
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 19 February 2025
Sec. Cancer Imaging and Image-directed Interventions
Volume 15 - 2025 | https://doi.org/10.3389/fonc.2025.1537688
Introduction: This study aimed to evaluate non-tumor liver parenchymal injury in advanced gastric cancer patients undergoing transarterial infusion chemotherapy (TAI) using imaging parameters and liver injury biomarkers, providing objective evidence for early detection of drug-induced liver injury.
Methods: A retrospective analysis was conducted on 52 advanced gastric cancer patients who received TAI at our center from July 2015 to July 2023. Abdominal CT images and laboratory data were collected before and after treatment. Imaging postprocessing software was used to measure the liver-to-spleen (L/S) attenuation ratio and spleen volume. Liver fibrosis indices (APRI, FIB-4) and liver function scores (Child-Pugh, ALBI) were calculated. Statistical analysis included Wilcoxon rank-sum test and paired t-test for pre- and post-treatment comparisons.
Results: Patients received an average of 2.80 TAI cycles over 8.3 weeks. Post-treatment, 76.92% (40/52) showed a significant reduction in L/S attenuation ratio (1.23 ± 0.13 vs. 1.12 ± 0.1, P < 0.01), and 73.1% (38/52) exhibited increased spleen volume (151,219.33 mm³ vs. 202, 171.32 mm³, P < 0.01). Liver fibrosis indices significantly increased: APRI (0.19 ± 0.15 vs. 0.37 ± 0.27) and FIB-4 (1.29 ± 0.88 vs. 2.24 ± 1.38) (P < 0.01). No significant changes were observed in ALBI (-2.7 ± 0.41 vs. -2.58 ± 0.43) or Child-Pugh scores (5.31 ± 0.47 vs. 5.38 ± 0.64) (P > 0.05).
Discussion: Transarterial infusion chemotherapy for advanced gastric cancer results in short-term damage to the non-tumor liver parenchyma, with imaging findings showing hepatic steatosis (reduction in L/S ratio) and splenomegaly. Serum markers suggest progression of hepatic fibrosis, consistent with the pathological features of hepatic sinusoidal obstruction syndrome. The combination of CT imaging and APRI/FIB-4 provides a sensitive method for detecting subclinical liver injury, reflecting pathological changes earlier than traditional liver function scores.
Gastric cancer is the fifth most common cancer worldwide and one of the three leading causes of cancer deaths (1). In China, early gastric cancer accounts for approximately 20% of all gastric cancers, and the majority of gastric cancer patients are already in advanced stages when they seek treatment, resulting in a 5-year survival rate of less than 50% (2). Chemotherapy is a common treatment for advanced gastric cancer, and improving its efficacy, especially with neoadjuvant chemotherapy and translational therapy, thus increasing the rate of surgical resection, is an important factor affecting patient survival. In recent years, neoadjuvant chemotherapy has been gradually applied in the treatment of advanced gastric cancer and has achieved excellent clinical efficacy (3). Neoadjuvant chemotherapy is effective in reducing the tumor lesion size, lowering the tumor stage, and increasing the rate of radical treatment (4). Early systemic therapy also provides rapid relief from symptoms, eliminates tumor micrometastases, and prevents intraoperative tumor spread and postoperative tumor recurrence.
Recently, with advancement of minimally invasive techniques, such as endovascular intervention, transarterial infusion chemotherapy has emerged as a new neoadjuvant chemotherapy method, which allows direct delivery of chemotherapy drugs into the target organ through blood supply arteries, increasing drug concentration in the tumor area and enhancing the therapeutic effect. Compared to intravenous infusion, transarterial infusion chemotherapy significantly increases local tumor drug concentrations and improves efficacy (5). Arterial infusion has a higher tumor response rate than that of intravenous infusion. FOLFOX regimen chemotherapy for progressive hepatocellular carcinoma has a tumor response rate of 8.15% for intravenous infusion compared to a 46% tumor response rate for arterial infusion (6, 7). In chemotherapy for colorectal cancer liver metastases, the tumor objective response rate to transarterial infusion of fluorouracil analogues was significantly higher than that to intravenous infusion (41 vs. 14%; P < 0.01) (8). The conventional transarterial infusion chemotherapy is most commonly used in liver cancer, and its use in hollow organ is rare. In recent years, with the advancement of interventional techniques, transarterial chemotherapy infusion has achieved promising results in the treatment of cancers of hollow organs such as gastric, colorectal, bladder, and ovarian cancers (9–11), with particularly significant effects observed in gastric cancer (12–16). Therefore, it is recommended in many current clinical guidelines for tumor treatment (17–19).
However, the adverse effects of transarterial infusion chemotherapy, especially the damage caused by high concentrations of chemotherapeutic agents to adjacent normal tissues and organs, have rarely been reported and are only briefly mentioned or summarized in the relevant literature as undetailed hepatotoxicity (8). Therefore, to address this phenomenon, this study investigated the impact of transarterial infusion chemotherapy on non-tumor liver parenchyma in 52 patients with advanced gastric cancer.
This single-center, retrospective, observational study was reviewed and approved by the institutional review board(JD-HG-2024-244). The requirement for informed consent was waived because this study was based on routinely collected claims data.
We retrospectively analyzed the data of 60 patients with locally advanced gastric cancer who received transarterial infusion chemotherapy in the interventional unit of our hospital between July 2015 and July 2023. Inclusion criteria: complete preoperative and postoperative plain CT and enhanced CT of the upper abdomen, complete relevant laboratory parameters, ECOG (physical status) score ≤ 1. Patients undergo a minimum number of infusion treatment cycles greater than or equal to 2. Exclusion criteria: patients involved in other therapeutic regimens, patients with a combination of other malignant tumors, patients with severe underlying liver disease such as various types of cirrhosis, viral hepatitis, and moderate-to-severe fatty liver. Fifty-two patients who met the criteria were screened. The baseline and tumor characteristics of the patients are presented in Table 1.
Chemotherapy regimen: oxaliplatin 100 mg/m ², etoposide 80 mg/m ², epimedium 30 mg/m ², and S-1 40–60 mg/m ².
Procedure: The 4F vascular sheath was inserted percutaneously into the femoral artery using the modified Seldinger technique. The RH catheter was then directed into the celiac axis and connected to an external high-pressure injector for high-pressure angiography. Based on the lesion location and the main responsible vessel, the microcatheter was advanced into the main vessel and left in place. The retention locations of the microcatheter are shown in Table 1.
Medication methods: On the first day, half of the chemotherapeutic drugs were infused via a microcatheter in the following order: oxaliplatin, etoposide, and epothilone; each drug was infused for 1 h. On the second day, the microcatheter was retreated to a 4F RH tube in the celiac trunk, and the remaining drugs were infused in the celiac axis in the same order and at the same time as before. Additionally, all patients received oral S-1, (40–60 mg, twice a day; the total dose depending on the patient’s body surface area as follows: <1.25 m2, 80 mg; 1.25–1.50 m2, 100 mg; and >1.50 m2, 120 mg; on days 1–14 of every 3-week cycle. Treatment cycles were performed every 3 weeks. Imaging and laboratory tests were reviewed after 2-3 cycles for evaluation.
