- 1Minjiang Road Community Health Service Center, Shinan District Medical Health Group, The Affiliated Hospital of Qingdao University, Qingdao, Shandong, China
- 2Department of Radiation Oncology, The Affiliated Hospital of Qingdao University, Qingdao, Shandong, China
- 3Medical Department, The Affiliated Hospital of Qingdao University, Qingdao, Shandong, China
- 4Department of Obstetrics and Gynecology, The Affiliated Hospital of Qingdao University, Qingdao, Shandong, China
Background: Wolffian adnexal tumor (WAT) is a rare neoplasm originating from the remnants of the Wolffian duct (mesonephric duct). Malignant WAT occurring in the ovary is exceptionally uncommon. This article presents a case of malignant WAT in the ovary, analyzing and discussing its histological features, diagnostic challenges, biological behavior, and treatment options in conjunction with relevant literature to enhance our understanding of this rare tumor.
Case presentation: A 64-year-old woman presented with an 8-month history of persistent abdominal pain and distension. An exploratory laparotomy revealed a small amount of pale-yellow ascites, a slightly atrophic uterus, and a left ovary without significant abnormalities. A solid mass measuring approximately 12 × 10 cm was observed between the left fallopian tube and ovary, displaying extensive dense adhesions to the posterior broad ligament and surrounding bowel. Frozen section pathology indicated a malignant tumor with necrotic areas suggestive of poorly differentiated carcinoma. The patient subsequently underwent a total hysterectomy, bilateral adnexectomy, omentectomy, pelvic lymphadenectomy, and pelvic adhesion release. Adjuvant chemotherapy with four cycles of paclitaxel and carboplatin (TC regimen) was administered, achieving normalization of tumor markers by the second cycle.
Conclusions: WAT is a rare entity within the spectrum of female reproductive system tumors, predominantly benign in nature. Due to its extremely low incidence, standardized treatment protocols remain elusive. Further research is warranted to establish effective management strategies and provide a reference for future cases.
Background
Wolffian adnexal tumor (WAT) is a rare neoplasm of the female reproductive system, with only a limited number of reported cases worldwide. It is believed to originate from mesonephric duct remnants, mostly found in the broad ligament, although cases have also been reported in the mesosalpinx, ovary, and retroperitoneum. Histopathological evaluation, including light microscopy, electron microscopy, and immunohistochemical analysis, reveals distinct features that differentiate WAT from Mullerian-origin tumors. First described by Kariminejad and Scully in 1973 as female adnexal tumors of probable Wolffian origin (FATWO) (1), WAT was later officially classified as a mesonephric duct remnant tumor in the WHO Classification of Tumours of Female Reproductive Organs (2). Although traditionally considered benign, malignant transformation of WAT is rare, yet some case reports have documented recurrence and metastasis (3). Due to the limited understanding of its biological behavior, further research is essential to establish standardized diagnostic and therapeutic strategies. Here, we report a case of malignant WAT with high proliferative activity, highlighting its histopathological and molecular characteristics, as well as its clinical management and follow-up outcomes.
