
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 05 March 2025
Sec. Cancer Imaging and Image-directed Interventions
Volume 15 - 2025 | https://doi.org/10.3389/fonc.2025.1498691
This article is part of the Research TopicMethods and Applications of Tumour Metabolic Imaging in the Preclinical and Clinical SettingView all 7 articles
 Xiaoyan Wang1
Xiaoyan Wang1 Yan Zhang1*
Yan Zhang1* Jingliang Cheng1
Jingliang Cheng1 Liangjie Lin2
Liangjie Lin2 Ying Hu1
Ying Hu1 Anfei Wang1
Anfei Wang1 Yong Zhang1
Yong Zhang1 Ruhua Wang1
Ruhua Wang1 Ying Li1
Ying Li1 Kun Zhang1
Kun Zhang1 Wenhua Zhang1
Wenhua Zhang1Purpose: This study aims to investigate the feasibility of cellular microstructural mapping by the diffusion MRI (IMPULSED, imaging microstructural parameters using limited spectrally edited diffusion) of breast tumors, and further to evaluate whether the MRI-derived microstructural features is associated with the prognostic factors in breast cancer.
Materials and methods: This prospective study collected 232 patients with suspected breast tumors from March to August 2023. The IMPULSED MRI scan included acquisitions of diffusion MRI using both pulsed (PGSE) and oscillating (OGSE) gradient spin echo with the oscillating frequencies up to 33 Hz. The OGSE and PGSE data were fitted by the IMPUSLED method using a two-compartment model to estimate mean cell diameter (dmean), intracellular fraction (fin), extracellular diffusivity (Dex), and cellularity index (fin/d) within breast tumor lesions. The apparent diffusion coefficients (ADCs) were calculated from the conventional diffusion weighted imaging, PGSE, and OGSE (17 Hz and 33 Hz) sequences (ADCDWI, ADCPGSE, ADC17Hz, and ADC33Hz). The independent samples test was used to compare the dmean, fin, Dex, cellularity index, and ADC values between benign and malignant breast tumors, and between breast cancer subgroups with different risk factors. The receiver operating characteristic (ROC) curve was used to access the diagnostic performance.
Results: 213 patients were finally included and divided into malignant (n=130) and benign (n=83) groups according to the histopathological results. The dmean (15.74 ± 2.68 vs. 14.28 ± 4.65 μm, p<0.001), fin (0.346 ± 0.125 vs. 0.279 ± 0.212, p<0.001) and cellularity index (21.19 ± 39.54 vs. 19.38 ± 14.87 ×10-3 um-1, p<0.005) values of malignant lesions were significantly higher than those of benign lesions, and the Dex (2.119 ± 0.395 vs. 2.378 ± 0.332 um2/ms, p<0.001) and ADCDWI (0.877 ± 0.148 vs. 1.453 ± 0.356 um2/ms, p<0.001) of malignant lesions were significantly lower than those of benign lesions. For differentiation between benign and malignant breast lesions, ADCDWI showed the highest AUC of 0.951 with the sensitivity of 80.49% and specificity of 98.28%. The combination of dmean, fin, Dex, and cellularity for differentiation between benign and malignant breast lesions showed AUC of 0.787 (sensitivity = 70.73%, and specificity = 77.86%), and the combination of IMPULSED-derived parameters with ADCs by PGSE and OGSE further improve the AUC to 0.897 (sensitivity = 81.93%, and specificity = 81.54%). The fin values of HER-2(+) tumors were significantly lower than those of HER-2(-) tumors (0.313 ± 0.100 vs. 0.371 ± 0.137, p=0.015), and the ADCDWI, ADC17Hz and ADC33Hz values of HER-2(+) tumors were significantly higher than those of HER-2(-) tumors (ADCDWI: 0.929 ± 0.115 vs. 0.855 ± 0.197 um2/ms, p=0.023; ADC17Hz: 1.373 ± 0.306 vs. 1.242 ± 0.301 um2/s, p =0.025; ADC33Hz: 2.042 ± 0.545 vs. 1.811 ± 0.392 um2/s, p = 0.008). The fin (0.377 ± 0.136 vs. 0.300 ± 0.917, p=0.001) and cellularity index (27.22 ± 12.02 vs. 21.66 ± 7.76 ×10-3 um-1, p=0.007) values of PR(+) tumors were significantly higher than those of PR(-) tumor. The ADC17Hz values of PR(+) tumors were significantly lower than those of PR(-) tumors(1.227 ± 0.299 vs. 1.404 ± 0.294 um2/s, p =0.002).The ADC17Hz and Dex values of ER(+) tumors were significantly lower than those of ER(-) tumors (ADC17Hz: 1.258 ± 0.313 vs. 1.400 ± 0.273 um2/s, p = 0.029; Dex: 2.070 ± 0.405 vs. 2.281 ± 0.331 um2/ms, p=0.011). For differentiation between ER(+) and ER(-), the ADC17Hz and Dex showed AUCs of 0.643 (sensitivity = 76.67%, and specificity = 47.06%) and 0.646 (sensitivity = 80.0%, and specificity = 45.98%), and the combination of Dex and ADC17Hz showed AUCs of 0.663 (sensitivity =93.33%, specificity = 36.78%). For differentiation of PR(+) and PR(-), the ADC17Hz, fin, and cellularity index showed AUCs of 0.666 (sensitivity = 68.18%, and specificity = 61.97%), 0.697 (sensitivity = 77.27%, and specificity = 60.27%) and 0.661 (sensitivity = 68.18%, and specificity = 61.64%), respectively, and their combination showed AUCs of 0.729 (sensitivity =72.73%, specificity = 65.75%). For differentiation of HER-2(+) and HER-2(-), the ADCDWI, ADC17Hz, and ADC33Hz, and fin showed AUCs of 0.625 (sensitivity = 59.42%, specificity = 63.04%), 0.632 (sensitivity = 43.66%, and specificity = 84.78%), 0.664 (sensitivity = 47.95%, and specificity = 82.67%) and 0.650 (sensitivity = 77.46%, and specificity = 56.52%), respectively, and their combination showed AUCs of 0.693 (sensitivity = 69.57%, specificity = 64.79%) of HER-2(+) and HER-2(-).
Conclusion: The IMPULSED method demonstrates promise for characterizing cellular microstructural features in breast tumors, which may be helpful for prognostic risk evaluation in breast cancer.
In China, no matter in urban or rural areas, breast cancer ranks first in the spectrum of female cancer incidence and top 4 in the spectrum of female cancer death, and is also the most common type of cancer after lung cancer (1). Breast cancer is associated with complex biological behavior, and the classification of molecular subtypes can provide a basis for the formulation of treatment strategies and prognosis assessment for breast cancer patients (2). Perou et al. (3) proposed that expression of estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor-2 (HER-2) and antigen identified by monoclonal antibody Ki-67 were the main factors determining the classification of breast cancer, which would guide the strategies for targeted therapy, endocrine therapy, or chemotherapy (4, 5). And efforts to identify molecular subtypes or prognostic factors of breast cancer using preoperative imaging have been ongoing.
Magnetic resonance imaging (MRI) is a non-invasive technique with exceptional soft tissue contrast and can provide anatomical and functional information on both normal and diseased tissues, such as tumors. MRI plays an important role in the diagnosis, treatment and prognosis assessment of breast diseases (6–8). However, traditional MRI can only reflect macroscopic features of a lesion, such as lesion size and morphology (9). Dynamic contrast-enhanced MRI (DCE-MRI) and diffusion-weighted imaging (DWI) based imaging biomarkers have been shown to be highly correlated with molecular subtypes and other prognostic and predictive factors in breast cancer (10). For DCE-MRI, due to the enhancement of background parenchyma and partial overlap of the time-intensity curves of benign and malignant lesions, the diagnosis by DCE-MRI is neither specific nor consistent (11). The conventional diffusion-weighted imaging (DWI) along with the derived apparent diffusion coefficient (ADC) has shown important diagnostic value in breast cancer, e.g., for discriminating malignancy. However, there is currently still no uniform standard of using ADC values for predicting the status of different tumor characteristic receptors (12). One of the key reasons may lie in that ADC is a general measurement of restricted diffusion rate that cannot pinpoint the underlying pathology; e.g., the change of cell size, cell density, and intra- or extra-cellular diffusivity (13).
