
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Oncol. , 13 March 2025
Sec. Neuro-Oncology and Neurosurgical Oncology
Volume 15 - 2025 | https://doi.org/10.3389/fonc.2025.1507940
Glioblastoma, a grade IV astrocytoma, typically has a poor prognosis, with most patients succumbing within eighteen months of diagnosis and few experiencing long-term survival. Focused ultrasound, an emerging localized therapy, has shown promising results in early-phase studies for glioblastoma by improving the uptake of temozolomide and carboplatin. The blood-brain barrier is critical to homeostasis by regulating the movement of substances between the bloodstream and the central nervous system. While this barrier helps prevent infections from bloodborne pathogens, it also hinders the delivery of cancer therapies to gliomas. Combining focused ultrasound with circulating microbubbles enhances local blood-brain barrier permeability, facilitating the intratumoral uptake of systemic cancer therapies. The purpose of this study was to identify promising new therapeutics in the treatment of glioblastoma for localized drug delivery via focused ultrasound. This review provides an overview of the current standard of care for newly diagnosed and recurrent glioblastoma, identifies current therapies indicated for the treatment, discusses key aspects of microbubble resonators, describes focused ultrasound devices under evaluation in human trials, and concludes with a perspective of emerging therapeutics for future studies.
The World Health Organization CNS5 classification categorizes adult glioblastoma multiform (GBM) as isocitrate dehydrogenase wild-type adult-type diffuse astrocytoma with one or more biomarkers, such as necrosis, microvascular proliferation, mutation of the TERT promoter gene, chromosomes +7/-10 copy number changes, or amplification of endothelial growth factor receptor (EGFR) genes (1). Newly diagnosed GBM elicits a poor prognosis with a 14–18 month median overall survival (mOS), a 2-year survival rate of 27%, and a 5-year survival rate of 6% following standard-of-care resection and chemoradiation (2–4). O6-methylguanine DNA-methyltransferase (MGMT) methylation presents with a more favorable prognosis, with a 23 month mOS and a 49% 2-year survival rate, relative to a 13 month mOS and 12% 2-year survival rate for MGMT-promoter unmethylated GBM (4).
Focused ultrasound (FUS) holds promise in improving GBM outcomes by enhancing the blood-brain barrier (BBB) permeability to facilitate the localized uptake of systemic therapies. This review explores current and emerging GBM therapies, along with ongoing FUS-enhanced research applications.
Newly diagnosed GBM is typically treated with tumor resection preceding concomitant temozolomide (TMZ) chemoradiation and subsequent maintenance (adjuvant) TMZ therapy (4–6). The United States Food and Drug Administration (FDA) has approved five pharmaceuticals and one device for GBM: TMZ, oral lomustine (CCNU), bevacizumab, intravenous carmustine (BCNU), carmustine wafers, and tumor-treating fields (TTFs) (7). Regorafenib and procarbazine hydrochloride-lomustine-vincristine sulfate (PCV) combination therapy are also listed in the United States National Comprehensive Cancer Network (NCCN) guidelines as preferred treatments for recurrent GBM (rGBM) (2, 8).
Resection is often limited to tumor debulking and histological sampling (4). GBM tumors can grow along vessels and fiber tracts microscopically several centimeters beyond the macroscopic tumor region (9, 10). Early glioma hemispherectomies saw recurrence in the contralateral hemisphere (11). Radiotherapy targets the excision cavity and remnant tumor sites, typically with 2 Gy fractions totaling 60 Gy over 6 weeks, concurrently with TMZ (2, 12).
TMZ is an alkylating agent activated at physiological alkalinity to 5-(3-methyl)-1-triazen-1-yl-imidazole-4-carboxamide (MTIC) within approximately 2 hr of oral administration, and passively diffuses across vascular endothelial cell membranes (13–17). TMZ levels in brain parenchyma are typically less than 20% of blood plasma (18–21). Improved outcomes occur with MGMT-promoter methylated GBM, where epigenetic silencing by methylation of CpG (5’—Cytosine—phosphate—Guanine—3’) sites within the MGMT gene promotor region reduces the reparation of TMZ-induced alkylation (22, 23).
TTFs with maintenance TMZ were FDA-approved and incorporated into NCCN guidelines after improving median progression-free survival (mPFS) and mOS for newly diagnosed GBM (NCT00916409) (2, 24, 25). The Optune Gio (Novocure, Haifa, Israel) is FDA-approved for recurrent and newly diagnosed GBM (26). Alternating electric fields of 0.7 V.cm-1 and 200 kHz create dielectrophoretic movement of charged organelles and dipolar macromolecules to induce cell death of proliferating tumor cells (27–30).
Recurrence rates and mOS are about 90% and 7–9 months, respectively (12, 31, 32). rGBM treatment options include further surgical resection, TMZ rechallenge, alkylating agents, PCV chemotherapy, re-irradiation, bevacizumab, TTFs, regorafenib, palliative care alone, and experimental techniques (2, 12). Molecular structures and pharmacological properties of GBM therapeutics are shown in Figure 1E and Table 1.
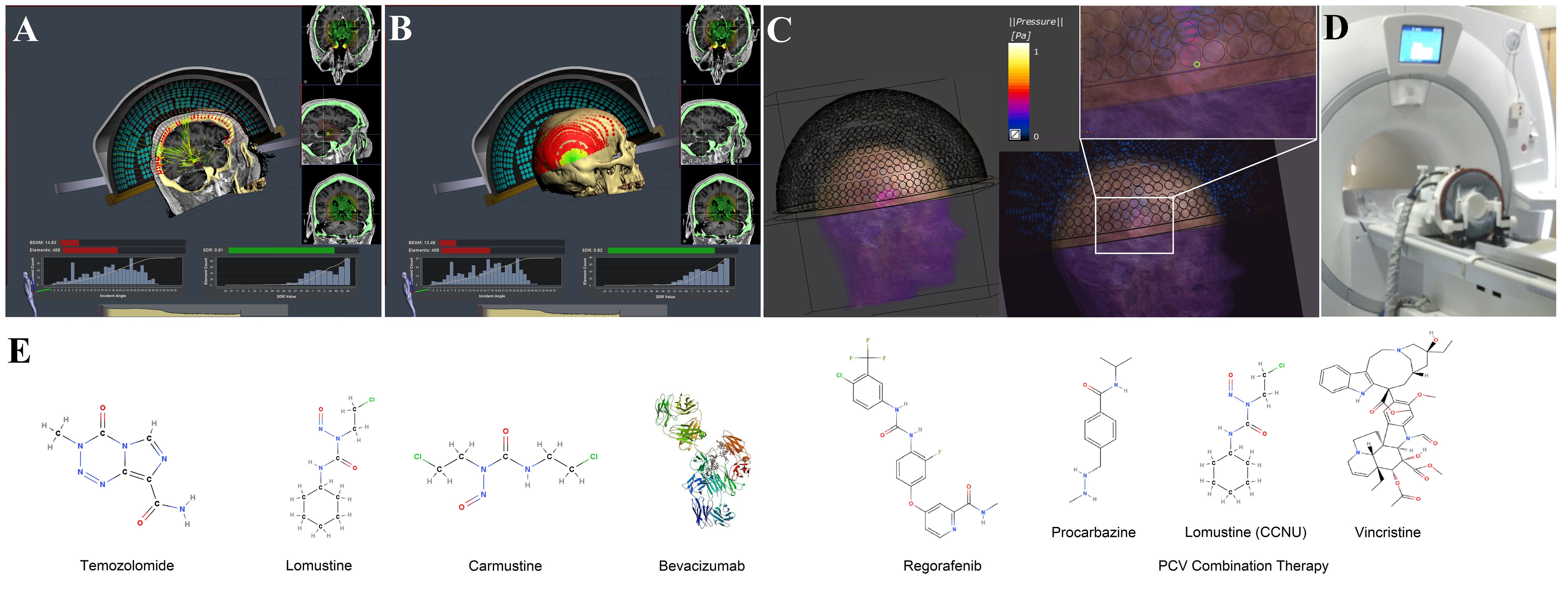
Figure 1. This illustration provides an overview of a hemispherical array used in blood-brain barrier (BBB) opening procedures, along with the molecular structures of selected glioblastoma therapies. (A) An example of a peripheral tumor site, which leads to high incidence angles for many elements during ray tracing. The application is intended for ablative treatments to deactivate elements, but illustrates the extent of incidence angles beyond 30°. (B) An illustration of the incidence angle distribution in relation to the skull surface. The red circles represent elements with incidence angles more than 30°, and the green circles represent those less than 30°. Figure made with Kranion and datasets from The Cancer Imaging Archive (182, 183). (C) A simulated normalized pressure field for a 220 kHz Exablate 4000 Type 2.0 transducer model, without aberration correction, recreated using settings described previously (184). (D) An image of an Exablate 4000 Type 1.0 transducer system used for ablative procedures. (E) The molecular structure of pharmaceuticals approved by the United States Food and Drug Administration (FDA) or recommended by the United States National Comprehensive Cancer Network (NCCN) for the treatment of glioblastoma (185, 186).
Regorafenib is an anti-angiogenic multi-kinase (VEGFR1-3, TIE2) inhibitor added to NCCN rGBM guidelines after improving mOS compared to lomustine (2, 33, 34). TTFs are a chemotherapy-free treatment option that can improve toxicity and quality of life (2, 35). Lomustine is the de facto standard, with improved outcomes for MGMT-promoter methylated GBM, and is frequently a control arm in clinical trials, with a 2 months mPFS, 20% 6-month PFS, and a mOS of 6–9 months (36). Anti-angiogenic bevacizumab is a monoclonal antibody that neutralizes circulating vascular endothelial growth factors (VEGF) (2, 37, 38). Debate exists regarding improved mOS, but the treatment can reduce steroid use and enhance quality of life (39).
The BBB provides an interface between the brain parenchyma and capillaries, regulating homeostasis by managing blood flow, oxygenation, glucose, essential amino acids, and other metabolite levels (40, 41). During progression, the BBB changes can be distinguished as the blood-tumor barrier (BTB), which features disrupted tight and adherens junctions, extensive BBB fenestration, and inhibition of receptor-mediated transcellular pathways (40, 42). Natural BTB disruption enhances BBB permeability, but drugs often remain less than ten times higher than in healthy brain tissue (40). The BTB allows the passage of small ions and molecules but restricts the entry of larger therapeutics (18, 40, 41). Lipinski’s rule of 5 predicts passive BBB permeability, indicating that no more than one of the following criteria can be violated: less than six hydrogen bond donors, less than eleven hydrogen bond acceptors, a molecular weight of less than 500 Da, and a lipophilicity octanol-water partition coefficient less than five (43, 44).
