
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 28 February 2025
Sec. Surgical Oncology
Volume 15 - 2025 | https://doi.org/10.3389/fonc.2025.1528036
Objective: Nosocomial infections are one of the severe postoperative complications that compromise perioperative safety in patients with colon cancer. However, there are limited studies on constructing visual risk prediction screening tools for nosocomial infections in these patients. The objective of this study is to construct a nomogram for predicting the risk of nosocomial infections among patients after colon cancer surgery.
Methods: Total 1146 patients after colon cancer surgery were selected and divided into a training set and a validation set. After identifying the most significant predictors through LASSO regression and logistic regression, the model was presented as static and dynamic nomogram. AUC was used to evaluate the discrimination of model. Calibration was evaluated by means of calibration curves. Decision and impact curves were applied to evaluate the clinical validity.
Results: 110 patients (9.60%) suffered nosocomial infections following colon cancer surgery. Peak temperature on the second postoperative day, Braden score on the first postoperative day, duration of retention of abdominal drains, ASA class, surgical type and postoperative complications were correlated with nosocomial infections. The nomogram composed of these predictors demonstrated good discrimination, calibration and clinical benefit in both the training and validation sets.
Conclusion: Risk predictors are important breakthroughs for healthcare workers in nosocomial infections prevention and control initiatives. The dynamic nomogram built in this study may be helpful for healthcare personnel to identify the risk of nosocomial infections among patients after colon cancer surgery.
Colon cancer is one of the common malignant tumors of the gastrointestinal tract worldwide. The proportion of colon cancer has been rising over the past few years. The Global Burden of Cancer 2020 reports that there will be more than 1.14 million new cases of colon cancer and more than 570,000 attributable deaths in 2020 (1). At present, many poor lifestyles and dietary structures further contribute to the younger age of onset of colon cancer (2).
The high morbidity and mortality rates of colon cancer have made it one of the diseases of great concern in the field of healthcare. At this stage, the main form of treatment is to remove the tumor by surgery. How to effectively guarantee the perioperative safety of patients has become the focus of healthcare professionals. In particular, prevention of nosocomial infections (NIs) is one of the most vital elements to ensure perioperative safety. Despite the existence of many interventions aimed at reducing the risk of NIs, NIs pose a number of adverse prognostic consequences for patients. Without effective control of NIs, patients may be at risk of continued deterioration and progression to infectious shock, which can ultimately lead to multiple organ dysfunction syndrome. Therefore, it is extremely important to prevent postoperative NIs among patients after colon cancer. NIs cause a significant disease and economic burden on global healthcare systems (3, 4). A study by MacLaurin A showed that the average annual number of NIs in Canada is about 220,000 (5). The situation of NIs in the United States is also not optimistic, as the annual average number of inpatient deaths due to NIs has long exceeded 100,000 (6). At the hospital level, the occurrence of NIs can lead to medical disputes and negatively affect hospitals’ operational capacity (7). At the patient level, NIs increase the severity of the patient’s condition, prolong length of stay, affect the outcome of in-hospital treatment and quality of life (8), and cause anxiety and distress to the patient.
Nomogram is a predictive model constructed on the basis of Logistic regression analysis, which visualizes graphs showing the extent to which each predictor contributes to the effect of the dependent variable (9). The presentations of nomogram includes both static and dynamic forms. Effective risk prediction models are essential aids to assist healthcare professionals in targeting those at high risk for NIs. However, there are few studies that constructed nomogram for predicting the risk of NIs in patients after colon cancer surgery. Given the severity of NIs, the aim of this study was to explore the potential predictors associated with NIs in patients after colon cancer surgery and to construct a dynamic nomogram.
In this study, colon cancer patients who underwent surgical treatment between 1 January 2020 and 31 December 2022 in a tertiary comprehensive teaching hospital affiliated to Shandong University were included as study subjects. Patients were included if they were (1) diagnosed with colon cancer, (2) underwent surgery, and (3) were older than 18 years. Patients who only underwent palliative surgeries such as exploratory laparotomy or colostomy, as well as patients who develop preoperative NIs, were excluded. This study was approved by the ethics committee on scientific research of Shandong University and conducted following the Declaration of Helsinki. Due to the retrospective nature of this study and anonymous data collection, informed consent is not required. The patient screening process is shown in Figure 1.
Patient data from 2020 to 2021 was used as a training set to construct the model, and patient data from 2022 was used as a validation set to validate the model. In order to achieve a predictive model that accurately estimates the overall risk of occurrence of the outcome event, we used the sample size calculation metric mentioned by Riley et al (10). The formula is as follows:
The “φ” represents the probability of occurrence of the ending event. The NIs rate of postoperative colon cancer patients in the pre-survey was about 10%, and based on clinical experience, we set the permissible error “δ” to be 0.03, which was calculated to obtain a sample size of 384 cases for the training set.
Definition of NIS cases according to the diagnostic criteria for NIs issued by the Centers for Disease Control and Prevention (11). Gastrointestinal clinicians made the NIs diagnosis by combining the patient’s clinical symptoms and ancillary test results. To ensure the rigor of the study, all included NIs cases were audited by professional NIs managers.
Hospital infection surveillance system and hospital information system were used to complete the collection of clinical data from patients by two independent researchers with professional training. Preoperative and postoperative laboratory values were measured within 24 hours of admission and within 24 hours after surgery, respectively. The postoperative complications that occurred in the patients collected in this study included intestinal obstruction, chyle leak, anastomotic leakage, incision dehiscence, deep venous thrombosis, pericardial effusion, hypokalemia, gastric emptying disability, heart failure, respiratory failure, and fistula of intestine. Since NIs being positive outcome events in this study, they were not included in the postoperative complications mentioned above. All predictor variables were collected prior to the outcome event.
We analyzed all data using SPSS version 26.0 and R 4.1.0 software. Categorical variables were statistically described using frequencies, rates or percentages (%). Inter group comparisons were analyzed with Pearson’s chi-square test or Fisher’s exact probability test. Continuous variables were described as mean ± standard deviation (SD) if normally distribution, otherwise as median (interquartile range). Inter group comparisons were analyzed with the Student’s t-test or Mann-Whitney U-test. P < 0.05(two-sided) were considered significant.
