- Department of Gynecology, Liaoning Cancer Hospital and Institute, Shenyang, China
Objective: To compare different intestinal reconstruction methods after intestinal resection for advanced ovarian malignancy.
Methods: Retrospective data of patients with advanced ovarian malignancy were collected and then assigned into three groups: primary intestinal anastomosis, protective enterostomy and colostomy. General clinical characteristics, intraoperative findings and postoperative outcomes were compared between the three groups.
Results: A total of 530 cases were included for final analysis. The colostomy group had a lower serum albumin level, larger volume of ascites, higher likelihood of multiple intestinal resections and lower likelihood of rectal resection, lower peritoneal cancer index, more intraoperative blood loss, transfusions and infusions, lower likelihood of optimal cytoreductive surgery and shorter interval time to chemotherapy than the other two groups (p < 0.05). The primary intestinal anastomosis group exhibited a larger blood transfusion volume, higher incidence rates of anastomotic leak and electrolyte disturbance, and longer times to first flatus, first feeding and drain removal than the other two groups (p < 0.05).
Conclusions: Colostomy can be adopted for advanced ovarian cancer patients with a large ascites volume, hypoproteinemia, large intraoperative blood and fluid loss volumes, multiple intestinal resections, anastomoses located below the peritoneal reflection, high PCI and suboptimal cytoreductive surgery. For patients with good intraoperative and postoperative outcomes, one anastomosis, an anastomosis located above the peritoneal reflection, low PCI or optimal cytoreductive surgery, intestinal anastomosis can be carried out to restore the normal physiological function of the intestine. For patients with a large volume of ascites (≥500 mL), multiple anastomoses or an anastomosis located below the peritoneal reflection, intestinal anastomosis combined with protective enterostomy has an advantage over intestinal anastomosis alone.
1 Introduction
Ovarian malignancies are one of the three major types of gynecological malignancies. Due to their insidious onset, over 70% of patients have advanced disease at the time of diagnosis (1). The primary malignancy is frequently accompanied by extensive metastases to the pelvis and abdominal cavity. The metastasis rate to the intestine is approximately 50%, the metastasis rate to the small intestine is 26%–33%, and the metastasis rate to the colorectum is 30%–39% (2, 3). Pelvic and rectal metastasis and extensive invasion to the rectouterine pouch are the most common sites of spread. For this reason, combined ovarian and intestinal resection has been increasingly applied in these cases (4). This approach aims to achieve optimal cytoreductive surgery, which can significantly prolong the survival time and improve the patient quality of life. Due to the high volume of concomitant resections, increasing attention has been paid to the method of intestinal tract reconstruction following intestinal resection.
The options of intestinal tract reconstruction after intestinal resection include intestinal anastomosis, enterostomy and colostomy. The methods for intestinal anastomosis include anastomosis alone (primary anastomosis, after partial resection of the rectum, free of the colon, and the remaining rectum is anastomosed with the colon without tension or blood supply obstacles at the anastomosis) and anastomosis plus protective enterostomy. Intestinal resection and anastomosis may lead to many postoperative complications, including anastomotic leak, anastomotic bleeding, anastomotic stenosis and intraabdominal infection. Leakage is the most severe and challenging complication. According to reports, the incidence rate of colorectal anastomotic leaks is 3%–24% (1, 5–8). The incidence rate of anastomotic leaks in colorectal or ileal-rectal anastomoses is much higher than that of colo-colic anastomoses. Patients with clinical anastomotic leak are associated with worse survival compared with patients without anastomotic leak (9, 10), and anastomotic leaks lead to death in 6%–26% of patients with colorectal cancer (8). Therefore, ways to prevent and/or mitigate the occurrence of anastomotic leaks are continually being explored by surgeons. The concept of protective stoma was first proposed in the low anterior resection of rectal cancer (11). Protective ostomy can prevent anastomotic fistula by transferring feces. Some studies have shown that the occurrence of anastomotic leaks is an event of small probability, and, even if they occur, anastomotic leaks can be clinically cured in most patients through conservative treatment methods such as fasting, intravenous nutrition and adequate drainage. A previous study has shown that protective enterostomy can prevent the occurrence of anastomotic leaks during intestinal surgery for advanced ovarian cancer (12). However, it has also been shown that protective enterostomy is not associated with anastomotic leaks during intestinal surgery for advanced ovarian cancer (13). As a result, the clinical significance of protective enterostomy remains controversial. Meanwhile, it is impossible to accurately predict the occurrence of anastomotic leaks in patients after intestinal anastomosis. The study on the risk factors related to anastomotic leakage shows that, the univariate analyses showed that male sex, the distance from the anal verge, and a duration of operation ≥140 min were associated with an increased incidence of anastomotic leakage in colorectal cancer (14), but preoperative serum albumin level <30 mg/dl, multiple bowel resections and primary cytoreduction have been identified as risk factors of anastomotic leakage in ovarian cancer (15). Because the biological behavior of ovarian cancer and colorectal cancer is inconsistent, the judgment of anastomotic leakage and which patients need protective ostomy cannot be completely based on intestinal cancer.
Enterostomies can be classified as either permanent or temporary. An example of a permanent ostomy is an end colostomy resulting from an abdominal-perineal resection in which the anus is excised through radical surgery for a low rectal malignancy. Temporary ostomies are generally protective enterostomies and usually ileostomies. Permanent enterostomies are generally adopted in circumstances where the anus cannot be preserved through radical surgery due to the low position of a malignant rectal tumor. However, in patients with ovarian malignancies complicated by intestinal metastasis, the lesions are located above the peritoneal reflection (in the rectum, rectouterine pouch, and sigmoid colon in most cases). Because the Douglas pouch serves as a line of defense, prohibiting tumor invasion beyond the peritoneum and infiltration into extraperitoneal tissues, colorectal anastomosis may be performed at the level of the mid-rectum or above the peritoneal reflection, and intestinal anastomosis or colostomy with preservation of the anus is possible. Therefore, a colostomy for ovarian cancer surgery is typically not a permanent enterostomy in the strictest sense, and like protective enterostomy, it can often be reversed. However, stoma reversal and postoperative complication rates may be higher in patients after protective enterostomy, while the quality of life of patients with protective enterostomy is poorer than that of patients with colostomy. Therefore, the selection, efficacy and clinical significance of different intestinal tract reconstruction methods after intestinal resection for advanced ovarian cancer are worthy of in-depth exploration.
In this work, the clinical data and follow-up data of patients with advanced ovarian cancer who underwent intestinal resection were retrospectively collected. The differences in clinical efficacy among different intestinal tract reconstruction methods were analyzed.
