fact that 18F-FDG is not a tumor-specific imaging agent, and the SUV value is the cumulative activity value of 18F-FDG (4), therefore, SUV is unable to accurately distinguish between tumors, inflammation and normal physiological metabolism.
Dynamic PET (dPET) can reflect the physiological and metabolic information of the tumor from the time of the injection of the imaging agent. This is achieved by extracting kinetic parameters through kinetic modeling, including the uptake rate constant Ki, the metabolic rate of FDG (MRFDG), the time activity curves (TACs), and other parameters. Quantitative assessment of the images can be achieved using dPET by avoiding the influence of various factors, such as body mass index and injection-imaging time. Nonetheless, some factors such as excessive noise, the requirement for arterial blood collection as an input function (5), and long scanning times limit the clinical application of dPET. Conventional PET scanners have a limited axial of view (aFOV) (about 25 cm), restricting dPET imaging of a single organ away from the blood pool, such as the lower abdomen, pelvis, and extremities. The recently established long-axis field-of-view whole-body PET systems have enhanced signal-to-noise ratios by utilizing 3D acquisition modes, high-efficiency bismuth germanate detector crystals, and lengthening the length of the system in the axial direction to capture more detection counts. Additionally, the advanced PET systems enhance spatial resolution by transitioning from large conventional photomultiplier tubes to compact solid-state silicon photomultipliers. The optimization of time-of-flight techniques and image reconstruction techniques, such as point diffusion, enhances the precise positioning of the annihilation point on the line of response, further improving the temporal resolution and reducing the image noise. These developments may facilitate pharmacokinetic studies and provide a foundation for the application of novel imaging agents to improve the acceptance of dynamic imaging in the clinical setting. This review aimed to describe the acquisition modalities of dPET and the relevant kinetic parameters, discuss the advantages of dynamic imaging, and provide an overview of the application of dynamic imaging in malignant tumors.
dPET acquisition captures the spatial distribution of the captured radiotracer changes over time. The dynamic scanning in the aFOV of conventional PET/CT 15 to 25 cm is mainly performed in two ways: step-and-shoot scan mode (SS) and continuous bed motion (CBM). In SS, the number of beds is fixed, and scans must be selected to fit within these beds, with adjacent beds having overlapping sections that can cause partial volume effects. In contrast, CBM does not have fixed beds, allowing for customizable scanning ranges. The absence of bed overlap reduces artifacts and enhances patient comfort, leading to shorter acquisition times and improved efficiency (6).
Conventional PET scanners cannot concurrently capture dynamic data at the far end of the body because of the limits of the short-axis field of view. Therefore, increased axial coverage in a whole-body PET scanner may achieve kinetic analysis on lesions outside of the traditional aFOV. This eliminates time gaps and improves sensitivity, thus eliminating the need for arterial blood collection, which can lead to smaller doses of medication. A new generation of PET scanners entered the market in late 2018, leveraging the most recent advancements in electronics and materials research to attain scanning fields of 1 to 2 meters. These scanners can complete a whole-body acquisition in less than 60 seconds, providing real-time insight into pathophysiologic processes (Figure 1).
SUV is the most commonly used quantitative indicator of radiopharmaceutical uptake. SUV is a simple semi-quantitative analytical technique obtained by sketching the concentration of activity in a lesion after a period of rest from injected radiopharmaceuticals. Ki images and DV images that have been kinetically modeled have better contrast compared to SUV images. Compartmental models are frequently used in the clinic for the evaluation of kinetic models, and the most commonly used compartmental models in PET are the two-tissue compartment model and the three-tissue compartment model, which describes the exchange of radiopharmaceuticals between the blood and tissue compartments, where the four transport rates are represented by the meanings of K1 related to the inflow of tracer from the blood compartment into the tissue compartments, K2 related to the efflux, K3 reflecting the rate of phosphorylation, and K4 reflects the rate of dephosphorylation. Whereas the parameter that allows the estimation of partial blood volume, also known as vascular density (Vb). Vb and K1 are correlated and are usually higher than the rate of phosphorylation(K3).This model differs from the model that does not consider K4 and Vb, as proposed by Sokoloff et al. The absence of K4 and Vb results in different values of K1 and K3, as K1 is dependent on Vb, whereas K3 is dependent on K4. In addition, the dephosphorylation rate (K4) of 18F-FDG may be low but cannot be ignored. Compartmental models have the advantage of directly visualizing different kinetic parameters, but it is computationally intensive and sensitive to noise. As an alternative to compartmental modeling, the Patlak model is a linear graphical method that can approximate Ki using the slope of the graphical curves of the blood input function and the tissue time-activity curve (TAC), and obtains intercepted images related to the volume fraction and the volume of distribution. For parametric imaging, the Patlak model has the advantages of computational efficiency and noise robustness. The associated PET kinetic modeling and parametric imaging flow is shown in Figure 2.
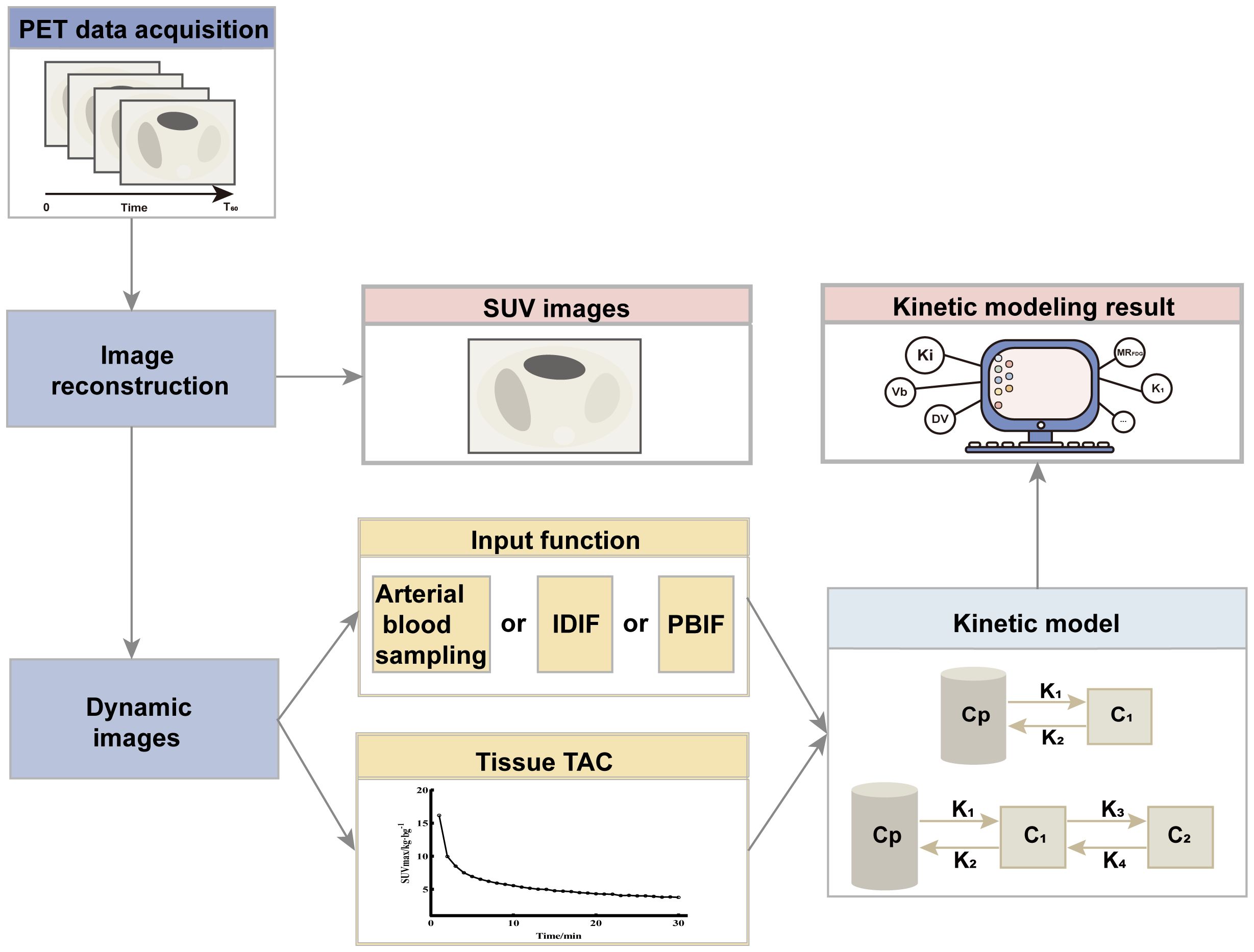
Figure 2. Flowchart of PET kinetic modeling and parametric imaging. PET images are acquired over a period of time (generally 0-60 min), and the 60th min data are reconstructed to obtain SUV images. Further all the data are reconstructed to get the dynamic image. For each image voxel or region of interest, the TAC is extracted from the dynamic sequence, and kinetic parameters (Ki, Vb, and MRFDG, etc.) are estimated using kinetic models and input functions. The input function can be measured invasively by arterial blood sampling, derived non-invasively from the dynamic images (Image-derived input function, IDIF) or a population-based input function (PBIF). Evaluation of kinetic modeling can be based on a two-tissue compartment model or a three-tissue compartment model. Cp is the plasma compartment; C1 and C2 are tissue compartments.
