- 1Mackenzie Cancer Research Group, Department of Pathology and Biomedical Science, University of Otago Christchurch, Christchurch, New Zealand
- 2Centre for Trace Element Analysis, Department of Geology, University of Otago, Dunedin, New Zealand
- 3Department of Immunobiochemistry, Medical Faculty, Mannheim Institute for Innate Immunoscience (MI3), Heidelberg University, Mannheim, Germany
- 4Oncogenic Transcription Laboratory, Olivia Newton-John Cancer Research Institute, Melbourne, VIC, Australia
- 5Mātai Hāora, Centre for Redox Biology and Medicine, Department of Pathology and Biomedical Science, University of Otago, Christchurch, New Zealand
- 6Department of Pathology, Dunedin School of Medicine, University of Otago, Dunedin, New Zealand
Introduction: Glioblastomas are an aggressive primary brain cancer, characterised by hypoxia and poor patient survival. Iron is the most abundant transition metal in the brain, yet data on the iron content of brain cancers is sparse. Ferrous iron is an essential cofactor for a super-family of enzymes, the iron- and 2-oxoglutarate-dependent dioxygenase enzymes (2-OGDD). These enzymes control the response to hypoxia via hydroxylation of the hypoxia-inducible factor-1α (HIF-1α), and DNA demethylation via hydroxylation of 5-methyl cytosines (5hmC).
Methods: This study used clinical glioblastoma samples from 40 patients to determine the relationship between 2-OGDD activity and iron. Elemental iron was measured using inductively coupled plasma mass spectrometry (ICP-MS) and ferrous iron was measured using the colorimetric ferrozine assay. Iron measurements were compared against patient survival and clinicopathological data, and 2-OGDD-dependent activity of HIF-1 activation and 5hmC.
Results and discussion: Elemental and ferrous iron levels were weakly related. Higher ferrous iron content of clinical glioblastoma tissue was associated with longer overall survival compared to lower ferrous iron content, but elemental iron showed no such relationship. Neither form of iron was related to clinicopathological data or markers of 2-OGDD activity. The impact of iron supplementation on the hypoxic response was assessed in three glioblastoma cell lines in vitro, similarly showing only a limited influence of iron on these 2-OGDD enzymes. Our data, together with prior studies in anaemic patients, highlight the importance of healthy iron levels in patients with glioblastoma, but further mechanistic studies are needed to elucidate the molecular pathways involved.
1 Introduction
Glioblastomas are highly aggressive primary brain cancers derived from glial cells located in the central nervous system (1, 2). Current clinical guidelines use isocitrate dehydrogenase (IDH) mutation status to separate glioblastoma (IDH wild type) from high-grade astrocytoma and oligodendrogliomas (IDH mutant) (3). Glioblastomas carry a poor prognosis, with a median survival of 15-31 months, and a high likelihood of recurrence (4, 5). Glioblastomas are particularly hypoxic which is related to their poor prognosis (6).
Iron is a vital micronutrient for biological processes in all cells, including for cell growth, oxygen transport, and metabolic processes (7). The ability of iron to reversibly convert from ferric iron (Fe3+) to ferrous iron (Fe2+) is essential for these biological processes (8, 9). Approximately 80% of the body’s iron stores is bound to the O2 transport proteins haemoglobin in erythrocytes and myoglobin in muscle. The remainder is either bound to Fe-containing haem proteins, such as peroxidases or cytochromes, non-haem iron proteins and enzymes, or stored in hepatocytes and macrophages and in the iron storage protein, ferritin (8, 9).
Ferrous iron is essential for a superfamily of enzymes, the iron- and 2-oxoglutarate-dependent dioxygenase enzymes (2-OGDD) (10–12). Besides iron, all 2-OGDD require ascorbate as cofactor, and molecular oxygen and 2-oxoglutarate as substrates. The human 2-OGDDs catalyse numerous hydroxylations, affecting vital biological mechanisms including DNA demethylation, cellular metabolism, and hypoxic adaptation (11, 12). The prolyl hydroxylases (PHD 1-3) and the asparagine hydroxylase factor-inhibiting hypoxia-inducible factor (FIH), control the hypoxic pathway by hydroxylating the hypoxia-inducible factor (HIF) regulatory α subunit, preventing activation of the HIF transcription factor (10, 13). The 2-OGDD enzyme family also includes enzymes that regulate gene expression. The ten-eleven-translocation enzymes (TET 1-3) hydroxylate 5-methyl cytosine (5mC) to 5-hydroxymethyl cytosine (5hmC) and further oxidation products, resulting in DNA demethylation and increased global gene expression (12, 14). Lysine and histidine demethylases that that modify histones, thus affecting global gene expression, are 2-OGDD family members (12, 15).
Iron has been investigated as a novel anti-tumour agent with respect to its capacity to induce ferroptosis, an iron-overload induced cell death (16), inhibition of cell migration (17), and promotion of anti-tumour immunity (18), among other potential functions. Macrophages, a major infiltrate in glioblastoma tumours, can store iron, especially under conditions of iron overload (19). Iron is the most abundant transition metal in the brain (20–22). Some regions within the brain, especially the iron-rich substantia nigra, caudate nucleus and globus pallidus, contain more iron than others (23). Of note, most gliomas arise in the frontal/temporal lobe, a region relatively low in iron. Astrocytes function as iron sensors to regulate and communicate the iron requirement of the brain via secreting hepcidin, which modulates the expression of iron regulatory proteins (9, 19, 24). Iron uptake into the brain is tightly regulated by endothelial cells and neighbouring astrocytes at the blood brain barrier (19, 24–26).
The rational above indicates that iron levels could exert a significant impact on cancer cell biology, yet it remains unclear whether iron content varies by location in the brain, or patient sex or age. The level of iron in glioblastoma tissue, and whether it impacts patient outcome, is unknown. It is not known whether iron plays a role in supporting 2-OGDD enzyme activity in glioblastoma cells. Our study aims to address these gaps in our knowledge. We have used clinical glioblastoma samples to measure iron content in tissues and determine associations with patient or tumour characteristics, and used glioblastoma cell lines grown in culture to ascertain the impact of iron levels on the activation of the hypoxic response.
2 Materials and methods
2.1 Materials
Unless stated explicitly, all chemicals were from Sigma-Aldrich (St Louis, MO, USA).
2.2 Human ethics, donor consent and glioblastoma samples
Most samples from this patient cohort have previously been analysed for ascorbate, IDH mutation status, hypoxic pathway activity and epigenetic analysis (27, 28). Human ethical approvals were from the University of Otago Ethics Committee (H19/163) and the national Health and Disability Ethics Committee (MEC/08/02/016), with approval for the use of samples from the Canterbury Tissue Bank Board (2001DPVR). Each donor provided written consent for the use of their sample and access to medical notes for research. After resection, samples excess to diagnosis were gifted to He Taonga Tapu Cancer Society Tissue Bank Christchurch or University of Otago Dunedin, and snap frozen (collection 2003-2019). Medical notes and pathology reports provided patient and tumour characteristics, as well as follow-up data (up to 2021). Consultation with Māori, Aotearoa/New Zealand’s indigenous people, was carried out through the University of Otago, with an option of sample disposal with karakia (blessing) offered to donors.
2.3 Glioblastoma sample processing
For ICP-Q-MS, clinical samples were cracked into small pieces in a pre-chilled mortar on dry ice, and carefully weighed. For the ferrozine assay, frozen tissue samples were placed into an Eppendorf tube with potassium phosphate buffer (pH 7.4) and homogenized using an Eppendorf micro-pestle. Following centrifugation, supernatant was used for the ferrozine assay.
2.4 Inductively coupled plasma-quadrupole-mass spectrometry
All chemicals were specifically ultra-trace analysis grade. After thawing, samples were digested in 14 N nitric acid and moved to precleaned perfluoroalkoxy-polymer (PFA) vessels (Savillex, USA) for digestion preparation. The PFA vessels were capped, and the acid reflux process was completed overnight at 105°C to completely digest the tissue samples. Subsequently, the solution was allowed to evaporate to dryness, followed by addition of 50 µL of hydrogen peroxide (30% vol/vol) and a further 50 µL of 14 N nitric acid to complete digestion of samples. Once digested and dried, 3 mL of 2% nitric acid (vol/vol) was added to the PFA vessel, the mixture was warmed to complete the solubilization process before being transferred into auto-sampler vials for ICP-Q-MS analysis. The concentrations of iron, sodium, magnesium, phosphorus, potassium, calcium, copper, and zinc in the glioblastoma tissue samples were measured using an Agilent 7900 ICP-Q-MS (Agilent, Santa Clara, USA). Predetermined cocktails of standard elements were used as internal controls and for quality assurance and standardization of the instrument; a calibration against serially diluted National Institute of Standards and Technology traceable standards was used. Elements of interest were measured in standards and samples with a helium collision gas tune to eliminate polyatomic interferences. To determine effect of extraction on the ICP-Q-MS measurements, an extraction blank was measured on five empty cryovials, and the average mass of each element of interest was subtracted from the samples. The density of solutions was known and the concentration of elements in the glioblastoma tissues was calculated using known volumes and the tissue weights that were initially recorded.