2 Methods
2.1 Study design
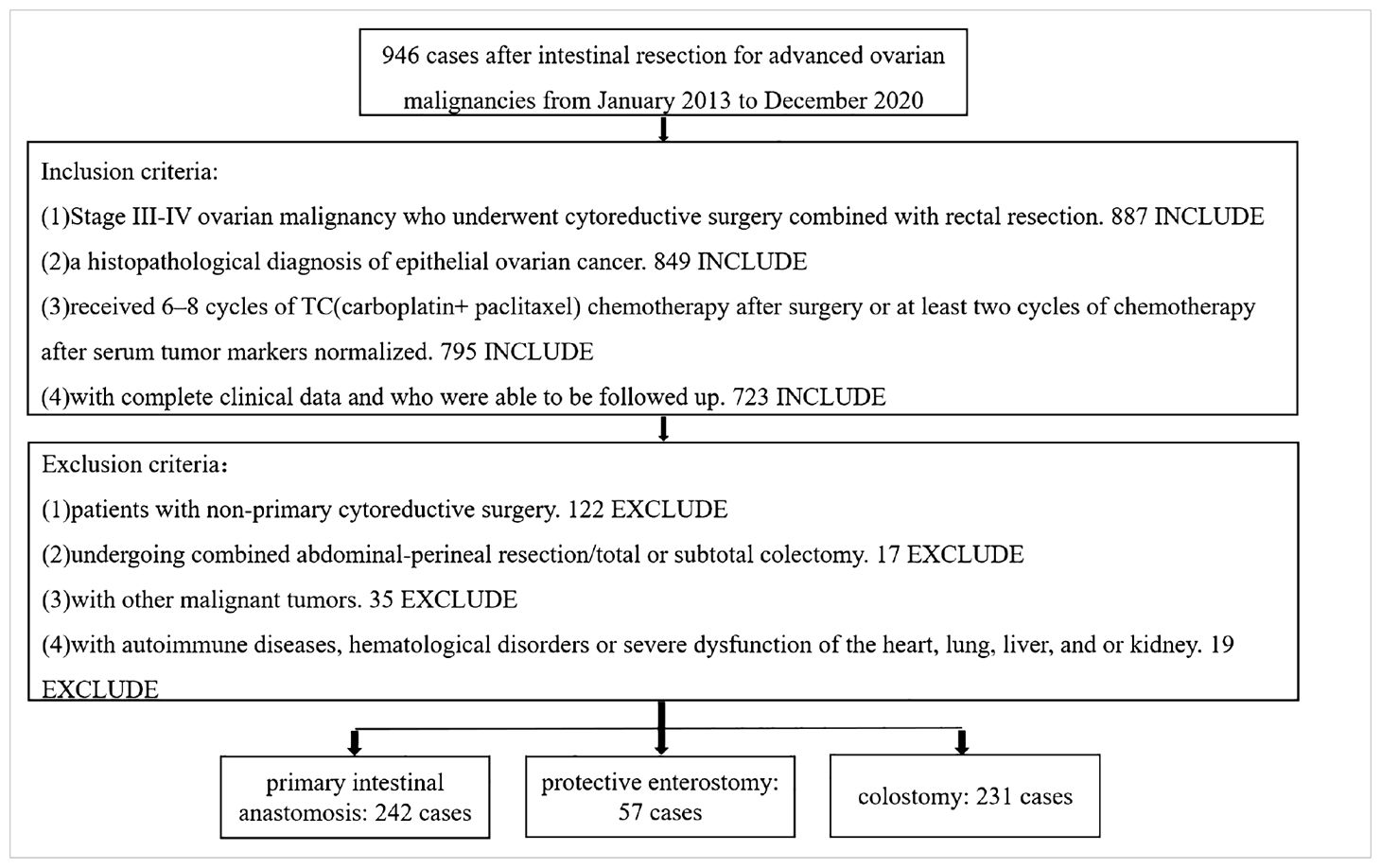
Data from patients with advanced ovarian malignancy undergoing enterectomy in the Department of Gynecology of Liaoning Cancer Hospital and Institute from January 2013 to December 2020 were retrospectively collected. The enrolled patients were grouped by intestinal tract reconstruction method as follows: primary intestinal anastomosis alone (n = 242), intestinal anastomosis + protective enterostomy (protective loop ileostomy) (n = 57) and colostomy (end-colostomy after Hartmann procedure) (n = 231) (Table 1). All intestinal resections and intestinal tract reconstructions were performed by experienced surgeons who each perform >50 intestinal resections per year. The intestinal anastomosis alone group was then subdivided into two groups based on whether anastomotic leaks occurred, and the groups were subanalyzed.
2.2 Data collection
All clinical datasets were complete, including age, body mass index (BMI), neoadjuvant chemotherapy, diabetes, stage, histologic subtype, differentiation, ascites, blood biochemical indexes, tumor markers, operation time, number and site of intestinal resection, peritoneal cancer index (PCI), level of cytoreduction achieved [complete (R0), optimal <1 cm (R1) and suboptimal (R2) (16)], complications, postoperative recovery, adjuvant therapy and stoma reversal. The final follow-up date was 31 December 2021. Medical information reviews, hospital follow-up and telephone follow-up were conducted at the same time point to ensure that the survival outcome at follow-up was accurate. After surgery, all patients were followed-up every 3 months for 2 years, and then every 6 months thereafter. Follow-up evaluations included survival status, recurrence time, recurrence site (local pelvic recurrence or distant recurrence), time of death (if applicable), and cause of death (tumor-related deaths or non-tumor deaths, if applicable). The study and its protocols were approved by the research ethics committee of Liaoning Cancer Hospital & Institute (No. 20220315G).
2.3 Statistical analysis
Data analysis was carried out with SPSS 26 software (IBM, Chicago, IL, USA). Continuous variables were analyzed by analysis of variance (ANOVA). The Chi squared test or Fisher’s Z correction exact test were used to compare categorical variables. OS and DFS were calculated by the Kaplan–Meier method and compared using the log-rank test. Multivariate analysis was performed using the logistic regression model. Results are presented as hazard ratios (HRs) with 95% confidence intervals (95% CIs). P values <0.05 were considered statistically significant.
3 Results
3.1 Preoperative status
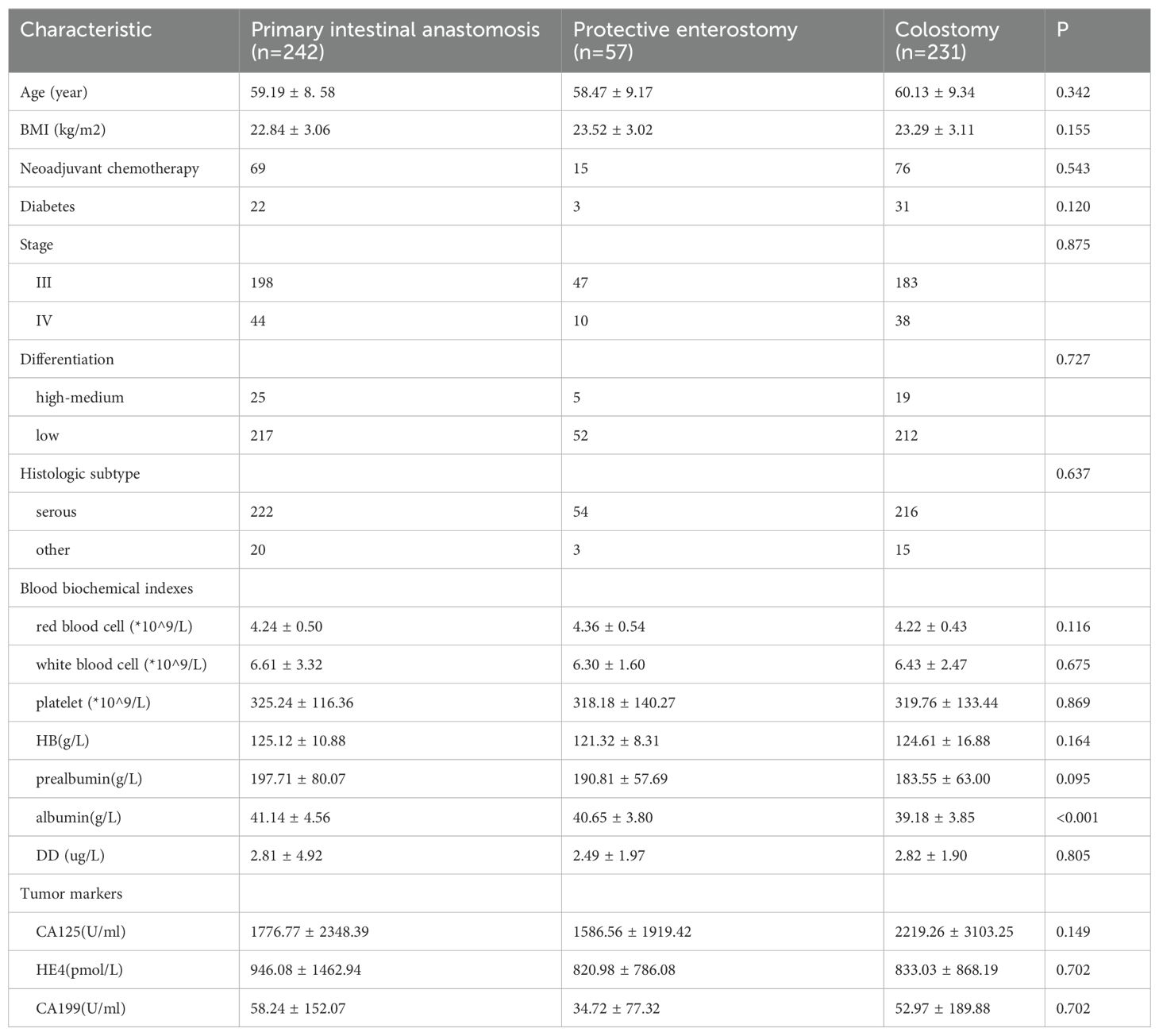
The serum albumin level in the colostomy group was lower than in the other two groups (p < 0.05). No significant differences were noted in age, BMI, diabetes, stage, histologic subtype, differentiation, blood biochemical indexes (red blood cells, white blood cells, platelets, hemoglobin (HB), prealbumin, D-dimer (DD)), tumor markers (CA125, HE4, CA199) in the three groups (all p > 0.05) (Table 2).
