
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 02 April 2025
Sec. Thoracic Oncology
Volume 15 - 2025 | https://doi.org/10.3389/fonc.2025.1514653
This article is part of the Research TopicHarnessing Molecular Insights for Enhanced Drug Sensitivity and Immunotherapy in CancerView all 28 articles
 Xiangran Feng1†
Xiangran Feng1† Ran Zeng1†
Ran Zeng1† Mengchen Lyu1†
Mengchen Lyu1† Xiaoyan Chen2
Xiaoyan Chen2 Ziwei Xu1
Ziwei Xu1 Yue Hu1
Yue Hu1 Zhiyao Bao1,3,4
Zhiyao Bao1,3,4 Xianwen Sun1,3,4
Xianwen Sun1,3,4 Jingya Zhao1,3,4
Jingya Zhao1,3,4 Ling Zhou1,3,4
Ling Zhou1,3,4 Jun Zhou1,3,4
Jun Zhou1,3,4 Beili Gao1,3,4
Beili Gao1,3,4 Lei Dong2*
Lei Dong2* Yi Xiang1,3,4*
Yi Xiang1,3,4*Background: The differences in clinical characteristics and treatment prognosis in NSCLC patients harboring primary and acquired BRAF mutations are still poorly understood.
Methods: From Oct 2017 to Dec 2023, 10, 211 lung cancer patients at Shanghai Ruijin Hospital were reviewed. 88 primary and 15 acquired BRAF-mutated NSCLC patients resistant to EGFR TKIs were included in the study.
Results: Primary BRAF-mutated patients preferentially occurred in the elderly (median age: 67 vs 61, p=0.015), males (53.4% vs 26.7%, p=0.056), former/current smokers (36.5% vs 6.7%, p=0.033), non-adenocarcinoma (11.4% vs 0%, P=0.351) compared to acquired BRAF-mutated patients. Significant differences in gender (33.3% vs 62.3%, p=0.012), smoking history (22.2% vs 43.1%, p=0.063), and adenocarcinomas (100% vs 83.6%, p=0.028) were observed between primary BRAF/EGFR co-mutated and non-co-mutated groups. While primary and acquired BRAF/EGFR co-mutated patients had similar clinical characteristics, with EGFR mutations being the most common coexisting oncogene (30.7% and 93.3%). The genotype of EGFR mutations differed, with acquired BRAF-mutated cases showing more complexity and a higher rate of dual EGFR mutations (35.7%) compared to primary cases. For primary BRAF/EGFR co-mutated patients, no matter what kinds of therapies, the EGFR 19del patients had a better prognosis than non-19del patients, and the first line mPFS was NR and 9.0 months (95% CI: 7.7-10.3 months) (p=0.0062), respectively. Dabrafenib and trametinib plus 3rd EGFR TKIs improved the prognosis of primary BRAF/EGFR non-19del co-mutated patients, achieving ORR and mPFS of 100% (3/3) and 12 months. For acquired co-mutated patients, the mPFS for 5 patients was 8.6 months (95% CI: 5.4-11.8 months). No new safety concerns and > grade 3 AEs were noted.
Conclusion: Together, our study demonstrates that primary and acquired BRAF-mutant patients show distinct differences in some clinical and molecular characteristics, but acquired BRAF/EGFR co-mutated and primary BRAF/EGFR non-19del co-mutated patients may both respond to triple-targeted therapy.
Lung cancer remains the leading cause of cancer mortality worldwide, owing to its high prevalence and 44.1% at advanced stage at the time of diagnosis (1, 2). The discovery of oncogenic driver alterations in non-small cell lung cancer (NSCLC) has revolutionized the treatment paradigm for patients with specific genomic alterations. Among these mutations, a significant proportion of epidermal growth factor receptor (EGFR) mutations are detected in 10%-15% of advanced NSCLC in Western populations and 40%-50% in Asians (3, 4). Third-generation EGFR tyrosine kinase inhibitors (TKIs) osimertinib has been approved as the standard treatment for EGFR-mutated NSCLC patients, with median progression-free survival (PFS) and overall survival (OS) reaching 18.9 months and 38.6 months, respectively (5). Previous studies have shown that patients with EGFR 19 deletions tend to have longer PFS and OS compared to those with L858R mutations after treatment with EGFR TKIs (6–8).
The V-Raf murine sarcoma viral oncogene homolog B1 (BRAF) is a key component in the mitogen-activated protein kinase (MAPK) pathway and has been identified as an oncogenic driver gene (9, 10). BRAF mutations were initially identified in melanoma with an occurrence rate of more than 60% (11). In NSCLC, BRAF mutations have been reported to occur in 3%-4% of Western populations (12–14) and 0.5%-2% of East Asian populations (15, 16). The BRAF V600E is the most common mutation, which accounts for 50%-56.8% of all BRAF mutations (12, 13). Notably, BRAF inhibitor vemurafenib or dabrafenib has achieved an objective response rate (ORR) of 33%–42% and mPFS of 5.5 to 7.3 months in BRAF V600E mutant NSCLC patients, respectively (17, 18). Dual-targeted BRAF/MEK inhibition with dabrafenib and trametinib improves therapeutic efficacy in BRAF V600E mutated NSCLC patients, achieving ORR and mPFS of 64% and 14.6 months (19).
With advancements in oncogenic driver gene detection technology, guidelines now recommend comprehensive genetic testing before treatment, resulting in the identification of an increasing number of co-mutations. The frequency of EGFR and BRAF co-mutations in treatment-naïve NSCLCs is reported to be 0.91% (15, 20). Due to the low frequency, little is known about the molecular biology of BRAF/EGFR co-mutations or the prognosis of EGFR TKIs monotherapy and EGFR plus BRAF inhibitors in BRAF/EGFR co-mutated NSCLC patients. BENEFIT study (21) showed that for advanced EGFR-mutated patients who received gefitinib, mPFS in EGFR-mutated alone vs EGFR with other oncogenes were 13.2 months (95%CI: 11.5–15.0) vs 4.7 months (9% CI: 1.9–9.3), which indicated that patients with concomitant oncogenes had a poor prognosis. In addition, acquired BRAF mutations have been identified as a resistance mechanism during EGFR-TKI treatment, occurring at a frequency of 1%-5% (22–24). Several studies have studied the efficacy of dabrafenib, trametinib plus osmertinib (triple-targeted therapy regimen) in these acquired BRAF/BRAF co-mutated patients, which has achieved an ORR of 58% to 80% and mPFS of 2 to 13 months (25, 26). These studies showed that triple-targeted therapy had better efficacy in acquired BRAF/EGFR co-mutated patients, but the regimen in primary BRAF/EGFR co-mutated patients has never been reported the efficacy of triple-targeted therapy regimen. Therefore, further research is required to investigate the efficacy and safety of this treatment regimen in primary and acquired BRAF/EGFR co-mutated patients.
Therefore, this study retrospectively analyzed the demographics and molecular characteristics between primary and acquired BRAF-mutated patients, as well as the triple-treatment regimen efficacy in these groups, and provided a new option for the treatment of primary and acquired BRAF-mutated NSCLC patients.
From Oct 2017 to Dec 2023, a total of 10, 211 lung cancer patients at Shanghai Ruijin Hospital were reviewed in our study. Primary BRAF-mutated patients tested BRAF mutation-positive before the first systematic treatment. Acquired BRAF-mutated patients were: 1) BRAF mutation-negative at baseline; 2) Detected BRAF mutation-positive after systematic treatment failure and subsequent rebiopsy gene testing. The inclusion criteria for patients were: (1) Histologically confirmed NSCLC; (2) BRAF and EGFR mutations detected by clinically approved sequencing platforms.
This retrospective study was approved by the Institutional Review Board of Ruijin Hospital (ID:2024-172) following the Declaration of Helsinki (revised in 2013).