2.5 Ferrozine assay
Ferrous iron was measured as previously described (29, 30). Frozen tissue samples were placed into an Eppendorf and homogenized on ice using an Eppendorf micro-pestle with 200 µL of ice-cold potassium phosphate buffer (pH 7.4). The sample was centrifuged at 10,000 rcf for 10 minutes at 4°C and 200 μL of the supernatant was added to 600 µL of ferrozine stock (4.6 mM sodium L-ascorbate, 17mM sodium acetate, 0.18 mM ferrozine (3-(2-pyridyl)-5,6-diphenyl-1,2,4-triazone 4’,4”-disulfonic acid sodium salt, Cat no 82950)). A standard curve was created using FeSO4 (0.5 µg/mL to 100 µg/mL in 0.01 N HCl; AnalR BDH Cat no 10112) (Supplementary Figure 1). The samples were measured at 550 nm using a Wallace 1420 Victor microplate reader (PerkinElmer Life and Analytical Sciences, USA). The ferrous iron concentrations of the glioblastoma samples were interpolated from the standard curve.
2.6 Cell culture
Human glioblastoma cells (U251MG, T98G, U87MG) were sourced from the European Collection of Authenticated Cell Cultures (ECACC, Sigma-Aldrich, St Louis, MO, USA). Cells were grown in minimum essential media (MEM, Thermo Fisher Scientific, Waltham, MA USA) substituted with 10% foetal bovine serum (FBS, Gibco, Thermo Fisher), 1% sodium pyruvate and 1% non-essential amino acids with 1% penicillin/streptomycin. Cells were maintained in a 37°C humidified incubator in air with 5% CO2, used at low passages (<20), and confirmed negative for mycoplasma (e-Myco™ Mycoplasma PCR Detection Kit, iNtRON Biotechnology, Korea).
2.7 Iron manipulation in vitro
Glioblastoma cells were exposed to ferrous sulphate (100 μM FeSO4, made fresh at 10 mM stock in MEM). Cells were either exposed to FeSO4 for 24 h in normoxia (air with 5% CO2), followed by exposure to normoxia or mild hypoxia for 24 h (5% O2, 5% CO2, balance N2, Xvivo System Model X3, BioSpherix). Cells were collected for western blotting.
2.8 Western blotting of in vitro samples
Western blotting was carried out as previously described (31). Following treatments, cells were lysed in samples buffer (60 mM Tris pH 4.8, 2% SDS, 20% glycerol, 0.1 M 1,4 dithiothreitol (DTT) with 1x cOmplete™ protease inhibitors and 0.02% bromophenol blue) for Western blot analysis. Proteins from the cell lysates were separated by SDS-PAGE using 4-12% gradient Bis-Tris Plus SDS gels (Invitrogen, Thermo Fisher, Auckland NZ) and transferred to polyvinylidene difluoride membranes. Proteins of interest were detected using primary anti-human antibodies (HIF-1α (1:250, 610959, BD Transduction Laboratories, USA), Bcl2-interacting protein 3 (BNIP3, 1:1000, AF4147, R&D Systems, Invitro Technologies, Auckland, NZ), carbonic anhydrase 9 (CA-IX, 1:1000, AF2188, R&D Systems, Invitro Technologies, Auckland, NZ), β-actin (1:10,000, A5316)), with suitable secondary antibodies and ECL Select Western Blotting Detection Reagent (GEHERPN2235, Bio-Strategy, Auckland, NZ). Proteins of interest were detected using the Alliance Q9 imaging software and measured using the Alliance Q9 software, with β-actin as loading control.
2.9 Statistics
Data were analysed using GraphPad Prism (V10) with significance set at p<0.05. The Schapiro-Wilks test was used to test normality, Mann-Whitney or unpaired t-tests to test associations, and paired t-test was used to compare means between FeSO4-treated and untreated groups. Spearman’s and Pearson’s were used to test correlations. Kaplan-Meier survival curves were analysed using Log rank tests. Cell culture experiments included ≥3 independent repeats.
3 Results
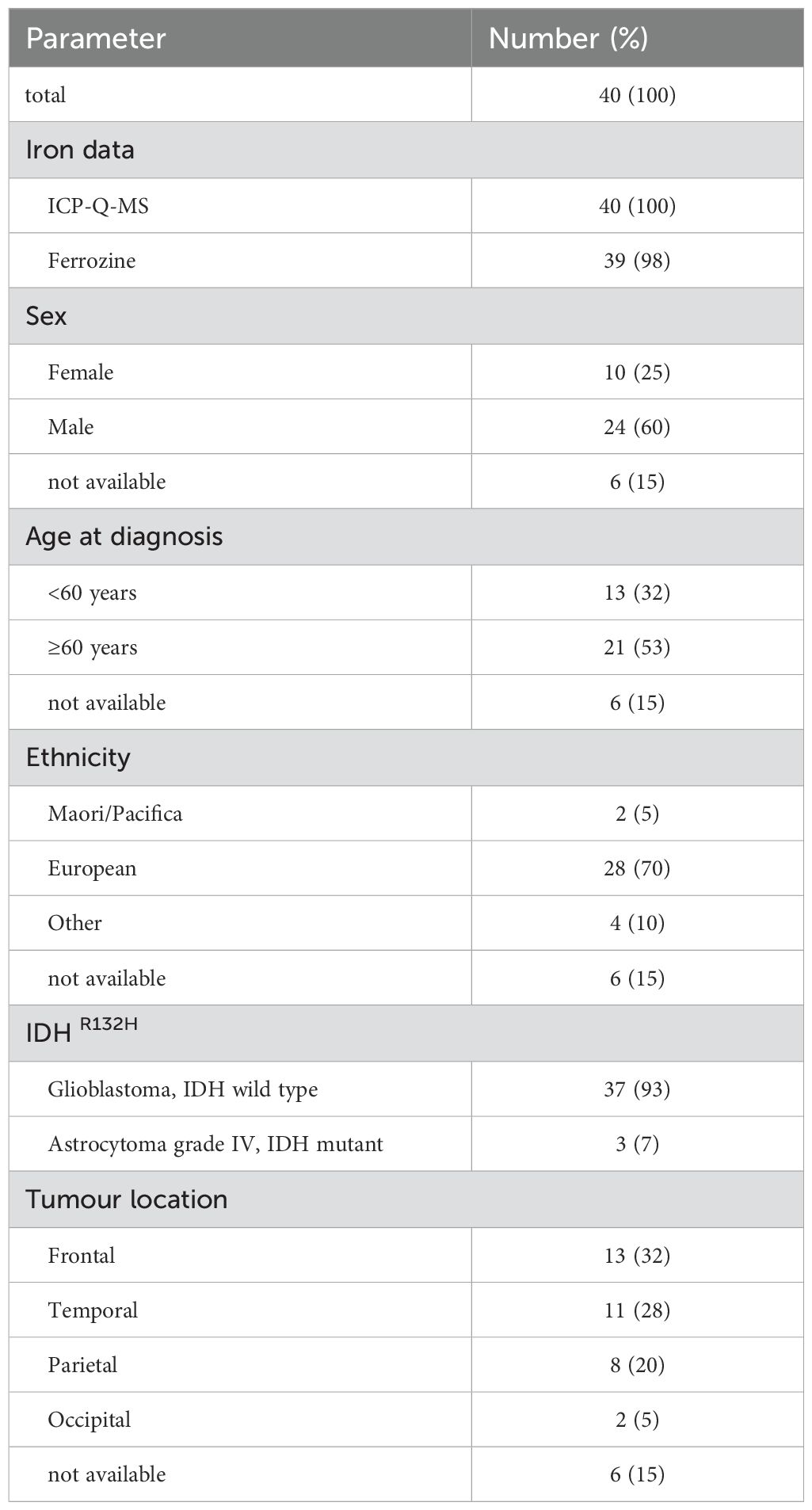
Glioblastoma samples from a total of 40 patients were available for analysis (Table 1). Elemental iron was measured in all samples, whereas ferrous iron could only be measured in 39 of the samples (Table 1). Most patients identified as European with only 2 identifying as Māori or Pacific peoples, and most were male with a median age of 59 years (Table 1). Although all tumours were initially described as glioblastoma, three were subsequently redefined as high-grade astrocytoma according to their IDHR132H mutation status. Similar numbers of tumours were located in the frontal, temporal and parietal lobes, with few located in the occipital part of the brain (Table 1). Analyses of ascorbate, hypoxic pathway and epigenetic levels of most of this cohort have been published (27, 28).
