
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 17 March 2025
Sec. Pediatric Oncology
Volume 15 - 2025 | https://doi.org/10.3389/fonc.2025.1535594
This article is part of the Research Topic Critical Complications In Pediatric Oncology and Hematopoietic Cell Transplant - Volume III View all 3 articles
Background: Lipoblastoma is a benign tumor of embryonal fat tissue that typically affects children under the age of 3 years. The impact of lipoblastoma on the body largely depends on the tumor’s location and its involvement with adjacent organs. Mediastinal lipoblastoma, a relatively rare form, often presents with dyspnea and difficulty breathing, however, airway involvement has not been previously reported.
Case presentation: We describe a 4-month-old female infant diagnosed with lipoblastoma in the mediastinum and endotracheal region, leading to progressive upper airway obstruction and dyspnea. Diagnosis was confirmed through imaging and histopathological analysis. The patient underwent a series of treatments, including bronchoscopic-guided endotracheal intubation with ventilator-assisted ventilation, ECMO-supported mass resection, and airway reconstruction. The patient ultimately achieved complete recovery, with no tumor recurrence observed at a 2-month follow-up.
Conclusion: Mediastinal lipoblastoma can be a significant cause of upper respiratory obstruction and respiratory distress in children. The potential for tracheal infiltration, as seen in this case, has not been previously reported and can pose a life-threatening risk. Recognizing and understanding this rare presentation is essential for timely diagnosis and effective management of this condition.
Lipoblastomas (LPBs) are rare, benign mesenchymal tumors originating from embryonic fat cells, typically occurring in infancy and early childhood. They are classified into two morphological forms: lipoblastoma and lipoblastomatosis (1). These tumors most frequently occur in the limbs and trunk, with only limited cases reported in the mediastinum (2, 3). To date, no cases of LPB have been reported exclusively within the mediastinum or the tracheal lumen, except for a single case involving both the neck and trachea (4). When located in the mediastinum, LPB can pose a significant risk of airway obstruction, often presenting with symptoms such as dyspnea, cough, and palpitations, which may lead to life-threatening complications. Complete surgical resection is critical to prevent recurrence. Recognizing LPB in these atypical locations is crucial for timely diagnosis and effective management. In this paper, we report a rare pediatric case of upper airway obstruction and dyspnea caused by mediastinal and endotracheal LPB, accompanied by a literature review to improve awareness and management strategies for pediatricians treating LPB.
A 4-month-old girl was admitted to our hospital with a persistent cough, stridor, nausea, and difficulty breathing persisting for one month. The patient had no significant medical history. A chest X-ray revealed only mild exudative changes (Figure 1A). Despite aggressive anti-infective therapy, her respiratory distress worsened. Upon admission to the Pediatric Intensive Care Unit (PICU), her initial vital signs were a heart rate of 165 beats per minute, a temperature of 36.6°C, a respiratory rate of 62 