1. L/S density ratio measurement and calculation: Hepatic and splenic ratios were measured in the unenhanced phase of CT scans using region-of-interest (ROI) cursors with an area of 1 mm² in the liver and spleen. The ROIs were located in the caudate, left, and right lobes of the liver. In the spleen, ROIs were located at the same level as the selected liver, and all measurements were manually obtained in regions of uniform parenchymal attenuation, with care taken to avoid vessels, artefacts, and other areas that might have spuriously increased or decreased the measurements. The average of the measurements of each segment of the liver and spleen was taken, and the L/S density ratio was calculated (Figure 1).
2. Splenic volume measurement: We collected all patients’ upper abdominal CT images in DICOM format and imported thin-layer plain CT images into the 3D-Slicer software (www.slicer.org). Two experienced radiologists identified the optimal levels for outlining the spleen’s ROIs. They traced the spleen at multiple levels, automatically generating 3D stereo images through level tracing and slice filling functions, finally calculating the spleen volume. (To ensure accuracy of the data, the shape and edges of the spleen were carefully observed during the sketching process).
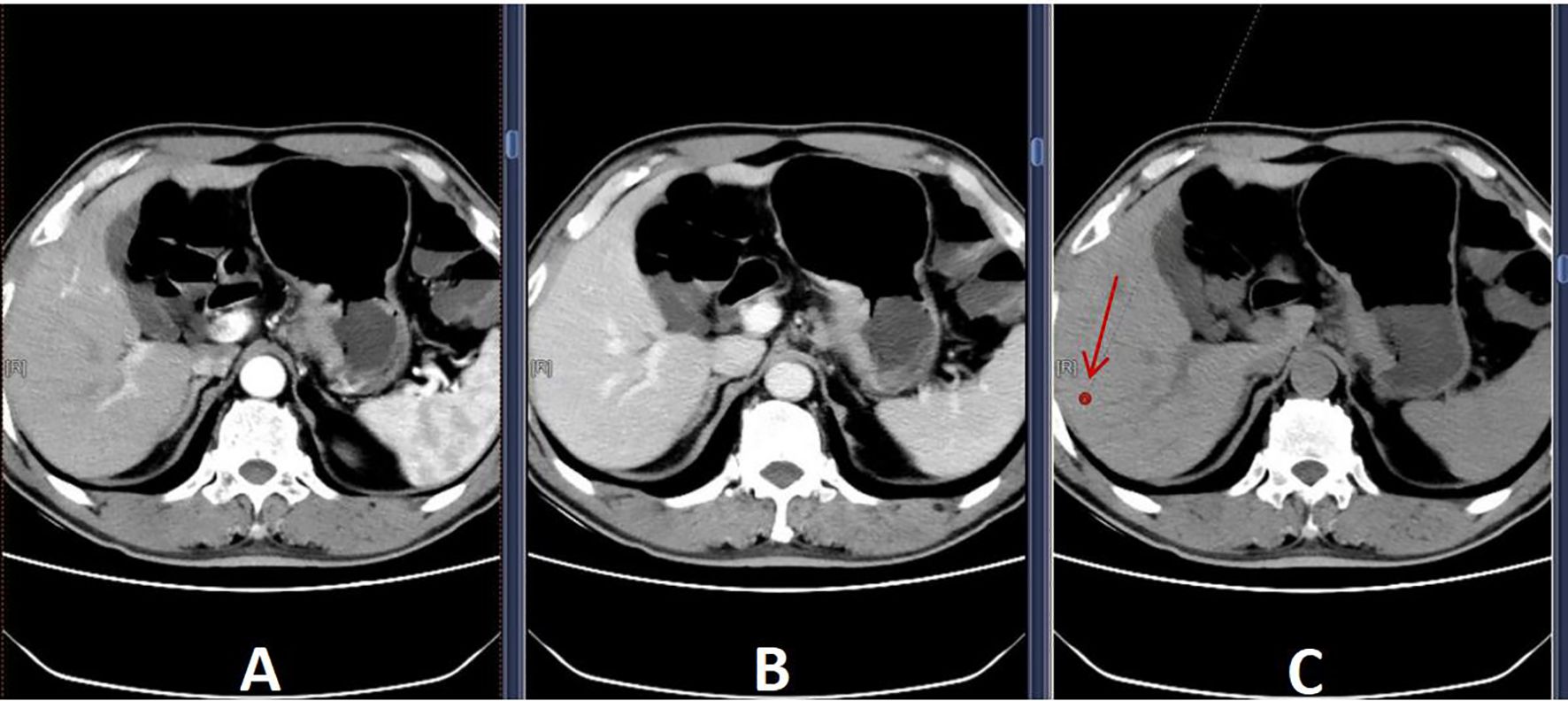
Figure 1. Selected ROIs (A) Arterial phase; (B) Delayed phase; (C) Unenhanced phase. To avoid blood vessels, bile ducts, and areas of heterogeneity, a 1 mm² region-of-interest (arrow) was selected in (C) to measure liver density.
1) APRI(aspartate aminotransferase-platelet ratio index)=
(20).
2) FIB-4(fibrosis index based on 4 factor)=
(20)
1) Liver functional enzyme profiles: ALT, AST, ALP, GGT and TBIL.
2) Albumin-Bilirubin Grade (ALBI)=
(21)
3) Child-Turcotte-Pugh score (22).
Statistical analysis was performed using SPSS 27, and the measurement data are expressed as mean ± S standard deviation. Data that conformed to normal distribution with chi-square were tested using paired t-test, and data that did not conform to normal distribution were tested using Wilcoxon signed rank sum test. To control the false discovery rate (FDR) in multiple comparisons, we used the Benjamini-Hochberg procedure. All P values were first ranked and then adjusted by the FDR method to control for FDR at the 0.05 level.
The success rate of the interventional procedure was 100%, with no complications related to the surgical procedure. Catheter retention positions were as follows: RH catheter was left in the celiac axis in 52 cases; The placement of microcatheters are determined based on angiography (details in Table 1). The average number of transarterial infusion chemotherapy cycles was 2.77 (X-ray images are shown in Figure 2).
