
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 31 January 2025
Sec. Hematologic Malignancies
Volume 15 - 2025 | https://doi.org/10.3389/fonc.2025.1506799
This article is part of the Research Topic Overcoming Resistance of Immune Checkpoint Inhibitors View all 3 articles
Background: The significance of soluble programmed death protein ligand-1 (PD-L1) in predicting the prognosis of diffuse large B-cell lymphoma (DLBCL) has been previously analyzed, but with conflicting results. This study investigated the effect of soluble PD-L1 (sPD-L1) expression on the prognosis of patients with DLBCL.
Methods: We comprehensively searched the Web of Science, PubMed, Embase, and CNKI databases between their inception and August 14, 2024. The value of sPD-L1 in predicting the overall survival (OS) and progression-free survival (PFS) of patients with DLBCL was analyzed by computing the combined hazard ratios (HRs) and 95% confidence intervals (CIs). Associations between sPD-L1 and the clinicopathological factors of DLBCL were explored by combining odds ratios (ORs) and 95%CIs.
Results: Seven articles involving 826 patients were included in this meta-analysis. Based on our pooled data, elevated sPD-L1 was closely related to poor OS (HR = 2.81, 95%CI = 1.99–3.95, p < 0.001) and inferior PFS (HR = 3.16, 95%CI = 1.41–7.08, p = 0.005) of DLBCL. Moreover, based on the pooled data, higher sPD-L1 was significantly related to the Eastern Cooperative Oncology Group Performance Status Scale (ECOG PS) ≥2 (OR=4.10, 95%CI=1.82-9.24, p=0.001), clinical stage III-IV (OR = 3.30, 95%CI = 1.48–7.39, p = 0.004), elevated lactate dehydrogenase (LDH) levels (OR = 2.14, 95%CI = 1.07–4.30, p = 0.032), and the International Prognostic Index (IPI) score 3–5 (OR = 3.83, 95%CI = 1.91–7.68, p < 0.001) in DLBCL.
Conclusion: According to our findings, a higher sPD-L1 level was a significant predictor of poor OS and PFS in patients with DLBCL. Elevated sPD-L1 levels are closely related to factors representing disease aggressiveness in DLBCL.
Diffuse large B-cell lymphoma (DLBCL) has the highest morbidity among lymphoid neoplasms, accounting for 30% of annual non-Hodgkin lymphoma (NHL) cases (1). DLBCL usually occurs in the elderly, and the median age at diagnosis is seven decades (2). Approximately 60% of DLBCL cases can be treated with regimens such as rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) (3). Although patients developing non-germinal center B cell (non-GCB) subtypes of DLBCL exhibit markedly worse prognosis than those with the GCB subtype, the R-CHOP regimen is the preferred choice for new DLBCL cases (4). Despite the effectiveness of chemotherapy in patients with DLBCL, the survival rate is less than 40%, with a 5-year survival of just 20–30% (1). Furthermore, approximately 40% of DLBCL patients relapse or develop resistance to treatment (5). Consequently, identifying efficient biomarkers for DLBCL prognosis is imperative.
A growing body of evidence suggests that programmed death protein-1 (PD-1)- and programmed death protein ligand-1 (PD-L1)-targeting immune checkpoint inhibitors (ICIs) are potent therapeutic options for many cancers (6). By interacting with PD-1, PD-L1 suppresses T-cell growth and activity, leading to immunological resistance (7). According to recent studies, soluble PD-L1 (sPD-L1) serves as a significant prognostic marker for various cancers, such as gastric cancer (8), hepatocellular carcinoma (9), prostate cancer (10), melanoma (11), and non-small cell lung cancer (NSCLC) (12). The efficiency of sPD-L1 in predicting DLBCL prognosis has been previously analyzed; however, inconsistent findings have been reported (13–19). For example, in some studies, higher sPD-L1 levels were significantly associated with a poor prognosis of DLBCL (14–16, 19). However, according to other researchers, sPD-L1 is not markedly related to survival outcomes of DLBCL (17). Consequently, a literature review was conducted before the present meta-analysis to analyze sPD-L1’s precise effect on prognosis in DLBCL. Moreover, the correlations between sPD-L1 and DLBCL clinicopathological factors were examined in this meta-analysis.
This study was conducted according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guideline (20).
We thoroughly searched PubMed, Web of Science, Embase, and CNKI between inception and August 14, 2024, using the search terms (soluble or serum or plasma) and (lymphoma large B-cell or diffuse large B-cell lymphoma or DLBCL or lymphoma) and programmed cell death ligand 1 (PD-L1). No limitations were placed on the language of publication. References from relevant studies were searched to identify additional related studies.
Articles on the following subjects were enrolled (1): those describing DLBCL diagnosed by pathology (2), those evaluating associations between sPD-L1 levels and survival outcomes in DLBCL (3), those with reported hazard ratios (HRs) and 95% confidence intervals (CIs), and (4) those with an available threshold sPD-L1. The following articles were excluded (1): reviews, case reports, comments, meeting abstracts, and letters (2); articles without available survival information; and (3) animal studies.
Two independent researchers (HL and LL) reviewed the literature and collected data from relevant studies. Disputes were resolved through negotiation with a third researcher (WD). The following information was collected: author, country, year, sample size, age, sex, study design, study center, study period, clinical stage, treatment, threshold, threshold determination method, specimen, follow-up, survival outcomes, survival analysis types, and HRs with 95%CIs. Primary and secondary survival endpoints included overall survival (OS) and progression-free survival (PFS), respectively. The Newcastle Ottawa Scale (NOS) was used to assess the quality of the selected articles (21). Notably, NOS evaluates quality in 3 domains, namely, selection, comparability, and outcome. The NOS scores range from 0–9, and scores ≥ 6 represent high-quality studies.
The effect of sPD-L1 in forecasting OS and PFS in DLBCL was estimated by combining HRs and 95%CIs. We assessed the heterogeneity between the included studies based on I2 statistics and the Cochrane Q test. High heterogeneities were judged based on I2 > 50% and p < 0.10 and in those cases a random-effects model was used. Otherwise, a fixed-effects model was adopted. Subgroup analyses based on various factors were performed to further investigate the prognostic value of sPD-L1 expression. Associations between sPD-L1 and clinicopathological factors of DLBCL were analyzed by combining odds ratios (ORs) with 95% confidence intervals (CIs). To assess the stability and robustness of the results, we performed a sensitivity analysis by eliminating one study and calculating new HRs and 95%CIs. Publication bias was evaluated using funnel plots, and Begg’s and Egger’s tests. Stata 12.0 (StataCorp, College Station, Texas, USA) was used for statistical analysis. P-values < 0.05 were considered statistically significant.