BRAF mutation and other gene alterations were mainly detected by amplification refractory mutation system polymerase chain reaction (ARMS-PCR) or next-generation sequencing (NGS). The ARMS molecular analysis of samples was conducted using the AmoyDx® Multi-Gene Mutations Detection Kit (Amoy Diagnostics, Xiamen, China). The experiments were performed according to the manufacturer’s instructions. This kit contains 118 hotspot mutations/fusions in EGFR, KRAS, NRAS, BRAF, ALK, ROS1, HER2, RET, MET and PIK3CA genes (27). The NGS platforms used in the study encompassed various clinically approved sequencing platforms, covering panels ranging from 68 to 196 genes including the 10 driver oncogenes as above. Tissue samples were primarily used for molecular testing in most cases, while blood-based circulating tumor DNA (ctDNA) analysis was employed when tissue samples were unavailable or insufficient. All sequencing procedures followed institutional ethical guidelines and manufacturer recommendations.
Baseline demographic and clinical characteristics including age, sex, pathology, smoking status, ECOG status, clinical stage, distant organ metastasis, PD-L1 tumor proportion score (TPS), molecular data, treatment regimen, efficacy, safety, and survival outcomes were extracted through a systematic review of electronic medical records. PD-L1 expression was assessed using the Dako 22C3 platform (Agilent, Santa Clara, CA, USA). Complete response (CR), partial response (PR), stable disease (SD), and progression disease (PD) were defined according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. The objective response rate (ORR) was defined as CR plus PR. The progression-free survival (PFS) was the time from treatment initiation to the disease progression or death date. The overall survival (OS) was defined as the time from the diagnosis of lung cancer to death. The disease staging was determined according to the American Joint Council on Cancer (AJCC) Staging System (Version 8). Treatment-related adverse events (TRAEs) were assessed according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0.
Descriptive statistics were used to characterize the patients. For categorical variables, the characteristics were described as frequency and percentages, and either the chi-square test or Fisher’s exact test was used for comparison. For continuous variables, median and interquartile were used, and the non-parametric Mann-Whitney U test was employed for comparison. Kaplan-Meier analysis and log-rank test were used to assess OS and PFS. The data were analyzed using SPSS 27.0 (IBM Corp., Armonk, New York, USA), GraphPad Prism 9.0 (GraphPad Software, San Diego, California, USA), and R software version 4.2.3 (R Foundation for Statistical Computing). The two-sided P < 0.05 was considered statistically significant. The last follow-up time was March 2024.
Between Oct 2017 and Dec 2023, 129 BRAF-mutated lung cancer patients were identified. After excluding patients with incomplete medical records(n=16), with synchronous second primary malignancies (n=9), a total of 104 BRAF-mutated patients were included. In this cohort, 88 (84.6%) patients had the primary BRAF mutation, and 16 (15.4%) patients had the acquired BRAF mutation, which included one ALK-TKIs resistant patient and 15 EGFR-TKIs resistant patients. Given that this study primarily focused on patients with secondary BRAF mutations following resistance to EGFR-TKIs, subsequent research excluded patients with ALK TKIs resistance. Among patients with primary BRAF mutations, there were 23 early-stage cases (26.1%) and 65 advanced or metastatic cases (73.9%). The scheme of this study is shown in Figure 1.
The median age was 67 years old (range: 28-89 years old), 32 (31.1%) had a former/current smoking history, and most were adenocarcinomas (93/103, 90.3%). All ten non-adenocarcinoma patients, including nine with squamous cell carcinoma and one with adenosquamous carcinoma, were in the primary cohort.
Primary BRAF-mutated patients had more elderly (median age: 67 versus 61, p=0.015), males (53.4% vs 26.7%, p=0.056), former/current smokers (36.5% vs 6.7%, p=0.033), non-adenocarcinoma (11.4% vs 0%, P=0.351) patients than the acquired BRAF-mutated cohort. The two groups had no differences in the distribution of BRAF V600E and non-V600E mutations.
The primary BRAF-mutated patients were divided into two subgroups, the BRAF/EGFR co-mutated cohort, and the BRAF/EGFR non-co-mutated cohort. There were significant differences in gender (males, 33.3% vs 62.3%, p=0.012), smoking history (22.2% vs 43.1%, p=0.063), and histological types (adenocarcinomas, 100% vs 83.6%, p=0.028). The proportion of PD-L1 expression TPS≥1% (41.7% vs 55.3%, p=0.411) and ≥50% (16.7% vs 23.7%, p=1.000) patients was slightly lower in primary BRAF/EGFR co-mutated patients compared to primary non-BRAF/EGFR co-mutated patients. The detailed data on clinical characteristics are summarized in Table 1.
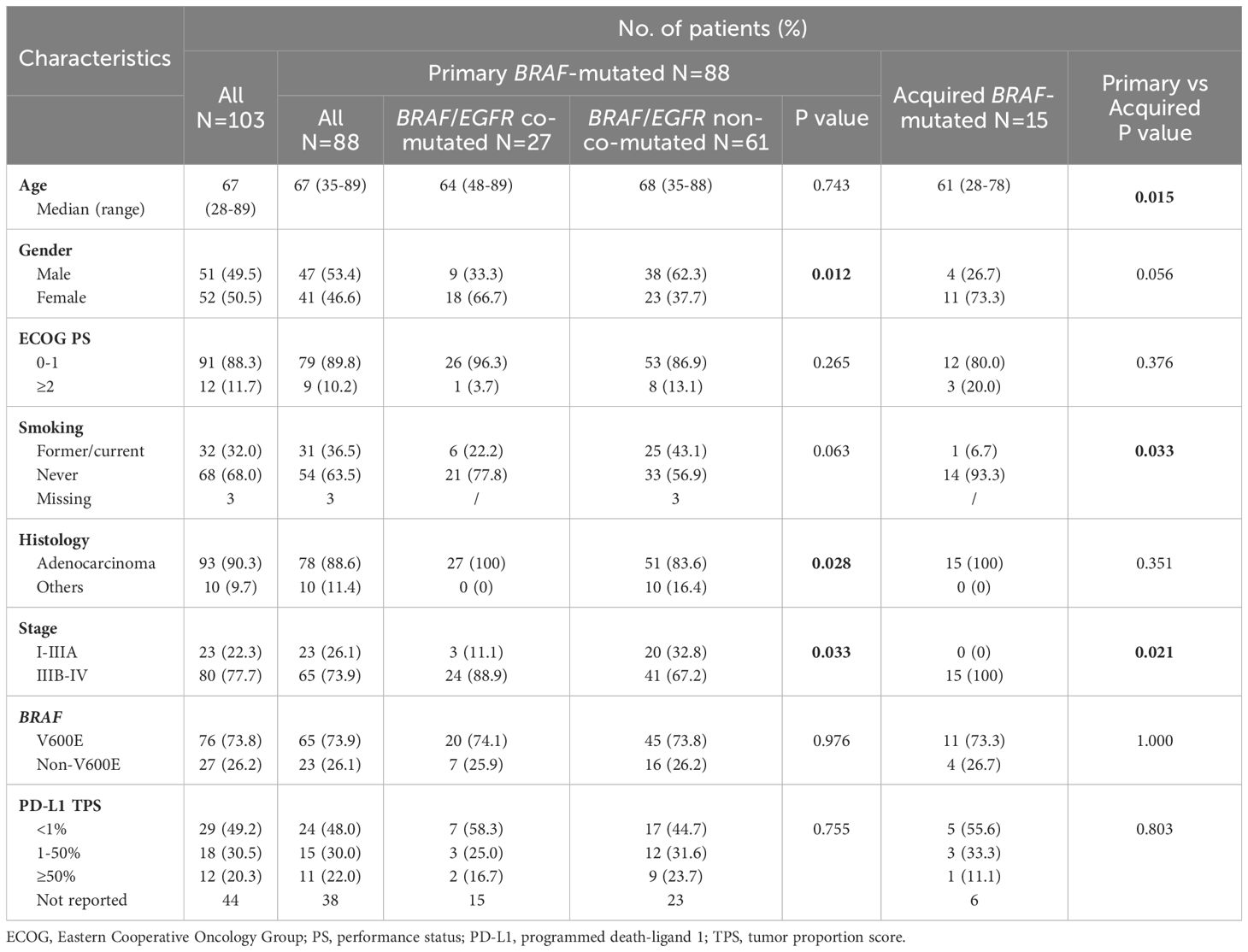
Table 1. Clinical and molecular characteristics between primary and acquired BRAF-mutated NSCLC patients.
