- 1Department of Radiology, Ningbo No. 2 Hospital, Ningbo, China
- 2Department of Radiotherapy and Chemotherapy, Ningbo No. 2 Hospital, Ningbo, China
- 3Cancer Radiochemotherapy Center, First Affiliated Hospital of Ningbo University, Ningbo, China
Background and objectives: Acute radiation hematologic toxicity may disturb the radiotherapy plan and thus decrease the treatment outcome. However, whether the dose map has enough prediction value for detecting hematologic toxicity (HT) is still unknown.
Methods: In this study, the pre-treatment CT images and the in-treatment dose map were collected from a discovery dataset of 299 patients and a validation dataset of 65 patients from another center. Then, the radiomic features of the clinical target volume (CTV) in the radiotherapy were extracted, and the least absolute shrinkage and selection operator (LASSO) algorithm was used for feature dimension deduction; three classifiers, that is, support vector machine (SVM) (rbf kernel), random forest, and CatBoost, were used to construct the HT classification model in rectal cancer patients. The model performance was evaluated by both the internal 20% dataset and the external multicenter dataset.
Results: The results revealed that CatBoost achieved the best model performance in almost all tasks and that CT images performed similarly with the dose map, although their combination model performed lower. In addition, gender, age, and some radiomic features from the decomposed image space were the most representative features for HT prediction.
Conclusion: Our study can confirm that the HT occurrence in locally advanced rectal cancer (LARC) patients was multifactorial, and combining effective features together can classify the high-risk patients with HT, thus timely preventing or detecting HT to improve the subsequent outcome.
1 Introduction
Radiation hematologic toxicity (HT) is a common complication when locally advanced rectal cancer (LARC) patients are undergoing radiotherapy or chemoradiotherapy (1–3). The appearance of acute HT may disturb the following treatment plan, thus influencing the treatment outcome and survival quality. Therefore, timely recognition of high-risk HT patients is essential for personalized precision treatment of rectal cancer.
In the early years, several studies have been conducted to explore the possibility of HT prediction with the dosimetric factors, and the dosimetric factors of specific regions of interest (ROIs) were consistently reported to be highly associated with HT in rectal cancer (1, 4, 5). However, the commonly used dosimetric factors are simple quantitative metrics, such as V5, V10, and V15, where Vx is the volume percentage with radiation dose more than x Gy. It is clear that the whole dose map contains more dose distribution information than these metrics, but whether the whole dose map can contribute to the HT prediction is still unknown.