The recently developed microstructural diffusion MRI methods, which captures the restriction of water diffusion at different diffusion length scales by varying diffusion times (td) and b values, have shown unique advantages in delineating cellular microstructures (14–16). In addition to the commonly used pulse gradient spin-echo (PGSE) sequence, which only allows td measurement on the order of tens of milliseconds on most clinical MRI scanners, the oscillating gradient spin-echo (OGSE) technique (15, 17) was usually introduced by microstructural diffusion MRI to achieve shorter td for probing microstructures at smaller scales. By incorporating the microstructural diffusion MRI with specific biophysical models, we can estimate important microstructural properties such as cell size, cell volume fraction, and transcytolemmal water exchange, which are closely related to the pathological changes of tumor (18). Among them, the IMPULSED (imaging microstructural parameters using limited spectrally edited diffusion) method (19) has been comprehensively validated using computer simulations in silico, cells in vitro, and animals in vivo. The MRI data acquisition for the IMPULSED method has also been successfully implemented in patients with breast (20) and prostate cancer (21–23) within clinically feasible scan times (eg, <7 minutes for breast imaging). Changes in cell sizes are typical features for both mitotic arrest (cell swelling) and apoptosis (cell shrinkage), for example, a cell in an early apoptotic stage may have a smaller diameter than a normal cell (22), therefore, measurements of cellular microstructures including cell size may provide a unique means for characterization of breast tumors associated with different kinds of risk factors.
The current study aims to evaluate the efficacy of microstructural mapping by the IMPULSED method in breast tumors, and further to evaluate whether the MRI-derived microstructural properties are associated with and can be used to predict the prognostic factors of breast cancer.
This is a prospective study, and all participants were approved by our Clinical Research Ethics Review Committee. A total of 236 patients with clinical diagnosis of breast tumors from March 2023 to August 2023 were collected for breast MRI imaging. Inclusion criteria: 1) Suspicious breast lesions detected by mammography and/or ultrasound examination; 2) Patients who did not undergo puncture, radiotherapy or chemotherapy before MRI examination; 3) No MRI contraindications. Exclusion criteria: 1) Lesion diameter <8 mm (10 cases, small lesions will reduce the reliability of signal measurement); 2) No clear pathological or immunohistochemical results obtained after MRI scans (5 cases); 3) poor MRI image quality (8 cases). All enrolled patients were excluded due to one single exclusion criterion. Finally, 213 cases were enrolled, and the participant flowchart is shown in Figure 1.
MRI was performed on a 3-T scanner (Ingenia Elition, Philips Healthcare, Best, the Netherlands) with the maximum gradient amplitude of 45 mT/m per axis, the maximum gradient slew rate of 220 mT/m/ms and a 7-channel breast coil. Routine pre-contrast MRI included T1WI, fat-suppressed T2WI, and conventional DWI. The DCE-MRI was used for anatomical reference. The IMPULSED MRI scan included acquisitions of diffusion MRI with both oscillating (OGSE) and pulsed (PGSE) gradient encoding using the oscillating frequencies up to 33 Hz. Table 1 shows detailed parameters for all scans.
The IMPULSED parameters, including the mean cell diameter (dmean), intracellular fraction (fin), extracellular diffusivity (Dex), and cellularity index (fin/dmean), were estimated using a two-compartment model, with the intracellular diffusivity (Din) fixed at 1.58 μm2/ms to ensure fitting stability according to the previous study (19). The parameters were constrained to 4<dmean<30 μm, 0<fin <1, and 0<Dex <3.5 μm2/ms based on physiologically relevant values. The fitting was performed using the least square curve fitting toolbox in MATLAB (Mathworks, Inc.) according to a previous study (20) with the code available at https://github.com/jzxu0622/mati. Additionally, the ADC values for DWI, PGSE, and OGSE sequences were fitted according to S/S0 = exp(−b×ADC) (13).
The regions-of-interest (ROIs) for breast tumors were manually delineated on the slice with the largest scale of the lesion with reference to the high b value DWI and DCE-MRI images by experienced radiologists (H.Y with 13 years of experience and W.X.Y with 10 years of experience), and necrotic area and/or surrounding tissues were carefully excluded. The fitted microstructural parameters were calculated in a voxel-wise manner and averaged within the tumor ROIs.
Two pathologists (with 8 and 12 years of experience, respectively) independently analyzed the hematoxylin and eosin staining and immunohistochemical results of the lesion specimens. Breast tumors were first divided into malignant and benign groups according to pathological results, and all the pathological results were obtained by operation. Besides, immunohistological staining of breast tumor excisions or biopsies provides the following information: hormone receptor (ER and PR) status, HER-2 status, and Ki-67 index. The criteria for positive expression of ER or PR were as follows: ER or PR were positive in ≥10% of tumor cells (24). The criteria for HER-2 status were as follows: samples of + and - signals were negative, and samples of +++ signals were positive; samples with a ++ signal were further hybridized in situ (samples with gene amplification were positive and samples without gene amplification were negative) (25). The criteria of Ki-67 expression were as follows: high expression was defined as staining positive in ≥14% of tumor cells, and low expression was defined as staining positive in < 14% of tumor cells (26). The concept of molecular typing of breast cancer was first proposed by Perou et al. (3), and breast cancer was divided into four main molecular subtypes through clustering analysis of gene expression profiles: Luminal Type A (ER+ and/or PR+, HER-2-), Luminal Type B (ER+ and/or PR+, HER-2+), HER-2 overexpression type (ER- and PR-, HER-2+), TN (triple-negative) type (ER- and PR-, HER-2-). The grade of invasive breast cancer (IBC) was evaluated according to pathological criteria, among which grade I was highly differentiated tumors; grade II, moderately differentiated tumor; and grade III, poorly differentiated tumor.
Statistical analyses were performed using Graphpad prism software (version 8.0, GraphPad Software, Inc., San Diego, CA, USA). Data homogeneity of variance was evaluated by Levene test. All quantitative measurements are expressed as mean ± standard deviation. The intraclass correlation coefficient (ICC) was used to evaluate the intra-observer reliability regarding the measurements of ADCs and cellular microstructural parameters. The independent samples t test was used to compare the dmean, fin, Dex, cellularity index, and ADC values between benign and malignant breast tumors, between breast cancer with different histological grading, between breast cancer with positive and negative expression of ER, PR, and HER-2, and between breast cancer with high and low expression of Ki-67, respectively. The receiver operating characteristic (ROC) curve was used to access the diagnostic performance of different imaging parameters in differentiation between benign and malignant tumors, as well as in recognition of different breast cancer risk factors. Logistic regression analyses were used to identify independent factors and combination diagnosis. P < 0.05 indicated that the difference was statistically significant.
213 patients (45.12 ± 12 years old) with 213 tumor lesions (83 benign and 130 malignant) were included in the final analysis. Basic demographic and clinical information of the patients are summarized in Table 2. Among the 130 malignant breast tumors, 117 cases were recognized as IBC. For the 117 cases of IBC, 87 out of 117 (74.36%) were identified ER-positive and 30/117 (25.64%) were negative, 73 out of 117 (62.39%) were identified PR-positive and 44/117 (37.61%) were negative, 46 out of 117 (39.32%) were identified HER-2-positive and 71/117 (60.68%) were negative, 104 out of 117 (88.89%) were identified high expression of Ki-67 and 13/117 (11.11%) were low expression of Ki-67. Among the IBC, there were 4 cases of grade I, 67 cases of grade II, and 46 cases of grade III.