We then analyzed the clinical characteristics of primary and acquired BRAF/EGFR co-mutated advanced patients. There were no apparent differences in gender (males, 29.2% vs 28.6%, p=1.000), smoking history (16.7% vs 7.1%, p=0.633), BRAF V600E (75% vs 78.6%, p=1.000) and PD-L1 TPS≥50% (10.0% vs 11.1%, p=1.000). The detailed data are shown in Table 2.
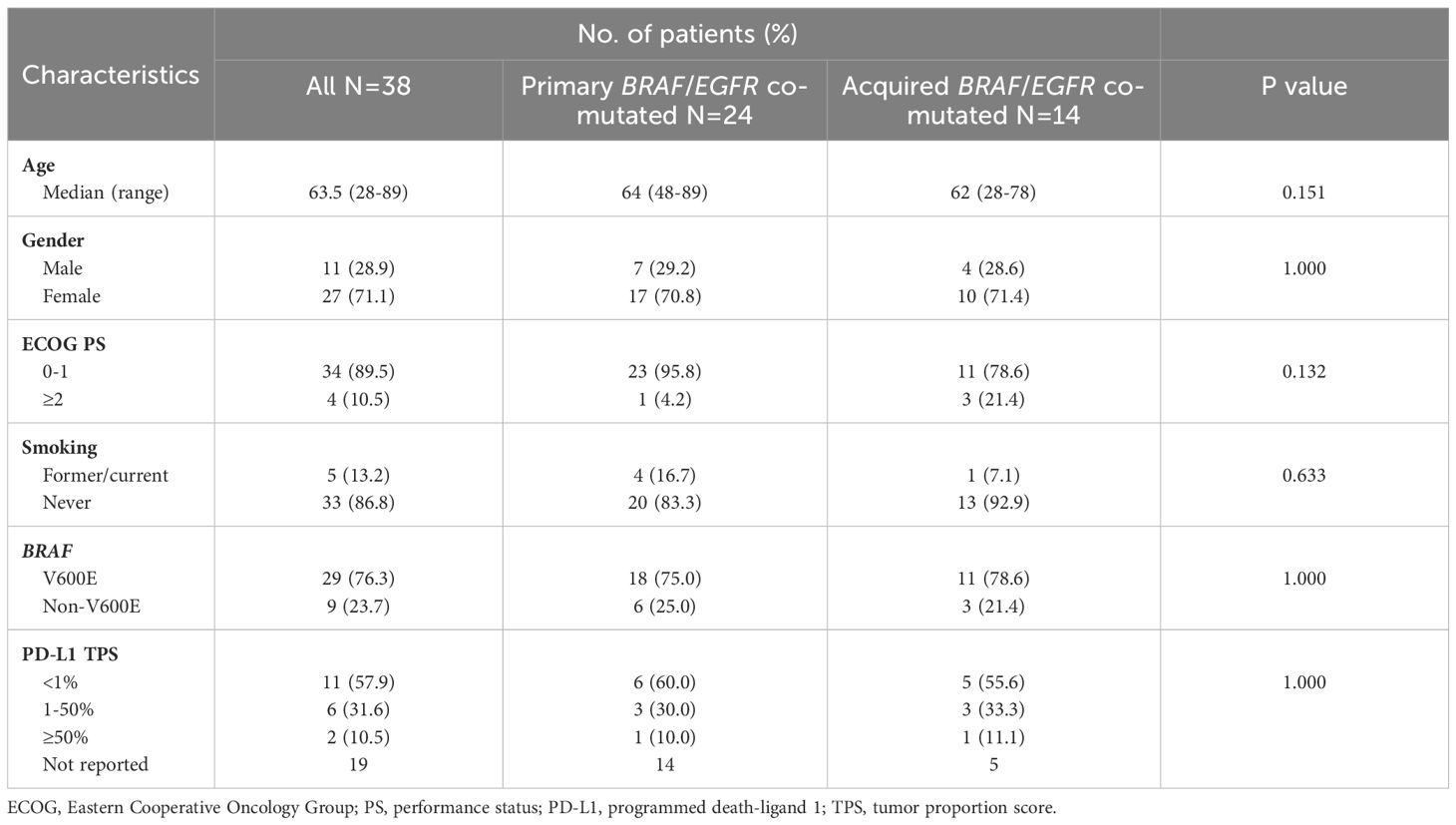
Table 2. Clinical and molecular characteristics between primary and acquired advanced BRAF/EGFR co-mutated NSCLC patients.
We then investigated the genomic landscape of BRAF-mutated patients. In our cohort, among the 88 primary patients, 26 (29.5%) were tested using NGS, while 62 (70.5%) were tested using PCR. In contrast, among the acquired patients, NGS was predominantly used (14/15, 93.3%), with only one patient tested by PCR. Among patients with primary BRAF mutations, the majority of molecular analyses were performed using tissue samples (81/88, 92.0%), with only 7 cases (8.0%) analyzed via blood samples. In contrast, for patients with acquired BRAF mutations, tissue and blood samples were utilized in 8/15 (53.3%) and 7/15 (46.7%) of cases, respectively. The frequency of concomitant gene mutations was 53.4% (55/103), with 46.6% (41/88) in primary BRAF-mutated patients and 93.3% (14/15) in acquired BRAF-mutated patients (p<0.001). (Figure 2A). Among the primary patients, the mutation rates for EGFR, KRAS, NRAS, HER2, ALK, MET, ROS1, and RET were 30.7%, 3.4%, 1.2%, 1.2%, 3.4%, 5.7%, 2.3%, and 2.4%, respectively. Among the acquired patients, the mutation rates were 93.3%, 6.7%, 0%, 0%, 0%, 6.7%, 0%, and 0%, respectively. In advanced or metastatic NSCLC patients, 52.3% (34/65) primary BRAF-mutated patients and 93.3% (14/15) acquired BRAF-mutated patients (p=0.003) had concomitant oncogenic driver genes. The most frequently coexisting oncogenes of primary and acquired BRAF-mutated NSCLC patients were EGFR mutations (30.7% and 93.3%) (Figure 2B). The genotype of concomitant EGFR mutation differed in the two BRAF/EGFR co-mutated groups. The primary BRAF-mutated cohorts had EGFR 19del (n=11, 45.8%), EGFR L858R (n=9, 37.5%), EGFR T790M (n=1, 4.2%), EGFR amplification (n=1, 4.2%), EGFR 19del+L858R (n=1, 4.2%) and EGFR T790M+L858R (n=1, 4.2%). In the acquired BRAF-mutated cohorts, the genotype of EGFR mutations mainly included EGFR19 deletions (n=8, 57.1%), dual EGFR mutations (n=5, 35.7%), and L858R mutation (n=1, 7.1%). The details are shown in Figures 2C, D.