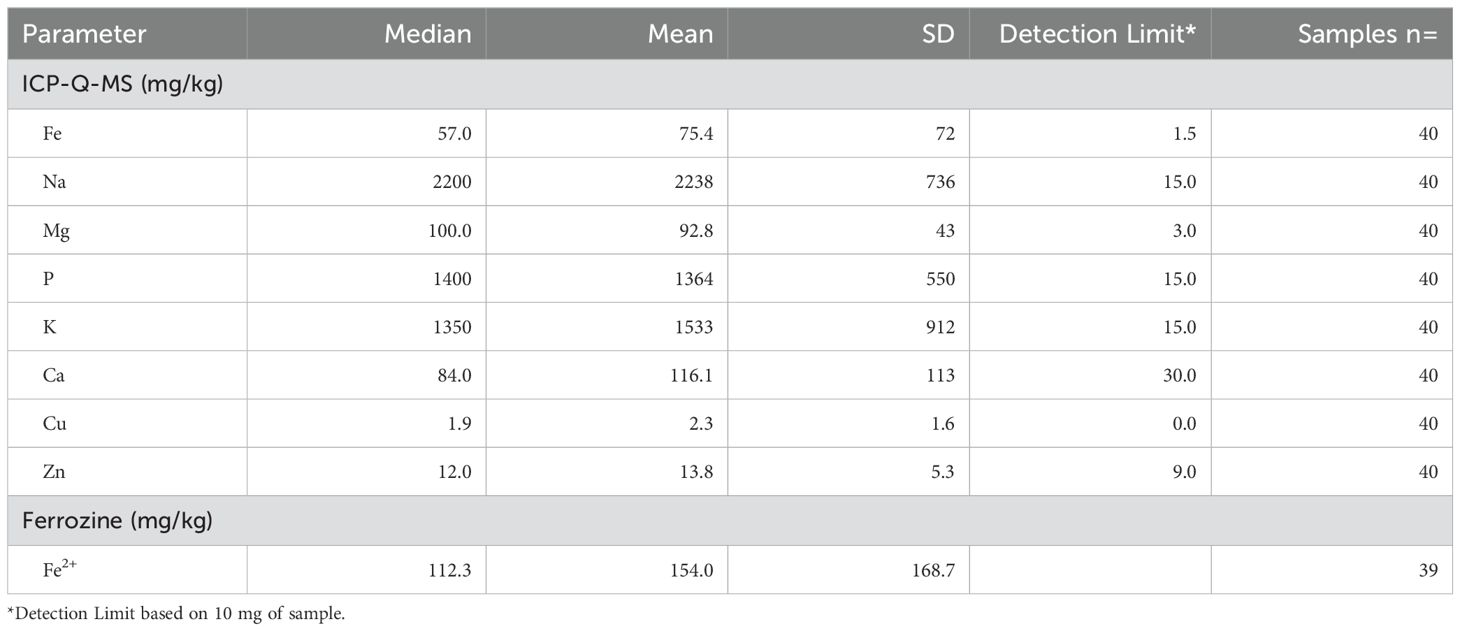
Elemental iron was measured by ICP-Q-MS, showing a median of 57 mg/kg and a mean of 75 ± 72 mg/kg (Table 2). Samples contained between 2.5 – 200 mg/kg of elemental iron, with one very high measurement (410 mg/kg, Figure 1). Ferrous iron was estimated using the ferrozine assay, showing a median 112 mg/kg and a mean of 154 ± 169 mg/kg, ranging from 0 to 558 mg/kg (Figure 1). There was only a weak correlation between elemental and ferrous iron (Figure 1, r=0.26, p=0.1). Elemental iron content was similar in IDH mutant and IDH wild type tumours, and did not vary significantly by tumour location, patient sex or patient age (Figure 2). Similarly, ferrous iron content did not vary significantly by IDH mutation status, location, sex or age (Figure 3).
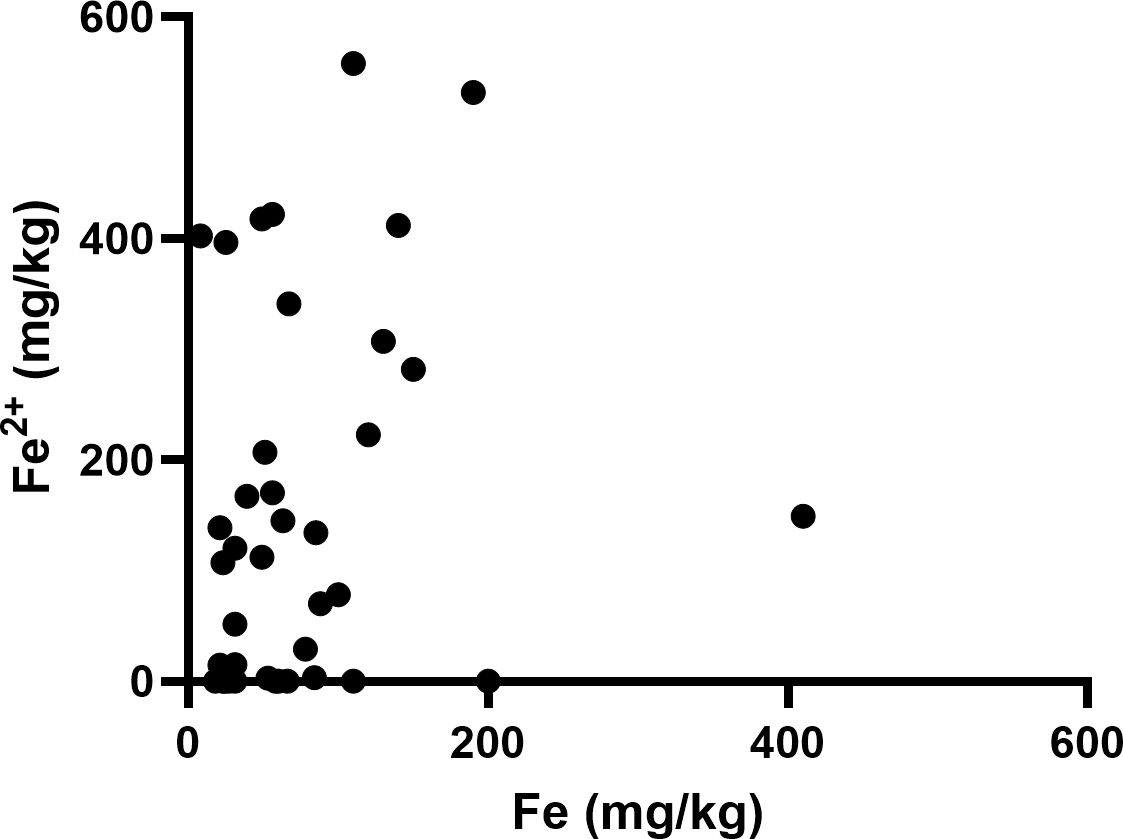
Figure 1. Iron content of glioblastoma samples. Correlation between ferrous iron measured by ferrozine assay and elemental iron measured by ICP-MS (Spearman’s correlation r=0.26, p=0.1, n=40).
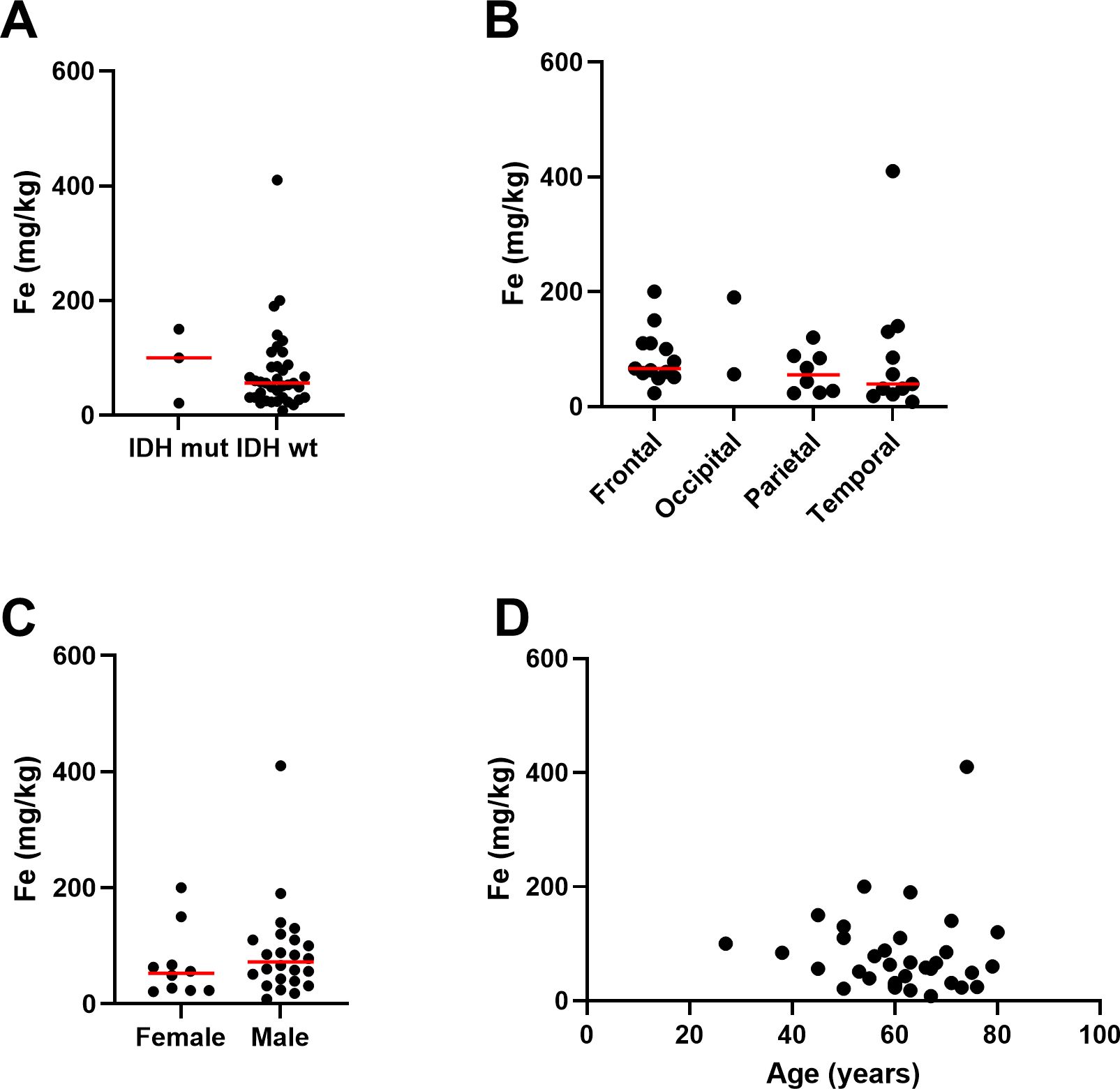
Figure 2. Associations between elemental iron and clinicopathological data. Associations between iron measured by ICP-Q-MS in glioblastoma samples and (A) IDH mutation status of glioblastoma (Mann Whitney p=0.63, n=40), (B) location of tumour in the brain (Kruskal-Wallis p=0.36, n=34), (C) sex of patient (Mann Whitney p=0.31, female n=10, male n=24), and (D) age of patient (Spearman’s correlation r-0.15, n=34). Median is shown as red line.
