
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 20 March 2025
Sec. Gastrointestinal Cancers: Gastric and Esophageal Cancers
Volume 15 - 2025 | https://doi.org/10.3389/fonc.2025.1527237
Background: Extra-gastrointestinal stromal tumor (EGIST) is a rare subtype of gastrointestinal stromal tumor, with limited information on its clinical characteristics and prognostic factors. This study aims to identify the risk factors affecting survival in EGIST patients and to develop a prognostic nomogram for predicting EGIST-specific survival (TSS).
Methods: Patients diagnosed with EGIST, aged 18 to 80, were enrolled from the Surveillance, Epidemiology, and End Results (SEER) Research Plus database, covering the years 2000 to 2019. Univariate and multivariate analyses were conducted to identify risk factors for developing a nomogram. The predictive accuracy of the nomogram was evaluated using time-dependent receiver operating characteristic curves, calibration plots, and the concordance index. Decision curve analysis (DCA) was performed to assess the nomogram’s clinical utility and net benefit for application in clinical practice. Additionally, the nomogram’s performance was compared with the tumor SEER stage.
Results: A total of 389 patients were included in this study. Univariate and multivariate analyses identified age, household income, surgery, tumor grade, tumor size, and tumor SEER stage as significant predictors of TSS (all P<0.05). These factors were incorporated into the prognostic nomogram. The nomogram demonstrated superior prognostic value compared to the tumor SEER stage alone, as evidenced by the area under the curve and concordance index. The calibration plot indicated a high level of accuracy in forecasting survival probabilities. Furthermore, DCA highlighted the nomogram’s clinical applicability and positive net benefit. The nomogram also proved more effective than the tumor SEER stage alone in identifying patients with poorer prognoses.
Conclusions: Age, household income, surgery, tumor grade, tumor size, and tumor SEER stage were identified as risk factors for TSS in patients with EGIST. We have developed and validated a prognostic nomogram to predict TSS in EGIST patients, which may improve patient management and guide personalized medical treatment for EGIST.
Gastrointestinal stromal tumor (GIST) is the most common subtype of sarcoma, with an incidence rate between 1.1 and 1.5 per 100,000 persons (1, 2). GIST predominantly originates in the digestive tract, with the stomach accounting for 55% of cases, the small intestine for 31%, and the colorectum for 6%. Fewer than 5% of gastrointestinal stromal tumors occur outside the digestive tract and are classified as extra-gastrointestinal stromal tumors (EGIST) (2). Although EGIST shares similar pathological characteristics with GIST, it presents a distinct spectrum of clinical features. EGIST cases exhibit a higher proportion of poorly differentiated tumors, and a higher proportion of patients with lymph node or distant metastasis (3, 4). Additionally, the majority of GISTs are caused by activating mutations in KIT (75%–80%) or PDGFRA (5%–10%). However, the incidence of KIT or PDGFRA mutation is only 40–50% in EGIST patients, the lower mutation rate of KIT or PDGFRA in EGIST patients may influence the TKI treatment effort for EGISTs (5). As the incidence of EGIST is comparatively low, with most cases reported individually, the genomic research on EGIST is limited compared with GIST, there is a lack of whole-genome sequencing, whole-exome sequencing, whole-transcriptome sequencing, and circulating tumor DNA sequencing analyses, which have been proved to predict the prognosis of patients and guiding precision treatment for GIST patients (6, 7). Despite the existing knowledge gap concerning EGIST, there is currently no specific guideline tailored for EGIST. The current understanding of EGIST is scarce, and potential prognostic factors are poorly elucidated, leading to poor prognostic outcomes for EGIST patients. Despite limited knowledge regarding EGIST’s clinical aspects, these tumors remain a significant subset of GISTs, representing a unique entity with distinct characteristics and outcomes that merit further investigation (8–10). Therefore, identifying the risk factors associated with EGISTs and developing predictive models is essential for assessing patient prognosis and informing treatment decisions.
Nomograms are widely used prognostic tools, especially for predicting tumor prognosis, as they provide personalized risk assessments and support treatment decisions, enhancing clinical management’s precision of clinical management (11). Presently, EGIST’s prognostic implications are not well understood. While some nomograms predict the prognosis of GIST patients, they generally include only cases of GIST occurring within the gastrointestinal system or a small proportion of EGIST cases. Given the evidence highlighting the heterogeneity in clinical characteristics and prognosis between GIST and EGIST, these existing nomograms are inadequate for accurately predicting outcomes in EGIST patients with EGIST (12–14). Therefore, it is crucial to develop models that address EGIST’s unique characteristics to improve patient management and guide personalized treatment strategies.
This study aims to identify the risk factors influencing survival in EGIST patients and to develop and validate a novel nomogram specifically tailored for EGIST. The Surveillance, Epidemiology, and End Results (SEER) database, a valuable resource for cancer research, includes approximately half of all cancer cases in the United States (15). Data for this study were selected from the SEER database. Our nomogram integrates key demographic, clinicopathological, and therapeutic variables to construct a comprehensive tool for estimating patient survival probabilities. This nomogram aids in guiding treatment decisions and optimizing clinical management, aiming to deepen our understanding of EGIST and enhance clinical decision-making for its effective treatment.
The data for this study were extracted from the SEER program (https://seer.cancer.gov), which provides comprehensive information from population-based cancer registries, including demographic data, tumor characteristics, antitumor therapy methods, and survival information. A data use agreement was executed to secure authorization for SEER database access. The dataset used for this investigation was SEER Research Data 17, submitted on November 12, and downloaded using SEER*Stat software (version 8.4.3, NY, USA).
A total of 686 EGIST patients were identified based on the following criteria: (1) tumors histologically confirmed as GIST according to the ICD-O-3 recode; (2) patients aged between 18 and 80 years; and (3) primary tumor site outside the digestive tract, including locations such as the esophagus, stomach, small intestine, appendix, colon, and rectum. Exclusions included 77 cases with unknown primary tumor locations, 116 patients with multiple cancers, 8 cases missing cause of death, 24 cases with unknown marital status, and 72 patients lacking tumor SEER stage information. Ultimately, 389 patients remained for analysis. Figure 1 illustrates the patient selection flowchart.