From primary literature retrieval, 820 articles were obtained, of which 604 were retained after eliminating duplicates (Figure 1). Through title and abstract screening, 589 articles were eliminated because of irrelevance. Later, the full-texts of 15 articles were analyzed, of which eight were removed because of irrelevance to sPD-L1 (n = 4) and no provided survival data (n = 4). Ultimately, seven articles comprising 826 cases (13–19) were included in the present study (Figure 1).
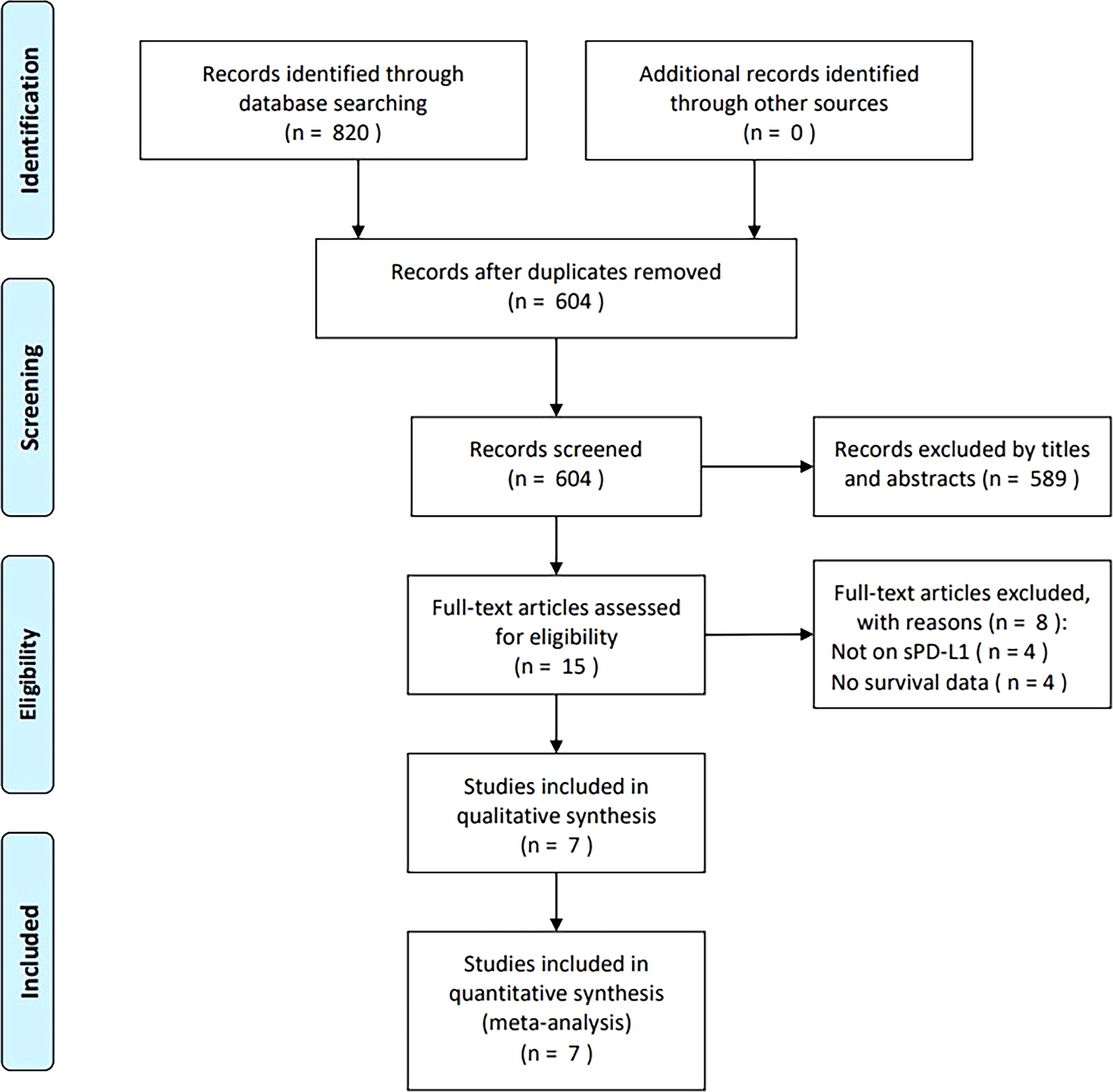
Figure 1. PRISMA flowchart of the study retrieval and selection process for this meta-analysis. From primary literature retrieval, 820 articles were obtained, among which, 604 were maintained following eliminating duplicates. By title- and abstract-screening, 589 articles were eliminated because of irrelevance. Later, full-texts in 15 articles were analyzed, among which, 8 were removed because of irrelevance to sPD-L1 (n=4) and no survival data provided (n=4). Eventually, seven articles were recruited in the present work.
Table 1 shows the baseline characteristics of all included studies (13–19) published between 2014–2023. Four studies were conducted in China (14, 16, 18, 19) and one each in France (13), South Korea (15), and Finland (17). Four articles were published in English (13, 15–17) and three were published in Chinese (14, 18, 19). Five studies had a retrospective design (14–16, 18, 19), and two had a prospective design (13, 17). Four were single-center studies (14, 16, 18, 19) and three were multicenter studies (13, 15, 17). The sample sizes ranged from 41–288 (median, 109). All studies included patients with stage I–IV DLBCL (13–19). Six studies indicated treatment of patients with chemotherapy (13, 14, 16–19) and one study indicated chemoradiotherapy (CRT) (15). Four studies reported sPD-L1 detection in plasma (13, 14, 16, 19), and three in sera (15, 17, 18). The median sPD-L1 threshold was 1.52 (range 0.432–4.57) ng/ml. The threshold was determined by using receiver operating characteristic (ROC) curves in five studies (13–15, 18, 19), and two studies used median values (16, 17). All seven studies reported the significance of sPD-L1 in predicting OS (13–19), whereas four studies reported a relationship between sPD-L1 and PFS (15–17, 19) in DLBCL. Five studies derived HRs and 95%CIs through univariate analysis (14–18), whereas two adopted multivariate analyses (13, 19). NOS scores ranged from 7–9, suggesting that the enrolled articles were of high quality (Table 1).
All seven studies with 826 patients (13–19) mentioned the effect of sPD-L1 in predicting the OS of DLBCL. A fixed-effects model was applied considering the insignificant heterogeneity (I2 = 27.0%, p = 0.223). Resulting values of HR = 2.81, 95%CI = 1.99–3.95, and p < 0.001 showed that a higher sPD-L1 level was markedly associated with poor OS in DLBCL (Table 2, Figure 2). Based on subgroup analyses, a higher sPD-L1 still significantly predicted poor OS regardless of region, sample size, study design, study center, treatment, cut-off value, or specimen (all p < 0.05; Table 2).
