
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 17 March 2025
Sec. Neuro-Oncology and Neurosurgical Oncology
Volume 15 - 2025 | https://doi.org/10.3389/fonc.2025.1510221
This article is part of the Research Topic Advancements in Meningioma Management: From Imaging Techniques to Personalized Medicine Approaches View all 4 articles
Primary intracranial-extracranial communicating leiomyosarcomas, capable of invading both intracranial and extracranial regions and involving complex anatomical structures, are exceedingly rare neoplasms. We present the case of a 37-year-old male initially presented with a subcutaneous mass on the left frontal vertex. Post-surgical intervention, a recurrent lump emerged on the left frontotemporal vertex. Symptoms, computed tomography (CT), and magnetic resonance imaging (MRI) revealed a mass on the left frontal vertex accompanied by an irregular abnormal lesion. Leiomyosarcoma diagnosis was confirmed on both occasions. The patient underwent leiomyosarcoma excision under general anesthesia. Recurrence was noted 2 years and 4 months post-surgery, necessitating an expanded excision. After 2 years of follow-up, no significant complications were observed, and the patient’s condition remains stable. Primary extracranial communicating leiomyosarcoma is exceptionally rare, with surgery as the primary treatment modality. The decision to excise the lesion should consider the patient’s age, tumor location, pathological features, and presence of distant metastases.
Primary intracranial leiomyosarcomas, rare smooth muscle sarcomas within the cranial cavity, account for merely 0.1% to 0.2% of all intracranial tumors (1). They can affect individuals of any age and gender, presenting various symptoms based on tumor location, such as memory loss, hemiparesis, and seizures (2). Imaging studies typically show an irregular mass with heterogeneous enhancement on MRI, originating from the dura mater or cerebrum (3).
Histologically, these tumors are similar to soft tissue leiomyosarcomas, with interwoven spindle-shaped cells, and occasional fence-like or perivascular epithelioid cell tumor-like arrangement (4). The cells are densely packed, sometimes showing fibrosis and myxoid alterations. Larger tumors often exhibit hyalinization and coagulative necrosis (5). The precise etiology remains elusive, but associations with immunosuppression and viral infections are reported (6). This neoplasm is generally aggressive with a poor prognosis.
Treatment strategies typically depend on the patient’s age, tumor location, pathological characteristics, and presence of distant metastases, including surgical resection, radiotherapy, chemotherapy, and biological therapy, with surgical resection being primary (7). These may include surgical resection, radiotherapy, chemotherapy, and biological therapy, with surgical resection serving as the primary treatment modality (8). With over 30 surgical treatment reports currently available, this case report discusses the clinical characteristics and treatment experience of a rare primary intracranial-extracranial communicating leiomyosarcoma. The patient underwent surgical resection in 2015, experienced a recurrence 2 years and 4 months later, treated with an expanded resection. As of 2023, no signs of recurrence were noted, underscoring the efficacy of surgical intervention as the main treatment approach.
A 37-year-old married male patient was admitted to our hospital on May 10, 2015, with a gradually enlarging subcutaneous mass on the left frontal vertex, present for 1 month. Examination revealed a pliable, non-tender mass measuring approximately 4 cm x 5 cm with a clear texture and well-defined borders, elevated about 0.7 cm above the normal skin margin.
CT indicated local lytic changes in the left frontal bone, with spindle-shaped hypo-to isodense shadows seen on the inner and outer aspects of the skull. The partial brain tissue was slightly compressed and shifted inward, with uneven density within and a CT value of about 38 HU. Uneven enhancement was observed at the edges.
MRI revealed a spindle-shaped lesion with equal T1 and long T2 signals in the left frontal vertex bone, measuring approximately 2.2 cm x 3.5 cm x 4.0 cm, centered on the skull bone with associated bone absorption and thinning. The lesion displayed uneven low signals indicating bone destruction, and abnormal uneven enhancement was observed after contrast administration, with significant enhancement on delayed scanning. The adjacent brain parenchyma was slightly compressed and shifted inward (Figures 1a–e).