Case presentation
A 64-year-old female patient presented with an 8-month history of abdominal pain and distension. Contrast-enhanced CT revealed a well-demarcated, soft-tissue mass in the left adnexal region, anterior to the uterus, measuring approximately 93.1 mm × 58.4 mm. The lesion demonstrated prominent peripheral enhancement with a non-enhanced center and mild dilation of the left ovarian vein. No enlarged lymph nodes were observed in the pelvis or around the bilateral iliac vessels (Figures 1A–C). Gynecological examination detected a mobile mass, approximately 9 cm in diameter, in the anterior uterine region. Gynecological ultrasound showed a hypoechoic, irregularly shaped mass measuring 8.4 cm × 7.9 cm × 6.8 cm in the left posterior region of the uterus, adjacent to a 1.7 cm × 1.2 cm structure resembling the left ovary, with indistinct borders between them and moderately increased internal blood flow signals (Figure 1D). Tumor marker analysis revealed cancer antigen 125 (CA125) at 38.6 U/mL, human epididymis protein 4 (HE4) at 83.8 pmol/L, and neuron-specific enolase (NSE) at 30.8 ng/mL. Exploratory laparotomy identified a small amount of pale-yellow ascites, a slightly smaller uterus, and a 12 cm × 10 cm mass between the left fallopian tube and ovary, with dense adhesions to the posterior broad ligament and surrounding bowel. The left ovary appeared unremarkable, while the right adnexa and omentum were normal in appearance with no visible nodules. The liver and spleen surfaces were smooth, with no detectable nodules. Frozen pathological examination of the mass revealed features of a poorly differentiated malignant tumor with necrosis, raising the possibility of germ cell tumors, sex cord-stromal tumors, or metastatic carcinoma. Given the ambiguous findings and the potential for microscopic lymph node metastasis, systematic pelvic lymphadenectomy was performed to ensure accurate staging and comprehensive tumor clearance. Consequently, total hysterectomy, bilateral salpingo-oophorectomy, omentectomy, pelvic lymphadenectomy, and adhesion release were performed.

Figure 1. Imaging findings of an adnexal mass in a 64-year-old female patient. (A–C) Contrast-enhanced CT images reveal a well-defined soft-tissue mass (93.1 mm × 58.4 mm) in the left adnexal region, anterior to the uterus, with peripheral enhancement, a non-enhancing center, and mild dilation of the left ovarian vein. No pelvic or iliac lymphadenopathy is noted. (D) Gynecological ultrasound shows a hypoechoic, irregular mass (8.4 cm × 7.9 cm × 6.8 cm) in the left posterior uterine region, adjacent to a 1.7 cm × 1.2 cm structure resembling the left ovary, with moderate internal blood flow signals.
Postoperative pathology revealed a poorly differentiated malignant tumor in the left adnexa (11 cm × 7 cm × 7 cm) with necrosis. Tumor cells were arranged in nests or trabeculae, exhibiting marked nuclear atypia and frequent mitotic figures (Figures 2A, B). Immunohistochemical analysis showed creatine kinase (CK) (+), estrogen receptor (ER) (+), GATA3 (−), Inhibin (−), Ki-67 (+), P16 (+), P53 (+, wild type), paired box 8 (Pax-8) (+), PMS2 (+), Progesterone receptor (PR) (++), SALL4 (−), synapsin (Syn) (+), Vimentin (focal +), and WT-1 (+), indicating an embryonic origin of the tumor (Figures 2C–P). Genetic testing confirmed a Breast cancer type 1 (BRCA1) systemic mutation with established clinical significance and a TP53 mutation with potential clinical relevance. The patient was diagnosed with Wolffian adnexal tumor (International Federation of Gynecology and Obstetrics (FIGO) stage IC) and underwent four cycles of adjuvant chemotherapy with paclitaxel and carboplatin (TC regimen). The patient has been regularly followed up post-treatment. At 9 months after surgery, pelvic CT and gynecological ultrasound revealed no signs of recurrence or metastasis (Figures 3A, B). Additionally, serial tumor marker assessments demonstrated a sustained decline in CA125 levels (Figure 3C).
