
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 21 February 2025
Sec. Cancer Imaging and Image-directed Interventions
Volume 15 - 2025 | https://doi.org/10.3389/fonc.2025.1511595
Pulmonary sclerosing pneumocytoma (PSP) is a relatively rare benign lung tumor, and it is difficult to obtain an accurate diagnosis before surgery. Herein, we present a case of 34-year-old woman who came to our hospital for medical help due to cough and sputum for one month. She underwent a chest computed tomography (CT) scan which revealed a circular soft tissue density shadow in the upper lobe of the left lung. A needle biopsy was subsequently performed which revealed a probable lung carcinoid. To further evaluate the nature of the mass and determine a treatment plan, the patient subsequently underwent dual nuclide tracer including fluorine-18 labeled fluorodeoxyglucose (18F-FDG) and gallium-68 labeled 1, 4, 7, 10-tetraazacyclododecane-1, 4, 7,10-tetraaceticacid -D-Phel-Tyr3-Thr8-OC (68Ga-DOTATATE) PET/CT imaging. The results showed that the lession presented increased both 18F-FDG and 68Ga-DOTATATE uptake, suggesting a neuroendocrine tumor. However, postoperative pathology confirmed that the lesion was PSP. Our case study suggests that PSP may presents varying degrees of increased 18F-FDG and 68Ga-DOTATATE uptake on positron emission tomography (PET)/CT imaging, which should be considered as one of the differential diagnoses for lung carcinoids.
Pulmonary sclerosing pneumocytoma (PSP) was previously thought to originate from endothelial and vascular tissue and was called sclerosing hemangioma. However, it was later shown to originate in type II alveolar epithelial cells, so in 2015 the World Health Organization renamed it PSP (1). PSP is most common in middle-aged women in Asia, where the incidence of women is five times that of men (2). PSP usually has no obvious clinical symptoms and is usually found by chance during routine imaging examinations. Some patients could complain of cough and chest and back pain, which may be caused by tumor compression of surrounding tissues (3). Due to the relative rarity of PSP and the non-specific clinical symptoms, it is difficult to obtain an accurate diagnosis before surgery, especially when the imaging findings are atypical. Herein, we present the diagnosis and treatment of a patient with PSP, focusing on the presentation of it on dual nuclide tracers including fluorine-18 labeled fluorodeoxyglucose (18F-FDG) and gallium-68 labeled 1, 4, 7, 10-tetraazacyclododecane-1, 4, 7,10-tetraaceticacid -D-Phel-Tyr3-Thr8-OC (68Ga-DOTATATE) positron emission tomography (PET)/computed tomography (CT) to increase the understanding of this relatively rare benign lung tumor.
A 34-year-old woman came to our hospital for medical help due to cough and sputum for one month. Now the patient has no other discomfort except cough and sputum. Physical examination revealed that no abnormal breathing sounds were heard in the patient’s lungs, and no positive signs such as tenderness, pleural friction sound and subcutaneous superficial lymph node enlargement were found. She and her family have denied any history of cancer or genetic disease, tuberculosis, hepatitis and other infectious or serious illnesses. Her blood routine, serum tumor markers and other laboratory results were negative. The patient underwent a chest CT scan, which showed a circular soft tissue density shadow in the upper lobe of the left lung, with spotted high-density calcifications visible inside. The lesion showed mild enhancement on contrast-enhanced CT scan (as shown in Figure 1). The patient subsequently underwent a CT-guided needle biopsy, which indicated the possibility of a neuroendocrine tumor, pulmonary carcinoid. To further evaluate the nature of the mass and determine a treatment plan, the patient subsequently underwent dual nuclide tracer including 18F-FDG (Figure 2) and 68Ga-DOTATATE PET/CT (Figure 3) imaging (The interval between the two tracers was 24 hours). The results revealed that the lession presented inhomogeneously increased both 18F-FDG and 68Ga-DOTATATE uptake. The peripheral area of the lesion showed higher uptake, while the central area of the lesion showed lower uptake, possibly due to necrosis. In addition, no hot spot lesions were observed in the rest of the body. Based on these imaging findings, the patient was initially suspected to have a carcinoid. Because the lesion was isolated and localized, the patient underwent surgical resection of the mass after a series of evaluations. Hematoxylin eosin staining (as shown in Figure 4) showed matrixes composed of circular cells within the tumor, with epithelial structures composed of cubic cells covering the surface, and some gaps filled with red blood cells. Immunohistochemical results showed that the tumor cells positively expressed epithelial membrane antigen (EMA), thyroid transcription factor 1 (TTF1), NapsinA (epithelial cells) and cytokeratin 7 (epithelial cells), while negatively expressed chromogranin A (CgA) and synaptophysin (Syn). Based on the morphology of the tumor under the microscope and immunohistochemical findings, the patient was ultimately diagnosed with PSP. Due to the benign tumor nature of PSP, the patient was discharged after 3 days of anti-inflammatory treatment following surgery. The patient has been followed for 9 months and she has not complained of any discomfort.