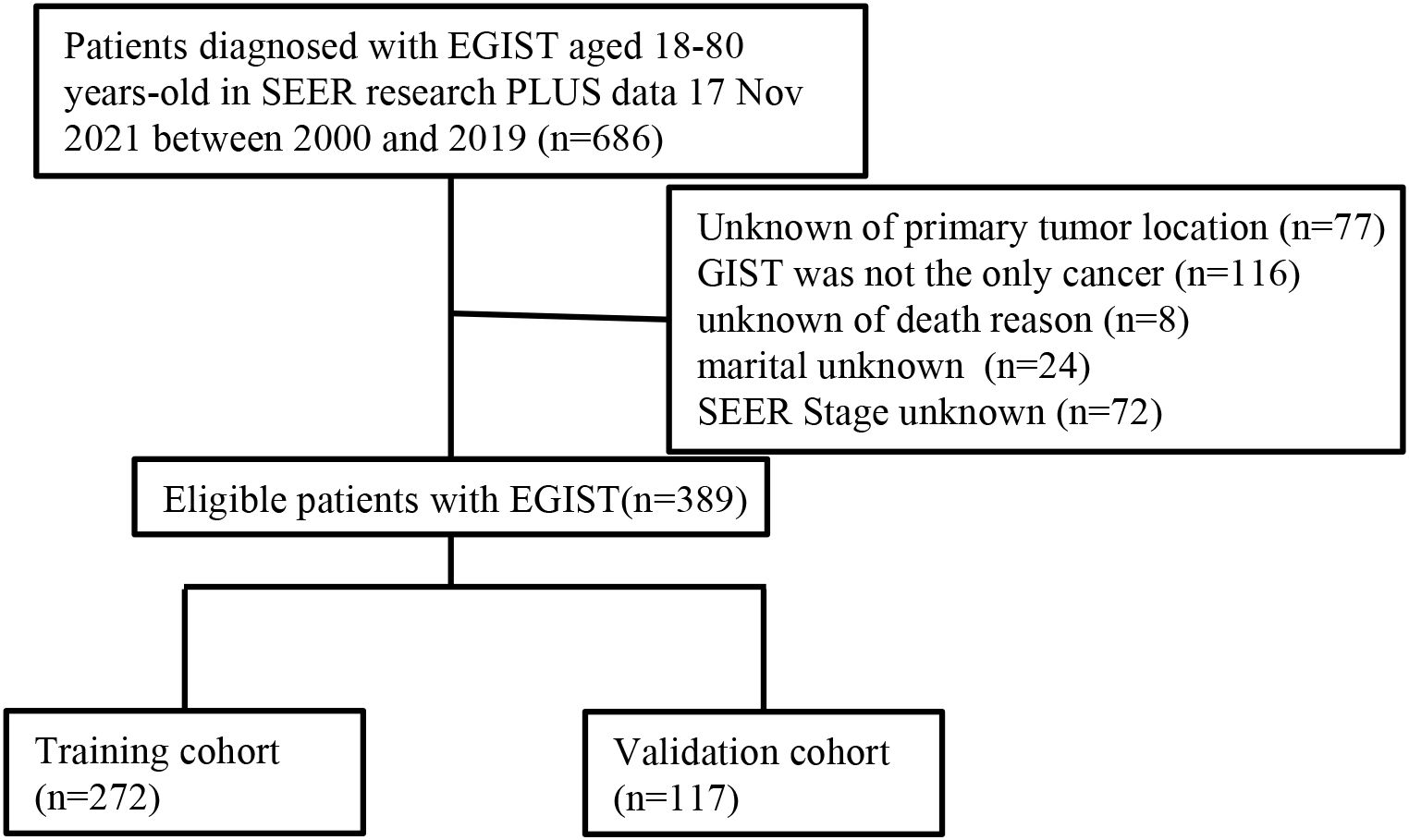
Figure 1. Flow diagram of the EGIST patients in the training and validation cohorts. EGIST: extra-gastrointestinal stromal tumors.
Demographic information, disease characteristics, and treatment details for EGIST patients were obtained from the SEER database. Age was categorized as young adults (18-60 years) and older adults (>60 years). Race was classified as white, and others were classified based on race recode. Median household income, adjusted to 2019, was divided into low-income (≤USD 60,000), middle-income (USD 60,000–74,999), and high-income (≥USD 75,000) groups. The tumor SEER stage was categorized as follows: (1) localized stage for tumors confined to the site of origin; (2) regional stage for tumors with direct extension or regional lymph node metastasis; and (3) distant stage for metastasis to distant sites or lymph nodes. Vital status and cause of death were obtained from SEER data, with tumor-specific mortality (TSM) defined as deaths directly due to EGIST. Tumor-specific survival (TSS) was recorded months after diagnosis.
The eligible EGIST patients were randomly divided into a training cohort (n=272) and a validation cohort (n=117) in a 7:3 ratio. Univariate and multivariate Cox regression analyses were conducted to identify risk factors significantly affecting TSS in EGIST patients. Variables with statistical and clinical relevance were identified as independent risk factors and included in the nomogram development.
The nomogram’s performance was evaluated using time-dependent receiver operating characteristic (ROC) curves, the concordance index (C-index), calibration curves, and decision curve analysis (DCA). The ROC curve assessed the model’s sensitivity and specificity, with the area under the curve (AUC) indicating accuracy. The C-index represented predictive accuracy, while DCA evaluated the model’s net benefit. Calibration curves were used to compare predicted TSS with observed outcomes, with the 45-degree line as a reference for actual outcomes. The C-index and ROC were also employed to evaluate the nomogram’s prognostic accuracy compared to the tumor staging system.
Based on nomogram scores, EGIST patients were categorized into high-risk and low-risk groups, with the median nomogram score of the cohort (n=296) serving as the cutoff. Kaplan-Meier curve analysis compared survival differences across risk groups and tumor SEER stages.
All analyses were performed using R software version 4.3.1 (http://www.r-project.org/). The “survminer,” “regplot,” “mstate,” “survival,” “cmprsk,” “Hmisc,” “timeROC,” “foreign,” “nricens,” “rmda,” and “DCA” packages in R were used to develop and validate the nomogram. Statistical distribution differences between the training and validation cohorts were assessed using the chi-square test. All p-values were two-tailed, with p<0.05 considered statistically significant.
A total of 389 EGIST patients were included, randomized into a training cohort (n=272) and a validation cohort (n=117) in a 7:3 ratio. Table 1 summarizes the demographic and clinical characteristics. Among the patients, 189 were young adults (48.59%), and 200 were older adults (51.41%). There were 201 male (51.67%) and 188 female patients (48.33%). The majority were white (67.87%, n=264). Tumor pathological grades were distributed as follows: grade I (7.71%), grade II (9.00%), grade III (5.91%), grade IV (7.46%), and unknown (69.72%). Regarding tumor size, 11.57% of patients had tumors <5 cm, 19.02% had tumors 5–10 cm, and 51.16% had tumors >10 cm. EGIST stage was localized in 34.19% of patients, regional in 25.96%, and distant in 39.85%. Surgical treatment was performed in 61.44% of patients, and 60.44% received chemotherapy. The marital status of 59.90% of patients were married. Low, middle, and high household incomes were observed in 24.94%, 43.44%, and 31.62% of patients, respectively. Comparison between the training and validation cohorts showed no significant differences in demographics and clinical characteristics (all P>0.05).
Table 2 presents the univariate and multivariate Cox regression analyses Older adults (HR=1.59, 95%CI=1.08-2.34), tumor grade IV (HR=4.33, 95%CI=1.56-12.0), tumor size>10cm (HR=2.72, 95%CI=1.30-5.67), regional tumor (HR=2.38, 95%CI=1.33-4.28), distant tumor (HR=3.59, 95%CI=2.09-6.16) were identified as independent risk factors for TSS in EGIST patients; surgery treatment (HR=0.52, 95%CI=0.32-0.83), middle-household income (HR=0.54, 95%CI=0.35-0.85), and high-household income (HR=0.57, 95%CI=0.35-0.94) were independent protective factors for TSS in EGIST patients (all p<0.05).