Microbubbles are used off-label as resonators for BBB opening. Optison (GE Healthcare, Chicago, IL, USA), SonoVue/Lumason (Bracco S.P.A., Milan, Italy), and Definity/Luminity (Lantheus Medical Imaging, North Billerica, MA, USA) have received FDA approval for contrast-enhanced ultrasonography (45, 46). Human FUS studies have often used Definity and SonoVue microbubbles (47).
Clinical FUS studies with the Exablate Neuro have been performed with both microbubble bolus doses (48) and infusion rates of 0.24–0.8 µL.kg-1.min-1 (49, 50), with possible treatment durations around 3 hr (50). Mechanical index (MI) thresholds for stable and inertial cavitation are temperature and tissue-dependent (51). The in vivo thresholds with Definity for FUS-enhanced BBB opening and inertial cavitation have been measured near 0.46 and 0.72–1.15, respectively (52, 53). The mean diameters for Definity microbubbles are 1.1–3.3 µm, with 98% less than 10 µm, and 100% less than 20 µm (54). Definity bolus doses exhibit a mean blood plasma half-life of 1.3–1.9 min, achieve intravascular equilibrium within 1 min, have a maximum serum concentration near 2 min, and become undetectable after 10–14 min (54–56). The C3F8 gas is inert, with low solubility, is eliminated non-metabolized through the lungs, and, in the presence of dissolved respiratory gas allows extended dissolution rates (57). The shell reduces perfluorocarbon gas diffusion, prevents coalescence, and reduces the immune response (57).
The microbubble mechanisms of BBB opening are believed to be independent of bulk heating and inertial cavitation (58). The acoustic radiation force propels the microbubbles toward the capillary walls, where microbubble oscillations trigger events, including shear stresses and microstreaming, that culminate in BBB opening (41, 58, 59). The enhanced drug uptake occurs largely through disturbance of the tight junctions, dysregulation of efflux transporters, and increased caveolae formation (60). BBB closure occurs over approximately 4–6 hr, with complete closure within 24 hr (58, 61). Influential factors include the MI, microbubble dose, duty cycle, vessel to bubble diameter ratio, frequency, tissue properties, and sonication duration (58, 62). Many cavitation-related bioeffects remain unknown, such as effects from microjetting, reactive oxygen species, ballistic motion, and bubble clusters (62).
Functionalizing microbubbles and altering their shape and size can prolong the circulatory half-life and improve drug delivery to smaller capillaries for more uniform concentrations (63). Nanobubbles increase disruption in smaller 2–6 µm rodent capillaries (64). Antibody-microbubble conjugates can target microscopic metastatic brain tumor sites for use with large-volume ultrasound fields (65). Perfluorocarbon droplets are similar colloids to microbubbles with a liquid rather than gaseous core and have shown potential for drug delivery (66). Droplets are size-tunable (67), integrate chemotherapeutics (68), prolong systemic circulation (69), increase inertial cavitation thresholds (70), enable 19F MRI (71), and potentially exhibit a unique cavitation mechanism (72). Other formulas incorporate metal chelates (73, 74), allow partial oxygen measurements in gliomas (75–77), can track macrophages after re-irradiation in glioma-bearing mice (78), and incorporate within clinical cell therapies for cell tracking (79, 80) and measuring apoptotic cell fraction (81). Nanodroplets have also exhibited the ability to permeabilize the BBB (82–84).
Devices and drugs for BBB disruption include FUS, laser ablation, mannitol, RMP-7, and regadenoson (85). Other localized drug delivery approaches include convection-enhanced delivery, intra-arterial catheter delivery, reservoir implants, stereotactic injections, and carmustine wafers placed in the resection cavity (86).
Thermoablative procedures are the only FDA-approved FUS modalities for neurological disease, and the 670 kHz Exablate Neuro 4000 Type 1.0/1.1 (InSightec, Haifa, Israel) is the only system both FDA-approved and Conformité Européenne (CE)-marked (87). Additional research applications include hyperthermia, sonothrombolysis, neuromodulation, histotripsy, sonodynamic therapy, and liquid biopsy (60, 87). At least three FUS devices have been used in early-phase clinical trials for BBB opening in GBM, including the NaviFUS (NaviFUS Corp., Taipei, Taiwan), Exablate Neuro 4000 Type 2.0 (InSightec, Haifa, Israel), and SonoCloud-9 implant (CarThera, Paris, Île-de-France, France) (47, 60, 88–90).
The 220 kHz Exablate Neuro 4000 Type 2.0 system is a hemispherical phased array transducer with ±25 mm electronic steering and treatment volumes beyond 30 cm2 (49, 91). Ray tracing aberration correction incorporates the shear sound speed with incidence angles beyond 30° (91). The transducer integrates with existing neuroablation systems and can treat conditions beyond GBM. Repeated BBB opening during maintenance TMZ has illustrated prolonged survival, with no adverse events or TMZ neurotoxicity (NCT03712293) (19, 48). Elevated concentrations have been observed with liposomal doxorubicin, TMZ, and fluorescein (NCT02343991, NCT03322813) (92, 93). The device has completed Phase 2 trials for sonodynamic therapy in newly diagnosed GBM (NCT04845919) and is ongoing for carboplatin monotherapy for rGBM (NCT04417088, NCT04440358). A safety and feasibility study was recently completed for maintenance TMZ in newly diagnosed GBM (NCT03551249) (49, 91, 94).
Hemispherical arrays are monitored with embedded acoustic receivers for microbubble harmonic emissions. Numerous approaches have been developed for feedback control (95). The Exablate algorithm is proprietary but generates a cavitation score from the harmonic emissions, and allows altered sonication duration, applied power, gain, and cavitation dose goal (49). Human GBM studies observed lower microbubble concentrations than in animals, the need for improved receiver sensitivity, and relatively hypovascular white matter targets that reduced microbubble concentrations (49). Sites near the skull surface can lead to standing waves, reflections, and impact focusing (49). Figures 1A–D illustrates the system and the incidence angle distributions at a peripheral target site.
The Sonocloud-9 is a 1 MHz MRI-compatible, minimally invasive, transcranial implant placed in the location of the bone flap after tumor resection or biopsy (96, 97). Clinical studies include carboplatin for rGBM (NCT03744026), checkpoint inhibitors for metastases (NCT04021420), carboplatin for pediatric gliomas (NCT05293197), nanoparticle albumin–bound paclitaxel (nab-paclitaxel) for rGBM (NCT04528680), anti-programmed cell death protein 1 (aPD-1) and anti-cytotoxic T-lymphocyte-associated protein 4 (aCTLA-4) monoclonal antibodies and liposomal doxorubicin in newly diagnosed GBM (NCT05864534), and adjuvant TMZ for newly diagnosed GBM (NCT04614493) (96, 98–100). The device can target large volumes (∼45 cm2), features short procedure times, and is not influenced by skull aberration (89). Thus avoiding aberration correction, MRI guidance, feedback control, and can be performed on an outpatient basis (88, 101–103). Safety and feasibility studies showed tolerability and evidence of improved mOS for carboplatin delivery in rGBM (NCT02253212, NCT03744026) (96, 98, 103–105), and a Phase 3 trial is underway (NCT05902169).
NaviFUS is a 500 kHz 256-channel neuronavigational phased array system attached to a mechanical arm designed to be used without a stereotactic headframe (NCT03626896, NCT04446416, NCT04988750) (106–109). Studies have used ramped-up feedback control at 0.5–0.68 MI (107, 109). Position sensors register the device to pretreatment imaging for 3D focal tracking. The system integrates intraoperative pressure simulations, lowers cost, increases portability, with treatment durations below 15 min, and negates intraoperative MRI guidance (88, 108, 109). The device has evaluated enhanced bevacizumab delivery for rGBM (NCT04446416) (109) and illustrated a possible synergistic effect with radiotherapy (NCT04988750) (107). An rGBM Phase 3 trial is evaluating bevacizumab delivery (NCT06496971).
Previously suggested technical improvements include whole-brain electronic steering, cavitation mapping, simulation-based focusing, and holography (91, 110). Passive acoustic mapping has been limited by axial resolution (109) but could be correlated with bioeffects, tumor response, and local drug concentrations (62, 111). The approach is feasible with neuronavigational systems (112, 113) and hemispherical arrays (114, 115). Receiver arrays within custom hemispherical transducers could enable MRI-free procedures (116, 117). Diagnostic extra-cranial systems have been adapted for acoustic mapping for drug delivery to colorectal liver metastases (ISRCTN17598292) (118).
Thorough lists of GBM clinical trials and preclinical studies evaluating a range of therapeutics are provided elsewhere (47, 88–90, 119–122). Briefly, therapeutics evaluated in animal models include TMZ, methotrexate, irinotecan, carboplatin, paclitaxel, carmustine, doxorubicin, cisplatin, etoposide, MGMT inactivators, targeted therapies like bevacizumab, and immunotherapies like checkpoint inhibitors and CAR T-cell therapy. Many of these drugs are used off-label and have been evaluated by systemic administration or loading within nanocarriers and microbubbles (119). Pharmaceuticals evaluated in clinical studies include TMZ, doxorubicin, liposomal doxorubicin, aPD-1 antibodies, aCTLA-4 antibodies, fluorescein, bevacizumab, paclitaxel, nab-paclitaxel, and carboplatin (90, 96, 99, 100, 120). FUS-mediated BBB opening is also being evaluated for Parkinson’s disease (50, 123, 124), Alzheimer’s disease (125–132), amyotrophic lateral sclerosis (133), and metastatic brain tumors (134).
FUS can modulate the innate immune response, improve the penetrance of targeted therapies and immunotherapies, and improve survival in rodents (99, 100, 119, 122). A number of immunotherapies are being evaluated clinically in combination with FUS for primary and secondary brain tumors. Balstilimab, botensilimab, and pembrolizumab are being studied for newly diagnosed and rGBM (NCT05864534) (99, 100). Pembrolizumab is being assessed in a Phase 3 trial for non-small cell lung cancer brain metastases (NCT05317858). Nivolumab, pembrolizumab, and ipilimumab are being evaluated for melanoma brain metastases (NCT04021420).