3.2 Intraoperative findings
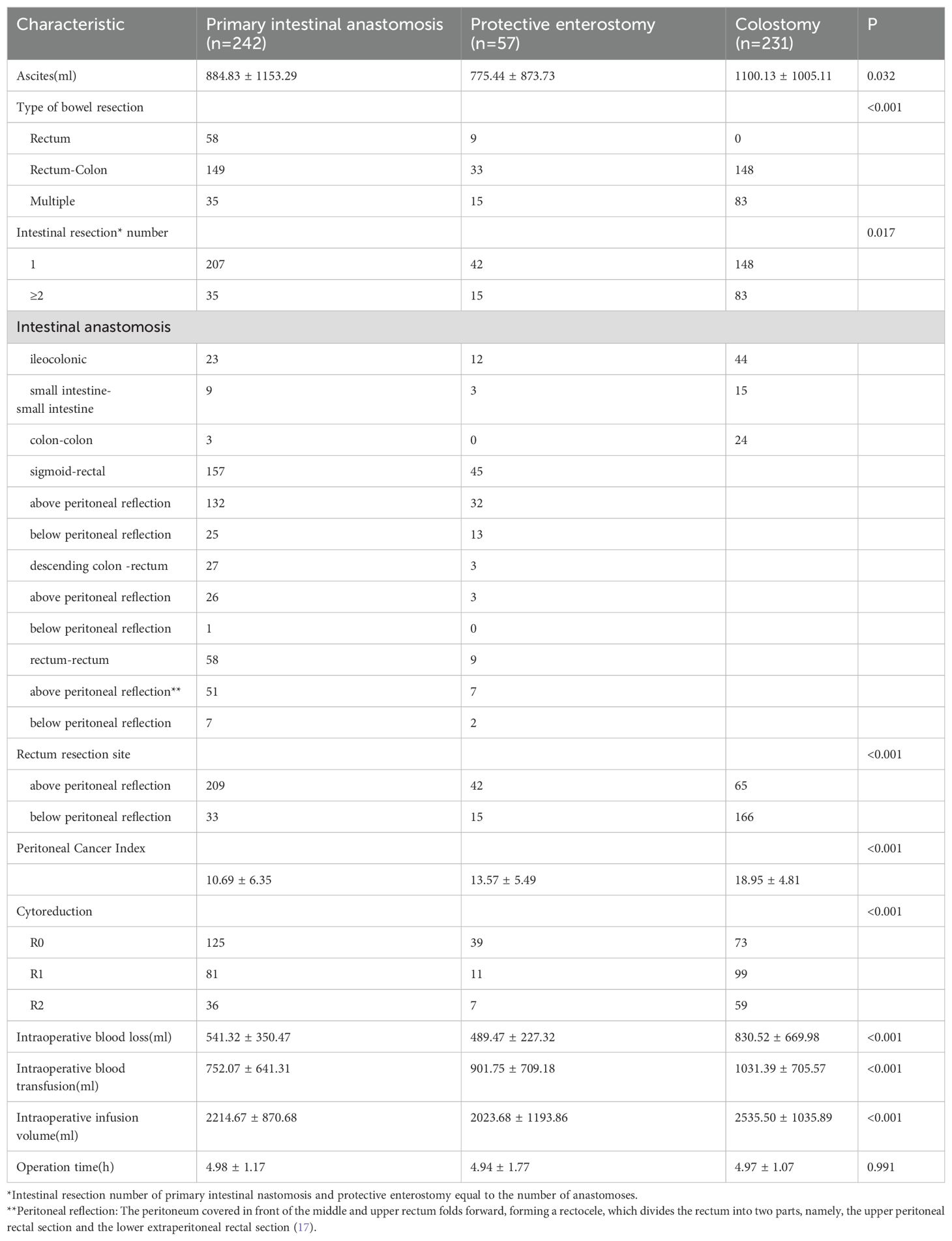
The proportion of patients with optimal cytoreductive surgery was significantly higher in the intestinal anastomosis + protective enterostomy and intestinal anastomosis alone groups than in the colostomy group (87.72%, 85.13% vs. 74.46%). No statistically significant difference was found between the intestinal anastomosis + protective enterotomy group and the intestinal anastomosis alone group. The volume of ascites was significantly larger in the colostomy group than in the intestinal anastomosis + protective enterostomy and intestinal anastomosis alone groups (P = 0.032). No significant difference was found between the intestinal anastomosis + protective enterostomy and the intestinal anastomosis alone groups (p > 0.05). The value of PCI was significantly larger in the colostomy group than in the intestinal anastomosis + protective enterostomy and intestinal anastomosis alone groups (P < 0.001). No significant difference was found between the intestinal anastomosis + protective enterostomy and the intestinal anastomosis alone groups (p > 0.05). The rate of only rectal resection was the lowest but multiple resection was the highest in the colostomy group compared to the other two groups (P < 0.001). The proportions of patients with multiple intestinal segments resected and with rectal resections below the peritoneal reflection (17) were the largest in the colostomy group, followed by the intestinal anastomosis + protective enterostomy and intestinal anastomosis alone groups (56.08% vs. 26.32%, 14.46%, and 71.86% vs. 26.32%, 13.63%), with significant differences among the three groups. However, no significant difference was found between the intestinal anastomosis + protective enterostomy and the intestinal anastomosis alone groups. Intraoperative blood loss, blood transfusion and infusion volumes were significantly larger in the colostomy group than in the intestinal anastomosis + protective enterostomy and intestinal anastomosis alone groups. No significant difference was found between the intestinal anastomosis + protective enterostomy and intestinal anastomosis alone groups (p > 0.05). No significant difference was found in operation time between the three groups (p > 0.05) (Table 3).