Based on univariate and multivariate Cox regression analyses, age, tumor grade, tumor size, tumor SEER stage, surgery, and household income were selected to construct the nomogram for predicting TSS in EGIST patients (Figure 2). The risk scores for each variable were calculated and aggregated to determine total scores, with the probability of 1-, 3-, and 5-year TSS estimated by drawing a straight line on the final three rows. The C-index for the training and validation cohorts was 0.75 and 0.73, respectively (Figures 3A, B). The ROC curves showed AUC values of 0.77, 0.78, and 0.77 for 1-, 3-, and 5-year TSS in the training cohort, and 0.80, 0.71, and 0.72 in the validation cohort, indicating good predictive performance (Figures 4A, B). DCA curves showed good clinical applicability and positive net benefit in both cohorts (Figure 5). The calibration curves showed agreement between predicted and observed TSS rates at 1, 3, and 5 years (Figure 6).
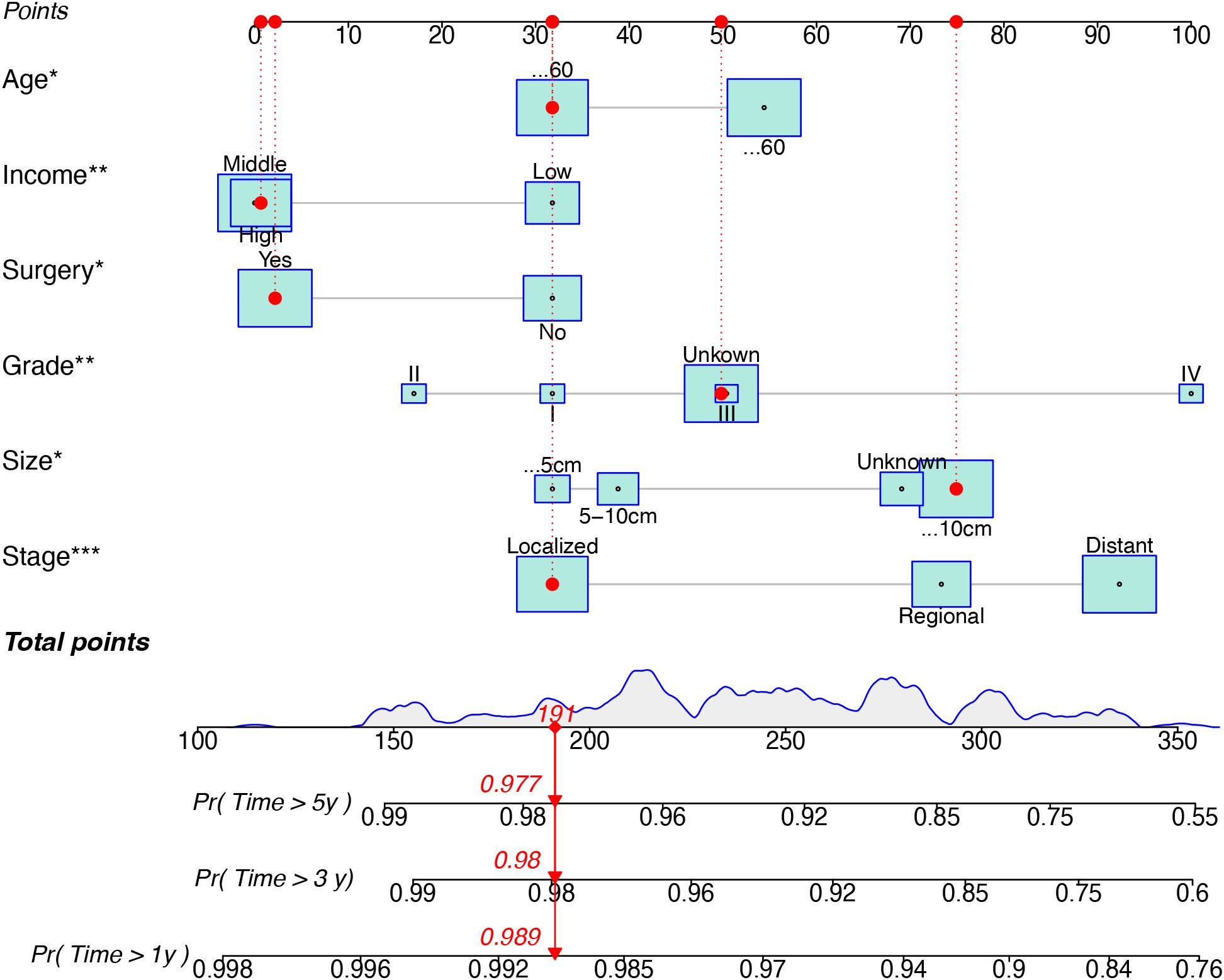
Figure 2. A nomogram for prediction TSS for EGIST patient. TSS, Extra-gastrointestinal stromal tumor-specific survival; EGIST, extra-gastrointestinal stromal tumors. *indicated p value < 0.05, **indicated p value < 0.01, and ***indicated p value < 0.001 in multivariate Cox regression analyses for predicting TSS for EGIST patients.