Drug-loaded microbubble and nanocarriers, along with drug conjugates, offer alternatives to systemic administration (111, 119). Nanoparticle therapeutics increase preclinical survival times and relative concentrations than systemic antibodies and chemotherapies (111). The nanocarrier hydrodynamic diameters ideally remain below 100 nm (119, 135), with a trade-off between increased permeation and clearance rates (136). Nanocarrier ligand groups target vascular or tumor surface receptors and allow internalized and externally activated drug release (119).
Overcoming the BBB is the main challenge to GBM therapies (137). Difficulties in focused ultrasound adoption include establishing standardized treatment settings and rigorous safety studies (138). Hypovascular white matter targets reduce drug delivery (49, 105). Further, concurrent anesthetic administration can alter hemodynamics, vasoactivity, and temperature to confound permeability and cavitation thresholds (63). New GBM animal models are needed to better account for surgical resection, recurrence, and immunological response (119). The disease is rare, with about 8–11% clinical trial participation (7), and only three pivotal studies between 2005–2022 prolonged survival (139, 140). With hemispherical arrays, individual patient characteristics can influence outcomes, such as skull characteristics on feedback control (49). Ablation is complicated by bone attenuation, impedance mismatch, skull heating, and bone heterogeneity (141, 142). These aspects are less problematic for BBB opening because the lower frequencies and powers reduce acoustic absorption, aberrations, and risk of thermal damage (142, 143).
At least two additional therapeutic regimens have improved mOS in Phase 3 trials in recent years, but had contentious trial designs (144, 145). Autologous tumor lysate-dendritic cell vaccine (DCVax-L) reported improved mOS for newly diagnosed and recurrent GBM (NCT00045968) (146, 147). Lomustine-TMZ combination therapy improved mOS for newly diagnosed MGMT-promoter methylated GBM compared to standard-of-care chemoradiation (NCT01149109) (148). TTFs with maintenance TMZ arguably provide the best survival rates in newly diagnosed GBM (24), and a pragmatic approach would be evaluating FUS-enhanced adjuvant TMZ with TTFs (119). TTFs with Withaferin A illustrated a synergistic effect, suggesting increased vulnerability to anti-mitotic chemotherapies (30, 149, 150). Immunotherapies have mostly lacked survival benefits in Phase 3 trials (151). New targets such as immunosuppressive CD73 myeloid cells have been proposed with anti-CD73 antibodies in combination with aCTLA-4 and aPD-1 therapies (152–154).
Theranostics can measure longitudinal pharmacokinetics, biodistribution, and drug concentrations for association with treatment response (155). R1 relaxation rates and volumetric transfer coefficients are surrogates for drug concentration (156–158). In vivo radiolabeled GBM therapeutics can be quantitatively imaged with nuclear imaging, with or without FUS, using 11C-TMZ (half-life: 20.3 min) (159), 68Ga-bevacizumab (half-life: 68 min) (160, 161), and 89Zr-cetuximab (half-life: 78.4 hr) (162, 163). 89Zr-bevacizumab has been evaluated without FUS in pediatric diffuse intrinsic pontine glioma (164), a condition under evaluation for FUS-mediated drug delivery (165). Radionuclide therapeutics for metastatic prostate cancer and somatostatin receptor-positive gastroenteropancreatic neuroendocrine tumors received regulatory approval with [177Lu]Lu-PSMA-617 and [177Lu]Lu-DOTA-TATE, respectively (166, 167). These therapies along with [131I]-IPA, [177Lu]Lu-NeoB, [177Lu]Lu-FF58, and [177Lu]Lu-6A10-Fab fragments are in clinical trials for GBM (166, 168, 169). Carrier-mediated L-type amino acid transporters (LAT-1) such as small molecule [131I]-IPA (NCT03849105, NCT05450744) have high BBB permeability and would allow quantitative comparison of drug delivery with FUS (137, 170–173). [14C]-regorafenib has been used in human pharmacokinetic studies (174), and 3.0 T and 9.4 T 19F-MRI has illustrated longitudinal measurements of trifluoro-methylated pharmaceuticals, similar to regorafenib, in murine models (175, 176). Fluorine-containing metastatic chemotherapies with less than two rule of 5 violations include abemaciclib (177), larotrectinib, encorafenib, and vemurafenib (178). Larotrectinib has no rule of 5 violations (178), has shown promise for pediatric neurotrophic tyrosine receptor kinase (NTRK) fusion-positive gliomas (179, 180), and in adults NTRK gene fusions are most frequently found in GBM (181).
While most studies have been to establish safety and feasibility, limiting inclusion criteria to MGMT-promoter methylated GBM for FUS-enhanced TMZ therapies could improve outcomes due to epigenetic silencing. TTFs, lomustine-TMZ combination therapy, and DCVax-L have demonstrated improved mOS in Phase 3 trials of newly diagnosed GBM, and have not been evaluated in conjunction with FUS. Studies of FUS-enhanced drug delivery with lomustine have been relatively limited. 19F-MRI might allow for longitudinal drug concentrations in preclinical survival studies, using regorafenib, larotrectinib, drug-loaded perfluorocarbon nanodroplets, or cell therapies labeled with perfluorocarbon emulsions. Radionuclide theranostics like LAT-1 [131I]-IPA or [177Lu]Lu-DOTA-TATE could be used similarly with nuclear imaging.
In conclusion, FUS-enhanced delivery of systemic therapies has demonstrated safety, tolerability, and evidence of efficacy in preclinical and early-phase clinical studies and presents a promising localized delivery technique with the potential to improve the standard-of-care management for GBM.
RH: Writing – original draft, Visualization, Writing – review & editing. NM: Writing – review & editing, Supervision, Project administration, Funding acquisition.
The author(s) declare that financial support was received for the research and/or publication of this article. RH was supported by the “Training in Image Guidance, Precision Diagnosis and Therapy” NIH T32 Fellowship (5 T32 EB 25823-4) in the Brigham and Women’s Hospital Department of Radiology. The work received support from NIH grant R01EB033307.
Thanks to the National Center for Image-guided Therapy at Brigham & Women’s Hospital and Harvard Medical School for providing the resources to compile the document. Thanks to the members of the Focused Ultrasound Laboratory for the helpful discussions regarding the article. Thanks to the members of Editage (http://www.editage.com) for editing and reviewing this manuscript. Figure 1E was made with MolView. Figures 1A, B was made with Kranion using datasets from The Cancer Imaging Archive. Figure 1C was made with Sim4Life.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro-Oncology. (2021) 23:1231–51. doi: 10.1093/neuonc/noab106
2. National Comprehensive Cancer Network. Nccn clinical practice guidelines in oncology: central nervous system cancers. nccn.org 2022, version 1.2022. Available online at: https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf (Accessed January 22, 2025).
3. National Institute of Health and Care Excellence. Brain tumours (primary) and brain metastases in over 16s (ng99). Available online at: https://www.nice.org.uk/guidance/NG99 (Accessed January 22, 2025).
4. Stupp R, Brada M, van den Bent MJ, Tonn JC, Pentheroudakis G. High-grade glioma: Esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2014) 25:iii93–iii101. doi: 10.1093/annonc/mdu050
5. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. (2005) 352:987–96. doi: 10.1056/NEJMoa043330
6. Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase iii study: 5-year analysis of the eortc-ncic trial. Lancet Oncol. (2009) 10:459–66. doi: 10.1016/S1470-2045(09)70025-7
7. Fisher JP, Adamson DC. Current fda-approved therapies for high-grade Malignant gliomas. Biomedicines. (2021) 9:1–13. doi: 10.3390/biomedicines9030324
8. Dominic A Solimando J, Waddell JA. Procarbazine, lomustine, and vincristine (pcv) regimen for central nervous system tumors. Hosp Pharm. (2017) 52:98–104. doi: 10.1310/hpj5202-98
9. Kantelhardt SR, Giese A. Strategies in glioma-surgery. chap. 20. In: Abujamra AL, editor. Diagnostic Techniques and Surgical Management of Brain Tumors. IntechOpen, Rijeka (2011). p. 385–402. doi: 10.5772/22085
11. Woodworth GF, Dunn GP, Nance EA, Hanes J, Brem H. Emerging insights into barriers to effective brain tumor therapeutics. Front Oncol. (2014) 4:126. doi: 10.3389/fonc.2014.00126
12. Wen PY, Weller M, Lee EQ, Alexander BM, Barnholtz-Sloan JS, Barthel FP, et al. Glioblastoma in adults: a society for neuro-oncology (sno) and european society of neuro-oncology (eano) consensus review on current management and future directions. Neuro-Oncology. (2020) 22:1073–113. doi: 10.1093/neuonc/noaa106
13. Temodar. Highlights of prescribing information. Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021029s031lbl.pdf (Accessed January 22, 2025).