Ki represents the uptake rate of radiopharmaceuticals into tissues and is a quantitative measure of metabolism from the Patlak slope. Ki can be determined using dynamic imaging to acquire the TAC and artery input function (AIF). Although SUV and Ki are not similar, they exhibit a correlation. SUV measures total activity (both metabolic and nonmetabolic) in a lesion, whereas Patlak analysis separates metabolic and non-metabolic activity and determines only metabolic uptake. Therefore, Ki is considered to be a more accurate indicator of glucose metabolic rate than SUV. Besides, Ki has a greater level of sensitivity than SUV images and thus has better sensitivity (from 92.5% to 95%) and accuracy (from 90.24% to 95.12%) in diagnosing malignant tumors (7) and potentially enhanced specificity. Fotis Kotasidis et al. conducted multichannel whole-body 18F-FDG PET parametric imaging in 8 patients with staged lung and liver lesions. They found that three malignant liver lesions, confirmed by contrast-enhanced CT, and one biopsy-confirmed hepatic malignant lesion, were not visible on static SUV or Patlak images. However, these lesions were clearly identified on K1 and K2 images, and the Ki images enhanced lesion detectability (8). Oncology Patlak Ki imaging complements standard SUV imaging to achieve equal or higher lesion detection rates, especially in tissue organs with a rich blood supply (e.g., liver), where Ki images significantly reduce background uptake, resulting in an increased tumor-to- background ratio(TBR), which makes it easier to detect lesions (9). Moreover, due to the higher TBR, Ki images are more accurate in outlining the radiotherapy target area and volume of interest. Ki images have similar (or lower) quality compared to SUV images. Also, significant motion artifacts may be apparent in Ki images during prolonged dynamic scanning.
In addition, the time it takes for tracers to reach different organs and lesions can vary significantly. Long axial field-of-view PET/CT, which does not require continuous bed movement, allows for precise capture of tracer pharmacokinetics in major organs and most lesions, offering high temporal resolution (10). Christos Sachpekidis et al. (11) used a two-tissue model for 38 cases of prostate cancer patients for whole-body dynamic imaging and found significantly higher parameter K3 values in prostate lesions and parotid glands than in the liver and spleen, reflecting higher tracer binding and internalization rates in diseased tissues. Further, rapid temporal sampling provides the opportunity to capture blood volume or blood flow (perfusion), which are potential biomarkers for predicting treatment response or survival. Data indicates that the mid-term dynamic 18F-FDG PET/CT Patlak parameter Kimax can predict the prognosis of patients with diffuse large B-cell lymphoma and that predictive modeling by Kimax and mid-term response to treatment permits accurate stratification of prognostic risk in diffuse large B-cell lymphoma (12).
MRFDG and Distribution volume of free FDG(DVFDG) are quantitative metrics that describe parametric images. MRFDG and DVFDG are calculated by fitting a linear regression function to the time-activity data and each pixel based on dPET data. The slope of the image indicates the metabolic rate of FDG entering the tissue based on the contrasting effect on the surrounding tissue. This information can be utilized to identify malignant lesions and determine the placement of volumes of interests (VOIs). The intercept of the image represents DVFDG, which can be used for better anatomical localization of the lesion. André H Dias et al. performed dynamic whole-body PET/CT scans of 103 patients using long axial field-of-view PET/CT and compared static whole-body 18F-FDG PET/CT with multiparametric Patlak images and found that MRFDG images can reduce false-positive rate, identify false-positive lesions misdiagnosed by the SUV images. They also showed that MRFDG images provide a higher TBR and contrast-to-noise ratio(CNR) (13). Similarly, Pedersen et al. (14) performed whole-body dynamic imaging of patients with locally advanced breast cancer and found that MRFDG images had better lesion visibility and 2.28 times higher TBR than SUV images, which effectively improved the detection rate of locally advanced breast cancer lesions. DVFDG images can identify small SUV artifact foci and improve the specificity of PET/CT. DVFDG images reflect free FDG, whereas MRFDG images reflect metabolic FDG. SUV images are equivalent to the “sum” of MRFDG and DVFDG images. Therefore, artifactual foci are usually not detected on MRFDG images but can be detected on DVFDG images and SUV images. In terms of benign and malignant differentiation, MRFDG and DVFDG may be robust indicators for identifying benign and malignant lung nodules. It has been demonstrated that most malignant lung nodules exhibit high uptake on MRFDG and SUV, while benign lesions exhibit low uptake on MRFDG but high uptake on DVFDG (15). Moreover, factors such as different pathological types, lesion sizes and methods of lesion delineation can influence MRFDG results (16). In a direct comparison of whole-body PET scans from 45 patients, Wu et al. (17) found that the improved image quality of MRFDG did not necessarily result in significant differences between benign and malignant lesions. This may be because MRFDG is particularly useful for lesions surrounded by high background activity, but it provides limited additional information for lesions with low uptake and low background activity.
Fractal dimension (FD) is used to measure the heterogeneity of the VOI in the non-compartmental model- FD can be determined by analyzing the time-activity data of each individual voxel within the VOI. The FD number ranges from 0 to 2, suggesting either a deterministic or chaotic distribution of tracer activity. A higher FD value indicates a more varied and uneven distribution of the tracer activity (18). Therefore, the FD value reflects the degree of benignity or malignancy of the tumor(malignant lesions have higher FD values than benign lesions). However, parametric images do not offer substantial additional insights into tumor heterogeneity when compared to static images. Meijer et al. delineated lesion volumes on both static and parametric images in 35 non-small cell lung cancer (NSCLC) cases for radiation treatment planning. They subsequently compared the volume measurements obtained from PET images with pathological volumes, as well as the volumes associated with different NSCLC tissue subtypes. The results showed that the pathological volume was larger than the PET volume, the glucose metabolism rate, 18F-FDG phosphorylation rate and heterogeneity of VB were lower in the adenocarcinoma group than in the squamous carcinoma group, and there was no difference in the parametric images and static images of NSCLC (19). Similarly, Tixier et al (20) extracted heterogeneity parameters from static and parametric images of 20 NSCLC patients and showed that the differences between static and parametric images in quantitative measures of intra-tumor heterogeneity were mainly attributed to variations in image features and noise, rather than to substantial differences in the spatial distribution of uptake within the actual tumor.
Kinetic modeling analysis of 18F-FDG using PET/CT requires AIF based on TAC image-derived AIF. Although arterial blood collection is the gold standard for obtaining AIF (21, 22), it is not the clinically preferred method due to its invasive nature during the procedure (23). Image-derived input function (IDIF) is currently the most commonly used input function. IDIF allows the selection of a dynamic scan of the initial blood pool (heart or aorta) in place of arterial blood collection. The ascending aorta is widely chosen to obtain IDIF because of the strong correlation between the results of the ascending aorta and arterial sampling. Besides, a relatively large ROI can be determined when using an ascending aorta with better statistical properties and less interference from adjacent myocardial spillovers. However, the accuracy of using ascending aorta is affected by body motion and partial volume effects (23). Zhu et al. used IDIF from small vascular regions (carotid artery) as a merging kernel of a priori information, stabilizing the performance of the simultaneous estimation of the input function method and significantly enhancing the accuracy of evaluating early time points of the input function (24). Besides IDIF, a template can be generated using a population-based input function (PBIF). PBIF can be obtained by averaging the arterial blood data collected from the subjects, predetermining the model parameters, and applying a scaling factor for normalization for each subject. Mika et al. compared PBIFs normalized by different scaling factors and found good accuracy and precision using a scaling factor consisting of the area under the curve of the time window and the initial volume of distribution of the developer (22). However, using a single model with a single input function from a single blood pool can lead to inaccurate kinetic parameter estimates for organs with dual blood supplies (liver and lungs) (25). Wang et al. proposed that dynamics can be modeled using an optimally derived dual-blood input function (DBIF). Compared with the traditional single-blood input function (SBIF) and PBIF, DBIF can significantly improve the fitting of liver time-activity curves without the need to invade the arteries for blood sampling or delineate additional regions of interest. Besides, the optimized model demonstrated that FDG blood-hepatic transit rate Ki is significantly correlated with the histological grading of liver inflammation, making it suitable for individual patients (26). Similarly, Yiran Wang et al. used high-temporal resolution dynamic imaging and demonstrated that the DBIF model can be used to better quantify the kinetic parameters and improve the quality of the fit of the efficiency curves under the influence of dual blood supply to the lungs. They also showed that this effect is particularly pronounced in lung tumors (27). In addition, no input function is required in the non-compartmental model to reflect the changes in tumor heterogeneity. The temporal activity data of the tracer in a single voxel within the ROI can be evaluated to show the non-homogeneity of the distribution of the tracer activity over time within the ROI, which is closely correlated with the pathological findings (5, 28, 29). It is also possible to acquire image IDIFs from multiple blood pools (e.g., ventricles and arteries) simultaneously by expanding the axial scanning field of view, such as with long axial PET/CT, and selecting the most relevant vascular IDIFs for kinetic modeling (30).
Although dynamic scanning allows continuous tracking of changes in tracer activity, it is associated with long scanning times, poor patient tolerance, and limited clinical application. The ultra-high-sensitivity whole-body PET scanners can shorten the time for dynamic acquisition. Feng et al. demonstrated that ultra-high sensitivity whole-body PET scanner can achieve whole-body parametric image reconstruction at an early stage of scanning (within 2 minutes of injection) (31). Wu et al. also demonstrated that a nonlinear estimation method can reduce the acquisition time of FDG PET Ki parametric imaging (32). However, Wu et al. also indicated that the shorter imaging time (10 min) is associated with a significantly increased computational cost. Several researchers have used simplified dynamic scanning protocols to effectively shorten the dynamic acquisition time. Compared with the 0-75 min standard parametric images, Hui Wang et al. found that using two short-duration dynamic multiparametric images of 0-6 min (input function) and 60-75 min (equilibrium activity) generated from 0-75 min standard parametric images provide good image quality. Dual time-point imaging can effectively improve gastrointestinal motility artifacts in standard parametric images due to long scanning times by leaving the scanner in the middle of the scanning process (33). Meanwhile, this needs the use of regions of interest from both aortas in both scans to generate the full IDIF and two CT scans to attenuate the two PET images for correction, increasing the radiation dose. Alternatively, PBIF can be used to scale the IDIF extracted from the later time frames of the scan, and a scaling curve can be used to generate the Patlak Ki values. However, the shape of the input curve must be assumed to be the same for each patient. Hasan Sari et al. explored the effect of different scanning cycles on the accuracy of sPBIF and found that sPBIF generated from 55-65 min of image data can accurately assess Ki (deviation <5%) and provide good image quality when it is used for Ki images generated from 20 min (45-65 min post-injection) dynamic scanning (34). Alternatively, a global scaling factor can be used to normalize the Patlak slopes obtained from the data in the later frames, thus eliminating the need for PBIF (35). Although some potential inaccuracies may occur, images obtained using post-scanning give better target-to-background ratios than static images, effectively reducing scanning time (Figure 3).
