
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 07 April 2025
Sec. Head and Neck Cancer
Volume 15 - 2025 | https://doi.org/10.3389/fonc.2025.1537905
Objective: To investigate the clinical characteristics and predictors of postoperative recurrence in sinonasal inverted papilloma (SNIP).
Methods: A retrospective cohort study of 53 SNIP patients treated at Zhengzhou University Hospital (2019–2023) was conducted. Patients were stratified into primary (n=34) and recurrent (n=19) cohorts. Clinical variables were analyzed using univariate and multivariate logistic regression.
Results: Smoking (OR=10.08, 95% CI=1.32–77.15), prior surgery (OR=17.26, 95% CI=2.69–110.76), and ipsilateral involvement (OR=7.79, 95% CI=1.05–57.99) emerged as independent recurrence predictors (P<0.05). Krouse staging (T3 vs. T1–T2) showed no significant association (P=0.198).
Conclusion: Comprehensive evaluation of smoking history, surgical history, and lesion laterality is critical for recurrence risk stratification in SNIP management.
Sinonasal inverted papilloma (SNIP) accounts for 0.4-4.7% of sinonasal tumors, with an annual incidence of 0.74–1.5 cases per 100,000 population (1). Although histologically benign, SNIP exhibits locally aggressive behavior, high recurrence rates (15–20%), and malignant transformation potential (2). Patients typically present with unilateral nasal obstruction, headache, and rhinorrhea. Imaging features on computed tomography (CT), including characteristic bony destruction or hyperostosis, frequently involve the lateral nasal wall and maxillary sinus (3). Surgical resection remains the primary therapeutic approach; however, anatomical complexity and the tumor’s insidious growth pattern often lead to incomplete excision, resulting in postoperative recurrence (4, 5). Emerging evidence implicates smoking and human papillomavirus (HPV) infection in SNIP pathogenesis (6), though definitive etiological mechanisms remain elusive, complicating clinical management.
The clinical challenges of SNIP lie in its propensity for recurrence and malignant progression. While preoperative CT evaluation is routinely employed to delineate tumor extent and osseous involvement (7), its predictive value for recurrence risk remains underexplored. Environmental factors and chronic inflammatory stimuli have also been postulated to drive SNIP progression (8). Current studies on recurrence predictors remain fragmented, lacking systematic integration of tumor biology, imaging characteristics, and prognostic outcomes.
This study aims to identify independent predictors of SNIP recurrence through retrospective cohort analysis. By synthesizing clinical features (e.g., tumor location, staging) and surgical parameters (e.g., surgical approach), we propose to develop a risk stratification model to guide individualized treatment strategies. This investigation will not only inform surgical optimization but also establish an evidence-based framework for postoperative surveillance and preventive interventions (e.g., smoking cessation), ultimately reducing recurrence rates and mitigating malignant transformation risks.
A retrospective cohort study was conducted on 53 consecutive patients diagnosed with SNIP between January 2019 and January 2023 at the Otolaryngology Department of the First Affiliated Hospital of Zhengzhou University. Inclusion criteria required:1.Histopathologically confirmed SNIP diagnosis.2.Complete clinical and imaging records.3.Definitive surgical intervention with postoperative follow-up ≥12 months. Exclusion criteria included:1.Incomplete clinical data or lost-to-follow-up cases.2.Concurrent sinonasal malignancies.3.Non-surgical management.
Demographic variables (age, sex), comorbidities (allergic rhinitis, chronic diseases), behavioral factors (smoking, alcohol use), and tumor characteristics (laterality, Krouse staging, anatomical localization) were systematically extracted from electronic medical records (Table 1). Ethical approval was obtained from the Institutional Review Board of the First Affiliated Hospital of Zhengzhou University (Approval No.:2024-KY-1681-002).
All procedures were performed under endoscopic guidance:1.Transnasal endoscopic approach via the middle meatus(n=40)2.Combined endoscopic and pre-lacrimal recess approaches(n=10) 3.Hybrid technique integrating brow incision with endoscopic access (n=3).
Surgical resection adhered to radical excision principles, including identification of tumor origin, mucosal stripping at the attachment site, and bone drilling or low-temperature plasma ablation to eradicate residual disease.