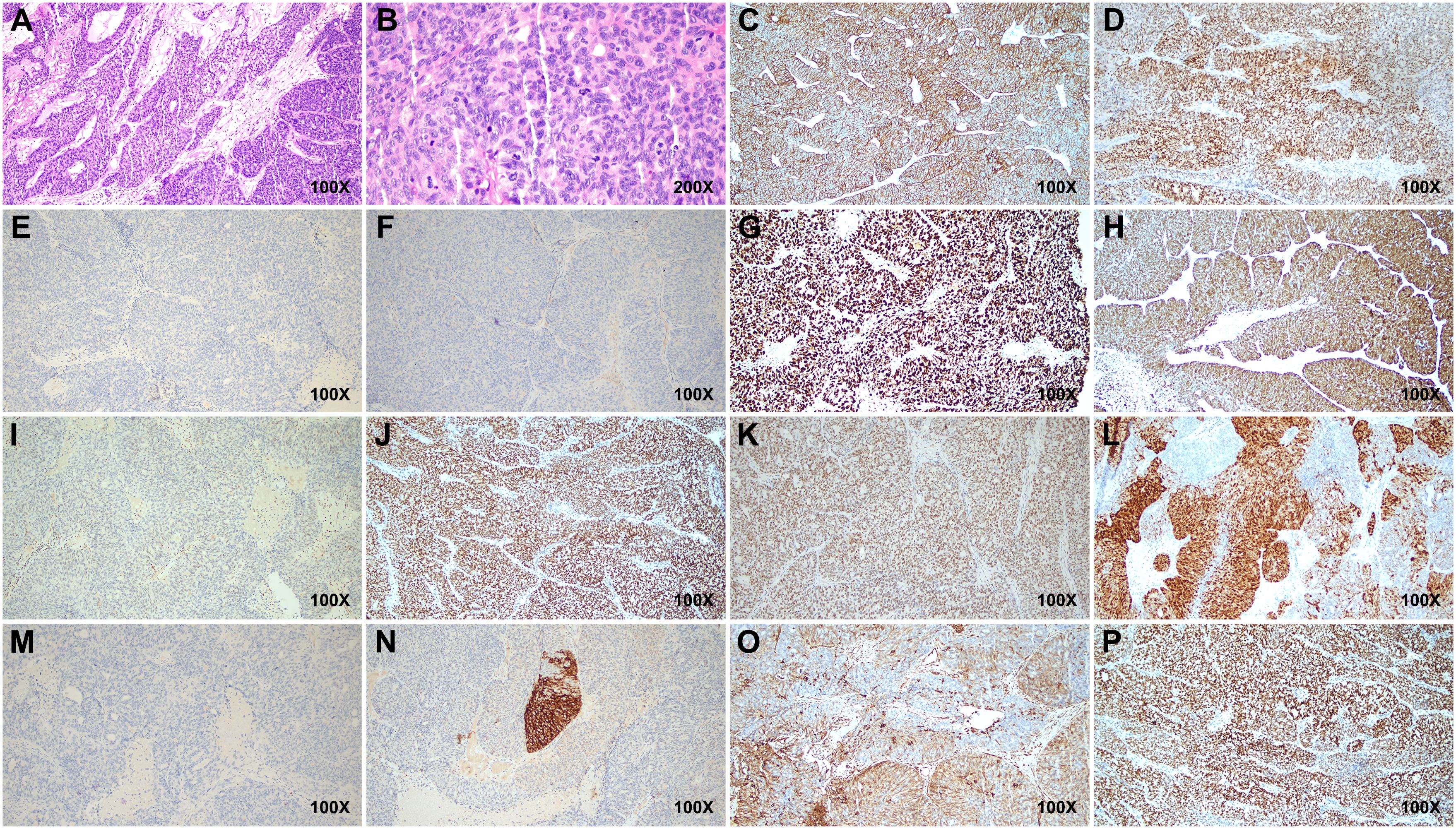
Figure 2. Histopathological and immunohistochemical features of the tumor. (A, B) Hematoxylin and eosin (H&E)-stained sections reveal tumor cells arranged in nests and trabeculae, showing marked nuclear atypia and frequent mitotic figures. (C–P) Immunohistochemical staining shows positive expressions for CK, ER, Ki-67, P16, Pax-8, PMS2, PR, Syn, Vimentin, and WT-1, while GATA3, Inhibin, and SALL4 are negative. P53 exhibits wild-type positivity. Scale bars indicate magnification. CK, creatine kinase; ER, estrogen receptor; Pax-8, paired box 8; Syn, synapsin.