The ICCs between the two observers for measurement of quantitative ADC and cellular microstructural parameters were all higher than 0.75, suggesting excellent reliability (Table 3). The microstructural parameters for benign and malignant tumors are shown in Table 4, and the representative images of patients in the two groups are shown in Figures 2, 3. The dmean, fin and cellularity index values of malignant lesions were significantly higher than those of benign lesions (15.74 ± 2.68 vs. 14.28 ± 4.65 μm, 0.346 ± 0.125 vs. 0.279 ± 0.212, 21.19 ± 39.54 vs. 19.38 ± 14.87 ×10-3 um-1, dmean and cellularity p<0.001, fin p<0.005), and the Dex and ADC values of malignant lesions were significantly lower than those of benign lesions (Dex: 2.119 ± 0.395 vs. 2.378 ± 0.332, ADCDWI: 0.877 ± 0.148 vs. 1.453 ± 0.356, ADCPGSE: 1.196 ± 0.379 vs. 0.853 ± 0.243, ADC17Hz: 1.582 ± 0.377 vs. 1.285 ± 0.468, and ADC33Hz: 2.180 ± 0.386 vs. 1.896 ± 0.473 um2/ms; all p<0.001). For both of benign and malignant breast lesions ADC33Hz >ADC17Hz > ADCPGSE (benign: 2.180 ± 0.386 vs. 1.582 ± 0.377 vs. 1.196 ± 0.379 um2/ms, malignant: 1.896 ± 0.473 vs. 1.285 ± 0.468 vs. 0.853 ± 0.243 um2/ms). For differentiation between benign and malignant breast lesions, ADCDWI showed the highest area under ROC curve (AUC, 0.951) (sensitivity = 80.49, and specificity = 98.28%). The ADC values by PGSE and OGSE sequences showed AUCs ranged from 0.728 to 0.753. The IMPULSED derived microstructural parameters, including dmean, fin, Dex and the cellularity index, showed the AUCs ranged from 0.630 to 0.700, and the diagnostic performance can be significantly improved with their combination (AUC = 0.787). The combination of IMPULSED-derived parameters and ADCs by PGSE and OGSE can further improve the AUC to 0.897 (sensitivity = 81.93%, and specificity = 81.54%).
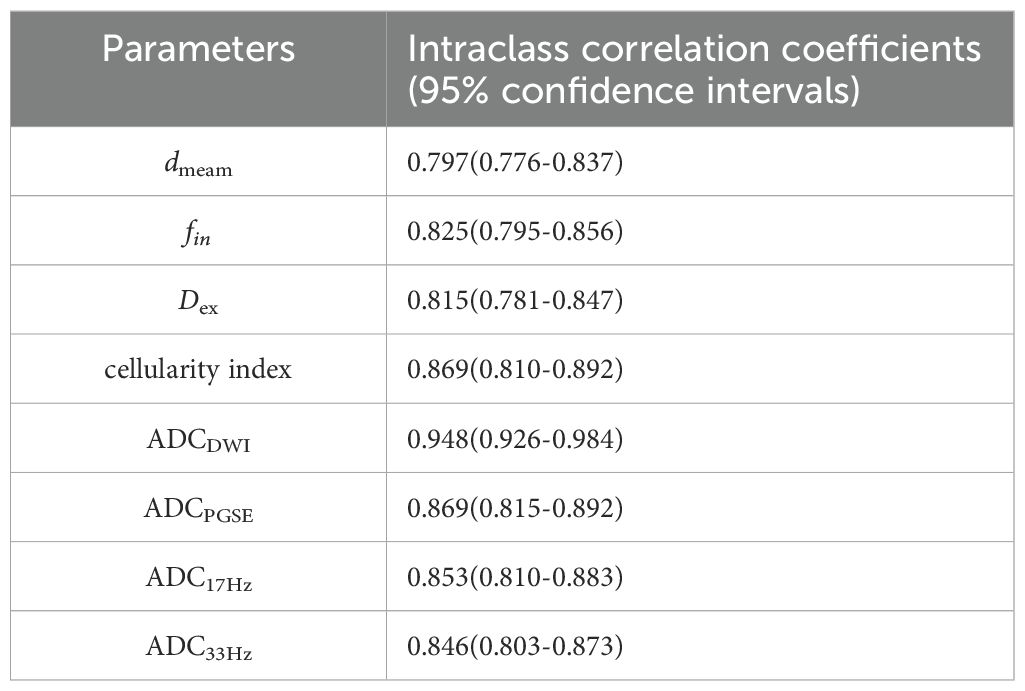
Table 3. The interclass correlation coefficient and 95% confidence intervals for dmean, fin, Dex, cellularity index, and ADCs measurements between observers.
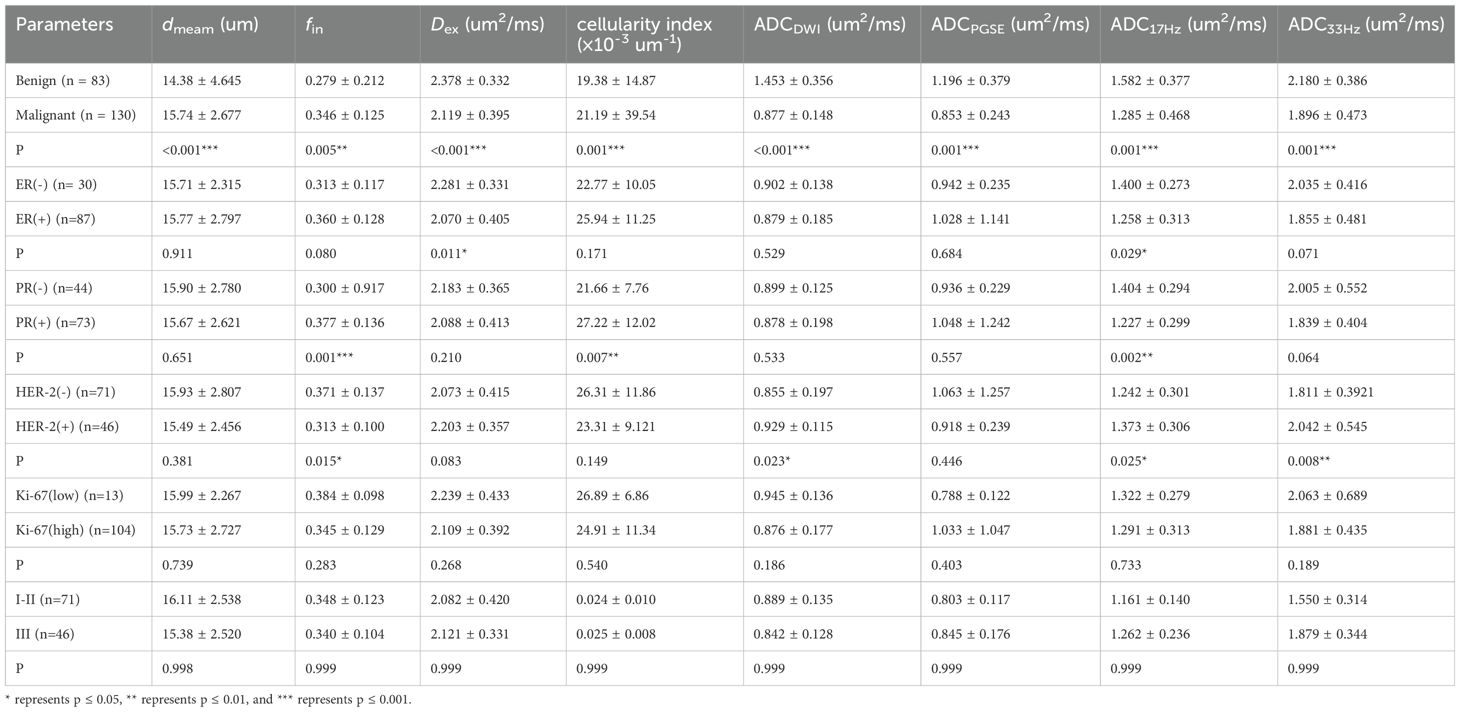
Table 4. Comparison of Microstructural diffusion MRI parameters between benign and malignant breast lesions, and between different subtypes or histological grades of breast cancer.