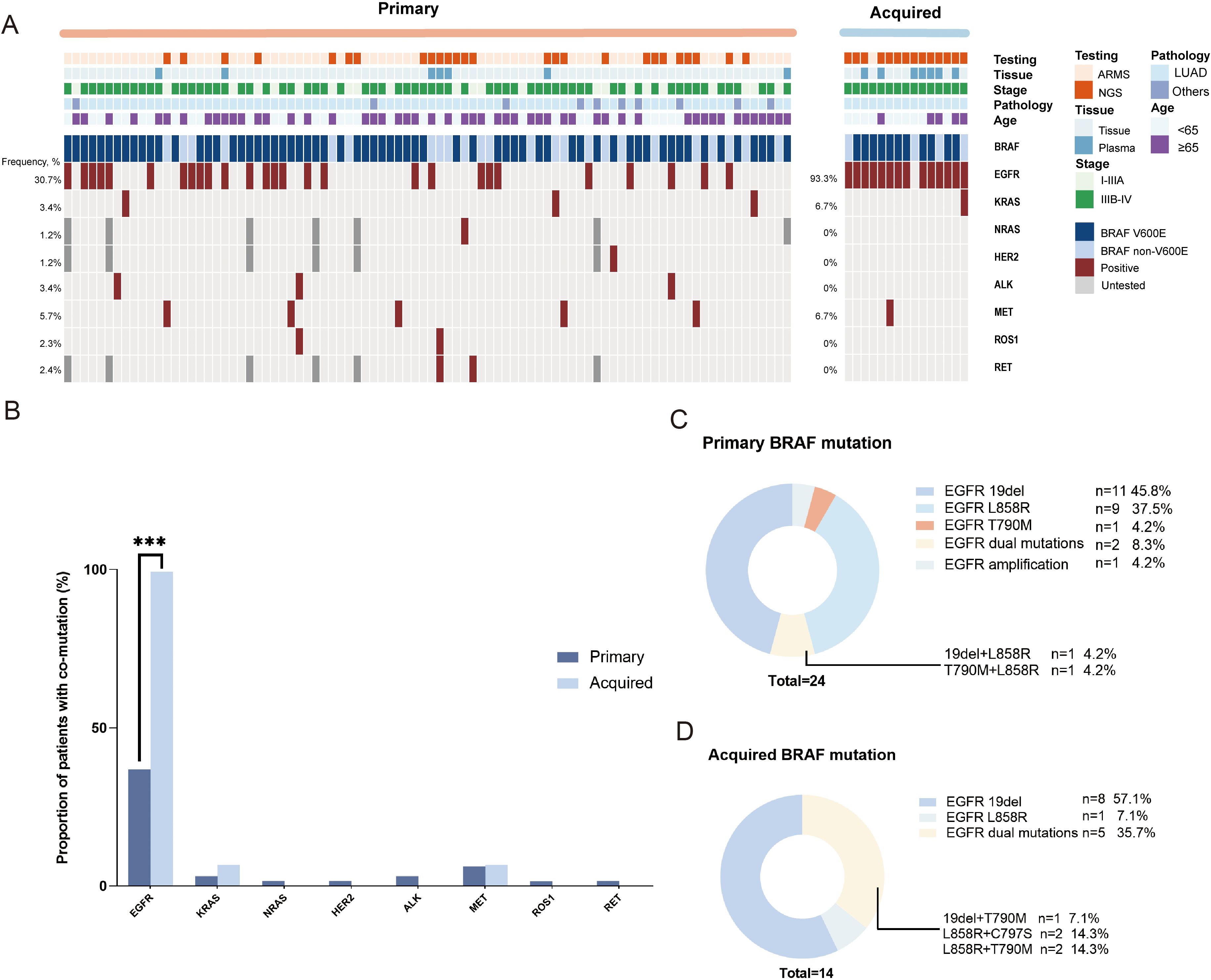
Figure 2. The genomic landscape of primary and acquired BRAF mutation resistant to EGFR TKIs NSCLC patients. (A) Oncogenic driver gene heatmap in the total patient cohort (N=103). (B) The bar graph of the oncogenic driver gene alterations in the advanced primary and acquired NSCLC patients. ***P < 0.001. (C) The type of the concomitant EGFR mutation in advanced primary BRAF-mutated NSCLC patients. (D) The type of the concomitant EGFR mutation in advanced acquired BRAF-mutated NSCLC patients.
The median follow-up time was 51.3 months (range: 11.2-153.4 months) for the 15 acquired BRAF-mutated patients. The acquired BRAF-mutated cohort included 11 (73.3%) V600E-mutated patients and 4 (26.7%) non-V600E-mutated patients. The median age detected BRAF mutation was 61 years old (range: 28-78). The median time from EGFR-TKIs treatment to BRAF mutation detection was 32.1 months, and the median treatment line at which BRAF mutation was acquired was 3 (range: 2-5). EGFR mutations were identified in 14 (93.3%) patients with EGFR 19del (n=8, 57.1%), EGFR L858R (n=1, 7.1%), EGFR L858R+C797S (n=2, 14.3%), EGFR L858R+T790M (n=2, 14.3%) and EGFR 19del+T790M (n=1, 7.1%). One patient lost the EGFR L858R mutation after using first-generation EGFR TKIs.
Five patients received dabrafenib, trametinib, and 3rd generation EGFR TKIs (osimertinib/furmonertinib) after acquiring BRAF mutation. The median treatment line was 4 (range: 2-5). And the mPFS of this triple-treatment regimen was 8.6 months (95% CI: 5.4-11.8 months). Detailed information about them is provided in Table 3.
All acquired BRAF-mutated patients who received at least one dose of triple-targeted therapy were evaluated for safety. The most common TRAEs of any grade were pyrexia (n=3, 30%), decreased appetite (n=3, 30%), rash (n=1, 10%), fatigue (n=1, 10%), nausea (n=1, 10%), and white blood cell count decrease (n=1, 10%). There were no fatalities attributed to TRAEs (Table 4).
Among the 88 patients with primary BRAF mutations, 65 (73.9%) patients carried V600E-mutated and 23 (26.1%) carried non-V600E mutations. Of those 65 advanced or metastatic patients, 49 patients had received systematic treatment, in which the first-line regimen included targeted therapy (32/49, 65.3%), immunotherapy (11/49, 22.4%), and chemotherapy alone (2/49, 4.1%) or in combination with bevacizumab (4/49, 8.2%). Among them, 22 (44.9%) patients were primary BRAF/EGFR co-mutated, and 27 (55.1%) were non-EGFR co-mutated group. In the BRAF/EGFR co-mutated group, 95.5% (21/22) received targeted therapy, and 4.5% (1/22) underwent chemotherapy with bevacizumab. In the non-EGFR co-mutated group, first-line regimens included targeted therapy (40.7%, 11/27), immunotherapy (40.7%, 11/27), chemotherapy alone (11.1%, 3/27), and chemotherapy with bevacizumab (7.4%, 2/27). The median follow-up time was 18.0 months (range: 3.0-70.0 months). The mPFS of first-line treatments was 18.0 months (95% CI: 10.3-25.7 months), and mOS was 49.7 months (NR) (Figures 3A, B). Seven primary BRAF-mutated patients (1 with EGFR amplification) received dabrafenib and trametinib as the first-line treatment. The mPFS was 9.0 months (95% CI: 3.8-14.2 months). The mPFS of BRAF V600E and non-V600E patients was 24.0 months and 9.0 months, respectively. Regardless of the treatment line, the mPFS of 10 patients receiving dabrafenib and trametinib was 8.6 months (95% CI: 5.5–11.7 months).