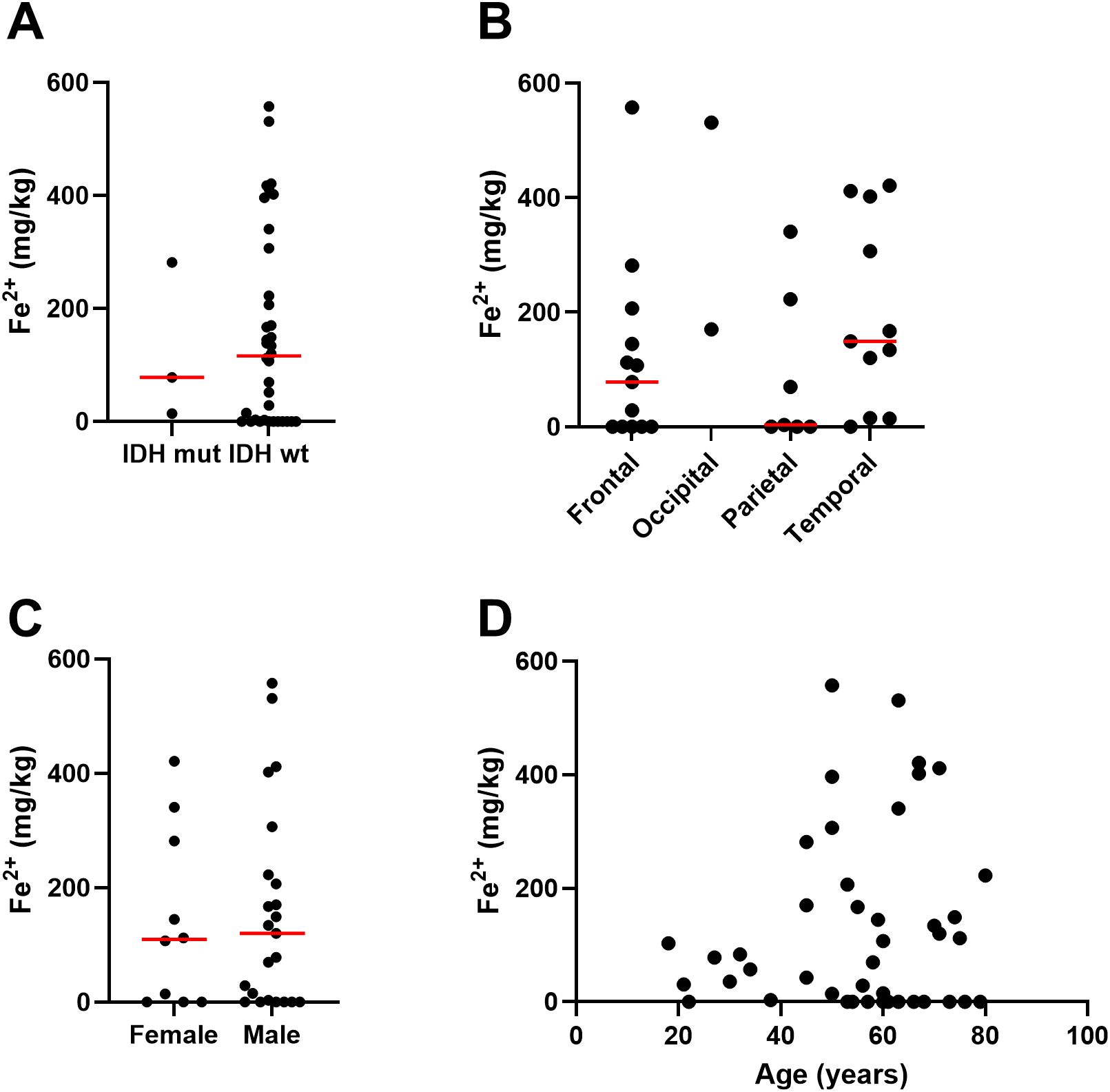
Figure 3. Associations between ferrous iron and clinicopathological data. Associations between iron measured by the ferrozine assay in glioblastoma samples and (A) IDH mutation status of glioblastoma (Mann Whitney p=0.97, n=39), (B) location of tumour in the brain (Kruskal-Wallis p=0.16, n=31), (C) sex of patient (Mann Whitney p=0.83, female n=10, male n=23), and (D) age of patient (Spearman’s correlation r 0.03, n=29). Median shown as red line.
We next determined whether iron content of glioblastoma samples was associated with patient outcome (Figure 4). Overall survival of patients with glioblastoma was associated with ferrous iron availability: survival was significantly shorter for those patients with glioblastoma tumours that contained below median ferrous iron, compared to those with above median ferrous iron content (Figure 4B). In contrast, survival did not vary by median elemental iron content (Figure 4A).
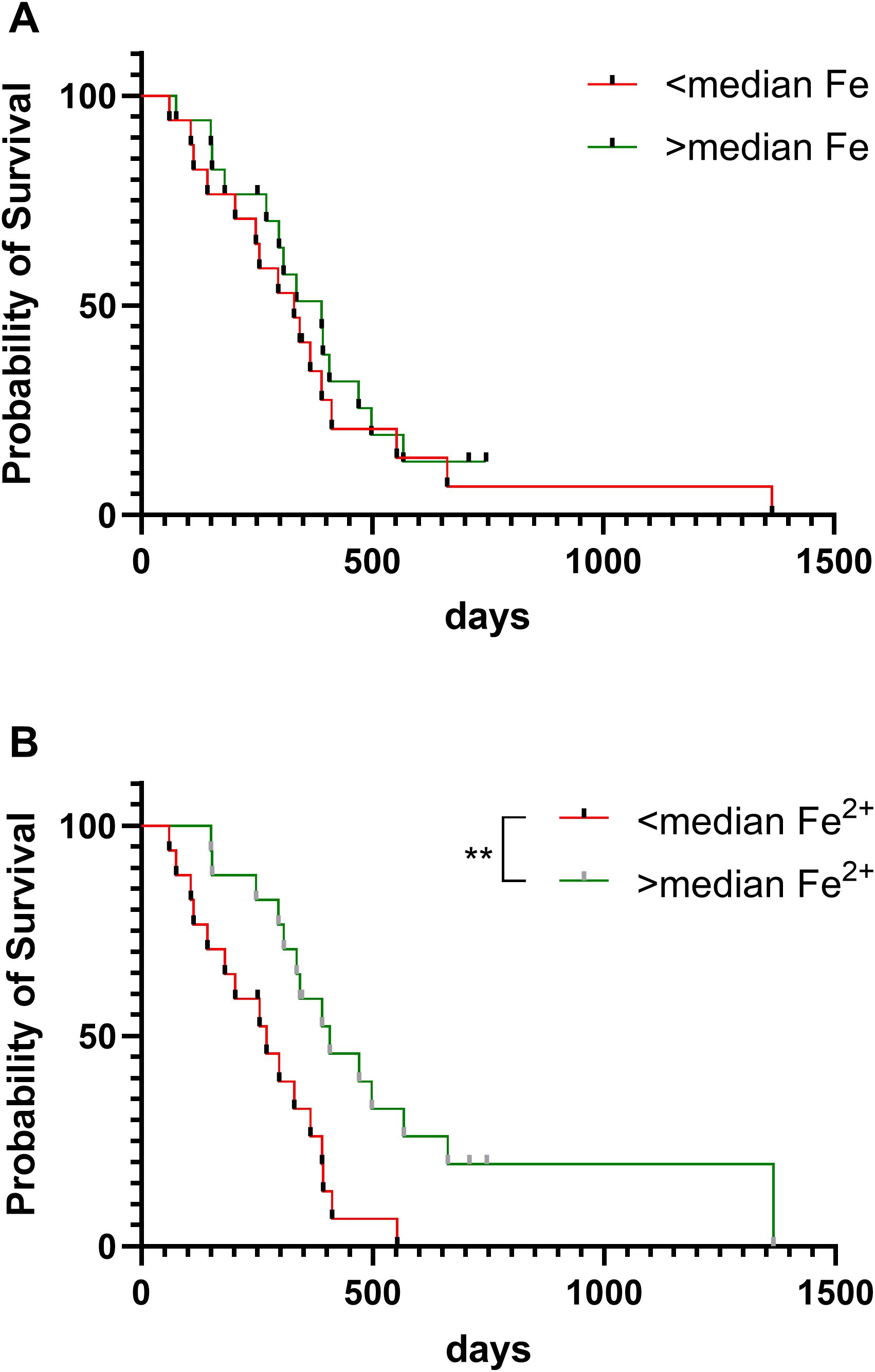
Figure 4. Patient survival according to iron content of glioblastoma tumours. Overall survival of patients with glioblastoma according to (A) elemental iron content of tumours (Log-rank test, p=0.50, n=34), and (B) ferrous iron of tumours (Log-rank p=0.005, n=33). Samples were divided into two groups of below or above median elemental iron (61.5 mg/kg) or median ferrous iron (112 mg/kg). ** p < 0.01.