The least absolute shrinkage and selection operator (LASSO) regression algorithm is an estimation method that enables the streamlining of indicator sets (12). LASSO is based on the principle of adding a penalty term to the least squares basis to compress the estimated parameters, making them zero when they are reduced to less than a threshold, and ultimately producing a set of independent variables with some correlation with the ending variables (12–15). LASSO is suitable for dealing with data that may be subject to collinearity and studies with a low number of outcome events (16). In LASSO regression, the adjustment of model complexity can be achieved by adjusting the value of “λ” (17). In this study, 10-fold cross-validation was used to determine the optimal “λ” value. Due to the large number of variables collected in this study, in order to reduce the impact of collinearity between them, we used LASSO to conduct preliminary screening of the variables. Afterwards, we used Logistic regression for final screening of independent risk predictors of NIs in patients after colon cancer, and finally developed a logic-model based NIs Nomogram. The above method of screening variables is referred to as lasso-logistic regression (18). P < 0.05(two-sided) were considered significant.
We used the independent risk predictors to construct a nomogram. Discrimination of the model can be represented by receiver operating characteristic (ROC) curve (19). Discrimination increases gradually as the AUC value approaches 1. We used the Hosmer-Lemeshow test and the calibration curve to evaluate calibration (19). The P > 0.05 of the Hosmer-Lemeshow test indicates a good calibration ability of the model. In addition, the clinical benefit of the nomogram in this study was assessed by decision curve analysis (DCA) and clinical impact curve (CIC). DCA can be used to evaluate the potential population impact of applying nomogram in clinical practice. The vertical axis shows standardized net benefit, while the horizontal axis represents the risk threshold. CIC is generated based on DCA. It can display the estimated number of people predicted as high-risk at each risk threshold and intuitively show the proportion of true positive patients (20).
Ultimately, this study comprised 1146 colon cancer patients who underwent surgery, with an average age of 59.79 ± 12.31 years. The number of patients in the training set was 762, with an average age of 60.11 ± 12.51 years. The number of patients in the validation set was 384, with an average age of 59.16 ± 11.89 years. In this study, a total of 110 patients developed NIs, with an NIs rate of 9.60%. Among the 110 patients with NIs, lower respiratory tract infections and surgical site infections were predominantly found in 38 (34.55%) and 34 (30.91%) cases, respectively. Other sites of NIs included abdominal infections in 4 cases (3.64%), urinary tract infections in 4 cases (3.64%), ascites infections in 2 cases (1.82%) and bloodstream infection in 1 case (0.91%). Additionally, there were 27 cases (24.55%) of multiple sites of infections. The NIs rates for the training and validation sets were 9.71% and 9.38%, respectively. Table 1 had displayed the baseline characteristics of study subjects in the training set and validation set.
We used LASSO regression for the initial screening of predictors of NIs. Figure 2 present the process of variable screening in LASSO regression. Based on LASSO regression, we ultimately selected seven features as the optimal variables.

Figure 2. Predictors selection using the least absolute shrinkage and selection operator (LASSO) binary logistic regression model. (A) The curve of the coefficient path of variables in the training set. Each curve indicated the trajectory of a variable coefficient change. (B) LASSO regression coefficients profiles of variables. The two vertical dashed vertical lines correspond to lambda.min (logarithm of the minimum mean error λ) and lambda.1se (logarithm of the doubled standard error λ). In order to obtain a well-performing and more parsimonious model, seven features with non-zero coefficients were selected as the best variables in this study based on lambda.1se.
After including the above seven variables in the multivariate logistic regression, a total of six independent predictors were finally obtained (Table 2). Figure 3 showed the nomogram for predicting the risk of NIs in postoperative colon cancer patients constructed on the basis of six independent predictors. In addition, for the convenience of application, we produced a dynamic nomogram(https://jiechangailiexiantu.shinyapps.io/ccDynNomapp/). This web page allows clinicians to automatically calculate the probability of a patient’s risk of postoperative NIs on-line by selecting or entering the values of the predictor variables based on the patient’s actual condition, which is easy to apply and highly efficient. Figure 4 is a screenshot of an example of the nomogram application. As can be seen from Figure 4, the risk of NIs in this colon cancer patient was 73.40%.
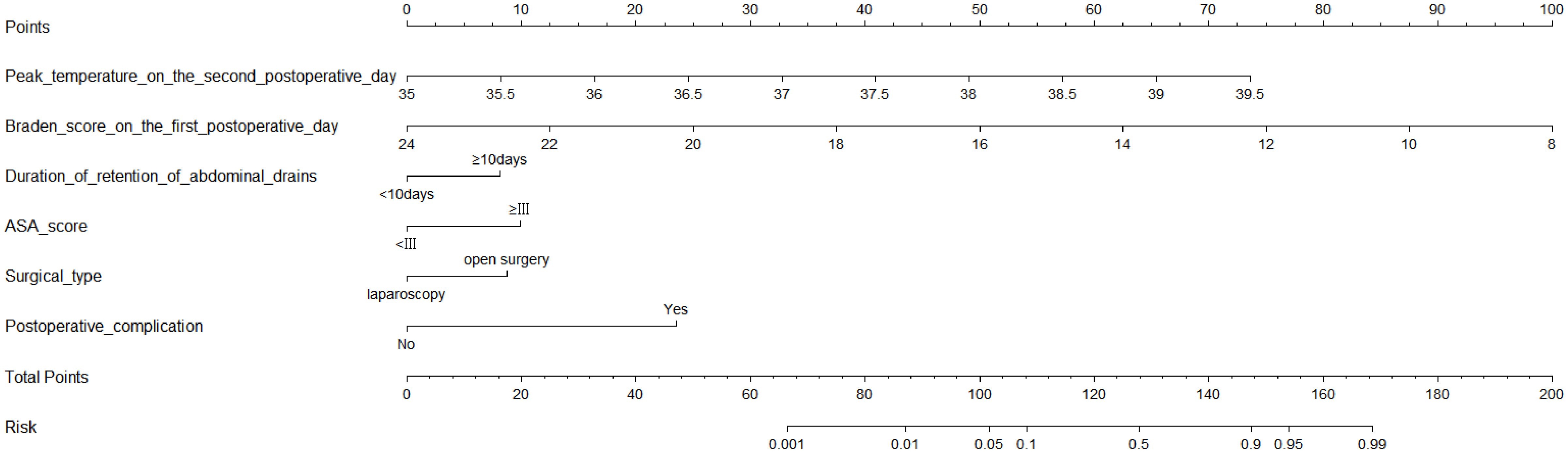
Figure 3. Nomogram for predicting the risk of NIs in patients undergoing colon cancer surgery. ASA, American Society of Anesthesiologists.