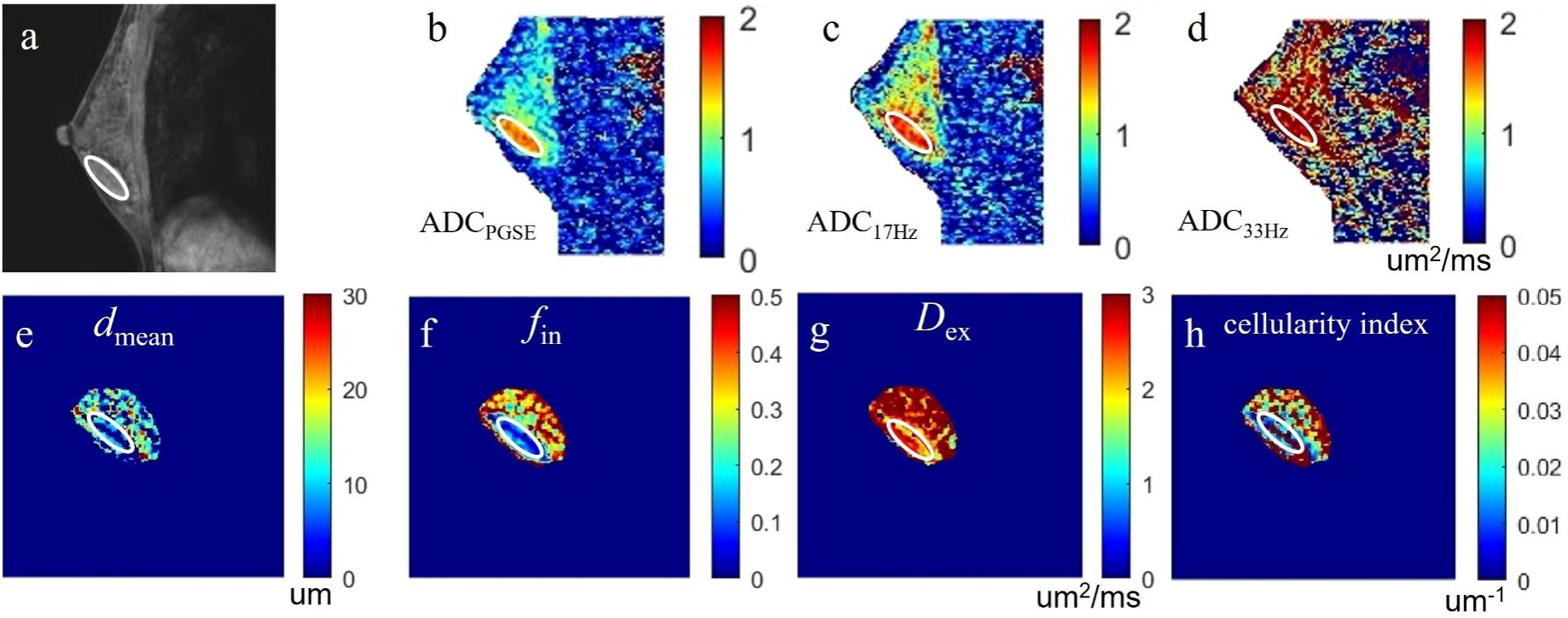
Figure 2. a case of fibroadenoma in the right breast: (A) the sagittal DCE-MRI image as reference; (B-D) the sagittal ADCPGSE, ADC17Hz, and ADC33Hz images with values for the lesion of 1.382, 1.589 and 2.523 um2/ms, respectively; (E-H) the dmean, fin, Dex, and cellularity index images around the lesion fitted by the IMPULSED method with values for the lesion of 10.38 um, 14.22%, 2.456 um2/ms, and 14.91×10-3 um-1, respectively. These microstructural parameters were only fitted at a limited region covering the lesion for saving of the post-processing time. The circles on the images are the ROIs of the lesion.
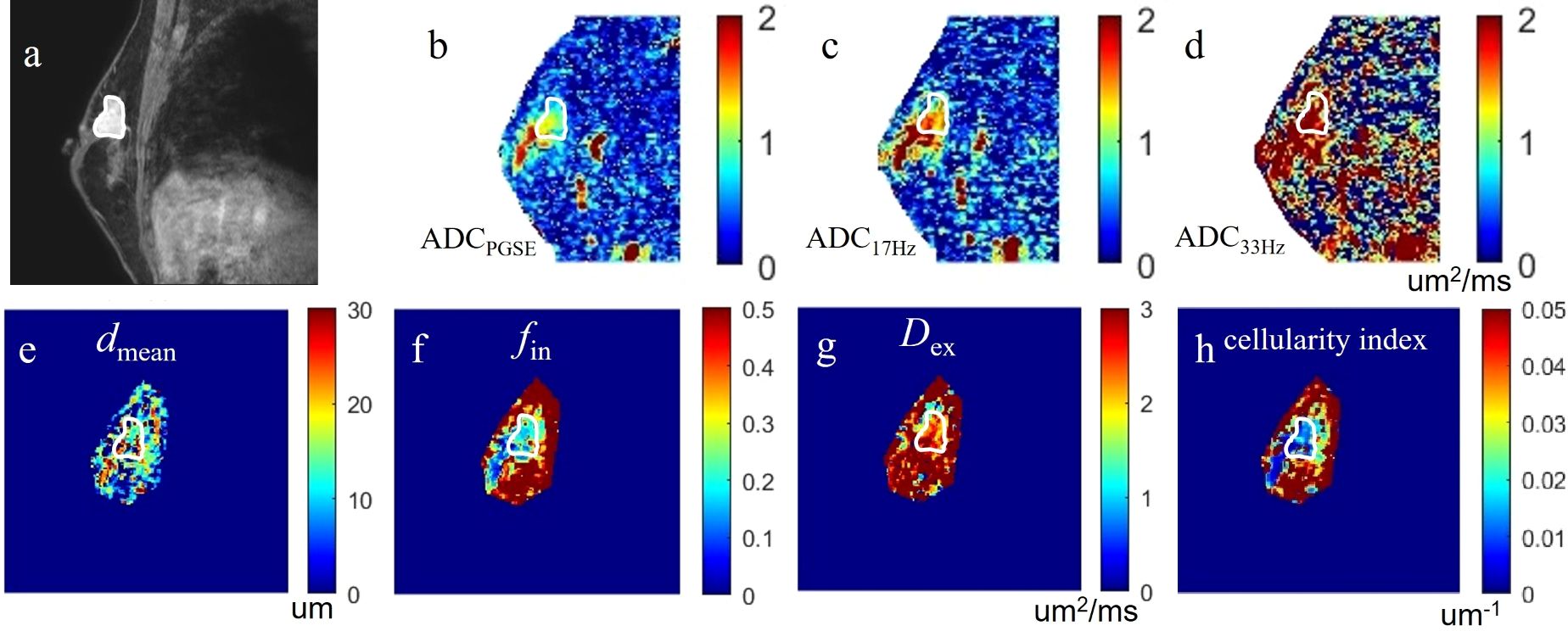
Figure 3. a case of invasive carcinoma in the right breast: (A) the DCE-MRI image as reference; (B-D) the ADCPGSE, ADC17Hz and ADC33Hz images with values for the lesion of 1.021, 1.409, and 2.018 um2/ms, respectively; (E-H) the dmean, fin, Dex, and cellularity index images around the lesion fitted by the IMPULSED method with values for the lesion of 13.86 um, 25.53%, 2.267 um2/ms, and 21.24×10-3 um-1, respectively. These microstructural parameters were only fitted at a limited region covering the lesion for saving of the post-processing time. The circles on the images are the ROIs of the lesion.