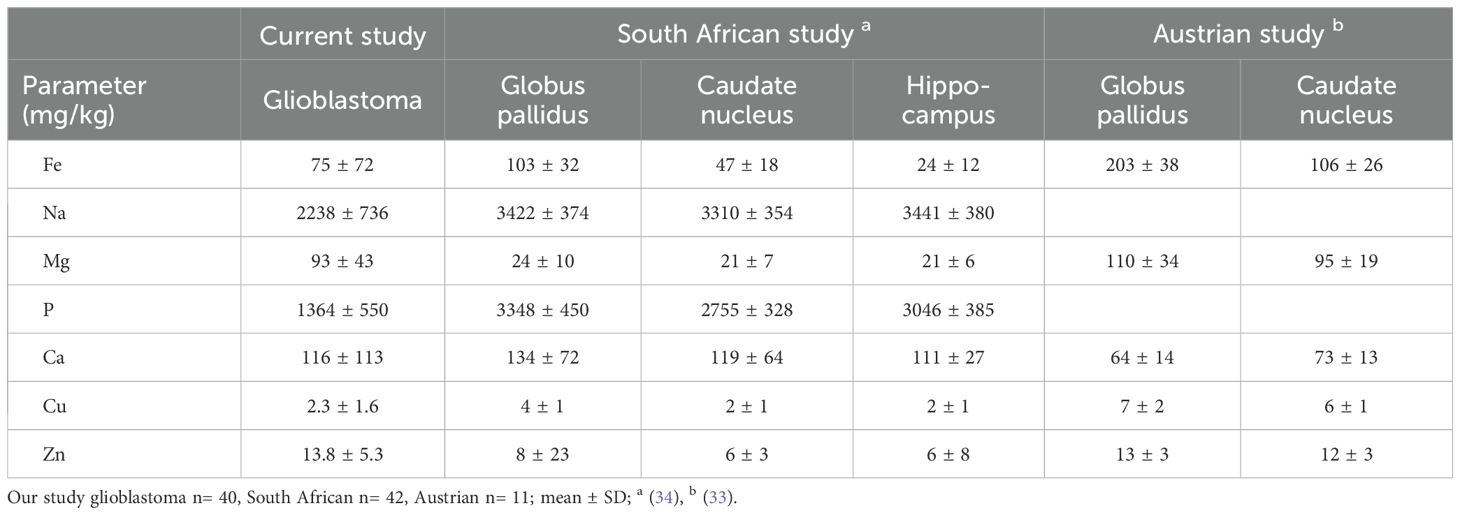
Other chemicals were measured by ICP-Q-MS in glioblastoma samples at the time of measuring elemental iron. Glioblastomas contained the following median levels: sodium (2200 mg/kg); phosphate (1400 mg/kg); potassium (1350 mg/kg); magnesium (100 mg/kg); calcium (84 mg/kg); copper (1.9 mg/kg) and zinc (12.0 mg/kg) (Table 2). We then compared data from glioblastoma samples in our study to two previously published studies that used ICP-MS to quantify elements in different regions of human brain (Table 3). Due to large variations between individual samples, there appeared to be no major difference between elements (Fe, Na, Mg, P, Ca, Cu, Zn) in glioblastoma tissue and regions in non-cancerous brain (iron-rich globus pallidus and caudate nucleus, hippocampus) (Table 3).
As ferrous iron is an integral part of the active site of all 2-OGDD enzymes, we investigated whether iron content was associated with 2-OGDD enzyme activity. This was done by analysing the relationships of elemental iron or ferrous iron content of glioblastoma samples with the relative HIF-score, as a measure of PHD and FIH activity (27), or percentage 5hmC, as a measure of TET activity (28) (Figure 5). The relative HIF-score was previously derived from protein data by combining HIF-1α and 6 HIF-regulated proteins into a single relative score for each glioblastoma sample (27). Similarly, the 5hmC data was previously measured using mass spectrometry in a selection of the glioblastoma samples and reported (28). The HIF-score was not significantly associated with elemental iron (p=0.50, Figure 5A), or ferrous iron (p=0.08, Figure 5B). Similarly, 5hmC data was not associated with either elemental or ferrous iron (p=0.54 and p=0.44, respectively, Figures 5C, D). This indicated no clear relationship between iron content and 2-OGDD enzyme activity in clinical glioblastoma samples.
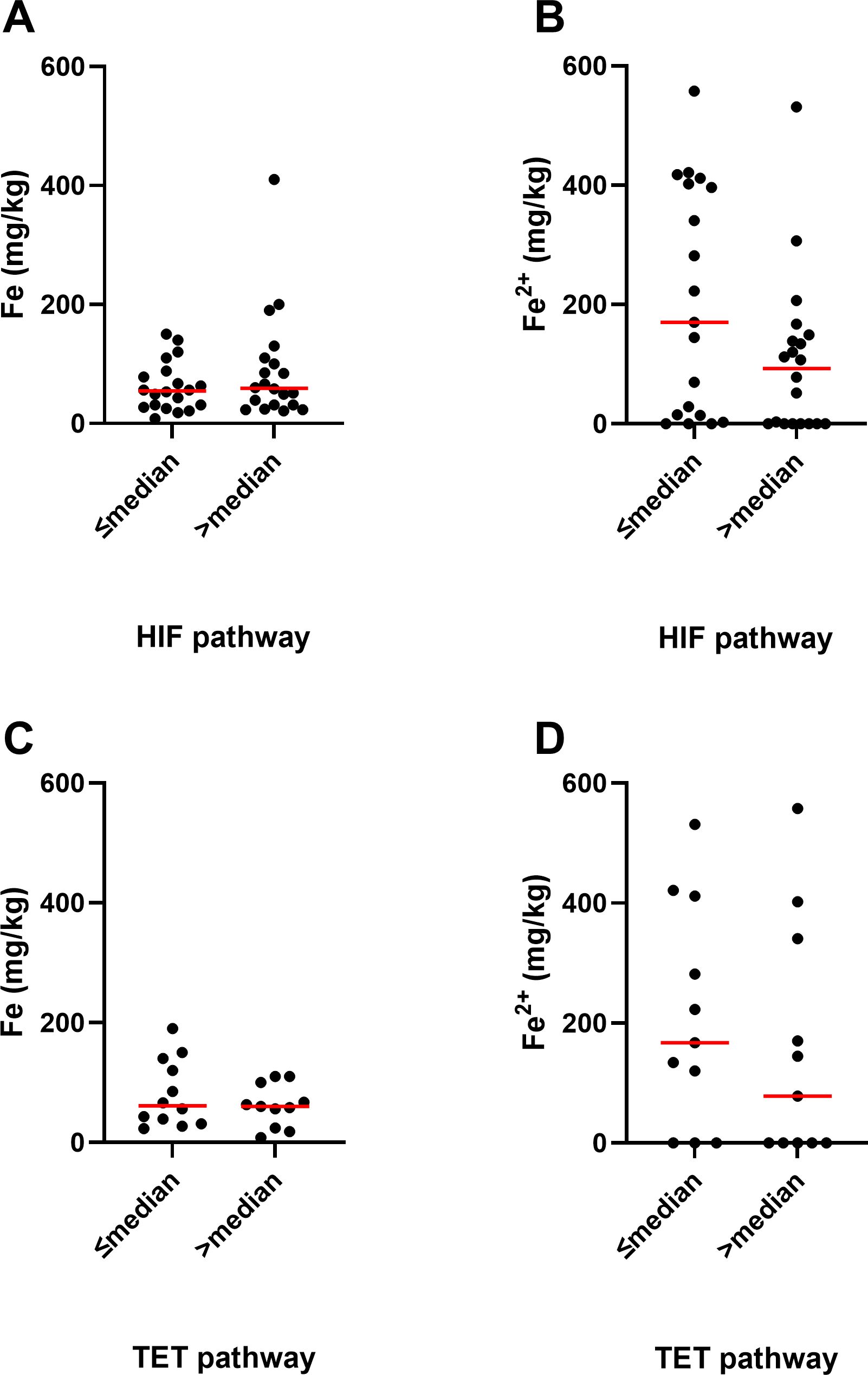
Figure 5. Association between iron and activity of 2-OGDDs in glioblastoma samples. Association between elemental iron and (A) above or below median relative HIF pathway (p=0.50, n=40), and (C) above or below median TET pathway (p=0.54, n=23). Association between ferrous iron and (B) above or below median relative HIF pathway (p=0.08, n=39), and (D) above or below median TET pathway (p=0.44, n=22). Associations were tested using Mann Whitney test. The median relative HIF pathway score was 2, and the median TET pathway score was 0.18% 5hmC. Median shown as red line.
To further explore the role of iron in 2-OGDD enzyme activity, we studied glioblastoma cells in vitro. Three glioblastoma cell lines (U251MG, T98G, U87MG) were exposed to FeSO4 with or without mild hypoxia (5% O2), and their hypoxic pathway response was analysed (Figure 6). In response to hypoxia, all three glioblastoma cell lines showed a robust stabilization of HIF-1α and an increase in BNIP3 protein, with T98G and U87MG also exhibiting an increase in CA-IX protein (Figures 6A, C, E). The addition of ferrous iron significantly reduced hypoxia-induced HIF-1α in U251MG cells and BNIP3 in T98G cells (Figures 6B, D), and did not significantly or consistently suppress the hypoxic pathway in vitro (Figures 6B, D, F). This suggests that the addition of ferrous iron did not affect the activity of the 2-OGDD enzymes PHD and FIH in glioblastoma cells in vitro.