In recent years, with the development of radiomics in radiology, the radiomic features that record the subtle characteristics from medical imaging have been widely used for the diagnosis and prognosis of cancer diseases (6–8). Yue et al. first explored the feasibility of pre-treatment CT imaging in HT prediction and revealed that the imaging features performed better than the conventional dosimetric factors (9). Even so, whether the CT imaging maintains better performance in HT prediction than the dose map needs further investigation.
In this study, we designed a comparative study that used a large-sample retrospective LARC dataset that collected both the pre-treatment CT imaging and dose map, which we analyzed using a standardized radiomic procedure. After that, we adopted three different machine learning classifiers to assess the HT prediction performance in both the internal testing dataset and external validation dataset. We infer the dose map may be more sensitive than the CT imaging in HT prediction.
2 Methods
2.1 Discovery dataset
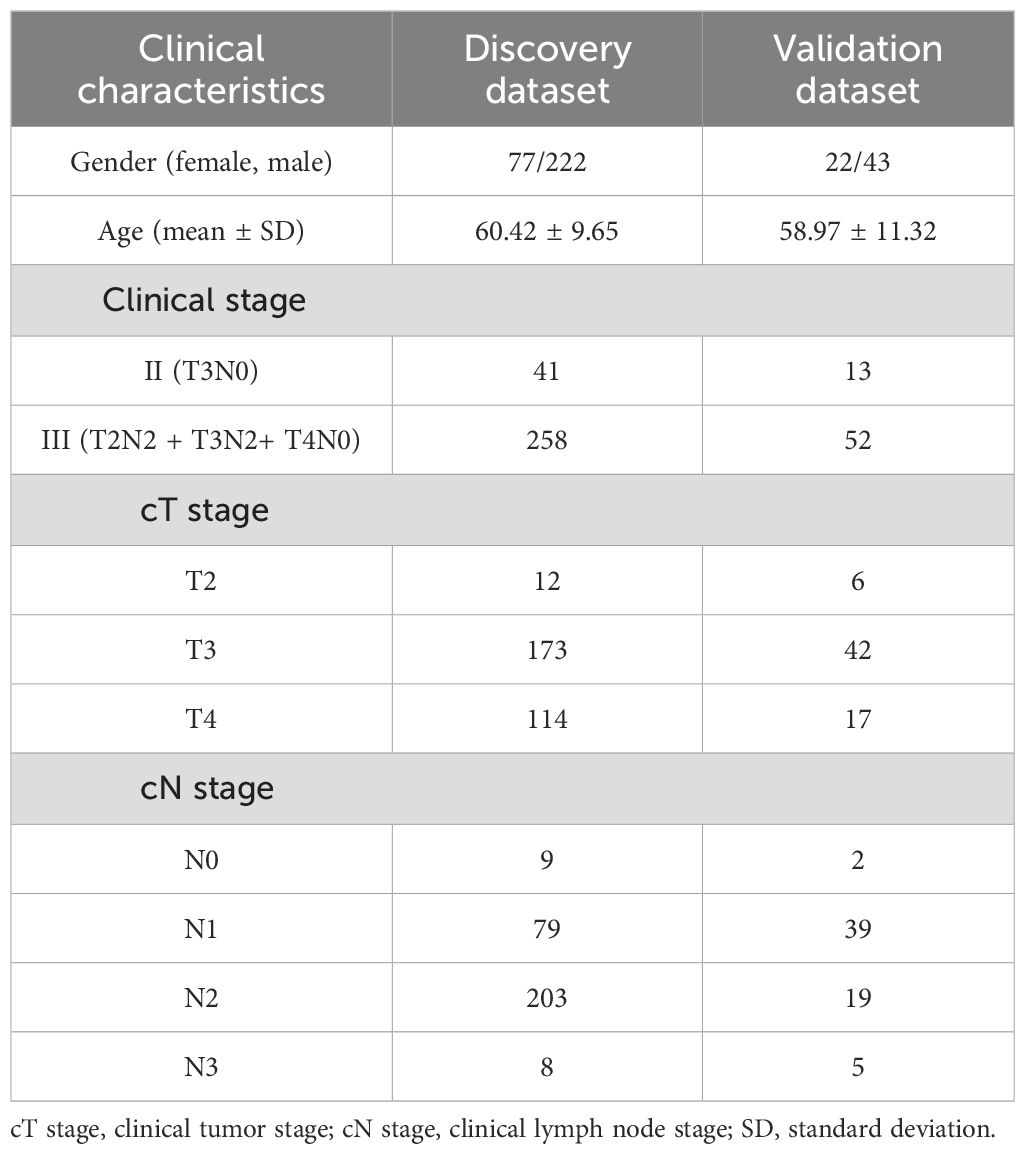
A total of 336 locally advanced rectal cancer patients from January 2019 to April 2024 were retrospectively included from Ningbo No. 2 Hospital. All patients should satisfy the following criteria: pathologically proven rectal carcinoma, first-time radiation therapy for rectal cancer, and entire blood tests during time points (i.e., within 1 week prior to radiotherapy, once a week during the radiotherapy, and within 1 week after radiotherapy). Patients were excluded if they had 1) a history of radiotherapy or chemoradiotherapy and 2) inconsistent prescription doses, incomplete blood data, or no full course of radiotherapy. A total of 299 patients were finally selected for this study, including 86 patients with acute HT symptoms. Table 1 provides all patients’ clinical characteristics, such as the clinical stage and cTN stage. This study was approved by the ethics committee of Ningbo No. 2 Hospital. HT was graded according to the Radiation Therapy Oncology Group’s (RTOG’s) acute radiation morbidity scoring criteria. The blood data of all patients were routinely examined 1 week before the radiotherapy and every week during the radiotherapy. Once the patient reached leukocyte grade ≥2, neutrophil grade ≥2, or thrombocytopenia grade ≥2 during radiotherapy, the sample was considered to have HT (9).
2.2 Validation dataset
To validate the generalization of the HT prediction model, a total of 65 LARC subjects from the First Affiliated Hospital of Ningbo University were also collected using the same inclusion and exclusion criteria, of which 25 patients had symptoms of HT. Table 1 summarizes the clinical characteristics of the patients, including the clinical stage and cTN stage.
The flowchart of this study is illustrated in Figure 1, and it mainly includes the following three parts: 1) multi-modal data collection, 2) radiomic feature calculation, and 3) machine learning model construction.