14. Stupp R, Gander M, Leyvraz S, Newlands E. Current and future developments in the use of temozolomide for the treatment of brain tumours. Lancet Oncol. (2001) 2:552–60. doi: 10.1016/S1470-2045(01)00489-2
15. Agarwala SS, Kirkwood JM. Temozolomide, a novel alkylating agent with activity in the central nervous system, may improve the treatment of advanced metastatic melanoma. Oncologist. (2000) 5:144–51. doi: 10.1634/theoncologist.5-2-144
16. Ramalho MJ, Andrade S, Álvaro Neto Coelho M, Loureiro JA, Pereira MC. Biophysical interaction of temozolomide and its active metabolite with biomembrane models: The relevance of drug-membrane interaction for glioblastoma multiforme therapy. Eur J Pharm Biopharm. (2019) 136:156–63. doi: 10.1016/j.ejpb.2019.01.015
17. Dutra JA, Luiz MT, Tavares AG, Di Filippo LD, Carvalho SG, Chorilli M. Temozolomide: An overview of biological properties, drug delivery nanosystems, and analytical methods. Curr Pharm Des. (2022) 28:2073–88. doi: 10.2174/1381612828666220603152918
18. Bunevicius A, McDannold NJ, Golby AJ. Focused ultrasound strategies for brain tumor therapy. Oper Neurosurg. (2020) 19:9–18. doi: 10.1093/ons/opz374
19. Park SH, Kim MJ, Jung HH, Chang WS, Choi HS, Rachmilevitch I, et al. One-year outcome of multiple blood–brain barrier disruptions with temozolomide for the treatment of glioblastoma. Front Oncol. (2020) 10:1663. doi: 10.3389/fonc.2020.01663
20. Portnow J, Badie B, Chen M, Liu A, Blanchard S, Synold TW. The neuropharmacokinetics of temozolomide in patients with resectable brain tumors: potential implications for the current approach to chemoradiation. Clin Cancer Res. (2009) 15:7092–8. doi: 10.1158/1078-0432.CCR-09-1349
21. Ostermann S, Csajka C, Buclin T, Leyvraz S, Lejeune F, Decosterd LA, et al. Plasma and cerebrospinal fluid population pharmacokinetics of temozolomide in Malignant glioma patients. Clin Cancer Res. (2004) 10:3728–36. doi: 10.1158/1078-0432.CCR-03-0807
22. Mansouri A, Hachem LD, Mansouri S, Nassiri F, Laperriere NJ, Xia D, et al. MGMT promoter methylation status testing to guide therapy for glioblastoma: refining the approach based on emerging evidence and current challenges. Neuro-Oncology. (2018) 21:167–78. doi: 10.1093/neuonc/noy132
23. Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. Mgmt gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. (2005) 352:997–1003. doi: 10.1056/NEJMoa043331
24. Stupp R, Taillibert S, Kanner A, Read W, Steinberg DM, Lhermitte B, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: A randomized clinical trial. JAMA. (2017) 318:2306–16. doi: 10.1001/jama.2017.18718
25. Stupp R, Taillibert S, Kanner AA, Kesari S, Steinberg DM, Toms SA, et al. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: A randomized clinical trial. JAMA. (2015) 314:2535–43. doi: 10.1001/jama.2015.16669
26. Optune. Pma p100034/s013 summary of safety and effectiveness data. Available online at: https://www.accessdata.fda.gov/cdrh_docs/pdf10/p100034s013b.pdf (Accessed January 22, 2025).
27. Davies AM, Weinberg U, Palti Y. Tumor treating fields: a new frontier in cancer therapy. Ann N Y Acad Sci. (2013) 1291:86–95. doi: 10.1111/nyas.12112
28. Hottinger AF, Pacheco P, Stupp R. Tumor treating fields: a novel treatment modality and its use in brain tumors. Neuro-Oncology. (2016) 18:1338–49. doi: 10.1093/neuonc/now182
29. Funk RH, Scholkmann F. The significance of bioelectricity on all levels of organization of an organism. part 1: From the subcellular level to cells. Prog Biophys Mol Biol. (2023) 177:185–201. doi: 10.1016/j.pbiomolbio.2022.12.002
30. Szklener K, Bilski M, Nieoczym K, Mańdziuk D, Mańdziuk S. Enhancing glioblastoma treatment through the integration of tumor-treating fields. Front Oncol. (2023) 13:1274587. doi: 10.3389/fonc.2023.1274587
31. Weller M, Cloughesy T, Perry JR, Wick W. Standards of care for treatment of recurrent glioblastoma—are we there yet? Neuro-Oncology. (2012) 15:4–27. doi: 10.1093/neuonc/nos273
32. Chaul-Barbosa C, Marques DF. How we treat recurrent glioblastoma today and current evidence. Curr Oncol Rep. (2019) 21:1–8. doi: 10.1007/s11912-019-0834-y
33. Lombardi G, De Salvo GL, Brandes AA, Eoli M, Rudà R, Faedi M, et al. Regorafenib compared with lomustine in patients with relapsed glioblastoma (regoma): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol. (2019) 20:110–9. doi: 10.1016/S1470-2045(18)30675-2
34. Stivarga. Highlights of prescribing information. Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/203085lbl.pdf (Accessed January 22, 2025).
35. Stupp R, Wong ET, Kanner AA, Steinberg D, Engelhard H, Heidecke V, et al. Novottf-100a versus physician’s choice chemotherapy in recurrent glioblastoma: A randomised phase iii trial of a novel treatment modality. Eur J Cancer. (2012) 48:2192–202. doi: 10.1016/j.ejca.2012.04.011
36. Weller M, Le Rhun E. How did lomustine become standard of care in recurrent glioblastoma? Cancer Treat Rev. (2020) 87:1–8. doi: 10.1016/j.ctrv.2020.102029
37. Wick W, Gorlia T, Bendszus M, Taphoorn M, Sahm F, Harting I, et al. Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med. (2017) 377:1954–63. doi: 10.1056/NEJMoa1707358
38. Avastin. Highlights of prescribing information. Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125085s332lbl.pdf (Accessed January 22, 2025).
39. Tan AC, Ashley DM, López GY, Malinzak M, Friedman HS, Khasraw M. Management of glioblastoma: State of the art and future directions. CA Cancer J Clin. (2020) 70:299–312. doi: 10.3322/caac.21613
40. Steeg PS. The blood–tumour barrier in cancer biology and therapy. Nat Rev Clin Oncol. (2021) 18:696–714. doi: 10.1038/s41571-021-00529-6
41. Burgess A, Shah K, Hough O, Hynynen K. Focused ultrasound-mediated drug delivery through the blood–brain barrier. Expert Rev Neurother. (2015) 15:477–91. doi: 10.1586/14737175.2015.1028369
42. Lamsam L, Johnson E, Connolly ID, Wintermark M, Gephart MH. A review of potential applications of mr-guided focused ultrasound for targeting brain tumor therapy. Neurosurg Focus FOC. (2018) 44:1–7. doi: 10.3171/2017.11.FOCUS17620
43. Dréan A, Goldwirt L, Verreault M, Canney M, Schmitt C, Guehennec J, et al. Blood-brain barrier, cytotoxic chemotherapies and glioblastoma. Expert Rev Neurother. (2016) 16:1285–300. doi: 10.1080/14737175.2016.1202761
44. Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Delivery Rev. (2012) 64:4–17. doi: 10.1016/j.addr.2012.09.019
45. Dietrich C, Albrecht T, Becher H, Harvey C, Jenssen C, Lim A, et al. History of contrast enhanced ultrasound (ceus). Med Ultrason. (2024) 26:405–16. doi: 10.11152/mu-4366
46. Barr RG, Wilson SR, Lyshchik A, McCarville B, Darge K, Grant E, et al. Contrast-enhanced Ultrasound—State of the art in north america: Society of radiologists in ultrasound white paper. Ultrasound Q. (2020) 36:S1–S39. doi: 10.1097/RUQ.0000000000000515
47. Zhu H, Allwin C, Bassous MG, Pouliopoulos AN. Focused ultrasound-mediated enhancement of blood–brain barrier permeability for brain tumor treatment: a systematic review of clinical trials. J Neurooncol. (2024) 170:235–52. doi: 10.1007/s11060-024-04795-z
48. Park SH, Kim MJ, Jung HH, Chang WS, Choi HS, Rachmilevitch I, et al. Safety and feasibility of multiple blood-brain barrier disruptions for the treatment of glioblastoma in patients undergoing standard adjuvant chemotherapy. J Neurosurg. (2021) 134:475–83. doi: 10.3171/2019.10.JNS192206
49. McDannold N, Wen PY, Reardon DA, Fletcher SM, Golby AJ. Cavitation monitoring, treatment strategy, and acoustic simulations of focused ultrasound blood-brain barrier disruption in patients with glioblastoma. J Control Release. (2024) 372:194–208. doi: 10.1016/j.jconrel.2024.06.036
50. Huang Y, Meng Y, Pople CB, Bethune A, Jones RM, Abrahao A, et al. Cavitation feedback control of focused ultrasound blood-brain barrier opening for drug delivery in patients with parkinson’s disease. Pharmaceutics. (2022) 14:1–14. doi: 10.3390/pharmaceutics14122607
51. Haar Gt. Chapter 3 - mechanisms for induction of bio-effects of focused ultrasound in tissue. In: Haar Gt, Rivens I, Wu F, editors. Image-guided focused ultrasound therapy: physics and clinical applications, 1st ed. Boca Raton, Florida, USA: CRC Press (2024). p. 11–8. doi: 10.1201/9780429162671-26
52. McDannold N, Vykhodtseva N, Hynynen K. Blood-brain barrier disruption induced by focused ultrasound and circulating preformed microbubbles appears to be characterized by the mechanical index. Ultrasound Med Biol. (2008) 34:834–40. doi: 10.1016/j.ultrasmedbio.2007.10.016
53. Arvanitis CD, Vykhodtseva N, Jolesz F, Livingstone M, McDannold N. Cavitation-enhanced nonthermal ablation in deep brain targets: feasibility in a large animal model. J Neurosurg. (2016) 124:1450–1459. doi: 10.3171/2015.4.JNS142862
54. Definity. Highlights of prescribing information. Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021064s011lbl.pdf (Accessed January 22, 2025).