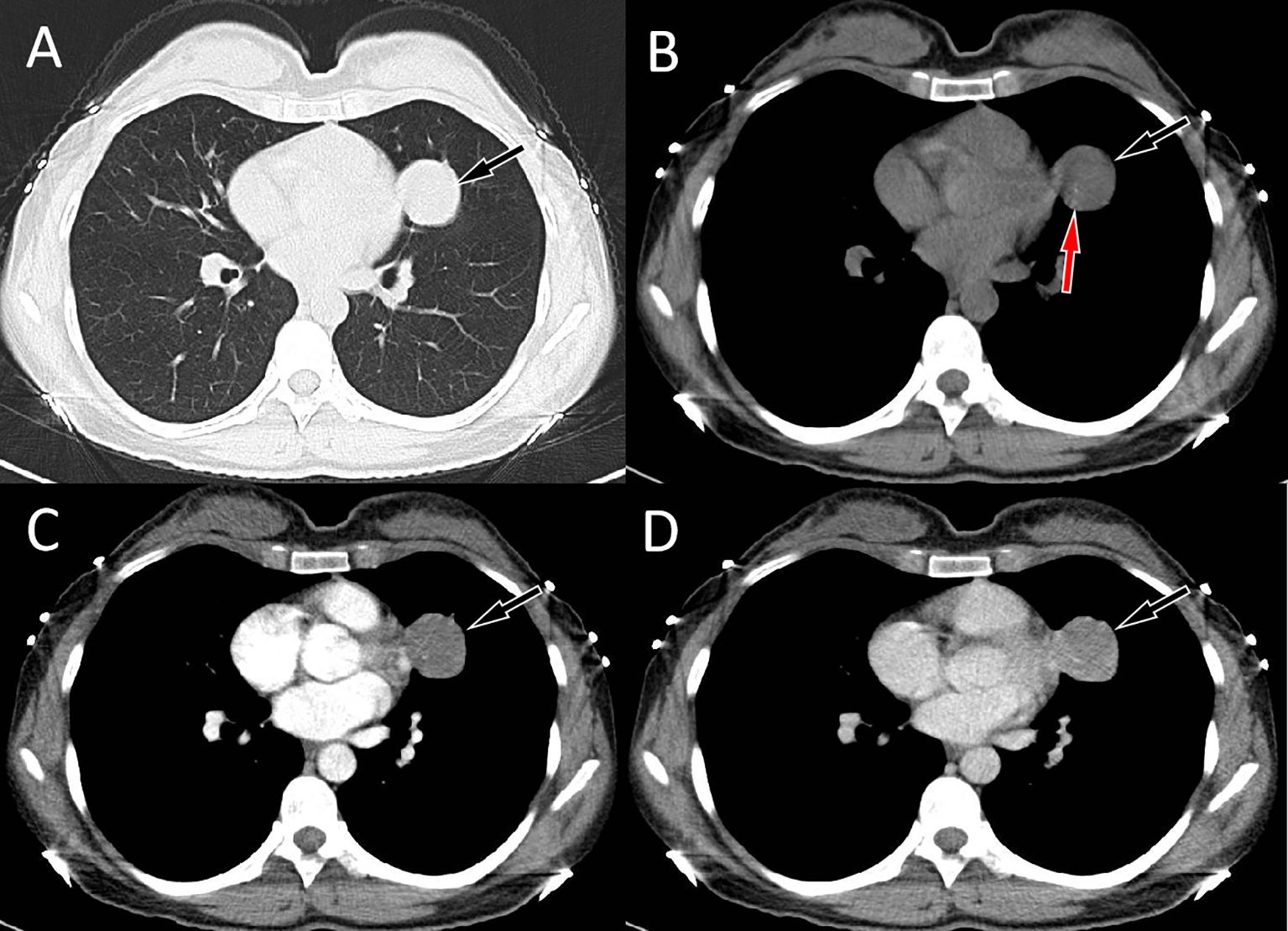
Figure 1. Chest computed tomography (CT) pulmonary window (A) and mediastinal window (B) revealed a soft tissue density mass (black arrows) about 3.4 cm×3.2 cm in size with punctate calcification (red arrow) can be seen on the upper lobe of the left lung; In the arterial phase (C) of contrast-enhanced CT, the lesion showed mild uniform enhancement (arrow); and in the vein phase (D), the lesion showed continuous uniform enhancement (arrow), and the degree of enhancement increased compared with the arterial phase.