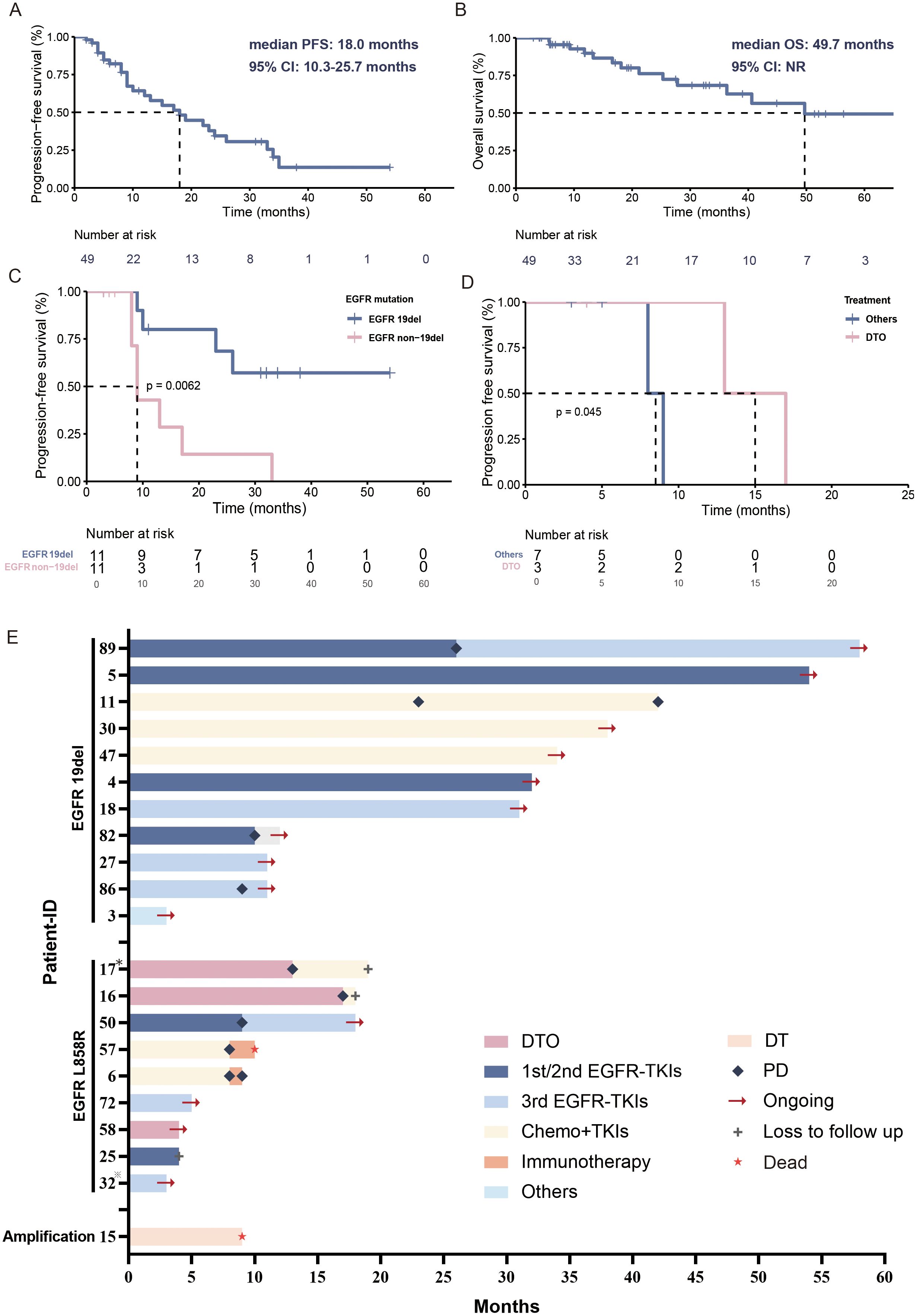
Figure 3. Treatment overview of primary BRAF-mutated patients. (A) Kaplan–Meier estimates of PFS of primary BRAF-mutated NSCLC patients. (B) Kaplan–Meier estimates of OS of primary BRAF-mutated NSCLC patients. (C) Kaplan–Meier estimates of PFS of primary BRAF/EGFR 19del co-mutated and BRAF/EGFR non-19del co-mutated NSCLC patients. (D) Kaplan–Meier estimates of PFS of primary BRAF/EGFR non-19del co-mutated NSCLC patients who received TKIs. (E) Swimming plot of treatment processes in primary BRAF/EGFR co-mutated NSCLC patients. *Patient 17 with EGFR L858R+T790M mutation; *Patient 32 with EGFR L858R+19del mutation; DTO, dabrafenib+ trametinib + osimertinib; PD, progressive disease; Others, EGFR TKIs/EGFR TKIs+chemotherapy/dabrafenib+ trametinib.
We further investigated the clinical outcome of those primary BRAF/EGFR co-mutated patients (Figure 3E). The BRAF/EGFR 19del co-mutated patients had better PFS than non-19del (NR versus 9.0 months, 95% CI: 7.7-10.3 months, p=0.0062) (Figure 3C).
For these BRAF/EGFR non-19del patients, three patients (2 with EGFR L858R and 1 with EGFR T790M+L858R) received dabrafenib and trametinib plus osimertinib (triple-targeted therapy). The mPFS was 12.0 months and ORR was 100% (3/3). Other patients treated with EGFR TKIs (4/7, 57.1%), chemotherapy+EGFR TKIs (2/7, 28.6%), and dabrafenib+ trametinib (1/7, 14.3%) as the first-line regimen. Their mPFS and ORR were 8.0 months and 71.4% (5/7) (Figure 3D). Despite the small number of patients and short follow-up time, this triple-targeted regimen showed even better efficacy than other EGFR TKI-based regimes. Patient 1 received chemotherapy combined with bevacizumab, so we excluded him from the Kaplan-Meier analysis to observe the treatment prognosis of TKIs.
The adverse events associated with this triple-targeted regimen included one case of alanine aminotransferase increase (grade 3), aspartate aminotransferase increase (grade 3), rash (grade 2), and peripheral edema (grade 1); one case of dyspnoea (grade 1); and one case of pyrexia (grade 2) (Table 4).
Patient 17 was a 48-year-old never-smoker male diagnosed with stage IVb lung adenocarcinoma with rib metastasis. EGFR L858R/T790M and BRAF V600E mutations were identified by ARMS PCR (Figure 4A). The triple treatment achieved a PFS of 12.2 months, with the best clinical efficacy being PR. The serum carcinoembryonic antigen (CEA) changes are shown in Figure 4B. Twelve months later, he had the progression of brain metastases. A rebiopsy was performed, and EGFR L858R/T790M and c-met mutations were detected. Based on the gene status alterations, treatment was changed to the third-generation EGFR TKI almolertinib and the multi-target tyrosine kinase inhibitor anlotinib. Two months later, due to intestinal perforation, a jejunectomy was performed. Postoperative pathology indicated metastasis of lung cancer, with genetic mutations showing EGFR L858R/T790M, PIK3CA C901F, and TP53 H193D. Additionally, liver metastasis was observed, thus subsequent treatment was changed to chemotherapy combined with almolertinib.
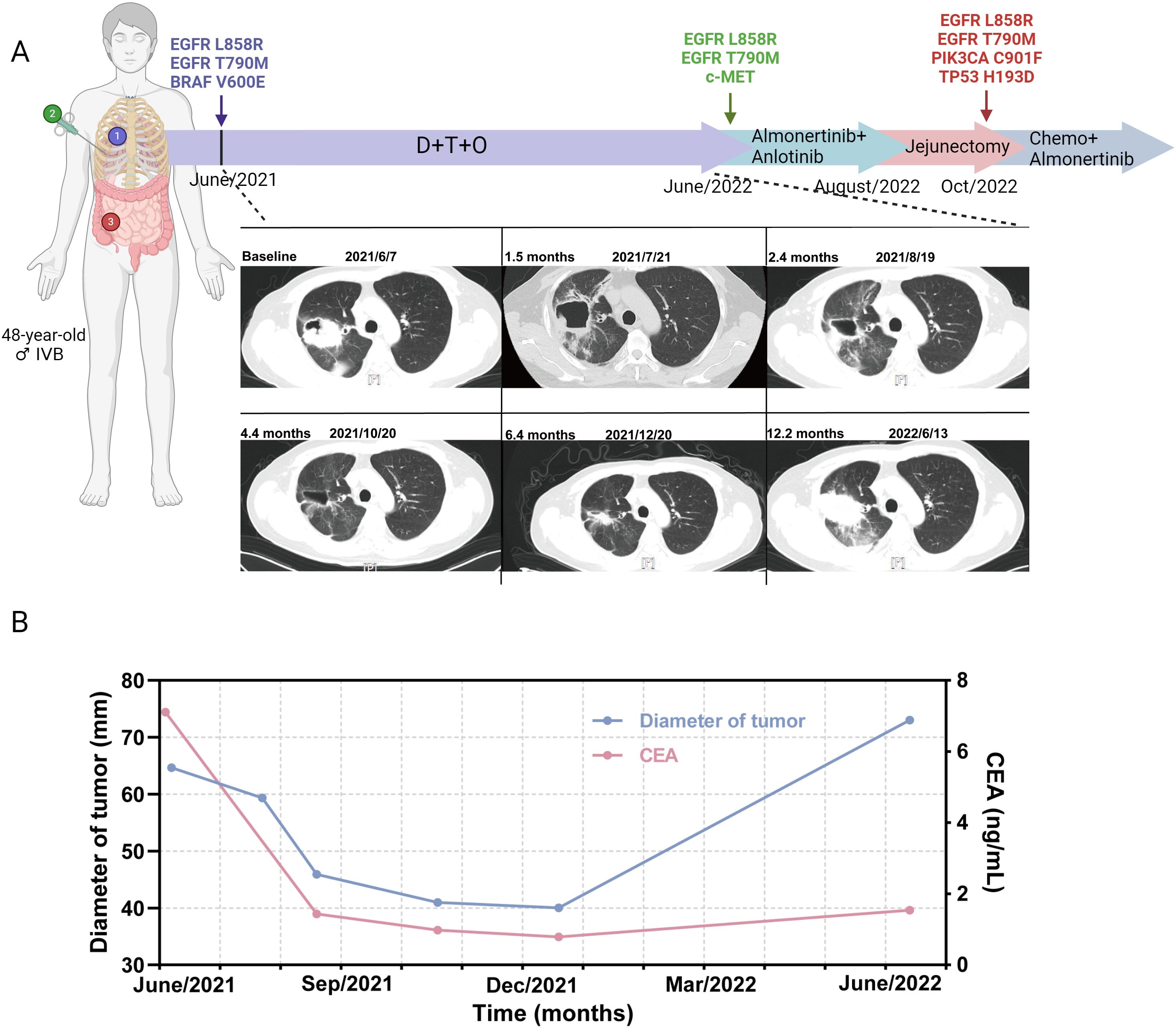
Figure 4. Treatment timeline and CT scans of a stage IVb adenocarcinoma patient receiving dabrafenib and trametinib plus osimertinib. (A) Treatment timeline and CT scans of the patient. (B) Tumor diameter (mm) changes and serum CEA changes during DTO treatment. CEA, carcinoembryonic antigen; D, dabrafenib; T, trametinib; O, osimertinib.