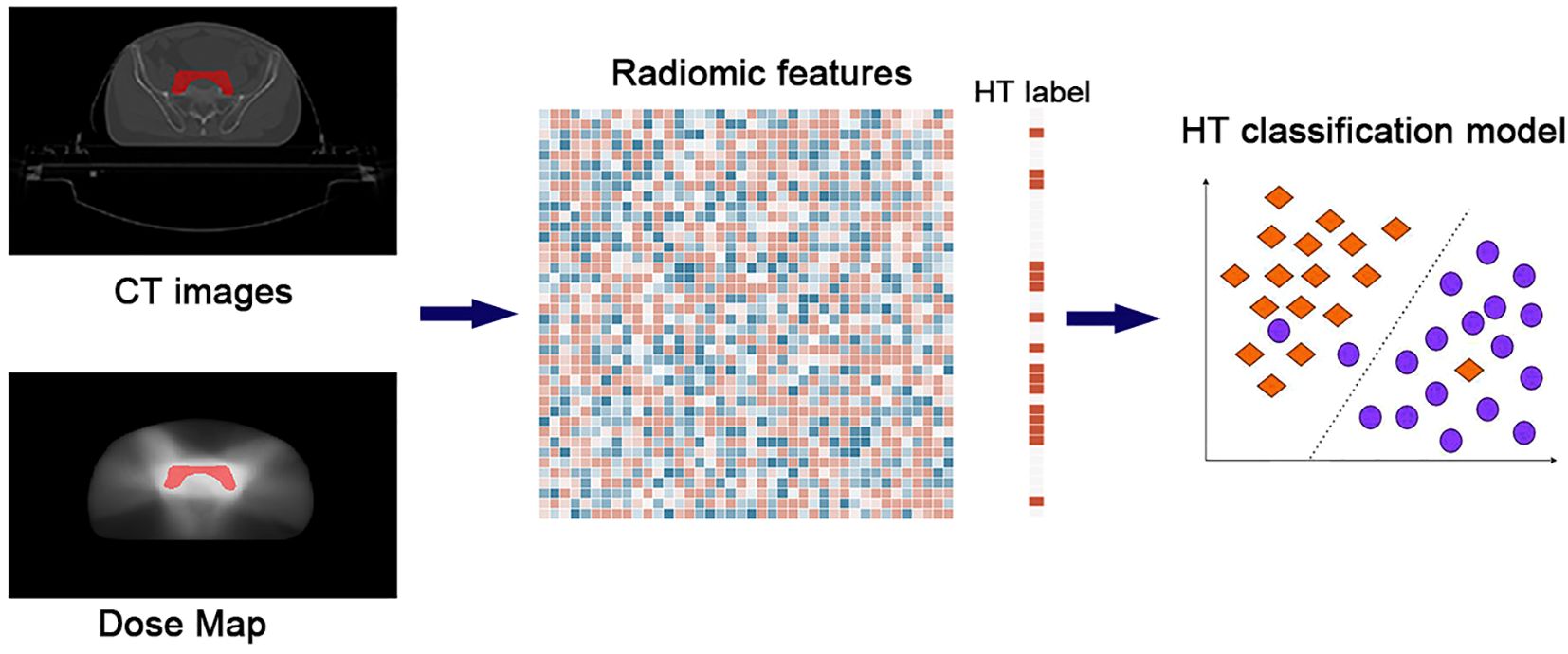
Figure 1. The flowchart of the study. The whole pipeline included three steps: 1) multi-modal data collection, 2) radiomic feature calculation, and 3) machine learning model construction.
2.3 Multi-modal data collection
2.3.1 Pre-treatment CT imaging
The CT images were acquired for simulation and irradiation in subsequent radiotherapy treatment plans by Siemens SOMATOM Sensation Open CT (Siemens, Erlangen, Germany). All patients were in a supine position with a full bladder by drinking 1 L of water 1 hour before the CT scanning, with the following parameters collected: 5-mm thickness, continuous slices without gap, and uniform slice matrix dimension (512 × 512) with voxel size (1.27 mm × 1.27 mm). The validation dataset shared the same scanning parameters as the discovery dataset.
2.3.2 Radiotherapy dose map
In both the discovery and validation datasets, the volumetric modulated arc therapy (VMAT) treatment plans were generated using the Eclipse treatment planning system (V.15.6). All patients received conventional fractionated VMAT with two dose levels simultaneously: 50.6 and 41.8 Gy, 22 or 34 fractions. Treatment was delivered five times per week with a single fraction per day, which was executed on the IX or TrueBeam (Varian Medical Systems, Palo Alto, CA, USA) medical linear accelerator. Finally, every patient would generate a dose map that records the dosimetry distribution at all voxels of abdominal CT images; i.e., the dose map had the same image dimension as the corresponding CT images.
2.4 Radiomic feature calculation
In this study, one key ROI used to develop treatment planning was selected for radiomic analysis: clinical target volume (CTV) (10). It was contoured by two experts (Y.L. and L.G.) based on the RTOG guidelines; if there were any disagreements between two experts, a consensus ROI would be obtained by discussion between them. A total of 1,200 radiomic features were extracted using the PyRadiomics software (Version 3.0.1) on treatment planning CT imaging and dose map. The calculated radiomic features were not only from the original image but also from the wavelet-filtered images and Laplacian of Gaussian-filtered images. The type of features mainly included the texture features, such as gray-level co-occurrence matrix, gray-level run-length matrix, gray-level size zone matrix, neighboring gray-tone difference matrix, and gray-level dependence matrix.