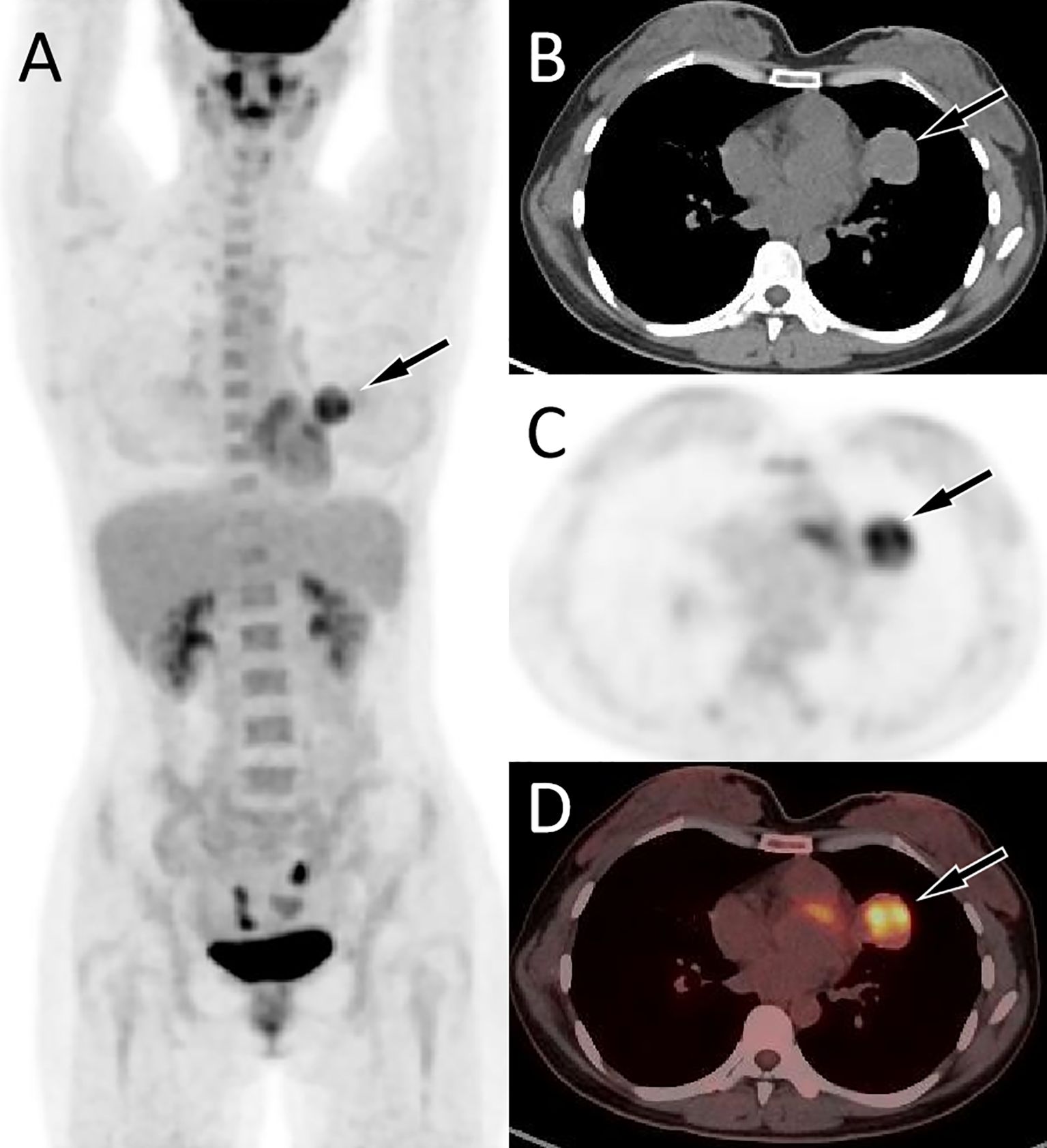
Figure 2. Fluorine-18 fluorodeoxyglucose (18F-FDG) positron emission tomography (PET)/CT imaging of the patient; The maximum intensity projection [MIP, (A)] showed an increased 18F-FDG uptake in the left upper lung (arrow). Axial CT (B) showed a soft tissue mass in the upper lobe of the left lung (arrow). The corresponding lesion presented increased 18F-FDG uptake on axial PET [(C), arrow] and PET/CT fusion [(D) arrow], with a maximum standardized uptake value (SUVmax) of 5.6.