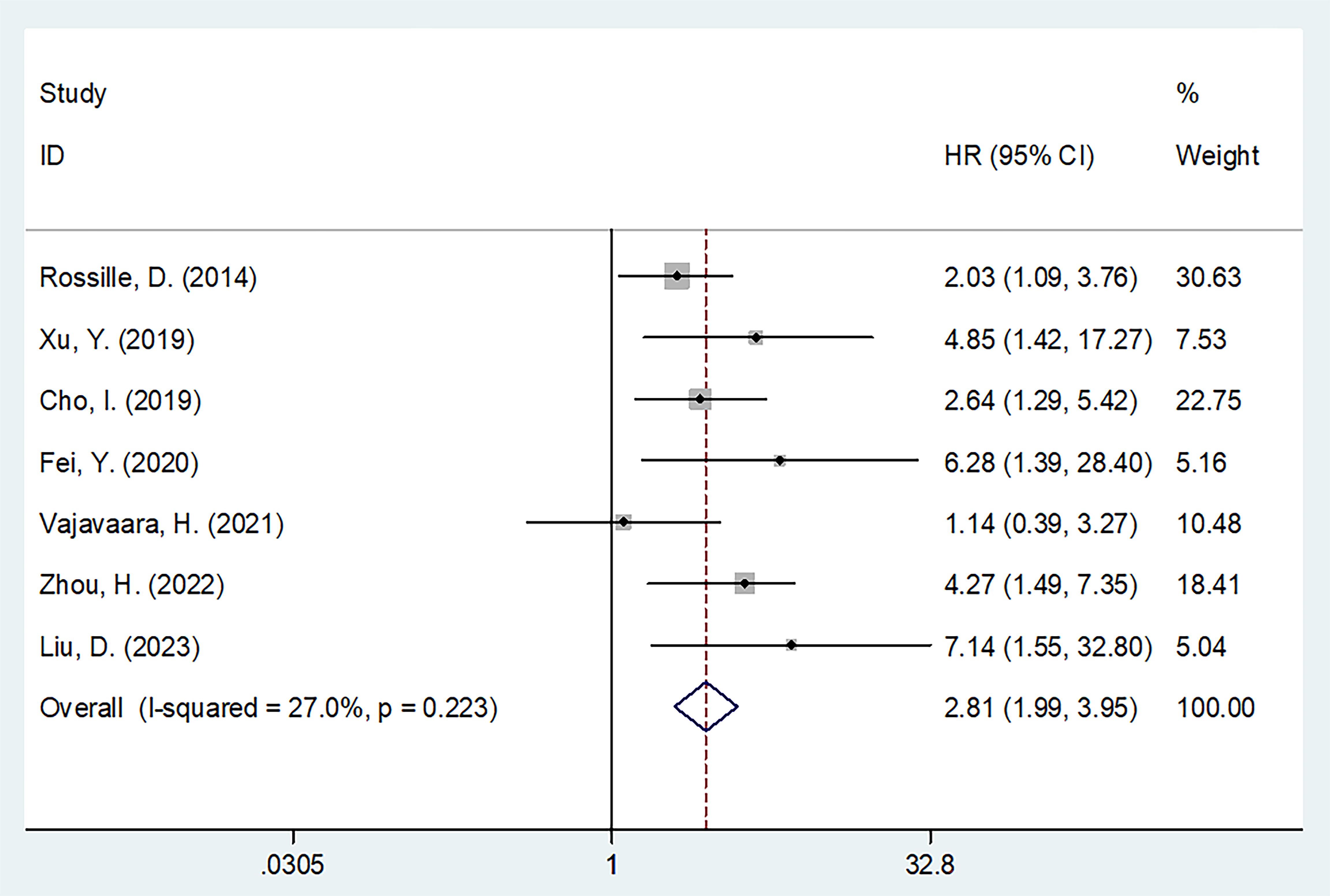
Figure 2. Forest plot of the association between sPD-L1 and OS in patients with DLBCL. HR=2.81, 95%CI=1.99-3.95, and p<0.001 could be acquired, which revealed that higher sPD-L1 level was markedly associated with dismal OS in DLBCL.
Four studies involving 385 patients reported a relationship between sPD-L1 levels and PFS (15–17, 19). Considering the heterogeneity (I2 = 66.5%, p = 0.035), this study utilized a random-effects model (Table 2). As a result, high sPD-L1 apparently estimated poor PFS of DLBCL (HR = 3.16, 95%CI = 1.41–7.08, p = 0.005; Table 3, Figure 3). As indicated by the subgroup analyses, the role of sPD-L1 in forecasting PFS remained unaffected by treatment, threshold, study center, specimen, or survival analysis (all p < 0.05; Table 3).
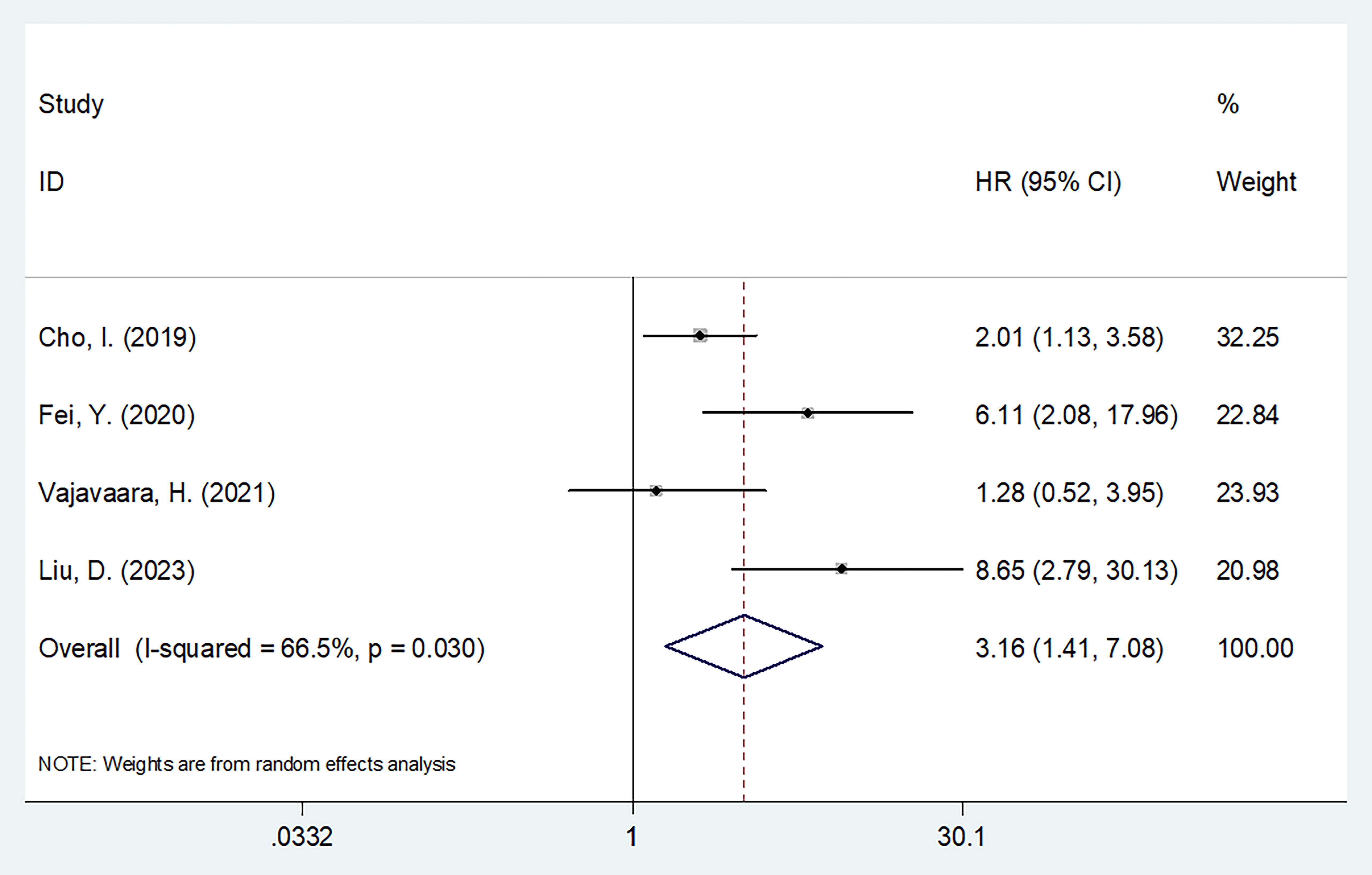
Figure 3. Forest plot of the association between sPD-L1 and PFS in patients with DLBCL. As a result, high sPD-L1 apparently estimated poor PFS of DLBCL (HR=3.16, 95%CI=1.41-7.08, p=0.005).