3.3 Postoperative outcomes
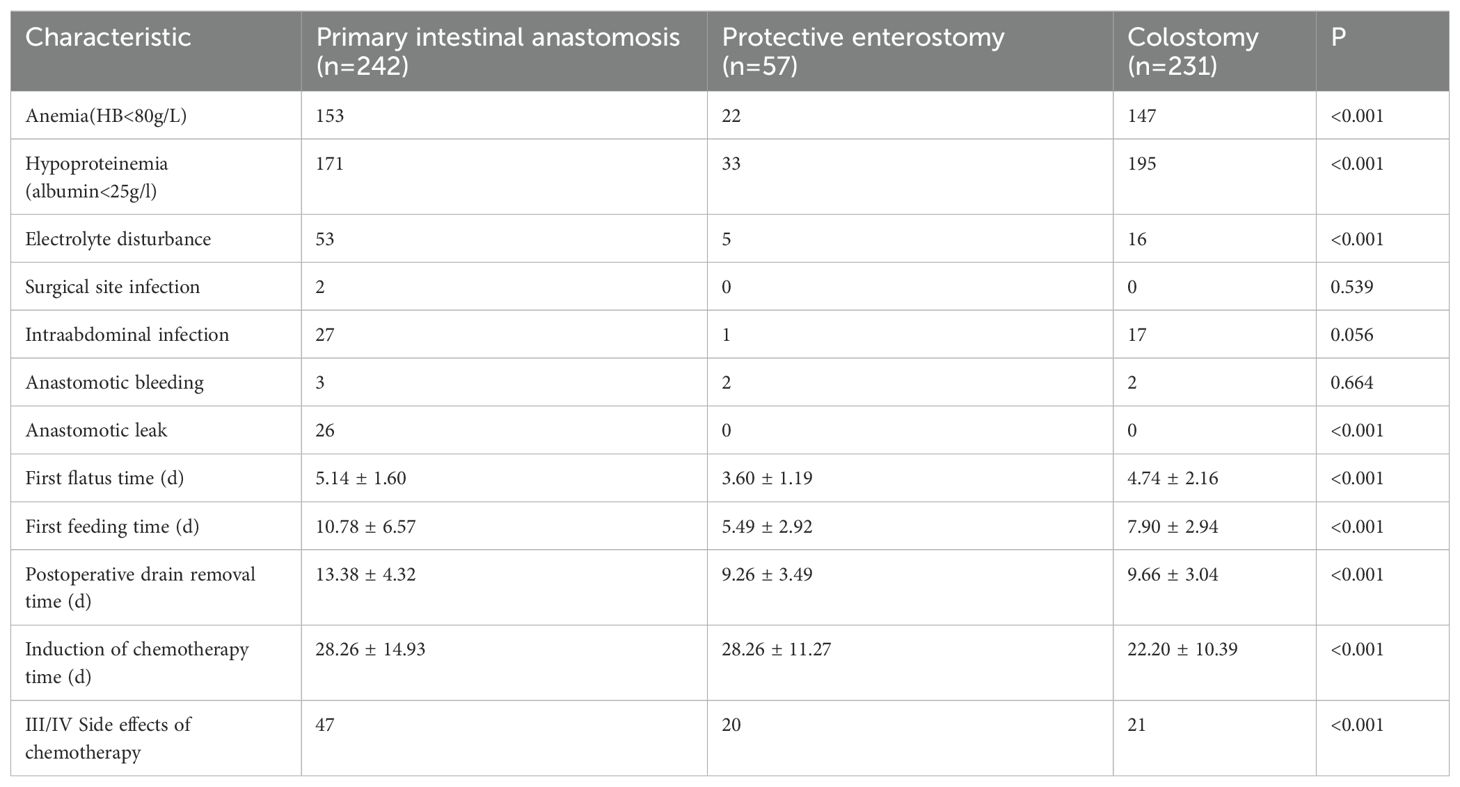
A total of 26 (10.43%) cases of anastomotic leaks were found in the intestinal anastomosis alone group, with statistically significant differences between the groups. The three groups showed no statistically significant differences in the rates of anastomotic bleeding and surgical site infection. The intestinal anastomosis alone group exhibited a significantly higher rate of intraabdominal infection than the intestinal anastomosis + protective enterostomy group (11.16% vs. 1.75%). The incidence of hypoproteinemia was the highest in the colostomy group, followed by the intestinal anastomosis alone and intestinal anastomosis + protective enterostomy groups (84.42% vs. 70.66%, 57.89%, respectively), and the differences were statistically significant. The incidence of anemia was significantly lower in the intestinal anastomosis + protective enterostomy group than in the intestinal anastomosis alone and colostomy groups (38.60% vs. 63.22%, 63.64%). No significant difference was noted between the intestinal anastomosis alone and colostomy groups. The rate of electrolyte disturbance was significantly higher in the intestinal anastomosis alone group than in the intestinal anastomosis + protective enterostomy and colostomy groups (21.90% vs. 8.77%, 6.93%). No significant difference was observed between the intestinal anastomosis + protective enterostomy and colostomy groups.
The time to first flatus and first feeding were the shortest in the intestinal anastomosis + protective enterostomy group, followed by the intestinal anastomosis alone and colostomy groups. The time to postoperative drain removal was shorter in the intestinal anastomosis + protective enterostomy and colostomy groups than in the intestinal anastomosis alone group. The incidence rate of grade III–IV myelosuppression was significantly higher in the intestinal anastomosis + protective enterostomy group than that in the intestinal anastomosis alone and colostomy groups (35.09% vs. 19.42%, 9.09%). The time to induction of chemotherapy was significantly shorter in the colostomy group than that in the intestinal anastomosis alone and intestinal anastomosis + protective enterostomy groups, and the differences were statistically significant (Table 4).
3.4 Analysis of risk factors related to anastomotic leak
3.4.1 Comparison of preoperative status and operation-related conditions between the two groups
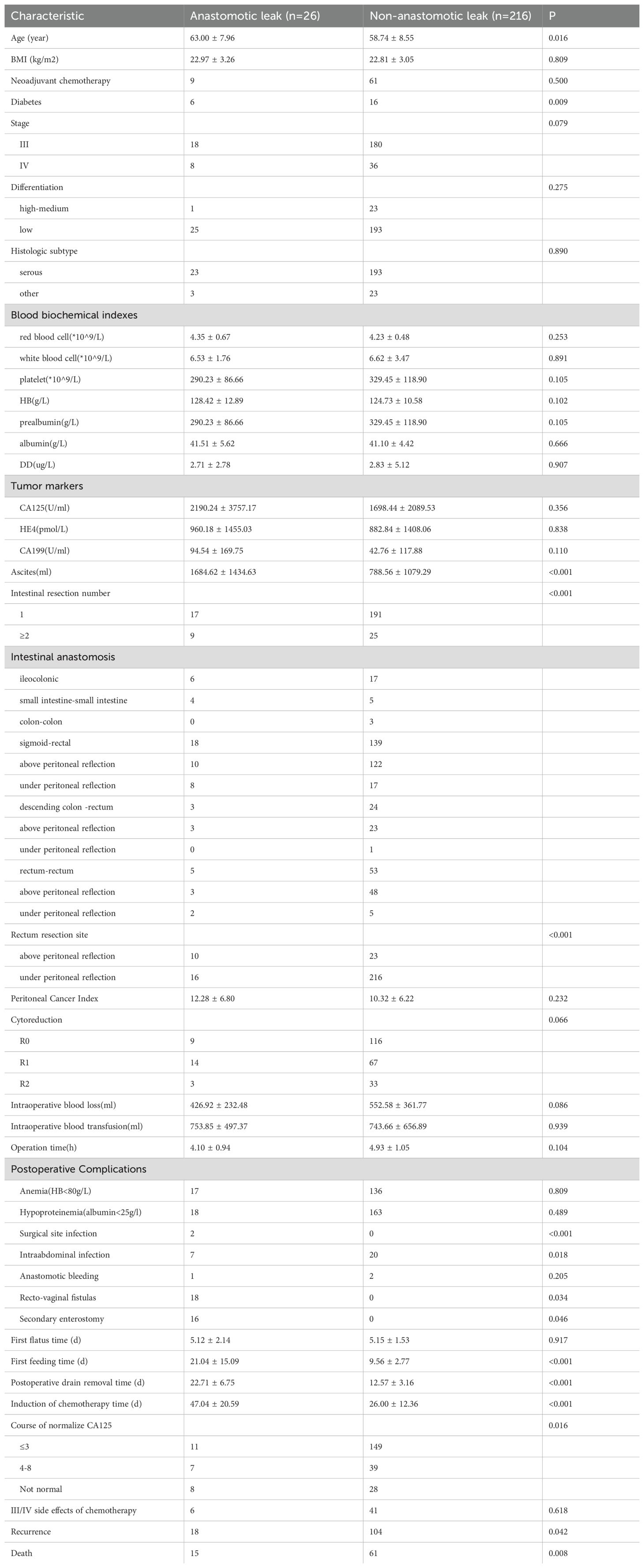
The subdivision of the intestinal anastomosis alone group demonstrated that 10.74% (n = 26) of the cohort experienced anastomotic leak and 89.26% (n = 216) did not. Patients in the anastomotic leak group were older and more likely to have diabetes mellitus (23.08% vs. 7.41%). No significant differences were noted in BMI, neoadjuvant chemotherapy, diabetes, stage, histologic subtype, differentiation, blood biochemical indexes (red blood cells, white blood cells, platelets, HB, prealbumin, albumin, DD), CA125, HE4 and CA199 between the two groups (all p > 0.05) (Table 5).