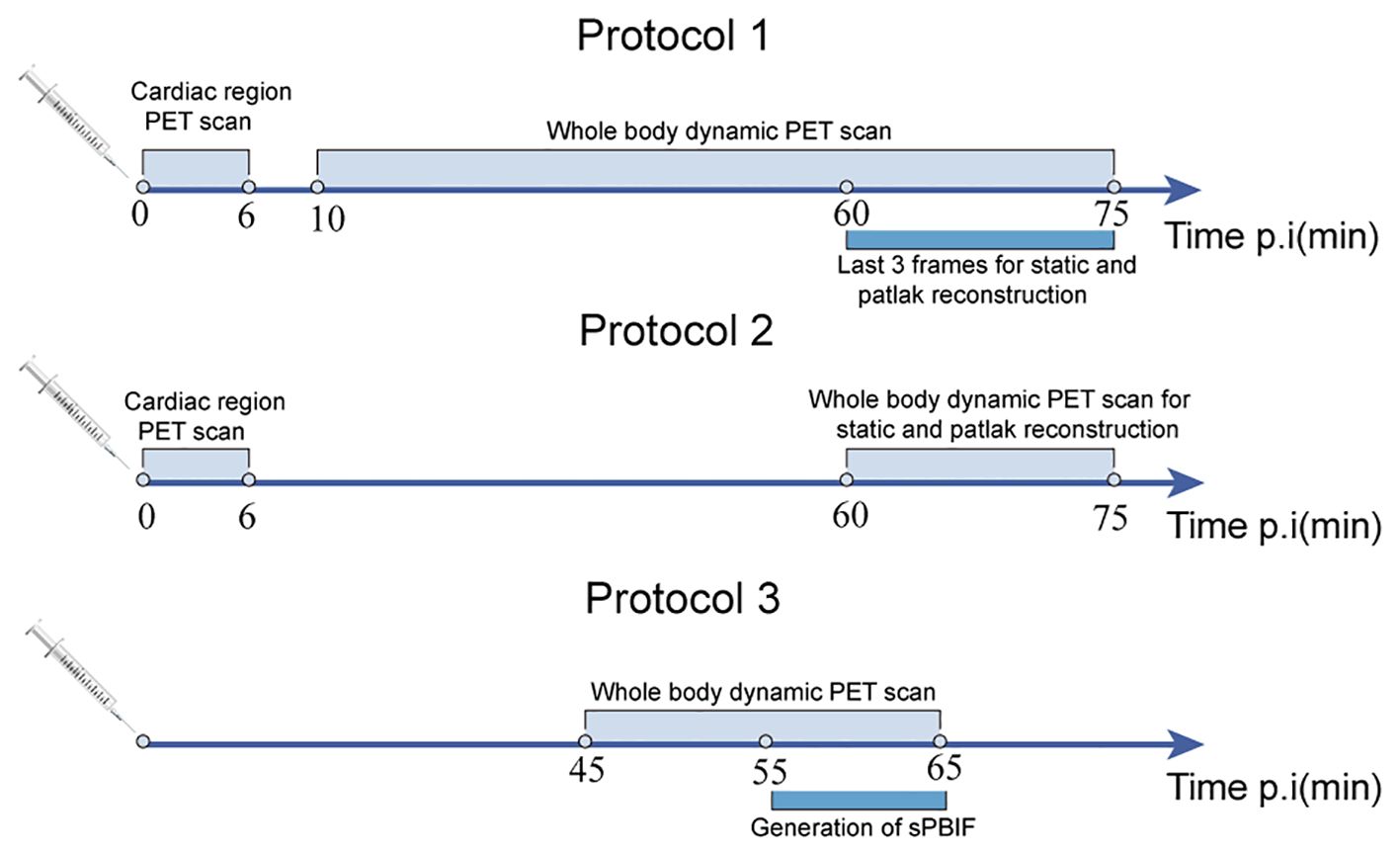
Figure 3. Three different imaging schemes for shortened scanning protocols: standard 75 min dynamic scanning (protocol 1), dual time-point imaging (protocol 2), and 20 min dynamic scanning based on population input function (protocol 3).
Whole-body dynamic scanning is mainly achieved by SS and CBM scanning modes in conventional PET scanners due to the limitations of the detector axial scanning field. The high sensitivity of the continuous bed motion listing mode is mainly reflected in the provision of continuous short-interval whole-body dynamic scanning. The whole-body dynamic images are acquired by multi-channel and multibed PET acquisition. Motoki Tomohito Kaji et al. found that the use of CBM scanning mode, which removes the frames with motion in the images, can improve the quality of image acquisition for patients who may move during the scanning process (36). In addition, it is sometimes difficult to identify lesions in the urinary tract and gastrointestinal tract using conventional static PET imaging. As a result, radiologic technicians are needed to distinguish whether the gastrointestinal tract is pathologically ingested or physiologically ingested through delayed imaging. Besides, Motoki Nishimura et al. found that continuous dynamic whole-body imaging can differentiate between pathologic and physiologic uptake based on changes in uptake shape, minimizing the need for such delayed imaging and reducing radiation dose (36, 37). Although multibed, multichannel approaches can provide dynamic imaging with extended AFOV, their temporal resolution is limited by bed motion and scanner sensitivity, which cannot rapidly capture tracer kinetics. In contrast, long-axis field-of-view PET/CT permits dynamic imaging of the entire body at high temporal resolution, acquisitions are no longer segmented by bed position. The long-axis field-of-view PET/CT eliminates the need for segmenting bed position during acquisitions. Besides, long-axis field-of-view PET/CT significantly improves sensitivity by increasing the number of detectors, allowing for dynamic whole-body acquisitions and improved image quality, increasing the detectability of microscopic lesions. Guillaume Fahrni et al. compared SUV and Ki images for 18 different oncologic indications (lesion characterization and staging) and found that Ki can improve sensitivity (from 92.5% to 95%) and accuracy (from 90.24% to 95.12%) compared with SUV imaging, especially for organs with high background uptake (liver). This is because Ki images suppress the non-specific 18F-FDG signal in the background, resulting in higher contrast in the region of abnormal uptake, improved lesion detectability, and reduced rate of false positives of lesions (7). In addition, the effect of respiratory motion in routine PET scans may lead to inaccurate description (tumor size, location, and morphology) and quantification of small lung nodules near the diaphragm. The use of breath-holding PET technology combined with the counting performance in the long aFOV can minimize motion artifacts and PET/CT image mismatches, especially for small nodules ≤10 mm from the pleura, effectively reducing the false-negative rate of early lung adenocarcinoma (38).
Dynamic imaging has had a significant impact on the diagnosis and management of tumor patients and has gained initial use worldwide. This article focuses on malignant solid tumors for which dynamic imaging has been more commonly used in clinical practice and summarizes the characteristics of the relevant studies (Table 1). Figure 4 summarizes the clinical applications of dPET in partial solid tumor studies.
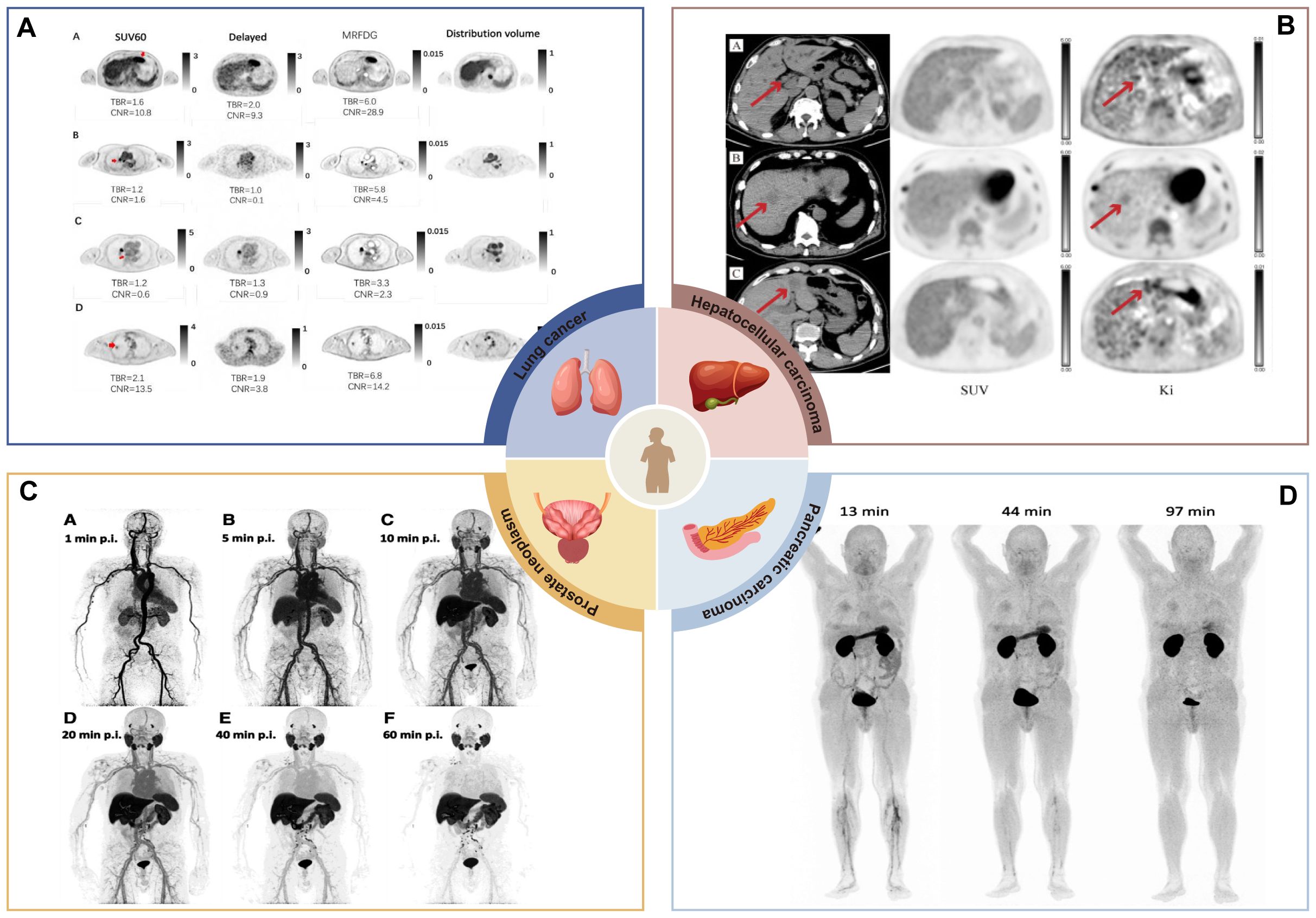
Figure 4. Clinical application of dPET in partial solid tumor studies. (A) MRFDG images improve the recognition of suspicious primary lesions. Primary suspicious lesions of hepatocellular carcinoma, mediastinal lesions, and lung cancer cannot be accurately identified in conventional static images, but they can be clearly distinguished in MRFDG images (17). Image taken from Yaping Wu et al. CC BY 4.0 License: http://creativecommons.org/licenses/by/4.0/. (B) K1 images were used to show positive lesions masked by physiologic background uptake. A case of hepatocellular carcinoma, the lesion was not metabolized in SUV images but was detected on Ki images (9). Image taken from Christos Sachpekidis et al. CC BY 4.0License: http://creativecommons.org/licenses/by/4.0/. (C) Dynamic PET was applied to study the systemic pharmacokinetics of 18F-PSMA-1007 in a group of prostate cancer patients. The figure shows dynamic PET acquisitions of a patient with PC biochemical recurrence at various time points. The patient had multiple iliac, retroperitoneal and supraclavicular manifestations of high uptake lymph node metastases. Of note, some retroperitoneal lymph node metastases were visualized within 10 minutes of tracer injection (11). Image taken from Christos Sachpekidis et al. CC BY 4.0License: http://creativecommons.org/licenses/by/4.0/. (D) Dynamic imaging was applied to estimate organ distribution and dosimetry data for a new contrast agent (68Ga-Trivehexin). In addition to the kidneys and bladder, there was a significant uptake in the stomach, which disappeared 1.5 hours after injection (74). Image taken from Neil Gerard Quigley et al. CC BY 4.0 License: http://creativecommons.org/licenses/by/4.0/.