Three studies incorporating 276 patients (15–17) provided information regarding the relationship between sPD-L1 expression and the clinicopathological features of DLBCL. As revealed by our pooled data, a higher sPD-L1 was significantly related to the Eastern Cooperative Oncology Group Performance Status (ECOG PS) ≥2 (OR = 4.10, 95%CI= 1.82–9.24, p = 0.001), clinical stage III–IV (OR= 3.30, 95%CI = 1.48–7.39, p = 0.004), elevated lactate dehydrogenase (LDH) levels (OR = 2.14, 95%CI = 1.07–4.30, p = 0.032), and the International Prognostic Index (IPI) score 3–5 (OR = 3.83, 95%CI= 1.91–7.68, p < 0.001) (Table 4, Figure 4). But sPD-L1 remained uncorrelated with sex (OR = 1.08, 95%CI = 0.64–1.82, p = 0.778) or age (OR= 1.78, 95%CI = 0.58–5.41, p = 0.312) in DLBCL (Table 4, Figure 4).
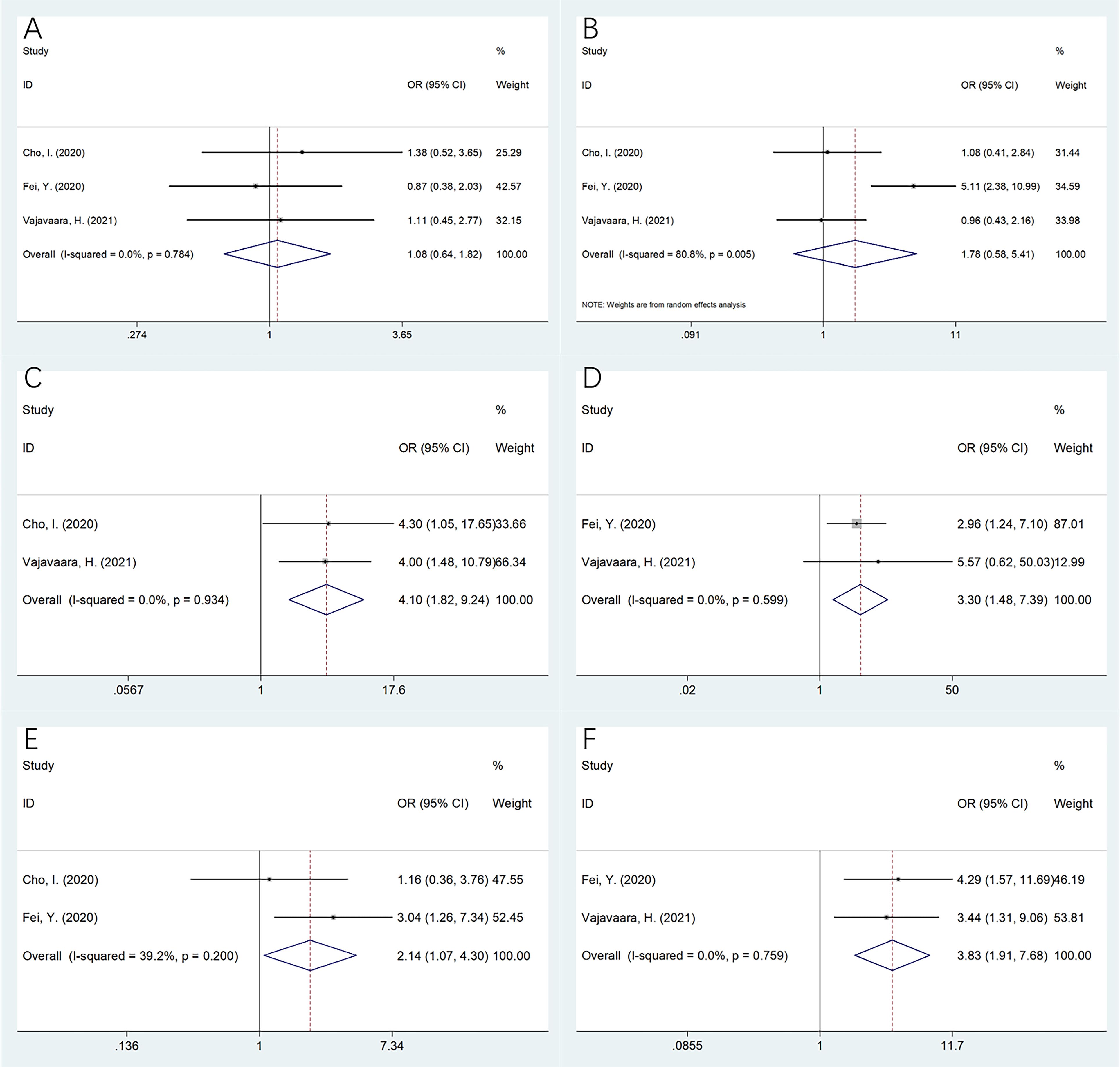
Figure 4. The relationship betweensPD-L1 and clinicopathological features in DLBCL. (A) Gender (male vs female); (B) Age (years) (≥60 vs <60); (C) ECOG PS (≥2 vs 0-1); (D) Clinical stage (III-IV vs I-II); (E) LDH level (elevated vs normal); and (F) IPI score (3-5 vs 0-2). As revealed by our pooling data, higher sPD-L1 was significantly related to ECOG PS≥2 (OR=4.10, 95%CI=1.82-9.24, p=0.001), clinical stage III-IV (OR=3.30, 95%CI=1.48-7.39, p=0.004), elevated LDH levels (OR=2.14, 95%CI=1.07-4.30, p=0.032), and IPI score 3-5 (OR=3.83, 95%CI=1.91-7.68, p<0.001). But sPD-L1 remained uncorrelated with gender (OR=1.08, 95%CI=0.64-1.82, p=0.778) or age (OR=1.78, 95%CI=0.58-5.41, p=0.312) in DLBCL.