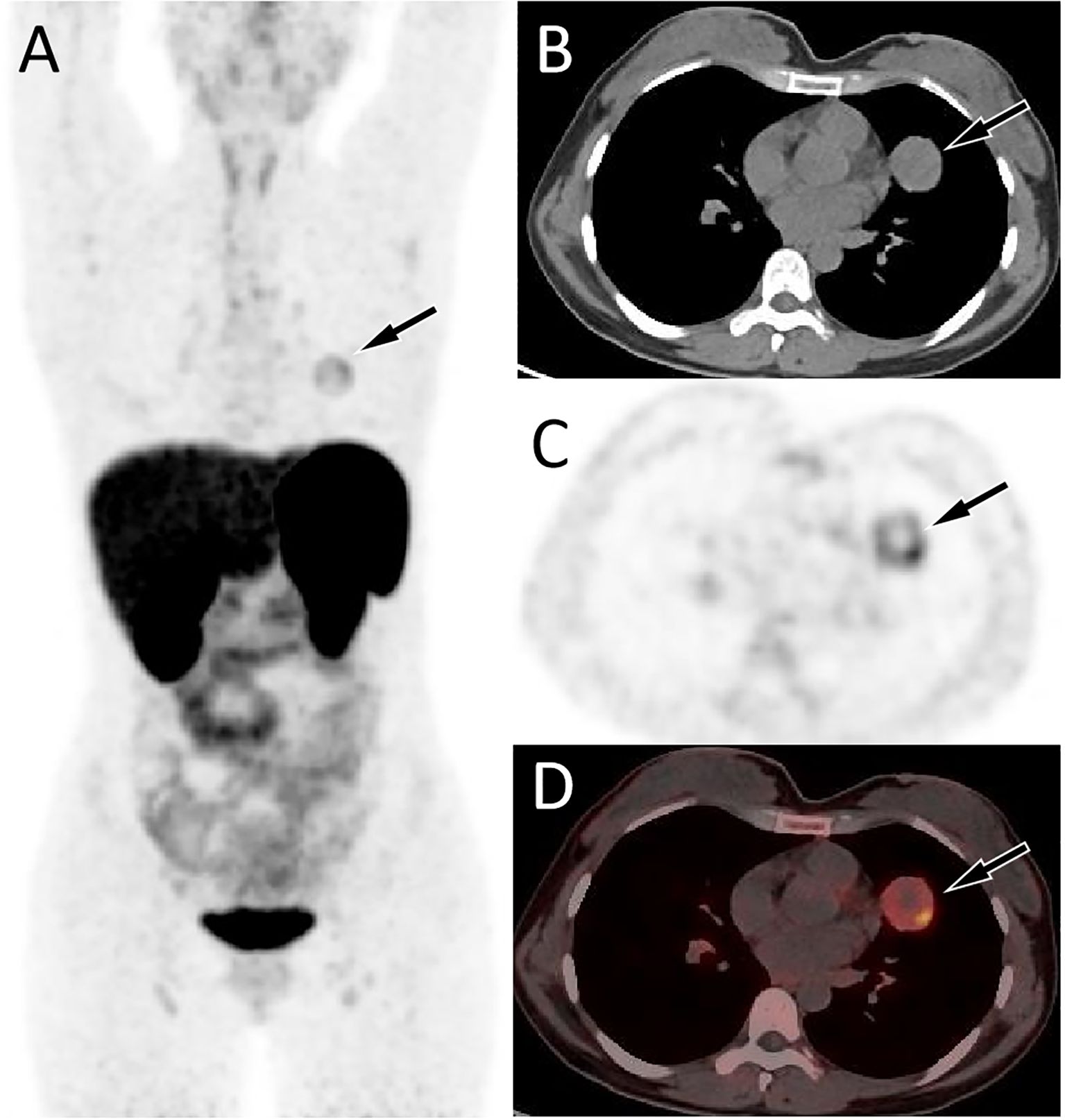
Figure 3. 68Ga-DOTATATE PET/CT imaging of the patient; The MIP (A) showed an increased 68Ga-DOTATATE uptake in the left upper lung (arrow). Axial CT (B), PET (C) and PET/CT fusion (D) showed this inhomogeneously increased focal uptake in the upper lobe of the left lung (arrow), with lower uptake areas in the center zome of the lesion due to necrotic changes, in the same location as the lesion shown on 18F-FDG PET/CT, with a SUVmax of 4.4.