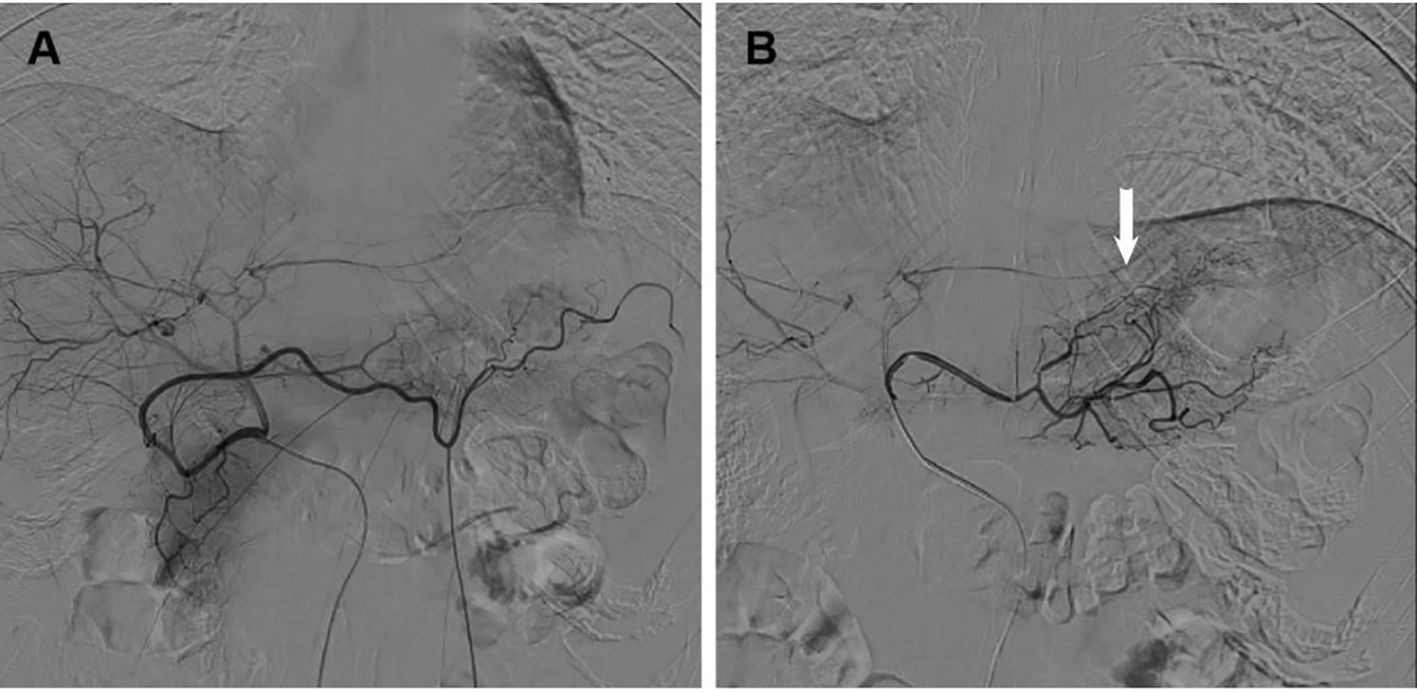
Figure 2. X-ray images of catheter retention positions (A) Angiography of the RH catheter in the celiac axis; (B) Angiography of microcatheter in tumor blood supplying arteries (Arrow: Tumor blood supplying arteries).
L/S density ratio decreased after transarterial infusion chemotherapy in 76.92% patients (40/52), of which 46.15% (24/52) showed decreased postoperative L/S density ratio (to ≤ 1.1). Compared with pre-treatment, the mean reduction in L/S density ratio after treatment was 0.11. After applying the Benjamini-Hochberg procedure for controlling the false discovery rate, the statistically significant difference persisted (1.23 ± 0.13 vs 1.12 ± 0.1, corrected P < 0.01).
Following transarterial infusion chemotherapy, spleen volume increased in 73.07% of patients (38/52), with 14 patients showing an increase of more than 30%, and 6 patients experiencing an increase of more than 50% (Figure 3). The mean increase in volume was 14% (range: -48.63%–77.78%). The median increase in spleen volume compared to pre-treatment was 50,951.97 mm³ (P < 0.05) (Table 2).
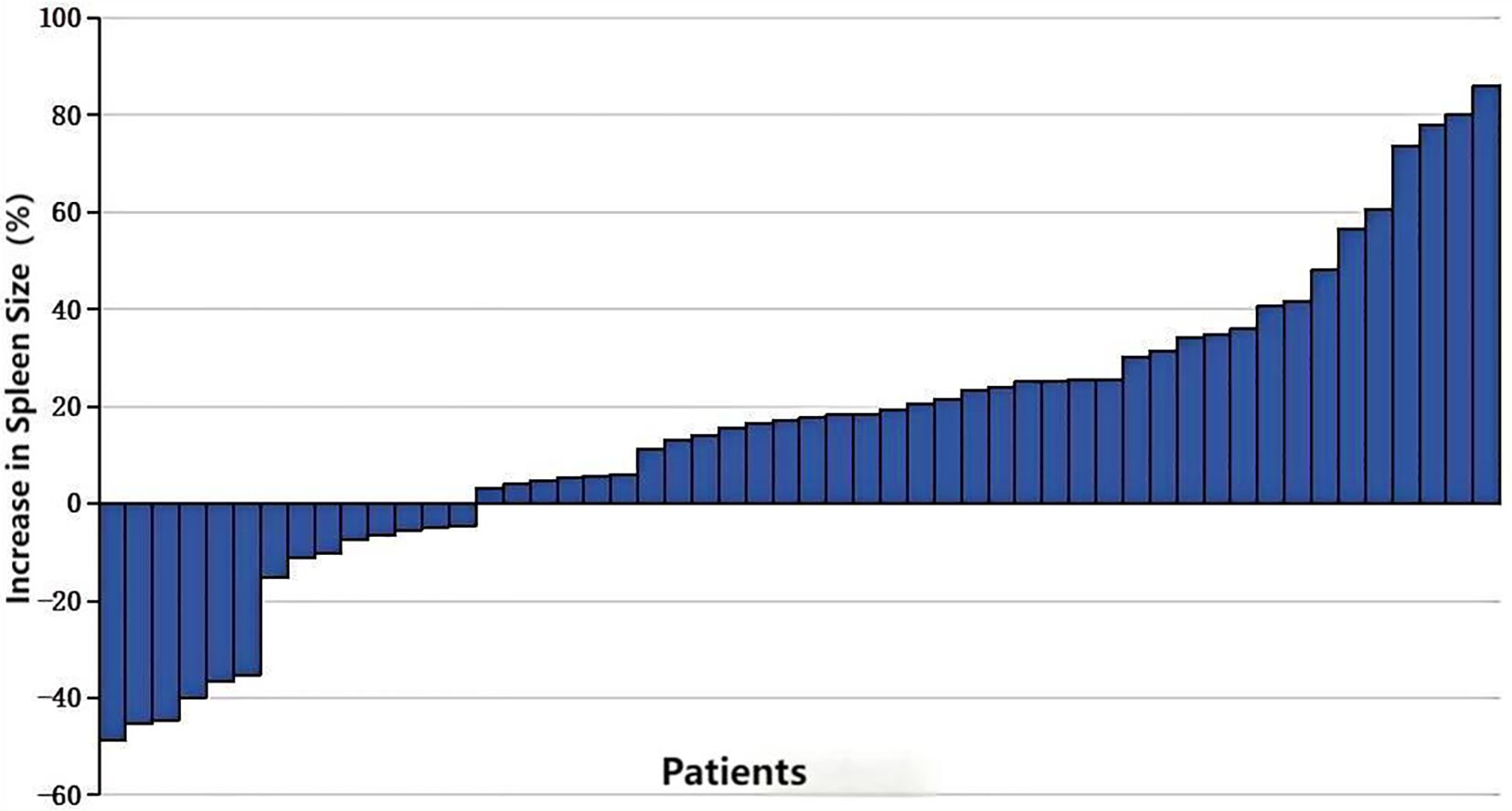
Figure 3. Percentage increase in splenic volume size in patients receiving 2-3 cycles of transarterial infusion chemotherapy.