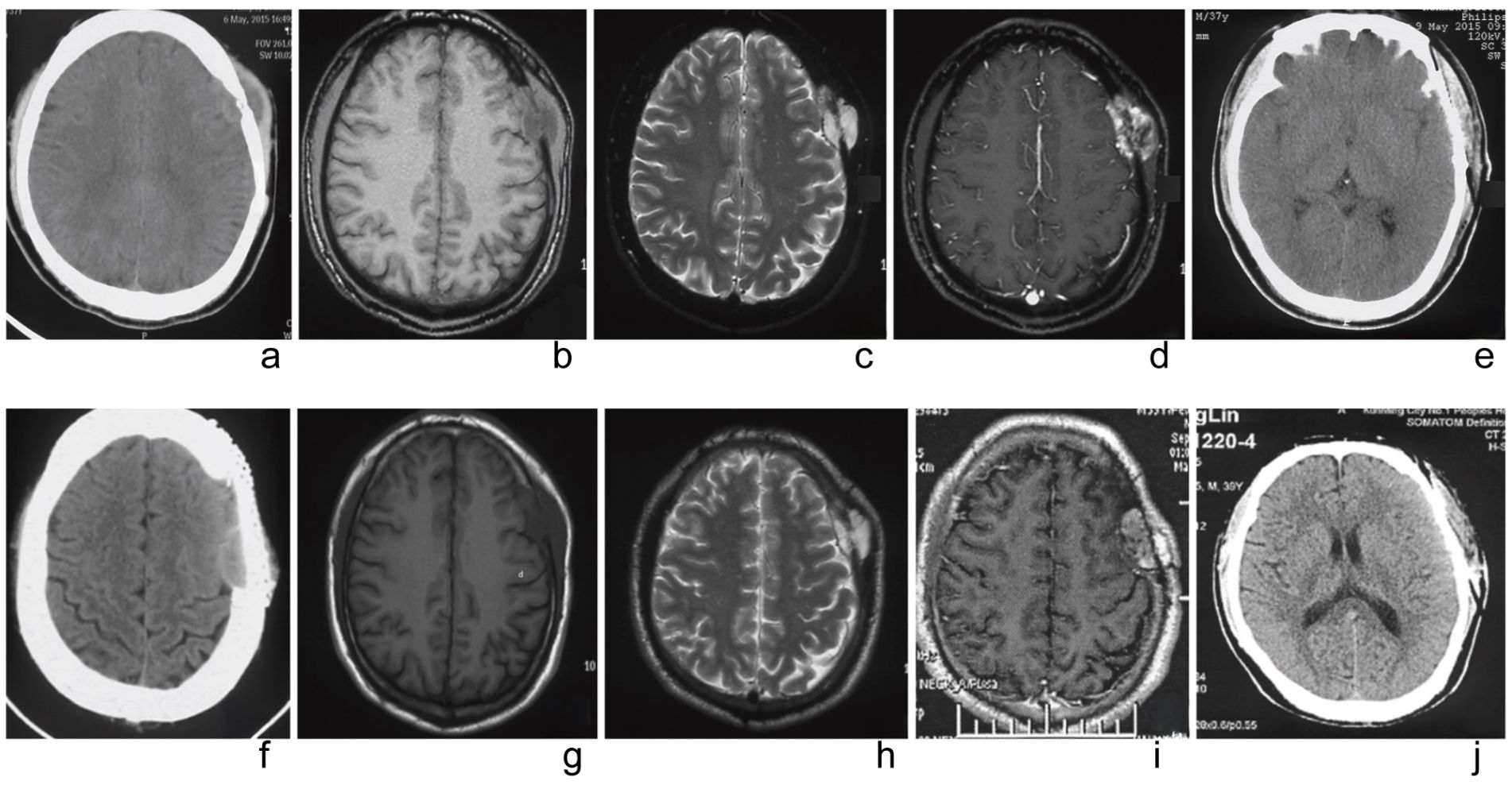
Figure 1. The imaging findings before and after the first surgery. (a) Preoperative CT showed local cranial bone with moth-eaten changes and a spindle-shaped to hypodense shadow on the lateral side of the skull, with uneven density inside. (b) Preoperative MRI (T1WI) displays an uneven signal density. (c) Preoperative MRI (T2WI) appears as an isointense to hyperintense signal. (d) Preoperative MRI enhancement shows ring-like enhancement. (e) A one-week postoperative follow-up cranial CT indicates complete tumor resection. The imaging findings before and after the second surgery. (f) Preoperative CT reveals a low-density lesion with a wide base and clear boundary. (g) Preoperative MRI (T1WI) shows the soft tissue as a lower-density shadow. (h) Preoperative MRI (T2WI) presents as an isointense to hyperintense signal. (i) Preoperative MRI enhancement demonstrates uneven enhancement. (j) A one-week postoperative follow-up cranial CT indicates complete tumor resection.