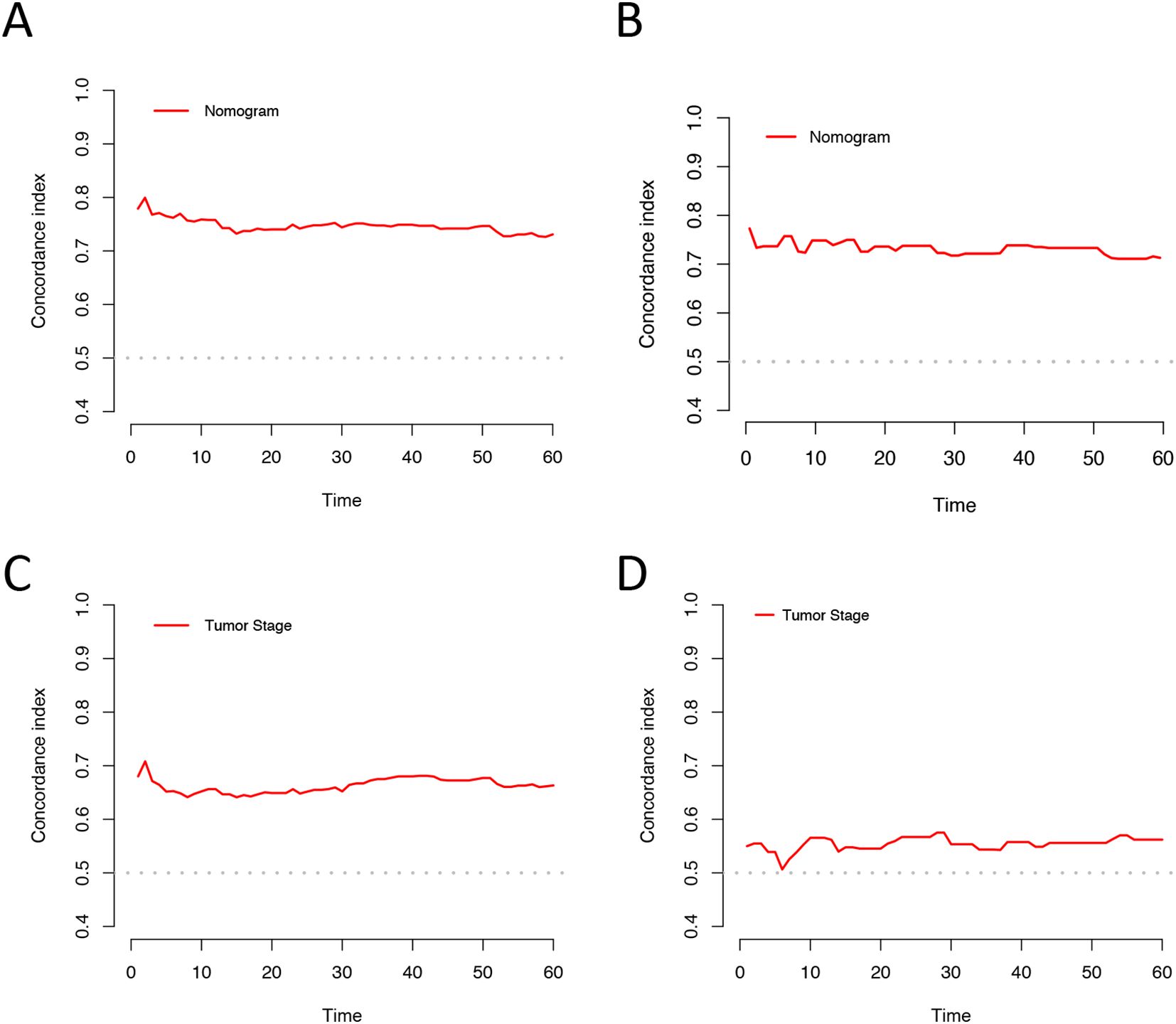
Figure 3. C-index analysis for the nomogram and tumor SEER stage prediction. (A) Training cohorts based on the nomogram. (B) Validation cohorts based on the nomogram. (C) Training cohorts based on the tumor SEER stage. (D) Validation cohorts based on the tumor SEER stage.
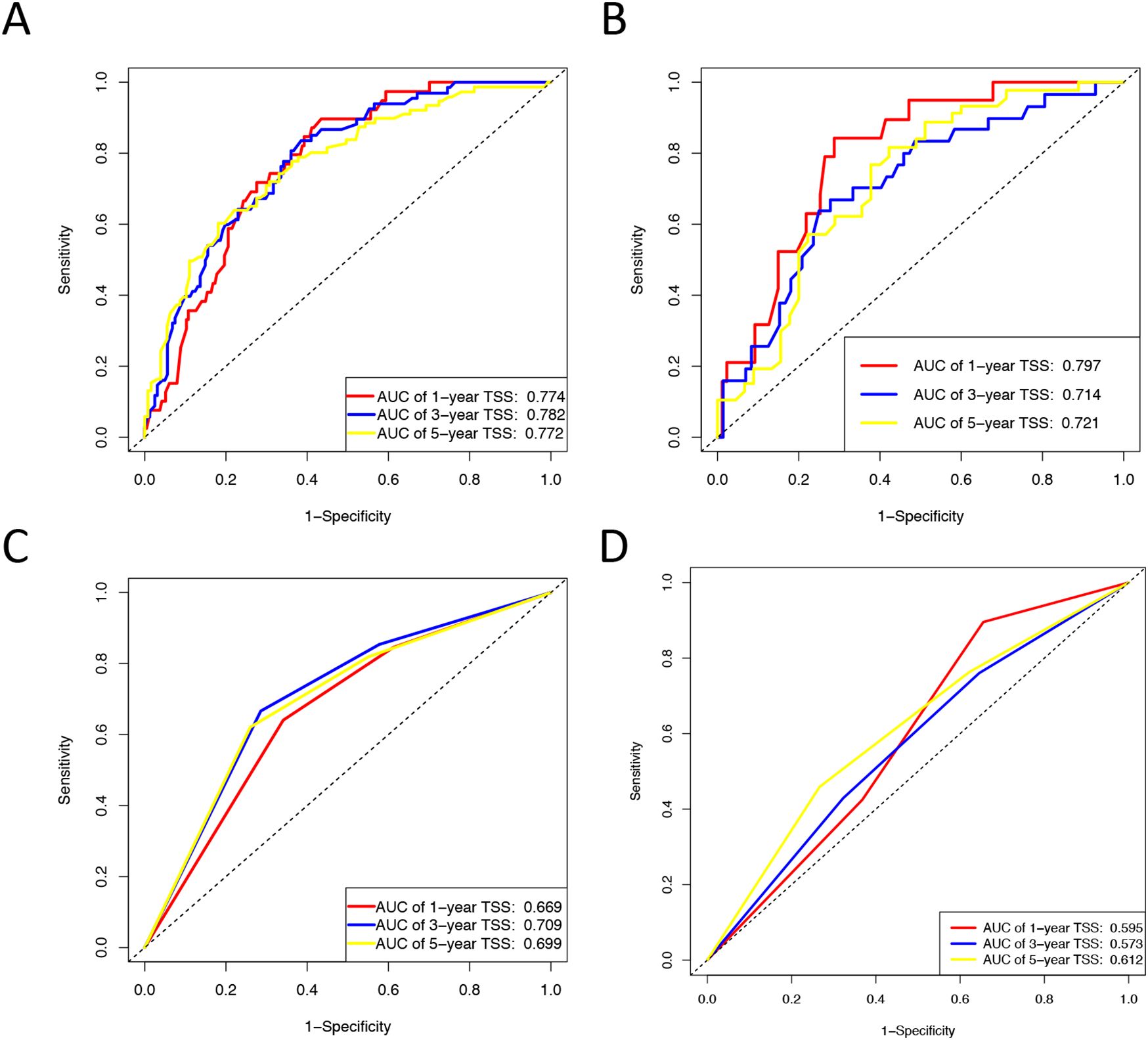
Figure 4. ROC curves for the nomogram and Tumor SEER Stage for 1-, 3-, and 5-year prediction. (A) Training cohorts based on the nomogram. (B) Validation cohorts based on the nomogram. (C) Training cohorts based on the tumor SEER stage. (D) Validation cohorts based on the tumor SEER stage.

Figure 5. Decision curve analysis. (A, C, E) DCA curves of 1-year, 3-year, and 5-year TSS in the training cohort. (B, D, F) DCA curves of 1-year, 3-year, and 5-year TSS in the validation cohort. DCA, decision curve analysis; TSS, Extra-gastrointestinal stromal tumor-specific survival.