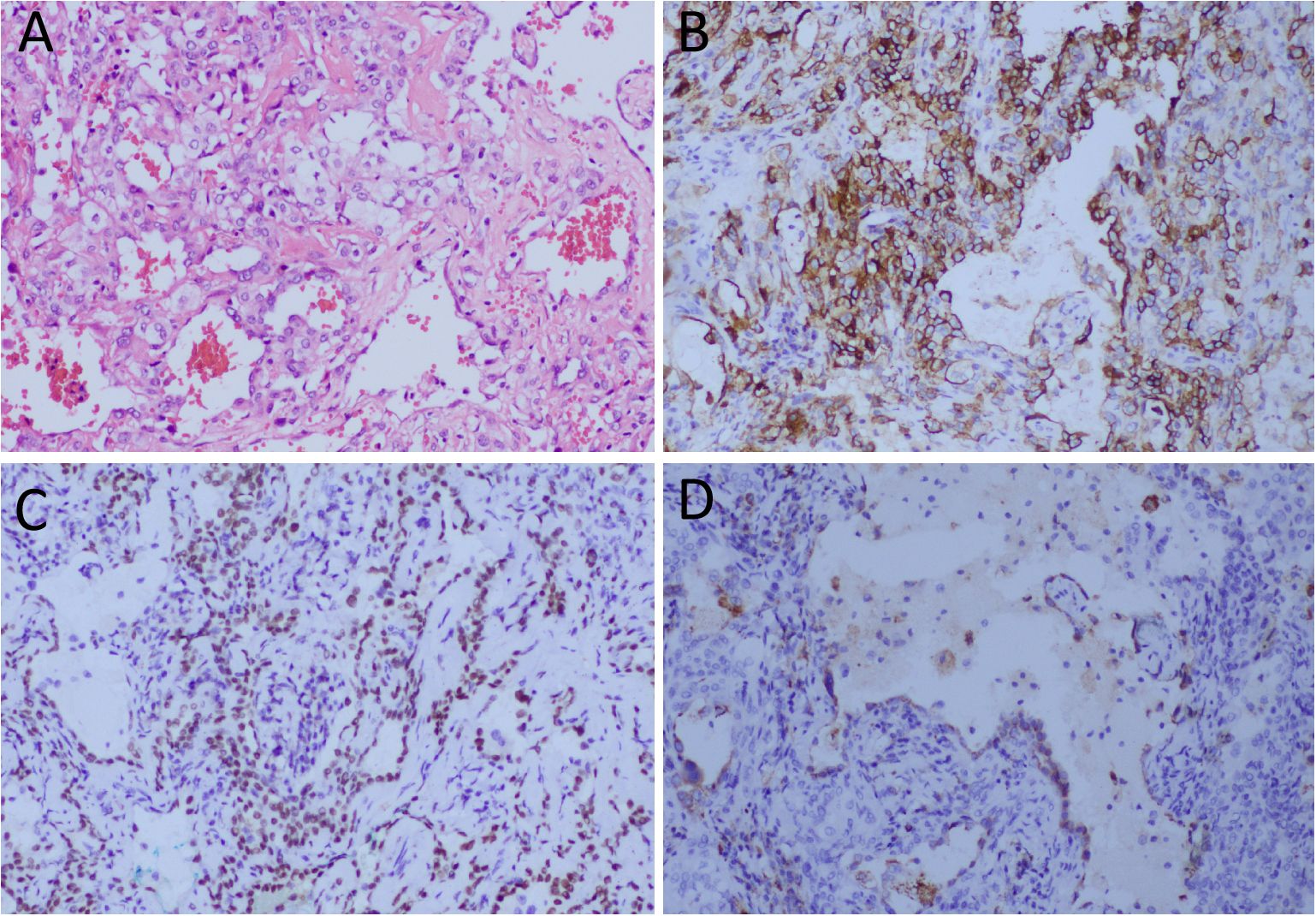
Figure 4. (A) Hematoxylin-eosin staining showing the cubed epithelial cells on the surface of the papillary structure and the rounded stromal cells between the solid area and the papillary structure are seen within in the tumor tissue. Immunohistochemical results showed that the tumor cells positively expressed EMA (B), TTF1 (C) and Napsin-A (D). Notes: SMA, epithelial membrane antigen; TTF1, thyroid transcription factor 1; All images are 100 times magnification.
PSP is a relatively rare benign tumor, accounting for only 2% to 3% of lung tumors, and is most common in women between the ages of 40 and 60 (4). Most PSP patients have no obvious clinical manifestations, and a few can present cough, sputum, shortness of breath, hemoptysis, chest pain, chest tightness and other atypical clinical manifestations (5). The patient we present is female, but younger than the preferred age for PSP. Due to cough and phlegm, she subsequently underwent a chest CT examination which revealed a lung nodule.