During surgery, a mass in the left frontal area was observed to have a fish-flesh appearance, firm texture, and no significant adhesion to the scalp. An intraoperative frozen section examination was performed. After fully exposing the extracranial tumor, the skull was drilled, and the bone was bitten along the tumor. A circular incision was made in the dura mater. Due to the lack of adhesion between the tumor and the brain, the entire tumor was removed (Figure 2). Following the intraoperative frozen section examination, which suggested a malignant tumor, the scope was expanded, and approximately 2 cm of surrounding tissue was removed. A range of about 8 cm x 8 cm of normal skull and dura mater was excised, and a phase-one artificial dura mater and titanium mesh repair were performed.
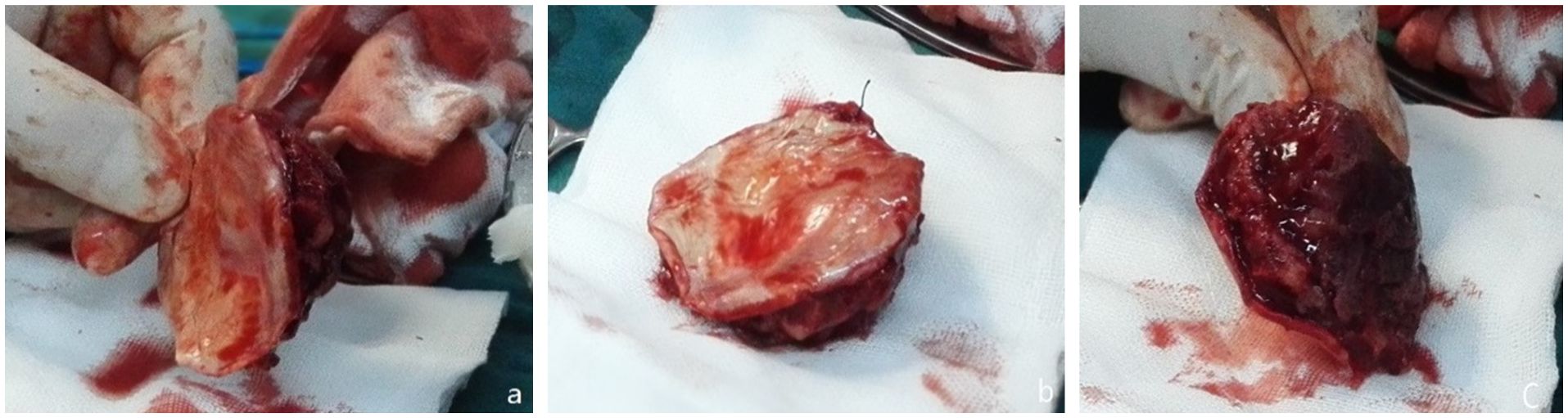
Figure 2. During the first surgery. (a) Leiomyosarcoma tissue excised (b) Affected dural surface. (c) The inner surface of the skull.
The diagnosis of leiomyosarcoma was confirmed by the presence of spindle-shaped and round cells irregularly arranged within the tumor, with abnormally enlarged, deeply stained nuclei showing significant atypia. A few vacuolated cells were observed, with abundant acidophilic cytoplasm and visible mitosis, forming diffuse plaques and some interstitial mucoid degeneration.