breaths per minute, and an oxygen saturation of 91% while on supplemental oxygen via nasal cannula. Physical examination revealed inspiratory stridor without cyanosis, paraparesis, or hemiparesis. Routine laboratory tests, including a complete blood count, C-reactive protein, and liver function tests, were within normal limits. Chest-enhanced computed tomography (CT) showed a fat-density soft tissue mass in the upper left mediastinum (Figures 1B, C) extending into the trachea. Magnetic resonance imaging (MRI) revealed a fat-dominant irregular mass in the same region, with high signal intensity on both T1- and T2-weighted images (Figure 1D). Bronchoscopy identified a smooth-surfaced obstructive mass within the upper tracheal lumen (Figure 1E). An esophageal examination found no abnormalities (Figure 1F). Screening tests for tuberculosis, TORCH infections, neuron-specific enolase, hepatitis viruses, β-human chorionic gonadotropin, alpha-fetoprotein, and bacterial and fungal cultures of the lavage fluid were negative. The patient was initially managed with non-invasive ventilation to correct hypoxia, with the ventilator settings including a positive end-expiratory pressure (PEEP) of 5 mmHg, peak inspiratory pressure (PIP) of 25 mmHg, and a fraction of inspired oxygen (FiO2) at 60%. However, despite these interventions, the patient experienced several episodes of cardiopulmonary arrest, which were temporarily resolved with emergency positive-pressure oxygenation to restore the heart rate. Given the recurrent episodes of respiratory distress, bradycardia, and decreased oxygen saturation, the decision was made to proceed with bronchoscopic-guided endotracheal intubation. Anesthesia induction was achieved with propofol at a dose of 2.5 mg/kg. Subsequently, invasive mechanical ventilation was initiated to provide more stable respiratory support. Meanwhile, Extracorporeal Membrane Oxygenation (ECMO) was kept on standby as a resuscitation option in case of further deterioration. After preoperative evaluation and confirmation of the lesion’s location, the patient underwent surgical resection and airway reconstruction with the support of ECMO. During the surgery, a soft tissue mass measuring 2.5×1.8×0.8 cm was identified (Figure 2A), which had encased the airway. Due to the infiltration of the tumor into the tracheal tissue, a combined resection of the tracheal segments along with the involved tracheal cartilage was performed, followed by end-to-end anastomosis. The entire surgical procedure lasted for 107 minutes. Postoperatively, the patient was successfully weaned off ECMO and continued on mechanical ventilation. The patient’s heart rate, oxygenation, and blood pressure were all maintained within normal limits. Histopathological analysis of the mass confirmed a diagnosis of lipoblastoma, with no encapsulated borders (Figures 2A, B). Immunohistochemistry revealed S-100 positivity in adipocytic cells, while MDM2 and P16 staining were negative (Figure 2C). Fluorescence in situ hybridization (FISH) analysis showed rearrangement of the pleomorphic adenoma gene 1 (PLAG1) (Figure 2D), supporting a final diagnosis of LPB. Given the tumor’s lack of encapsulation and its infiltrative growth pattern, it was more consistent with lipoblastomatosis, a rarer form of lipoblastoma, as described in the literature (1). The postoperative course was uneventful, and the patient did not require further ventilatory support. Bronchoscopy showed no residual foreign body (Figure 1G). At a two-month follow-up, there was no evidence of tumor recurrence (Figure 1H), and the patient remains under regular surveillance.