The ADC17Hz and Dex values of ER(+) tumors were significantly lower than those of ER(-) tumors (ADC17Hz: 1.258 ± 0.313 vs. 1.400 ± 0.273 mm2/s, p = 0.029; Dex: 2.070 ± 0.405 vs. 2.281 ± 0.331 um2/ms, p=0.011) (Table 4). The fin (0.377 ± 0.136 vs. 0.300 ± 0.917, p=0.001) and cellularity index (27.22 ± 12.02 vs. 21.66 ± 7.76 ×10-3 um-1, p=0.007) values of PR(+) tumors were significantly higher than those of PR(-) tumor. The ADC17Hz values of PR(+) tumors were significantly lower than those of PR(-) tumors(1.227 ± 0.299 vs. 1.404 ± 0.294 mm2/s, p =0.002). The fin values of HER-2(+) tumors were significantly lower than those of HER-2(-) tumors (0.313 ± 0.100 vs. 0.371 ± 0.137, p=0.015), and the ADCDWI, ADC17Hz and ADC33Hz values of HER-2(+) tumors were significantly higher than those of HER-2(-) tumors (ADCDWI: 0.929 ± 0.115 vs. 0.855 ± 0.197 mm2/ms, p=0.023; ADC17Hz: 1.373 ± 0.306 vs. 1.242 ± 0.301, mm2/s, p =0.025; ADC33Hz: 2.042 ± 0.545 vs. 1.811 ± 0.392 mm2/s, p = 0.008). For differentiation between ER(+) and ER(-), the ADC17Hz and Dex showed AUCs of 0.643 (sensitivity = 76.67%, and specificity = 47.06%) and 0.646 (sensitivity = 80.0%, and specificity = 45.98%), and the combination of Dex and ADC17Hz showed a AUC of 0.663 (sensitivity =93.33%, specificity = 36.78%). For differentiation of PR(+) and PR(-), the ADC17Hz, fin, and cellularity index showed AUCs of 0.666 (sensitivity = 68.18%, and specificity = 61.97%), 0.697 (sensitivity = 77.27%, and specificity = 60.27%) and 0.661 (sensitivity: 68.18%, and specificity: 61.64%), respectively, and their combination showed a AUC of 0.729 (sensitivity =72.73%, specificity = 65.75%). For differentiation of HER-2(+) and HER-2(-), the ADCDWI, ADC17Hz, and ADC33Hz, and fin showed AUCs of 0.625 (sensitivity = 59.42%, specificity = 63.04%), 0.632 (sensitivity = 43.66%, and specificity = 84.78%), 0.664 (sensitivity = 47.95%, and specificity = 82.67%) and 0.650 (sensitivity = 77.46%, and specificity = 56.52%), respectively, and their combination showed a AUC of 0.693 (sensitivity = 69.57%, specificity = 64.79%) (Table 5, Figures 4, 5). There was no significant difference in the ADCs and quantitative microstructural parameters between breast cancer with low-to-moderate (I and II) and high (III) histological grade, as well as between breast tumors with high and low expression of Ki-67.
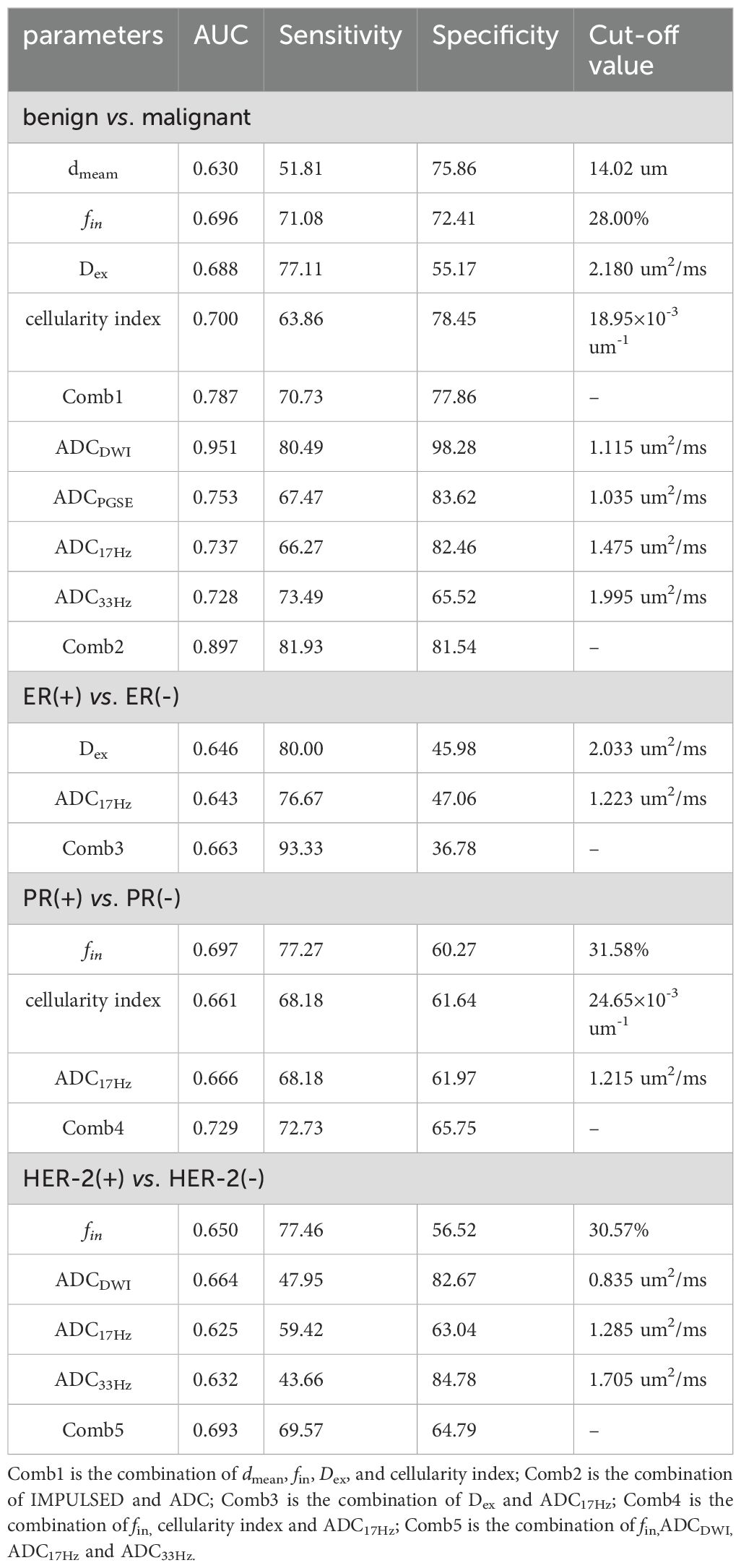
Table 5. Performance of the cellular microstructural parameters derived by IMPULSED in differentiation between benign and malignant breast lesions, as well as between different subtypes of breast cancer.