55. Li P, Du P, Peng J, Zhao Z, Li H, Yu W, et al. Pharmacokinetics and pharmacodynamics of perfluoropropane after intra-venous bolus injection of perflutren lipid microsphere injection (DEFINITY®) in healthy chinese volunteers. BMC Pharmacol Toxicol. (2024) 25:1–12. doi: 10.1186/s40360-023-00729-z
56. Khalili K, Atri M, Kim TK, Jang HJ. Recognizing the role of the reticuloendothelial system in the late phase of us contrast agents. Radiology. (2021) 298:287–91. doi: 10.1148/radiol.2020203245
57. Borden MA, Song KH. Reverse engineering the ultrasound contrast agent. Adv Colloid Interface Sci. (2018) 262:39–49. doi: 10.1016/j.cis.2018.10.004
58. Aryal M, Arvanitis CD, Alexander PM, McDannold N. Ultrasound-mediated blood–brain barrier disruption for targeted drug delivery in the central nervous system. Adv Drug Delivery Rev. (2014) 72:94–109. doi: 10.1016/j.addr.2014.01.008
59. Vykhodtseva N, McDannold N, Hynynen K. Progress and problems in the application of focused ultrasound for blood–brain barrier disruption. Ultrasonics. (2008) 48:279–96. doi: 10.1016/j.ultras.2008.04.004
60. Meng Y, Hynynen K, Lipsman N. Applications of focused ultrasound in the brain: from thermoablation to drug delivery. Nat Rev Neurol. (2021) 17:7–22. doi: 10.1038/s41582-020-00418-z
61. Todd N, Angolano C, Ferran C, Devor A, Borsook D, McDannold N. Secondary effects on brain physiology caused by focused ultrasound-mediated disruption of the blood–brain barrier. J Control Release. (2020) 324:450–9. doi: 10.1016/j.jconrel.2020.05.040
62. Stride E, Coussios C. Nucleation, mapping and control of cavitation for drug delivery. Nat Rev Phys. (2019) 1:495–509. doi: 10.1038/s42254-019-0074-y
63. López-Aguirre M, Castillo-Ortiz M, Viña-González A, Blesa J, Pineda-Pardo JA. The road ahead to successful bbb opening and drug-delivery with focused ultrasound. J Control Release. (2024) 372:901–13. doi: 10.1016/j.jconrel.2024.07.006
64. Gattegno R, Arbel L, Riess N, Shinar H, Katz S, Ilovitsh T. Enhanced capillary delivery with nanobubble-mediated blood-brain barrier opening and advanced high resolution vascular segmentation. J Control Release. (2024) 369:506–16. doi: 10.1016/j.jconrel.2024.04.001
65. Johanssen VA, Ruan JL, Vince O, Thomas A, Peeters S, Soto MS, et al. Targeted opening of the blood-brain barrier using vcam-1 functionalised microbubbles and “whole brain” ultrasound. Theranostics. (2024) 14(10):4076–89. doi: 10.7150/thno.93172
66. Holman R, Lorton O, Guillemin PC, Desgranges S, Contino-Pépin C, Salomir R. Perfluorocarbon emulsion contrast agents: A mini review. Front Chem. (2022) 9:810029. doi: 10.3389/fchem.2021.810029
67. Desgranges S, Lorton O, Gui-Levy L, Guillemin P, Celicanin Z, Hyacinthe JN, et al. Micron-sized pfob liquid core droplets stabilized with tailored-made perfluorinated surfactants as a new class of endovascular sono-sensitizers for focused ultrasound thermotherapy. J Mater Chem B. (2019) 7:927–39. doi: 10.1039/C8TB01491D
68. Al Rifai N, Desgranges S, Le-Guillou-Buffello D, Giron A, Urbach W, Nassereddine M, et al. Ultrasound-triggered delivery of paclitaxel encapsulated in an emulsion at low acoustic pressures. J Mater Chem B. (2020) 8:1640–8. doi: 10.1039/C9TB02493J
69. Bérard C, Desgranges S, Dumas N, Novell A, Larrat B, Hamimed M, et al. Perfluorocarbon nanodroplets as potential nanocarriers for brain delivery assisted by focused ultrasound-mediated blood-brain barrier disruption. Pharmaceutics. (2022) 14:1–25. doi: 10.3390/pharmaceutics14071498
70. Holman R, Lorton O, Guillemin PC, Desgranges S, Santini F, Preso DB, et al. Perfluorocarbon emulsion enhances mr-arfi displacement and temperature in vitro: Evaluating the response with mri, nmr, and hydrophone. Front Oncol. (2023) 12:1025481. doi: 10.3389/fonc.2022.1025481
71. Lorton O, Hyacinthe JN, Desgranges S, Gui L, Klauser A, Celicanin Z, et al. Molecular oxygen loading in candidate theranostic droplets stabilized with biocompatible fluorinated surfactants: Particle size effect and application to in situ 19f mri mapping of oxygen partial pressure. J Magn Reson. (2018) 295:27–37. doi: 10.1016/j.jmr.2018.07.019
72. Pfeiffer P, Shahrooz M, Tortora M, Casciola CM, Holman R, Salomir R, et al. Heterogeneous cavitation from atomically smooth liquid–liquid interfaces. Nat Phys. (2022) 18:1431–5. doi: 10.1038/s41567-022-01764-z
73. Jahromi AH, Wang C, Adams SR, Zhu W, Narsinh K, Xu H, et al. Fluorous-Soluble metal chelate for sensitive fluorine-19 magnetic resonance imaging nanoemulsion probes. ACS Nano. (2019) 13:143–51. doi: 10.1021/acsnano.8b04881
74. Kislukhin AA, Xu H, Adams SR, Narsinh KH, Tsien RY, Ahrens ET. Paramagnetic fluorinated nanoemulsions for sensitive cellular fluorine-19 magnetic resonance imaging. Nat Mater. (2016) 15:662–8. doi: 10.1038/nmat4585
75. Kadayakkara DKK, Janjic JM, Pusateri LK, Young WB, Ahrens ET. In vivo observation of intracellular oximetry in perfluorocarbon-labeled glioma cells and chemotherapeutic response in the cns using fluorine-19 mri. Magn Reson Med. (2010) 64:1252–9. doi: 10.1002/mrm.22506
76. Zhong J, Sakaki M, Okada H, Ahrens ET. In vivo intracellular oxygen dynamics in murine brain glioma and immunotherapeutic response of cytotoxic t cells observed by fluorine-19 magnetic resonance imaging. PloS One. (2013) 8:1–7. doi: 10.1371/journal.pone.0059479
77. Chapelin F, Leach BI, Chen R, Lister D, Messer K, Okada H, et al. Assessing oximetry response to chimeric antigen receptor t-cell therapy against glioma with 19f mri in a murine model. Radiol: Imaging Cancer. (2021) 3:1–9. doi: 10.1148/rycan.2021200062
78. Croci D, Méndez RS, Temme S, Soukup K, Fournier N, Zomer A, et al. Multispectral fluorine-19 mri enables longitudinal and noninvasive monitoring of tumor-associated macrophages. Sci Transl Med. (2022) 14:eabo2952. doi: 10.1126/scitranslmed.abo2952
79. Ahrens ET, Helfer BM, O’Hanlon CF, Schirda C. Clinical cell therapy imaging using a perfluorocarbon tracer and fluorine-19 mri. Magn Reson Med. (2014) 72:1696–701. doi: 10.1002/mrm.25454
80. Darçot E, Colotti R, Brennan D, Deuchar GA, Santosh C, van Heeswijk RB. A characterization of abl-101 as a potential tracer for clinical fluorine-19 mri. NMR BioMed. (2020) 33:e4212. doi: 10.1002/nbm.4212
81. Ahrens ET, Helfer BM, O’Hanlon CF, Lister DR, Bykowski JL, Messer K, et al. Method for estimation of apoptotic cell fraction of cytotherapy using in vivo fluorine-19 magnetic resonance: pilot study in a patient with head and neck carcinoma receiving tumor-infiltrating lymphocytes labeled with perfluorocarbon nanoemulsion. J Immunother Cancer. (2023) 11:1–10. doi: 10.1136/jitc-2023-007015
82. Dauba A, Spitzlei C, Bautista KJB, Jourdain L, Selingue E, VanTreeck KE, et al. Low-boiling-point perfluorocarbon nanodroplets for adaptable ultrasound-induced blood-brain barrier opening. J Control Release. (2024) 376:441–56. doi: 10.1016/j.jconrel.2024.10.023
83. Wu SY, Fix SM, Arena CB, Chen CC, Zheng W, Olumolade OO, et al. Focused ultrasound-facilitated brain drug delivery using optimized nanodroplets: vaporization efficiency dictates large molecular delivery. Phys Med Biol. (2018) 63:035002. doi: 10.1088/1361-6560/aaa30d
84. Chen CC, Sheeran PS, Wu SY, Olumolade OO, Dayton PA, Konofagou EE. Targeted drug delivery with focused ultrasound-induced blood-brain barrier opening using acoustically-activated nanodroplets. J Control Release. (2013) 172:795–804. doi: 10.1016/j.jconrel.2013.09.025
85. Karmur BS, Philteos J, Abbasian A, Zacharia BE, Lipsman N, Levin V, et al. Blood-brain barrier disruption in neuro-oncology: Strategies, failures, and challenges to overcome. Front Oncol. (2020) 10:563840. doi: 10.3389/fonc.2020.563840
86. van Solinge TS, Nieland L, Chiocca EA, Broekman MLD. Advances in local therapy for glioblastoma — taking the fight to the tumour. Nat Rev Neurol. (2022) 18:221–36. doi: 10.1038/s41582-022-00621-0
87. State of the field report. Focused ultrasound foundation(2024). Available online at: https://www.fusfoundation.org/the-foundation/foundation-reportsSOTF (Accessed January 22, 2025).