The FIB-4 index was elevated in 42 patients (80.77%) after transarterial perfusion chemotherapy, including twelve patients with FIB-4 > 3.25; meanwhile, 42 patients (80.77%) had elevated APRI values post-procedure, with three having an APRI > 0.79. A strong correlation was observed between the magnitude of increase in FIB-4 and APRI, with a correlation coefficient of 0.93 (P < 0.05) (Figure 4). The hepatic fibrosis indices FIB-4 and APRI of patients after transarterial infusion chemotherapy were significantly greater than pre-treatment, with the differences being statistically significant after applying the Benjamini-Hochberg procedure for controlling the false discovery rate (adjusted P < 0.01) for multiple comparisons (Table 3).
Changes in liver function indicators before and after treatment, as assessed using the drug indices of liver damage (including glutamic aminotransferase, glutamic alanine aminotransferase, total bilirubin, and serum albumin), were not statistically significant after applying the Benjamini-Hochberg procedure for controlling the false discovery rate (adjusted P > 0.05) (Figure 5).
In recent years, several studies have highlighted the promising clinical outcomes of transarterial infusion chemotherapy in the treatment of progressive gastric cancer. The treatment regimen used in this study was based on a previous investigation by Li et al. (23–25). The arterial chemotherapeutic agents used included oxaliplatin, etoposide, and epirubicin, combined with oral S-1 chemotherapy. Although this treatment regimen has demonstrated promising therapeutic efficacy in clinical settings, there are limited reports on its potential side effects, particularly damage to adjacent organs, such as the liver. In recent years, with the advancement of medical imaging technology, radiomics, particularly quantitative imaging analysis, has become increasingly widespread. As an effective evaluation method, quantitative imaging analysis has been extensively applied in the clinical monitoring of functional and structural changes in the liver and spleen (26, 27). Changes in the L/S density ratio have been shown to be significantly correlated with chemotherapy-induced liver injury, while an increase in splenic volume is closely associated with the degree of systemic inflammatory response and liver damage. Therefore, in this study, based on imaging parameters such as the L/S density ratio and splenic volume, in combination with corresponding hepatic fibrosis markers, we identified latent liver damage caused to the normal hepatic parenchyma during chemotherapy in the progression of advanced gastric cancer.
The L/S density ratio is currently the main noninvasive method for diagnosing fatty liver and assessing the presence of steatosis in donor livers prior to liver transplantation. The diagnosis of fatty liver is based on the principle that fat accumulation in the liver leads to decreased liver density and lower CT values. A liver density lower than that of the spleen indicates higher diagnostic specificity, with an L/S density ratio of <1 being the recognized criterion. As this ratio decreases, the severity of fatty liver worsens (28, 29). Hepatocellular steatosis is a key indicator for assessing the quality of living donor livers before liver transplantation, with optimal donors having steatosis below moderate levels (< 30%). Thus, noninvasive and quantitative assessment of hepatic steatosis ≥ 30% has become a research focus. In previous studies on CTL/S(the liver-to-spleen attenuation ratio. It was calculated as L/S, where L is the hepatic attenuation and S is the splenic attenuation) in the quantitative diagnosis of hepatic steatosis (29, 30), the specificity and sensitivity of the diagnosis of hepatic steatosis >30% were 100% and 82%, 97% and 79%, and 83% and 82% when the critical values of L/S density ratio were set to 0.80, 0.90, and 1.10, respectively. Hence, the L/S density ratio is an important method for noninvasively quantifying hepatic steatosis, helping to avoid unnecessary liver biopsies. Previous studies have reported chemotherapy-associated liver injury, with pathologic changes including hepatocellular steatosis (31–33). For example, Vauthey et al. found that a 17.32% incidence of moderate-to-severe hepatic steatosis-like changes in patients with colorectal cancer liver metastases who received preoperative intravenous chemotherapy (34). Additionally, Paul Ryan et al. (35) reported that 9.90% of 334 patients with liver recurrence after colorectal cancer surgery had significant steatosis (>33%) following intravenous adjuvant chemotherapy prior to hepatectomy. A limitation of these studies is that all patients had liver lesions pre-surgery, and although the pathology samples were taken from non-tumor liver tissue, baseline characteristics varied. To further investigate the impact of chemotherapeutic agents on non-tumor liver parenchyma, our study selected patients with advanced gastric cancer who had normal liver function before surgery. We utilized a noninvasive method (L/S density ratio) to assess liver parenchyma damage and evaluate the presence of steatosis or steatohepatitis (36, 37). In this study, the postoperative L/S density ratio of the patients was significantly lower than that in the preoperative period (P<0.05). A study conducted by Kanae Miyake and colleagues investigated the effects of oral 5-fluorouracil (5-FU) therapy on liver fat content in patients with colorectal cancer. The findings revealed a significant reduction in liver CT values following adjuvant chemotherapy with oral 5-FU. We have shown a statistically significant decrease in hepatic CT values associated with adjuvant chemotherapy using oral 5-FU drugs in patients with colon cancer, which was observed even without abnormalities in laboratory data (38).Our findings align with these results, suggesting that the observed reduction in postoperative liver CT attenuation values is attributable to the oral administration of S-1. This reduction indicates an increase in liver fat content, which may contribute to the frequent occurrence of hepatic steatosis in these patients. Tegio is a fluorouracil derivative that is usually administered orally. The oral administration of 5-FU analogs has been associated with increased hepatic fat content in patients. A kinetic study employing magnetic resonance spectroscopy (MRS) revealed that during intra-arterial or intravenous infusion of 5-FU, the drug itself cleared from circulation relatively quickly. However, FBAL( (Alpha-fluoro-beta-alanine)), a major metabolite of 5-FU, gradually accumulated in the liver, leading to an increase in liver fat content in affected patients (39).Potential mechanisms underlying the increase in liver fat content may include: 1. Exposure to FBAL, a fluorinated compound, which induces significant alterations in lipid-related metabolites, including lysophosphatidylethanolamine and lysophosphatidylcholine, thereby impacting hepatic lipid metabolism and contributing to an elevation in hepatic fat content (40); 2. The accumulation of FBAL may disrupt mitochondrial membrane integrity and decrease membrane potential, impairing mitochondrial function. These effects can contribute to liver injury by interfering with key cellular processes and energy production (41).