Lung cancer is the leading cause of morbidity and mortality worldwide, according to data released by CA in 2024 (39). Therefore, early detection, accurate diagnosis, and development of individualized treatment plans can improve the survival of lung cancer patients. The dynamic metabolic parameter Ki can be used to improve specificity when differentiating benign and malignant lung nodules. Studies have shown that the Ki parameter is lower in benignancies than in malignancies. Besides, the diagnostic specificity of Ki can be further improved (about 0.830) when the critical value of Ki is 0.0250 ml/g/min, compared with that of SUVmax (40), effectively reducing unnecessary invasive treatment. The Ki value is more sensitive than conventional SUV in identifying early lymph node metastasis. Besides, the use of the Ki and Ki/K1 values has higher diagnostic specificity in cases where conventional static PET may not accurately identify suspicious lymph nodes (lymph nodes located in the hilar and mediastinal regions), complementing the lower specificity of SUVmax (41).
In addition, dynamic imaging is a valuable non-invasive screening method that can be used to assess treatment effectiveness and predict prognosis. Furthermore, SUV and Ki values are significantly higher in squamous carcinomas than in adenocarcinomas, possibly due to the differentiation of tumor cells combined with GULT-1 and GULT-3 overexpression (42). Ki values are lower in EGFR-expressing adenocarcinoma patients than in patients not expressing EGFR. Also, Ki can improve the differentiation in some NSCLC patients who did not undergo EGFR testing (40). Besides Ki, the tumor load parameters MTV and TLG are reliable overall survival prognostic markers for NSCLC patients receiving platinum-based chemotherapy (43). Notably, the efficacy of a treatment in adjuvant trials is not determined until several years after determining disease-free survival and overall survival. Neoadjuvant trials allow for efficacy endpoints, such as clinical and pathologic response, to be determined in months. Studies have shown that neoadjuvant chemoimmunotherapy can improve long-term survival and increase the chances of cure in patients with unresectable NSCLC. However, there are no suitable predictive imaging markers that can be used to comprehensively characterize the response to neoadjuvant treatment. DaQuan Wang et al. recently demonstrated that the uEXPLORER system can be used for whole-body 18F-FDG PET/CT dynamic imaging of patients with locally-advanced NSCLC. The results found that Patlak-Ki values were associated with response to induction immunochemotherapy in unresectable locally-advanced NSCLC. Furthermore, they showed that higher FDG Ki values have a better response to induction therapy and a higher level of immune cell infiltration in the primary tumor (44). DaQuan Wang et al. further performed repetitive dynamic scanning of patients during combined immuno-radiotherapy and found that the metabolic profile of the high FDG Ki group after induction immunochemotherapy was significantly reduced during the treatment period. They also showed that the Ki value of the primary foci was significantly correlated with the efficacy and survival of combined immuno-radiotherapy in locally advanced NSCLC patients (45). In summary, dynamic imaging provides non-invasive biomarkers that can predict treatment response in patients with unresectable locally advanced NSCLC.
Hepatocellular carcinoma (HCC) is one of the leading causes of cancer deaths worldwide. Dynamic imaging, dynamic perfusion imaging, and novel imaging agents have emerged as complementary techniques for HCC diagnosis. The blood input function is crucial for precise analysis in dPET kinetic modeling. Early studies used SBIF from the hepatic artery or portal vein for hepatic kinetic modeling (46). However, recent studies have shown that using DBIF is reasonable because of the dual blood supply to liver tissue from the hepatic artery and portal vein (47). Notably, only a few studies have reported the use of DBIF, and no perfect kinetic modeling has been developed yet. Studies have shown that a reversible dual-tissue compartment model for kinetic modeling using DBIF and optimally derived blood-supply fraction hepatic artery can effectively differentiate malignant lesions from healthy liver tissues. Moreover, the model can be used to further distinguish between lesions of intrahepatic cholangiocarcinoma and HCC (48). Dynamic perfusion imaging focuses on the liver and reveals information about the spatial distribution and temporal dynamics of the tumor through dynamic blood flow parameters, such as time to peak (TTP), blood flow, and hepatic perfusion index starting from the injection of 18F-FDG. Therefore, dynamic perfusion imaging enables quantitative interpretation of PET data (49). Studies have shown that TTP and perfusion index can be used to distinguish HCC from background liver tissue, significantly improving the detection rate of poorly differentiated and moderately differentiated HCC (49, 50).
Contrast-enhanced CT or magnetic resonance imaging can also be utilized for HCC screening. The two imaging are associated with many advantages, including multiphase enhancement characteristics and straightforward data gathering. However, the accuracy of these methods may be compromised when the lesion size is less than 2 cm (51). Also, the sensitivity of 18F-FDG in detecting HCC is limited to about 36%-68% (52). This is mainly because increased glucose transporter protein expression and hexokinase activity are found in highly differentiated HCC, reducing FDG accumulation in the lesions (53). Some scholars also found that 68Ga-FAPI-04 has a higher sensitivity in detecting intrahepatic HCC lesions than 18F-FDG, especially for tumors with a diameter of less than 2 cm(diagnostic efficiency: about 68.8%) (54). In addition, 68Ga-FAPI-04 showed high sensitivity in identifying liver malignant tumors and metastatic lymph nodes (55, 56).
Breast cancer (BC) has become the most common cancer in women, surpassing lung cancer, with an estimated 2.3 million new cases reported worldwide each year, and is the leading cause of cancer deaths in women (57). It is classified into four subtypes, including Luminal A, Luminal B, HER-2 overexpression, and triple-negative, based on the expression of cell proliferation markers (Ki-67), the estrogen (ER), progesterone (PR), and the HER-2 receptor (58). Lack of ER and PR expression in BC correlates with poor overall prognosis, whereas positivity for human epidermal growth factor receptor 2c-erbB2 and Ki-67 are considered good indicators of the invasiveness and metastastic potential of tumors (59). Kornélia Kajáry et al. conducted dynamic PET/CT imaging on 35 primary breast cancers. Their findings revealed that highly aggressive tumors, characterized by high differentiation, hormone receptor negativity (ER and/or PR), and rapid proliferation (high Ki-67), exhibited increased cellular uptake and phosphorylation of FDG (60), and these kinetic parameters can be used to characterize residual lesions following neoadjuvant therapy in locally advanced BC because they less dependent on pre-treatment FDG uptake as static parameters. Mid-treatment evaluation of changes in glucose delivery (K1) and metabolic rate (MRFDG) during neoadjuvant chemotherapy can predict recurrence-free survival (RFS). Specifically, a greater reduction in metabolism-to-perfusion ratios (MRFDG/peak PE and MRFDG/peak SER) compared to pretreatment levels is associated with longer RFS (61), suggesting a potential new biomarker for therapeutic response in breast cancer. Axillary lymph node metastasis is a critical prognostic factor in locally advanced breast cancer. Traditionally, lymph node status has been assessed invasively through sentinel lymph node biopsy and axillary lymph node dissection (62). These procedures often lead to edema, numbness, pain, and long-term complications (63). The study demonstrated that the TBR and CNR of MR parametric images generated using 6 min single bed dynamic scan and 64 min dynamic whole body PET scan were superior to SUV images, which significantly improved lesion detectability (14).