Immunohistochemistry results indicated positive silver staining, B-cell lymphoma-2 (Bcl-2), CD34, FLI-1, FN, and SMA, with scattered CD68, and Ki-67 at 30%. The specimen’s margins contained normal tissue, displaying tumor cells arranged in irregular spindle shapes with enlarged nuclei and some showing a vacuolar appearance (Figures 3a, b).
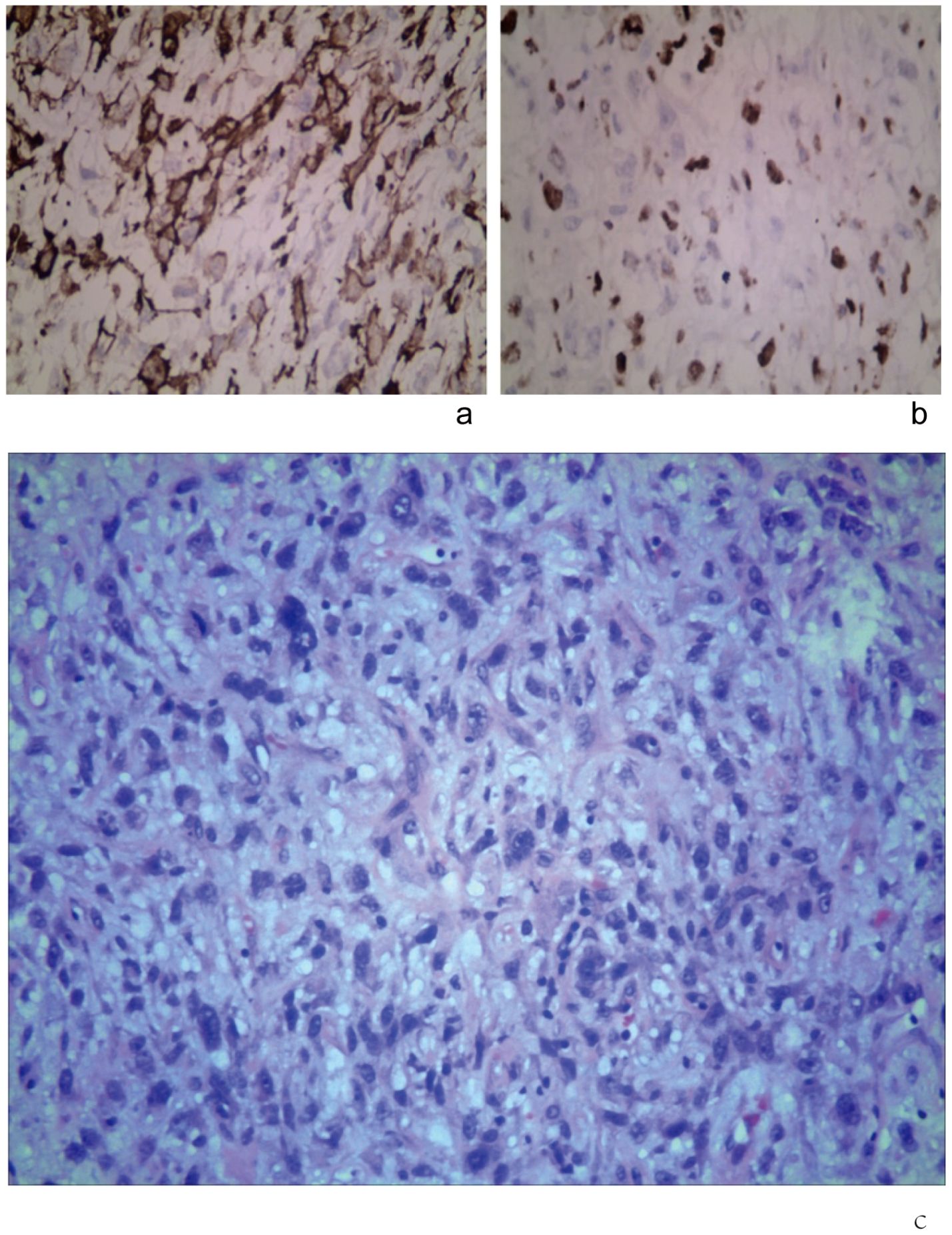
Figure 3. Immunohistochemical staining (original magnification, × 400). (a) SMA (+). (b) Ki-67 (+). (c) Tumor cells were under the microscope after the second surgery, with deeply stained nuclei, significant pleomorphism, and evident mitotic figures (HE, ×400).