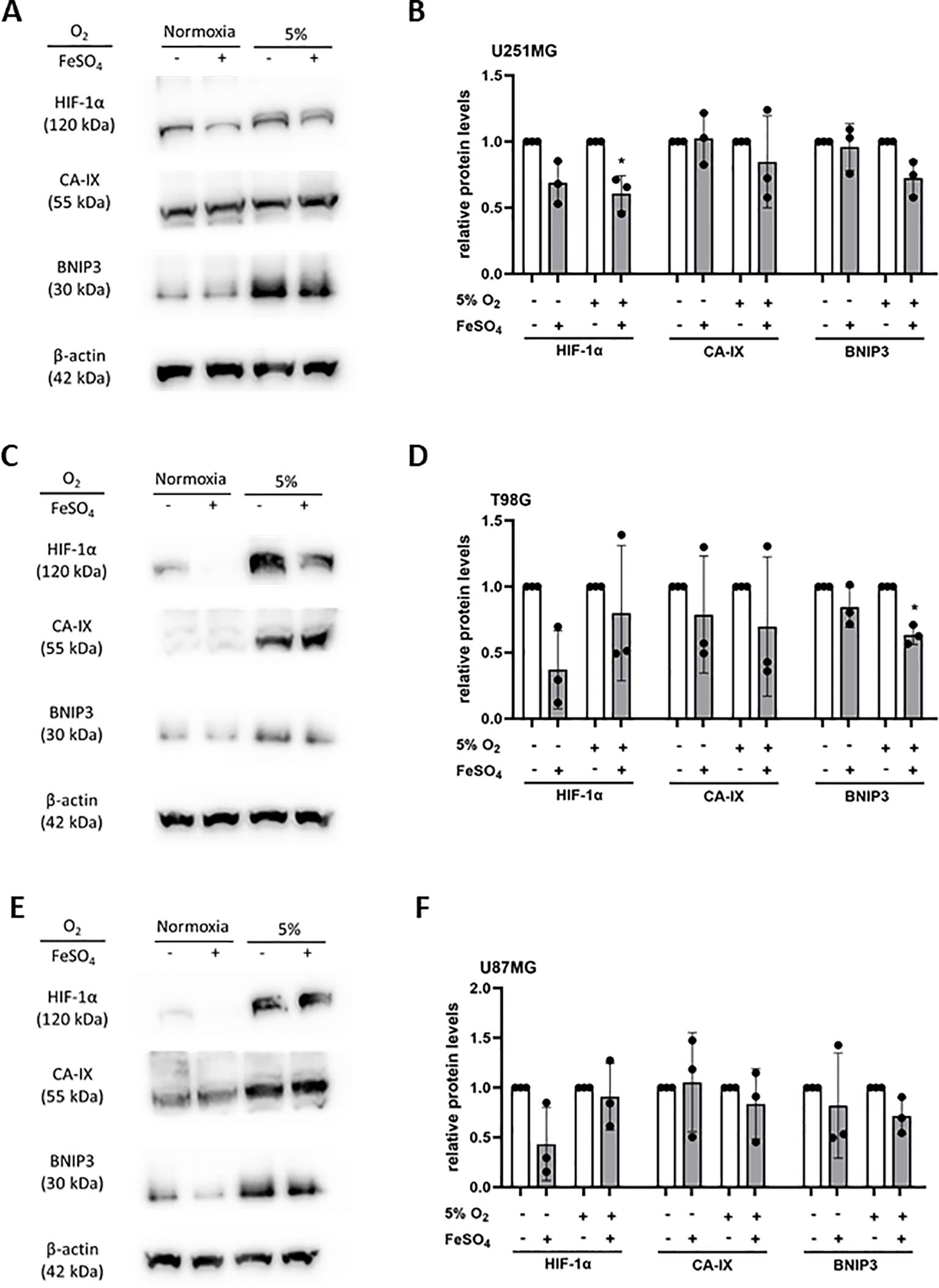
Figure 6. Hypoxic pathway response of glioblastoma cells exposed to iron supplementation. Cells were exposed to FeSO4 (100 μM) in normoxia, or exposed to FeSO4 in normoxia, then incubated in mild hypoxia (5% O2). Lysates of (A) U251MG, (C) T98G and (E) U87MG were collected for western blotting. Relative levels of HIF-1α, CA-IX and BNIP3 were used to analyse the hypoxic pathway, with β-actin used as loading control. Protein bands on western blots were quantified for relative protein levels in (B) U251MG, (D) T98G and (F) U87MG cells (with highest expression set as 1). Paired t-test; mean ± SD; n=3; * p<0.05.
4 Discussion
Elemental and ferrous iron content varied across clinical glioblastoma samples. There were no apparent associations between iron content and clinicopathological data. However, higher ferrous iron content of tumours was associated with improved overall survival of patients with glioblastoma. The iron content of clinical samples was not associated with 2-OGDD enzyme activity, and the lack of association between ferrous iron and the hypoxic response was confirmed in vitro.
Elemental and ferrous iron correlated only weakly, with Fe2+ appearing to be at a higher concentration than Fe. As elemental iron should encompass all forms of iron, irrespective of ionization, this was unexpected. Both ICP-Q-MS and ferrozine methods have been described as sensitive and accurate (29, 32). To our knowledge, ferrous iron has never been measured using the ferrozine assay in glioblastoma tissue. Our data was similar to elemental iron content reported for non-cancerous brain (33, 34). We did not have access to normal, uninvolved brain tissue from our patient cohort as that is not routinely resected during brain cancer surgery in New Zealand. The previously reported data was obtained from formalin-fixed tissue post-mortem analysed by MCP-MS (33), compared to our fresh-frozen tissue; how this may affect the comparative data is unclear.
A variety of techniques are used to measure iron within the brain, including X-ray fluorescence and emission iron mapping, magnetic resonance imaging (MRI), matrix-assisted laser desorption/ionization mass spectrometry, and inductively coupled plasma mass spectrometry (ICP-MS) (33–38), with very few reporting iron content of glioma tumours. In an MRI imaging study, 59 patients with glioma were scanned and quantitative susceptibility maps were constructed that enable estimation of the concentration of iron in an area of tissue (39). The study found that the mean basal ganglia susceptibility, or iron content, increased with glioma grade (39). X-ray fluorescence has been used to estimate iron content in glioma, but data was inconclusive due to small cohorts (40, 41).
Only one study has previously measured elemental iron in brain tumour tissue samples using ICP-MS (42). The study included tumour tissue (n=22, including n=7 glioblastoma and n=8 meningioma) and non-tumour brain tissue from a subset of the patients (n=6), and suggested that non-tumour brain tissue tended to have higher median iron content than tumour tissue, a finding we could not test. A study using X-ray fluorescence to measure a range of trace elements in both gliomas and normal brain tissue, reported that lower concentrations of iron were present in the glioma tissue when compared to the normal brain (40). The discrepancies may be influenced by the regions of brain that were sampled, as different regions are known to vary in iron content (20, 21, 23), and specifically, which brain region the glioma originated from. We saw no clear association between iron content and brain region of the tumours. Patients with glioblastoma may develop aplastic anaemia, especially after treatment with temozolomide, a standard treatment for patients with glioblastoma (43). This would influence iron measurements of brain tumours; however, our samples were all from patients prior to treatment. The blood iron status of our patients was not determined, and may have influenced tissue content, and account for the large inter-individual variations.
Follow-up data from the patient cohort showed a striking association between ferrous iron content of glioblastoma tissue and overall patient survival, although elemental iron appeared to be unrelated to outcome. The reason for the association with ferrous iron is unclear. Our previous study, that included all patients with glioblastoma in this study, had shown a significant association between poor overall survival and an activated hypoxic pathway (27). However, as our current data shows little or no relationship between ferrous iron and the hypoxic pathway, this association with patient survival was unexpected.
We propose that tissue levels of ferrous iron reflect global iron status, such that low tissue levels may indicate anaemia. As anaemia information is not available for our glioblastoma cohort, we were unable to determine associations between tissue iron levels and blood status. Anaemia in patients with glioblastoma has been associated with poor survival in most (44–48), but not all studies (49). A recent study reported that anaemia was associated with poor survival predominantly in male, but not female, patients with glioblastoma (48). Our survival data was not driven by sex bias, as both cohorts (above and below median ferrous iron) had similar male/female ratios. Anaemia, reflected in reduced haemoglobin or haematocrit readings (48), is associated with hypoxemia, a negative prognostic marker for glioblastoma (6). Whether there are subtle differences between hypoxemia, a reduction in blood oxygenation, and hypoxia, a localised drop in tissue oxygen levels, and their effect on the HIF-pathway is unclear.
We hypothesised that the concentrations of iron in glioblastoma tissue would correlate with 2-OGDD activity. These enzymes require ferrous iron and ascorbate as cofactors (11, 12), and we have previously detected strong correlations between tissue ascorbate content and 2-OGDD activity in glioblastoma (27, 28). The enzyme family include the HIF hydroxylases (PHDs and FIH) and TETs, with TETs having among the highest Km for iron of the known 2-OGDDs, ten to 100-fold above the HIF-hydroxylases (11, 50). This would imply that a reduction in iron could specifically reduce TET activity, leading to lower 5hmC levels. Yet, we saw no relationship between 5hmC levels and iron content in clinical glioblastoma samples. Interestingly, cancer-associated TET mutants display markedly lower Km values towards iron, compared to non-mutated TET enzymes (50), suggesting tighter iron binding by these enzymes and a reduced dependency on an extraneous iron source. Previous data had shown that the activity of TET enzymes was reduced in glioma compared to normal brain (51), and that decreased TET activity and lower 5hmC were associated with poor patient prognosis (52, 53).