To our knowledge, this is the first retrospective study to explore the clinical characteristics, molecular profiles, and treatment outcomes of primary and acquired BRAF-mutated patients with concomitant EGFR mutations. BRAF V600E has been identified as an oncogenic driver gene in NSCLC (18, 28), and the frequency of EGFR and BRAF co-mutation is 0.91% (20) in NSCLC patients. Moreover, multiple resistance mechanisms of EGFR TKIs for activating EGFR-mutant NSCLC patients have been elucidated, with BRAF being one of them (24, 29). Nowadays, several case reports and only a few retrospective studies have shown that dabrafenib and trametinib plus osimertinib might be an appropriate treatment option for acquired BRAF-mutated NSCLC patients. However, the clinical efficacy and safety of this triple-regimen therapy still need more clinical study. Moreover, whether this therapy regimen has better clinical efficacy on primary BRAF/EGFR co-mutated patients has never been reported.
In this study, the clinical characteristics, molecular profiles, therapeutic strategies, and prognosis of primary and acquired BRAF-mutated patients were analyzed. When we analyzed the clinicopathologic features, we observed several differences in the clinical features between primary and acquired BRAF-mutated cohorts. It has been shown that EGFR mutations are more frequent in non-smokers and females (30). In this study, primary BRAF-mutated patients were more likely to be current/former smokers, males, elderly, more complex histological types, and higher PD-L expression compared to acquired BRAF-mutated patients. Similar differences in clinical characteristics were observed between primary BRAF/EGFR co-mutated patients and primary BRAF/EGFR non-co-mutated patients. The primary BRAF/EGFR co-mutated patients showed similar demographics and clinical characteristics to acquired BRAF/EGFR co-mutated patients resistant to EGFR TKIs. Previous studies found that pre-existing T790M may exist in many TKI-naive NSCLCs, and may become the dominant tumor population as a result of drug pressure in EGFR TKIs resistant NSCLC patients (31). Based on this theory, primary BRAF/EGFR co-mutated patients have a higher propensity to develop dominant BRAF-mutated tumor clones following resistance to EGFR TKIs treatment. This indicated that the primary and acquired BRAF/EGFR co-mutated patients may have a common origin.
A previous large multi−center study had reported that 28 primary BRAF-mutated patients (11.8%, 28/238) had concomitant sensitizing EGFR mutations (32). In a retrospective study (33), which analyzed 53 BRAF-V600E mutant advanced NSCLC patients, 9 patients (9/53, 17.0%) had concomitant EGFR mutation, with 5 EGFR 19del, 3 EGFR L858R and 1 EGFR T790M. We observed that primary BRAF-mutated patients exhibited a high prevalence of EGFR mutations (27/88, 30.7%), indicating a significant co-mutation rate. This finding emphasizes the critical need to incorporate these co-mutations into first-line clinical treatment strategies. In vitro study (26) showed that the triple-targeted therapy of osmertinib + darafenib+ trametinib has a lower IC50 value and stronger anti-tumor effect compared with the two targeted combination regimens of osmertinib+ vemurafenib and osmertinib+ encorafenib+ cetuximab, as well as the combination of pemetrexed+ carboplatin. The tumor growth inhibition rates of these four regimens were 99.36%, 99.25%, 98.92%, and 62.83%, respectively. Hence, the triple regimen has shown good antitumor efficacy. Moreover, a Phase Ib study (34) suggests that multi-segment blockade of the RAS/RAF/ERK pathway may offer significant antitumor efficacy in patients with advanced and metastatic KRAS or BRAF-mutant non-small cell lung cancer. However, the sample size was small and the data were therefore not representative of this patient group. The effectiveness of this therapy regimen in this patient group requires further in-depth research.
Previous studies have revealed that patients with EGFR 19 deletions are associated with longer PFS and OS than patients with L858R after EGFR TKIs (6–8), and this may be due to the different phosphotyrosine patterns between the two mutations (35). Our study confirmed this result, showing that BRAF concomitant EGFR 19del patients had the longest PFS. Even with concomitant BRAF mutations, patients with EGFR 19del mutations exhibited better prognoses compared to those with non-19del mutations. In real-world practice, first-line treatment for BRAF/EGFR 19del mutated patients predominantly involves EGFR TKI-based therapies without selecting BRAF-targeted agents. Despite the insufficient follow-up period for first-line PFS data, favorable treatment outcomes are still observed. Further investigation is needed to determine if a triple-targeted regimen would offer superior efficacy compared to current treatments. For BRAF/EGFR non-19del patients, triple-targeted therapy demonstrates better outcomes than other treatment strategies (EGFR TKIs, EGFR TKIs+ chemotherapy, or dabrafenib+ trametinib). Therefore, triple therapy might be a more promising treatment approach for BRAF/EGFR non-19del patients.
However, oncogenic driver genes were previously thought to be mutually exclusive (36). BRAF and EGFR are mutually resistant mechanisms to EGFR TKIs or BRAF TKIs (29, 37). And BRAF mutations are considered a negative prognostic factor (12, 38). Nevertheless, research has shown that patients harboring both EGFR and BRAF mutations can benefit from treatment with EGFR TKIs and BRAF TKIs (39). This indicates that these patients might have varied responses to EGFR TKIs and BRAF TKIs. We hypothesize that the effectiveness of these drugs may depend on the activated oncogene abundance when the tumor is driven by two distinct driver genes, detecting the abundance of EGFR mutations and BRAF mutations is crucial for optimizing the selection of TKIs in clinical practice. Additionally, liquid biopsy (40) can effectively address the issue of limited biopsy sites and tumor heterogeneity in advanced patients, and can also serve as an additional option for screening gene mutations in patients, especially for patients who are unable to tolerate tissue biopsy or have poor-quality samples. Therefore, for gene mutations with high BRAF mutation abundance and relatively poor prognosis EGFR mutations, triple therapy can be considered as a treatment option.
In addition, we compared the EGFR mutation genotypes between the primary and acquired BRAF-mutated groups. The results indicated that the genotypes of coexisting EGFR mutations differed, with the acquired group predominantly having a complex genotype of EGFR mutations and more dual EGFR mutations (5/15, 33.3%) compared to primary BRAF/EGFR co-mutated patients. Due to the involvement of both on-target EGFR kinase domain mutations and bypass pathway activations in the resistance mechanisms following EGFR TKI treatment (24), the presence of these two mechanisms has been rarely reported. Our study demonstrated the existence of both mechanisms with a relatively high proportion. Therefore, clinical treatment needs to address both resistance mechanisms simultaneously. In the emergence of acquired resistance to EGFR TKIs, BRAF mutations have been identified as a potential alternative mechanism (29), but the subsequent therapy reports are still limited. Our study included 15 acquired BRAF-mutated patients after EGFR-TKI treatments. Previous studies have shown dabrafenib and trametinib plus osimertinib showed substantial efficacy among these EGFR TKIs resistant BRAF V600E mutant NSCLC patients, with PFS ranging from 2 to 13 months (41). 5 patients in our study received this triple-regimen treatment after acquiring BRAF mutations. And the mPFS of this treatment was 16.0 months (range: 2-16 months) until July 2024. One patient achieved a PFS of 16 months, and the adverse side effects were manageable. Consistent with previous studies, these results suggest the treatment can be an appropriate option for these patients.