2.5 Machine learning model construction
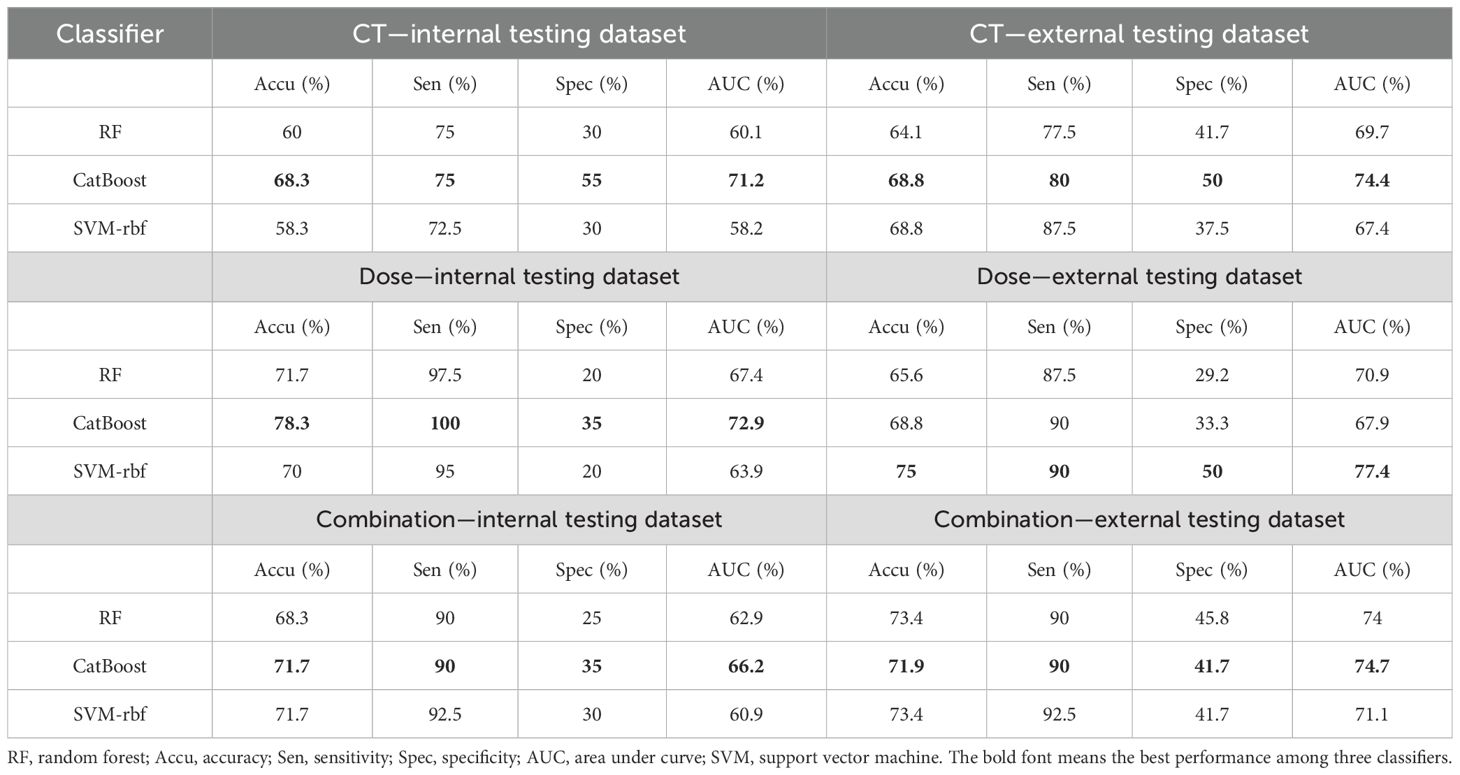
In addition to the radiomic features, two demographic (age and gender) features were combined together to construct the HT classification model. Here, only age and gender were selected for the model construction because of their consistently reported roles in HT occurrence (5, 9). To eliminate the dependence on the performance of specific classifiers, three widely used machine learning models were constructed, that is, the support vector machine (rbf kernel), random forest, and CatBoost. All these models were conducted by the scikit-learn package (Version 1.4.2) in Python. Concerning the high-dimensional feature sets, the least absolute shrinkage and selection operator (LASSO) was first used for feature reduction in the training dataset, which was then input into three classifiers to obtain the optimized model. Specifically, the hyperparameters of the LASSO and three classifiers were optimized by an inner fivefold cross-validation in the training dataset. In this study, 80% of the discovery data were used as training data, while the remaining 20% of the data were seen as internal testing data; the multicenter data were deemed as external testing data. The model performance was compared according to accuracy, sensitivity, specificity, and area under the curve (AUC) among the single CT imaging model, the single dose map model, and their combination model.
3 Results
Figure 2 summarizes the HT model performance of three different classifiers, and the CatBoost model achieved the best AUC in almost all conditions. The dose map performed similarly with the CT imaging in HT prediction, while their combination had lower performance. Table 2 shows the detailed performance metric of every classifier in all groups, and it should be emphasized that the specificity in these models was not high enough, which may explain the current challenge in HT prediction.