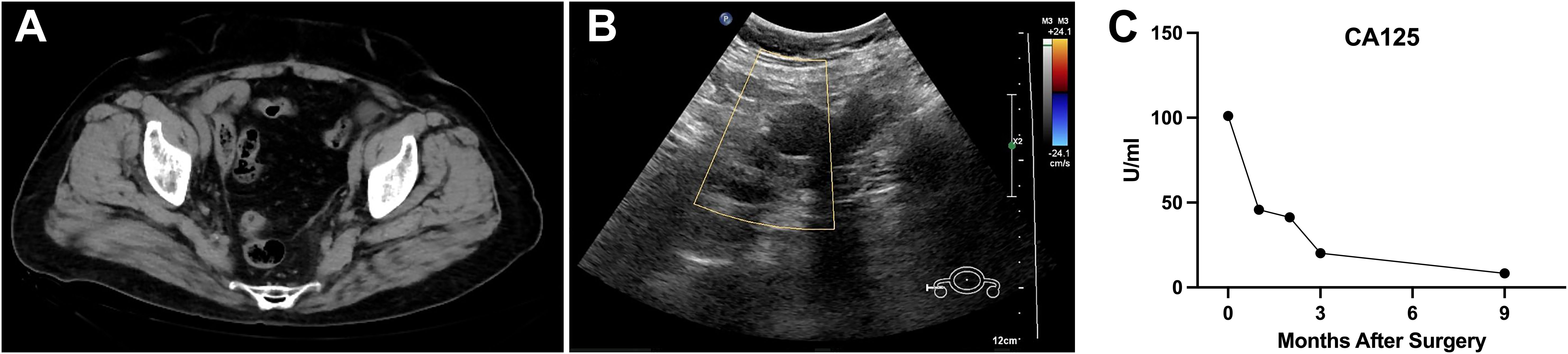
Figure 3. Postoperative follow-up imaging and tumor marker trends indicating no recurrence or metastasis. (A, B) Postoperative follow-up pelvic CT and gynecological ultrasound performed 9 months after surgery revealed no signs of recurrence or metastasis in the pelvic region. (C) Serial measurements of the tumor marker CA125 demonstrated a continuous downward trend post-surgery. CA125, cancer antigen 125.
Discussion and conclusion
WAT is an exceptionally rare entity, with fewer than 90 cases reported in the literature to date (4). The age of onset ranges widely from 15 to 83 years, with a mean age of approximately 50 (5). WAT typically presents without specific clinical symptoms and is often an incidental finding during surgery (6). In some cases, patients may experience non-specific symptoms such as abdominal pain, distension, or an abdominal mass and may experience pressure symptoms like vaginal bleeding, urinary frequency, or constipation. WATs are most often found as unilateral adnexal masses, frequently on the right side (7). While specific tumor markers are lacking, some cases may show elevated CA125 and estradiol levels, with histopathological examination remaining the definitive diagnostic standard (8, 9).
Currently, there is no consensus on the diagnostic criteria for malignant WAT. WATs tend to arise in specific locations, including the broad ligament, mesosalpinx, fallopian tube, ovary, and retroperitoneum, where mesonephric duct remnants may be found. Tumor sizes vary considerably, from 0.8 to 25 cm (4, 10). Grossly, WATs are encapsulated and well-demarcated, with gray-yellow or light-brown cut surfaces, and often have a solid, occasionally nodular or cystic appearance with focal hemorrhage or cystic necrosis. Microscopically, WAT cells typically exhibit mild atypia and cribriform or glandular patterns and rarely show mitotic figures (11). Immunohistochemical markers play a pivotal role in diagnosing WAT, distinguishing it from other pelvic tumors. WAT typically expresses CK, PAX8, and GATA3, markers indicative of Wolffian duct origin. TTF-1 and Vimentin may also be positive, while CD10 and α-inhibin are inconsistently expressed (12, 13). Negative or weak expression of ER and PR further differentiates WAT from endometrial and sex cord-stromal tumors (14–16). In this case, the tumor exhibited CK (+), PAX8 (+), WT1 (+), and focal expression of Vimentin, supporting its Wolffian origin. The absence of GATA3, Inhibin, and SALL4 ruled out mesonephric carcinoma and germ cell tumors. Additionally, the presence of wild-type P53 and a Ki-67 index above 30% highlighted the tumor’s malignant potential.