In this study, patients showed a significant increase in spleen volume postoperatively (P<0.05), which we consider to be another manifestation of hepatic damage induced by chemotherapeutic agents infused through the transcatheter trunk artery. A prospective study enrolling 50 patients with malignant tumors receiving intravenous chemotherapy containing oxaliplatin showed that 37 patients (74%) had an increase in splenic volume after chemotherapy, with a median increase of 31% and a maximum increase of 119% at 3 months (42). In another retrospective study of 161 patients receiving oxaliplatin-containing intravenous chemotherapy, CT imaging assessed spleen size after 6 months of treatment. An increase in splenic volume was observed in 104 patients (64.60%), with 19.43% of the patients having an increase of ≥30%, and 8.10% having an increase of ≥50% (43). Toi H et al. (44) investigated the relationship between the FOLFOX regimen and spleen volume, showing that after 6 months of FOLFOX intravenous chemotherapy, spleen volume (measured on CT images) increased from 229 cm³ to 323 cm³ (P<0.01), with 44.41% of patients showing an increase of >50%. The increase in splenic volume and hepatic sinusoidal injury due to chemotherapeutic agents, especially oxaliplatin, are closely related. Rubbia-Brandt et al. (33) retrospectively analyzed 153 patients with liver metastases from colorectal cancer; 44 of 87 patients (50.57%) who received preoperative oxaliplatin-containing chemotherapy developed pathological changes of hepatic sinusoidal damage, whereas the 66 patients who did not receive preoperative chemotherapy presented with normal livers. This study noted that liver pathology after surgical resection in patients receiving preoperative intravenous chemotherapy with oxaliplatin showed hepatic sinusoidal damage. Manifestations included sinusoidal dilatation, hemorrhage, thickening of small hepatic veins, fibrosis, and luminal narrowing or occlusion, occurring in 51%-78% of cases. Another study found that in patients with liver metastases treated preoperatively with 5-FU analogues, fluoropyrimidine, and oxaliplatin, both univariate (P=0.03) and multivariate (P=0.02) analyses showed increased spleen volume was associated with hepatic sinusoidal injury and was an independent predictor of severe injury (45). Oxaliplatin chemotherapy is associated with histologic changes in the liver characterized by dilated hepatic sinusoids, congestion, and centrilobular necrosis suggestive of hepatic sinusoidal obstruction syndrome. These changes are not clinically significant in the acute phase and are not accompanied by markedly elevated serum enzyme profiles or clinically significant liver injury (46). Our study is consistent with the findings of this experiment. However, long-term administration of oxaliplatin results in endothelial damage to the hepatic sinusoids within the liver tissue, which leads to non-cirrhotic portal hypertension(NCPH). This condition arises from obstruction of the hepatic sinusoids and the subsequent development of nodular regenerative hyperplasia(NRH). NCPH typically progresses gradually and remains asymptomatic for an extended period until complications such as variceal bleeding and splenomegaly manifest (47). The developmental time for such changes is usually 6 to 18 months (46). In this study, we observed radiological evidence of splenomegaly as early as 2-3 cycles (with an average of 6.8 weeks) post-treatment. We hypothesize that this may be attributed to regional portal vein underperfusion following the intra-arterial infusion of high concentrations of chemotherapeutic agents via the coeliac trunk and hepatic artery. Insufficient blood perfusion induces hepatocyte atrophy and apoptosis, while the perfusion of surrounding acinar cells increases. In areas with excessive perfusion, there is an elevation of cell growth activators, which, acting as autocrine or paracrine peptides, contribute to the formation of NRH. This process leads to the development of splenomegaly following the onset of secondary NCPH (48).
To reduce drug-induced liver injury, the most crucial approach is to minimize the dosage of the drug used. To ensure that the tumor site reaches the same drug concentration, intra-arterial infusion, compared to intravenous administration, can reduce the drug dosage. More prospective studies should be conducted to further optimize the use of drug doses. For example, reducing the duration of drug infusion (49), or using balloon catheters to lower the arterial infusion pressure, can slow down the blood flow rate, thereby reducing the drug dosage while maintaining the same drug concentration and infusion time. In patients with metastatic colorectal cancer who received oxaliplatin-based chemotherapy combined with bevacizumab, a longer median time to splenomegaly and a lower incidence of thrombocytopenia were observed (50). These approaches may delay liver function damage.
The histological manifestations associated with drug-induced liver injury are complex and varied, and the progression can be as severe as that of hepatic fibrosis and cirrhosis (51). FIB-4 and APRI indices are correlated and consistent with the degree of hepatic fibrosis and are commonly used to noninvasively assess the degree of hepatic fibrosis and cirrhosis in patients with chronic viral hepatitis. Rubbia-Brandt et al. (33) reported that 48% of liver pathological tissues obtained after intravenous chemotherapy exhibited steatosis and hepatic fibrosis. Therefore, to noninvasively assess chemotherapy-related hepatic fibrosis, indicators such as FIB-4, APRI, and ALBI have attracted the attention of researchers (52). In a study by Mukai et al. (43), the median FIB-4 index was significantly higher after 6 months of intravenous chemotherapy (1.28 vs 2.50, P<0.01), and nine of these patients developed portal hypertension with portal collateral circulation formation; the authors concluded that the FIB-4 index can be used as a useful predictor of portal hypertension with portal collateral circulation formation. The results of a study by Xuelin et al. on liver injury associated with intravenous chemotherapy for colorectal cancer showed a good correlation between the increase in FIB-4 and APRI (P<0.05) and a positive correlation between the increase in spleen volume and the increase in FIB-4 and APRI (P<0.01), with correlation coefficients of 0.36 and 0.58, respectively (53). Combined with related studies, we believe that FIB-4 and APRI can be used as noninvasive biological indices to monitor tumor chemotherapy-related liver injury and can reflect early hepatic sinusoidal injury more sensitively. In this study, the FIB-4 and APRI indices before and after transarterial infusion chemotherapy were significantly higher (P<0.01), suggesting the development of hepatic fibrosis in the short term after transarterial infusion chemotherapy. In conjunction with related studies, Gu et al. (54)observed that epothilone-treated FGF1 knockout mice exhibited worsening of intestinal fibrosis, accompanied by increased upregulation of connective tissue growth factor expression, a well-established marker of fibrosis. Similarly, a study by I.L. Ke Nalbantoglu et al. reported that the livers of patients treated with oxaliplatin chemotherapy demonstrated capillarization, hepatic sinusoidal fibrosis, and hepatocellular hyperplasia following hepatectomy (55). Based on these findings, we hypothesize that the liver fibrosis observed may result from the combined effects of oxaliplatin and epothilone. However, the exact underlying mechanism remains unclear, and further animal experiments will be conducted to investigate the mechanism of injury in greater detail.