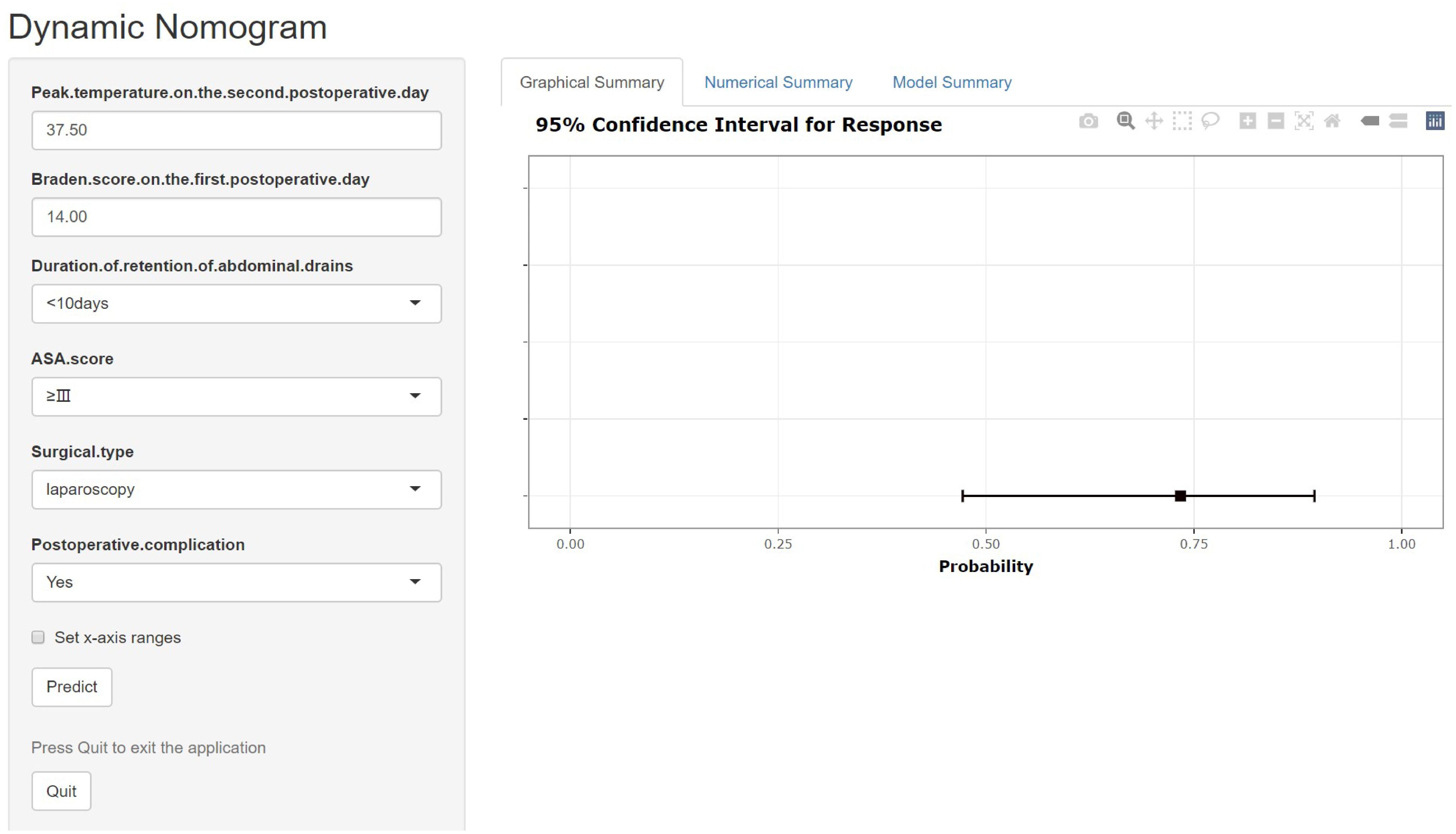
Figure 4. Dynamic nomogram for predicting the risk of NIs in patients undergoing colon cancer surgery. ASA, American Society of Anesthesiologists.
As illustrated in Figure 5, The AUC of the training set and validation set were 0.881 (95% CI: 0.856~0.903) and 0.813 (95% CI: 0.770~0.851), respectively, indicating that the model has good discriminative ability. Figure 6 showed the calibration curves of the training and validation sets. The bias correction curves in both sets were close to the ideal curves. In addition, the P of the Homer Lemeshow test results for the training and validation sets were 0.490 and 0.179, respectively. All the above results showed that the model is well calibrated. The DCA for the training and validation sets were represented in Figure 7. The DCA in our study revealed good net benefits of the nomogram in both the training and validation sets. Figure 8 demonstrated the CIC of the training and validation sets. As shown by the curves in the Figure, the number of patients with NIs predicted by the nomogram was close to the number of patients with NIs that actually occurred. The CIC showed that the nomogram model has good clinical utility.