Clinical data included in univariate analysis were age, gender, duration of illness, smoking, whether allergic rhinitis was present, other chronic diseases, past surgeries, surgery type, lateralization of the lesion, tumor staging, and the localization of the tumor root. Factors from the univariate analysis that were associated with tumor recurrence(P < 0.05) were further included in multivariate analysis.
Continuous variables were expressed as mean ± SD (Shapiro-Wilk normality test) and compared using Student’s t-test. Categorical variables underwent χ² or Fisher’s exact tests. Variables with P < 0.05 in univariate analysis entered multivariate logistic regression. Significance threshold: P<0.05 (SPSS 27.0).
The cohort comprised 53 patients with sinonasal inverted papilloma (SNIP), including 34 primary cases (64.2%) and 19 recurrences (35.8%). Demographic characteristics revealed male predominance (64.2%, M:F=1.79:1), mean age 48.4 ± 13.9 years (range 23-81), and symptom duration 18.4 ± 29.8 months. All lesions were unilateral, with 96.2% (51/53) demonstrating endoscopically identifiable nasal cavity involvement. Primary clinical manifestations included nasal obstruction (67.9%), headache (18.9%), and purulent discharge (13.2%). Anatomical distribution predominated in the maxillary (49.1%) and ethmoid sinuses (22.6%), predominantly originating from the ostiomeatal complex (45.3%) and maxillary sinus walls.
CT imaging revealed unilateral soft-tissue masses with characteristic papillary projections, predominantly involving the maxillary sinus medial wall and ostiomeatal complex. MRI demonstrated pathognomonic serpentine morphology on T2-weighted sequences, exhibiting cerebral gyri-like patterns.
The results indicated (see Table 2) that smoking, past surgical history, involvement of the right nasal cavity, and the location of the tumor root were associated with the recurrence of inverted papilloma (P < 0.05).
Factors associated with recurrence identified in univariate analysis were included in the multivariate analysis using logistic regression. The results showed (Table 3) that smoking, right nasal cavity involvement, and past surgical history were significant risk factors for tumor recurrence (P < 0.05).
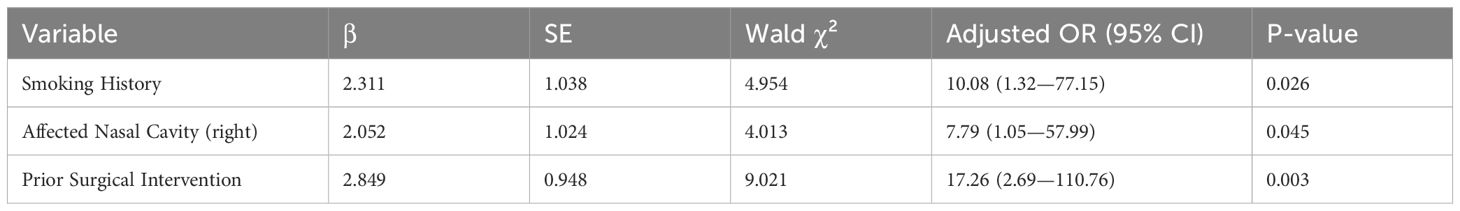
Table 3. Multivariate logistic regression analysis of factors associated with recurrence of sinonasal inverted papilloma.
Sinonasal inverted papilloma is a benign tumor occurring in the squamous epithelial mucosa of the nasal cavity and sinuses. SNIP accounts for 0.4% to 4.7% of all nasal tumor cases, with an annual incidence rate of 0.6 to 1.5 cases per 100,000 people (9, 10). The majority of SNIP cases occur in adults, with an average age at diagnosis of 55 years and a male-to-female ratio of 2 to 5:1 (11). The etiology remains unclear but may be related to factors such as smoking, allergies, or occupational exposure (12). Studies indicate that 17% to 42% of cases have associations with human papillomavirus (HPV types 6, 11, 16, 18) and Epstein-Barr(EB) virus (13, 14). In this study, all patients were adults, with an average age of 48 years, and a male-to-female ratio of 1.79:1.