The criteria proposed by Sivridis, including tumor size (>100 mm), hypercellularity, capsular invasion, and tumor implants, are often applied in clinical studies to guide diagnosis (17). A systematic diagnostic approach integrating morphology and Immunohistochemistry (IHC) findings is crucial for differentiating WAT, particularly malignant variants. Key indicators of malignancy include necrosis, capsular invasion, and increased mitotic activity. Differential diagnosis of pelvic masses is particularly challenging in cases like this, and the unique morphological and immunohistochemical profiles of WAT play a crucial role in distinguishing it from other neoplasms. Sertoli stromal tumors may exhibit tubular structures similar to WAT; however, WAT is characterized by solid spindle-cell areas and cystic structures. Additionally, Sertoli stromal tumors primarily arise in the ovary and are often associated with endocrine symptoms, whereas WAT typically originates from mesonephric duct remnants in extra-ovarian locations, such as the broad ligament and mesosalpinx (18). Granulosa cell tumors also commonly originate in the ovary and display coffee bean-like nuclei with Call–Exner bodies, features absent in WAT. Furthermore, Periodic Acid Schiff (PAS) staining of granulosa cell tumors reveals peritubular basement membrane structures, whereas WAT lacks these findings (19). Endometrioid adenocarcinoma exhibits greater cellular atypia, a high mitotic index, and frequent squamous metaplasia. Immunohistochemically, it is positive for Epithelial membrane antigen (EMA), PR, and ER, with a high Ki-67 index, whereas WAT typically lacks these markers or exhibits lower expression (20). Serous papillary adenocarcinoma is often cystic and fragile, with complex papillary structures and psammoma bodies. It expresses CKpan (AE1/3), CK7, EMA, and WT1, whereas WAT lacks α-inhibin expression, further aiding differentiation (21).
The diagnostic accuracy for malignant WAT is reported to be as low as 17% (22). Histopathological features indicative of malignancy include intratumoral necrosis, capsular invasion, high mitotic activity, and marked cellular atypia (8). Malignant WAT has an estimated recurrence rate of 11%, with a median interval of 48 months, primarily affecting the lungs and liver (22, 23). Given the rarity of malignant WAT and the limited prognostic value of morphological and immunohistochemical markers, long-term follow-up is essential, yet no standardized treatment guidelines exist (24). Existing case reports often extrapolate treatment strategies from malignancies of similar anatomical origin. Prognosis is primarily determined by clinicopathologic staging, cellular atypia, and proliferation indices (3). Complete surgical resection, including hysterectomy, bilateral adnexectomy, and cytoreductive surgery, remains the preferred primary treatment for malignant WAT (6). In the present case, due to the poorly differentiated nature of the tumor, systematic lymphadenectomy was performed despite the absence of intraoperative evidence of lymph node involvement. This approach ensured accurate staging and ruled out microscopic nodal metastases, aligning with oncological principles for malignancies with uncertain biological behavior. The role of chemotherapy and radiotherapy remains controversial. Subsequently, the patient received four cycles of paclitaxel and carboplatin (TC regimen). Regular follow-up post-treatment revealed no evidence of recurrence or metastasis at 9 months, as confirmed by pelvic CT and gynecological ultrasound (Figures 3A, B). Additionally, serial CA125 measurements demonstrated a continuous decline post-surgery, further supporting the absence of residual or recurrent disease (Figure 3C). Long-term follow-up should be conducted to continuously monitor disease status, allowing for the timely detection and management of any recurrence or metastasis.