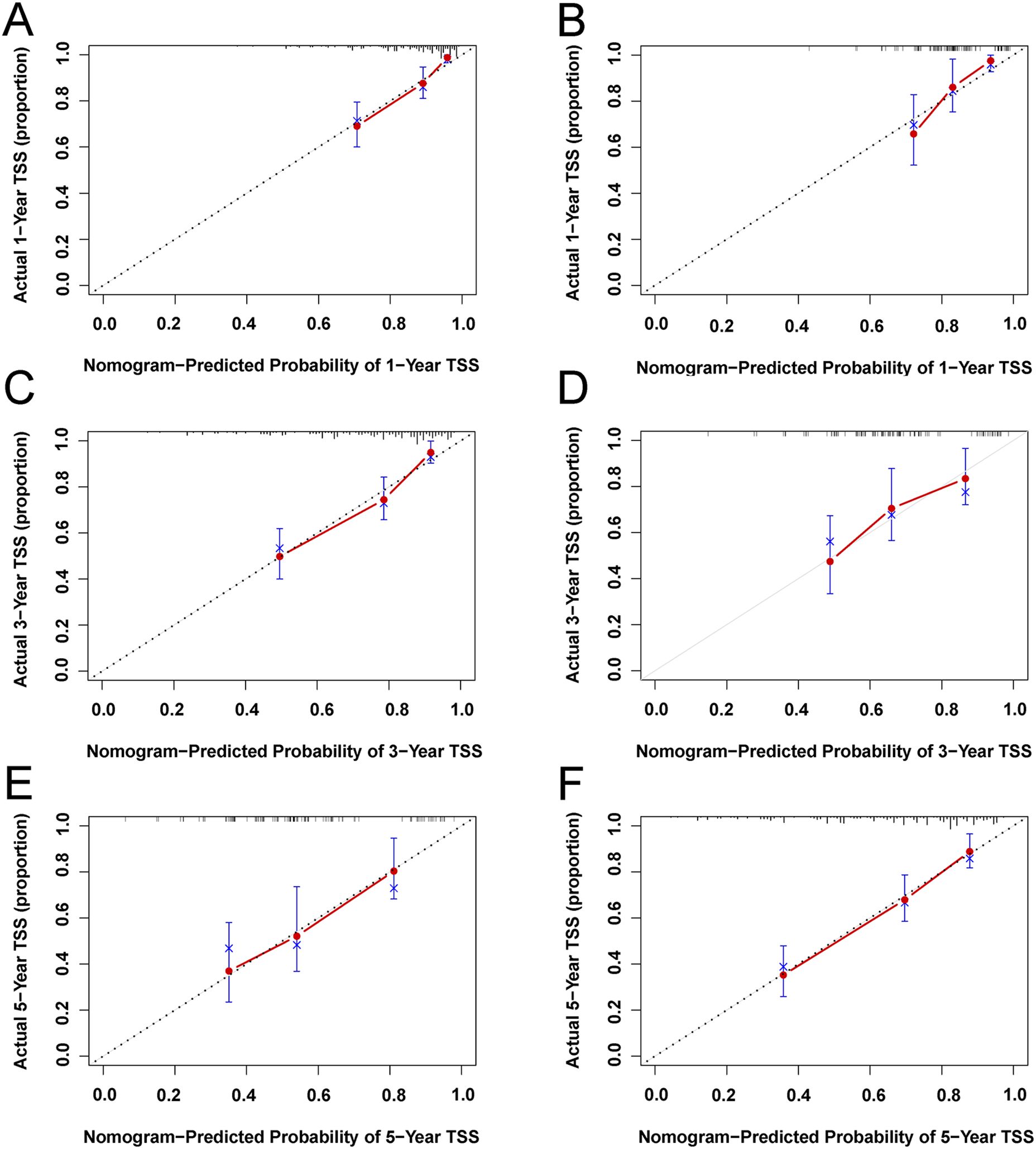
Figure 6. Calibration plots of 1-year, 3-year, and 5-year TSS for EGIST patients. (A, C, E) Calibration plots of 1-year, 3-year, and 5-year TSS in the training cohort. (B, D, F) Calibration plots of 1-year, 3-year, and 5-year CSS in the training cohort. TSS, Extra-gastrointestinal stromal tumor-specific survival.
The C-index and ROC were used to compare the accuracy of the nomogram and tumor staging system. The C-index of the nomogram was 0.75 and 0.73 in the training and validation cohorts, respectively, while the tumor SEER stage system had C-index values of 0.66 and 0.55 (Figure 3). The 1-, 3-, and 5-year ROC curves for the nomogram in the training cohort had AUC values of 0.77, 0.78, and 0.77, compared to 0.67, 0.71, and 0.70 for the staging system. In the validation cohort, the nomogram’s AUC values were 0.80, 0.71, and 0.72, whereas the staging system had AUC values of 0.60, 0.57, and 0.61 (Figure 4). The higher AUC and C-index values demonstrate that the nomogram provides superior TSS prediction accuracy compared to the tumor staging system.
Patients with EGIST were stratified into low-risk (total points <296) and high-risk (total points ≥296) groups according to the nomogram. Kaplan-Meier analysis showed a significant difference in TSS between the low-risk and high-risk groups. In contrast, the tumor staging system had limited capacity (63%) to differentiate patients with poor prognoses in training and validation cohorts (Figure 7).