Previous in vitro studies have shown that iron chelation (DFO) or iron displacement (NiCl2 or CoCl2) resulted in the suppression of 2-OGDD activity, resulting in decreased HIF-hydroxylase activity, with subsequent increased levels of HIF-1α and its downstream targets (31, 54). These reagents poison the enzyme active site by removal or displacement of the active ferrous iron (31, 55). We had therefore hypothesized that an increased iron supply would be associated with increased hydroxylase enzyme activity, but neither our clinical nor in vitro data supported this hypothesis.
The brain is known to have a high iron content (21, 22), and therefore iron may not be a limiting factor for 2-OGDD activity, unlike ascorbate (27, 28). We confirmed this lack of association between iron and 2-OGDD activity in glioblastoma cells in culture, where supplementation with FeSO4 did not consistently suppress the hypoxic pathway. Increasing iron concentration in vitro was challenging, because standard cell culture medium contains 10% bovine serum, that may provide additional iron. It is therefore plausible that both clinical tissues and cells in culture have sufficient bioavailable (intracellular) ferrous iron for the activity of 2-OGDDs.
This study has focused on high-grade primary brain cancer, and it remains to be investigated whether lower-grade primary brain cancers (28), or brain metastases (56), have similar iron content, or relationship between ferrous iron and 2-OGDD, or patient survival.
The main limitation of our study, besides a relatively small cohort size, is that iron-related proteins were not assessed. Information on transferrin, ferroportin, ferroreductase, transferrin receptor, ferritin and others would have provided a more complete picture of the iron pathways in glioblastoma tumours. In addition, further mechanistic studies in serum-free cell culture are required to confirm the role ferrous iron for 2-OGDD activity, specifically for TET activity.
In conclusion, iron content varied greatly between individuals, and ferrous iron content of glioblastoma tumours may be associated with patient outcome, but iron appears not to be a limiting factor for 2-OGDD activities in glioblastoma clinical samples or cell culture. Our data, together with prior studies in anaemic patients, highlight the importance of healthy iron levels in patients with glioblastoma, but further mechanistic studies will be needed to elucidate the molecular pathways involved.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by University of Otago Ethics Committee (H19/163) and the national Health and Disability Ethics Committee (MEC/08/02/016). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
GD: Conceptualization, Formal analysis, Funding acquisition, Supervision, Writing – original draft, Writing – review & editing. CP: Investigation, Methodology, Writing – original draft, Writing – review & editing. EB-S: Investigation, Methodology, Writing – original draft. MR: Investigation, Methodology, Writing – review & editing. EB: Investigation, Writing – review & editing. RC: Investigation, Writing – review & editing. JR: Resources, Writing – review & editing. TS: Resources, Writing – review & editing. EP: Conceptualization, Funding acquisition, Investigation, Methodology, Supervision, Writing – review & editing. MV: Conceptualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Funding was provided by the University of Otago Research Grants and the Mackenzie Charitable Foundation.
Acknowledgments
We would like to thank the patients who donated brain tumour samples for research We would like to acknowledge Helen Morrin and the tissue bank for curating the sample collection and retrieving patient data, and Bridget Robinson for providing clinical advice.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1536549/full#supplementary-material
Abbreviations
2-OGDD, ferrous iron- and 2-oxoglutarate dependent dioxygenase; BNIP3, BCL2/adenovirus E1B 19 kDa protein-interacting protein 3; FIH, factor inhibiting HIF; HIF, hypoxia inducible factor; ICP-Q-MS, inductively coupled-quadrupole-plasma mass spectrometry; IDH, isocitrate dehydrogenase; PHD, prolyl hydroxylase.
References
1. Ostrom QT, Gittleman H, Liao P, Vecchione-Koval T, Wolinsky Y, Kruchko C, et al. CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro Oncol. (2017) 19:v1–v88. doi: 10.1093/neuonc/nox158
2. Lapointe S, Perry A, Butowski NA. Primary brain tumours in adults. Lancet. (2018) 392:432–46. doi: 10.1016/S0140-6736(18)30990-5
3. Weller M, van den Bent M, Preusser M, Le Rhun E, Tonn JC, Minniti G, et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. (2021) 18:170–86. doi: 10.1038/s41571-020-00447-z
4. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. (2016) 131:803–20. doi: 10.1007/s00401-016-1545-1
5. Wesseling P, Capper D. WHO 2016 classification of gliomas. Neuropathol Appl Neurobiol. (2018) 44:139–50. doi: 10.1111/nan.2018.44.issue-2
6. Huang WJ, Chen WW, Zhang X. Glioblastoma multiforme: Effect of hypoxia and hypoxia inducible factors on therapeutic approaches. Oncol Lett. (2016) 12:2283–8. doi: 10.3892/ol.2016.4952
7. McDowell LR. Minerals in Animal and Human Nutrition. Iron (2003) p. p203–233. doi: 10.1016/B978-0-444-51367-0.X5001-6
8. Minotti G, Aust SD. Redox cycling of iron and lipid peroxidation. Lipids. (1992) 27:219–26. doi: 10.1007/BF02536182
9. Lane DJ, Merlot AM, Huang ML, Bae DH, Jansson PJ, Sahni S, et al. Cellular iron uptake, trafficking and metabolism: Key molecules and mechanisms and their roles in disease. Biochim Biophys Acta. (2015) 1853:1130–44. doi: 10.1016/j.bbamcr.2015.01.021
10. Islam MS, Leissing TM, Chowdhury R, Hopkinson RJ, Schofield CJ. 2-oxoglutarate-dependent oxygenases. Annu Rev Biochem. (2018) 87:585–620. doi: 10.1146/annurev-biochem-061516-044724
11. Losman JA, Koivunen P, Kaelin WG Jr. 2-Oxoglutarate-dependent dioxygenases in cancer. Nat Rev Cancer. (2020) 20:710–26. doi: 10.1038/s41568-020-00303-3
12. Crake RLI, Burgess ER, Royds JA, Phillips E, Vissers MCM, Dachs GU. The role of 2-oxoglutarate dependent dioxygenases in gliomas and glioblastomas: a review of epigenetic reprogramming and hypoxic response. Front Oncol. (2021) 11:619300. doi: 10.3389/fonc.2021.619300
13. Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. (2004) 5:343–54. doi: 10.1038/nrm1366
14. Kohli RM, Zhang Y. TET enzymes TDG. and the dynamics of DNA demethylation. Nature. (2013) 502:472–9. doi: 10.1038/nature12750
15. Dong C, Zhang H, Xu C, Arrowsmith CH, Min J. Structure and function of dioxygenases in histone demethylation and DNA/RNA demethylation. IUCrJ. (2014) 1:540–9. doi: 10.1107/S2052252514020922
16. Zhang C, Liu X, Jin S, Chen Y, Guo R. Ferroptosis in cancer therapy: a novel approach to reversing drug resistance. Mol Cancer. (2022) 21:47. doi: 10.1186/s12943-022-01530-y
17. Shenoy G, Kheirabadi S, Ataie Z, Sahu AP, Palsa K, Wade Q, et al. Iron inhibits glioblastoma cell migration and polarization. FASEB J. (2023) 37:e23307. doi: 10.1096/fj.202202157RR
18. Costa da Silva M, Breckwoldt MO, Vinchi F, Correia MP, Stojanovic A, Thielmann CM, et al. Iron induces anti-tumor activity in tumor-associated macrophages. Front Immunol. (2017) 8:1479. doi: 10.3389/fimmu.2017.01479
19. Shenoy G, Connor JR. A closer look at the role of iron in glioblastoma. Neuro Oncol. (2023) 25:2136–49. doi: 10.1093/neuonc/noad136
20. Völkl A, Ule G. Spurenelemente im menschlichen Gehirn. Altersabhängigkeit der Eisenkonzentration in 13 verschiedenen Hirnregionen [Trace elements in human brain. Iron concentration of 13 brain areas as a function of age. Z Neurol. (1972) 202:331–8.