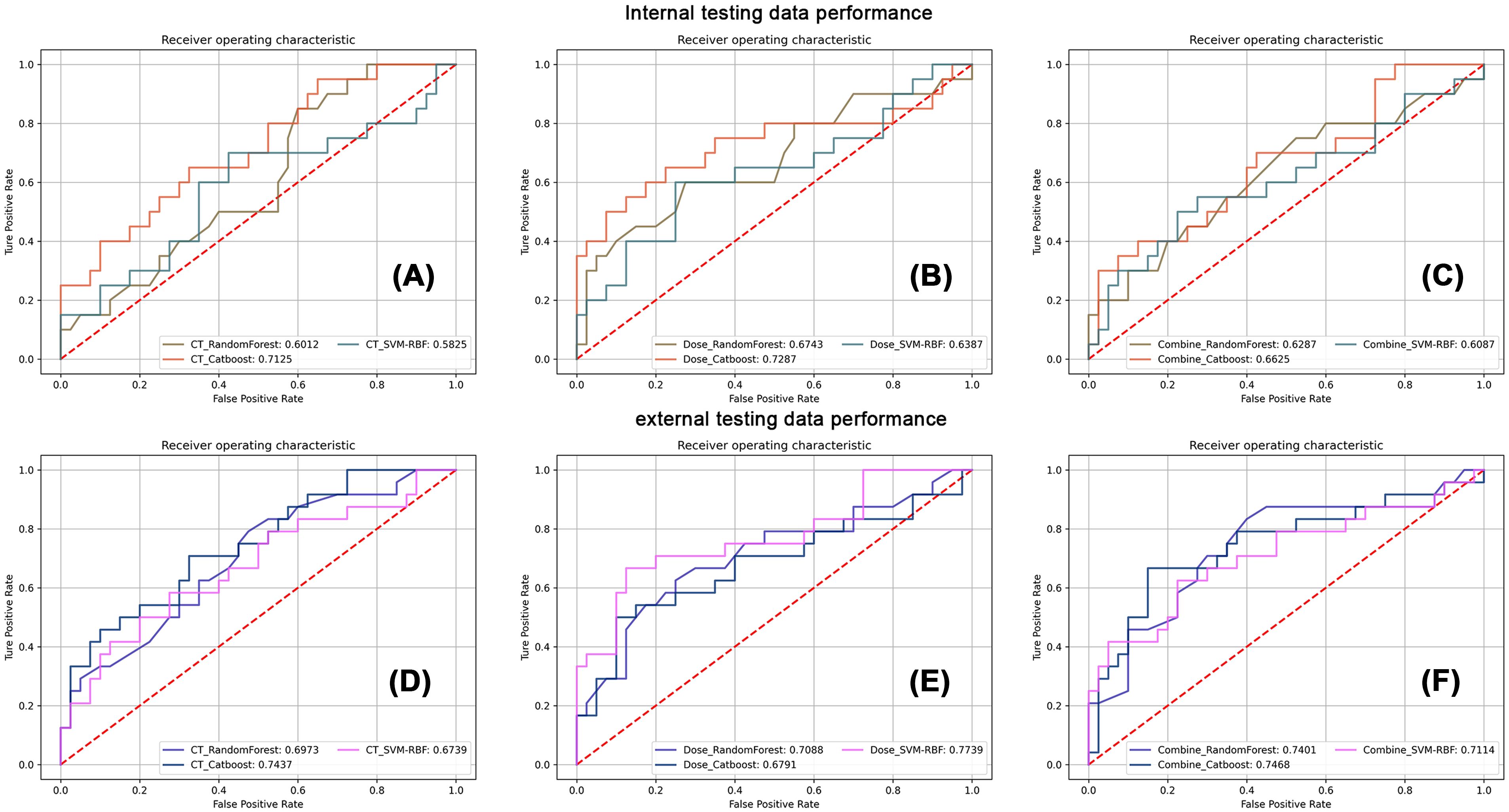
Figure 2. The corresponding receiver operating characteristic analysis of three classifiers in CT imaging, dose map, and their combination models. The first row displays the classification performance of three classifiers in internal testing dataset using CT images (A), the dose map (B), and their combination (C). The second row displays the classification performance of three classifiers in external testing dataset using CT images (D), the dose map (E), and their combination (F).
Figure 3 listed the top 10 features used in the CT imaging and dose map models, and sex was found to be a key demographic factor in both groups. In addition, age was related to HT prediction in the CT imaging model. In addition to the demographic features, the other features were mainly from the texture features of wavelet-transformed images, especially the type of first-order features.