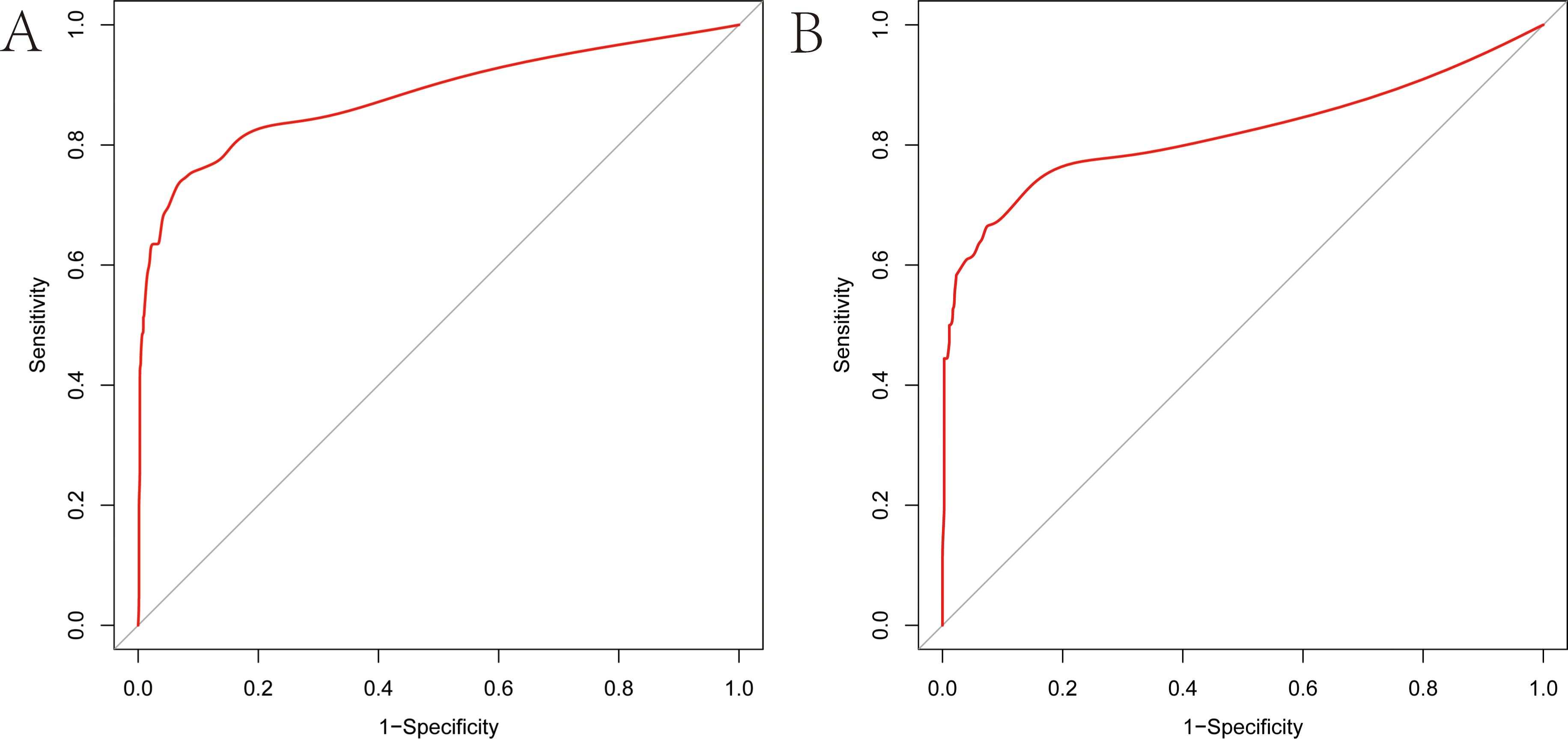
Figure 5. Receiver operating characteristic (ROC) curves of the nomogram (A) ROC curve of the training set. (B) ROC curve of the validation set. The vertical axis represents the true positive rate, and the horizontal axis represents the false positive rate. The higher the convexity of the red curve, the higher the AUC is demonstrated.
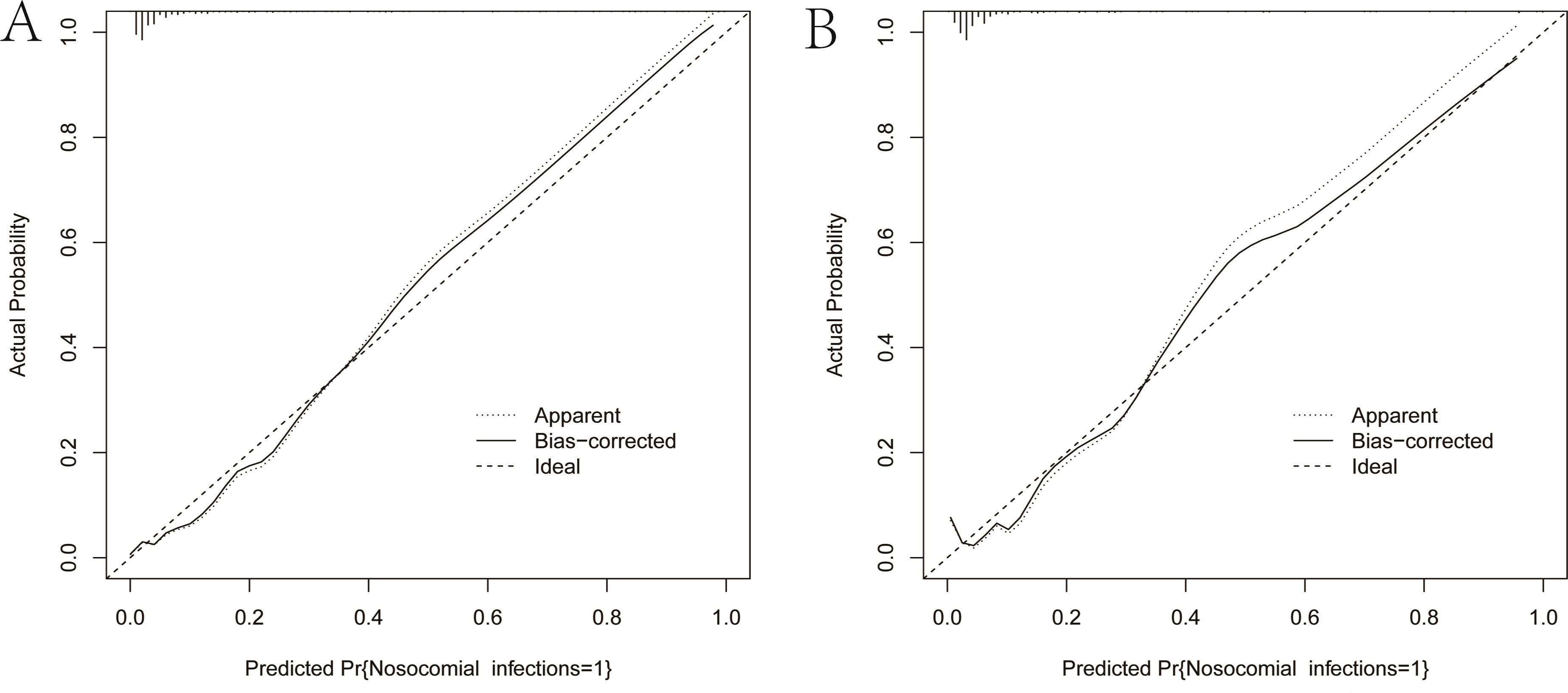
Figure 6. Calibration curves of the nomogram. (A) Calibration curve of the training set. (B) Calibration curve of the validation set. The solid black line is the result of bias correction by bootstrap resamples (1000 repetitions). The closer the solid black line is to the diagonal dashed line, the better the calibration of the nomogram.