One article was eliminated during the sensitivity analyses of OS and PFS each time to analyze the effect of this study on the overall results. These results intuitively demonstrated their robustness (Figure 5).
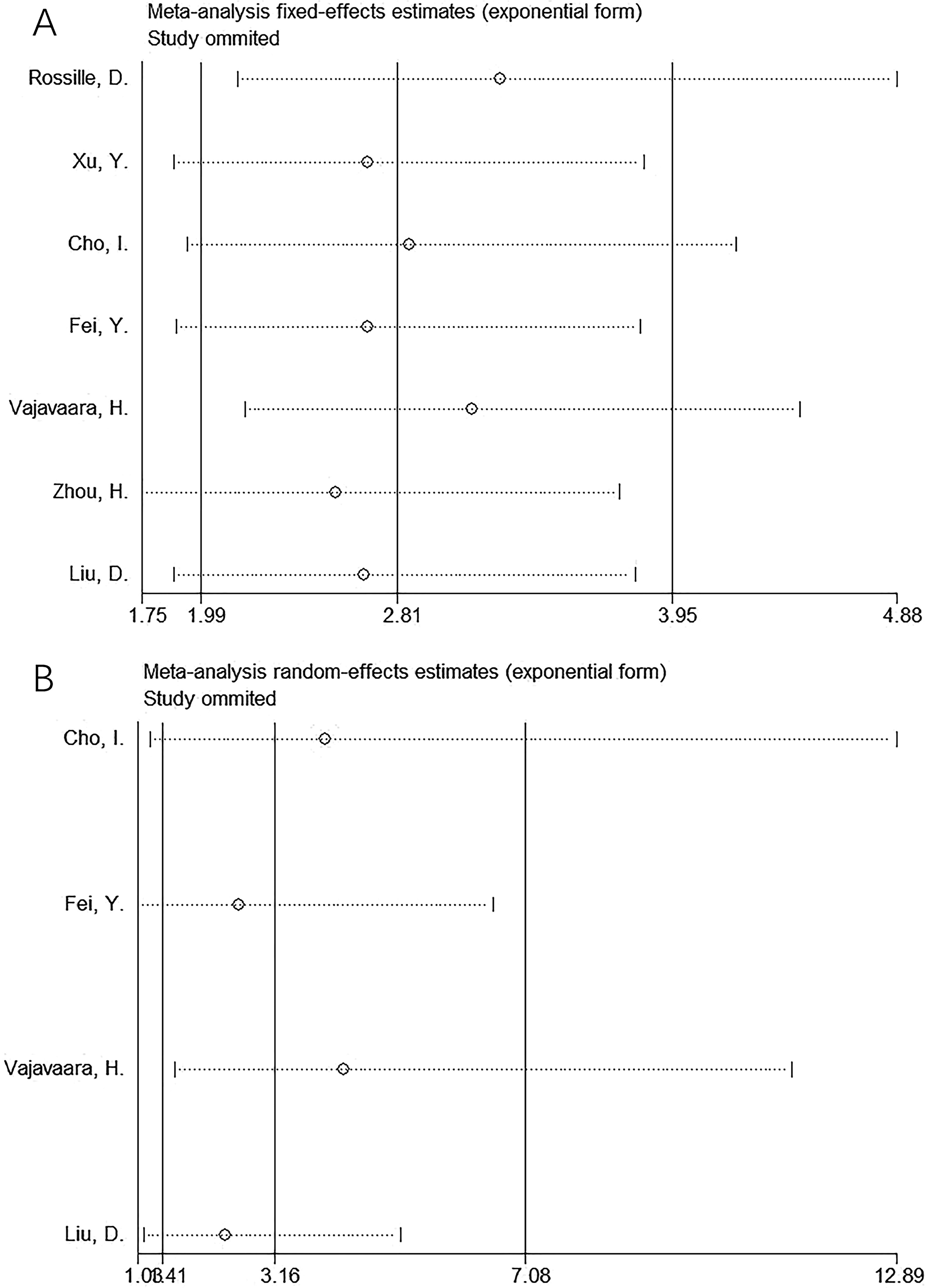
Figure 5. Sensitivity analysis. (A) OS and (B) PFS. One article was eliminated each time during sensitivity analyses on OS and PFS for analyzing the effect of this study on whole results. As a result, the results intuitively showed their robustness.
Begg’s and Egger’s tests were conducted to evaluate publication bias, which revealed no publication bias for OS (p = 0.133 and 0.219 in Begg’s and Egger’s tests, respectively) or PFS (p = 0.308 and 0.399 in Begg’s and Egger’s tests, respectively) (Figure 6).