88. Hughes A, Khan DS, Alkins R. Current and emerging systems for focused ultrasound-mediated blood–brain barrier opening. Ultrasound Med Biol. (2023) 49:1479–90. doi: 10.1016/j.ultrasmedbio.2023.02.017
89. Beccaria K, Canney M, Bouchoux G, Desseaux C, Grill J, Heimberger AB, et al. Ultrasound-induced blood-brain barrier disruption for the treatment of gliomas and other primary cns tumors. Cancer Lett. (2020) 479:13–22. doi: 10.1016/j.canlet.2020.02.013
90. Epstein JE, Pople CB, Meng Y, Lipsman N. An update on the role of focused ultrasound in neuro-oncology. Curr Opin Neurol. (2024) 37:682–292. doi: 10.1097/WCO.0000000000001314
91. McDannold N, Zhang Y, Fletcher SM, Wen PY, Reardon DA, Golby AJ, et al. Non-invasive blood-brain barrier disruption using acoustic holography with a clinical focused ultrasound system. IEEE Trans BioMed Eng. (2024) 71:3046–54. doi: 10.1109/TBME.2024.3407678
92. Mainprize T, Lipsman N, Huang Y, Meng Y, Bethune A, Ironside S, et al. Blood-brain barrier opening in primary brain tumors with non-invasive mr-guided focused ultrasound: A clinical safety and feasibility study. Sci Rep. (2019) 9:1–7. doi: 10.1038/s41598-018-36340-0
93. Anastasiadis P, Gandhi D, Guo Y, Ahmed AK, Bentzen SM, Arvanitis C, et al. Localized blood-brain barrier opening in infiltrating gliomas with mri-guided acoustic emissions-controlled focused ultrasound. Proc Natl Acad Sci. (2021) 118:e2103280118. doi: 10.1073/pnas.2103280118
94. Woodworth GF, Anastasiadis P, Chen C, Gandhi D, Ozair A, Meng Y, et al. Acoustic emissions dose and spatial control of blood-brain barrier opening with focused ultrasound. Cell Press Sneak Peek. (2025) 0:1–42. doi: 10.2139/ssrn.5080353
95. Mondou P, Mériaux S, Nageotte F, Vappou J, Novell A, Larrat B. State of the art on microbubble cavitation monitoring and feedback control for blood-brain-barrier opening using focused ultrasound. Phys Med Biol. (2023) 68:18TR03. doi: 10.1088/1361-6560/ace23e
96. Carpentier A, Stupp R, Sonabend AM, Dufour H, Chinot O, Mathon B, et al. Repeated blood–brain barrier opening with a nine-emitter implantable ultrasound device in combination with carboplatin in recurrent glioblastoma: a phase I/II clinical trial. Nat Commun. (2024) 15:1–12. doi: 10.1038/s41467-024-45818-7
97. Goldwirt L, Canney M, Horodyckid C, Poupon J, Mourah S, Vignot A, et al. Enhanced brain distribution of carboplatin in a primate model after blood–brain barrier disruption using an implantable ultrasound device. Cancer Chemother Pharmacol. (2016) 77:211–6. doi: 10.1007/s00280-015-2930-5
98. Sonabend AM, Gould A, Amidei C, Ward R, Schmidt KA, Zhang DY, et al. Repeated blood–brain barrier opening with an implantable ultrasound device for delivery of albumin-bound paclitaxel in patients with recurrent glioblastoma: a phase 1 trial. Lancet Oncol. (2023) 24:509–22. doi: 10.1016/S1470-2045(23)00112-2
99. Arrieta VA, Gould A, Kim KS, Habashy KJ, Dmello C, Vázquez-Cervantes GI, et al. Ultrasound-mediated delivery of doxorubicin to the brain results in immune modulation and improved responses to PD-1 blockade in gliomas. Nat Comm. (2024) 15:1–19. doi: 10.1038/s41467-024-48326-w
100. Kim KS, Habashy K, Gould A, Zhao J, Najem H, Amidei C, et al. Fc-enhanced anti-ctla-4, anti-pd-1, doxorubicin, and ultrasound-mediated blood–brain barrier opening: A novel combinatorial immunotherapy regimen for gliomas. Neuro-Oncology. (2024) 26:2044–60. doi: 10.1093/neuonc/noae135
101. Sonabend AM, Stupp R. Overcoming the blood–brain barrier with an implantable ultrasound device. Clin Cancer Res. (2019) 25:3750–2. doi: 10.1158/1078-0432.CCR-19-0932
102. Horodyckid C, Canney M, Vignot A, Boisgard R, Drier A, Huberfeld G, et al. Safe long-term repeated disruption of the blood-brain barrier using an implantable ultrasound device: a multiparametric study in a primate model. J Neurosurg. (2017) 126:1351–1361. doi: 10.3171/2016.3.JNS151635
103. Carpentier A, Canney M, Vignot A, Reina V, Beccaria K, Horodyckid C, et al. Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci Transl Med Sci. (2016) 8:343re2–2. doi: 10.1126/scitranslmed.aaf6086
104. Idbaih A, Canney M, Belin L, Desseaux C, Vignot A, Bouchoux G, et al. Safety and feasibility of repeated and transient blood–brain barrier disruption by pulsed ultrasound in patients with recurrent glioblastoma. Clin Cancer Res. (2019) 25:3793–801. doi: 10.1158/1078-0432.CCR-18-3643
105. Asquier N, Bouchoux G, Canney M, Martin C, Law-Ye B, Leclercq D, et al. Blood-brain barrier disruption in humans using an implantable ultrasound device: quantification with mr images and correlation with local acoustic pressure. J Neurosurg. (2020) 132:875–883. doi: 10.3171/2018.9.JNS182001
106. Chen KT, Lin YJ, Chai WY, Lin CJ, Chen PY, Huang CY, et al. Neuronavigation-guided focused ultrasound (navifus) for transcranial blood-brain barrier opening in recurrent glioblastoma patients: Clinical trial protocol. Ann Transl Med. (2020) 8:1–7. doi: 10.21037/atm-20-344
107. Chen KT, Huang CY, Pai PC, Yang WC, Tseng CK, Tsai HC, et al. Focused ultrasound combined with radiotherapy for Malignant brain tumor: a preclinical and clinical study. J Neuro Oncol. (2023) 165:535–45. doi: 10.1007/s11060-023-04517-x
108. Chen KT, Chai WY, Lin YJ, Lin CJ, Chen PY, Tsai HC, et al. Neuronavigation-guided focused ultrasound for transcranial blood-brain barrier opening and immunostimulation in brain tumors. Sci Adv. (2021) 7:eabd0772. doi: 10.1126/sciadv.abd0772
109. Lafond M, Payne A, Lafon C. Therapeutic ultrasound transducer technology and monitoring techniques: a review with clinical examples. Int J Hyperthermia. (2024) 41:2389288. doi: 10.1080/02656736.2024.2389288
110. Hynynen K, Jones RM. Image-guided ultrasound phased arrays are a disruptive technology for non-invasive therapy. Phys Med Biol. (2016) 61:R206. doi: 10.1088/0031-9155/61/17/R206
111. Schoen S, Kilinc MS, Lee H, Guo Y, Degertekin FL, Woodworth GF, et al. Towards controlled drug delivery in brain tumors with microbubble-enhanced focused ultrasound. Adv Drug Delivery Rev. (2022) 180:1–25. doi: 10.1016/j.addr.2021.114043
112. Bae S, Liu K, Pouliopoulos AN, Ji R, Jiménez-Gambín S, Yousefian O, et al. Transcranial blood–brain barrier opening in alzheimer’s disease patients using a portable focused ultrasound system with real-time 2-d cavitation mapping. Theranostics. (2024) 14(11):4519–35. doi: 10.7150/thno.94206
113. Bae S, Liu K, Pouliopoulos AN, Ji R, Konofagou EE. Real-time passive acoustic mapping with enhanced spatial resolution in neuronavigation-guided focused ultrasound for blood–brain barrier opening. IEEE Trans BioMed Eng. (2023) 70:2874–85. doi: 10.1109/TBME.2023.3266952
114. Arvanitis CD, McDannold N. Integrated ultrasound and magnetic resonance imaging for simultaneous temperature and cavitation monitoring during focused ultrasound therapies. Med Phys. (2013) 40:112901. doi: 10.1118/1.4823793
115. Arvanitis CD, Livingstone MS, McDannold N. Combined ultrasound and mr imaging to guide focused ultrasound therapies in the brain. Phys Med Biol. (2013) 58:4749. doi: 10.1088/0031-9155/58/14/4749
116. Deng L, Yang SD, O’Reilly MA, Jones RM, Hynynen K. An ultrasound-guided hemispherical phased array for microbubble-mediated ultrasound therapy. IEEE Trans BioMed Eng. (2022) 69:1776–87. doi: 10.1109/TBME.2021.3132014
117. O’Reilly MA, Jones RM, Hynynen K. Three-dimensional transcranial ultrasound imaging of microbubble clouds using a sparse hemispherical array. IEEE Trans BioMed Eng. (2014) 61:1285–94. doi: 10.1109/TBME.2014.2300838
118. Masiero M, Boulos P, Crake C, Rowe C, Coviello CM. Ultrasound-induced cavitation and passive acoustic mapping: Sonotran platform performance and short-term safety in a large-animal model. Ultrasound Med Biol. (2022) 48:1681–90. doi: 10.1016/j.ultrasmedbio.2022.03.010
119. Bérard C, Truillet C, Larrat B, Dhermain F, Estève MA, Correard F, et al. Anticancer drug delivery by focused ultrasound-mediated blood-brain/tumor barrier disruption for glioma therapy: From benchside to bedside. Pharmacol Ther. (2023) 250:108518. doi: 10.1016/j.pharmthera.2023.108518
120. Fletcher SM, Kobus T, Sutton J, McDannold NJ. Chapter 26 - transcranial ultrasound-mediated blood-brain barrier disruption for targeted drug delivery. In: Haar Gt, Rivens I, Wu F, editors. Image-guided focused ultrasound therapy: physics and clinical applications, 1st ed. Boca Raton, Florida, USA: CRC Press (2024). p. 394–419. doi: 10.1201/9780429162671-26
121. Mungur R, Zheng J, Wang B, Chen X, Zhan R, Tong Y. Low-intensity focused ultrasound technique in glioblastoma multiforme treatment. Front Oncol. (2022) 12:903059. doi: 10.3389/fonc.2022.903059
122. Brighi C, Salimova E, de Veer M, Puttick S, Egan G. Translation of focused ultrasound for blood-brain barrier opening in glioma. J Control Release. (2022) 345:443–63. doi: 10.1016/j.jconrel.2022.03.035