21. Ward RJ, Zucca FA, Duyn JH, Crichton RR, Zecca L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. (2014) 13:1045–60. doi: 10.1016/S1474-4422(14)70117-6
22. Schonberg DL, Miller TE, Wu Q, Flavahan WA, Das NK, Hale JS, et al. Preferential iron trafficking characterizes glioblastoma stem-like cells. Cancer Cell. (2015) 28:441–55. doi: 10.1016/j.ccell.2015.09.002
23. Berg D, Hochstrasser H. Iron metabolism in Parkinsonian syndromes. Mov Disord. (2006) 21:1299–310. doi: 10.1002/mds.21020
24. Beydoun R, Hamood MA, Gomez Zubieta DM, Kondapalli KC. Na+/H+ Exchanger 9 regulates iron mobilization at the blood-brain barrier in response to iron starvation. J Biol Chem. (2017) 292:4293–301. doi: 10.1074/jbc.M116.769240
25. Duck KA, Simpson IA, Connor JR. Regulatory mechanisms for iron transport across the blood-brain barrier. Biochem Biophys Res Commun. (2017) 494:70–5. doi: 10.1016/j.bbrc.2017.10.083
26. Malecki EA, Devenyi AG, Beard JL, Connor JR. Existing and emerging mechanisms for transport of iron and manganese to the brain. J Neurosci Res. (1999) 56:113–22. doi: 10.1002/(SICI)1097-4547(19990415)56:2<113::AID-JNR1>3.0.CO;2-K
27. Burgess ER, Crake RLI, Phillips E, Morrin HR, Royds JA, Slatter TL, et al. Increased ascorbate content of glioblastoma is associated with a suppressed hypoxic response and improved patient survival. Front Oncol. (2022) 12:829524. doi: 10.3389/fonc.2022.829524
28. Crake RLI, Burgess ER, Wiggins GAR, Magon NJ, Das AB, Vissers MCM, et al. Ascorbate content of clinical glioma tissues is related to tumour grade and to global levels of 5-hydroxymethyl cytosine. Sci Rep. (2022) 12:14845. doi: 10.1038/s41598-022-19032-8
29. Flores SE, Day AS, Keenan JI. Measurement of total iron in Helicobacter pylori-infected gastric epithelial cells. Biometals. (2015) 28:143–50. doi: 10.1007/s10534-014-9810-z
30. Phillips E, Horniblow RD, Poole V, Bedford M, Ward DG, Kirkham AJ, et al. A potential role for hepcidin in obesity-driven colorectal tumourigenesis. Oncol Rep. (2018) 39:392–400. doi: 10.3892/or.2017.6062
31. Kuiper C, Dachs GU, Currie MJ, Vissers MC. Intracellular ascorbate enhances hypoxia-inducible factor (HIF)-hydroxylase activity and preferentially suppresses the HIF-1 transcriptional response. Free Radic Biol Med. (2014) 69:308–17. doi: 10.1016/j.freeradbiomed.2014.01.033
32. Yip YC, Chu HS, Chan KC, Chan KK, Cheung PY, Sham WC. Determination of cadmium in oyster tissue using isotope dilution inductively coupled plasma mass spectrometry: comparison of results obtained in the standard and He/H2 cell modes. Anal Bioanal Chem. (2006) 386:1475–87. doi: 10.1007/s00216-006-0712-y
33. Krebs N, Langkammer C, Goessler W, Ropele S, Fazekas F, Yen K, et al. Assessment of trace elements in human brain using inductively coupled plasma mass spectrometry. J Trace Elem Med Biol. (2014) 28:1–7. doi: 10.1016/j.jtemb.2013.09.006
34. Cilliers K, Muller CJF. Multi-element analysis of brain regions from South African cadavers. Biol Trace Elem Res. (2021) 199:425–41. doi: 10.1007/s12011-020-02158-z
35. Stoeckli M, Chaurand P, Hallahan DE, Caprioli RM. Imaging mass spectrometry: a new technology for the analysis of protein expression in mammalian tissues. Nat Med. (2001) 7:493–6. doi: 10.1038/86573
36. Schenck JF. Magnetic resonance imaging of brain iron. J Neurol Sci. (2003) 207:99–102. doi: 10.1016/S0022-510X(02)00431-8
37. Zheng W, Nichol H, Liu S, Cheng YC, Haacke EM. Measuring iron in the brain using quantitative susceptibility mapping and X-ray fluorescence imaging. Neuroimage. (2013) 78:68–74. doi: 10.1016/j.neuroimage.2013.04.022
38. Holmes-Hampton GP, Chakrabarti M, Cockrell AL, McCormick SP, Abbott LC, Lindahl LS, et al. Changing iron content of the mouse brain during development. Metallomics. (2012) 4:761–70. doi: 10.1039/c2mt20086d
39. Reith TP, Prah MA, Choi EJ, Lee J, Wujek R, Al-Gizawiy M, et al. Basal ganglia iron content increases with glioma severity using quantitative susceptibility mapping: a potential biomarker of tumor severity. Tomography. (2022) 8:789–97. doi: 10.3390/tomography8020065
40. Wandzilak A, Czyzycki M, Radwanska E, Adamek D, Geraki K, Lankosz M. X-ray fluorescence study of the concentration of selected trace and minor elements in human brain tumours. Spectrochim Acta Part B At Spectrosc. (2015) 114:52–7. doi: 10.1016/j.sab.2015.10.002
41. Cilliers K, Muller CJF, Page BJ. Trace element concentration changes in brain tumors: A review. Anat Rec (Hoboken). (2020) 303:1293–9. doi: 10.1002/ar.24254
42. Gaman L, Radoi MP, Delia CE, Luzardo OP, Zumbado M, Rodríguez-Hernández Á, et al. Concentration of heavy metals and rare earth elements in patients with brain tumours: Analysis in tumour tissue, non-tumour tissue, and blood. Int J Environ Health Res. (2021) 31:741–54. doi: 10.1080/09603123.2019.1685079
43. Park AK, Waheed A, Forst DA, Al-Samkari H. Characterization and prognosis of temozolomide-induced aplastic anemia in patients with central nervous system Malignancies. Neuro Oncol. (2022) 24:964–73. doi: 10.1093/neuonc/noab240
44. Lutterbach J, Sauerbrei W, Guttenberger R. Multivariate analysis of prognostic factors in patients with glioblastoma. Strahlenther Onkol. (2003) 179:8–15. doi: 10.1007/s00066-003-1004-5
45. Odrazka K, Petera J, Kohlova T, Dolezel M, Vaculikova M, Zouhar M, et al. Prognostic impact of hemoglobin level prior to radiotherapy on survival in patients with glioblastoma. Strahlenther Onkol. (2003) 179:615–9. doi: 10.1007/s00066-003-1097-x
46. Céfaro GA, Genovesi D, Vinciguerra A, Trignani M, Taraborrelli M, Augurio A, et al. Prognostic impact of hemoglobin level and other factors in patients with high-grade gliomas treated with postoperative radiochemotherapy and sequential chemotherapy based on temozolomide: a 10-year experience at a single institution. Strahlenther Onkol. (2011) 187:778–83. doi: 10.1007/s00066-011-1129-x
47. Kaisman-Elbaz T, Elbaz Y, Merkin V, Dym L, Noy A, Atar-Vardi M, et al. Hemoglobin levels and red blood cells distribution width highlights glioblastoma patients subgroup with improved median overall survival. Front Oncol. (2020) 10:432. doi: 10.3389/fonc.2020.00432
48. Shenoy G, Slagle-Webb B, Khunsriraksakul C, Pandya Shesh B, Luo J, Khristov V, et al. Analysis of anemia and iron supplementation among glioblastoma patients reveals sex-biased association between anemia and survival. Sci Rep. (2024) 14:2389. doi: 10.1038/s41598-024-52492-8
49. Lutterbach J, Weigel P, Guttenberger R, Hinkelbein W. Accelerated hyperfractionated radiotherapy in 149 patients with glioblastoma multiforme. Radiother Oncol. (1999) 53:49–52. doi: 10.1016/S0167-8140(99)00128-0
50. Laukka T, Mariani CJ, Ihantola T, Cao JZ, Hokkanen J, Kaelin WG Jr, et al. Fumarate and succinate regulate expression of hypoxia-inducible genes via TET enzymes. J Biol Chem. (2016) 291:4256–65. doi: 10.1074/jbc.M115.688762
51. Chen X, Zhao C, Zhao Z, Wang H, Fang Z. Specific glioma prognostic subtype distinctions based on DNA methylation patterns. Front Genet. (2019) 10:786. doi: 10.3389/fgene.2019.00786
52. Chen B, Lei Y, Wang H, Dang Y, Fang P, Wang J, et al. Repression of the expression of TET2 by ZEB1 contributes to invasion and growth in glioma cells. Mol Med Rep. (2017) 15:2625–32. doi: 10.3892/mmr.2017.6288
53. Orr BA, Haffner MC, Nelson WG, Yegnasubramanian S, Eberhart CG. Decreased 5-hydroxymethylcytosine is associated with neural progenitor phenotype in normal brain and shorter survival in Malignant glioma. PloS One. (2012) 7:e41036. doi: 10.1371/journal.pone.0041036
54. Burgess ER, Praditi C, Phillips E, Vissers MCM, Robinson BA, Dachs GU, et al. Role of sodium-dependent vitamin c transporter-2 and ascorbate in regulating the hypoxic pathway in cultured glioblastoma cells. J Cell Biochem. (2025) 126(1):e30658. doi: 10.1002/jcb.30658
55. Rose NR, McDonough MA, King ON, Kawamura A, Schofield CJ. Inhibition of 2-oxoglutarate dependent oxygenases. Chem Soc Rev. (2011) 40:4364–97. doi: 10.1039/c0cs00203h
Keywords: brain cancer, survival, elemental iron, ferrous iron, hypoxia, glioblastoma
Citation: Praditi C, Beverley-Stone E, Reid M, Burgess ER, Crake RL, Vissers MCM, Royds JA, Slatter TL, Dachs GU and Phillips E (2025) Iron content of glioblastoma tumours and role of ferrous iron in the hypoxic response in vitro. Front. Oncol. 15:1536549. doi: 10.3389/fonc.2025.1536549
Received: 29 November 2024; Accepted: 17 February 2025;
Published: 07 March 2025.
Edited by:
Jose R. Pineda, University of the Basque Country, SpainReviewed by:
Roman Sankowski, University of Freiburg Medical Center, GermanyXingjiang Yu, Hust, China
Copyright © 2025 Praditi, Beverley-Stone, Reid, Burgess, Crake, Vissers, Royds, Slatter, Dachs and Phillips. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gabi U. Dachs, gabi.dachs@otago.ac.nz
†These authors have contributed equally to this work and share first authorship
 Citra Praditi1†
Citra Praditi1†