The patient was followed up 9 days after discharge. Since the tumor was completely excised and was not sensitive to radiotherapy and chemotherapy, these treatments were not administered. Routine follow-ups over 2 years and 3 months until August 2017 showed no recurrence, and PET-CT scans during this period did not reveal any recurrence or distant metastasis. However, at 2 years and 4 months postoperatively, a subcutaneous mass appeared on the left frontotemporal vertex, which was considered a recurrence of the leiomyosarcoma.
The patient was readmitted on September 27, 2017, due to a lump on the left frontotemporal vertex, present for 20 days. Examination revealed an original surgical scar on the left frontotemporal vertex and a subcutaneous mass approximately 3 cm in diameter, with a pliable texture, clear boundaries, no tenderness, redness, or skin ulceration, and elevated about 0.5 cm above the normal skin margin. No vascular murmur was detected within the mass. Enlarged lymph nodes were palpable on both sides of the neck, with the largest measuring about 3 cm in diameter.
CT scans indicated the absence of bone substance in the left frontotemporal vertex, revealing a broad-based segment of low-density shadow with clear boundaries, measuring approximately 3.2 cm × 1.3 cm, and a CT value of about 39 HU. MRI demonstrated a mass and post-surgical changes in the left frontal vertex, showing an irregular abnormal lesion in the inner table of the left parietal bone. This lesion presented as equal T1 and slightly long T2 signals, measuring about 3.5 cm × 1.6 cm × 3.5 cm, with uneven enhancement after contrast administration and significant mass effect (Figures 1f–j).
During the surgery, a mass of approximately 3 cm × 4 cm was observed attached to the three-dimensional titanium mesh on the left frontal vertex, with no clear demarcation from the surrounding tissue. The mass was removed in sections. After the titanium mesh was removed, a 4 cm mass was noted on the left parietal vertex. The excised mass had a fish-flesh appearance with clear demarcation. The artificial dura mater, tumor tissue, and affected skull in that area were excised. The tumor originated from the dura mater at the vertex. An additional 3 cm of skull, dura mater, periosteum, and subcutaneous tissue towards the vertex were removed. Towards the frontotemporal area, 4 cm from the tumor, proliferative tissue, artificial dura mater, brain tissue, and skull were excised, along with a skin flap measuring 2 cm × 5 cm at the tumor site. The surgical procedure involved a phase-one repair with artificial dura mater and titanium mesh.
The diagnosis confirmed leiomyosarcoma (WHO Grade III). The tumor contained spindle-shaped and round cells irregularly arranged, with deeply stained and enlarged nuclei. Hematoxylin and Eosin (HE) staining revealed significant pleomorphism, a few vacuolated cells, and conspicuous mitotic figures diffusely distributed in sheets, with some interstitial mucoid degeneration (Figure 3c). Immunohistochemistry studies showed positive results for silver immersion staining and SMA. No tumors were found in the surrounding tissues, and cytology from needle aspiration biopsy of the neck lymph nodes showed no tumor cells.
The patient was discharged 10 days after surgery. Given the recurrence of leiomyosarcoma without metastasis, no radiotherapy or chemotherapy was administered before or after the surgery. The patient has been regularly returning for check-ups, and as of June 1, 2020, has been followed up for over 2 years and 8 months, with no significant complications reported. Follow-up continues.