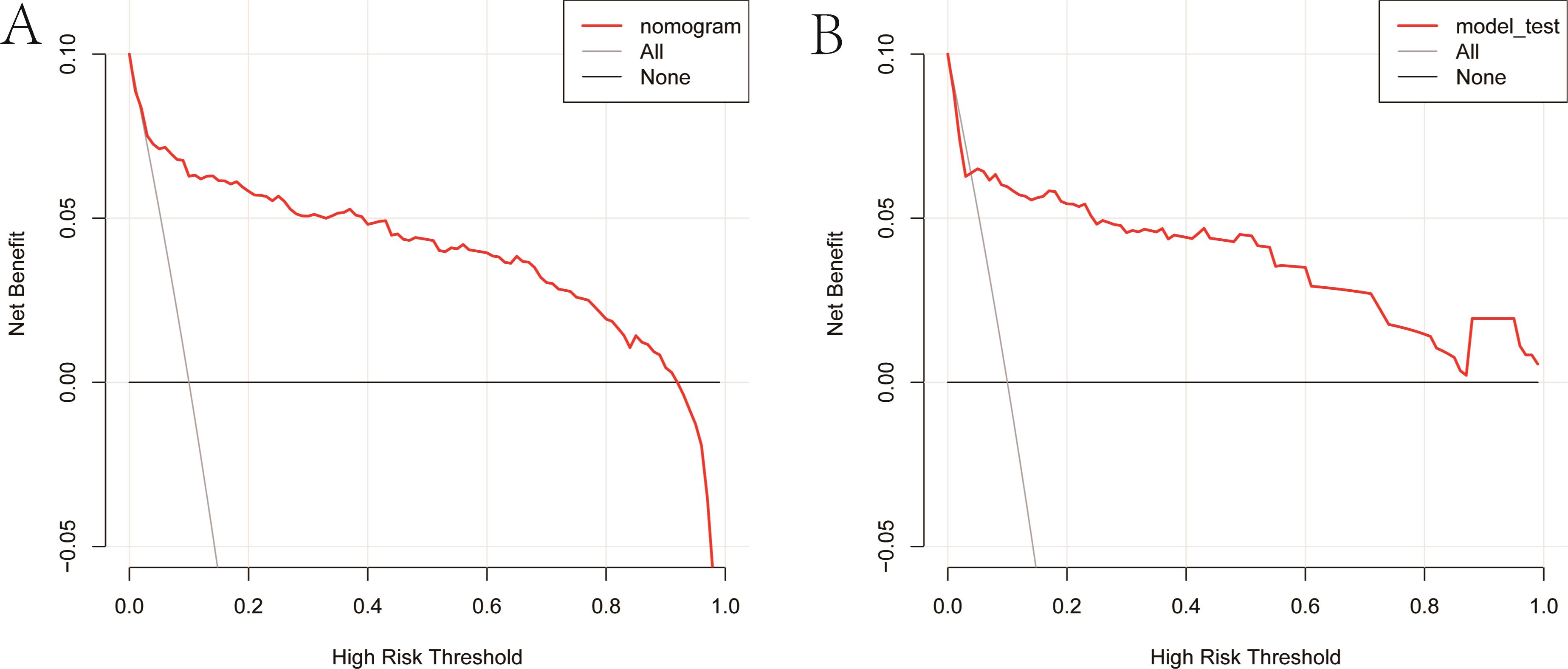
Figure 7. Clinical decision curves of the nomogram. (A) Clinical decision curve of the training set. (B) Clinical decision curve of the validation set. The gray diagonal line in the Figure represented the intervention performed on all patients. When the red curve did not coincide with the gray diagonal line and lies above the black horizontal line, it indicated that the nomogram has a net benefit.
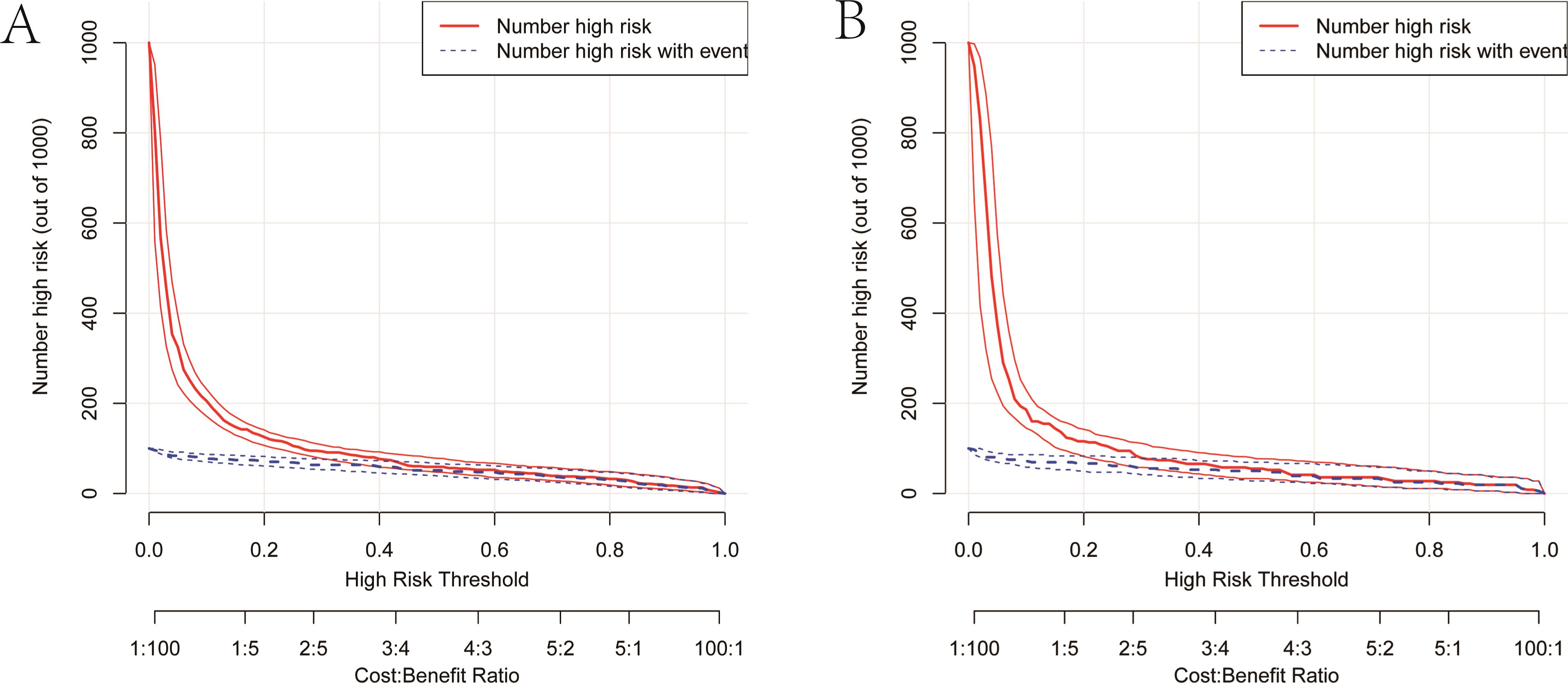
Figure 8. Clinical impact curves of the nomogram. (A) Clinical impact curve of the training set. (B) Clinical impact curve of the validation set. Among 1,000 patients, the red solid line displays the total number of people considered high-risk at each risk threshold. The blue dashed line represents the true NIs patients among them.