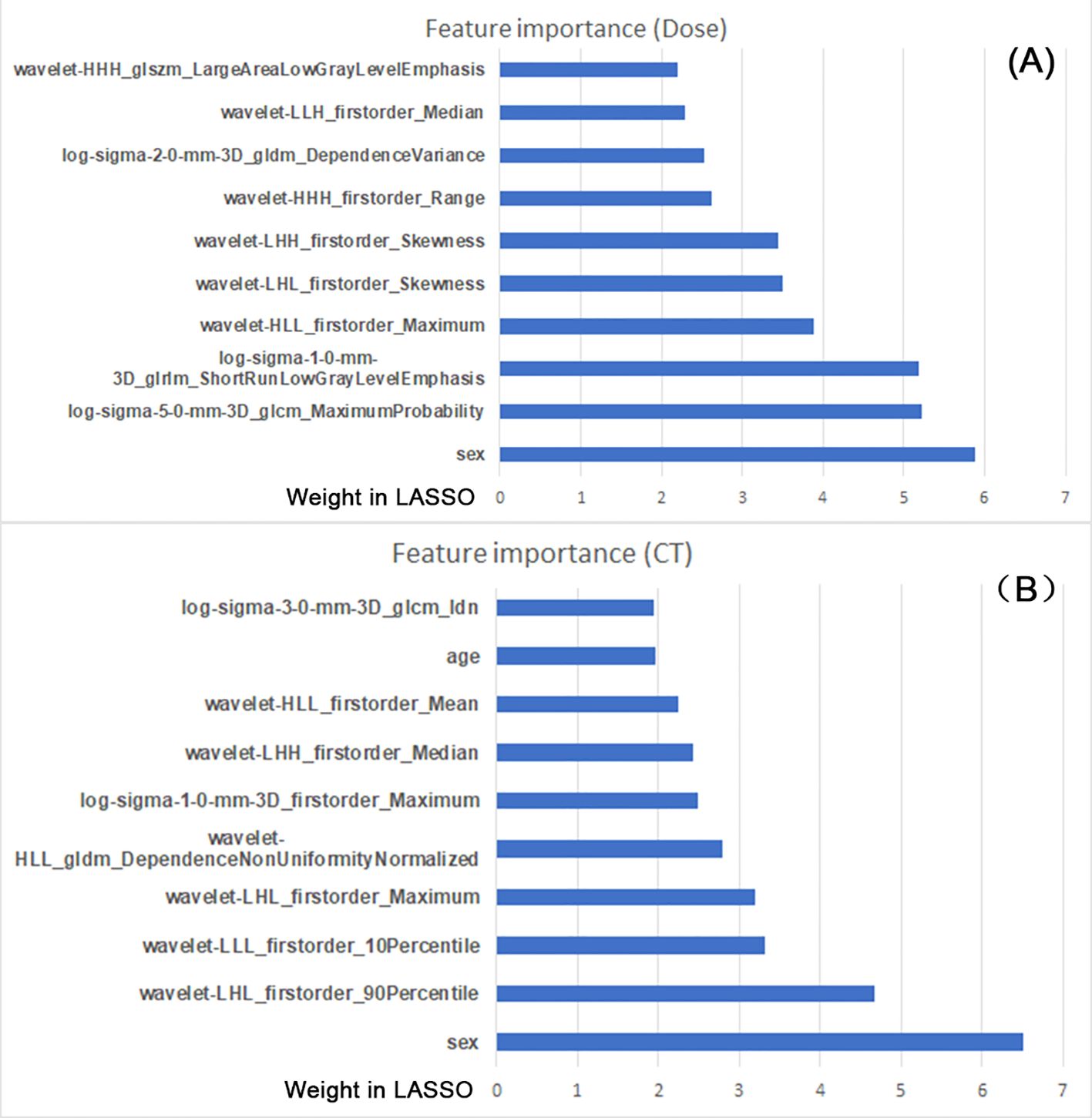
Figure 3. The top 10 features from the constructed models used in the CT images (A) and dose map (B). The feature importance was sorted by the weight coefficient in LASSO model (X-axis); the feature names are listed (Y-axis).
4 Discussion
In this study, we compared the HT prediction performance using the radiomic features of CTV in two imaging modalities with three classifiers and found that single dose map performed similarly with single pre-treatment CT images, but their combination performed worse than any single one. In addition, the model performance was dependent on the selection of the classifier, and sex is a key demographic feature in HT prediction. Our study can strengthen the understanding of the contribution factors to HT occurrence; i.e., the initial status of rectal cancer (CT images) and the dosage prescription (dose map) are both effective indicators, which may shed light on the timely diagnosis of high-risk HT patients and thus improve the subsequent individualized treatment response.
However, precise prediction of HT in rectal cancer patients during radiotherapy is still challenging. Our study revealed that the pre-treatment CT imaging performed similarly to the dose map of radiotherapy. The phenomenon may indicate that HT’s existence was related to multiplex factors; both pre-treatment status and radiotherapy treatment plan may contribute to HT. Even so, the classification accuracy was still not high; we infer that there may be some other latent factors that may influence the model performance, such as the treatment regimen (11), although tumor heterogeneity may be another invisible factor. Compared to a previous study (9), our study emphasized that using the dose map instead of the dosimetric features could be better for HT prediction. Especially, the previous study adopted eight different ROIs for HT prediction, and the most sensitive ROI was bone marrow, while CTV performed relatively low. In contrast, our study only used the tumor-related CTV as ROI, and its performance was acceptable in both CT imaging and dose map groups. A feasible interpretation was that we utilized three classifiers, which may have decreased the performance dependence of a single classifier in a previous study.