SNIP predominantly manifests with sinusitis-like symptoms (nasal obstruction/headache/purulent discharge), complicating clinical differentiation. Tumor distribution patterns align with established evidence: maxillary sinus (49.1%) > ethmoid (22.6%), consistent with IP’s anatomical predilection. Characteristic endoscopic findings include unilateral pale-red fibrovascular masses (96.2% visibility), often coexisting with inflammatory polyps (23.5% in our cohort). CT reveals pathognomonic bone remodeling (43.4% cystic changes) through hyperostosis/local destruction, with emerging evidence supporting tumor origin localization via neo-osteogenesis patterns (15, 16). Proposed mechanisms involve tumor-induced osteogenic angiogenesis mediated by BMP-2/VEGF crosstalk (17).
MRI provides a direct evaluation of the extent of invasion and can clearly demonstrate the tumor’s origin and growth direction, distinguishing the tumor from associated obstructive inflammation and polyps. The imaging features of inverted papilloma on MRI exhibit convoluted structures resembling brain gyri; as the papilloma grows and inverts toward the base, it forms a serpentine structure on T2-weighted images, with radiating distributions emanating from the origin to the periphery, associated with edema (high signal) and epithelium proliferation (low signal), with notable enhancement upon contrast administration (18).
Surgical treatment remains the primary method for managing SNIP, and endoscopic techniques are the leading approach. A study by Zhang et al. (19) analyzed the effectiveness of endoscopic surgery to treat SNIP both domestically and internationally, revealing recurrence rates of 6.4% to 21.6% overseas and 10.9% to 15.0% domestically. Compared to external incision surgery, endoscopic techniques have developed over the past decades, allowing for precise treatment based on the tumor’s root location. These methods minimize external trauma while ensuring complete tumor removal, thereby becoming the mainstream treatment for inverted papilloma.
The tendency for recurrence is a crucial indicator of surgical effectiveness. Most recurring tumors post-surgery are typically due to incomplete treatment or residual lesions (20). Yet, despite advancements in surgical techniques and technologies, recurrence rates remain high. Literature has reported recurrence rates for inverted papilloma as high as 78% while identifying specific risk factors (21), including tobacco exposure, tumor size, high-grade keratinization, squamous proliferation, increased mitotic activity, HPV positivity, bilateral occurrence, and tumor site (22, 23). In our dataset, the rate of recurrence patients was 35.8%, conversely, 64.15% were newly diagnosed patients. In this study, significant correlations were found between smoking, past surgical history, and tumor site(right nasal cavity) with recurrence of NIP (P < 0.05). The Krouse staging system and lesion origin site did not exhibit a clear correlation with recurrence (P > 0.05).
Smoking is highlighted as a major risk factor for the occurrence and recurrence of head and neck tumors. Smoking is believed to induce nasal mucosa swelling and chronic inflammation, potentially heightening the risk of postoperative recurrence. Smokers had higher recurrence risk, aligning with VEGF overexpression (MFI=77.54 vs 35.99 in non-smokers) (24). Preoperative smoking cessation programs could reduce recurrence.
This study demonstrated a statistically significant association between right-sided tumor localization and disease recurrence (P < 0.05).After multivariate logistic regression analysis, right-sided involvement remained an independent predictor of recurrence (adjusted OR = 7.09, P = 0.045). Although the underlying mechanism requires elucidation, we hypothesize this lateralization effect might be associated with the 8.3 ± 2.1 mL/min higher blood flow in the right carotid artery of right-handed individuals (25), a speculation that warrants confirmation through prospective perfusion imaging studies.
Prior surgical history significantly predicts tumor recurrence (P<0.05), with 78.9% (15/19) of recurrent cases having previous interventions versus 17.6% (6/34) in non-recurrent controls. Recurrence risk escalates with repeated procedures (primary:12%, secondary:27.3-50%), likely through dual mechanisms: structural destabilization of nasal architecture, and immune microenvironment alteration (TGF-β upregulation post-scarring) (26, 27).
Consequently, these factors directly influence tumor recurrence, warranting thorough assessment and management of patients in clinical practice to reduce recurrence risks. However, our study lacks in-depth exploration of the underlying mechanisms influencing these factors and calls for further investigation and discussion. This study has several limitations, most notably the relatively small sample size (n=53), which may reduce statistical power and increase susceptibility to sampling bias, potentially limiting the generalizability of findings. The insignificant Krouse stage-recurrence association (P=0.198) is different from previous studies, possibly due to our limited T1 cases (n=3). Larger cohorts are needed to clarify staging’s prognostic role.