Delayed diagnosis of NIs leads to prolonged hospitalization and recovery time for patients, and even re-operation. NIs also bring unnecessary medical expenses to patient families, and waste medical resources. Therefore, to achieve better clinical outcomes for colon cancer patients, it is necessary to screen out high-risk patients for NIs in advance and take preventive measures as early as possible to reduce the incidence of NIs. It is worth noting that the dynamic nomogram developed in this study resembles a network calculator. In this dynamic nomogram, we can enter the values of the predictors on the page to automatically calculate the predicted values of the ending events (21).
After analysis, this study concluded that peak temperature on the second postoperative day, Braden score on the first postoperative day, duration of retention of abdominal drains≥10days, ASA class ≥III, surgical type and postoperative complication were significant predictors of NIs. Nomograms generated using the above predictors have good discriminatory power, calibration and clinical validity, and to some extent it can predict the risk of NIs after resection of colon cancer.
Regular postoperative monitoring of patients’ vital signs can help healthcare professionals detect some postoperative infectious complications in a timely manner. Among them, temperature change is the first signal of infection perceived by the patient, and an elevated trend suggests that the patient may have potential inflammation in the organism (22). The postoperative body temperature data of colon cancer patients is easily obtainable in clinical setting. The nomogram of this study shows that the peak temperature on the second postoperative day is an essential factor affecting the occurrence of NIs in colon cancer patients, which further emphasizes the importance of monitoring patients’ postoperative temperatures on schedule. Zheng S et al. (22) reported that the average body temperature on the three postoperative day of patients exceeded 37°C may be a critical sign of surgical site infection.
Postoperative complications were shown to be one of the risk factors for NIs in patients in this study. Colon cancer surgery involves anastomosis between intestinal incisions. Anastomotic leakage can likely occur postoperatively if the patient has problems such as poor overall preoperative nutritional status, insufficient intestinal cleanliness, and poor blood flow to the postoperative intestinal anastomosis. Anastomotic leakage is a potentially serious complication that occurs after colon cancer surgery, which will increase the tumor recurrence rate and mortality rate of patients (23, 24). A meta-analysis by Lawler J. et al. showed that anastomotic leakage had a negative impact on the overall survival of colorectal cancer patients (25).
The surgical procedures performed on colon cancer patients in this study included open and laparoscopic. A meta-analysis synthesizing the results of several randomized controlled studies showed that laparoscopic colectomy has similar disease-free and overall survival rates to open colectomy and that the procedure is safe (25–27). Four randomized controlled trials on colorectal cancer surgery have confirmed that laparoscopic surgery has significant advantages over open surgery in terms of less intraoperative blood loss, less postoperative pain, faster recovery of intestinal function, and shorter length of hospital stay (28). The results of our study showed that the rate of NIs was significantly lower in laparoscopic surgery than in the group of patients who underwent open surgery, which is in line with the results of previous studies (29–33). This is further evidence that expanding the adoption of laparoscopic surgery may reduce the rate of NIs in colon cancer surgery.
In this study, the level of postoperative Braden score was inversely associated with the risk of NIs in colon cancer patients. The Braden Scale is a tool recommended by the U.S. Agency for Healthcare Policy Research in 1987 to predict the risk of pressure ulcers. Many studies in recent years have confirmed that the Braden Scale has other uses. Lovicu E et al. (34) found that admission Braden score was inversely associated with the risk of in-hospital mortality in COVID-19 patients (OR=0.76). Cohen RR et al. found that lower Braden scores predicted postoperative complications (OR=1.30) in elderly surgical patients (34). Although the Braden Scale is a widely used tool for routine nursing assessment of pressure ulcer events, it has been proposed by a previous researcher to be used as a frailty assessment tool because it simultaneously assesses several frailty-related indicators, such as nutrition, cognition, and function (35). The lower the Braden score of a colon cancer patient means that the patient’s physical mobility and nutritional intake, among other things, are likely to be poorer, which can lead to a low level of physical resistance and an increased chance of infection.
In this study, ASA class ≥ III (OR=3.041) was a predictor of NIs after colon cancer surgery, which was similar to the findings of Yang J et al. (36). The ASA class is a method of representing patient operative risk on a scale of I-VI (37). The ASA class ≥ III often implies that the patient is in poorer health and at higher risk of NIs. Therefore, clinical healthcare professionals should actively adjust the physical status of colon cancer patients, and adopt multidisciplinary consultation when necessary in order to minimize the risk of NIs in patients (22).
Similar to the results of this study, several previous studies have reported a correlation between indwelling prophylactic abdominal drains and the development of retrograde infectious complications, which may be related to the retrograde entry of pathogenic bacteria into the abdominal cavity through the line (38, 39). Clinical practice guidelines in the United States clearly state that it is recommended that routine use of abdominal drains should be avoided after colorectal surgery, given that there are no data to support the benefit of routine use of abdominal drains in the identification and prophylactic treatment of anastomotic fistulas, but rather the potential to lead to the development of drainage-associated complications such as extra-intestinal fistulas (40).