In this study, only two demographic features were included in the HT prediction model. On the one hand, age and gender are usually collected for LARC patients in the clinical practice of our hospital, while the other demographic information was not available for every patient. On the other hand, age and gender have been consistently reported in the HT prediction model (5, 9), and the main purpose of this study was to ascertain whether the dose map also contributes to HT prediction like CT imaging. In this study, gender was found as a key demographic feature for HT classification in both CT imaging and dose map models, implying that the HT occurrence was selective to gender in the initial tumor status (CT image) and the treatment plan (dose map). In our population, the sample size of male LARC patients was obviously larger than that of female patients, while the existence ratio of HT was higher in female patients. Additionally, the CT images also revealed that age was also an important factor, thus suggesting the pre-treatment factors were dependent on both gender and age. These findings add to the knowledge about the course of HT for LARC patients, implying that multi-modal fusion may serve as the essential avenue for improving HT prediction.
In addition to the demographic features, the radiomic features were also highly associated with the HT occurrence. Radiomic features reflect the microscopic characteristics within images (12, 13), and different imaging modalities adopted specific features, indicating that CT images and dose map had different participating roles in HT. Moreover, most features were from the wavelet transformed and Laplacian of Gaussian filtered images, again prompting that the most informative features were not from the original images. In addition, when combining the features of two modalities together, the model performance was slightly decreased, and this may be explained by the correlative features between the two modalities.
Finally, three different classifiers displayed a non-consistent tendency in HT classification. CatBoost performed the best in comparison to support vector machine-rbf (SVM-rbf) and random forest in this study. CatBoost is composed of gradient-boosted decision trees and shows low risk for overfitting, robustness to outliers, and fast training speed. In previous studies, it had also achieved good model performance in different tasks using radiomic features (7, 8, 14). In contrast, SVM-rbf and random forest performed clearly worse than CatBoost, although they are also widely used machine learning classifiers. This phenomenon underscores the essentials of selecting multiple classifiers in radiomic studies, which may more effectively unveil the application significance of machine learning models in clinical diagnosis.
Several limitations were available in this study: 1) we only compare the radiomic model of one ROI (CTV), and some other ROIs may be also sensitive to HT classification, which needs further studies in the future. 2) The models’ performance may be also influenced by the feature selection manner, and we only utilized the LASSO, while other feature selection algorithms may perform better. 3) The main purpose of this study is not to improve the HT prediction performance using radiomics but to confirm whether the dose map contributes to HT prediction like CT images, so there are many other unexplored factors that may be also important for HT occurrence, which can be explored in the future. 4) The dose intensification in rectal cancer (15) may be also influential to HT, which can be explored in future studies.
5 Conclusion
In this study, we found the radiomic features of CTV from the dose map could be used for HT prediction in LARC patients like CT images, although their combinations were not good enough. In addition, sex and age were also related to HT occurrence, and CatBoost gained the best model performance. Our study could deepen the understanding of the contributing factors and their complex interacting roles in HT prediction, which may be helpful to timely detect high-risk HT patients and make a targeted treatment strategy for these patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the IRB of Ningbo No.2 Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
YL: Conceptualization, Data curation, Formal Analysis, Writing – original draft, Writing – review & editing. LG: Formal Analysis, Funding acquisition, Investigation, Writing – original draft, Writing – review & editing. YW: Resources, Validation, Writing – original draft, Writing – review & editing. QX: Project administration, Visualization, Writing – original draft, Writing – review & editing. JZ: Methodology, Writing – original draft, Writing – review & editing. XM: Data curation, Investigation, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by HwaMei Research Foundation of Ningbo No. 2 Hospital (No. 2023HMKY30), Medical Scientific Research Foundation of Zhejiang Province (No. 2021KY298), Ningbo Clinical Research Center for Medical Imaging (No. 2021L003), and Ningbo Leading Medical & Health Discipline (No. 2022-S02).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Huang W, Dang J, Li Y, Cui HX, Lu WL, Jiang QF. Effect of pelvic bone marrow sparing intensity modulated radiation therapy on acute hematologic toxicity in rectal cancer patients undergoing chemo-radiotherapy. Front Oncol. (2021) 11:646211. doi: 10.3389/fonc.2021.646211