Microvessel density and tumor angiogenesis can influence the tumor growth and metastasis (64, 65). In contrast, patients with locally advanced breast cancer often exhibit a mismatch between blood supply and metabolic rate within the tumor. This imbalance, potentially caused by inadequate blood flow and/or elevated tumor metabolism, can create a hypoxic microenvironment linked to treatment resistance. This phenomenon is particularly prevalent in triple-negative breast cancer (65). Methods based on a 2-minute half-life 15O are effective for perfusion assessment (66), however the use of cyclotrons limits its clinical application. Neree Payan et al. used a first-pass scanning approach with a 2-minute dynamic acquisition centered on the chest immediately after injection of 18F-FDG, followed by a static acquisition 90 min later, which correlated well with 15O-water measurements of tumor perfusion. In patients with lymph node involvement, primary tumors exhibited high perfusion and K1 values (67), which may explain the extensive angiogenesis that may promote cancer metastasis.
Pancreatic cancer is a malignant tumor the primarily originates from pancreatic ductal epithelial and follicular cells, with high degree of malignancy and insidious onset. Among its various subtypes, pancreatic ductal adenocarcinoma cancer (PDAC) is the most common type, accounting for about 90% of the cases (68). Intraductal papillary mucinous neoplasm (IPMN) is the precursor of malignant transformation of PDAC (69). Although many IPMNs are benign, differentiating between these and those with malignant potential is crucial. Matthias Lang et al. demonstrated that dPET parameters (TTP, K1, K2, K3, K4) can effectively distinguish between high-grade and low-grade IPMNs. With 80% sensitivity and specificity, this approach offers a promising tool for predicting IPMN subtypes and potentially avoiding unnecessary surgery for non-malignant lesions (70). In terms of staging and prognosis of PDCA, the dual imaging with 68Ga-FAPI and 18F-FDG allowed precise stratification of postoperative patients, with 18.4% and 10.2% of patients experiencing a change in staging, respectively, but 68Ga-FAPI had a lower specificity in identifying primary lesions (71) that was mainly attributed to the overlapping uptake intensity of primary pancreatic tumors and tumor-induced obstructive pancreatitis of the pancreatic parenchyma. Unfortunately, dynamic 68Ga-FAPI-04 PET/CT did not effectively differentiate between PDAC and distal obstructive pancreatitis based on Ki values (72). However, dual time-point imaging with a 3-hour delay successfully distinguished between tumor and inflammation-induced fibrosis (72).
Currently, there are limited studies on the dynamics of PET/CT in pancreatic cancer, especially with the low sensitivity or specificity of 18F-FDG (73), the development of new imaging agents is imperative, and the use of 68Ga-labeled trimerized αvβ6 integrin-selective nonpeptide 68Ga-Trivehexin has been shown to be superior in PDAC metastases, especially in cancers with low metabolic conversion and/or high stromal ratios, such as liver (74), brain tissue and retroperitoneum (75). In addition, researchers are actively seeking innovative strategies to enhance pancreatic cancer treatment outcomes. A primary focus of targeted therapy involves identifying new targets through the administration of short-lived, highly radioactive radionuclides. In the study by Tadashi Watabe et al., dPET was employed to evaluate 64Cu and 225Ac to label mice with human pancreatic cancer xenografts using a FAP inhibitor (FAPI) and found that it significantly inhibited tumor growth (76), while Yuwei Liu et al. discovered that administration of 177Lu-FAPI-46 and 225Ac-FAPI-46 to PANC-1 xenograft mice decreased the tumor growth. However, 177Lu-FAPI-46 exhibited a less intense but more prolonged therapeutic effect compared with 225Ac-FAPI-46 (77). These studies suggest that β-therapy and α-therapy targeting FAP may offer effective treatment of pancreatic cancer, but it is currently in the clinical translational phase and further investigations using dPET are needed to determine the best mix of rapid FAP kinetics and physical radionuclide decay. There is a compelling need to develop combinatorial treatment strategies focused on tumor cell elimination.
Current statistics indicate that prostate neoplasm has surpassed lung cancer as the most common tumor in men worldwide and is the leading cause of cancer deaths in men (57). Since 2020, when 68Ga-PSMA-11 was approved by the FDA (78), prostate-specific membrane antigen (PSMA)-targeted PET imaging of prostate cancer employing 68Ga-labelled complexes is increasingly being adopted in routine clinical treatment worldwide. Studies have shown that the kinetic behavior of 68Ga-PSMA-11 varies across different organs and lesions. The irreversible two-tissue compartmental (2T4k) model is more suitable for describing the kinetics in pathological lesions, while the reversible two-tissue compartmental (2T4k) model better fits normal tissue kinetics. In terms of kinetic metrics, the K3 value effectively distinguished between abnormal lesions and healthy organs with increased absorption of 68Ga-PSMA-11, and is expected to gain broader clinical application as an imaging biomarker for differentiating pathological and non-specific 68Ga-PSMA-11 uptake in prostate cancer patients (79). Currently, delayed imaging with 68Ga-PSMA PET/CT can improve the identification of non-specific PSMA ingestion in patients with prostate cancer (80). In addition, Ki images collected using whole-body PET/CT scanning for 60 min are less noisy and have sharper lesions than SUV images, making them ideal for early detection of small lesions (81). The lengthy 60-min scan time can lead to significant bladder activity, hindering the detection of small lesions like local recurrences or lymph node metastases near the bladder. A combination of a 6-min early dynamic imaging and static imaging protocol (91), as proposed by Jun Wen et al., effectively reduces bladder interference and shortens the PET/CT acquisition time to 45 min. While the improvement in image quality is modest, it enhances patient comfort (82).
The longer half-life of 18F compared to 68Ga offers great convenience in terms of transportation and can be used in delayed imaging protocols. Evidence indicates that 18F-DCFPyL and 68Ga-PSMA-11 exhibit similar biodistribution patterns including the bladder uptake, and that 18F-DCFPyL has a higher overall detection rate although it did not show a higher detection rate in terms of localized foci (83), this may be due to the better spatial resolution of the images provided by 18F. A 22-min dynamic 18F-DCFPyL PET scan effectively detected dominant intraprostatic lesions. Kinetic parameters Ki and K4 derived from this scan accurately differentiated between tumor and benign tissue (84). These investigations suggest that 18F-DCFPyL is the most favorable substitute for 68Ga-labeled drugs. Moreover, the transition from 68Ga-labeled PSMA-targeted compounds to 18F-labeled PSMA-targeted compounds is expected to gain broader clinical acceptance.
Hypoxia is a common feature of head and neck cancer and a predictor of poor prognosis following radiotherapy (85). 18F-Fluoromisonidazole (FMISO) has been extensively investigated as a radiotracer for hypoxic PET with good performance (86). Tumor-to-Blood Ratio and Tumor-to-Muscle Ratios are measurements obtained from static FMISO scans and are frequently employed as substitute biomarkers for tumor hypoxia, depending on the specific moment of image capture. Furthermore, tumor-to-blood ratio and tumor-to-muscle ratios values are influenced by the distribution of FMISO within and among tumors. Consequently, this method may inaccurately assess the presence and severity of hypoxia. Milan Grkovski et al. performed a 30-min single-bed dynamic imaging of 120 patients with head and neck cancer, and found that 18F-FMISO dPET provided data required for parametric mapping of tumor hypoxia, perfusion, and radiotracer distribution volumes, and K3 was identified as a direct biomarker of hypoxia-mediated 18F-FMISO accumulation, and K3 was not dependent on the volume of distribution and time of acquisition with FMISO (87).
Tumor perfusion is a critical factor influencing drug delivery. Changes in perfusion can serve as a prognostic indicator for the effectiveness of both conventional and targeted therapies, such as anti-angiogenic treatments (88). Studies have reported that tumor perfusion and hypoxia can help to predict response to treatment (89). The 20-min truncated 18F-FMISO dPET scans, i.e., by the kinetic parameters K1 and K3 reacting to tumor hypoxia and perfusion can identify alternative markers of hypoxia and perfusion. Currently, two hypotheses have been proposed for the relationship between K1 and K3, one is a negative correlation, whereby hypoxia occurs in a hypoperfused tumor volume with a disorganized and dysfunctional microvascular structure. It is hypothesized that the increased separation between tumor cells and functional blood vessels results in diminished oxygen delivery to distal cells, causing diffusion-limited hypoxia. The other is a positive correlation, which implies that areas with good perfusion may experience hypoxia (89). This may reflect the various forms of hypoxia (patchy, banded, and mixed) described in head and neck tumors by Ljungkvist et al, who reported that hypoxia and perfusion can simultaneously occur in very close proximity under the microscope (90). Notably, hypoxia may promote angiogenesis, hyperperfusion and perfusion heterogeneity.
Radiotracer-based kinetic modeling and dynamic imaging provide data that cannot be obtained by conventional static PET/CT, and have been successfully applied in various malignant tumors. They provide kinetic scanning parameters are of superior value for diagnosis, differential diagnosis, and assessment of treatment response and prognosis in these diseases than the visual and semiquantitative analysis of conventional static imaging. The practical application of kinetic imaging is hindered by issues related to data volume, scan duration, and the sophistication required for data analysis. With the advent of state-of-the-art PET/CT scanners, these problems can be ameliorated with the optimization of existing clinical applications of whole-body PET/CT, such as sequential dPET imaging of all relevant organ structures to map the pharmacokinetic behavior of (new) tracers. Moreover, exploring the potential of artificial intelligence in image analysis could lead to advanced multiparametric methods and uncover new avenues for research.