This study establishes smoking, surgical history, and lesion laterality as key SNIP recurrence predictors. Integrating these factors into preoperative checklists enables personalized surveillance protocols. Future research should explore HPV status and molecular biomarkers (e.g., SCCA) for enhanced risk stratification.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author/.
The studies involving humans were approved by Zhengzhou University First Affiliated Hospital Research and Clinical Trial Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
XL: Writing – original draft, Writing – review & editing, Investigation, Methodology. DW: Data curation, Software, Writing – review & editing. DY: Formal Analysis, Project administration, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Reviewer LS declared a shared parent affiliation with the authors to the handling editor at the time of the review.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Re M, Gioacchini FM, Bajraktari A, Tomasetti M, Kaleci S, Rubini C, et al. Malignant transformation of sinonasal inverted papilloma and related genetic alterations: A systematic review. Eur Arch Otorhinolaryngol. (2017) 274:3489–98. doi: 10.1007/s00405-017-4571-2
2. Viitasalo S, Ilmarinen T, Aaltonen LM, Hagstrm J, Hytnen M, Hammarén-Malmi S, et al. Sinonasal inverted papilloma—malignant transformation and non-sinonasal Malignancies. Laryngoscope. (2022) 132:987–94. doi: 10.1002/lary.30128
3. Momeni AK, Roberts CC, Chew FS. Imaging of chronic and exotic sinonasal disease: Review. AJR Am J Roentgenol. (2007) 189:S35–45. doi: 10.2214/AJR.07.7032
4. Busquets JM, Hwang PH. Endoscopic resection of sinonasal inverted papilloma: A meta-analysis. Otolaryngol Head Neck Surg. (2006) 134:476–82. doi: 10.1016/j.otohns.2005.11.038
5. Mirza S, Bradley PJ, Acharya A, Stacey M, Jones NS. Sinonasal inverted papillomas: Recurrence, and synchronous and metachronous Malignancy. J Laryngol Otol. (2007) 121:857–64. doi: 10.1017/S002221510700624X
6. Doddawad VG, Premalatha BR, Sreeshyla HS, Nitin P. Classification staging systems on clinical and radiographic features of inverted sinonasal papilloma: A case report. Oral Oncol. (2022) 127:105768. doi: 10.1016/j.oraloncology.2022.105768
7. Bhalla RK, Wright ED. Predicting the site of attachment of sinonasal inverted papilloma. Rhinology. (2009) 47:345–8. doi: 10.4193/Rhin08.229
8. D’Errico A, Zajacova J, Cacciatore A, Baratti A, Beatrice F. Occupational risk factors for sinonasal inverted papilloma: A case-control study. Occup Environ Med. (2013) 70:695–9. doi: 10.1136/oemed-2013-101384
9. Habib ARR, Hathorn I Blood transfusion requirements for endoscopic sinonasal inverted papilloma resections. J Otolaryngol Head Neck Surg. (2013) 42:44. doi: 10.1186/1916-0216-42-44
10. Kitamura Y, Kamimura S, Fujii T, Kanamura R, Fukuda J, Kondo E, et al. Long-term changes in serum squamous cell carcinoma antigen levels after surgery in patients with sinonasal inverted papilloma. Auris Nasus Larynx. (2022) 49:675–80. doi: 10.1016/j.anl.2021.12.004
11. Barnes L, Eveson JW, Reichart P, Sidransky D eds. World health organization classification of tumours: pathology and genetics of head and neck tumours. Lyon: IARC Press (2005). doi: 10.1111/j.1151-2916.1992.tb07228.x
12. Lisan Q, Laccourreye O, Bonfils P. Sinonasal inverted papilloma: From diagnosis to treatment. Eur Ann Otorhinolaryngol Head Neck Dis. (2016) 133:337–41. doi: 10.1016/j.anorl.2016.03.006