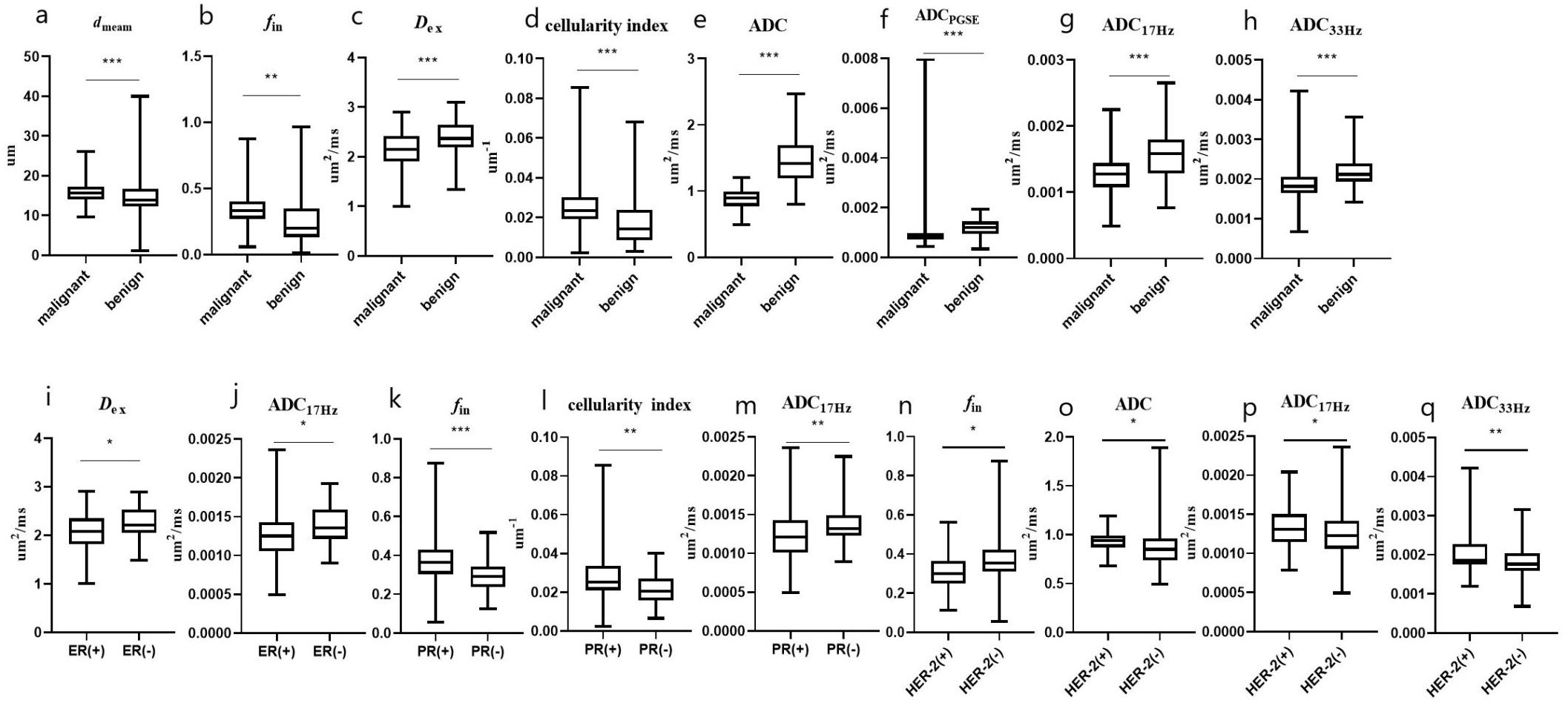
Figure 4. The microstructural parameters measured by the IMPUSED method with significant differences between benign and malignant tumors, or between different subtypes of breast cancer. The dmean (A), fin (B) and cellularity index (D) of malignant lesions were significantly higher than those of benign lesions, and the Dex (C) and ADCs (E–H) of malignant lesions were significantly lower than those of benign lesions; the Dex and ADC17Hz were lower in the ER(+) than in ER(-) group (I, J); the fin and cellularity index were higher in the PR(+) than in PR(-) group (K, L); the ADC17Hz values of PR(+) tumors were significantly lower than those of PR(-) tumors (M); and the fin, ADCDWI, ADC17Hz and ADC33Hz values were higher in the HER-2(+) than in HER-2(-) group (N–Q). * represents p ≤ 0.05, ** represents p ≤ 0.01, and *** represents p ≤ 0.001.
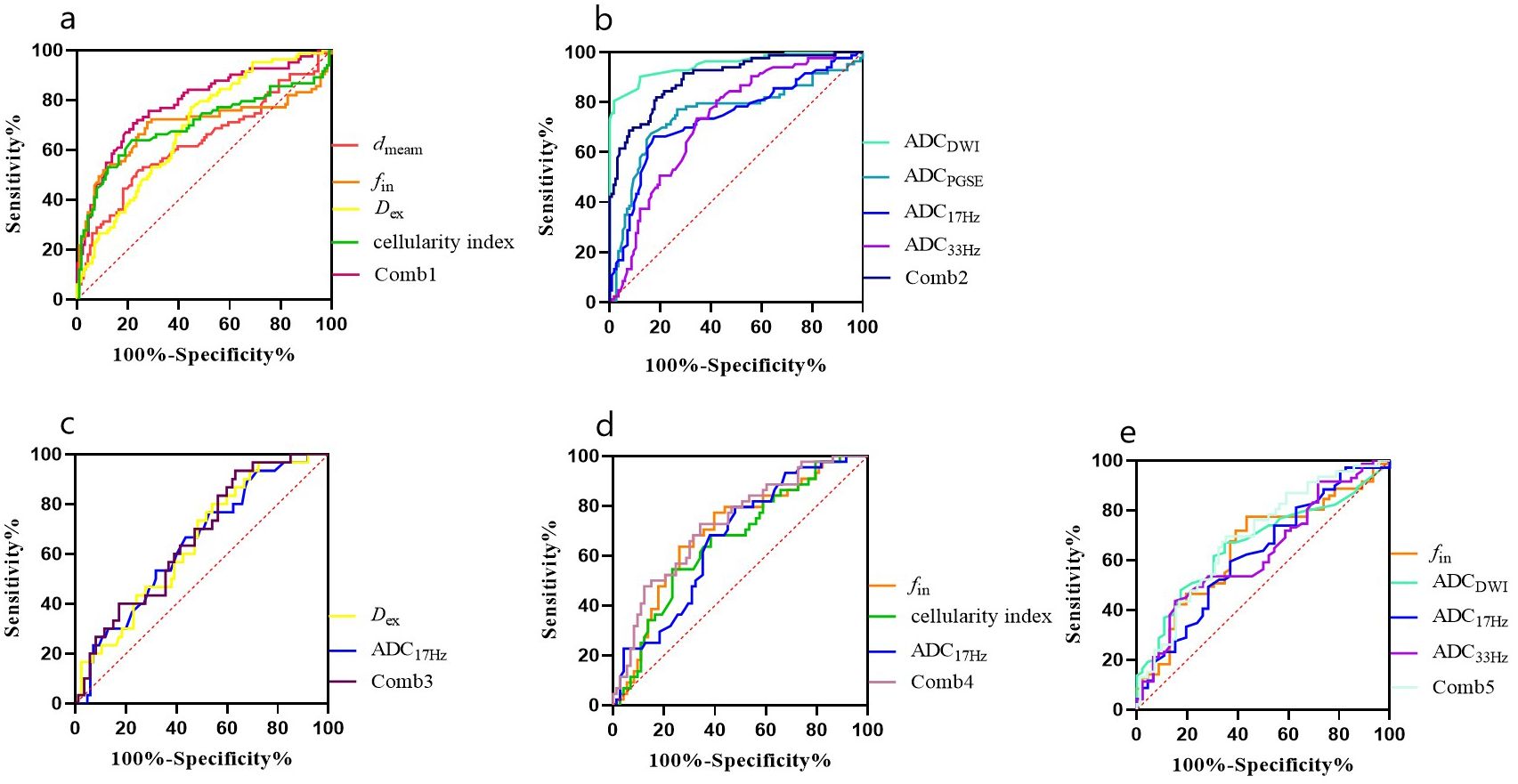
Figure 5. ROC curves for different parameters (A, B) for differentiation between malignant and benign breast lesions (Comb1: the combination of IMPULSED derived parameters, and Comb2: the combination of IMPULSED derived parameters and ADCs by PGSE and OGSE), and ROC curves of different parameters for differentiation between different subtypes of breast cancer (C: ER, D: PR, and E: HER-2).