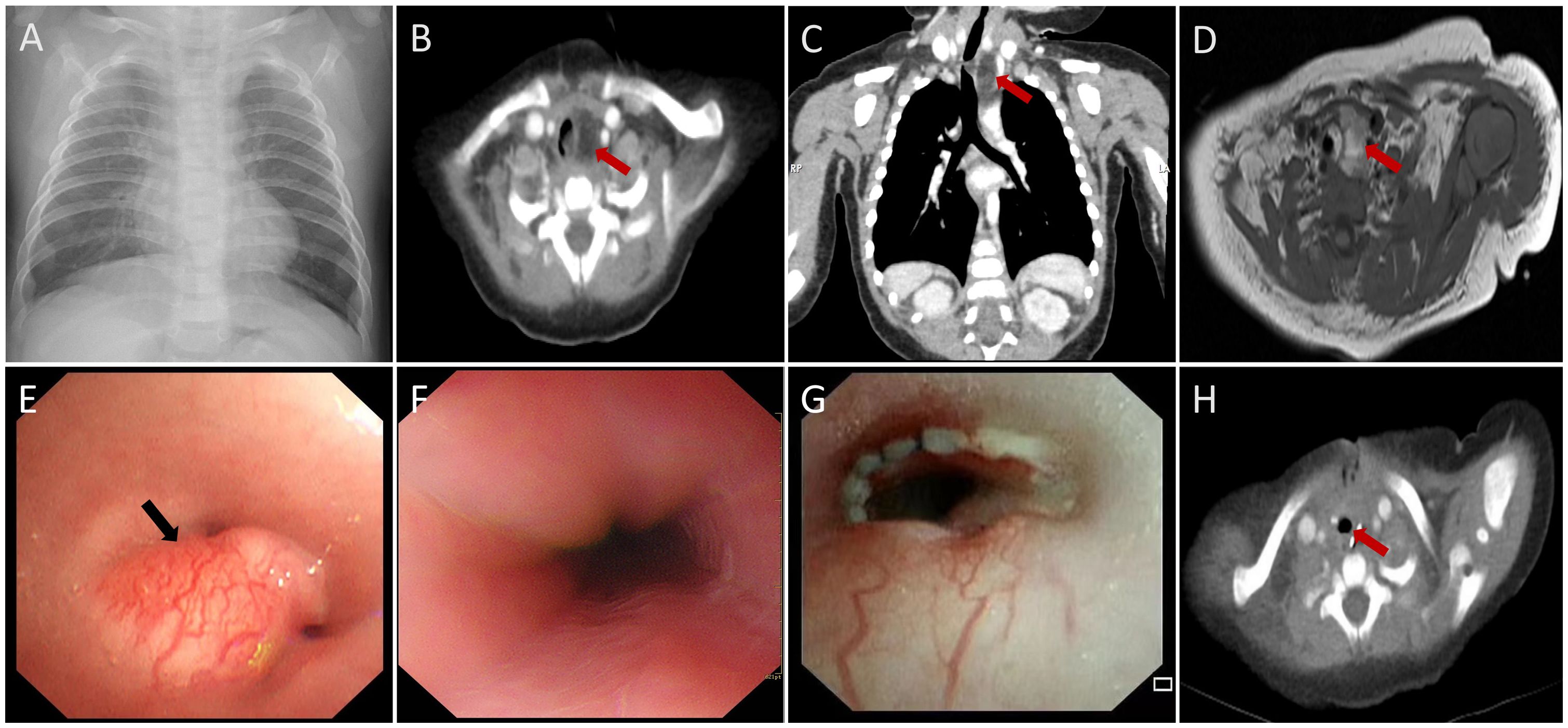
Figure 1. Imaging of mediastinal and endotracheal mass (A) Chest radiography demonstrated no obvious mediastinal mass. (B-D) CT and MRI revealed a mediastinal and endotracheal fatty mass measuring 1.4×1.2×2.3 cm. The trachea was markedly compressed, and the mass protruded into the tracheal lumen, resulting in significant tracheal stenosis (red arrows). (E) Bronchoscopic examination revealed a smooth-surfaced obstructing mass in the upper segments of the tracheal lumen, surrounded by prominent blood vessels (black arrow). (F) Esophageal examination showed no abnormalities. (G, H) MRI scans taken two months postoperatively and postoperative bronchoscopy revealed no recurrence of the mass (red arrows).

Figure 2. Pathological findings of the mass (A) Macroscopic view of a lobular mass with a myxoid appearance measuring 2.5×1.8×0.8 cm. (B) Microphotograph (hematoxylin and eosin stain, ×40) demonstrating mature adipose tissue arranged in lobules. (C) Immunohistochemical staining showed that the tumor cells were negative for MDM2 (×40). (D) FISH analysis detected PLAG1 breakage and rearrangement using a two-color separation probe in lipoblastoma.
LPB predominantly affects children under three years of age (2), with mediastinal LPB cases being rare in pediatric populations (5). There are no documented cases involving both the mediastinum and intraluminal trachea, although one report describes combined endotracheal and neck LPB in a 7-month-old girl (4). To our knowledge, this is the first documented case of LPB concurrently in mediastinum and trachea. It can be life-threatening due to local compression (6), especially when the tumor extends into the trachea. In our patient, the mass extended into the tracheal lumen, resulting in localized stenosis, which caused severe dyspnea and respiratory failure. This case is noteworthy not only due to its rarity but also due to the complexity presented in such a young patient.
Although extremely rare, mediastinal and endotracheal LPB should be considered in patients with recurrent cough or stridor that fails to respond to standard treatments. In this case, the patient’s symptoms of stridor, respiratory failure, and recurrent cardiopulmonary arrest during non-invasive mechanical ventilation raised initial clinical suspicion. Early imaging is essential for diagnosis. Muraoka et al. (7) found that 80% of LPB cases demonstrated abnormal chest radiographic findings, underscoring the value of timely detection through X-rays. However, chest radiography alone cannot accurately locate or measure the mass (8). As observed in our patient, early chest X-rays revealed no masses or space-occupying lesions. While ultrasound can aid in diagnosing LPB, it is less effective in assessing mediastinal or endotracheal involvement. CT and MRI are the preferred imaging modalities, offering comprehensive information on the tumor’s location, size, composition, and relationship to surrounding structures, which is critical for surgical planning.