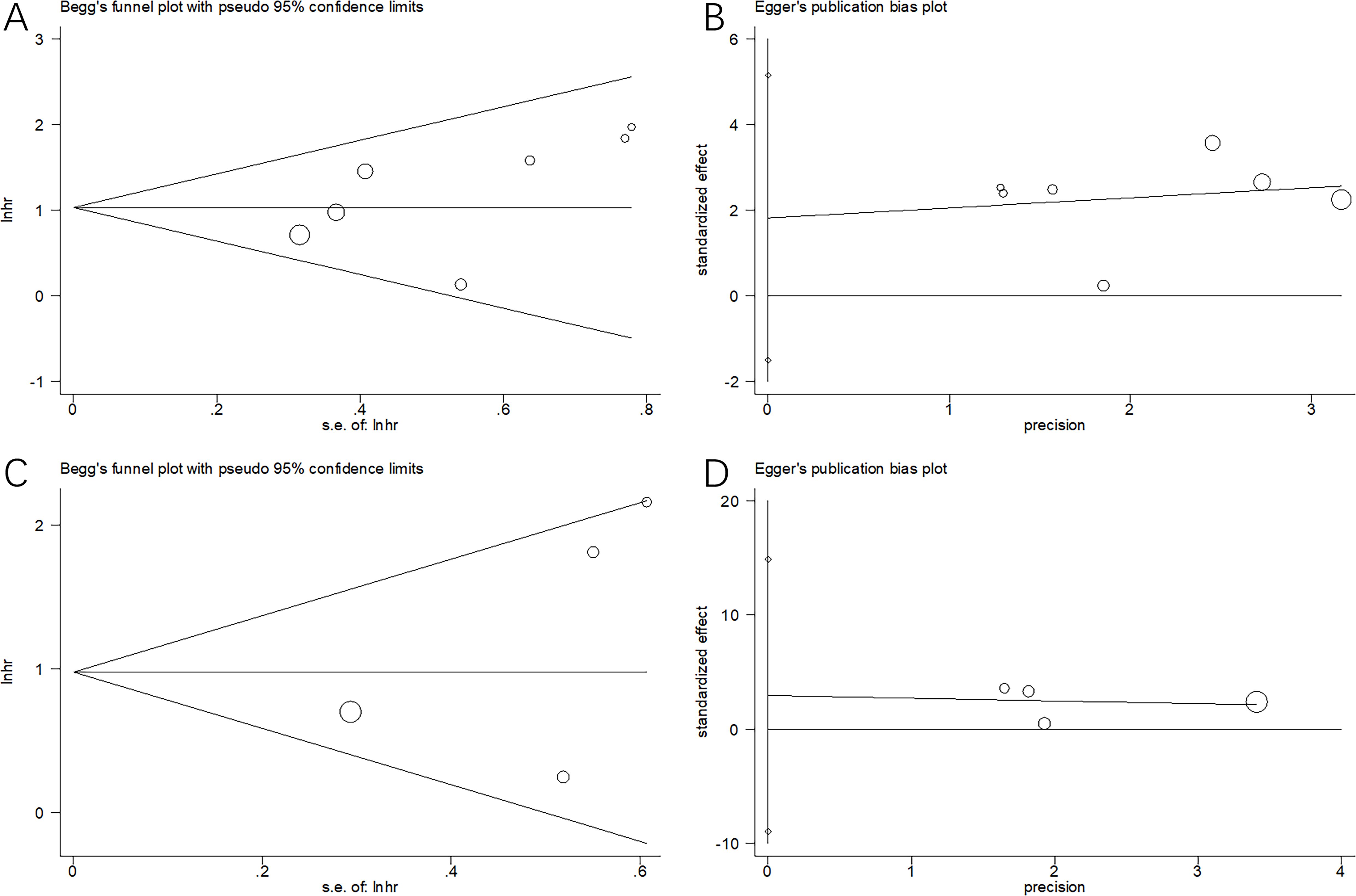
Figure 6. Publication bias test in this meta-analysis. (A) Begg’s test for OS, p=0.133; (B) Egger’s test for OS, p=0.219; (C) Begg’s test for PFS, p=0.308; and (D) Egger’s test for PFS, p=0.399. We conducted Begg’s and Egger’s tests to evaluate publication bias, which revealed no publication bias of OS or PFS.
The effect of sPD-L1 in DLBCL prognosis has been widely examined; however, inconsistent results have been reported. Data were collected from seven articles with 826 cases (13–19) to analyze how sPD-L1 affects DLBCL prognosis. Based on our data, elevated sPD-L1 levels were markedly associated with unfavorable OS and shortened PFS in patients with DLBCL. Additionally, a higher sPD-L1 was also apparently connected to ECOG PS ≥ 2, advanced clinical stage, elevated LDH levels, and IPI score 3–5 in DLBCL. Sensitivity, subgroup, and publication-bias tests were used to verify the stability of the results. Collectively, high sPD-L1 levels remarkably predicted inferior short- and long-term survival in patients with DLBCL. To our knowledge, this is the first meta-analysis to explore the effect of sPD-L1 expression on DLBCL prognosis.
This study showed that sPD-L1 has a prognostic significance in DLBCL. The potential mechanisms underlying sPD-L1’s prognostic value for DLBCL are as follows: First, sPD-L1 can reduce cyclin A, ERK (p-ERK), and Akt; reduce adenosine triphosphate production; and attenuate T-cell respiration (22). By inhibiting the antitumor action of T cells by binding to PD-1 on their surface, sPD-L1 induces T cell death, thus promoting the immune escape of cancer cells (23). Second, CD274 encodes sPD-L1, a type I transmembrane glycoprotein. Its transcription generates several PD-L1 splice variants, each differing in length. In particular, exon 4-enriched variants produce PD-L1, which is secreted by the body (24). Third, in a mouse model of triple-negative breast cancer, a senescent tumor-cell vaccine expressing sPD-1 retarded tumor occurrence and inhibited tumor development (25).
Our meta-analysis showed that sPD-L1 is an important factor in predicting poor OS and PFS outcomes in patients with DLBCL. Therefore, the PD-1/PD-L1 axis may be an effective target for DLBCL treatment. Two antibodies targeting PD-1, nivolumab, and pembrolizumab, and three antibodies targeting PD-L1, durvalumab, atezolizumab, and avelumab, have been approved for B-cell lymphoma (26, 27). Atezolizumab is a monoclonal antibody of the humanized immunoglobulin G1 type that targets PD-L1, and has shown antitumor activity in several types of tumors. Younes et al. assessed the safety and effectiveness of atezolizumab combined with R-CHOP in patients with newly diagnosed DLBCL and found that the complete remission rates were enhanced when compared to those in the control group (28). PFS was achieved with a relatively shorter follow-up period than OS. Our meta-analysis showed consistent prognostic efficiency of sPD-L1 in both OS and PFS, indicating that sPD-L1 could be used to monitor long-term survival outcomes in DLBCL.
This meta-analysis suggests that sPD-L1 is a significant prognostic marker for DLBCL. This study had several strengths. To our knowledge, this is the first meta-analysis to investigate the prognostic value of sPD-L1 in DLBCL. Previous studies showed the prognostic effect of sPD-L1 in lymphoma (29); however, lymphoma is a heterogeneous disease with various entities. This meta-analysis focused on DLBCL, which accounts for 30% of all NHL cases. Second, this study not only investigated the prognostic value of sPD-L1 in DLBCL but also showed a correlation between sPD-L1 expression and several clinicopathological factors of DLBCL. These results provide a comprehensive understanding of the biological role of sPD-L1 in DLBCL. Third, our results were verified using sensitivity analysis and publication bias tests, and were reliable.
Recent studies have revealed advances in the prognostic function of sPD-L1 in cancer (30). A meta-analysis of 1,054 patients showed that elevated sPD-L1 levels were significantly associated with poor OS in patients with ICI-treated cancer (31). Mazzaschi et al. conducted a study on 109 patients with NSCLC, analyzing the pretreatment levels of soluble PD-L1, circulating PD1+ CD8+ cells, and NK cells as biomarkers for predicting ICI response (32). With the advent of precision medicine, liquid biopsies and circulating biomarkers have become essential for identifying the best treatment options for patients. Circulating biomarkers show potential for forecasting immunotherapy outcomes; however, they face hurdles before being integrated into standard clinical practice. First, no standardized pipelines are available for the isolation and analysis of circulating analytes. Second, no optimal cut-offs have been universally established for stratifying patients and predicting their response to ICI. Third, circulating biomarkers are more relevant for patients with advanced or metastatic disease, as those patients are more likely to release tumor-derived cells, EVs, and DNA fragments into the blood. The analysis of circulating biomarkers may not be beneficial in patients with localized tumors (33). Future studies should investigate the standard cut-off value of sPD-L1 in various cancers.