YW: Funding acquisition, Writing – original draft. YY: Funding acquisition, Writing – review & editing. JL: Data curation, Methodology, Writing – review & editing. DC: Formal Analysis, Methodology, Writing – review & editing. HX: Investigation, Supervision, Writing – review & editing. JH: Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Province Key Laboratory of Molecular Imaging, Grant (No. 2023fzyx025), Jingzhou Healthcare Science and Technology Program(2023HC07 to YY), and the College Students Innovative Entrepreneurial Training Program in Yangtze University (Yz202308 to YW).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
SUV, Standardized uptake value; PET/CT, Positron emission tomography/computed tomography; ROI, Region of interest; DPET, Dynamic PET; MRFDG, Metabolic rate of FDG; TACs, Time activity curves; AFOV, Axial of view; SS, Step-and-shoot scan mode; CBM, Continuous bed motion; AIF, Artery input function; DVFDG, Distribution volume of free FDG; VOIs, Volumes of interests; TBR, Tumor-to- background ratio; CNR, Contrast-to-noise ratio; MTV, Metabolic tumor volume; TLG, Total lesion glycolysis; FD, Fractal dimension; IDIF, Image-derived input function; PBIF, Population-based input function; DBIF, Dual-blood input function; SBIF, Single-blood input function; NSCLC, Non-small cell lung cancer; HCC, Hepatocellular carcinoma; TTP, Time to peak; BC, Breast cancer; PDAC, Pancreatic ductal adenocarcinoma cancer; IPMN, Intraductal papillary mucinous neoplasm.
1. Sarikaya I, Sarikaya A. Assessing pet parameters in oncologic 18F-FDG studies. J Nucl Med Technol. (2020) 483:278–82. doi: 10.2967/jnmt.119.236109
2. Yang M, Lin Z, Xu Z, Li D, Lv W, Yang S, et al. Influx rate constant of 18F-FDG increases in metastatic lymph nodes of non-small cell lung cancer patients. Eur J Nucl Med Mol Imaging. (2020) 475:1198–208. doi: 10.1007/s00259-020-04682-5
3. Zhang L, Chen J, Xu C, Zhang Y, Qi L, Zhang X. The value of 4D fluorine-18-fluorodeoxyglucose positron emission tomography/computed tomography in the diagnosis of lung lesions. Nucl Med Commun. (2020) 4112:1306–12. doi: 10.1097/mnm.0000000000001289
4. Dias AH, Pedersen MF, Danielsen H, Munk OL, Gormsen LC. Clinical feasibility and impact of fully automated multiparametric PET imaging using direct Patlak reconstruction: evaluation of 103 dynamic whole-body 18F-FDG PET/CT scans. Eur J Nucl Med Mol Imaging. (2021) 483:837–50. doi: 10.1007/s00259-020-05007-2
5. Dimitrakopoulou-Strauss A, Pan L, Sachpekidis C. Kinetic modeling and parametric imaging with dynamic PET for oncological applications: General considerations, current clinical applications, and future perspectives. Eur J Nucl Med Mol Imaging. (2021) 481:21–39. doi: 10.1007/s00259-020-04843-6
6. Schatka I, Weiberg D, Reichelt S, Owsianski-Hille N, Derlin T, Berding G, et al. A randomized, double-blind, crossover comparison of novel continuous bed motion versus traditional bed position whole-body PET/CT imaging. Eur J Nucl Med Mol Imaging. (2016) 434:711–7. doi: 10.1007/s00259-015-3226-z
7. Fahrni G, Karakatsanis NA, Di Domenicantonio G, Garibotto V, Zaidi H. Does whole-body patlak 18F-FDG PET imaging improve lesion detectability in clinical oncology? Eur Radiol. (2019) 299:4812–21. doi: 10.1007/s00330-018-5966-1
8. Zaker N, Kotasidis F, Garibotto V, Zaidi H. Assessment of lesion detectability in dynamic whole-body PET imaging using compartmental and patlak parametric mapping. Clin Nucl Med. (2020) 455:e221–e31. doi: 10.1097/rlu.0000000000002954
9. Cai D, He Y, Yu H, Zhang Y, Shi H. Comparative benefits of Ki and SUV images in lesion detection during PET/CT imaging. EJNMMI Res. (2024) 141:98. doi: 10.1186/s13550-024-01162-x
10. Leung EK, Berg E, Omidvari N, Spencer BA, Li E, Abdelhafez YG, et al. Quantitative accuracy in total-body imaging using the uEXPLORER PET/CT scanner. Phys Med Biol. (2021) 66:205008. doi: 10.1088/1361-6560/ac287c
11. Sachpekidis C, Pan L, Groezinger M, Strauss DS, Dimitrakopoulou-Strauss A. Combined whole-body dynamic and static PET/CT with low-dose 18F-PSMA-1007 in prostate cancer patients. Eur J Nucl Med Mol Imaging. (2024) 517:2137–50. doi: 10.1007/s00259-024-06620-1
12. Yin J, Wang H, Zhu G, Chen N, Khan MI, Zhao Y. Prognostic value of whole-body dynamic 18F-FDG PET/CT Patlak in diffuse large B-cell lymphoma. Heliyon. (2023) 99:e19749. doi: 10.1016/j.heliyon.2023.e19749
13. Dias AH, Pedersen MF, Danielsen H, Munk OL, Gormsen LC. Correction to: Clinical feasibility and impact of fully automated multiparametric pet imaging using direct patlak reconstruction: Evaluation of 103 dynamic whole-body 18F-FDG PET/CT scans. Eur J Nucl Med Mol Imaging. (2021) 483:954. doi: 10.1007/s00259-021-05225-2
14. Pedersen MA, Dias AH, Hjorthaug K, Gormsen LC, Fledelius J, Johnsson AL, et al. Increased lesion detectability in patients with locally advanced breast cancer-A pilot study using dynamic whole-body [18F]FDG PET/CT. EJNMMI Res. (2024) 141:31. doi: 10.1186/s13550-024-01096-4
15. Zhao Y, Lv T, Xu Y, Yin J, Wang X, Xue Y, et al. Application of dynamic 18F-FDG PET/CT multiparametric imaging leads to an improved differentiation of benign and Malignant lung lesions. Mol Imaging Biol. (2024) 265:790–801. doi: 10.1007/s11307-024-01942-w
16. Zhang L, Zhang J, Miao J, Zhu G, Su X, Wang H. Characteristics of whole-body dynamic 18F-FDG PET/CT Patlak multi-parametric imaging in lung cancer and the influence of different delineation methods on quantitative parameters. Quant Imaging Med Surg. (2024) 141:291–304. doi: 10.21037/qims-23-862
17. Wu Y, Fu F, Meng N, Wang Z, Li X, Bai Y, et al. The role of dynamic, static, and delayed total-body PET imaging in the detection and differential diagnosis of oncological lesions. Cancer Imaging. (2024) 241:2. doi: 10.1186/s40644-023-00649-5
18. Dimitrakopoulou-Strauss A, Pan L, Strauss LG. Quantitative approaches of dynamic FDG-PET and pet/ct studies (dPET/CT) for the evaluation of oncological patients. Cancer Imaging. (2012) 121:283–9. doi: 10.1102/1470-7330.2012.0033
19. Meijer TWH, de Geus-Oei LF, Visser EP, Oyen WJG, Looijen-Salamon MG, Visvikis D, et al. Tumor delineation and quantitative assessment of glucose metabolic rate within histologic subtypes of non-small cell lung cancer by using dynamic 18F fluorodeoxyglucose pet. Radiology. (2017) 2832:547–59. doi: 10.1148/radiol.2016160329
20. Tixier F, Vriens D, Cheze-Le Rest C, Hatt M, Disselhorst JA, Oyen WJ, et al. Comparison of tumor uptake heterogeneity characterization between static and parametric 18F-FDG PET images in non-small cell lung cancer. J Nucl Med. (2016) 577:1033–9. doi: 10.2967/jnumed.115.166918
21. Liu G, Xu H, Hu P, Tan H, Zhang Y, Yu H, et al. Kinetic metrics of 18F-FDG in normal human organs identified by systematic dynamic total-body positron emission tomography. Eur J Nucl Med Mol Imaging. (2021) 488:2363–72. doi: 10.1007/s00259-020-05124-y
22. Naganawa M, Gallezot JD, Shah V, Mulnix T, Young C, Dias M, et al. Assessment of population-based input functions for patlak imaging of whole body dynamic 18F-FDG PET. EJNMMI Phys. (2020) 71:67. doi: 10.1186/s40658-020-00330-x
23. Sarikaya I, Albatineh AN, Sarikaya A. Revisiting weight-normalized suv and lean-body-mass-normalized suv in pet studies. J Nucl Med Technol. (2020) 482:163–7. doi: 10.2967/jnmt.119.233353
24. Zhu Y, Tran Q, Wang Y, Badawi RD, Cherry SR, Qi J, et al. Optimization-derived blood input function using a kernel method and its evaluation with total-body PET for brain parametric imaging. Neuroimage. (2024) 293:120611. doi: 10.1016/j.neuroimage.2024.120611
25. Liu G, Yu H, Shi D, Hu P, Hu Y, Tan H, et al. Short-time total-body dynamic pet imaging performance in quantifying the kinetic metrics of 18F-FDG in healthy volunteers. Eur J Nucl Med Mol Imaging. (2022) 498:2493–503. doi: 10.1007/s00259-021-05500-2
26. Wang G, Corwin MT, Olson KA, Badawi RD, Sarkar S. Dynamic PET of human liver inflammation: Impact of kinetic modeling with optimization-derived dual-blood input function. Phys Med Biol. (2018) 6315:155004. doi: 10.1088/1361-6560/aac8cb
27. Wang Y, Abdelhafez YG, Spencer BA, Verma R, Parikh M, Stollenwerk N, et al. High-temporal-resolution kinetic modeling of lung tumors with dual-blood input function using total-body dynamic PET. J Nucl Med. (2024) 655:714–21. doi: 10.2967/jnumed.123.267036
28. Dimitrakopoulou-Strauss A, Pan L, Strauss LG. Parametric imaging: A promising approach for the evaluation of dynamic PET-18F-FDG studies - the dkfz experience. Hell J Nucl Med. (2010) 131:18–22.