2. Takeda K, Umezawa R, Yamamoto T, Takahashi N, Suzuki Y, Kishida K, et al. Acute hematologic toxicity of radiation therapy - a comprehensive analysis and predictive nomogram. J Radiat Res. (2023) 64:954–61. doi: 10.1093/jrr/rrad069
3. Yazbeck V, Alesi E, Myers J, Hackney MH, Cuttino L, Gewirtz DA. An overview of chemotoxicity and radiation toxicity in cancer therapy. Adv Cancer Res. (2022) 155:1–27. doi: 10.1016/bs.acr.2022.03.007
4. Li N, Liu X, Zhai F, Liu B, Cao X, Li S, et al. Association between dose-volume parameters and acute bone marrow suppression in rectal cancer patients treated with concurrent chemoradiotherapy. Oncotarget. (2017) 8:92904–13. doi: 10.18632/oncotarget.21646
5. Wan J, Liu K, Li K, Li G, Zhang Z. Can dosimetric parameters predict acute hematologic toxicity in rectal cancer patients treated with intensity-modulated pelvic radiotherapy? Radiat Oncol. (2015) 10:162. doi: 10.1186/s13014-015-0454-0
6. Liu H, Jiao Z, Han W, Jing B. Identifying the histologic subtypes of non-small cell lung cancer with computed tomography imaging: a comparative study of capsule net, convolutional neural network, and radiomics. Quant Imaging Med Surg. (2021) 11:2756–65. doi: 10.21037/qims-20-734
7. Amudala Puchakayala PR, Sthanam VL, Nakhmani A, Chaudhary MFA, Kizhakke Puliyakote A, Reinhardt JM, et al. Radiomics for improved detection of chronic obstructive pulmonary disease in low-dose and standard-dose chest CT scans. Radiology. (2023) 307:e222998. doi: 10.1148/radiol.222998
8. Evrimler S, Ali Gedik M, Ahmet Serel T, Ertunc O, Alperen Ozturk S, Soyupek S. Bladder urothelial carcinoma: machine learning-based computed tomography radiomics for prediction of histological variant. Acad Radiol. (2022) 29:1682–9. doi: 10.1016/j.acra.2022.02.007
9. Yue H, Geng J, Gong L, Li Y, Windsor G, Liu J, et al. Radiation hematologic toxicity prediction for locally advanced rectal cancer using dosimetric and radiomics features. Med Phys. (2023) 50:4993–5001. doi: 10.1002/mp.16308
10. Valentini V, Gambacorta MA, Barbaro B, Chiloiro G, Coco C, Das P, et al. International consensus guidelines on Clinical Target Volume delineation in rectal cancer. Radiother Oncol. (2016) 120:195–201. doi: 10.1016/j.radonc.2016.07.017
11. Yue H, Li X, You J, Feng P, Du Y, Wang R, et al. Acute hematologic toxicity prediction using dosimetric and radiomics features in patients with cervical cancer: does the treatment regimen matter? Front Oncol. (2024) 14:1365897. doi: 10.3389/fonc.2024.1365897
12. Abbaspour E, Karimzadhagh S, Monsef A, Joukar F, Mansour-Ghanaei F, Hassanipour S. Application of radiomics for preoperative prediction of lymph node metastasis in colorectal cancer: a systematic review and meta-analysis. Int J Surg. (2024) 110:3795–813. doi: 10.1097/JS9.0000000000001239
13. Li Z, Huang H, Zhao Z, Ma W, Mao H, Liu F, et al. Development and validation of a nomogram based on DCE-MRI radiomics for predicting hypoxia-inducible factor 1alpha expression in locally advanced rectal cancer. Acad Radiol. (2024) 31(12):4923–33. doi: 10.1016/j.acra.2024.05.015
14. Cui E, Li Z, Ma C, Li Q, Lei Y, Lan Y, et al. Predicting the ISUP grade of clear cell renal cell carcinoma with multiparametric MR and multiphase CT radiomics. Eur Radiol. (2020) 30:2912–21. doi: 10.1007/s00330-019-06601-1
15. Caravatta L, Lupattelli M, Mantello G, Gambacorta MA, Chiloiro G, Rosa C, et al. A. Gastrointestinal study group of, treatment volume, dose prescription and delivery techniques for dose-intensification in rectal cancer: A national survey. Anticancer Res. (2021) 41:1985–95. doi: 10.21873/anticanres.14966
Keywords: dose map, radiomics, radiation hematologic toxicity, CatBoost, rectal cancer
Citation: Liu Y, Guo L, Wang Y, Xu Q, Zhang J and Meng X (2025) Radiation hematologic toxicity prediction in rectal cancer: a comparative radiomics-based study on CT image and dose map. Front. Oncol. 15:1516855. doi: 10.3389/fonc.2025.1516855
Received: 25 October 2024; Accepted: 14 February 2025;
Published: 04 March 2025.
Edited by:
Xiaodong Wu, The University of Iowa, United StatesReviewed by:
Corrado Spatola, University of Catania, ItalyLiviu Bilteanu, Carol Davila University of Medicine and Pharmacy, Romania
Copyright © 2025 Liu, Guo, Wang, Xu, Zhang and Meng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xianyun Meng, eGlhbnl1bm1lbmcwOUAxNjMuY29t
†These authors have contributed equally to this work
 Yingpeng Liu
Yingpeng Liu Liping Guo1†
Liping Guo1† Yi Wang
Yi Wang