The strength of this study is that the constructed dynamic nomogram is based on clinically available predictors that can be used to guide healthcare professionals in developing strategies for NIs prevention and control. Compared with static nomograms, dynamic nomograms interactive interface is more convenient for clinicians to make personalized diagnosis and treatment decisions, which can simplify the complexity of the clinical practice of nomograms, and improve the efficiency of the use of nomograms.
There are some limitations to our study. First, the nomogram was not validated using data from other hospitals, which may limit the generalizability of the nomogram. Second, we only collected and compared patients’ NIs during their stay in the hospital, but failed to follow them long-term after discharge. Therefore, multicenter, large sample as well as prospective analyses should be conducted in the future.
We developed a dynamic nomogram of NIs risk with good discrimination, calibration, and clinical validity. This web-based online risk calculator may help healthcare professionals to identify patients at high risk of NIs early.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Ethics Committee of School of Nursing and Rehabilitation, Shandong University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
XY: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Writing – original draft, Writing – review & editing. SW: Formal analysis, Methodology, Supervision, Writing – original draft, Writing – review & editing. AL: Investigation, Supervision, Writing – original draft, Writing – review & editing. YX: Formal analysis, Methodology, Supervision, Writing – original draft, Writing – review & editing. NL: Formal analysis, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We would like to express our sincere gratitude to the professionals in the Infection Prevention and Control Department and all the healthcare professionals in the Colorectal Surgery Department for their excellent work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, et al. Colorectal cancer statistics, 2017. CA: Cancer J Clin. (2017) 67:177–93. doi: 10.3322/caac.21395
3. Suetens C, Latour K, Kärki T, Ricchizzi E, Kinross P, Moro ML, et al. Prevalence of healthcare-associated infections, estimated incidence and composite antimicrobial resistance index in acute care hospitals and long-term care facilities: results from two European point prevalence surveys, 2016 to 2017. Euro surveillance: Bull Europeen sur les maladies transmissibles = Eur communicable Dis Bull. (2018) 23. doi: 10.2807/1560-7917.ES.2018.23.46.1800516
4. Guest JF, Keating T, Gould D, Wigglesworth N. Modelling the annual NHS costs and outcomes attributable to healthcare-associated infections in England. BMJ Open. (2020) 10:e033367. doi: 10.1136/bmjopen-2019-033367
5. MacLaurin A, Amaratunga K, Couris C, Frenette C, Galioto R, Hansen G, et al. Measuring and monitoring healthcare-associated infections: A canadian collaboration to better understand the magnitude of the problem. Healthcare Q (Toronto Ont.). (2020) 22:116–28. doi: 10.12927/hcq.2020.26040
6. Lancet Infectious D. Health-care associated infection: USA on the right track. Lancet Infect Dis. (2013) 13:377. doi: 10.1016/S1473-3099(13)70115-4
7. Wilson P, Gurusamy KS, Morley R, Whiting C, Maeso B, FitzGerald G, et al. Top research priorities in healthcare-associated infection in the UK. J Hosp infection. (2019) 103:382–7. doi: 10.1016/j.jhin.2019.08.013
8. Storr J, Twyman A, Zingg W, Damani N, Kilpatrick C, Reilly J, et al. Core components for effective infection prevention and control programmes: new WHO evidence-based recommendations. Antimicrobial resistance infection control. (2017) 6:6. doi: 10.1186/s13756-016-0149-9
9. Wang S, Yang L, Ci B, Maclean M, Gerber DE, Xiao G, et al. Development and validation of a nomogram prognostic model for SCLC patients. J Thorac oncology: Off Publ Int Assoc Study Lung Cancer. (2018) 13:1338–48. doi: 10.1016/j.jtho.2018.05.037
10. Riley RD, Ensor J, Snell KIE, Harrell FE Jr., Martin GP, Reitsma JB, et al. Calculating the sample size required for developing a clinical prediction model. BMJ. (2020) 368:m441. doi: 10.1136/bmj.m441
11. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J infection control. (2008) 36:309–32. doi: 10.1016/j.ajic.2008.03.002
12. Tibshirani RB. Regression shrinkage and selection via the lasso. J Roy Stat Soc B. (1996) 58:267–88. doi: 10.1111/j.2517-6161.1996.tb02080.x
13. Kang J, Choi YJ, Kim IK, Lee HS, Kim H, Baik SH, et al. LASSO-based machine learning algorithm for prediction of lymph node metastasis in T1 colorectal cancer. Cancer Res Treat. (2021) 53:773–83. doi: 10.4143/crt.2020.974
14. Ohri N, Duan F, Snyder BS, Wei B, Machtay M, Alavi A, et al. Pretreatment 18F-FDG PET textural features in locally advanced non-small cell lung cancer: secondary analysis of ACRIN 6668/RTOG 0235. J Nucl medicine: Off publication Soc Nucl Med. (2016) 57:842–8. doi: 10.2967/jnumed.115.166934
15. Pavlou M, Ambler G, Seaman SR, Guttmann O, Elliott P, King M, et al. How to develop a more accurate risk prediction model when there are few events. BMJ. (2015) 351:h3868. doi: 10.1136/bmj.h3868
16. Bunea F, She Y, Ombao H, Gongvatana A, Devlin K, Cohen R. Penalized least squares regression methods and applications to neuroimaging. Neuroimage. (2011) 55:1519–27. doi: 10.1016/j.neuroimage.2010.12.028
17. Kidd AC, McGettrick M, Tsim S, Halligan DL, Bylesjo M, Blyth KG. Survival prediction in mesothelioma using a scalable Lasso regression model: instructions for use and initial performance using clinical predictors. BMJ Open Respir Res. (2018) 5:e000240. doi: 10.1136/bmjresp-2017-000240
18. Gu Z, Du Y, Wang P, Zheng X, He J, Wang C, et al. Development and validation of a novel nomogram to predict postoperative pancreatic fistula after pancreatoduodenectomy using lasso-logistic regression: an international multi-institutional observational study. Int J Surg. (2023) 109:4027–40. doi: 10.1097/JS9.0000000000000695