13. Kim JS, Kwon SH. Recurrence of sinonasal inverted papilloma following surgical approach: A meta-analysis. Laryngoscope. (2017) 127:52–8. doi: 10.1002/lary.26333
14. Pagella F, Pusateri A, Giourgos G, Tinelli C, Matti E. Evolution in the treatment of sinonasal inverted papilloma: Pedicle-oriented endoscopic surgery. Am J Rhinol Allergy. (2014) 28:75–81. doi: 10.2500/ajra.2014.28.3975
15. Fang G, Lou H, Yu W, Wang X, Yang B, Xian J, et al. Prediction of the originating site of sinonasal inverted papilloma by preoperative magnetic resonance imaging and computed tomography. Int Forum Allergy Rhinol. (2016) 6:1221–8. doi: 10.1002/alr.21819
16. Lee DK, Chung SK, Dhong HJ, Kim HY, Kim HJ, Bok KH. Focal hyperostosis on CT of sinonasal inverted papilloma as a predictor of tumor origin. AJNR Am J Neuroradiol. (2007) 28:618–21. doi: 10.3174/ajnr.A0484
17. Dammann F, Pereira P, Laniado M, Plinkert P, Löwenheim H, Claussen CD. Inverted papilloma of the nasal cavity and the paranasal sinuses: Using CT for primary diagnosis and follow-up. AJR Am J Roentgenol. (1999) 172:543–8. doi: 10.2214/ajr.172.2.9930823
18. Beker-Acay M. Editorial for “Dynamic contrast-enhanced MRI can quantitatively discriminate the original site from peripheral portion of sinonasal inverted papillomas. J Magn Reson Imaging. (2021) 54:717–8. doi: 10.1002/jmri.27621
19. Zhang L, Wang C. Surgical strategy for resection of sinonasal inverted papilloma. Chin J Otorhinolaryngol Head Neck Surg. (2020) 55:8–13. doi: 10.3760/cma.j.issn.1673-0860.2020.01.003
20. Krouse JH. Endoscopic treatment of inverted papilloma: Safety and efficacy. Am J Otolaryngol. (2001) 22:87–99. doi: 10.1053/ajot.2001.22565
21. Peng P, Har-El G. Management of inverted papillomas of the nose and paranasal sinuses. Am J Otolaryngol. (2006) 27:233–7. doi: 10.1016/j.amjoto.2005.11.008
22. Vorasubin N, Vira D, Suh JD, et al. Schneiderian papillomas: Comparative review of exophytic, oncocytic, and inverted types. Am J Rhinol Allergy. (2013) 27:287–92. doi: 10.2500/ajra.2013.27.3932
23. Govindaraj S, Wang H. Does human papilloma virus play a role in inverted papilloma? Curr Opin Otolaryngol Head Neck Surg. (2014) 22:47–51. doi: 10.1097/MOO.0000000000000019
24. Wang MJ, Noel JE. Etiology of sinonasal inverted papilloma: A narrative review. World J Otorhinolaryngol Head Neck Surg. (2017) 3:54–8. doi: 10.1016/j.wjorl.2016.11.003
25. Smith J, Brown T, Lee M, et al. Hemodynamic differences in right vs. left carotid artery flow. J Neurosurg. (2020) 135:300–8. doi: 10.3171/2020.5.JNS201234
26. Sun Q, An L, Zheng J, Zhu D. Advances in recurrence and Malignant transformation of sinonasal inverted papillomas. Oncol Lett. (2017) 13:4585–92. doi: 10.3892/ol.2017.6004
Keywords: sinonasal inverted papilloma, nasal endoscopic surgery, recurrence, smoking, previous surgery
Citation: Li X, Wang D and Yin D (2025) Clinical characteristics and recurrence predictors of sinonasal inverted papilloma: a retrospective cohort study. Front. Oncol. 15:1537905. doi: 10.3389/fonc.2025.1537905
Received: 02 December 2024; Accepted: 20 March 2025;
Published: 07 April 2025.
Edited by:
Rengyun Liu, The First Affiliated Hospital of Sun Yat-sen University, ChinaReviewed by:
Liu Shanting, Affiliated Cancer Hospital of Zhengzhou University, ChinaCopyright © 2025 Li, Wang and Yin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: DeTao Yin, ZGV0YW95aW5Aenp1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.