The volume of ascites, number of patients with multiple resections and/or with anastomoses below the peritoneal reflection were significantly higher in the anastomotic leak group than in the non-anastomotic leak group (33.33% vs. 10.68%, 38.46% vs. 9.50%). No statistically significant differences were found in PCI value, operation satisfaction, intraoperative blood loss, blood transfusion, infusion volumes and operation time between the two groups (p > 0.05) (Table 5).
The incidence rates of postoperative surgical site and intra-abdominal infection were significantly higher in the anastomotic leak group than in the non-anastomotic leak group (29.17% vs. 9.22%). The time to first feeding and postoperative drain removal was longer in the anastomotic leak group than in the non-anastomotic leak group. No statistically significant difference was found in anemia, hypoproteinemia, anastomotic bleeding and first flatus time between the two groups (p > 0.05) (Table 5).
3.4.2 Prognosis
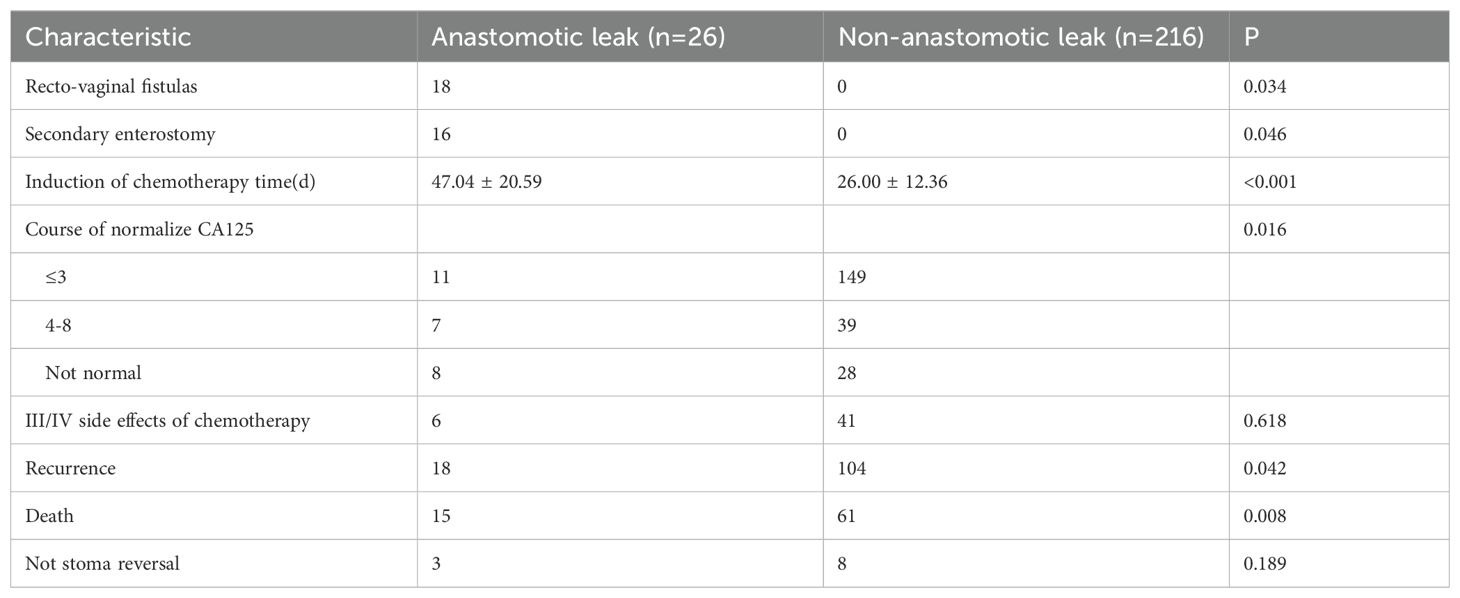
In the anastomotic leak group (n = 26), 18 cases (69.23%) of recto-vaginal fistulas were found. Sixteen (88.89%, 16/18) of these patients required a second enterostomy for their recto-vaginal fistulas. Fourteen (77.78%, 14/18) underwent a second procedure for their recto-vaginal fistulas. The interval time to the commencement of chemotherapy after surgery in the anastomotic leak group was significantly shorter than in the non-anastomotic leak group. The number of treatments to normalize tumor marker (CA125) levels was significantly lower in the non-anastomotic leak group than in the anastomotic leak group. In addition, 19 cases (73.08%) of recurrence and 15 (57.69%) deaths occurred in the anastomotic leak group. These rates were significantly higher than those in the non-anastomotic leak group [104 cases (48.15%) vs 61 cases (28.24%)]. Univariate Kaplan–Meier analysis demonstrated significantly shorter DFS and OS in the anastomotic leak group than in the non-anastomotic leak group (median time 12 vs. 36 months, 33 vs. 38 months). The incidence rate of grade III–IV side effects of chemotherapy was comparable between the two groups (Table 6, Figure 1).