The molecular characteristics of WAT remain incompletely defined; however, they may provide valuable diagnostic and therapeutic insights. In our case, the presence of a BRCA1 mutation suggests a potential hereditary predisposition. Further investigation of molecular markers, including c-KIT and TP53, may improve diagnostic accuracy and facilitate personalized treatment strategies. Integrating immunohistochemical profiling with molecular analysis offers a comprehensive approach to WAT diagnosis. Currently, Poly (ADP-ribose) Polymerase (PARP) inhibitors are recommended for patients with BRCA mutations in advanced epithelial ovarian cancer (25), yet their efficacy in WAT remains unexplored. In contrast, targeted therapies for TP53 mutations are still under clinical investigation. Notably, Steed et al. (26) reported a case of malignant WAT in a 15-year-old female patient who was refractory to surgery and chemotherapy but responded to Gleevec, a tyrosine kinase inhibitor targeting CD117 (27, 28). These findings suggest that molecular-targeted therapies based on mutations such as c-KIT, BRCA, and TP53 could represent a promising treatment avenue for malignant WAT, warranting further research to validate their efficacy (29, 30).
In conclusion, WAT presents significant diagnostic and therapeutic challenges due to its rarity, non-specific clinical manifestations, and the absence of standardized diagnostic criteria. A multidisciplinary approach integrating comprehensive morphological assessment, immunohistochemical profiling, and molecular analysis is crucial for accurate diagnosis and differentiation from other pelvic tumors. Advances in elucidating the molecular characteristics of WAT, particularly mutations in genes such as BRCA1 and TP53, provide novel insights into its pathogenesis and potential therapeutic targets. Given the limited number of reported cases, long-term follow-up and further research are essential to establish evidence-based management strategies and to evaluate the efficacy of molecular-targeted therapies in improving clinical outcomes.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethical Committee of The Affiliated Hospital of Qingdao University. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from primarily isolated as part of your previous study for which ethical approval was obtained. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
CC: Data curation, Investigation, Writing – original draft, Project administration. GL: Writing – review & editing, Formal Analysis, Validation. ZZ: Methodology, Writing – review & editing, Software. XW: Funding acquisition, Resources, Writing – review & editing, Investigation. XL: Data curation, Investigation, Project administration, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The present study was funded by the 2021 Shandong Medical Association Clinical Research Fund (Qilu Special Project: YXH2022ZX02147).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
WAT, Wolffian adnexal tumor; CA125, cancer antigen 125; HE4, human epididymis protein 4; NSE, neuron-specific enolase; CK, creatine kinase; ER, estrogen receptor; Syn, synapsin; Pax-8, paired box 8; P16, (MTS) multiple tumor suppressor 1; FATWO, female adnexal tumors of probable Wolffian origin.
References
1. Kariminejad MH, Scully RE. Female adnexal tumor of probable wolffian origin. A distinctive pathologic entity. Cancer. (1973) 31:671–7. doi: 10.1002/1097-0142(197303)31:3<671::aid-cncr2820310328>3.0.co;2-k
2. Kurman RJ, Carcangiu M, Herrington CS. World Health Organisation Classification of Tumours of the Female Reproductive Organs. 4th Revised ed. International Agency for Research on Cancer. (2014).