Our study has some limitations. The first is due to the single-center retrospective nature of this study which may introduce a selection bias. The second is the small size of BRAF-mutated patients due to the low prevalence of BRAF mutations in Asian people (0.5%-1.7%) (15, 16), which hindered the possibility of stratified analysis to some extent. Third, patients were mainly detected by the 10-genes ARMS-PCR (70.5%) rather than NGS in the baseline, so the proportion of BRAF V600E mutation (73.9%) in the primary BRAF-mutated cohort was higher than other studies (50%-56.8%) (12, 13). Additionally, the use of PCR-based methods may have introduced false-negative results, as these techniques are less comprehensive compared to NGS in detecting low-frequency or non-canonical mutations. Finally, the co-mutations analyzed in our study are mainly oncogenic driver genes, the non-driven mutations still need fully investigated. Testing for more potentially predictive and prognostic alterations is expected in future study designs.
Our study indicated the primary and acquired BRAF-mutated NSCLC patients had a high frequency of coexisting EGFR mutations. The primary and acquired BRAF/EGFR co-mutated patients showed similar clinical characteristics and may have a common origin. Triple-targeted therapy (dabrafenib, trametinib plus 3rd EGFR TKIs) could be considered the preferential treatment options for acquired BRAF/EGFR co-mutated and primary BRAF/EGFR non-19del co-mutated NSCLC patients. As for the primary BRAF/EGFR 19del co-mutated patients, the preferred first-line treatments still are EGFR TKIs-based target therapies in real-world clinical practice. Prospective randomized controlled clinical trials or larger sample-sized real-world studies are needed to confirm the effectiveness of triple-targeted therapy in primary BRAF/EGFR 19del co-mutated patients.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by institutional review board of Ruijin Hospital (Approval No. 2024-172). The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from a by- product of routine care or industry. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
XF: Conceptualization, Data curation, Formal Analysis, Writing – original draft. RZ: Data curation, Formal Analysis, Writing – original draft. ML: Conceptualization, Data curation, Writing – original draft. XC: Data curation, Writing – review & editing. ZX: Data curation, Writing – review & editing. YH: Data curation, Writing – review & editing. ZB: Data curation, Writing – review & editing. XS: Data curation, Writing – review & editing. JiZ: Data curation, Writing – review & editing. LZ: Data curation, Writing – review & editing. JuZ: Data curation, Writing – review & editing. BG: Data curation, Writing – review & editing. LD: Conceptualization, Supervision, Writing – review & editing. YX: Conceptualization, Investigation, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Key R&D Program of China (Grant No.2018YFC1311902), Shanghai Key Laboratory of Emergency Prevention, Diagnosis and Treatment of Respiratory Infectious Diseases (20dz2261100), Shanghai Municipal Key Clinical Specialty(shslczdzk02202), and the National Natural Science Foundation of China (No. 81672271).
We express our gratitude to Yimin Wu from the Information Department of Ruijin Hospital for providing invaluable support in data entry. The authors also thank the Shanghai Municipal Hospital Respiratory and Critical Care Medicine Specialist Alliance.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1514653/full#supplementary-material
EGFR, epidermal growth factor receptor; BRAF and V-Raf murine sarcoma viral oncogene homolog; NSCLC, non-small cell lung cancer; TKIs, tyrosine kinase inhibitors; IQR, interquartile range; PFS, progression-free survival; OS, overall survival; ORR, objective response rate; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ECOG, Eastern Cooperative Oncology Group; PS, performance status.
1. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
2. Ganti AK, Klein AB, Cotarla I, Seal B, Chou E. Update of incidence, prevalence, survival, and initial treatment in patients with non-small cell lung cancer in the US. JAMA Oncol. (2021) 7:1824–32. doi: 10.1001/jamaoncol.2021.4932
3. Boustany Y, Laraqui A, El Rhaffouli H, Bajjou T, El Mchichi B, El Anaz H, et al. Prevalence and patterns of EGFR mutations in non-small cell lung cancer in the middle east and north africa. Cancer Control. (2022) 29:1–9. doi: 10.1177/10732748221129464
4. Gahr S, Stoehr R, Geissinger E, Ficker J H, Brueckl W M, Gschwendtner A, et al. EGFR mutational status in a large series of Caucasian European NSCLC patients: data from daily practice. Br J Cancer. (2013) 109:1821–8. doi: 10.1038/bjc.2013.511
5. Yan K. Osimertinib in EGFR-mutated advanced NSCLC. N Engl J Med. (2020) 382:1863. doi: 10.1056/NEJMc2001514
6. Sheng M, Wang F, Zhao Y, Li S, Wang X, Shou T, et al. Comparison of clinical outcomes of patients with non-small-cell lung cancer harbouring epidermal growth factor receptor exon 19 or exon 21 mutations after tyrosine kinase inhibitors treatment: a meta-analysis. Eur J Clin Pharmacol. (2016) 72:1–11. doi: 10.1007/s00228-015-1966-0
7. Won YW, Han JY, Lee GK, Park SY, Lim KY, Yoon KA, et al. Comparison of clinical outcome of patients with non-small-cell lung cancer harbouring epidermal growth factor receptor exon 19 or exon 21 mutations. J Clin Pathol. (2011) 64:947–52. doi: 10.1136/jclinpath-2011-200169
8. Riely GJ, Pao W, Pham D, Li AR, Rizvi N, Venkatraman ES, et al. Clinical course of patients with non-small cell lung cancer and epidermal growth factor receptor exon 19 and exon 21 mutations treated with gefitinib or erlotinib. Clin Cancer Res. (2006) 12:839–44. doi: 10.1158/1078-0432.Ccr-05-1846
9. Comprehensive genomic characterization of squamous cell lung cancers. Nature. (2012) 489:519–25. doi: 10.1038/nature11404
10. Comprehensive molecular profiling of lung adenocarcinoma. Nature. (2014) 511:543–50. doi: 10.1038/nature13385
11. Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. (2002) 417:949–54. doi: 10.1038/nature00766
12. Marchetti A, Felicioni L, Malatesta S, Grazia Sciarrotta M, Guetti L, Chella A, et al. Clinical features and outcome of patients with non-small-cell lung cancer harboring BRAF mutations. J Clin Oncol. (2011) 29:3574–9. doi: 10.1200/jco.2011.35.9638
13. Cardarella S, Ogino A, Nishino M, Butaney M, Shen J, Lydon C, et al. Clinical, pathologic, and biologic features associated with BRAF mutations in non-small cell lung cancer. Clin Cancer Res. (2013) 19:4532–40. doi: 10.1158/1078-0432.Ccr-13-0657
14. Lim GHT, Balbi KJ, Poskitt B, Bennett P, Moore DA. Prevalence and breakdown of non-small cell lung cancer BRAF driver mutations in a large UK cohort. Lung Cancer. (2022) 173:71–4. doi: 10.1016/j.lungcan.2022.09.008
15. Li S, Li L, Zhu Y, Huang C, Qin Y, Liu H, et al. Coexistence of EGFR with KRAS, or BRAF, or PIK3CA somatic mutations in lung cancer: a comprehensive mutation profiling from 5125 Chinese cohorts. Br J Cancer. (2014) 110:2812–20. doi: 10.1038/bjc.2014.210