In our study, we used the microstructural diffusion MRI (IMPULSED) to evaluate the microscopic characteristics of breast tumors and found that the microstructural parameters and ADC values showed significant differences between malignant and benign lesions. The microstructural parameters and/or ADC values also showed potential for non-invasive prediction of different prognostic risk factors in breast cancer.
Our results showed that the dmean, fin and cellularity index values of malignant lesions were significantly higher than those of benign lesions, and the Dex and ADCs of malignant lesions were significantly lower than those of benign lesions, which is mostly in agreement with studies by Xu et al. (20) and Wu et al (21). Previous studies have shown that the ADC value is an effective parameter in differentiating benign and malignant breast lesions (27). Malignant tumors usually have lower ADC values than benign lesions due to their high cell density (28, 29), at the same time, the restriction of cell biofilm and the adsorption of macromolecules such as proteins on water molecules are also enhanced. The combined effect of these factors prevents the effective movement of water molecules in malignant tumors, thus reducing the ADC value, consistent with our findings. Lima et al. (30) found that ADC values of breast tumors increased with the shortening of diffusion time (increasing of gradient oscillation frequency) (ADCPGSE<ADCOGSE), and all the ADC values (by PGSE and OGSE) of malignant breast tumors were lower than those of benign breast tumors, which is consistent with our study. The combination (AUC = 0.897) of IMPULSED-derived parameters and ADCs by PGSE and OGSE show significant improvement in the diagnostic performance when compared to results by individual parameters (AUC = 0.630-0.753). Compared with benign lesions, the proliferation rate of malignant lesions was faster, the cell density was higher, and the extracellular space was reduced, which explained that dmean, fin and cellularity index were higher in malignant lesions than in benign lesions. When distinguishing benign and malignant lesions, our results showed that ADC had the best performance among all the quantitative measurements, followed by the different microstructural features. Wu et al. (21) showed that the cellularity index had an AUC of 0.96 in distinguishing clinically significant from clinically insignificant prostate tumors, which is better than traditional ADC measurements. The lower AUCs of the IMPULSED derived microstructural parameters (compared to ADC) in our study may be related to the complex tissue composition of breast lesions. The advanced and complex model fitting of the IMPULSED method may also suffer from lower image quality and contribute to greater intra-group variation.
In breast cancer, the status of IHC (Immunohistochemistry) tumor receptors determines the subtype of breast cancer and is closely related to the cellular, vascular, and aggressive nature of the tumor (18). HER-2 is a transmembrane tyrosine kinase receptor, and its overexpression in breast cancer is a major factor in tumor progression and metastasis (31, 32). Her2-positive cells have a more malignant phenotype that stimulates excessive cell proliferation, invasion, and metastasis (33). Our research showed that the fin was significantly lower in the HER-2(+) group compared to its negative counterpart, while the ADCDWI, ADC17Hz and ADC33Hz were significantly higher, which is in line with the previous reports by Catalano et al (34). The positive expression of HER-2 may lead to increased microcirculation perfusion in tumor tissue, and the limited diffusion of water molecules in tissue and increased blood perfusion may jointly affect the ADC value of tumor, resulting in increased ADC value in HER-2 positive tumors. The lower fin was observed in HER-2(+) than in HER-2(-) tumors, which may be related to the increase of water exchange across the membrane in HER-2-overexpressing breast tumors. Previous studies (35) found that if transmembrane water exchange could not be ignored, the intracellular volume fraction would be essentially underestimated for any biophysical diffusion method that assumes no water exchange (including the IMPLUSE method). Besides, the reduced fin may also indicate the more presence of necrotic core in HER-2(+) tumors, which is mainly composed of fluid and cell debris with reduced diffusion limitation (36).
ER and PR are hormone receptors that are known to be good prognostic factors, and in the presence of both receptors, treatment is effective for adjuvant or palliative hormone therapy. The cellularity index and fin were significantly higher in the PR(+) groups compared to their negative counterparts. This is consistent with the results of fin increase in the PR(+) group in the previous study by BaR et al. (13). The cellularity index is calculated as the quotient of intracellular volume fraction and IMPULSED-derived cell diameter, and thus is proportional to the intracellular volume fraction. Our results also show that the ADC17Hz value of PR(+) is lower than that of PR(-), which is basically consistent with the previous study by Ba et al. (13), which may be related to the differences in membrane permeability between different PR-expressing tumors. The Dex was significantly lower in the ER(+) group compared to its negative counterpart. The previous study has reported that ER-positive tumors were highly cellular (37). Animal model studies have also shown that angiogenic markers were inhibited when ER was overexpressed. All of these may result in the reduced Dex values (38) in ER(+) tumors. The results of this study show that the ADC17Hz value of ER(+) tumors is lower than that of ER(-) tumors, which may be due to the inhibitory effect of high level of ER expression on the angiogenic pathway of breast cancer (39).
Our study showed no statistical significance in quantitative microstructural diffusion MRI parameters and ADC values between low and high grade histological classification of malignant breast lesions, which is consistent with previous studies demonstrating no direct relationship between cell number and tumor grade (40, 41). Ki-67 index in tumor tissue is currently recognized as a marker of aggressive behavior in breast cancer. The microstructural diffusion MRI parameters and ADC values showed no significant difference between breast tumors with high and low expression of Ki-67. This is consistent with the results in the previous study by BaR, et al (13).
The current study has several limitations. First, although the consistency between IMPULSED-derived parameters and pathological results have been verified in previous studies (13, 20), it is still necessary for the current study to present such verification. However, the original pathological data for patients were unavailable to us, therefore, the related comparison was not presented in this study. Secondly, the number of some pathological type tumors was still relatively small, further investigation in a larger population is needed to verify the results of this study.
In conclusion, we have demonstrated the diagnostic potential of microstructural diffusion MRI based on the IMPULSED method for non-invasive exploration of cellular microstructural features in breast cancer in a clinical setting, and the feasibility of IMPULSED-derived parameters in differentiating breast cancer immunophenotypes. Results showed significant potential of microstructural diffusion MRI in discrimination of breast cancer immunophenotypes including the different expression status of ER, PR and HER-2.
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
The studies involving humans were approved by Ethics Committee of the First Affiliated Hospital of Zhengzhou University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
XW: Writing – original draft. YaZ: Conceptualization, Writing – review & editing. JC: Conceptualization, Writing – review & editing. LL: Conceptualization, Writing – review & editing, Formal Analysis. YH: Investigation, Writing – review & editing, Formal Analysis. AW: Investigation, Writing – review & editing, Formal Analysis. YoZ: Conceptualization, Writing – review & editing, Formal Analysis. RW: Investigation, Writing – review & editing. YL: Conceptualization, Writing – review & editing. KZ: Investigation, Writing – review & editing. WZ: Investigation, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research study was supported by the Natural Science Foundation of China (Nos. 81601467, 81871327, and 81601472) Medical science and technology research project of Henan province (201701011). We are grateful to our patients and their families for their continued support for our study.
The authors thank Zhigang Wu and Peng Sun from Philips Healthcare for their technical support on the implementation of microstructural diffusion MRI.