3. Gibbard E, Cochrane DR, Pors J, Negri GL, Colborne S, Cheng AS, et al. Whole-proteome analysis of mesonephric-derived cancers describes new potential biomarkers. Hum Pathol. (2021) 108:1–11. doi: 10.1016/j.humpath.2020.10.005
4. Hou Y, Yang B, Zhang G. Female adnexal tumor of probable wolffian origin. Arch Pathol Lab Med. (2022) 146:166–71. doi: 10.5858/arpa.2020-0432-OA
5. Cui C, Cui D, Pan J, Zhou S, Zheng X. Magnetic resonance imaging findings of a case with wolffian tumor and related literature review. Asian BioMed (Res Rev News). (2024) 18:81–6. doi: 10.2478/abm-2024-0012
6. Armstrong DK, Alvarez RD, Bakkum-Gamez JN, Barroilhet L, Behbakht K, Berchuck A, et al. Ovarian cancer, version 2.2020, nccn clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2021) 19:191–226. doi: 10.6004/jnccn.2021.0007
7. Fanghong L, Szallasi A, Young RH. Wolffian tumor of the ovary with a prominent spindle cell component: report of a case with brief discussion of unusual problems in differential diagnosis, and literature review. Int J Surg Pathol. (2008) 16:222–5. doi: 10.1177/1066896907307034
8. da Silva EM, Fix DJ, Sebastiao APM, Selenica P, Ferrando L, Kim SH, et al. Mesonephric and mesonephric-like carcinomas of the female genital tract: molecular characterization including cases with mixed histology and matched metastases. Mod Pathol. (2021) 34:1570–87. doi: 10.1038/s41379-021-00799-6
9. Shalaby A, Shenoy V. Female adnexal tumor of probable wolffian origin: A review. Arch Pathol Lab Med. (2020) 144:24–8. doi: 10.5858/arpa.2019-0152-RA
10. Qazi M, Movahedi-Lankarani S, Wang BG. Cytohistopathologic correlation of ovarian mesonephric-like carcinoma and female adnexal tumor of probable wolffian origin. Diagn Cytopathol. (2021) 49:E207–e13. doi: 10.1002/dc.24675
11. Long GG. Apparent mesonephric duct (Rete anlage) origin for cysts and proliferative epithelial lesions in the mouse ovary. Toxicol Pathol. (2002) 30:592–8. doi: 10.1080/01926230290105785
12. Pors J, Cheng A, Leo JM, Kinloch MA, Gilks B, Hoang L. A comparison of gata3, ttf1, cd10, and calretinin in identifying mesonephric and mesonephric-like carcinomas of the gynecologic tract. Am J Surg Pathol. (2018) 42:1596–606. doi: 10.1097/pas.0000000000001142
13. Bennett JA, Ritterhouse LL, Furtado LV, Lastra RR, Pesci A, Newell JM, et al. Female adnexal tumors of probable wolffian origin: morphological, immunohistochemical, and molecular analysis of 15 cases. Mod Pathol. (2020) 33:734–47. doi: 10.1038/s41379-019-0375-9
14. Goyal A, Yang B. Differential patterns of pax8, P16, and er immunostains in mesonephric lesions and adenocarcinomas of the cervix. Int J Gynecol Pathol. (2014) 33:613–9. doi: 10.1097/pgp.0000000000000102
15. Tiltman AJ, Allard U. Female adnexal tumours of probable wolffian origin: an immunohistochemical study comparing tumours, mesonephric remnants and paramesonephric derivatives. Histopathology. (2001) 38:237–42. doi: 10.1046/j.1365-2559.2001.01086.x
16. Howitt BE, Emori MM, Drapkin R, Gaspar C, Barletta JA, Nucci MR, et al. Gata3 is a sensitive and specific marker of benign and Malignant mesonephric lesions in the lower female genital tract. Am J Surg Pathol. (2015) 39:1411–9. doi: 10.1097/pas.0000000000000471
17. Sivridis E, Giatromanolaki A, Koutlaki N, Anastasiadis P. Malignant female adnexal tumour of probable wolffian origin: criteria of Malignancy. Histopathology. (2005) 46:716–8. doi: 10.1111/j.1365-2559.2005.02035.x