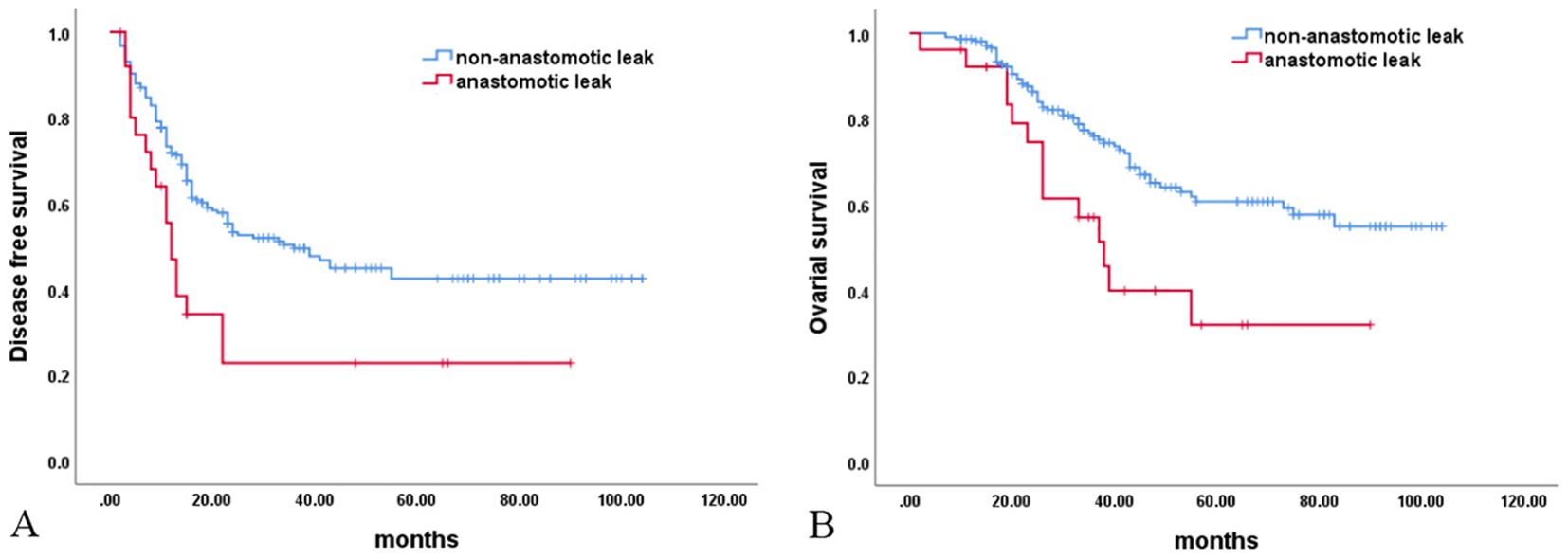
Figure 1. Survival analysis. (A) Disease-free survival (P = 0.005); (B) Overall survival (P = 0.006).
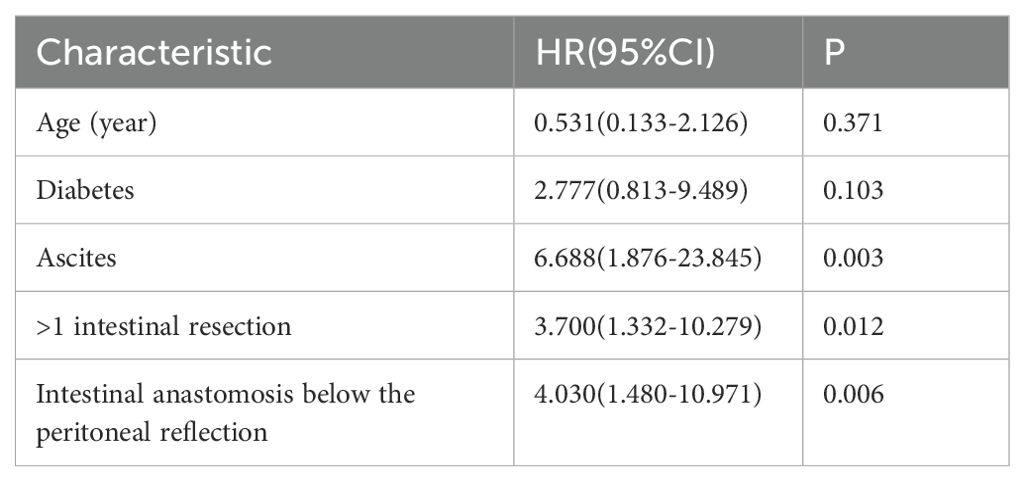
Multivariate logistic analysis was conducted on the factors associated with anastomotic leaks found in the univariate analysis (age, diabetes mellitus, ascites volume, number of intestinal segments resected, and intestinal resection site). Ascites, resection of >1 intestinal segments and intestinal anastomosis below the peritoneal reflection were independent risk factors for anastomotic leaks (Table 7).
4 Discussion
In this study, the clinical data of advanced ovarian cancer patients undergoing colostomy, intestinal anastomosis alone and intestinal anastomosis + protective enterostomy were retrospectively analyzed. It was found that the colostomy group had more comorbidities, which was reflected by a high incidence of preoperative hypoproteinemia due to a poor nutritional status and a large ascitic volume. Additionally, high PCI and extensive lesions gave rise to a large range of intestinal resections and multi-segment intestinal resections and a lower number of rectal resections. The time of the first chemotherapy after permanent enterostomy was significantly shorter than that of intestinal anastomosis in this study. Because the prognosis of ovarian cancer is related to the satisfaction of surgery and chemotherapy, if satisfactory cytoreductive surgery cannot be achieved, immediate chemotherapy is likely to improve the survival time of patients (18, 19). While the recovery time of patients after intestinal anastomosis will be long and the commencement of chemotherapy after surgery will be delayed, which may affect the survival time of patients, suboptimal cytoreduction may be an influencing factor for surgeons that choose permanent enterostomy. Non-optimal cytoreductive surgery may be an important factor when the surgeon is considering permanent enterostomy. The incidence rate of anastomotic leaks after intestinal anastomosis alone was higher than after protective enterectomy and permanent enterectomy. In the case of anastomotic leaks, feces flows into the abdominal cavity through the leak, resulting in intraabdominal infection. During conservative treatment or secondary surgery for anastomotic leaks, patients experience prolonged fasting times, and thus, have increased rates of anemia and electrolyte imbalances as well as larger postoperative blood transfusion volumes. In cases of both protective enterostomy and permanent colostomy, feces do not travel through the intestinal anastomosis. As a result, the incidence rates of postoperative fasting and electrolyte disturbance are significantly reduced. In addition, the time to flatus is short, after which patients can begin to eat. Moreover, the postoperative recovery is quicker and the interval time to first chemotherapy is shorter. Consequently, the prognosis of patients is improved.
The data of 299 patients receiving intestinal resection and anastomosis were subjected to stratification analysis. Compared with the intestinal anastomosis group, the protective enterostomy group had higher optimal cytoreduction rates and numbers of intraoperative intestinal anastomoses and anastomoses located below the peritoneal reflection. They also had notably decreased rates of anastomotic leaks, anastomotic bleeding, intra-abdominal infections, anemia, hypoproteinemia and ion disturbances and shorter times to flatus and eating after surgery. A reason why the surgeon may choose protective enterostomy may be that patients with optimal cytoreduction have the best prognosis and the possibility of reversing the stoma is high.
Advanced ovarian malignancies exhibit different biological behaviors from malignant colorectal tumors. First, adverse patient factors, such as a poor nutritional status, a large volume of ascites, and extensive intraperitoneal metastasis are often found in patients with advanced ovarian malignancies. As a result, such patients have an increased rate of anastomotic leaks. Secondly, resection of the greater omentum and abdominopelvic cavity peritoneum are typically performed during surgery for advanced ovarian cancer. This weakens the wrapping and protective effects of the omentum and peritoneum on the anastomosis, thereby increasing the risk of anastomotic leaks. Lastly, the standard procedure of cytoreductive surgery for ovarian cancer typically includes hysterectomy, and thus recto-vaginal fistulas, severe abdominal infection and other fatal complications may develop once anastomotic leaks occur, delaying chemotherapy and affecting the prognosis of patients. Therefore, the choice of the intestinal tract reconstruction method after intestinal resection for ovarian malignancy cannot be generalized to that of colorectal cancer. In the present study, 26 cases (10.74%) of anastomotic leaks were detected in the intestinal anastomosis alone group. This is significantly higher than the incidence found in anastomoses for intestinal cancer (i.e., 2%) (20). Eighteen cases of recto-vaginal fistulas were found in the anastomotic leak group, accounting for 69.23% (18/26) of the cohort, of which 16 cases required secondary enterostomy, corresponding to 88.89% (16/18) of the recto-vaginal fistula patient group. Fourteen cases (78%) required a second operation for recto-vaginal fistulas. As a result, patients had a prolonged hospitalization, increased cost, poor prognosis and shortened survival time due to the significantly prolonged interval time to chemotherapy after surgery. This further proves the importance of protective enterostomy for intestinal resection and anastomosis in advanced ovarian cancer.