19. Alba AC, Agoritsas T, Walsh M, Hanna S, Iorio A, Devereaux PJ, et al. Discrimination and calibration of clinical prediction models: users' Guides to the medical literature. JAMA. (2017) 318:1377–84. doi: 10.1001/jama.2017.12126
20. Kerr KF, Brown MD, Zhu K, Janes H. Assessing the clinical impact of risk prediction models with decision curves: guidance for correct interpretation and appropriate use. J Clin oncology: Off J Am Soc Clin Oncol. (2016) 34:2534–40. doi: 10.1200/JCO.2015.65.5654
21. Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. (2015) 16:e173–80. doi: 10.1016/S1470-2045(14)71116-7
22. Garami A, Steiner AA, Romanovsky AA. Fever and hypothermia in systemic inflammation. Handb Clin Neurol. (2018) 157:565–97. doi: 10.1016/B978-0-444-64074-1.00034-3
23. Gozalichvili D, Binquet C, Boisson C, Guiraud A, Facy O, Ortega-Deballon P. Early detection of anastomotic leak with C-reactive protein increases the chances of anastomotic salvage. Colorectal disease: Off J Assoc Coloproctology Great Britain Ireland. (2023) 25:728–37. doi: 10.1111/codi.16399
24. Zarnescu EC, Zarnescu NO, Costea R. Updates of risk factors for anastomotic leakage after colorectal surgery. Diagnostics (Basel Switzerland). (2021) 11:2382. doi: 10.3390/diagnostics11122382
25. Lawler J, Choynowski M, Bailey K, Bucholc M, Johnston A, Sugrue M. Meta-analysis of the impact of postoperative infective complications on oncological outcomes in colorectal cancer surgery. BJS Open. (2020) 4:737–47. doi: 10.1002/bjs5.50302
26. van der Pas MH, Haglind E, Cuesta MA, Fürst A, Lacy AM, Hop WC, et al. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. (2013) 14:210–8. doi: 10.1016/S1470-2045(13)70016-0
27. Kang SB, Park JW, Jeong SY, Nam BH, Choi HS, Kim DW, et al. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol. (2010) 11:637–45. doi: 10.1016/S1470-2045(10)70131-5
28. Tanis PJ, Buskens CJ, Bemelman WA. Laparoscopy for colorectal cancer. Best Pract Res Clin Gastroenterol. (2014) 28:29–39. doi: 10.1016/j.bpg.2013.11.017
29. Kagawa Y, Yamada D, Yamasaki M, Miyamoto A, Mizushima T, Yamabe K, et al. The association between the increased performance of laparoscopic colon surgery and a reduced risk of surgical site infection. Surg Today. (2019) 49:474–81. doi: 10.1007/s00595-019-1760-1
30. Zhang X, Yang Y, Liu P, Wang P, Li X, Zhu J, et al. Identification of risk factors and phenotypes of surgical site infection in patients after abdominal surgery. Ann Surg. (2023) 278:e988–94. doi: 10.1097/SLA.0000000000005939
31. Caroff DA, Chan C, Kleinman K, Calderwood MS, Wolf R, Platt R, et al. Combined laparoscopic and open colon surgery rankings fail to accurately rank hospitals by surgical-site infection rate. Infection control Hosp Epidemiol. (2022) 44:624–30. doi: 10.1017/ice.2022.153
32. Caroff DA, Chan C, Kleinman K, Calderwood MS, Wolf R, Wick EC, et al. Association of open approach vs laparoscopic approach with risk of surgical site infection after colon surgery. JAMA Netw Open. (2019) 2:e1913570. doi: 10.1001/jamanetworkopen.2019.13570
33. Imai E, Ueda M, Kanao K, Kubota T, Hasegawa H, Omae K, et al. Surgical site infection risk factors identified by multivariate analysis for patient undergoing laparoscopic, open colon, and gastric surgery. Am J infection control. (2008) 36:727–31. doi: 10.1016/j.ajic.2007.12.011
34. Lovicu E, Faraone A, Fortini A. Admission braden scale score as an early independent predictor of in-hospital mortality among inpatients with COVID-19: A retrospective cohort study. Worldviews Evidence-Based Nurs. (2021) 18:247–53. doi: 10.1111/wvn.12526
35. Cohen RR, Lagoo-Deenadayalan SA, Heflin MT, Sloane R, Eisen I, Thacker JM, et al. Exploring predictors of complication in older surgical patients: a deficit accumulation index and the Braden Scale. J Am Geriatr. Soc. (2012) 60:1609–15. doi: 10.1111/j.1532-5415.2012.04109.x
36. Yang J, Zhang T, Feng D, Dai X, Lv T, Wang X, et al. A new diagnostic index for sarcopenia and its association with short-term postoperative complications in patients undergoing surgery for colorectal cancer. Colorectal disease: Off J Assoc Coloproctology Great Britain Ireland. (2019) 21:538–47. doi: 10.1111/codi.2019.21.issue-5
37. Hackett NJ, De Oliveira GS, Jain UK, Kim JY. ASA class is a reliable independent predictor of medical complications and mortality following surgery. Int J Surg. (2015) 18:184–90. doi: 10.1016/j.ijsu.2015.04.079
38. Shen Z, Lin Y, Ye Y, Jiang K, Xie Q, Gao Z, et al. The development and validation of a novel model for predicting surgical complications in colorectal cancer of elderly patients: Results from 1008 cases. Eur J Surg oncology: J Eur Soc Surg Oncol Br Assoc Surg Oncol. (2018) 44:490–5. doi: 10.1016/j.ejso.2018.01.007
39. Menahem B, Vallois A, Alves A, Lubrano J. Prophylactic pelvic drainage after rectal resection with extraperitoneal anastomosis: is it worthwhile? A meta-analysis of randomized controlled trials. Int J Colorectal Dis. (2017) 32:1531–8. doi: 10.1007/s00384-017-2891-8
40. Carmichael JC, Keller DS, Baldini G, Bordeianou L, Weiss E, Lee L, et al. Clinical practice guidelines for enhanced recovery after colon and rectal surgery from the american society of colon and rectal surgeons and society of american gastrointestinal and endoscopic surgeons. Dis colon rectum. (2017) 60:761–84. doi: 10.1097/DCR.0000000000000883
Keywords: colon cancer, nosocomial infections, prediction, nomogram, model
Citation: Yao X, Wang S, Lu A, Xu Y and Li N (2025) A dynamic nomogram predicting nosocomial infections in patients after colon cancer surgery. Front. Oncol. 15:1528036. doi: 10.3389/fonc.2025.1528036
Received: 14 November 2024; Accepted: 10 February 2025;
Published: 28 February 2025.
Edited by:
Sharon R. Pine, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Tommaso Sciortino, University at Buffalo, United StatesCopyright © 2025 Yao, Wang, Lu, Xu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Na Li, ZmxhbWV3eHl6QDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.