Leiomyosarcoma is a rare malignant tumor originating from mesodermal tissues, commonly found in the uterus, gastrointestinal tract, and retroperitoneum. Head and neck leiomyosarcomas account for 1% to 4% of all leiomyosarcomas (1, 9). Primary intracranial-extracranial communicating leiomyosarcomas are exceedingly rare, often originating from the meningeal interstitium, and are unrelated to meningeal epithelial cells (10). These tumors can occur at any age and show no significant gender difference, typically appearing in individuals with compromised immune function (11), especially in HIV-positive patients carrying the EB virus (12). Intracranial sarcomas are mostly found supratentorial and very rarely in the cerebellum or spinal cord, with symptoms varying depending on the tumor location. Extracranial leiomyosarcomas often present as subcutaneous masses, while intracranial leiomyosarcomas commonly cause symptoms such as headaches, vomiting, and secondary epilepsy. In this case, the patient presented with a subcutaneous mass without related intracranial symptoms.
Several case reports and studies have documented similar cases of primary intracranial-extracranial communicating leiomyosarcomas, though their rarity makes comprehensive comparison challenging. Li et al. (2019) reported a case of primary intracranial leiomyosarcoma in an immunocompetent patient, emphasizing the importance of radiological features in diagnosis and surgical planning. Their case shared similar imaging characteristics with ours, particularly the pattern of bone invasion and enhancement on MRI (6). The 5-year survival achieved in our case is particularly noteworthy, as previous studies have reported varying survival rates. For instance, Zhang et al. (2020) reviewed 15 cases of primary intracranial leiomyosarcomas, finding a median survival time of 23 months, making our patient’s outcome relatively favorable in comparison (13).
Clinical diagnosis relies on the imaging characteristics of the tumor and pathological immunohistochemistry results. On CT, leiomyosarcomas typically appear as medium-sized soft tissue masses with clear boundaries, although some parts may exhibit infiltrative growth with blurred edges. MRI usually shows uneven signals with some cystic changes and necrosis, and ring-like enhancement at the edges after contrast (12). In this case, the sarcoma was clearly demarcated from the normal brain tissue, with only mild edema at the edges, uneven internal signals, and obvious uneven enhancement after MRI contrast, with non-enhanced flaky areas visible inside. Pathological examination revealed that intracranial-extracranial communicating leiomyosarcomas, similar to soft tissue leiomyosarcomas elsewhere, are primarily arranged in irregular spindle cell bundles. Microscopically, they exhibit significant pleomorphism, elongated and variable nuclei with deep staining, easily visible mitotic figures, acidophilic or pale cytoplasm, and a common vacuolated appearance. Immunohistochemistry typically shows strong diffuse positivity for SMA, calponin, and desmin, with approximately 30% Ki-67 positivity (14).
Primary intracranial-extracranial communicating leiomyosarcomas need to be differentiated from related intracranial and extracranial lesions, such as leiomyosarcoma (LMS), brain metastasis (BM), hemangiopericytoma (HPC), epithelioid glioblastoma (EGB), osteosarcoma (OS), gliosarcoma (GS), and fibrosarcoma (FS) (Supplementary Table 1) (15, 16).
The primary treatment for leiomyosarcoma remains surgical excision, removing all visible tumor tissue and ensuring negative pathological margins intraoperatively, followed by expanding the excision to include more than 2 cm of normal tissue (17, 18). Late-stage tumors are highly malignant, with a short disease-free survival period, typically between 6 to 24 months (19). Complete removal via surgery is challenging, so radiotherapy may be added before or after surgery (20). Studies have shown that bevacizumab can effectively treat certain soft tissue sarcomas (21), but insufficient clinical data supports its use for primary intracranial-extracranial communicating leiomyosarcomas.
In this case, the patient had bone destruction, and the first expanded excision surgery was effective. After recurrence, two additional surgeries further expanded the excision range by 3 to 4 cm. As of June 1, 2020, follow-ups showed no recurrence, and the patient has survived for 5 years since the first surgery, indicating that surgical excision remains the main treatment method for this tumor. Currently, there is no uniform standard for the range of surgical excision, and it is recommended that even if the intraoperative rapid pathological examination shows negative margins, the excision range should still be expanded by more than 2 cm.