123. Meng Y, Pople CB, Huang Y, Jones RM, Ottoy J, Goubran M, et al. Putaminal recombinant glucocerebrosidase delivery with magnetic resonance–guided focused ultrasound in parkinson’s disease: A phase i study. Mov Disord. (2022) 37:2134–9. doi: 10.1002/mds.29190
124. Gasca-Salas C, Fernández-Rodríguez B, Pineda-Pardo JA, Rodríguez-Rojas R, Obeso I, Hernández-Fernández F, et al. Blood-brain barrier opening with focused ultrasound in parkinson’s disease dementia. Nat Commun. (2021) 12:1–7. doi: 10.1038/s41467-021-21022-9
125. Rezai AR, D’Haese PF, Finomore V, Carpenter J, Ranjan M, Wilhelmsen K, et al. Ultrasound blood–brain barrier opening and aducanumab in alzheimer’s disease. New Engl J Med. (2024) 390:55–62. doi: 10.1056/NEJMoa2308719
126. Lipsman N, Meng Y, Bethune AJ, Huang Y, Lam B, Masellis M, et al. Blood–brain barrier opening in alzheimer’s disease using MR-guided focused ultrasound. Nat Commun. (2018) 9:1–8. doi: 10.1038/s41467-018-04529-6
127. Meng Y, Goubran M, Rabin JS, McSweeney M, Ottoy J, Pople CB, et al. Blood–brain barrier opening of the default mode network in alzheimer’s disease with magnetic resonance-guided focused ultrasound. Brain. (2023) 146:865–72. doi: 10.1093/brain/awac459
128. Mehta RI, Carpenter JS, Mehta RI, Haut MW, Ranjan M, Najib U, et al. Blood-brain barrier opening with mri-guided focused ultrasound elicits meningeal venous permeability in humans with early alzheimer disease. Radiology. (2021) 298:654–62. doi: 10.1148/radiol.2021200643
129. D’Haese PF, Ranjan M, Song A, Haut MW, Carpenter J, Dieb G, et al. B-amyloid plaque reduction in the hippocampus after focused ultrasound-induced blood–brain barrier opening in alzheimer’s disease. Front Hum Neurosci. (2020) 14:593672. doi: 10.3389/fnhum.2020.593672
130. Rezai AR, Ranjan M, Haut MW, Carpenter J, D’Haese PF, Mehta RI, et al. Focused ultrasound–mediated blood-brain barrier opening in alzheimer’s disease: long-term safety, imaging, and cognitive outcomes. J Neurosurg. (2023) 139:275–283. doi: 10.3171/2022.9.JNS221565
131. Rezai AR, Ranjan M, D’Haese PF, Haut MW, Carpenter J, Najib U, et al. Noninvasive hippocampal blood-brain barrier opening in alzheimer’s disease with focused ultrasound. Proc Natl Acad Sci USA. (2020) 117:9180–2. doi: 10.1073/pnas.2002571117
132. Epelbaum S, Burgos N, Canney M, Matthews D, Houot M, Santin MD, et al. Pilot study of repeated blood-brain barrier disruption in patients with mild alzheimer’s disease with an implantable ultrasound device. Alzheimer’s Res Ther. (2022) 14:1–13. doi: 10.1186/s13195-022-00981-1
133. Abrahao A, Meng Y, Llinas M, Huang Y, Hamani C, Mainprize T, et al. First-in-human trial of blood–brain barrier opening in amyotrophic lateral sclerosis using MR-guided focused ultrasound. Nat Commun. (2019) 10:1–9. doi: 10.1038/s41467-019-12426-9
134. Meng Y, Reilly RM, Pezo RC, Trudeau M, Sahgal A, Singnurkar A, et al. Mr-guided focused ultrasound enhances delivery of trastuzumab to her2-positive brain metastases. Sci Transl Med. (2021) 13:eabj4011. doi: 10.1126/scitranslmed.abj4011
135. Marty B, Larrat B, Landeghem MV, Robic C, Robert P, Port M, et al. Dynamic study of blood–brain barrier closure after its disruption using ultrasound: A quantitative analysis. J Cereb Blood Flow Metab. (2012) 32:1948–58. doi: 10.1038/jcbfm.2012.100
136. Ohta S, Kikuchi E, Ishijima A, Azuma T, Sakuma I, Ito T. Investigating the optimum size of nanoparticles for their delivery into the brain assisted by focused ultrasound-induced blood–brain barrier opening. Sci Rep. (2020) 10:18220. doi: 10.1038/s41598-020-75253-9
137. Tolboom N, Verger A, Albert NL, Brendel M, Cecchin D, Fernandez PA, et al. EANM position paper: theranostics in brain tumours—the present and the future. Eur J Nucl Med Mol Imaging. (2023) 51:202–5. doi: 10.1007/s00259-023-06425-8
138. Song KH, Harvey BK, Borden MA. State-of-the-art of microbubble-assisted blood-brain barrier disruption. Theranostics. (2018) 8(16):4393–408. doi: 10.7150/thno.26869
139. Pöhlmann J, Weller M, Marcellusi A, Grabe-Heyne K, Krott-Coi L, Rabar S, et al. High costs, low quality of life, reduced survival, and room for improving treatment: an analysis of burden and unmet needs in glioma. Front Oncol. (2024) 14:1368606. doi: 10.3389/fonc.2024.1368606
140. Oster C, Schmidt T, Agkatsev S, Lazaridis L, Kleinschnitz C, Sure U, et al. Are we providing best-available care to newly diagnosed glioblastoma patients? systematic review of phase iii trials in newly diagnosed glioblastoma 2005–2022. Neuro Oncol Adv. (2023) 5:vdad105. doi: 10.1093/noajnl/vdad105
141. Ghanouni P, Pauly KB, Elias WJ, Henderson J, Sheehan J, Monteith S, et al. Transcranial mri-guided focused ultrasound: A review of the technologic and neurologic applications. AJR Am J Roentgenol. (2015) 205:150–9. doi: 10.2214/AJR.14.13632
142. Kyriakou A, Neufeld E, Werner B, Paulides MM, Szekely G, Kuster N. A review of numerical and experimental compensation techniques for skull-induced phase aberrations in transcranial focused ultrasound. Int J Hyperthermia. (2014) 30:36–46. doi: 10.3109/02656736.2013.861519
143. Hasgall PA, Di Gennaro F, Baumgartner C, Neufeld E, Lloyd B, Gosselin MC, et al. It’is database for thermal and electromagnetic parameters of biological tissues, version 4.1, feb 22, 2022. doi: 10.13099/vip21000-04-1.
144. Weller M, van den Bent M, Preusser M, Le Rhun E, Tonn JC, Minniti G, et al. Eano guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. (2021) 18:170–86. doi: 10.1038/s41571-020-00447-z
145. Oronsky B, Reid TR, Oronsky A, Sandhu N, Knox SJ. A review of newly diagnosed glioblastoma. Front Oncol. (2021) 10:574012. doi: 10.3389/fonc.2020.574012
146. Preusser M, van den Bent M. Autologous tumor lysate-loaded dendritic cell vaccination (dcvax-l) in glioblastoma: Breakthrough or fata morgana? Neuro-Oncology. (2022) 25:631–4. doi: 10.1093/neuonc/noac281
147. Liau LM, Ashkan K, Brem S, Campian JL, Trusheim JE, Iwamoto FM, et al. Association of autologous tumor lysate-loaded dendritic cell vaccination with extension of survival among patients with newly diagnosed and recurrent glioblastoma: A phase 3 prospective externally controlled cohort trial. JAMA Oncol. (2023) 9:112–21. doi: 10.1001/jamaoncol.2022.5370
148. Herrlinger U, Tzaridis T, Mack F, Steinbach JP, Schlegel U, Sabel M, et al. Lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated mgmt promoter (ceteg/noa–09): a randomised, open-label, phase 3 trial. Lancet. (2019) 393:678–88. doi: 10.1016/S0140-6736(18)31791-4
149. Germano IM, Ziu M, Wen P, Ormond DR, Olson JJ. Congress of neurological surgeons systematic review and evidence-based guidelines update on the role of cytotoxic chemotherapy and other cytotoxic therapies in the management of progressive glioblastoma in adults. J Neuro Oncol. (2022) 158:225–253. doi: 10.1007/s11060-021-03900-w
150. Chang E, Pohling C, Beygui N, Patel CB, Rosenberg J, Ha DH, et al. Synergistic inhibition of glioma cell proliferation by withaferin a and tumor treating fields. J Neuro Oncol. (2017) 134:259–68. doi: 10.1007/s11060-017-2534-5
151. McFaline-Figueroa JR, Wen PY. Negative trials over and over again: How can we do better? Neuro-oncology. (2023) 25:1–3. doi: 10.1093/neuonc/noac226
152. Sharma P, Siddiqui BA, Anandhan S, Yadav SS, Subudhi SK, Gao J, et al. The next decade of immune checkpoint therapy. Cancer Discovery. (2021) 11:838–57. doi: 10.1158/2159-8290.CD-20-1680
153. Goswami S, Walle T, Cornish AE, Basu S, Anandhan S, Fernandez I, et al. Immune profiling of human tumors identifies cd73 as a combinatorial target in glioblastoma. Nat Med. (2020) 26:39–46. doi: 10.1038/s41591-019-0694-x
154. Sharma P, Goswami S, Raychaudhuri D, Siddiqui BA, Singh P, Nagarajan A, et al. Immune checkpoint therapy—current perspectives and future directions. Cell. (2023) 186:1652–69. doi: 10.1016/j.cell.2023.03.006
155. Ding H, Wu F. Image guided biodistribution and pharmacokinetic studies of theranostics. Theranostics. (2012) 2(11):1040–53. doi: 10.7150/thno.4652
156. McDannold N, Zhang Y, Supko JG, Power C, Sun T, Vykhodtseva N, et al. Blood-brain barrier disruption and delivery of irinotecan in a rat model using a clinical transcranial mri-guided focused ultrasound system. Sci Rep. (2020) 10:1–19. doi: 10.1038/s41598-020-65617-6
157. Shen Y, Goerner FL, Snyder C, Morelli JN, Hao D, Hu D, et al. T1 relaxivities of gadolinium-based magnetic resonance contrast agents in human whole blood at 1.5, 3, and 7 t. Invest Radiol. (2015) 50:330–8. doi: 10.1097/rli.0000000000000132
158. Aryal M, Park J, Vykhodtseva N, Zhang YZ, McDannold N. Enhancement in blood-tumor barrier permeability and delivery of liposomal doxorubicin using focused ultrasound and microbubbles: evaluation during tumor progression in a rat glioma model. Phys Med Biol. (2015) 60:2511–27. doi: 10.1088/0031-9155/60/6/2511
159. Saleem A, Brown GD, Brady F, Aboagye EO, Osman S, Luthra SK, et al. Metabolic activation of temozolomide measured in vivo using positron emission tomography. Cancer Res. (2003) 63:2409–15.