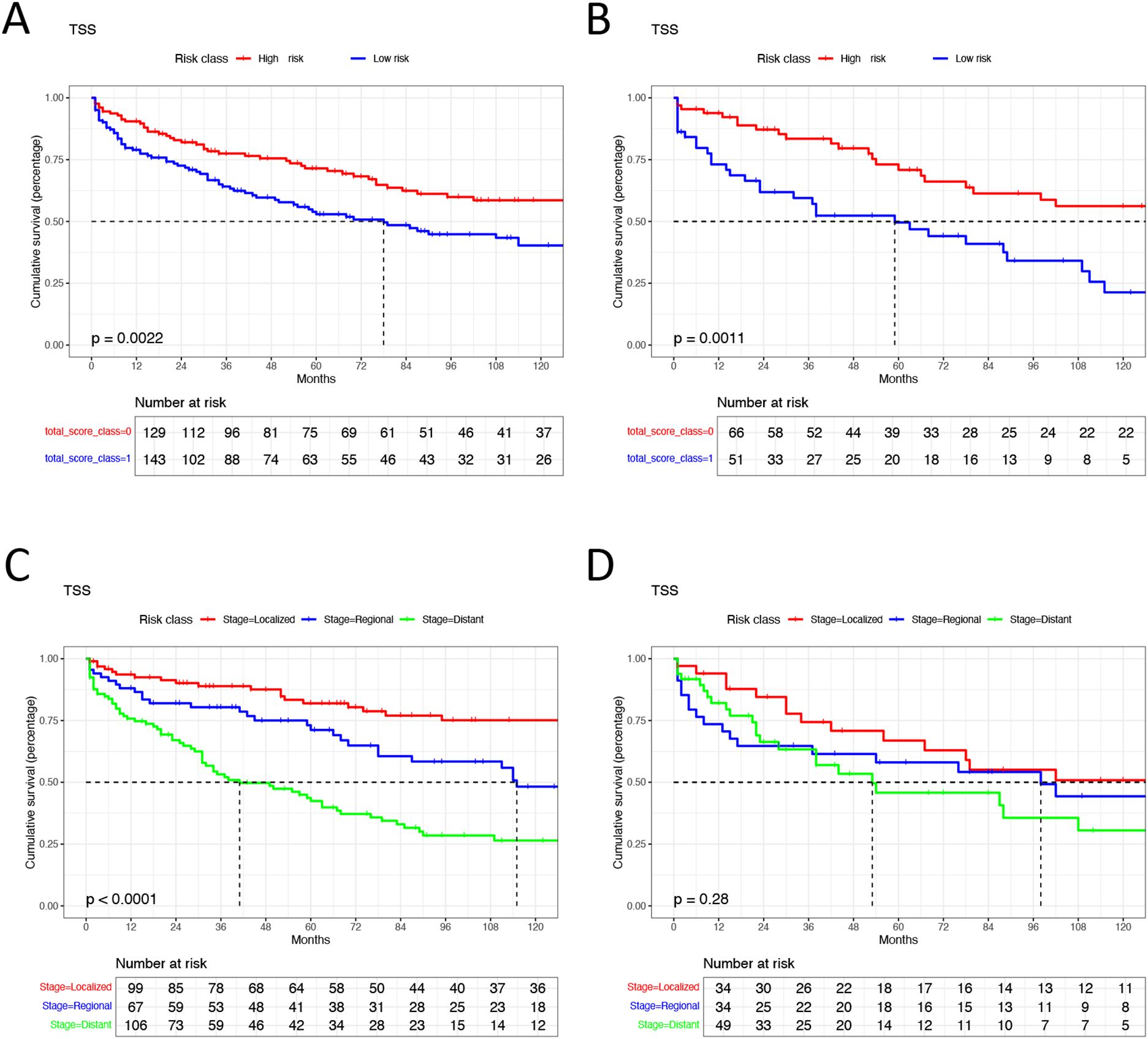
Figure 7. Kaplan-Meier analysis in different groups. (A) high risk group and low risk group in training cohorts. (B) high risk group and low risk group in validation cohorts. (C) different tumor SEER stage group in training cohorts. (D) different tumor SEER stage group in validation cohorts. TSS, Extra-gastrointestinal stromal tumor-specific survival.
In this study, we identified patient age, tumor grade, tumor size, tumor SEER stage, surgical treatment, and household income as risk factors significantly associated with TSS in patients diagnosed with EGIST. Using data from the SEER database, we developed a nomogram to predict TSS for EGIST patients. This prognostic tool demonstrated robust performance in predicting patient prognosis and effectively-identified high-risk groups with poorer outcomes who may require closer monitoring and intervention. Our nomogram represents a step forward in understanding EGIST and supports the advancement of personalized medicine for patients with this rare tumor type.
EGIST, a rare form of GIST occurring outside the gastrointestinal tract, comprises approximately 5% of all GIST cases. While EGIST shares common c-kit and PDGFR-α mutations with GIST, it displays distinct clinical behaviors and prognostic characteristics (16). EGIST has received attention due to its aggressive nature and unique clinical outcomes compared to GIST within the gastrointestinal tract. Most existing studies on EGIST consist of case reports, with limited systematic analyses of clinical characteristics and prognostic factors. Using the SEER database, our study gathered a large cohort of EGIST patients, enabling us to systematically summarize clinical features and evaluate risk factors.
Our study identified several key sociodemographic characteristics among patients with EGIST. Socioeconomic factors, such as household income, have been shown to influence survival outcomes across various cancers (17). Our study further identified that low-household income is an independent risk factor for the survival of patients with EGIST, a finding that aligns with previous research on GIST (13). However, socioeconomic distributions varied significantly across different regions, given these disparities, it is essential to conduct cross-national comparative studies to explore the experiences and lessons learned by different countries and regions in addressing socioeconomic inequalities. Despite these variations, our study underscores the importance of public health strategies that target EGIST patients, particularly those with lower socioeconomic status. While gender has been identified as a prognostic factor for GIST survival, with evidence indicating that female GIST patients tend to have better survival outcomes than male patients, our research findings are consistent with prior EGIST studies, showing no significant difference in survival by gender (18–20). Further studies are needed to elucidate the detailed reasons. Furthermore, racial and marital status did not significantly affect EGIST survival, unlike GIST, where such factors have been influential (20, 21). The differences of sociodemographic characteristics between EGIST and GIST patients remain notable. The management of EGIST differs from that of GIST patients, which requires specific strategies. Developing personalized treatment plans and psychological support programs specifically designed for EGIST is crucial for improving the prognosis of these patients.
The oncologic characteristics of EGIST also differed from GIST. A clinical study comparing 62 EGIST cases with 570 GIST cases demonstrated that EGIST patients had a higher incidence of larger tumor sizes (>10 cm), more frequent signs of tumor necrosis, and higher mitotic rates, all of which correlate with poor prognosis (19). Our study also shows that 51.2% EGIST have tumor larger than 10cm, 44.4% were grade III-IV, 65.8% have a regional or distant tumor at diagnosis, which indicated EGIST have a higher degree of malignancy. EGIST’s origin remains controversial, with some researchers viewing it as a distinct subtype of GIST and others regarding it as a form of metastasis from the gastrointestinal tract (3, 5, 22). However, EGIST’s unique clinical features, prognostic factors, and outcomes support its classification as a separate subtype rather than a metastatic variant of GIST. Furthermore, our study excluded individuals with multiple tumors. The evidence of non-gastrointestinal origin further reinforces the notion that EGIST represents a distinct subtype of GIST. The higher degree of malignancy and poorer prognosis observed in EGIST underscore the limitations of current understanding of this tumor. To bridge the knowledge gap concerning EGIST, it is essential to explore its genomic characteristics and identify novel therapeutic biomarkers. Such efforts will enhance our comprehension of the pathogenesis underlying EGIST and ultimately contribute to improving its prognosis.
The treatment modalities for EGIST are typically based on GIST protocols, mainly include surgical resection, targeted therapy, and comprehensive treatment strategies. Surgery remains the primary treatment option for both GIST and EGIST (10, 23–25). However, high-risk EGIST patients often have poorer outcomes than GIST patients after surgery, indicating that adopting GIST-based strategies for EGIST may lead to suboptimal treatment (19). Radiotherapy has shown benefits for metastatic or progressive GIST (26). However, in two retrospective studies on EGIST, no patients received radiotherapy, and there was only one EGIST patient received radiotherapy in our study, making it difficult to evaluate its potential as a treatment option for EGIST (19, 27). More studies are needed to determine whether, and which subgroups of EGIST patients, will benefit from radiotherapy. For unresectable or metastatic EGIST, targeted therapies remain the preferred treatment option. However, when used as primary treatment, targeted therapies achieve a median overall survival of only 3.1 years for EGIST patients, compared to 6.9 years for GIST patients (27, 28). Given the absence of EGIST-specific treatment guidelines and large randomized controlled trials, treatment strategies for EGIST based on GIST protocols lead to poor outcome, further study should focus on the personalized and precision treatments for EGIST.