Protective enterostomy enables early feeding and early chemotherapy (21, 22); however, it also has many disadvantages. In contrast to large intestinal stomas, small intestinal stomas may affect the absorption of nutrients. Additionally, the incidence rates of other enterostomy complications, such as dehydration, electrolyte disturbance and dermatitis in stomas, is high. A second operation is required 3–6 months later to reverse the stoma, which can cause psychological and physical trauma to patients and increases medical costs (23). For patients with colorectal cancer, stoma repair is conducted within 3 months after surgery in most cases (24, 25). The response rate of first-line chemotherapy for ovarian cancer exceeds 70%, thus emphasizing the importance of initiating chemotherapy promptly following surgery for ovarian malignant tumors. It is generally recommended to commence chemotherapy within 2 weeks after surgical recovery of gastrointestinal function, preferably not exceeding a duration of 4 weeks. Timing of cytotoxic treatment [≤ 28 days vs. >28 days] was a significant prognostic factor for overall survival in multivariate analysis (19).Stoma reversal is delayed in most patients with ovarian cancer to facilitate chemotherapy. In this study, 11 patients (19.30%) in the protective enterostomy group ultimately ended up with permanent stomas, as they could not undergo stoma reversal due to primary platinum chemotherapy resistance, cancer progression during chemotherapy and/or short-term recurrence. Although studies have shown that 90% of patients with malignant intestinal tumors do not benefit from protective enterostomy (5), if enterostomy is rashly implemented in all advanced ovarian cancer patients undergoing intestinal resection, this may place an unnecessary burden on some patients and lead to unnecessary surgery and trauma. Therefore, it is particularly important to master the indications for protective enterostomy during intestinal anastomosis for advanced ovarian cancer.
Furthermore, in this study, a subgroup analysis was carried out on patients with intestinal anastomosis alone to further investigate the indications for preventive enterostomy. Univariate analysis demonstrated that age, diabetes mellitus, a large ascitic volume, multiple anastomoses and anastomoses located below the peritoneal reflection were implicated in the development of anastomotic leaks. The results of further multivariate logistic analysis revealed that an ascitic volume ≥500 mL, multiple anastomoses and anastomoses located below the peritoneal reflection were independent risk factors for anastomotic leaks. A previous study has shown that ascitic volume is an independent risk factor for poor prognosis in ovarian malignancy (26). In this study, we demonstrated for the first time that an ascitic volume ≥500 mL was an independent risk factor for anastomotic leaks after intestinal anastomosis for ovarian malignancy. This may be due to the fact that the inflammatory microenvironment and extracellular matrix remodeling caused by TNF-αand MMP-2 in ascitic fluid of patients with ovarian cancer may be related to poor healing of intestinal anastomosis (27). Most patients with colorectal cancer have one anastomosis, so the relationship between the number of intestinal segments resected and the occurrence of anastomotic leaks in colorectal cancer remains poorly studied (28). The results of this study showed that resection of multiple intestinal segments was an independent risk factor for anastomotic leaks after intestinal resection and anastomosis for ovarian malignancy. This is mainly attributed to the following factors. First, multiple anastomoses suggest longer operation times, which is also an independent risk factor for anastomotic leaks. According to previous studies on the association between anastomotic location and anastomotic leaks in colorectal cancer, anastomoses close to the anus are a high-risk factor for leaks (28, 29). A ‘safe’ anastomosis should have low tension. In patients with ovarian malignancies, the low location of rectal anastomoses and the resection of multiple intestinal segments can result in increased anastomotic tension, giving rise to an increased risk of anastomotic leaks. Moreover, compared to colorectal cancer patients, those with advanced ovarian cancer also have an increased risk of infection due to hysterectomy. A previous study has reported that pelvic and intra-abdominal infection is also a high-risk factor for anastomotic leaks (30). In this study, the incidence rate of postoperative intra-abdominal infection in the anastomotic leak group was significantly higher than that in the non-anastomotic leak group.
In this study, there were few cases in the anastomotic leak group, so it was necessary to further increase the sample size to make the results more reliable. Additionally, some imaging data were incomplete due to the long review time and the fact that imaging data of some patients came from other hospitals. The complexity of the disease was not analyzed by the peritoneal metastasis score and Suidan CT score, which is also the focus of follow-up research. Some patients with advanced ovarian cancer with primary resistance to platinum chemotherapy or rapid disease progression, even if intestinal anastomosis and protective enterostomy are performed, may not be suitable for stoma reversal. Additionally, protective small intestinal enterostomy is inferior to permanent large intestinal enterostomy in nutrient absorption. Direct permanent enterostomy may have an advantage over intestinal anastomosis and protective enterostomy for such patients. Hence, the identification of patients with primary resistance to platinum and refractory disease and the selection of intestinal resection and reconstruction methods warrant further investigation.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The study and its protocols were approved by the research ethics committee of Liaoning Cancer Hospital & Institute (No. 20220315G). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
HW: Conceptualization, Funding acquisition, Investigation, Software, Writing – original draft. XL: Data curation, Methodology, Supervision, Writing – original draft. YJ: Formal analysis, Project administration, Writing – original draft. JC: Validation, Writing – original draft. RC: Resources, Visualization, Writing – original draft. JZ: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by a grant from the Liaoning Science and Technology Plan Joint Plan (Natural Science Foundation Project) (2024-MSLH-275) and Health and healthy development promotion project (tumor research project)(KC2023-JX-0186-RQ068).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
OS, overall survival; DFS, disease-free survival; BMI, body mass index; ANOVA, analysis of variance; HR, hazard ratio; 95% CI, 95% confidence interval; HB, hemoglobin; DD, D-dimer.
References
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. (2015) 136:E359–86. doi: 10.1002/ijc.v136.5
2. Philip CA, Pelissier A, Bonneau C, Hequet D, Rouzier R, Pouget N. Impact of neoadjuvant chemotherapy on the rate of bowel resection in advanced epithelial ovarian cancer. Anticancer Res. (2016) 36:4865–71. doi: 10.21873/anticanres.11050
3. Shao T, Chen XW, Zhang ZZ. An analysis of prognosis and risk factors of ovarian cancer with intestinal tract metastasis. China cancer& Jouranal Chin Oncol. (2014) 023:790–4.