According to the literature (56), chemotherapy-associated liver injury is routinely detected and diagnosed using laboratory tests for liver function, which typically include elevated ALT, ALP, and bilirubin levels, decreased albumin levels, or poor coagulation. In this study, the preoperative and postoperative laboratory indices of liver function, which were within the normal range, failed to meet the common diagnostic criteria for drug-induced liver injury. Simultaneously, there was no significant difference between the ALBI and traditional Child-Pugh scores pre- and post-operatively. Therefore, we believe that liver damage from transarterial infusion chemotherapy is predominantly chronic, and this insidious pharmacological liver damage can be detected by comparing preoperative and postoperative L/S density ratio, spleen volume, and noninvasive hepatic fibrosis assessment indices (FIB-4, APRI). Serum enzyme profiles, ALBI scores, and Child-Pugh scores are not sensitive to this liver injury, particularly in the early stages.
Our study had several major shortcomings. Firstly, this was a single-center, retrospective study that included a small sample size. And there were too many subsequent treatment factors to allow for further follow-up, resulting in a shorter follow-up period. Longer follow-up periods will provide a more complete assessment of the long-term effects of treatment on the liver. Secondly, the chemotherapy drugs selected in this study are rarely used alone in clinical practice but rather administered in combination. Therefore, the mechanism of liver injury caused by these drugs are complex, and it is difficult to determine whether a particular drug causes the abovementioned liver injury. Because the patients in this study had advanced gastric cancer and normal liver function before surgery, we could not obtain ethical approval for liver biopsy, limiting out ability to acquire definitive pathological tissues and clarify the type of liver damage. To address these limitations, further animal studies will be conducted to determine which drug caused liver damage and the nature of that damage.
Transarterial infusion chemotherapy can lead to subtle liver damage within a relatively short period, including hepatic steatosis, hepatic sinusoidal obstruction-like changes, and fibrosis. CT imaging and hepatic injury indicators may detect drug-induced hepatic damage before laboratory abnormalities or clinical manifestations become apparent. It is crucial for interventional radiologists to be aware of this potential side effect when monitoring patients undergoing transarterial infusion chemotherapy, as they may be the first to identify it and optimize therapeutic management.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Ethics Committee of the Second Affiliated Hospital of Soochow University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because This study is a retrospective analysis utilizing previously collected clinical and medical records. Given the nature of the study, which involves the use of anonymized patient data, the waiver of informed consent does not affect the rights, health, or privacy of the patients. The data used in this research were obtained in accordance with ethical standards.
YiJ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. JJ: Writing – review & editing, Data curation, Methodology, Formal analysis, Project administration, Resources. XS: Writing – review & editing, Data curation, Methodology, Formal analysis, Software. YoJ: Conceptualization, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing. XB: Conceptualization, Formal analysis, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer NJ declared a shared parent affiliation with the authors to the handling editor at the time of review.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Han B, Zheng R, Zeng H, Wang S, Sun K, Chen R, et al. Cancer incidence and mortality in China, 2022. J Natl Cancer Center. (2024) 4:47–53. doi: 10.1016/j.jncc.2024.01.006
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
2. Digklia A. Advanced gastric cancer: current treatment landscape and future perspectives. World J Gastroenterol. (2016) 22:2403–14. doi: 10.3748/wjg.v22.i8.2403
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
3. Coccolini F, Nardi M, Montori G, Ceresoli M, Celotti A, Cascinu S, et al. Neoadjuvant chemotherapy in advanced gastric and esophago-gastric cancer. Meta-Analysis Randomized Trials Int J Surg. (2018) 51:120–7. doi: 10.1016/j.ijsu.2018.01.008
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
4. Coccolini F, Nardi M, Montori G, Ceresoli M, Celotti A, Cascinu S, et al. Neoadjuvant chemotherapy in advanced gastric and esophago-gastric cancer. Meta-analysis of randomized trials. Int J Surg. (2018) 51:120–7. doi: 10.1016/j.ijsu.2018.01.008
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
5. Zhao M, Guo Z, Zou YH, Li X, Yan ZP, Chen MS, et al. Arterial chemotherapy for hepatocellular carcinoma in China: consensus recommendations. Hepatol Int. (2024) 18:4–31. doi: 10.1007/s12072-023-10599-6
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
6. Qin S, Bai Y, Lim HY, Thongprasert S, Chao Y, Fan J, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from asia. Am Soc Clin Oncol. (2013) 28:47–53. doi: 10.1200/JCO.2012.44.5643
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
7. Li Q-J, He M-K, Chen H-W, Fang W-Q, Zhou Y-M, Xu L, et al. Hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin versus transarterial chemoembolization for large hepatocellular carcinoma: A randomized phase iii trial. J Clin Oncol. (2022) 40:150–60. doi: 10.1200/jco.21.00608
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
8. Zervoudakis A, Boucher T, Kemeny NE. Treatment options in colorectal liver metastases: hepatic arterial infusion. Visceral Med. (2017) 33:47. doi: 10.1159/000454693
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
9. Zhou Y, Chen J, Chen P, Tang Y, Wu S, Cai S, et al. Super-selective transcatheter vesical arterial chemoembolization with drug-loaded beads for muscle-invasive bladder cancer with hematuria. Abdom Radiol (NY). (2023) 48:780–6. doi: 10.1007/s00261-022-03748-2
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
10. Cao Y, Yu D, Wu Y, Zhu W. Regional intra-arterial vs. Systemic chemotherapy for the treatment of advanced pancreatic cancer: A systematic review and meta-analysis. Front Oncol. (2024) 14:1197424. doi: 10.3389/fonc.2024.1197424
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
11. Meng W, Yang B, Huang B, Chen C, Zhu J, Jian D, et al. Impact of preoperative transcatheter rectal arterial chemoembolization with concurrent chemoradiotherapy on surgery and prognosis of patients with locally advanced rectal cancer. J Surg Oncol. (2021) 124:1451–8. doi: 10.1002/jso.26673
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
12. Li B, Tang R, Zhang G, Cheng J, Chao M, Ding K. The efficacy and safety of continuous intra-arterial infusion neoadjuvant chemotherapy with surgery for locally advanced gastric cancer: A preliminary pilot study. J Gastrointestinal Oncol. (2022) 13:968–76. doi: 10.21037/jgo-22-304
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
13. Lin W, Huang Z, Du Z, Wang Y, Zuo T. Case report: clinical application of continuous arterial infusion chemotherapy in neoadjuvant therapy for locally advanced gastric cancer. Front Oncol. (2023) 13:1214599. doi: 10.3389/fonc.2023.1214599
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
14. Bang YJ, Van Cutsem E, Fuchs CS, Ohtsu A, Tabernero J, Ilson DH, et al. Keynote-585: phase iii study of perioperative chemotherapy with or without pembrolizumab for gastric cancer. Future Oncol. (2019) 15(9):943–52. doi: 10.2217/fon-2018-0581
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
15. Ma YE, Liang X, Liu LI, Gao D, Yang TAO, Hoffman RM, et al. Survival response of patients with gastric cancer treated with regional arterial-perfusion chemotherapy is correlated with borrmann classification. Anticancer Res. (2021) 41:5507–15. doi: 10.21873/anticanres.15364
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
16. Liu S, Xu K, Min J, Hou W, Zhang L, Lei J. Effect of transarterial chemotherapy on the outcome and prognosis of patients with locally advanced proximal gastric cancer. Am J Transl Res. (2023) 15:1309–17.