18. Nann D, Gahlen S, Keul H, Voigt H, Fend F, Staebler A. Tumor of the mesosalpinx with unclear differentiation. Der Pathol. (2016) 37:84–7. doi: 10.1007/s00292-015-0128-6
19. Czernobilsky B, Lifschitz-Mercer B, Trejo L, Atlas I. Granulosa cell tumor of the broad ligament: report of a case with emphasis on the differential diagnosis with female adnexal tumor of probable wolffian origin. Int J Surg Pathol. (2011) 19:783–6. doi: 10.1177/1066896909356104
20. Fukunaga M, Bisceglia M, Dimitri L. Endometrioid carcinoma of the fallopian tube resembling a female adnexal tumor of probable wolffian origin. Adv Anat Pathol. (2004) 11:269–72. doi: 10.1097/01.pap.0000138141.88763.6a
21. Karpathiou G, Chauleur C, Corsini T, Venet M, Habougit C, Honeyman F, et al. Seromucinous ovarian tumor a comparison with the rest of ovarian epithelial tumors. Ann Diagn Pathol. (2017) 27:28–33. doi: 10.1016/j.anndiagpath.2017.01.002
22. Heatley MK. Is female adnexal tumour of probable wolffian origin a benign lesion? A systematic review of the english literature. Pathology. (2009) 41:645–8. doi: 10.3109/00313020903273084
23. Syriac S, Durie N, Kesterson J, Lele S, Mhawech-Fauceglia P. Female adnexal tumor of probable wolffian origin (Fatwo) with recurrence 3 years postsurgery. Int J Gynecol Pathol. (2011) 30:231–5. doi: 10.1097/PGP.0b013e3182005340
24. Piciu A, Cainap C, Sur D, Havasi A, Fetica B, Balacescu O, et al. Rare Malignant female adnexal tumor of wolffian origin (Fatwo) with multiple relapses and chemotherapy regimens. Acta Endocrinol (Bucharest). (2021) 17:259. doi: 10.4183/aeb.2021.259
25. Konstantinopoulos PA, Matulonis UA. Clinical and translational advances in ovarian cancer therapy. Nat Cancer. (2023) 4:1239–57. doi: 10.1038/s43018-023-00617-9
26. Steed H, Oza A, Chapman WB, Yaron M, De Petrillo D. Female adnexal tumor of probable wolffian origin: A clinicopathological case report and a possible new treatment. Int J Gynecol Cancer. (2004) 14:546–50. doi: 10.1111/j.1048-891x.2004.014319.x
27. Chen Q, Shen Y, Xie C. Recurrent and metastatic female adnexal tumor of probable wolffian origin: A case report and review of the literature. Med (Baltimore). (2021) 100:e25377. doi: 10.1097/md.0000000000025377
28. Mirkovic J, Dong F, Sholl LM, Garcia E, Lindeman N, MacConaill L, et al. Targeted genomic profiling of female adnexal tumors of probable wolffian origin (Fatwo). Int J Gynecol Pathol. (2019) 38:543–51. doi: 10.1097/pgp.0000000000000545
29. Pors J, Ho J, Prentice L, Thompson E, Cochrane D, Gibbard E, et al. C-kit analysis and targeted molecular sequencing of mesonephric carcinomas of the female genital tract. Am J Surg Pathol. (2020) 44:495–502. doi: 10.1097/pas.0000000000001403
Keywords: Wolffian adnexal tumor (WAT), female reproductive system, ovarian tumor, treatment, case report
Citation: Chi C, Li G, Zheng Z, Wang X and Liu X (2025) Malignant Wolffian adnexal tumor in the ovary: a case report and literature review. Front. Oncol. 15:1526030. doi: 10.3389/fonc.2025.1526030
Received: 11 November 2024; Accepted: 25 February 2025;
Published: 18 March 2025.
Edited by:
Robert Fruscio, University of Milano Bicocca, ItalyReviewed by:
José Luis Sánchez Iglesias, Gynecology Oncology, SpainLjiljana Vučković, University of Montenegro, Montenegro
Copyright © 2025 Chi, Li, Zheng, Wang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiangyu Liu, bGl1eGlhbmd5dUBxZHUuZWR1LmNu
 Cheng Chi
Cheng Chi Guoliang Li
Guoliang Li Zian Zheng3
Zian Zheng3 Xiangyu Liu
Xiangyu Liu