4. Bacalbasa N, Balescu I, Diaconu C, Iliescu L, Filipescu A, Pop C, et al. Right upper abdominal resections in advanced stage ovarian cancer. In Vivo. (2020) 34:1487–92. doi: 10.21873/invivo.11934
5. Degiuli M, Elmore U, De Luca R, Paola D, Mariano T, Alberto B, et al. Risk factors for anastomotic leakage after anterior resection for rectal cancer (RALAR study): A nationwide retrospective study of the Italian Society of Surgical Oncology Colorectal Cancer Network Collaborative Group. Colorectal Dis. (2022) 24:264–76. doi: 10.1111/codi.15997
6. Jae HJ, Hee CK, Jung WH, Park YA, Cho YB, Yun SH, et al. Anastomotic leak does not impact oncologic outcomes after preoperative chemoradiotherapy and resection for rectal cancer. Ann Surg. (2019) 269:678–85. doi: 10.1097/SLA.0000000000002582
7. Mirnezami A, Mirnezami R, Chandrakumaran K, Sasapu K, Sagar P, Finan P. Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: systematic review and meta-analysis. Ann Surg. (2011) 253:890–9. doi: 10.1097/SLA.0b013e3182128929
8. Matsubara N, Miyata H, Gotoh M, Naohiro T, Hideo B, Wataru K, et al. Mortality after commonrectal surgery in Japan:a study on low anterior resection from anewly established nationwide large-scale clinical database. Dis Colon Rectum. (2014) 57:1075–81. doi: 10.1097/DCR.0000000000000176
9. James DS, Jean MB, Martin RW, Michael I, Philip BP, Larissa K, et al. Anastomotic leak following low anterior resection in stage IV rectal cancer is associated with poor survival. Ann Surg Oncol. (2013) 20:2641–6. doi: 10.1245/s10434-012-2854-9
10. Melissa NN, Nynke GG, Sarah B, Pauline AJ, Rob HA, Richard P, et al. Long-term oncological outcomes after colorectal anastomotic leakage: A retrospective dutch population-based study. Ann Surg. (2022) 276:882–9. doi: 10.1097/SLA.0000000000005647
12. Debra LR, Andrea M, William AC. Risk factors for anastomotic leak after recto-sigmoid resection for ovarian cancer. Gynecol Oncol. (2006) 103:667–72. doi: 10.1016/j.ygyno.2006.05.003
13. Gaetano V, Amerigo V, Matteo M, Giorgio G, Francesco S, Fabrizio S, et al. Risks factors for anastomotic leakage in advanced ovarian cancer: A systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. (2022) 269:3–15. doi: 10.1016/j.ejogrb.2021.12.007
14. Sciuto A, Merola G, De Palma GD, Sodo M, Pirozzi F, Bracale U, et al. Predictive factors for anastomotic leakage after laparoscopic colorectal surgery. World J Gastroenterol. (2018) 24:2247–60. doi: 10.3748/wjg.v24.i21.2247
15. Restaino S, Schierano S, Arcieri M, Barbara C, Alice P, Pregnolato S, et al. Surgical management of anastomotic leakage related to ovarian cancer surgery: a narrative review. Front Surg. (2024) 11:1434730. doi: 10.3389/fsurg.2024.1434730
16. Phillips A, Sundar S, Singh K, Nevin J, Elattar A, Kehoe S, et al. Complete cytoreduction afterfive or more cycles of neo-adjuvant chemotherapy confers a survival benefit in advanced ovarian cancer. Eur J Surg Oncol. (2018) 44:760–5. doi: 10.1016/j.ejso.2018.01.097
18. Mahner S, Eulenburg C, Staehle A, Wegscheider K, Reuss A, Pujade-Lauraine E, et al. Prognostic impact of the time interval between surgery and chemotherapy in advanced ovarian cancer: analysis of prospective randomised phase III trials. Eur J Cancer (Oxford England: 1990). (2013) 49:142–9. doi: 10.1016/j.ejca.2012.07.023
19. Hofstetter G, Concin N, Braicu I, Chekerov R, Sehouli J, Cadron I, et al. The time interval from surgery to start of chemotherapy significantly impacts prognosis in patients with advanced serous ovarian carcinoma - analysis of patient data in the prospective OVCAD study. Gynecol Oncol. (2013) 131:15–20. doi: 10.1016/j.ygyno.2013.07.086
20. Laurie H, Jamshid D, Sami J, Amy LL, Stefan H, Scott RS, et al. Timing and outcome of right- vs left-sided colonic anastomotic leaks: Is there a difference? Am J Surg. (2022) 223:493–5. doi: 10.1016/j.amjsurg.2021.12.019
21. Shiomi A, Ito M, Maeda K, Hino H, Manabe S, Yamaoka Y, et al. Effects of a diverting stoma onsymptomatic anastomotic leakage after low anterior resection forrectal cancer:a propensity score matching analysis of 1,014consecutive patients. J Am Coll Surg. (2015) 220:186–94. doi: 10.1016/j.jamcollsurg.2014.10.017
22. Yao H, An Y, Zhang Z. The application of defunctioning stomasafter low anterior resection of rectal cancer. Surg Today. (2019) 49:451–9. doi: 10.1007/s00595-018-1736-6
23. Walma MS, Kornmann VN, Boerma D, Marnix A, Henderik L. Predictors of fecal incontinence and related quality of life after a total mesorectal excision with primary anastomosis for patients with rectal cancer. Ann Coloproctol. (2015) 31:23⁃28. doi: 10.3393/ac.2015.31.1.23
24. Bausys A, Kuliavas J, Dulskas A, Kryzauskas M, Pauza K, Kilius A, et al. Early versus standard closure of temporary ileostomy in patients with rectal cancer: a randomized controlled trial. J Surg Oncol. (2019) 120:294⁃299. doi: 10.1002/jso.v120.2
25. Snijders HS, van Leersum NJ, Henneman D, de Vries AC, Tollenaar RA, Stiggelbout AM, et al. Optimal treatment strategy in rectal cancer surgery: should we be cowboys or chickens? Ann Surg Oncol. (2015) 22:3582⁃3589. doi: 10.1245/s10434-015-4385-7
26. Dimitrios N, Maureen B, Emily MK, Haggerty AF, Cory L, Giuntoli IiRL, et al. Ascites volume at the time of primary debulking and overall survival of patients with advanced epithelial ovarian cancer. Int J Gynecol Cancer. (2021) 31:1579–83. doi: 10.1136/ijgc-2021-002978
27. El-Hussuna A, Krag A, Olaison G, Bendtsen F, Gluud LL. The effect of anti-tumor necrosis factor alpha agents on postoperative anastomotic complications in Crohn’s disease: a systematic review. Dis Colon Rectum. (2013) 56:1423–33. doi: 10.1097/DCR.0b013e3182a48505
28. Lago V, Fotopoulou C, Chiantera V, Minig L, Gil-Moreno A, Cascales-Campos PA, et al. Risk factors for anastomotic leakage after colorectal resection in ovarian cancer surgery: A multi-centre study. Gynecol Oncol. (2019) 153:549–54. doi: 10.1016/j.ygyno.2019.03.241
29. Eugenia CZ, Narcis OZ, Radu C. Updates of risk factors for anastomotic leakage after colorectal surgery. Diagnostics (Basel). (2021) 11:2382. doi: 10.3390/diagnostics11122382
Keywords: advanced ovarian cancer, intestinal resection, intestinal reconstruction, protective enterostomy, anastomotic leak
Citation: Wang H, Li X, Jiang Y, Chen J, Cao R and Zhang J (2025) Clinical analysis of different intestinal reconstruction methods after primary cytoreductive surgery combined with rectal resection for advanced ovarian cancer. Front. Oncol. 15:1500042. doi: 10.3389/fonc.2025.1500042
Received: 22 September 2024; Accepted: 09 January 2025;
Published: 27 January 2025.
Edited by:
Stefano Restaino, Ospedale Santa Maria della Misericordia di Udine, ItalyReviewed by:
Paul Willemsen, Hospital Network Antwerp (ZNA), BelgiumGiulia Pellecchia, KU Leuven, Belgium
Copyright © 2025 Wang, Li, Jiang, Chen, Cao and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingru Zhang, emhhbmdqaW5ncnUyMDIwMTBAMTYzLmNvbQ==
 Huimin Wang
Huimin Wang Xiaocen Li
Xiaocen Li