17. Wang FH, Zhang XT, Li YF, Wang C, Liu H, Qiu M, et al. The chinese society of clinical oncology (Csco): clinical guidelines for the diagnosis and treatment of gastric cancer, 2023. Cancer Commun. (2024) 44:127–72. doi: 10.1002/cac2.12516
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
18. Gordan JD, Kennedy EB, Abou-Alfa GK, Beg MS, Rose MG. Systemic therapy for advanced hepatocellula carcinoma: asco guideline. J Clin Oncol. (2020) 38:JCO.20.02672. doi: 10.1200/JCO.23.02745
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
19. Cho Y, Choi JW, Kwon H, Kim KY, Lee BC, Chu HH, et al. Transarterial chemoembolization for hepatocellular carcinoma: 2023 expert consensus-based practical recommendations of the korean liver cancer association. Korean J Radiol. (2023) 24:606–25. doi: 10.3348/kjr.2023.0385
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
20. Lee J, Vali Y, Boursier J, Spijker R, Zafarmand H. Prognostic accuracy of fib-4, nafld fibrosis score, and apri for nafld-related events: A systematic review. Liver international: Off J Int Assoc Study Liver. (2020) 41:261–70. doi: 10.1111/liv.14669
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
21. Toyoda H, Johnson PJ. The albi score: from liver function in patients with hcc to a general measure of liver function. JHEP Rep. (2022) 4:100557. doi: 10.1016/j.jhepr.2022.100557
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
22. Peng Y, Qi X, Guo X. Child-pugh versus meld score for the assessment of prognosis in liver cirrhosis: A systematic review and meta-analysis of observational studies. Medicine. (2016) 95:e2877. doi: 10.1097/MD.0000000000002877
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
23. Xiang XS, Su Y, Li GL, Ma L, Ma RF. Phase ii study of preoperative intra-arterial epirubicin, etoposide, and oxaliplatin combined with oral S-1 chemotherapy for the treatment of borrmann type 4 gastric cancer. J Gastric Cancer. (2020) 20:395. doi: 10.5230/jgc.2020.20.e40
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
24. He Q, Li Y, Ma L, Ji X, Li G. Application of fleeox preoperative chemotherapy via intra-arterial and intravenous administration in treatment of unresectable locally advanced gastric cancer. J Gastrointestinal Surg. (2016) 20:1421–7. doi: 10.1007/s11605-016-3153-8
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
25. Li Y, Chen J, He Q, Ji X, Wang X, Fan C, et al. Clinical efficacy of neoadjuvant chemotherapy regimens fleeox vs. Xelox in patients with initially unresectable advanced gastric cancer: A propensity score analysis. Oncotarget. (2017) 8. doi: 10.18632/oncotarget.19004
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
26. Jin Z, Chen L, Zhong B, Zhou H, Zhu H, Zhou H. Machine-learning analysis of contrast-enhanced computed tomography radiomics predicts patients with hepatocellular carcinoma who are unsuitable for initial transarterial chemoembolization monotherapy: A multicenter study. Trans Oncol. (2021) 14:101034. doi: 10.1016/j.tranon.2021.101034
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
27. Jin ZC, Zhou JW, Chen JJ, Ding R, Scheiner B, Wang SN, et al. Longitudinal body composition identifies hepatocellular carcinoma with cachexia following combined immunotherapy and target therapy (Chance2213). J Cachexia Sarcopenia Muscle. (2024) 15:2705–16. doi: 10.1002/jcsm.13615
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
28. Park SH, Kim PN, Kim KW, Lee SW, Yoon SE, Park SW, et al. Macrovesicular hepatic steatosis in living liver donors: use of ct for quantitative and qualitative assessment. Radiology. (2006) 239:105–12. doi: 10.1148/radiol.2391050361
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
29. Julien R, Stephanie R, Francois C, Biais M, Quinart A, Revel P, et al. Noninvasive assessment of macrovesicular liver steatosis in cadaveric donors based on computed tomography liver-to-spleen attenuation ratio. Liver transplantation: Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. (2015) 21(5):690–5. doi: 10.1002/lt.24105
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
30. Iwasaki M, Takada Y, Hayashi M, Minamiguchi S, Tanaka K. Noninvasive evaluation of graft steatosis in living donor liver transplantation. Transplantation. (2004) 78:1501–5. doi: 10.1097/01.tp.0000140499.23683.0d
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
31. Viganò L, Capussotti L, Rosa GD, Saussure WOD, Rubbia-Brandt L. Liver resection for colorectal metastases after chemotherapy: impact of chemotherapy-related liver injuries, pathological tumor response, and micrometastases on long-term survival. Ann Surg. (2013) 258:731–42. doi: 10.1097/SLA.0b013e3182a6183e
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
32. Zeiss J, Merrick HW, Savolaine ER, Woldenberg LS, Kim K, Schlembach PJ. Fatty liver change as a result of hepatic artery infusion chemotherapy. Am J Clin Oncol. (1990) 13:156–60. doi: 10.1097/00000421-199004000-00013
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
33. Rubbia-Brandt L, Audard V, Sartoretti P, Roth AD, Terris B. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. (2004) 15:460–6. doi: 10.1093/annonc/mdh095
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
34. Vauthey J-N, Pawlik TM, Ribero D, Wu T-T, Zorzi D, Hoff PM, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. (2006) 24:2065–72. doi: 10.1200/jco.2005.05.3074
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
35. Ryan P, Nanji S, Pollett A, Moore M, Moulton CA, Gallinger S, et al. Chemotherapy-induced liver injury in metastatic colorectal cancer: semiquantitative histologic analysis of 334 resected liver specimens shows that vascular injury but not steatohepatitis is associated with preoperative chemotherapy. Am J Surg Pathol. (2010) 34:784–91. doi: 10.1097/PAS.0b013e3181dc242c
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
36. Schwenzer NF, Springer F, Schraml C, Stefan N, Machann J, Schick F. Non-invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J Hepatol. (2009) 51:433–45. doi: 10.1016/j.jhep.2009.05.023
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
37. Wang C, Huang Y, Liu C, Liu F, Hu X, Kuang, et al. Diagnosis of clinically significant portal hypertension using ct- and mri-based vascular model. Radiology. (2023) 307:e221648. doi: 10.1148/radiol.221648