Due to the high risk associated with tracheal intubation under general anesthesia, and given the likelihood of airway obstruction and the risk of rupture and bleeding caused by the bronchial mass, endotracheal intubation was performed under bronchoscopic guidance. This approach ensured effective respiratory management, emphasizing the importance of bronchoscopy-guided tracheal intubation and ventilation in the prompt and systematic treatment of LPB with such critical symptoms. In our case, fine-needle biopsy was considered inappropriate for diagnosing lipoblastoma due to the significant risk of non-representative sampling and the potential for substantial bleeding. Therefore, this is not recommended. Given the high-risk location of the mass, involving the endotracheal space and extending into the mediastinum, we opted to perform a thoracotomy for definitive management.
The pathogenesis of LPB involves amplification and rearrangement of chromosome 8q11-13, often accompanied by involvement of the PLAG1 gene, which serves as a surrogate marker for PLAG1 fusions commonly seen in LPB (9). MDM2 is typically positive in liposarcomas but negative in LPB (2, 10), aiding in the differential diagnosis. Considering the patient was only 4 months old, the possibility of malignancy in the endotracheal mass could not be excluded due to its invasive growth. For this patient, with elevating levels of human chorionic gonadotropin and α-fetoprotein, and other further screenings were all negative, which helps rule out malignancy, the final definitive diagnosis relied on histopathological analysis, that immunohistochemical staining showed that the tumor cells were negative for MDM2 and FISH analysis detected PLAG1 breakage and rearrangement. Histopathological analysis revealed a non-encapsulated adipose tissue border with an infiltrative growth pattern. Therefore, although imaging and histopathology confirmed LPB, the findings indicate that lipoblastomatosis, a rare variant, may be a more accurate classification for this case. Similar to our case, both Safavi et al. (11) and de Carvalho et al. (12) have reported cases of mediastinal lipoblastoma causing local airway compression. However, neither study described the infiltrative growth of the tumor, especially into the tracheal tissue.
Besides, surgery remains the primary treatment for LPB to preserve vital structures. Complete resection leads to a lower recurrence rate than partial resection (11, 12). Long-term follow-up for 5 to 10 years is recommended due to the risk of late recurrence (13). In our case, no recurrence was observed at the two-month postoperative follow-up, and the patient did not require additional respiratory support.
Mediastinal and endotracheal LPB should be considered in pediatric patients presenting with upper airway obstruction and dyspnea. Lipoblastomatosis, especially in its infiltrative form, is a rare benign tumor that can be invasive. Due to the atypical clinical presentation and imaging characteristics, lipoblastomatosis is often overlooked or misdiagnosed. An accurate diagnosis requires a combination of MRI, histopathological analysis, and immunohistochemistry. The preferred treatment for LPB is complete surgical excision. Additionally, close postoperative follow-up recommended monitoring for possible recurrence.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
This study was approved by the Ethics Committee of West China Second Hospital of Sichuan University. And informed consent from all the patient's parents had been obtained, including the patient's clinical and imaging details in the manuscript for the purpose of publication.
YC: Writing – original draft, Writing – review & editing. LQ: Writing – review & editing. WK: Writing – review & editing. ZL: Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by grants from the Science and Technology Bureau of Sichuan province (No.2019YFS0241). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors would like to thank the family for participating and supporting this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
LPBs, Lipoblastomas; PICU, Pediatric Intensive Care Unit; CT,computed tomography; MRI, Magnetic resonance imaging; PEEP, positive end-expiratory pressure; PIP, peak inspiratory pressure; FiO2, fraction of inspired oxygen; ECMO, Extracorporeal Membrane Oxygenation; FISH, Fluorescence in situ hybridization; PLAG1, pleomorphic adenoma gene.