This case report is significant for documenting a rare primary intracranial-extracranial communicating leiomyosarcoma with detailed imaging, surgical, and follow-up data, with our patient’s exceptional 5-year survival following aggressive surgical management exceeding the typically reported median survival of 23 months, while also demonstrating successful management of tumor recurrence through expanded surgical resection. Our experience has important clinical implications, suggesting that aggressive surgical resection with margins exceeding 2 cm may improve outcomes even in recurrent cases, while the successful outcome without adjuvant therapy indicates surgery alone may suffice in selected cases with complete resection; furthermore, our case emphasizes the necessity of long-term surveillance, as recurrence occurred at 2 years and 4 months post-initial surgery. While the study’s main limitation is its nature as a single case report involving an immunocompetent patient, which limits generalizability, its strengths include comprehensive documentation of imaging, pathological findings, and surgical approach, along with extended follow-up data showing successful outcome—most notably, the patient’s 5-year survival provides evidence that aggressive surgical management can achieve favorable outcomes in these rare tumors.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Kunming Provincial First People’s Hospital of Kunming, China. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
KP: Writing – original draft. NZ: Writing – original draft, Writing – review & editing. TW: Data curation, Writing – review & editing. ZL: Investigation, Writing – review & editing. XL: Software, Writing – review & editing. JW: Conceptualization, Writing – review & editing. DS: Resources, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Yunnan Provincial Science and Technology Plan Project (202301AY070001-286), Yunnan Provincial Department of Education Scientific Research Fund Project (2024J0374), and Kunming Municipal Health Research Project (2023-04-04-004).
The authors are grateful for the patient’s consent to be part of this study. The authors would like to acknowledge Dr. Zhiwei Cao.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1510221/full#supplementary-material
1. Cohen N, Fedewa S, Chen AY. Epidemiology and demographics of the head and neck cancer population. Oral Maxillofac Surg Clinics. (2018) 30:381–95. doi: 10.1016/j.coms.2018.06.001
2. Adelman MR. The morcellation debate: the history and the science. Clin obstetrics gynecology. (2015) 58:710–7. doi: 10.1097/GRF.0000000000000150
3. Zhao L, Jiang Y, Wang Y, Bai Y, Sun Y, Li Y. Primary intracranial leiomyosarcoma secondary to glioblastoma: case report and literature review. Front Oncol. (2021) 11:642683. doi: 10.3389/fonc.2021.642683
4. Marko J, Wolfman DJ. Retroperitoneal leiomyosarcoma from the radiologic pathology archives. Radiographics /: Rev Publ Radiological Soc North America Inc. (2018) 38:1403–20. doi: 10.1148/rg.2018180006
5. Singh DK, Singh N, Parihar A, Singh R. Craniopharyngioma and epidermoid tumor in the same child: a rare association. BMJ Case Rep. (2013) 2013:1–4. doi: 10.1136/bcr-2013-009421
6. Li XL, Ren J, Niu RN, Jiang X, Xu GH, Zhou P, et al. Primary intracranial leiomyosarcoma in an immunocompetent patient: Case report with emphasis on imaging features. Med (Baltimore). (2019) 98:e15269. doi: 10.1097/MD.0000000000015269
7. Canisius J, Wagner A, Bunk EC, Spille DC, Stögbauer L, Grauer O, et al. Expression of decitabine-targeted oncogenes in meningiomas in vivo. Neurosurgical Rev. (2022) 45:2767–75. doi: 10.1007/s10143-022-01789-1
8. Zhu J, Wang H, Huang YQ, Song W, Li YF, Wang WJ, et al. Comprehensive analysis of a long non-coding RNA-associated competing endogenous RNA network in glioma. Oncol letters. (2020) 20:63. doi: 10.3892/ol.2020.11924