A nomogram designed for EGIST could address this need by predicting patient survival and categorizing risk levels, aiding treatment decisions, and potentially improving outcomes.
In GIST research, several nomograms have been developed to predict survival outcomes, incorporating age, race, tumor location, mitotic rate, chemotherapy, tumor size, stage, grade, and more (29–31). Sun et al. (32) created a nomogram for GIST patients post-surgery, while Bertsimas et al. (33, 34) developed prognostic models to predict the recurrence of GIST and assist in selecting patient groups suitable for adjuvant imatinib therapy. However, since patients with EGIST exhibit distinct prognostic risk factors compared to those with GIST, the existing nomograms are not applicable to EGIST patients. Our study used prognostic factors including age, tumor grade, size, tumor SEER stage, surgery, and household income to develop a nomogram predicting TSS in EGIST patients. The ROC and C-index values demonstrated the nomogram’s accuracy, and calibration curves indicated high concordance with observed outcomes. DCA further supported the nomogram’s clinical applicability with a positive net benefit. To our knowledge, our study involved the largest cohort of EGIST patients and introduced the first nomogram explicitly designed for this tumor type. Additionally, we stratified patients into high- and low-risk groups using the nomogram, with the high-risk group showing significantly poorer TSS outcomes. While the SEER staging system was limited in identifying patients with poor prognoses, our risk stratification approach effectively differentiated these patients, potentially guiding personalized and targeted treatment.
Our study has several strengths. It included the largest known cohort of EGIST patients, allowing for a comprehensive evaluation of clinical characteristics and risk factors. Additionally, we restricted the study to patients with a single primary tumor located outside the gastrointestinal tract, reducing the potential for misclassification of EGIST as metastatic GIST. There are also some limitations in our study, the SEER database spans multiple decades, and data gaps exist for some variables. Additionally, some patients were missing key characteristics such as tumor grade and tumor size. Moreover, given the extremely low incidence rate of EGIST, it is hard to collect sufficient external validation data. Therefore, we used internal validation data to assess the reliability and reproducibility of the nomogram. Despite these limitations, the high AUC and C-index values indicate that our nomogram is accurate and practical, supporting its use in predicting prognosis and guiding treatment.
We identified age, household income, surgical treatment, tumor grade, tumor size, and tumor SEER stage as significant risk factors for TSS in EGIST patients. Our prognostic nomogram offers a valuable tool for predicting TSS, enhancing patient management, and guiding personalized medical treatment for EGIST patients.
Publicly available datasets were analyzed in this study. This data can be found here: The data used in this study can be accessed through the SEER program (https://seer.cancer.gov/).
The SEER database is publicly accessible, with data usage governed by a data use agreement. No Institutional Review Board (IRB) approval or participant consent was required for this study.
GJ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Software, Validation, Writing – original draft. XL: Validation, Visualization, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Natural Science Foundation of Hubei Province (2023AFB218) and the Fundamental Research Funds for the Central Universities (2042022kf1109).
The authors thank all contributors for their dedication and responsible engagement.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Serrano C, Martin-Broto J, Asencio-Pascual JM, Lopez-Guerrero JA, Rubio-Casadevall J, Bague S, et al. 2023 GEIS Guidelines for gastrointestinal stromal tumors. Ther Adv Med Oncol. (2023) 15:17588359231192388. doi: 10.1177/17588359231192388
2. Soreide K, Sandvik OM, Soreide JA, Giljaca V, Jureckova A, Bulusu VR. Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol. (2016) 40:39–46. doi: 10.1016/j.canep.2015.10.031
3. Liu L, Feng Y, Ye Y, Wang Z, Xu X. Survival analysis of extragastrointestinal stromal tumors based on the SEER database: a population-based study. Surg Endosc. (2023) 37:8498–510. doi: 10.1007/s00464-023-10433-y
4. Guller U, Tarantino I, Cerny T, Schmied BM, Warschkow R. Population-based SEER trend analysis of overall and cancer-specific survival in 5138 patients with gastrointestinal stromal tumor. BMC Cancer. (2015) 15:557. doi: 10.1186/s12885-015-1554-9
5. Sawaki A. Rare gastrointestinal stromal tumors (GIST): omentum and retroperitoneum. Transl Gastroenterol Hepatol. (2017) 2:116. doi: 10.21037/tgh.2017.12.07
6. Xie F, Luo S, Liu D, Lu X, Wang M, Liu X, et al. Genomic and transcriptomic landscape of human gastrointestinal stromal tumors. Nat Commun. (2024) 15:9495. doi: 10.1038/s41467-024-53821-1
7. Serrano C, Bauer S, Gomez-Peregrina D, Kang YK, Jones RL, Rutkowski P, et al. Circulating tumor DNA analysis of the phase III VOYAGER trial: KIT mutational landscape and outcomes in patients with advanced gastrointestinal stromal tumor treated with avapritinib or regorafenib. Ann Oncol. (2023) 34:615–25. doi: 10.1016/j.annonc.2023.04.006
8. Tian YT, Liu H, Shi SS, Xie YB, Xu Q, Zhang JW, et al. Malignant extra-gastrointestinal stromal tumor of the pancreas: report of two cases and review of the literature. World J Gastroenterol. (2014) 20:863–8. doi: 10.3748/wjg.v20.i3.863
9. Becker EC, Ozcan G, Wu GY. Primary hepatic extra-gastrointestinal stromal tumors: molecular pathogenesis, immunohistopathology, and treatment. J Clin Transl Hepatol. (2023) 11:942–8. doi: 10.14218/JCTH.2022.00173
10. Lin J, Liao W, Wang J, Li W, Tang X, Li H, et al. Primary extra-gastrointestinal stromal tumor of retroperitoneum: Clinicopathologic characteristics and prognosis of six cases. Front Oncol. (2023) 13:1033598. doi: 10.3389/fonc.2023.1033598
11. Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. (2015) 16:e173–80. doi: 10.1016/S1470-2045(14)71116-7