1. Abera MT, Yaynishet YA, Mammo BG, Jiffar AD, Salah FO. Intrathoracic extension of a chest wall Lipoblastoma in an infant: A rare case report. Radiol Case Rep. (2024) 19:3076–9. doi: 10.1016/j.radcr.2024.04.045
2. Ameloot E, Cordier F, Van Dorpe J, Creytens D. Update of pediatric lipomatous lesions: A clinicopathological, immunohistochemical and molecular overview. J Clin Med. (2022) 11:1938. doi: 10.3390/jcm11071938
3. Coffin CM, Lowichik A, Putnam A. Lipoblastoma (LPB): a clinicopathologic and immunohistochemical analysis of 59 cases. Am J Surg Pathol. (2009) 33:1705–12. doi: 10.1097/PAS.0b013e3181b76462
4. Torre M, Borel C, Saitua F, Ossandon F, Latorre JJ, Varela P. Lipoblastoma with unique localization requiring tracheal and esophageal resection. J Pediatr Surg. (2010) 45:e21–3. doi: 10.1016/j.jpedsurg.2010.06.034
5. Bigley TM, Song Z, Orscheln RC, Wilson DB, Shakhsheer BA, He M. X-linked chronic granulomatous disease presenting with mediastinal lipoblastoma and adjacent pneumonia. J Clin Immunol. (2021) 41:1969–71. doi: 10.1007/s10875-021-01129-w
6. Ghosh P, Das RN, Ghosh R, Chatterjee U, Datta C, Mishra PK. Lipoblastoma and lipoblastomatosis: A clinicopathological study of six cases. J Cancer Res Ther. (2015) 11:1040. doi: 10.4103/0973-1482.176135
7. Muraoka M, Oka T, Akamine S, Nagayasu T, Iseki M, Suyama N. Endobronchial lipoma: review of 64 cases reported in Japan. Chest. (2003) 123:293–6. doi: 10.1378/chest.123.1.293
8. María Santos C, Patricio Herrera O. Mediastinal lipoblastoma in paediatrics. Rev Chil Pediatr. (2020) 91:246–50. doi: 10.32641/rchped.v91i2.1223
9. Warren M, Tiwari N, Sy S, Raca G, Schmidt RJ, Pawel B. PLAG1 immunohistochemical staining is a surrogate marker for PLAG1 fusions in lipoblastomas. Pediatr Dev Pathol. (2022) 25:134–40. doi: 10.1177/10935266211043366
10. Lopez-Nunez O, Alaggio R, Ranganathan S, Schmitt L, John I, Church AJ. New molecular insights into the pathogenesis of lipoblastomas: clinicopathologic, immunohistochemical, and molecular analysis in pediatric cases. Hum Pathol. (2020) 104:30–41. doi: 10.1016/j.humpath.2020.07.016
11. Safavi M, Akhlaghi N, Ashjaei B. Large intrathoracic lipoblastoma. Asian Cardiovasc Thorac Ann. (2021) 29:858–61. doi: 10.1177/02184923211038407
12. de Carvalho VB, Dias JL, Nunes A. Paediatric mediastinal lipoblastoma. J Belgian Soc Radiology. (2023) 107:53, 1–3. doi: 10.5334/jbsr.3166
Keywords: lipoblastoma, mediastinal and endotracheal mass, MRI, airway obstruction, children
Citation: Chen Y, Qiu L, Kou W and Liu Z (2025) Upper airway obstruction and dyspnea in an infant caused by mediastinal and endotracheal lipoblastoma: a case report. Front. Oncol. 15:1535594. doi: 10.3389/fonc.2025.1535594
Received: 27 November 2024; Accepted: 27 February 2025;
Published: 17 March 2025.
Edited by:
Asya Agulnik, St. Jude Children’s Research Hospital, United StatesReviewed by:
Saad Ghafoor, St. Jude Children’s Research Hospital, United StatesCopyright © 2025 Chen, Qiu, Kou and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhongqiang Liu, bGl1X3poX3FpYW5nQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.