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
38. Miyake K, Hayakawa K, Nishino M, Morimoto T, Mukaihara S. Effects of oral 5-fluorouracil drugs on hepatic fat content in patients with colon cancer. Acad Radiol. (2005) 12:722–7. doi: 10.1016/j.acra.2005.02.010
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
39. Sadahiro S. Pharmacokinetics of 5-fluorouracil following hepatic intra-arterial infusion in a vx2 hepatic metastasis model. Japanese J Clin Oncol. (2003) 33:377. doi: 10.1093/jjco/hyg077
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
40. Liu S, Liu Y, Zhang D, Li H, Shao X, Xie P, et al. Novel insights into perfluorinated compound-induced hepatotoxicity: chronic dietary restriction exacerbates the effects of pfbs on hepatic lipid metabolism in mice. Environ Int. (2023) 181:108274. doi: 10.1016/j.envint.2023.108274
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
41. Grivicich I, Regner A, Da Rocha AB, Kayser GB, Schunemann DP, Grass LB. The irinotecan/5-fluorouracil combination induces apoptosis and enhances manganese superoxide dismutase activity in ht-29 human colon carcinoma cells. Chemotherapy. (2005) 51:93–102. doi: 10.1159/000085617
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
42. Alissar EC, Ali H, Ayman H, Sarah AM, Lara H, Deborah M, et al. Increase in spleen volume as a predictor of oxaliplatin toxicity. Ther Clin Risk Manage. (2018) 14:653–7. doi: 10.2147/TCRM.S150968
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
43. Mukai K, Nishida T, Matsumoto K, Kitanaka T, Hosokawa K, Sakamoto N, et al. Predictive factors of collateral vessel development induced by oxaliplatin-based chemotherapy. Int J Clin Oncol. (2022) 28:280–8. doi: 10.1007/s10147-022-02280-z
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
44. Toi H, Morita T, Nakamura T, Muranaga S, Hase T. Splenomegaly associated with folfox therapy. Gan to Kagaku Ryoho Cancer Chemotherapy. (2011) 38:1457–60.
45. Overman MJ, Maru DM, Charnsangavej C, Loyer EM, Wang H, Pathak P, et al. Oxaliplatin-mediated increase in spleen size as a biomarker for the development of hepatic sinusoidal injury. J Clin Oncol. (2010) 28:2549–55. doi: 10.1200/JCO.2009.27.5701
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
46. National Institute of Diabetes and Digestive and Kidney Diseases. Oxaliplatin. In: LiverTox: Clinical and research information on drug-induced liver injury. United States: U.S. National Library of Medicine (2020). Available at: https://www.ncbi.nlm.nih.gov/books/NBK547852/.
47. Ganta N, Prasad A, Aknouk M, Ghodasara K, Nair A, Taqvi Z, et al. A case report of nodular regenerative hyperplasia and non-cirrhotic portal hypertension post oxaliplatin chemotherapy. Cureus. (2022) 14(9):e28740. doi: 10.7759/cureus.28740
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
48. Sarin SK, Kumar A, Chawla YK, Baijal SS, Dhiman RK, Jafri W, et al. Noncirrhotic portal fibrosis/idiopathic portal hypertension: apasl recommendations for diagnosis and treatment. Hepatol Int. (2007) 1:398–413. doi: 10.1007/s12072-007-9010-9
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
49. Lai Z, Huang Y, Wen D, Lin X, Kan A, Li Q, et al. One day versus two days of hepatic arterial infusion with oxaliplatin and fluorouracil for patients with unresectable hepatocellular carcinoma. BMC Med. (2022) 20:415. doi: 10.1186/s12916-022-02608-6
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
50. Overman MJ, Ferrarotto R, Raghav K, George B, Qiao W, MaChado KK, et al. The addition of bevacizumab to oxaliplatin-based chemotherapy: impact upon hepatic sinusoidal injury and thrombocytopenia. J Natl Cancer Inst. (2018) 110:888–94. doi: 10.1093/jnci/djx288
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
51. Chair C P G P, Members P, et al. EASL Clinical Practice Guidelines: Drug-induced liver injury. J Hepatol. (2019) 70:1222–61. doi: 10.1016/j.jhep.2019.02.014
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
52. Pereyra D, Rumpf B, Ammann M, Perrodin SF, Tamandl D, Haselmann C, et al. The combination of apri and albi facilitates preoperative risk stratification for patients undergoing liver surgery after neoadjuvant chemotherapy. Ann Surg Oncol. (2019) 26(3):791–9. doi: 10.1245/s10434-018-07125-6
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
53. Fu X-L, Jun D, Xiao-Ning H, Wu-Gen L, Bi-Bo P, Chun-Lai L, et al. Analysis of changes in spleen volume and liver fibrosis index in patients with hepatic sinus obstruction syndrome induced by oxaliplatin. Liver. (2021) 05:1008–704. doi: 10.14000/j.cnki.issn.1008-1704.2021.05.022
54. Gu C, Liu Z, Li Y, Yi M, Wang S, Fan X, et al. Endogenous fgf1 deficiency aggravates doxorubicin-induced hepatotoxicity. Toxics. (2023) 11:925. doi: 10.3390/toxics11110925
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
55. Nalbantoglu I, Tan BR, Linehan DC, Gao F, Brunt EM. Histological features and severity of oxaliplatin nduced liver injury and clinical associations. J Digestive Dis. (2014) 15:553–60. doi: 10.1111/1751-2980.12177
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
56. Björnsson HK, Björnsson ES. Drug-induced liver injury: pathogenesis, epidemiology, clinical features, and practical management. Eur J Internal Med. (2022) 97:26–31. doi: 10.1016/j.ejim.2021.10.035
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
Keywords: transarterial infusion chemotherapy, non-tumor liver parenchyma damage, CT imaging, hepatic injury indicators, advanced gastric cancer
Citation: Jing Y, Jing J, Sun X, Jin Y and Bai X (2025) Assessment of non-tumor liver parenchyma damage in advanced gastric cancer treatment with transarterial infusion chemotherapy: a study using imaging and hepatic injury indicators. Front. Oncol. 15:1537688. doi: 10.3389/fonc.2025.1537688
Received: 01 December 2024; Accepted: 28 January 2025;
Published: 19 February 2025.
Edited by:
Francesco Vasuri, Azienda Ospedaliero-Universitaria di Bologna, ItalyReviewed by:
Zhi-Cheng Jin, Southeast University, ChinaCopyright © 2025 Jing, Jing, Sun, Jin and Bai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuming Bai, MjAwNWJhaXh1bWluZ0AxNjMuY29t; Yong Jin, amlueW9uZ0BzdWRhLmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.