Previous imaging studies on PSP have focused on CT findings. Most of the CT findings of PSP show the imaging features of benign lung tumors with isolated masses or nodules with smooth edges and clear boundaries, which may be accompanied by calcification and cystic degeneration (6). Especially, the typical CT signs of PSP include vascular patching sign, air crescent sign, pulmonary artery main sign, tail sign, halo sign and so on (7, 8). On contrast enhanced CT scan, different degrees of enhancement can occur according to the different size of the lesion and the proportion of tissue components, and progressive and delayed continuous enhancement are the relative characteristics (9). The CT findings of the patient we reported showed a circular soft tissue density nodule with a small amount of calcification. The contrast enhanced scan showed moderate enhancement, which was characteristic of a benign lung tumor, but could not exclude the possibility of lung carcinoid cancer. Due to the benign tumor nature of PSP, there have been few studies on PET/CT imaging of it. 18F-FDG is one of the most commonly used tracers for PET-C, and its level of energy uptake often reflects the malignancy of the tumor. Studies have shown that PSP usually presents with a slightly to moderately increased 18F-FDG uptake, the intensity of uptake is positively correlated with tumor size, and symptomatic patients show a higher average uptake of 18F-FDG than asymptomatic patients (10, 11). The patient in our current report showed significantly increased 18F-FDG uptake on PET/CT, which may be related to the relatively large tumor size (greater than 3.0cm). Moreover, the patient underwent 68Ga-DOTATATE PET/CT imaging due to the high probability of the needle biopsy indicating lung carcinoid cancer. Lung carcinoids are neuroendocrine tumors (NET), most of which express somatostatin receptors (SSTR), especially type 2. 68Ga-DOTATATE has a relatively high affinity for SSTR and can be used as a tracer in PET/CT imaging for targeted diagnosis of SSTR. It can not only determine the location of the primary tumor, but also detect some small metastases that are difficult to find, which is of great significance for improving the accuracy of NET diagnosis and determining the tumor stage, and thus improving the prognosis (12, 13). However, interestingly, although the patient we reported was ultimately diagnosed with PSP, non NETs, it also showed increased 68Ga-DOTATATE uptake on somatostatin receptor PET/CT imaging. Due to the rarity of similar studies in the past, the reasons for the increased 68Ga-DOTATATE uptake in PSP need further investigation.
According to the imaging findings and clinical features of PSP, it should be distinguished from pulmonary carcinoid, peripheral pulmonary adenocarcinoma, tuberculous spheres. Lung carcinoids, especially peripheral carcinomas, often appear as solitary round or quasi-round soft tissue masses on CT. About 30% of the tumors may have spotty or lamellar calcifications, showing moderate to significant enhancement on contrast-enhanced CT (14). Moreover, lung carcinoids may also show varying degrees of increased uptake of 18F-FDG or 68Ga-DOTATATE on PET imaging (15, 16), making it difficult to distinguish them from PSP. A previous study revealed that the “iceberg sign”, that is, the small tumor is manifested as a bronchial lumen lesion, or the small bronchial lumen nodule is fused with a large extracellular lung lesion, is a characteristic manifestation of lung carcinoid (14), and can be distinguished from other diseases, but the occurrence rate of this sign is rare. Peripheral lung adenocarcinoma often shows burrs, lobulation, pleural depression, and less calcification, which often shows obvious uneven enhancement on contrast-enhanced scan, and usually significantly increased 18F-FDG uptake on PET imaging (17, 18). However, PSP usually presents a round-like soft tissue mass, which usually shows uniform enhancement on contrast-enhanced scan, and there was a mildly to moderately increased 18F-FDG uptake on PET, so identification of the two was relatively easy. Tuberculomas can have fever, night sweat and other toxic symptoms, and CT mostly also shows circular nodules with calcification and necrosis, while it is often accompanied by satellite foci around, and the enhancement degree of tuberculomas is weaker than that of PSP on contrast-enhanced CT scan (19). Other differential diagnoses include hamartoma, inflammatory pseudotumor, adenoma and fungal infection, which can be distinguished according to the clinical manifestations and imaging characteristics of patients.
The diagnosis of PSP is confirmed by histopathological examination. Under the microscope, the tumor tissue structure of PSP is relatively complex, including four regions consisting of nipple area, solid area, hardened area, and hemorrhagic area, as well as two basic types of tumor cells: surface cuboidal epithelial cells and circular stromal cells (20). Immunohistochemical results showed that TTF-1 and EMA were positive in epithelial and stromal cells of almost all tumor tissues (2). Moreover, CK7 and Napsin-A, CD56, Syn were also expressed positively in some tumor tissues (21). The pathological features of this patient reported by us were consistent with the above literature, and both epithelial cells and stromal cells of the tumor had positive expressions of EMA and TTF1, and CK7 and Napsin-A were also positively expressed in epithelial cells.