Recent meta-analyses have suggested that sPD-L1 is important in forecasting the prognostic outcomes of different cancers (34–38). According to Cui et al., higher sPD-L1 expression before treatment was markedly associated with poor OS and PFS in NSCLC patients in a meta-analysis of 928 patients (35). As reported by Scirocchi et al., higher sPD-L1 expression in the blood correlated with poor OS and PFS in tumor patients receiving immunotherapy in a meta-analysis of 12 articles (36). A recent meta-analysis of 1,188 cases showed that higher sPD-L1 levels were markedly associated with worse OS and PFS in patients with lung cancer receiving ICIs therapy (37). According to Li et al., higher sPD-L1 expression is associated with worse OS and other survival endpoints in different cancers in a meta-analysis of 21 studies (38). Our results are consistent with studies investigating other cancers.
The present study had some limitations. First, most eligible studies were conducted in Asia. Therefore, our findings are likely to be applicable to Asian patients with DLBCL. Second, most of the included studies had retrospective designs, and therefore, inherent heterogeneity was possible. Third, the threshold sPD-L1 level was not consistent among the eligible studies, which may have caused selection bias. Owing to these limitations, multicenter prospective trials with uniform thresholds should be conducted to validate the prognostic role of sPD-L1 in DLBCL.
In summary, high sPD-L1 levels are a significant predictor of poor OS and PFS in patients with DLBCL. Elevated sPD-L1 levels are related to factors representing disease aggressiveness in DLBCL.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
HL: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Writing – original draft. LL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Validation, Visualization, Writing – original draft. JM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – original draft. MS: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Resources, Validation, Visualization, Writing – original draft. HW: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Visualization, Writing – original draft. ZW: Conceptualization, Formal analysis, Methodology, Project administration, Resources, Visualization, Writing – original draft. WD: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Visualization, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We would like to thank Editage (www.editage.com) for English language editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sehn LH, Salles G. Diffuse large B-cell lymphoma. N Engl J Med. (2021) 384:842–58. doi: 10.1056/NEJMra2027612
2. Bataillard EJ, Cheah CY, Maurer MJ, Khurana A, Eyre TA, El-Galaly TC. Impact of R-CHOP dose intensity on survival outcomes in diffuse large B-cell lymphoma: a systematic review. Blood Adv. (2021) 5:2426–37. doi: 10.1182/bloodadvances.2021004665
3. Feugier P, Van Hoof A, Sebban C, Solal-Celigny P, Bouabdallah R, Fermé C, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d’Etude des Lymphomes de l’Adulte. J Clin oncology: Off J Am Soc Clin Oncol. (2005) 23:4117–26. doi: 10.1200/jco.2005.09.131
4. Miao Y, Medeiros LJ, Li Y, Li J, Young KH. Genetic alterations and their clinical implications in DLBCL. Nat Rev Clin Oncol. (2019) 16:634–52. doi: 10.1038/s41571-019-0225-1
5. Vaidya R, Witzig TE. Prognostic factors for diffuse large B-cell lymphoma in the R(X)CHOP era. Ann oncology: Off J Eur Soc Med Oncol. (2014) 25:2124–33. doi: 10.1093/annonc/mdu109
6. Doroshow DB, Sanmamed MF, Hastings K, Politi K, Rimm DL, Chen L, et al. Immunotherapy in non-small cell lung cancer: facts and hopes. Clin Cancer research: an Off J Am Assoc Cancer Res. (2019) 25:4592–602. doi: 10.1158/1078-0432.Ccr-18-1538
7. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. (2012) 12:252–64. doi: 10.1038/nrc3239
8. Chivu-Economescu M, Herlea V, Dima S, Sorop A, Pechianu C, Procop A, et al. Soluble PD-L1 as a diagnostic and prognostic biomarker in resectable gastric cancer patients. Gastric cancer: Off J Int Gastric Cancer Assoc Japanese Gastric Cancer Assoc. (2023) 26:934–46. doi: 10.1007/s10120-023-01429-7
9. Yang Z, Liu X, Zhou P, Mao Y, Li J, Mao X. Clinical significance of soluble programmed cell death-ligand 1 in hepatitis B virus-related hepatocellular carcinoma. MedComm (2020). (2023) 4:e225. doi: 10.1002/mco2.225
10. Zvirble M, Survila Z, Bosas P, Dobrovolskiene N, Mlynska A, Zaleskis G, et al. Prognostic significance of soluble PD-L1 in prostate cancer. Front Immunol. (2024) 15:1401097. doi: 10.3389/fimmu.2024.1401097
11. Oya K, Nakamura Y, Shen LT, Ishizuki S, Matsusaka S, Fujisawa Y. Soluble PD-L1 predicts tumor response and immune-related adverse events in patients with advanced melanoma treated with anti-PD-1 antibodies. J Dermatol. (2024) 51:807–15. doi: 10.1111/1346-8138.17183
12. Himuro H, Nakahara Y, Igarashi Y, Kouro T, Higashijima N, Matsuo N, et al. Clinical roles of soluble PD-1 and PD-L1 in plasma of NSCLC patients treated with immune checkpoint inhibitors. Cancer immunology immunotherapy: CII. (2023) 72:2829–40. doi: 10.1007/s00262-023-03464-w
13. Rossille D, Gressier M, Damotte D, Maucort-Boulch D, Pangault C, Semana G, et al. High level of soluble programmed cell death ligand 1 in blood impacts overall survival in aggressive diffuse large B-Cell lymphoma: results from a French multicenter clinical trial. Leukemia. (2014) 28:2367–75. doi: 10.1038/leu.2014.137
14. Xu Y. The clinical value of peripheral blood sPD-L1 in predicting the prognosis of invasive non-hodgkin lymphoma. Pract J Cancer. (2019) 34:339–42. doi: 10.3969/j.issn.1001-5930.2019.02.045
15. Cho I, Lee H, Yoon SE, Ryu KJ, Ko YH, Kim WS, et al. Serum levels of soluble programmed death-ligand 1 (sPD-L1) in patients with primary central nervous system diffuse large B-cell lymphoma. BMC Cancer. (2020) 20:120. doi: 10.1186/s12885-020-6612-2