160. Arif WM, Elsinga PH, Gasca-Salas C, Versluis M, Martínez-Fernández R, Dierckx RA, et al. Focused ultrasound for opening blood-brain barrier and drug delivery monitored with positron emission tomography. J Control Release. (2020) 324:303–16. doi: 10.1016/j.jconrel.2020.05.020
161. Liu HL, Hsu PH, Lin CY, Huang CW, Chai WY, Chu PC, et al. Focused ultrasound enhances central nervous system delivery of bevacizumab for Malignant glioma treatment. Radiology. (2016) 281:99–108. doi: 10.1148/radiol.2016152444
162. Tran VL, Novell A, Tournier N, Gerstenmayer M, Schweitzer-Chaput A, Mateos C, et al. Impact of blood-brain barrier permeabilization induced by ultrasound associated to microbubbles on the brain delivery and kinetics of cetuximab: An immunopet study using 89zr-cetuximab. J Control Release. (2020) 328:304–12. doi: 10.1016/j.jconrel.2020.08.047
163. Porret E, Kereselidze D, Dauba A, Schweitzer-Chaput A, Jegot B, Selingue E, et al. Refining the delivery and therapeutic efficacy of cetuximab using focused ultrasound in a mouse model of glioblastoma: An 89zr-cetuximab immunopet study. Eur J Pharm Biopharm. (2023) 182:141–51. doi: 10.1016/j.ejpb.2022.12.006
164. Jansen MH, Veldhuijzen van Zanten SE, van Vuurden DG, Huisman MC, Vugts DJ, Hoekstra OS, et al. Molecular drug imaging: 89zr-bevacizumab pet in children with diffuse intrinsic pontine glioma. J Nucl Med. (2017) 58:711–6. doi: 10.2967/jnumed.116.180216
165. Keating GF, Chesney KM, Patel N, Kilburn L, Fonseca A, Packer RJ, et al. Mr-guided focused ultrasound in pediatric neurosurgery: current insights, technical challenges, and lessons learned from 45 treatments at children’s national hospital. Neurosur Focus. (2024) 57:E6. doi: 10.3171/2024.6.FOCUS24332
166. Weller M, Albert NL, Galldiks N, Bink A, Preusser M, Sulman EP, et al. Targeted radionuclide therapy for gliomas: Emerging clinical trial landscape. Neuro-Oncology. (2024) 26:S208–14. doi: 10.1093/neuonc/noae125
167. Lapi SE, Scott PJH, Scott AM, Windhorst AD, Zeglis BM, Abdel-Wahab M, et al. Recent advances and impending challenges for the radiopharmaceutical sciences in oncology. Lancet Oncol. (2024) 25:e236–49. doi: 10.1016/S1470-2045(24)00030-5
168. Nemati R, Shooli H, Rekabpour SJ, Ahmadzadehfar H, Jafari E, Ravanbod MR, et al. Feasibility and therapeutic potential of peptide receptor radionuclide therapy for High-Grade gliomas. Clin Nucl Med. (2021) 46:389–95. doi: 10.1097/RLU.0000000000003599
169. Albert NL, Le Rhun E, Minniti G, Mair MJ, Galldiks N, Tolboom N, et al. Translating the theranostic concept to neuro-oncology: disrupting barriers. Lancet Oncol. (2024) 25:e441–51. doi: 10.1016/S1470-2045(24)00145-1
170. Tolboom N, Verger A, Albert NL, Fraioli F, Guedj E, Traub-Weidinger T, et al. Theranostics in neurooncology: Heading toward new horizons. J Nucl Med. (2024) 65:167–73. doi: 10.2967/jnumed.123.266205
171. Jarkko Rautio MG, Laine K. Lat1-mediated prodrug uptake: a way to breach the blood–brain barrier? Ther Delivery. (2013) 4:281–4. doi: 10.4155/tde.12.165
172. Pichler J, Traub-Weidinger T, Spiegl K, Imamovic L, Braat AJAT, Snijders TJ, et al. Results from a phase i study of 4-l-[131i]iodo-phenylalanine ([131i]ipa) with external radiation therapy in patients with recurrent glioblastoma (ipax-1). Neuro Oncol Adv. (2024) 6:vdae130. doi: 10.1093/noajnl/vdae130
173. Wen PY, Preusser M, Albert NL. Design and conduct of theranostic trials in neuro-oncology: Challenges and opportunities. Neuro-Oncology. (2024) 26:S199–207. doi: 10.1093/neuonc/noae162
174. Gerisch M, Hafner FT, Lang D, Radtke M, Diefenbach K, Cleton A, et al. Mass balance, metabolic disposition, and pharmacokinetics of a single oral dose of regorafenib in healthy human subjects. Cancer Chemother Pharmacol. (2018) 81:195–206. doi: 10.1007/s00280-017-3480-9
175. Prinz C, Starke L, Millward JM, Fillmer A, Delgado PR, Waiczies H, et al. In vivo detection of teriflunomide-derived fluorine signal during neuroinflammation using fluorine mr spectroscopy. Theranostics. (2021) 11(6):2490–504. doi: 10.7150/thno.47130
176. Starke L, Millward JM, Prinz C, Sherazi F, Waiczies H, Lippert C, et al. First in vivo fluorine-19 magnetic resonance imaging of the multiple sclerosis drug siponimod. Theranostics. (2023) 13(4):1217–34. doi: 10.7150/thno.77041
177. Rahman R, Trippa L, Lee EQ, Arrillaga-Romany I, Fell G, Touat M, et al. Inaugural results of the individualized screening trial of innovative glioblastoma therapy: A phase ii platform trial for newly diagnosed glioblastoma using bayesian adaptive randomization. J Clin Oncol. (2023) 41:5524–5535. doi: 10.1200/JCO.23.00493
178. Roskoski R. Rule of five violations among the fda-approved small molecule protein kinase inhibitors. Pharmacol Res. (2023) 191:1–12. doi: 10.1016/j.phrs.2023.106774
179. Ziegler DS, Wong M, Mayoh C, Kumar A, Tsoli M, Mould E, et al. Brief report: Potent clinical and radiological response to larotrectinib in TRK fusion-driven high-grade glioma. Br J Cancer. (2018) 119:693–6. doi: 10.1038/s41416-018-0251-2
180. Doz F, van Tilburg CM, Geoerger B, Højgaard M, Øra I, Boni V, et al. Efficacy and safety of larotrectinib in trk fusion-positive primary central nervous system tumors. Neuro-Oncology. (2021) 24:997–1007. doi: 10.1093/neuonc/noab274
181. Weller M, Le Rhun E, Van den Bent M, Chang SM, Cloughesy TF, Goldbrunner R, et al. Diagnosis and management of complications from the treatment of primary central nervous system tumors in adults. Neuro-Oncology. (2023) 25:1200–24. doi: 10.1093/neuonc/noad038
182. Clark K, Vendt B, Smith K, Freymann J, Kirby J, Koppel P, et al. The cancer imaging archive (TCIA): Maintaining and operating a public information repository. J Digit Imaging. (2013) 26:1045–57. doi: 10.1007/s10278-013-9622-7
183. Sammartino F, Beam DW, Snell J, Krishna V. Kranion, an open-source environment for planning transcranial focused ultrasound surgery: technical note. J Neurosurg. (2020) 132:1249–55. doi: 10.3171/2018.11.JNS181995
184. Kyriakou A, Neufeld E, Werner B, Székely G, Kuster N. Full-wave acoustic and thermal modeling of transcranial ultrasound propagation and investigation of skull-induced aberration correction techniques: a feasibility study. J Ther Ultrasound. (2015) 3:1–18. doi: 10.1186/s40349-015-0032-9
185. Kim S, Chen J, Cheng T, Gindulyte A, He J, He S, et al. PubChem 2023 update. Nucleic Acids Res. (2022) 51:D1373–80. doi: 10.1093/nar/gkac956
186. Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. (2017) 46:D1074–82. doi: 10.1093/nar/gkx1037
187. EMEA/H/C/001124 - Temomedac EPAR Product Information. European medicines agency (2023). Available online at: https://www.ema.europa.eu/en/medicines/human/EPAR/temomedac (Accessed January 22, 2025).
188. Ozawa T, Rodriguez M, Zhao G, Yao TW, Fischer WN, Jandeleit B, et al. A novel blood-brain barrierpermeable chemotherapeutic agent for the treatment of glioblastoma. Cureus. (2021) 13:e17595. doi: 10.7759/cureus.17595
189. EMEA/H/C/000582 - Avastin EPAR Product Information. European medicines agency (2023). Available online at: https://www.ema.europa.eu/en/medicines/human/EPAR/avastin (Accessed January 22, 2025).
190. Gojo J, Sauermann R, Knaack U, Slavc I, Peyrl A. Pharmacokinetics of bevacizumab in three patients under the age of 3 years with cns Malignancies. Drugs R D. (2017) 17:469–74. doi: 10.1007/s40268-017-0190-z
191. Braghiroli MI, Sabbaga J, Hoff PM. Bevacizumab: overview of the literature. Expert Rev Anticancer Ther. (2012) 12:567–80. doi: 10.1586/era.12.13
192. Wu JY, Wu XN, Ding L, Zhao YB, Ai B, Li Y, et al. Phase i safety and pharmacokinetic study of bevacizumab in chinese patients with advanced cancer. Chin Med J. (2010) 123:901–6. doi: 10.3760/cma.j.issn.0366-6999.2010.07.025
193. Gleostine. Highlights of prescribing information. Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/017588s042lbl.pdf (Accessed January 22, 2025).
194. Lomustine. Summary of product characteristics. Available online at: https://www.medicines.org.uk/emc/product/1401 (Accessed January 22, 2025).
195. Lee FYF, Workman P, Roberts JT, Bleehen NM. Clinical pharmacokinetics of oral ccnu (lomustine). Cancer Chemother Pharmacol. (1985) 14:125–31. doi: 10.1007/BF00434350
196. Puyo S, Montaudon D, Pourquier P. From old alkylating agents to new minor groove binders. Crit Rev Oncol Hematol. (2014) 89:43–61. doi: 10.1016/j.critrevonc.2013.07.006
197. BiCNU. Highlights of prescribing information. Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/017422s055lbl.pdf (Accessed January 22, 2025).
198. Carmustine. Highlights of prescribing information. Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215000s000lbl.pdf (Accessed January 22, 2025).
199. Henner WD, Peters WP, Eder JP, Antman K, Schnipper L, Frei E 3rd. Pharmacokinetics and immediate effects of high-dose carmustine in man. Cancer Treat Rep. (1986) 70:877–80.
200. Gerson SL, Caimi PF, William BM, Creger RJ. Chapter 57 - pharmacology and molecular mechanisms of antineoplastic agents for hematologic Malignancies. In: Hematology, Seventh edition. Amsterdam, Netherlands: Elsevier Inc (2018). p. 849–912. doi: 10.1016/B978-0-323-35762-3.00057-3
Keywords: drug delivery, focused ultrasound, glioblastoma, glioma, blood-brain barrier, chemotherapy, brain tumors
Citation: Holman R and McDannold N (2025) Identifying new therapeutics for focused ultrasound-enhanced drug delivery in the management of glioblastoma. Front. Oncol. 15:1507940. doi: 10.3389/fonc.2025.1507940
Received: 14 October 2024; Accepted: 07 February 2025;
Published: 13 March 2025.
Edited by:
John Bianco, Princess Maxima Center for Pediatric Oncology, NetherlandsReviewed by:
Carlotta Pucci, Sant’Anna School of Advanced Studies, ItalyCopyright © 2025 Holman and McDannold. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ryan Holman, cmhvbG1hbjJAYndoLmhhcnZhcmQuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.