The treatment of PSP is mainly thoracoscopic resection, as the nature of the benign tumor, further mediastinal lymph node dissection is not necessary (5). The prognosis of PSP after surgical resection is generally good, although mediastinal lymph node metastasis, pleural metastasis and even distant metastasis have been reported in the literature, but it does not affect the survival of patients (22–26). There was no evidence of tumor recurrence after surgical resection of the tumor in the patient we reported at present. The most recent telephone follow-up was 9 months after discharge, and the patient did not report any discomfort.
PSP is a relatively rare benign lung tumor, and it is difficult to obtain an accurate diagnosis before surgery. Our case study suggests that PSP presents varying degrees of increased 18F-FDG and 68Ga-DOTATATE uptake on PET/CT imaging, which should be considered as one of the differential diagnoses for lung carcinoids.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
RY: Investigation, Methodology, Project administration, Writing – original draft. WZ: Data curation, Software, Validation, Writing – original draft. YY: Conceptualization, Project administration, Supervision, Writing – review & editing. XH: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Guizhou Province Science and Technology Plan Project (grant numbers: Qiankehe-ZK(2024)-329) and Zunyi Science and Technology Joint Fund (grant number: HZ-2023-284).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, et al. The 2015 world health organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. (2015) 10:1243–60. doi: 10.1097/JTO.0000000000000630
2. Devouassoux-Shisheboran M, Hayashi T, Linnoila RI, Koss MN, Travis WD. A clinicopathologic study of 100 cases of pulmonary sclerosing hemangioma with immunohistochemical studies: TTF-1 is expressed in both round and surface cells, suggesting an origin from primitive respiratory epithelium. Am J Surg Pathol. (2000) 24:906–16. doi: 10.1097/00000478-200007000-00002
3. Zhou L, Sun C, Huang Y, Li Q, Tang H, Wang Y. Pulmonary sclerosing hemangioma with a rare symptom: A case report and review of the literature. Mol Clin Oncol. (2017) 6:221–4. doi: 10.3892/mco.2016.1114
4. Xu Y, Li Q, Wang C, Yang X. Pulmonary sclerosing pneumocytoma. Asian J Surg. (2023) 46:3362–3. doi: 10.1016/j.asjsur.2023.03.083
5. Zheng Q, Zhou J, Li G, Man S, Lin Z, Wang T, et al. Pulmonary sclerosing pneumocytoma: clinical features and prognosis. World J Surg Oncol. (2022) 20:140. doi: 10.1186/s12957-022-02603-4
6. Shin SY, Kim MY, Oh SY, Lee HJ, Hong SA, Jang SJ, et al. Pulmonary sclerosing pneumocytoma of the lung: CT characteristics in a large series of a tertiary referral center. Med (Baltimore). (2015) 94:e498. doi: 10.1097/MD.0000000000000498
7. Han J, Xiang H, Ridley WE, Ridley LJ. Tail sign: Pulmonary sclerosing pneumocytoma. J Med Imaging Radiat Oncol. (2018) 62 Suppl 1:49. doi: 10.1111/1754-9485.35_12785
8. Cheung YC, Ng SH, Chang JW, Tan CF, Huang SF, Yu CT. Histopathological and CT features of pulmonary sclerosing haemangiomas. Clin Radiol. (2003) 58:630–5. doi: 10.1016/S0009-9260(03)00177-6
9. Luo C, Song Y, Liu Y, Wang R, Gao J, Yue S, et al. Analysis of the value of enhanced CT combined with texture analysis in the differential diagnosis of pulmonary sclerosing pneumocytoma and atypical peripheral lung cancer: a feasibility study. BMC Med Imaging. (2022) 22:16. doi: 10.1186/s12880-022-00745-1
10. Liu H, Dang H, Wang R, Yao S, Wu Y, Xu B. Analysis of the F-18 FDG PET/CT features of pulmonary sclerosing pneumocytoma. Nucl Med Commun. (2021) 42:665–71. doi: 10.1097/MNM.0000000000001374
11. Jiang L, Huang Y, Tang Q, Zhao Q, Li Y, Wu X, et al. (18)F-FDG PET/CT characteristics of pulmonary sclerosing hemangioma vs. pulmonary hamartoma. Oncol Lett. (2018) 16:660–5. doi: 10.3892/ol.2018.8660