16. Fei Y, Yu J, Li Y, Li L, Zhou S, Zhang T, et al. Plasma soluble PD-L1 and STAT3 predict the prognosis in diffuse large B cell lymphoma patients. J Cancer. (2020) 11:7001–8. doi: 10.7150/jca.47816
17. Vajavaara H, Mortensen JB, Leivonen SK, Hansen IM, Ludvigsen M, Holte H, et al. Soluble PD-1 but not PD-L1 levels predict poor outcome in patients with high-risk diffuse large B-cell lymphoma. Cancers (Basel). (2021) 13(3):398. doi: 10.3390/cancers13030398
18. Zhou H, Wang Y, Peng Y. Relationship of serum TTR and PD-L1 levels with the pathological characteristics and prognosis of patients with relapsed/refractory diffuse large B-cell lymphoma. Pract J Cancer. (2022) 37:758–62. doi: 10.3969/j.issn.1001-5930.2022.05.016
19. Liu D, Wang X, Sun Y, Zheng W, She X, Lian S, et al. Peripheral blood soluble PD-L1 as a biomarker for prognosis assessment of patients with Malignant B-cell lymphoma. J Dalian Med Univ. (2023) 45:113–8. doi: 10.11724/jdmu.2023.02.03
20. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. (2009) 62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005
21. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
22. Jalali S, Price-Troska T, Paludo J, Villasboas J, Kim HJ, Yang ZZ, et al. Soluble PD-1 ligands regulate T-cell function in Waldenstrom macroglobulinemia. Blood Adv. (2018) 2:1985–97. doi: 10.1182/bloodadvances.2018021113
23. Gu D, Ao X, Yang Y, Chen Z, Xu X. Soluble immune checkpoints in cancer: production, function and biological significance. J Immunother Cancer. (2018) 6:132. doi: 10.1186/s40425-018-0449-0
24. Hassounah NB, Malladi VS, Huang Y, Freeman SS, Beauchamp EM, Koyama S, et al. Identification and characterization of an alternative cancer-derived PD-L1 splice variant. Cancer immunology immunotherapy: CII. (2019) 68:407–20. doi: 10.1007/s00262-018-2284-z
25. Chen Z, Hu K, Feng L, Su R, Lai N, Yang Z, et al. Senescent cells re-engineered to express soluble programmed death receptor-1 for inhibiting programmed death receptor-1/programmed death ligand-1 as a vaccination approach against breast cancer. Cancer Sci. (2018) 109:1753–63. doi: 10.1111/cas.13618
26. Sokołowski M, Sokołowska A, Mazur G, Butrym A. Programmed cell death protein receptor and ligands in hematological Malignancies - Current status. Crit Rev oncology/hematology. (2019) 135:47–58. doi: 10.1016/j.critrevonc.2019.01.003
27. Ribatti D, Cazzato G, Tamma R, Annese T, Ingravallo G, Specchia G. Immune checkpoint inhibitors targeting PD-1/PD-L1 in the treatment of human lymphomas. Front Oncol. (2024) 14:1420920. doi: 10.3389/fonc.2024.1420920
28. Younes A, Burke JM, Cheson BD, Diefenbach CS, Ferrari S, Hahn UH, et al. Safety and efficacy of atezolizumab with rituximab and CHOP in previously untreated diffuse large B-cell lymphoma. Blood Adv. (2023) 7:1488–95. doi: 10.1182/bloodadvances.2022008344
29. Ding Y, Sun C, Hu L, Xiong S, Zhai Z. Prognostic value of soluble programmed cell death ligand-1 (sPD-L1) in lymphoma: a systematic review and meta-analysis. Ann Hematol. (2023) 102:2425–34. doi: 10.1007/s00277-023-05325-z
30. Holder AM, Dedeilia A, Sierra-Davidson K, Cohen S, Liu D, Parikh A, et al. Defining clinically useful biomarkers of immune checkpoint inhibitors in solid tumors. Nat Rev Cancer. (2024) 24:498–512. doi: 10.1038/s41568-024-00705-7
31. Széles Á, Fazekas T, Váncsa S, Váradi M, Kovács PT, Krafft U, et al. Pre-treatment soluble PD-L1 as a predictor of overall survival for immune checkpoint inhibitor therapy: a systematic review and meta-analysis. Cancer immunology immunotherapy: CII. (2023) 72:1061–73. doi: 10.1007/s00262-022-03328-9
32. Mazzaschi G, Minari R, Zecca A, Cavazzoni A, Ferri V, Mori C, et al. Soluble PD-L1 and circulating CD8+PD-1+ and NK cells enclose a prognostic and predictive immune effector score in immunotherapy treated NSCLC patients. Lung Cancer (Amsterdam Netherlands). (2020) 148:1–11. doi: 10.1016/j.lungcan.2020.07.028
33. Goh KY, Cheng TY, Tham SC, Lim DW. Circulating biomarkers for prediction of immunotherapy response in NSCLC. Biomedicines. (2023) 11(2):508. doi: 10.3390/biomedicines11020508
34. Shimizu T, Inoue E, Ohkuma R, Kobayashi S, Tsunoda T, Wada S. Soluble PD-L1 changes in advanced non-small cell lung cancer patients treated with PD-1 inhibitors: an individual patient data meta-analysis. Front Immunol. (2023) 14:1308381. doi: 10.3389/fimmu.2023.1308381
35. Cui Q, Li W, Wang D, Wang S, Yu J. Prognostic significance of blood-based PD-L1 analysis in patients with non-small cell lung cancer undergoing immune checkpoint inhibitor therapy: a systematic review and meta-analysis. World J Surg Oncol. (2023) 21:318. doi: 10.1186/s12957-023-03215-2
36. Scirocchi F, Strigari L, Di Filippo A, Napoletano C, Pace A, Rahimi H, et al. Soluble PD-L1 as a prognostic factor for immunotherapy treatment in solid tumors: systematic review and meta-analysis. Int J Mol Sci. (2022) 23(22):14496. doi: 10.3390/ijms232214496
37. Cheng Y, Wang C, Wang Y, Dai L. Soluble PD-L1 as a predictive biomarker in lung cancer: a systematic review and meta-analysis. Future Oncol (London England). (2022) 18:261–73. doi: 10.2217/fon-2021-0641
Keywords: PD-L1, circulating, survival, biomarker, meta-analysis
Citation: Lu H, Luo L, Mi J, Sun M, Wang H, Wang Z and Ding W (2025) Prognostic and clinicopathological role of soluble programmed cell death ligand-1 in patients with diffuse large B-cell lymphoma: a meta-analysis. Front. Oncol. 15:1506799. doi: 10.3389/fonc.2025.1506799
Received: 06 October 2024; Accepted: 16 January 2025;
Published: 31 January 2025.
Edited by:
Ling Li, City of Hope, United StatesReviewed by:
Yuan Tian, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, ChinaCopyright © 2025 Lu, Luo, Mi, Sun, Wang, Wang and Ding. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenwen Ding, dmFuZWRpbmdkaW5nQDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.