9. Workman AD, Farquhar DR, Brody RM, Parasher AK, Carey RM, Purkey MT, et al. Leiomyosarcoma of the head and neck: A 17-year single institution experience and review of the National Cancer Data Base. Head neck. (2018) 40:756–62. doi: 10.1002/hed.25054
10. Rizzo A, Nigro MC, Ramponi V, Gallo C, Perrone AM, De Iaco P, et al. Skull metastasis from uterine leiomyosarcoma, a rare presentation for a rare tumor: A case report and review of the literature. Front Oncol. (2020) 10:869. doi: 10.3389/fonc.2020.00869
11. Gallagher SJ, Rosenberg SA, Francis D, Salamat S, Howard SP, Kimple RJ. Primary intracranial leiomyosarcoma in an immunocompetent patient: Case report and review of the literature. Clin Neurol Neurosurgery. (2018) 165:76–80. doi: 10.1016/j.clineuro.2017.12.014
12. Sivendran S, Vidal CI, Barginear MF. Primary intracranial leiomyosarcoma in an HIV-infected patient. Int J Clin Oncol. (2011) 16:63–6. doi: 10.1007/s10147-010-0110-5
13. Zhang L, Li H, Yu J, Lu Z, Zhang G, Zhang H, et al. Primary intracranial leiomyosarcomas: A retrospective study of 15 cases and review of the literature. World Neurosurgery. (2020) 134:e733–44. doi: 10.1007/s10143-020-01422-z
14. Gaetke-Udager K, McLean K, Sciallis AP, Alves T, Maturen KE, Mervak BM, et al. Diagnostic accuracy of ultrasound, contrast-enhanced CT, and conventional MRI for differentiating leiomyoma from leiomyosarcoma. Acad radiology. (2016) 23:1290–7. doi: 10.1016/j.acra.2016.06.004
15. Wang N, Osswald M. Meningiomas: overview and new directions in therapy. Semin neurology. (2018) 38:112–20. doi: 10.1055/s-0038-1636502
16. Shankar JJS, Hodgson L, Sinha N. Diffusion-weighted imaging may help differentiate intracranial hemangiopericytoma from meningioma. J neuroradiology. (2019) 46:263–7. doi: 10.1016/j.neurad.2018.11.002
17. Alshawwa K, Hijazi J, Jaber B, AlHassan H, Abu-Amer T, Salahaldeen R, et al. Resection of primary renal leiomyosarcoma involving the inferior vena cava (IVC) with IVC resection and reconstruction. Case Rep surgery. (2022) 2022:6037890. doi: 10.1155/2022/6037890
18. Kadouri Y, Lachkar S, Dergamoun H, El Sayegh H, Benslimane L, Nouini Y. Management of the uncommon bladder cancers: A single-center experience over 10 years. Adv urology. (2020) 2020:7563703. doi: 10.1155/2020/7563703
19. Hussain S, Nanda A, Fowler M, Ampil FL, Burton GV. Primary intracranial leiomyosarcoma: report of a case and review of the literature. Sarcoma. (2006) 2006:52140. doi: 10.1155/SRCM/2006/52140
20. Boșoteanu M, Vodă RI, Așchie M, Bosoteanu LA, Bălțătescu GI. Morphological and ancillary features of uterine leiomyosarcoma: case report. Clin Pathol (Thousand Oaks Ventura County Calif). (2022) 15:2632010x221105224. doi: 10.1177/2632010X221105224
21. Agulnik M, Yarber JL, Okuno SH, von Mehren M, Jovanovic BD, Brockstein BE, et al. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncology: Off J Eur Soc Med Oncol. (2013) 24:257–63. doi: 10.1093/annonc/mds237
Keywords: primary intracranial tumor, leiomyosarcoma, clinical features, surgical removal, case report
Citation: Pu K, Wang T, Li Z, Lin X, Wu J, Shao D and Zhao N (2025) Surgical treatment of primary intracranial and extracranial communicating leiomyosarcoma: a case report. Front. Oncol. 15:1510221. doi: 10.3389/fonc.2025.1510221
Received: 12 October 2024; Accepted: 24 February 2025;
Published: 17 March 2025.
Edited by:
Ning Pan, South-Central Minzu University, ChinaReviewed by:
Yichang Hao, Peking University Third Hospital, ChinaCopyright © 2025 Pu, Wang, Li, Lin, Wu, Shao and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nan Zhao, MTk3OHpoYW9uYW5Ac2luYS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.