29. Sachpekidis C, Hillengass J, Goldschmidt H, Wagner B, Haberkorn U, Kopka K, et al. Treatment response evaluation with 18F-FDG PET/CT and 18F-NAF PET/CT in multiple myeloma patients undergoing high-dose chemotherapy and autologous stem cell transplantation. Eur J Nucl Med Mol Imaging. (2017) 441:50–62. doi: 10.1007/s00259-016-3502-6
30. Zhang X, Xie Z, Berg E, Judenhofer MS, Liu W, Xu T, et al. Total-body dynamic reconstruction and parametric imaging on the uEXPLORER. J Nucl Med. (2020) 612:285–91. doi: 10.2967/jnumed.119.230565
31. Feng T, Zhao Y, Shi H, Li H, Zhang X, Wang G, et al. Total-body quantitative parametric imaging of early kinetics of 18F-FDG. J Nucl Med. (2021) 625:738–44. doi: 10.2967/jnumed.119.238113
32. Wu Y, Feng T, Zhao Y, Xu T, Fu F, Huang Z, et al. Whole-body parametric imaging of 18F-FDG PET using uEXPLORER with reduced scanning time. J Nucl Med. (2022) 634:622–8. doi: 10.2967/jnumed.120.261651
33. Wang H, Miao Y, Yu W, Zhu G, Wu T, Zhao X, et al. Improved clinical workflow for whole-body patlak parametric imaging using two short dynamic acquisitions. Front Oncol. (2022) 12:822708. doi: 10.3389/fonc.2022.822708
34. Sari H, Eriksson L, Mingels C, Alberts I, Casey ME, Afshar-Oromieh A, et al. Feasibility of using abbreviated scan protocols with population-based input functions for accurate kinetic modeling of 18F-FDG datasets from a long axial FOV PET scanner. Eur J Nucl Med Mol Imaging. (2023) 502:257–65. doi: 10.1007/s00259-022-05983-7
35. Zuo Y, Qi J, Wang G. Relative patlak plot for dynamic pet parametric imaging without the need for early-time input function. Phys Med Biol. (2018) 6316:165004. doi: 10.1088/1361-6560/aad444
36. Kaji T, Osanai K, Nakata T, Tamaki N. Dynamic whole-body 18F-FDG PET for minimizing patient motion artifact. Clin Nucl Med. (2020) 4511:880–2. doi: 10.1097/rlu.0000000000003253
37. Nishimura M, Tamaki N, Matsushima S, Kiba M, Kotani T, Bamba C, et al. Dynamic whole-body 18F-FDG PET for differentiating abnormal lesions from physiological uptake. Eur J Nucl Med Mol Imaging. (2020) 4710:2293–300. doi: 10.1007/s00259-020-04726-w
38. Cheng Z, Chen L, Wang X, Wang Y, Zhao M, Zan K, et al. Role of breath-hold lung PET in stage ia pulmonary adenocarcinoma. Insights Imaging. (2023) 141:100. doi: 10.1186/s13244-023-01446-1
39. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 743:229–63. doi: 10.3322/caac.21834
40. Wumener X, Zhang Y, Zang Z, Du F, Ye X, Zhang M, et al. The value of dynamic FDG PET/CT in the differential diagnosis of lung cancer and predicting EGFR mutations. BMC Pulm Med. (2024) 241:227. doi: 10.1186/s12890-024-02997-9
41. Wumener X, Zhang Y, Wang Z, Zhang M, Zang Z, Huang B, et al. Dynamic FDG-PET imaging for differentiating metastatic from non-metastatic lymph nodes of lung cancer. Front Oncol. (2022) 12:1005924. doi: 10.3389/fonc.2022.1005924
42. De Geus-Oei LF, Van Krieken JH, Aliredjo RP, Krabbe PF, Frielink C, Verhagen AF, et al. Biological correlates of fdg uptake in non-small cell lung cancer. Lung Cancer. (2007) 551:79–87. doi: 10.1016/j.lungcan.2006.08.018
43. Sharma A, Mohan A, Bhalla AS, Sharma MC, Vishnubhatla S, Das CJ, et al. Role of various metabolic parameters derived from baseline 18F-FDG PET/CT as prognostic markers in non-small cell lung cancer patients undergoing platinum-based chemotherapy. Clin Nucl Med. (2018) 431:e8–e17. doi: 10.1097/rlu.0000000000001886
44. Wang D, Qiu B, Liu Q, Xia L, Liu S, Zheng C, et al. Patlak-Ki derived from ultra-high sensitivity dynamic total body 18F-FDG PET/CT correlates with the response to induction immuno-chemotherapy in locally advanced non-small cell lung cancer patients. Eur J Nucl Med Mol Imaging. (2023) 5011:3400–13. doi: 10.1007/s00259-023-06298-x
45. Wang D, Mo Y, Liu F, Zheng S, Liu H, Li H, et al. Repeated dynamic 18F-FDG PET/CT imaging using a high-sensitivity PET/CT scanner for assessing non-small cell lung cancer patients undergoing induction immuno-chemotherapy followed by hypo-fractionated chemoradiotherapy and consolidative immunotherapy: Report from a prospective observational study (GASTO-1067). Eur J Nucl Med Mol Imaging. (2024) 51(13):4083–98. doi: 10.1007/s00259-024-06819-2
46. Geist BK, Wang J, Wang X, Lin J, Yang X, Zhang H, et al. Comparison of different kinetic models for dynamic 18F-FDG PET/CT imaging of hepatocellular carcinoma with various, also dual-blood input function. Phys Med Biol. (2020) 654:045001. doi: 10.1088/1361-6560/ab66e3
47. Munk OL, Bass L, Roelsgaard K, Bender D, Hansen SB, Keiding S. Liver kinetics of glucose analogs measured in pigs by pet: Importance of dual-input blood sampling. J Nucl Med. (2001) 425:795–801.
48. Wang J, Shao Y, Liu B, Wang X, Geist BK, Li X, et al. Dynamic 18F-FDG PET imaging of liver lesions: Evaluation of a two-tissue compartment model with dual blood input function. BMC Med Imaging. (2021) 211:90. doi: 10.1186/s12880-021-00623-2
49. Zhang Y, Dong Y, Yu W, Chen S, Yu H, Li B, et al. Combined early dynamic 18F-FDG PET/CT and conventional whole-body 18F-FDG PET/CT in hepatocellular carcinoma. Abdom Radiol (NY). (2023) 4810:3127–34. doi: 10.1007/s00261-023-03986-y
50. Wang S, Li B, Li P, Xie R, Wang Q, Shi H, et al. Feasibility of perfusion and early-uptake 18F-FDG PET/CT in primary hepatocellular carcinoma: A dual-input dual-compartment uptake model. Jpn J Radiol. (2021) 3911:1086–96. doi: 10.1007/s11604-021-01140-6
51. Osho A, Rich NE, Singal AG. Role of imaging in management of hepatocellular carcinoma: Surveillance, diagnosis, and treatment response. Hepatoma Res. (2020) 6:55. doi: 10.20517/2394-5079.2020.42
52. Lee SM, Kim HS, Lee S, Lee JW. Emerging role of 18F-fluorodeoxyglucose positron emission tomography for guiding management of hepatocellular carcinoma. World J Gastroenterol. (2019) 2511:1289–306. doi: 10.3748/wjg.v25.i11.1289
53. Wu HB, Wang QS, Li BY, Li HS, Zhou WL, Wang QY. F-18 FDG in conjunction with 11C-choline pet/ct in the diagnosis of hepatocellular carcinoma. Clin Nucl Med. (2011) 3612:1092–7. doi: 10.1097/RLU.0b013e3182335df4
54. Wang H, Zhu W, Ren S, Kong Y, Huang Q, Zhao J, et al. 68GA-FAPI-04 versus 18F-FDG PET/CT in the detection of hepatocellular carcinoma. Front Oncol. (2021) 11:693640. doi: 10.3389/fonc.2021.693640
55. Guo W, Pang Y, Yao L, Zhao L, Fan C, Ke J, et al. Imaging fibroblast activation protein in liver cancer: A single-center post hoc retrospective analysis to compare 68Ga-FAPI-04 PET/CT versus mri and 18F-FDG PET/CT. Eur J Nucl Med Mol Imaging. (2021) 485:1604–17. doi: 10.1007/s00259-020-05095-0
56. Zhang J, Jiang S, Li M, Xue H, Zhong X, Li S, et al. Head-to-head comparison of 18F-FAPI and 18F-FDG PET/CT in staging and therapeutic management of hepatocellular carcinoma. Cancer Imaging. (2023) 231:106. doi: 10.1186/s40644-023-00626-y
57. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. (2024) 741:12–49. doi: 10.3322/caac.21820
58. Vaz SC, Woll JPP, Cardoso F, Groheux D, Cook GJR, Ulaner GA, et al. Joint EANM-SNMMI guideline on the role of 2-18F-FDG PET/CT in no special type breast cancer: (endorsed by the ACR, ESSO, ESTRO, EUSOBI/ESR, and EUSOMA). Eur J Nucl Med Mol Imaging. (2024) 51(9):2706–32. doi: 10.1007/s00259-024-06696-9
59. Yaghan R, Stanton PD, Robertson KW, Going JJ, Murray GD, McArdle CS. Oestrogen receptor status predicts local recurrence following breast conservation surgery for early breast cancer. Eur J Surg Oncol. (1998) 245:424–6. doi: 10.1016/s0748-7983(98)92341-1
60. Kajáry K, Lengyel Z, Tőkés AM, Kulka J, Dank M, Tőkés T. Dynamic FDG-PET/CT in the initial staging of primary breast cancer: Clinicopathological correlations. Pathol Oncol Res. (2020) 262:997–1006. doi: 10.1007/s12253-019-00641-0
61. Kazerouni AS, Peterson LM, Jenkins I, Novakova-Jiresova A, Linden HM, Gralow JR, et al. Multimodal prediction of neoadjuvant treatment outcome by serial FDG PET and MRI in women with locally advanced breast cancer. Breast Cancer Res. (2023) 251:138. doi: 10.1186/s13058-023-01722-4