12. Zhao L, Pang Y, Wang Y, Chen J, Zhuang Y, Zhang J, et al. Somatostatin receptor imaging with [(68)Ga]Ga-DOTATATE positron emission tomography/computed tomography (PET/CT) in patients with nasopharyngeal carcinoma. Eur J Nucl Med Mol Imaging. (2022) 49:1360–73. doi: 10.1007/s00259-021-05587-7
13. Hu X, Li D, Wang R, Wang P, Cai J. Comparison of the application of 18F-FDG and 68Ga-DOTATATE PET/CT in neuroendocrine tumors: A retrospective study. Med (Baltimore). (2023) 102:e33726. doi: 10.1097/MD.0000000000033726
14. Meisinger QC, Klein JS, Butnor KJ, Gentchos G, Leavitt BJ. CT features of peripheral pulmonary carcinoid tumors. AJR Am J Roentgenol. (2011) 197:1073–80. doi: 10.2214/AJR.10.5954
15. Park H, Subramaniam RM. Diagnosis and treatment of lung neuroendocrine neoplasms: somatostatin receptor PET imaging and peptide receptor radionuclide therapy. PET Clin. (2023) 18:223–31. doi: 10.1016/j.cpet.2022.11.005
16. Al-Toubah T, Montilla-Soler J, El-Haddad G, Haider M, Strosberg J. Somatostatin receptor expression in lung neuroendocrine tumors: an analysis of DOTATATE PET scans. J Nucl Med. (2023) 64:1895–8. doi: 10.2967/jnumed.123.266185
17. Li S, Zhao B, Wang X, Yu J, Yan S, Lv C, et al. Overestimated value of (18)F-FDG PET/CT to diagnose pulmonary nodules: Analysis of 298 patients. Clin Radiol. (2014) 69:e352–7. doi: 10.1016/j.crad.2014.04.007
18. Sun L, Qin C, Fu Q, Hu S, Zhao W, Li H. Comparison of the detection rates of different diagnostic methods for primary peripheral lung cancer. Front Oncol. (2021) 11:696239. doi: 10.3389/fonc.2021.696239
19. Yin YH, Qi YG, Wang B. Differential diagnosis of pulmonary nodular mucinous adenocarcinoma and tuberculoma with dynamic CT: a retrospective study. J Thorac Dis. (2022) 14:1225–31. doi: 10.21037/jtd-22-372
20. Yang CH, Lee LY. Pulmonary sclerosing pneumocytoma remains a diagnostic challenge using frozen sections: a clinicopathological analysis of 59 cases. Histopathology. (2018) 72:500–8. doi: 10.1111/his.2018.72.issue-3
21. Maleki Z, Muller S, Layfield L, Siddiqui MT, Rekhtman N, Pantanowitz L. Pulmonary sclerosing pneumocytoma: Cytomorphology and immunoprofile. Cancer Cytopathol. (2020) 128:414–23. doi: 10.1002/cncy.v128.6
22. Wang X, Zhang L, Wang Y, Jia X, Wang J, Zhang H. Sclerosing pneumocytoma with metastasis to the mediastinal and regional lymph nodes. Indian J Pathol Microbiol. (2018) 61:407–9. doi: 10.4103/IJPM.IJPM_98_17
23. Pokharel S, Dhillon SS, Ylagan L, George S, Yendamuri S. Sclerosing pneumocytoma with lymph node metastasis. J Thorac Oncol. (2016) 11:1802–4. doi: 10.1016/j.jtho.2016.06.005
24. Suzuki H, Saitoh Y, Koh E, Hoshino H, Kase D, Kasei Y, et al. Pulmonary sclerosing hemangioma with pleural dissemination: report of a case. Surg Today. (2011) 41:258–61. doi: 10.1007/s00595-009-4220-5
25. Bae YS, Ro JY, Shim HS, Hong SW, Yoon SO. Pulmonary sclerosing haemangioma with metastatic spread to stomach. Histopathology. (2012) 60:1162–4. doi: 10.1111/j.1365-2559.2012.04213.x
Keywords: pulmonary sclerosing pneumocytoma, lung carcinoid, 18F-FDG, PET/CT, 68Ga-DOTATATE
Citation: Yu R, Zhao W, Yu Y and Hu X (2025) Case Report: Pulmonary sclerosing pneumocytoma mimicking as a neuroendocrine tumor on 18F-FDG and 68Ga-DOTATATE PET/CT: a case presentation. Front. Oncol. 15:1511595. doi: 10.3389/fonc.2025.1511595
Received: 15 October 2024; Accepted: 05 February 2025;
Published: 21 February 2025.
Edited by:
Georgios S. Limouris, National and Kapodistrian University of Athens, GreeceReviewed by:
Carmelo Caldarella, Fondazione Policlinico Universitario A. Gemelli IRCCS, ItalyCopyright © 2025 Yu, Zhao, Yu and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xianwen Hu, NTQxNzU3MDkxQHFxLmNvbQ==; Yonglin Yu, eXV5b25nbGluMjE0QDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.