12. Joensuu H, Vehtari A, Riihimaki J, Nishida T, Steigen SE, Brabec P, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. (2012) 13:265–74. doi: 10.1016/S1470-2045(11)70299-6
13. Li X, You L, Liu Q, He W, Cui X, Gong W. A nomogram for predicting survival in patients with gastrointestinal stromal tumor: a study based on the surveillance, epidemiology, and end results database. Front Med (Lausanne). (2024) 11:1403189. doi: 10.3389/fmed.2024.1403189
14. Li Y, Zhang Y, Fu Y, Yang W, Wang X, Duan L, et al. Development and validation of a prognostic model to predict the prognosis of patients with colorectal gastrointestinal stromal tumor: A large international population-based cohort study. Front Oncol. (2022) 12:1004662. doi: 10.3389/fonc.2022.1004662
15. Che WQ, Li YJ, Tsang CK, Wang YJ, Chen Z, Wang XY, et al. How to use the Surveillance, Epidemiology, and End Results (SEER) data: research design and methodology. Mil Med Res. (2023) 10:50. doi: 10.1186/s40779-023-00488-2
16. Zheng S, Huang KE, Tao DY, Pan YL. Gene mutations and prognostic factors analysis in extragastrointestinal stromal tumor of a Chinese three-center study. J Gastrointest Surg. (2011) 15:675–81. doi: 10.1007/s11605-010-1292-x
17. Afshar N, English DR, Milne RL. Factors explaining socio-economic inequalities in cancer survival: A systematic review. Cancer Control. (2021) 28:10732748211011956. doi: 10.1177/10732748211011956
18. Rong J, Chen S, Song C, Wang H, Zhao Q, Zhao R, et al. The prognostic value of gender in gastric gastrointestinal stromal tumors: a propensity score matching analysis. Biol Sex Differ. (2020) 11:43. doi: 10.1186/s13293-020-00321-8
19. Feng H, Hu W, Zheng C, Wang W, Zheng G, Feng X, et al. Clinical features of extragastrointestinal stromal tumor compared with gastrointestinal stromal tumor: A retrospective, multicenter, real-world study. J Oncol. (2021) 2021:1460131. doi: 10.1155/2021/1460131
20. Guller U, Tarantino I, Cerny T, Ulrich A, Schmied BM, Warschkow R. Revisiting a dogma: similar survival of patients with small bowel and gastric GIST. A population-based propensity score SEER analysis. Gastric Cancer. (2017) 20:49–60. doi: 10.1007/s10120-015-0571-3
21. Alvarez CS, Piazuelo MB, Fleitas-Kanonnikoff T, Ruhl J, Perez-Fidalgo JA, Camargo MC. Incidence and survival outcomes of gastrointestinal stromal tumors. JAMA Netw Open. (2024) 7:e2428828. doi: 10.1001/jamanetworkopen.2024.28828
22. Miettinen M, Felisiak-Golabek A, Wang Z, Inaguma S, Lasota J. GIST manifesting as a retroperitoneal tumor: clinicopathologic immunohistochemical, and molecular genetic study of 112 cases. Am J Surg Pathol. (2017) 41:577–85. doi: 10.1097/PAS.0000000000000807
23. Long KB, Butrynski JE, Blank SD, Ebrahim KS, Dressel DM, Heinrich MC, et al. Primary extragastrointestinal stromal tumor of the pleura: report of a unique case with genetic confirmation. Am J Surg Pathol. (2010) 34:907–12. doi: 10.1097/PAS.0b013e3181d9f18f
24. Kanamori K, Yamagata Y, Honma Y, Date K, Wada T, Hayashi T, et al. Extra-gastrointestinal stromal tumor arising in the lesser omentum with a platelet-derived growth factor receptor alpha (PDGFRA) mutation: a case report and literature review. World J Surg Oncol. (2020) 18:183. doi: 10.1186/s12957-020-01961-1
25. Qian XH, Yan YC, Gao BQ, Wang WL. Prevalence, diagnosis, and treatment of primary hepatic gastrointestinal stromal tumors. World J Gastroenterol. (2020) 26:6195–206. doi: 10.3748/wjg.v26.i40.6195
26. Cuaron JJ, Goodman KA, Lee N, Wu AJ. External beam radiation therapy for locally advanced and metastatic gastrointestinal stromal tumors. Radiat Oncol. (2013) 8:274. doi: 10.1186/1748-717X-8-274
27. Yi JH, Park BB, Kang JH, Hwang IG, Shin DB, Sym SJ, et al. Retrospective analysis of extra-gastrointestinal stromal tumors. World J Gastroenterol. (2015) 21:1845–50. doi: 10.3748/wjg.v21.i6.1845
28. Haller V, Reiff C, Hamacher R, Kostbade K, Kaths M, Treckmann J, et al. Overall survival of patients with KIT-mutant metastatic GIST in the era of multiple kinase inhibitor availability. J Cancer Res Clin Oncol. (2024) 150:489. doi: 10.1007/s00432-024-05965-2
29. Bischof DA, Kim Y, Behman R, Karanicolas PJ, Quereshy FA, Blazer DG 3rd, et al. A nomogram to predict disease-free survival after surgical resection of GIST. J Gastrointest Surg. (2014) 18:2123–9. doi: 10.1007/s11605-014-2658-2
30. Khoo CY, Chai X, Quek R, Teo MCC, Goh BKP. Systematic review of current prognostication systems for primary gastrointestinal stromal tumors. Eur J Surg Oncol. (2018) 44:388–94. doi: 10.1016/j.ejso.2017.12.006
31. Liu R, Wu Y, Gong J, Zhao R, Li L, Wan Q, et al. Development and external validation of a nomogram for individualized adjuvant imatinib duration for high-risk gastrointestinal stromal tumors: A multicenter retrospective cohort study. Cancer Med. (2022) 11:3093–105. doi: 10.1002/cam4.v11.16
32. Sun Q, Chen Y, Li T, Zhu X, Zhu G, Ni B, et al. Prognostic nomogram for gastrointestinal stromal tumors after surgery based on the SEER database. BioMed Res Int. (2022) 2022:5639174. doi: 10.1155/2022/5639174
33. Bertsimas D, Margonis GA, Tang S, Koulouras A, Antonescu CR, Brennan MF, et al. An interpretable AI model for recurrence prediction after surgery in gastrointestinal stromal tumour: an observational cohort study. EClinicalMedicine. (2023) 64:102200. doi: 10.1016/j.eclinm.2023.102200
34. Bertsimas D, Margonis GA, Sujichantararat S, Koulouras A, Ma Y, Antonescu CR, et al. Interpretable artificial intelligence to optimise use of imatinib after resection in patients with localised gastrointestinal stromal tumours: an observational cohort study. Lancet Oncol. (2024) 25:1025–37. doi: 10.1016/S1470-2045(24)00259-6
Keywords: extra-gastrointestinal stromal tumors, nomogram, survival, prognostic model, risk factor
Citation: Jia G and Li X (2025) Establishment and validation of a prognostic nomogram for extra-gastrointestinal stromal tumors. Front. Oncol. 15:1527237. doi: 10.3389/fonc.2025.1527237
Received: 13 November 2024; Accepted: 25 February 2025;
Published: 20 March 2025.
Edited by:
Ulrich Ronellenfitsch, Medical Faculty of the Martin-Luther-University Halle-Wittenberg, GermanyReviewed by:
Ting Gong, University of Hawaii at Manoa, United StatesCopyright © 2025 Jia and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiangpan Li, Uk0wMDEyMjdAd2h1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.