16. Ding X, Zhang Z, Jiang T, Li X, Zhao C, Su B, et al. Clinicopathologic characteristics and outcomes of Chinese patients with non-small-cell lung cancer and BRAF mutation. Cancer Med. (2017) 6:555–62. doi: 10.1002/cam4.1014
17. Hyman DM, Puzanov I, Subbiah V, Faris JE, Chau I, Blay JY, et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med. (2015) 373:726–36. doi: 10.1056/NEJMoa1502309
18. Planchard D, Kim TM, Mazieres J, Quoix E, Riely G, Barlesi F, et al. Dabrafenib in patients with BRAF(V600E)-positive advanced non-small-cell lung cancer: a single-arm, multicentre, open-label, phase 2 trial. Lancet Oncol. (2016) 17:642–50. doi: 10.1016/s1470-2045(16)00077-2
19. Planchard D, Smit EF, Groen HJM, Mazieres J, Besse B, Helland Å, et al. Dabrafenib plus trametinib in patients with previously untreated BRAF(V600E)-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol. (2017) 18:1307–16. doi: 10.1016/s1470-2045(17)30679-4
20. Peng P, Lv G, Hu J, Wang K, Lv J, Guo G. Co-mutations of epidermal growth factor receptor and BRAF in Chinese non-small cell lung cancer patients. Ann Transl Med. (2021) 9:1321. doi: 10.21037/atm-21-3570
21. Wang Z, Cheng Y, An T, Gao H, Wang K, Zhou Q, et al. Detection of EGFR mutations in plasma circulating tumour DNA as a selection criterion for first-line gefitinib treatment in patients with advanced lung adenocarcinoma (BENEFIT): a phase 2, single-arm, multicentre clinical trial. Lancet Respir Med. (2018) 6:681–90. doi: 10.1016/s2213-2600(18)30264-9
22. Westover D, Zugazagoitia J, Cho BC, Lovly CM, Paz-Ares L. Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors. Ann Oncol. (2018) 29:i10–9. doi: 10.1093/annonc/mdx703
23. Ricordel C, Friboulet L, Facchinetti F, Soria JC. Molecular mechanisms of acquired resistance to third-generation EGFR-TKIs in EGFR T790M-mutant lung cancer. Ann Oncol. (2018) 29:i28–37. doi: 10.1093/annonc/mdx705
24. Cooper AJ, Sequist LV, Lin JJ. Third-generation EGFR and ALK inhibitors: mechanisms of resistance and management. Nat Rev Clin Oncol. (2022) 19:499–514. doi: 10.1038/s41571-022-00639-9
25. Meng P, Koopman B, Kok K, Ter Elst A, Schuuring E, van Kempen LC, et al. Combined osimertinib, dabrafenib and trametinib treatment for advanced non-small-cell lung cancer patients with an osimertinib-induced BRAF V600E mutation. Lung Cancer. (2020) 146:358–61. doi: 10.1016/j.lungcan.2020.05.036
26. Chengdi Weng KT, Jin S, Zhou C, Yao Y, Su J-w, Chen H, et al. Triple-targeted therapy of dabrafenib, trametinib and osimertinib for the treatment of acquired BRAF V600E mutation after progression on EGFR-TKIs in advanced EGFR-mutant NSCLC. ESMO Asia. (2023) 34:560P. doi: 10.1016/j.annonc.2023.10.638
27. Wang S, Qu X, Cao L, Hu X, Hou K, Liu Y, et al. Assessment of nine driver gene mutations in surgically resected samples from patients with non-small-cell lung cancer. Cancer Manag Res. (2020) 12:4029–38. doi: 10.2147/cmar.S250822
28. Planchard D, Besse B, Groen HJM, Souquet PJ, Quoix E, Baik CS, et al. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol. (2016) 17:984–93. doi: 10.1016/s1470-2045(16)30146-2
29. Ho CC, Liao WY, Lin CA, Shih JY, Yu CJ, Yang JC. Acquired BRAF V600E mutation as resistant mechanism after treatment with osimertinib. J Thorac Oncol. (2017) 12:567–72. doi: 10.1016/j.jtho.2016.11.2231
30. Dearden S, Stevens J, Wu YL, Blowers D. Mutation incidence and coincidence in non small-cell lung cancer: meta-analyses by ethnicity and histology (mutMap). Ann Oncol. (2013) 24:2371–6. doi: 10.1093/annonc/mdt205
31. Inukai M, Toyooka S, Ito S, Asano H, Ichihara S, Soh J\ , et al. Presence of epidermal growth factor receptor gene T790M mutation as a minor clone in non-small cell lung cancer. Cancer Res. (2006) 66:7854–8. doi: 10.1158/0008-5472.Can-06-1951
32. Lin Q, Zhang H, Ding H, Qian J, Lizaso A, Lin J, et al. The association between BRAF mutation class and clinical features in BRAF-mutant Chinese non-small cell lung cancer patients. J Transl Med. (2019) 17:298. doi: 10.1186/s12967-019-2036-7
33. Qu J, Shen Q, Li Y, Kalyani FS, Liu L, Zhou J, et al. Clinical characteristics, co-mutations, and treatment outcomes in advanced non-small-cell lung cancer patients with the BRAF-V600E mutation. Front Oncol. (2022) 12:911303. doi: 10.3389/fonc.2022.911303
34. Planchard D, Wolf J, Solomon B, Sebastian M, Wermke M, Heist RS, et al. A phase Ib study of the combination of naporafenib with rineterkib or trametinib in patients with advanced and metastatic KRAS- or BRAF-mutant non-small cell lung cancer. Lung Cancer. (2024) 197:107964. doi: 10.1016/j.lungcan.2024.107964
35. Okabe T, Okamoto I, Tamura K, Terashima M, Yoshida T, Satoh T, et al. Differential constitutive activation of the epidermal growth factor receptor in non-small cell lung cancer cells bearing EGFR gene mutation and amplification. Cancer Res. (2007) 67:2046–53. doi: 10.1158/0008-5472.Can-06-3339
36. Skoulidis F, Heymach JV. Co-occurring genomic alterations in non-small-cell lung cancer biology and therapy. Nat Rev Cancer. (2019) 19:495–509. doi: 10.1038/s41568-019-0179-8
37. Spagnolo F, Ghiorzo P, Orgiano L, Pastorino L, Picasso V, Tornari E, et al. BRAF-mutant melanoma: treatment approaches, resistance mechanisms, and diagnostic strategies. Onco Targets Ther. (2015) 8:157–68. doi: 10.2147/ott.S39096
38. Chen M, Xu Y, Zhao J, Zhong W, Zhang L, Bi Y, et al. Concurrent driver gene mutations as negative predictive factors in epidermal growth factor receptor-positive non-small cell lung cancer. EBioMedicine. (2019) 42:304–10. doi: 10.1016/j.ebiom.2019.03.023
39. Wei XW, Deng JY, Xu CR, Chen ZH, Zhu DQ, Wu Q, et al. Characteristics of and treatment strategies for advanced EGFR-mutant NSCLC with concomitant BRAF variations. JTO Clin Res Rep. (2022) 3:100348. doi: 10.1016/j.jtocrr.2022.100348
40. Canale M, Pasini L, Bronte G, Delmonte A, Cravero P, Crinò L, et al. Role of liquid biopsy in oncogene-addicted non-small cell lung cancer. Transl Lung Cancer Res. (2019) 8:S265–s279. doi: 10.21037/tlcr.2019.09.15
Keywords: BRAF, EGFR, primary, acquired, non-small cell lung cancer, co-mutation
Citation: Feng X, Zeng R, Lyu M, Chen X, Xu Z, Hu Y, Bao Z, Sun X, Zhao J, Zhou L, Zhou J, Gao B, Dong L and Xiang Y (2025) Clinical and molecular characteristics, therapeutic strategies, and prognosis of non-small cell lung cancer patients harboring primary and acquired BRAF mutations. Front. Oncol. 15:1514653. doi: 10.3389/fonc.2025.1514653
Received: 21 October 2024; Accepted: 14 March 2025;
Published: 02 April 2025.
Edited by:
Haixia Zhu, Nantong Tumor Hospital, ChinaReviewed by:
Songxiao Xu, University of Chinese Academy of Sciences, ChinaCopyright © 2025 Feng, Zeng, Lyu, Chen, Xu, Hu, Bao, Sun, Zhao, Zhou, Zhou, Gao, Dong and Xiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Xiang, eGlhbmd5aWh0QDE2My5jb20=; Lei Dong, REwxMTk2OEByamguY29tLmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.