62. Brackstone M, Baldassarre FG, Perera FE, Cil T, Chavez Mac Gregor M, Dayes IS, et al. Management of the axilla in early-stage breast cancer: Ontario health (cancer care ontario) and asco guideline. J Clin Oncol. (2021) 3927:3056–82. doi: 10.1200/jco.21.00934
63. Verbelen H, Tjalma W, Meirte J, Gebruers N. Long-term morbidity after a negative sentinel node in breast cancer patients. Eur J Cancer Care (Engl). (2019) 285:e13077. doi: 10.1111/ecc.13077
64. Weidner N. Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res Treat. (1995) 362:169–80. doi: 10.1007/bf00666038
65. Mankoff DA, Dunnwald LK, Partridge SC, Specht JM. Blood flow-metabolism mismatch: Good for the tumor, bad for the patient. Clin Cancer Res. (2009) 1517:5294–6. doi: 10.1158/1078-0432.Ccr-09-1448
66. Laking G, Price P. Radionuclide imaging of perfusion and hypoxia. Eur J Nucl Med Mol Imaging. (2010) 37:20–9. doi: 10.1007/s00259-010-1453-x
67. Payan N, Presles B, Brunotte F, Coutant C, Desmoulins I, Vrigneaud JM, et al. Biological correlates of tumor perfusion and its heterogeneity in newly diagnosed breast cancer using dynamic first-pass 18F-FDG PET/CT. Eur J Nucl Med Mol Imaging. (2020) 475:1103–15. doi: 10.1007/s00259-019-04422-4
68. Yang J, Liu Y, Liu S. The role of epithelial-mesenchymal transition and autophagy in pancreatic ductal adenocarcinoma invasion. Cell Death Dis. (2023) 148:506. doi: 10.1038/s41419-023-06032-3
69. Pollini T, Adsay V, Capurso G, Dal Molin M, Esposito I, Hruban R, et al. The tumour immune microenvironment and microbiome of pancreatic intraductal papillary mucinous neoplasms. Lancet Gastroenterol Hepatol. (2022) 712:1141–50. doi: 10.1016/s2468-1253(22)00235-7
70. Lang M, Spektor AM, Hielscher T, Hoppner J, Glatting FM, Bicu F, et al. Static and dynamic 68GA-FAPI PET/CT for the detection of Malignant transformation of intraductal papillary mucinous neoplasia of the pancreas. J Nucl Med. (2023) 642:244–51. doi: 10.2967/jnumed.122.264361
71. Ding J, Qiu J, Hao Z, Huang H, Liu Q, Liu W, et al. Comparing the clinical value of baseline 68Ga-FAPI-04 PET/CT and 18F-FDG PET/CT in pancreatic ductal adenocarcinoma: additional prognostic value of the distal pancreatitis. Eur J Nucl Med Mol Imaging. (2023) 5013:4036–50. doi: 10.1007/s00259-023-06297-y
72. Pang Y, Zhao L, Shang Q, Meng T, Zhao L, Feng L, et al. Positron emission tomography and computed tomography with [68Ga]Ga-fibroblast activation protein inhibitors improves tumor detection and staging in patients with pancreatic cancer. Eur J Nucl Med Mol Imaging. (2022) 494:1322–37. doi: 10.1007/s00259-021-05576-w
73. Rijkers AP, Valkema R, Duivenvoorden HJ, van Eijck CH. Usefulness of F-18-fluorodeoxyglucose positron emission tomography to confirm suspected pancreatic cancer: A meta-analysis. Eur J Surg Oncol. (2014) 407:794–804. doi: 10.1016/j.ejso.2014.03.016
74. Quigley NG, Steiger K, Hoberück S, Czech N, Zierke MA, Kossatz S, et al. PET/CT imaging of head-and-neck and pancreatic cancer in humans by targeting the “Cancer Integrin” αvβ6 with Ga-68-Trivehexin. Eur J Nucl Med Mol Imaging. (2022) 494:1136–47. doi: 10.1007/s00259-021-05559-x
75. Rehm J, Winzer R, Notni J, Hempel S, Distler M, Folprecht G, et al. Concomitant metastatic head-and-neck cancer and pancreatic cancer assessed by αvβ6-integrin PET/CT using 68Ga-Trivehexin: Incidental detection of a brain metastasis. Eur J Nucl Med Mol Imaging. (2024) 51(11):3469–71. doi: 10.1007/s00259-024-06750-6
76. Watabe T, Liu Y, Kaneda-Nakashima K, Shirakami Y, Lindner T, Ooe K, et al. Theranostics targeting fibroblast activation protein in the tumor stroma: 64Cu- and 225Ac-labeled FAPI-04 in pancreatic cancer xenograft mouse models. J Nucl Med. (2020) 614:563–9. doi: 10.2967/jnumed.119.233122
77. Liu Y, Watabe T, Kaneda-Nakashima K, Shirakami Y, Naka S, Ooe K, et al. Fibroblast activation protein targeted therapy using 177Lu-FAPI-46 compared with 225Ac-FAPI-46 in a pancreatic cancer model. Eur J Nucl Med Mol Imaging. (2022) 493:871–80. doi: 10.1007/s00259-021-05554-2
78. Zhao R, Ke M, Lv J, Liu S, Liu Y, Zhang J, et al. First-in-human study of PSMA-targeting agent, [18F]AlF-P16-093: Dosimetry and initial evaluation in prostate cancer patients. Eur J Nucl Med Mol Imaging. (2024) 516:1753–62. doi: 10.1007/s00259-024-06596-y
79. Chen R, Ng YL, Yang X, Zhu Y, Li L, Zhao H, et al. Assessing dynamic metabolic heterogeneity in prostate cancer patients via total-body 68Ga-PSMA-11 PET/CT imaging: quantitative analysis of 68Ga-PSMA-11 uptake in pathological lesions and normal organs. Eur J Nucl Med Mol Imaging. (2024) 513:896–906. doi: 10.1007/s00259-023-06475-y
80. Xiao L, Su M, Li Y. Diagnostic value of dual-time point 68Ga-PSMA PET/CT image for benign and Malignant lesions in patients with prostate cancer. Abdom Radiol (NY). (2024) 49(9):3214–19. doi: 10.1007/s00261-024-04269-w
81. Chen R, Ng YL, Yang X, Zhu Y, Li L, Zhao H, et al. Comparison of parametric imaging and SUV imaging with 68Ga-PSMA-11 using dynamic total-body PET/CT in prostate cancer. Eur J Nucl Med Mol Imaging. (2024) 512:568–80. doi: 10.1007/s00259-023-06456-1
82. van der Sar ECA, Viol SLM, Braat A, van Rooij R, Lam M, de Jong H, et al. Impact of uptake time on image quality of 68Ga-PSMA-11 PET/CT. Med Phys. (2023) 5012:7619–28. doi: 10.1002/mp.16429
83. Ferreira G, Iravani A, Hofman MS, Hicks RJ. Intra-individual comparison of 68Ga-PSMA-11 and 18F-dcfpyl normal-organ biodistribution. Cancer Imaging. (2019) 191:23. doi: 10.1186/s40644-019-0211-y
84. Yang DM, Li F, Bauman G, Chin J, Pautler S, Moussa M, et al. Kinetic analysis of dominant intraprostatic lesion of prostate cancer using quantitative dynamic 18F-dcfpyl-pet: Comparison to 18F-fluorocholine-PET. EJNMMI Res. (2021) 111:2. doi: 10.1186/s13550-020-00735-w
85. Bigos KJ, Quiles CG, Lunj S, Smith DJ, Krause M, Troost EG, et al. Tumour response to hypoxia: Understanding the hypoxic tumour microenvironment to improve treatment outcome in solid tumours. Front Oncol. (2024) 14:1331355. doi: 10.3389/fonc.2024.1331355
86. Sommat K, Tong AKT, Ong ALK, Hu J, Sin SY, Lam WWC, et al. 18F-FMISO PET-guided dose escalation with multifield optimization intensity-modulated proton therapy in nasopharyngeal carcinoma. Asia Pac J Clin Oncol. (2023) 20(5):611–9. doi: 10.1111/ajco.13953
87. Grkovski M, Schöder H, Lee NY, Carlin SD, Beattie BJ, Riaz N, et al. Multiparametric imaging of tumor hypoxia and perfusion with 18F-fluoromisonidazole dynamic pet in head and neck cancer. J Nucl Med. (2017) 587:1072–80. doi: 10.2967/jnumed.116.188649
88. Johnson GB, Harms HJ, Johnson DR, Jacobson MS. PET imaging of tumor perfusion: A potential cancer biomarker? Semin Nucl Med. (2020) 506:549–61. doi: 10.1053/j.semnuclmed.2020.07.001
89. Rickard AG, Palmer GM, Dewhirst MW. Clinical and pre-clinical methods for quantifying tumor hypoxia. Adv Exp Med Biol. (2019) 1136:19–41. doi: 10.1007/978-3-030-12734-3_2
90. Ljungkvist AS, Bussink J, Rijken PF, Kaanders JH, van der Kogel AJ, Denekamp J. Vascular architecture, hypoxia, and proliferation in first-generation xenografts of human head-and-neck squamous cell carcinomas. Int J Radiat Oncol Biol Phys. (2002) 541:215–28. doi: 10.1016/s0360-3016(02)02938-3
Keywords: dynamic PET, dynamic acquisition, quantification, oncology, clinical application
Citation: Wang Y, Yang Y, Li J, Cheng D, Xu H and Huang J (2025) Dynamic FDG PET/CT imaging: quantitative assessment, advantages and application in the diagnosis of malignant solid tumors. Front. Oncol. 15:1539911. doi: 10.3389/fonc.2025.1539911
Received: 05 December 2024; Accepted: 24 March 2025;
Published: 14 April 2025.
Edited by:
Wen Shen, Tianjin First Central Hospital, ChinaReviewed by:
Sikandar Shaikh, Shadan Hospital and Institute of Medical Sciences, IndiaCopyright © 2025 Wang, Yang, Li, Cheng, Xu and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinbai Huang, WVpqaW5iYWlodWFuZ0AxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.