LL was employed by the company Philips Healthcare.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interes.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Zheng R, Zhang S, Zeng H, Wang S, Sun K, Chen R, et al. Cancer incidence and mortality in China, 2016. J Natl Cancer Cent. (2022) 2:1–9. doi: 10.1016/j.jncc.2022.02.002
2. Huang G, Du S, Gao S, Guo L, Zhao R, Bian X, et al. Molecular subtypes of breast cancer identified by dynamically enhanced MRI radiomics: the delayed phase cannot be ignored. Insights Imaging. (2024) 15:127. doi: 10.1186/s13244-024-01713-9
3. Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. (2000) 406:747–52. doi: 10.1038/35021093
4. Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. (2004) 10:5367–74. doi: 10.1158/1078-0432.CCR-04-0220
5. Blows FM, Driver KE, Schmidt MK, Broeks A, van Leeuwen FE, Wesseling J, et al. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies. PloS Med. (2010) 7:e1000279. doi: 10.1371/journal.pmed.1000279
6. Bakker MF, de Lange SV, Pijnappel RM, Mann RM, Peeters PHM, Monninkhof EM, et al. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. (2019) 381:2091–102. doi: 10.1056/NEJMoa1903986
7. Sumkin JH, Berg WA, Carter GJ, Bandos AI, Chough DM, Ganott MA, et al. Diagnostic performance of MRI, molecular breast imaging, and contrast-enhanced mammography in women with newly diagnosed breast cancer. Radiology. (2019) 293:531–40. doi: 10.1148/radiol.2019190887
8. Jahani N, Cohen E, Hsieh MK, Weinstein SP, Pantalone L, Hylton N, et al. Prediction of treatment response to neoadjuvant chemotherapy for breast cancer via early changes in tumor heterogeneity captured by DCE-MRI registration. Sci Rep. (2019) 9:12114. doi: 10.1038/s41598-019-48465-x
9. Torii C, Hida Y, Shindoh M, Akiyama K, Ohga N, Maishi N, et al. Vasohibin-1 as a novel prognostic factor for head and neck squamous cell carcinoma. Anticancer Res. (2017) 37:1219–25. doi: 10.21873/anticanres.11437
10. Yuan C, Jin F, Guo X, Zhao S, Li W, Guo H. Correlation analysis of breast cancer DWI combined with DCE-MRI imaging features with molecular subtypes and prognostic factors. J Med Syst. (2019) 43:83. doi: 10.1007/s10916-019-1197-5
11. Lin Z, Zhang X, Guo L, Wang K, Jiang Y, Hu X, et al. Clinical feasibility study of 3D intracranial magnetic resonance angiography using compressed sensing. J Magn Reson Imaging. (2019) 50:1843–51. doi: 10.1002/jmri.26752
12. Zhang N, Kang J, Wang H, Liu A, Miao Y, Ma X, et al. Differentiation of fibroadenomas versus Malignant breast tumors utilizing three-dimensional amide proton transfer weighted magnetic resonance imaging. Clin Imaging. (2022) 81:15–23. doi: 10.1016/j.clinimag.2021.09.002
13. Ba R, Wang X, Zhang Z, Li Q, Sun Y, Zhang J, et al. Diffusion-time dependent diffusion MRI: effect of diffusion-time on microstructural mapping and prediction of prognostic features in breast cancer. Eur Radiol. (2023) 33:6226–37. doi: 10.1007/s00330-023-09623-y
14. Novikov DS, Fieremans E, Jespersen SN, Kiselev VG. Quantifying brain microstructure with diffusion MRI: theory and parameter estimation. NMR BioMed. (2019) 32:e3998. doi: 10.1002/nbm.v32.4
15. Gore JC, Xu J, Colvin DC, Yankeelov TE, Parsons EC, Does MD. Characterization of tissue structure at varying length scales using temporal diffusion spectroscopy. NMR BioMed. (2010) 23:745–56. doi: 10.1002/nbm.v23:7
16. Novikov DS, Jensen JH, Helpern JA, Fieremans E. Revealing mesoscopic structural universality with diffusion. Proc Natl Acad Sci U.S.A. (2014) 111:5088–93. doi: 10.1073/pnas.1316944111
17. Baron CA, Beaulieu C. Oscillating gradient spin-echo (OGSE) diffusion tensor imaging of the human brain. Magn Reson Med. (2014) 72:726–36. doi: 10.1002/mrm.24987
18. Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ. Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer. Ann Oncol. (2011) 22:1736–47. doi: 10.1093/annonc/mdr304
19. Jiang X, Li H, Xie J, McKinley ET, Zhao P, Gore JC, et al. In vivo imaging of cancer cell size and cellularity index using temporal diffusion spectroscopy. Magn Reson Med. (2017) 78:156–64. doi: 10.1002/mrm.26356
20. Xu J, Jiang X, Li H, Arlinghaus LR, McKinley ET, Devan SP, et al. Magnetic resonance imaging of mean cell size in human breast tumors. Magn Reson Med. (2020) 83:2002–14. doi: 10.1002/mrm.28056
21. Wu D, Jiang K, Li H, Zhang Z, Ba R, Zhang Y, et al. Time-dependent diffusion mri for quantitative microstructural mapping of prostate cancer. Radiology. (2022) 303(3):578–87. doi: 10.1148/radiol.211180
22. Jiang X, McKinley ET, Xie J, Li H, Xu J, Gore JC. In vivo magnetic resonance imaging of treatment-induced apoptosis. Sci Rep. (2019) 9:9540. doi: 10.1038/s41598-019-45864-y
23. Jiang X, Li H, Zhao P, Xie J, Khabele D, Xu J, et al. Early detection of treatment-induced mitotic arrest using temporal diffusion magnetic resonance spectroscopy. Neoplasia. (2016) 18:387–97. doi: 10.1016/j.neo.2016.04.006
24. Hammond ME. Commentary: improving breast cancer testing for patients-the secret sauce is collaboration. J Oncol Pract. (2010) 6:198. doi: 10.1200/JOP.777012
25. Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. (2013) 31:3997–4013. doi: 10.1200/JCO.2013.50.9984
26. Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. (2013) 24:2206–23. doi: 10.1093/annonc/mdt303
27. Guo Y, Cai YQ, Cai ZL, Gao YG, An NY, Ma L, et al. Differentiation of clinically benign and Malignant breast lesions using diffusion-weighted imaging. J Magnn Reson Imaing. (2002) 16:172–8. doi: 10.1002/jmri.10140
28. Suo S, Zhang K, Cao M, Suo X, Hua J, Geng X, et al. Characterization of breast masses as benign or Malignant at 3.0T MRI with whole-lesion histogram analysis of the apparent diffusion coefficient. J Magnn Reson Imaing. (2016) 43:894–902. doi: 10.1002/jmri.25043
29. Liu HL, Zong M, Wei H, Lou JJ, Wang SQ, Zou QG, et al. Differentiation between Malignant and benign breast masses: combination of semiquantitative analysis on DCE-MRI and histogram analysis of ADC maps. Clin Radiol. (2018) 73:460–6. doi: 10.1016/j.crad.2017.11.026
30. Iima M, Kataoka M, Honda M, Ohashi A, Ohno Kishimoto A, Ota R, et al. The rate of apparent diffusion coefficient change with diffusion time on breast diffusion-weighted imaging depends on breast tumor types and molecular prognostic biomarker expression. Invest Radiol. (2021) 56:501–8. doi: 10.1097/RLI.0000000000000766
31. Toikkanen S, Helin H, Isola J, Joensuu H. Prognostic significance of HER-2 oncoprotein expression in breast cancer: a 30-year follow-up. J Clin Oncol. (1992) 10:1044–8. doi: 10.1200/JCO.1992.10.7.1044
32. Giatromanolaki A, Koukourakis MI, Simopoulos C, Polychronidis A, Gatter KC, Harris AL, et al. c-erbB-2 related aggressiveness in breast cancer is hypoxia inducible factor-1alpha dependent. Clin Cancer Res. (2004) 10:7972–7. doi: 10.1158/1078-0432.CCR-04-1068
33. Akiyama T, Sudo C, Ogawara H, Toyoshima K, Yamamoto T. The product of the human c-erbB-2 gene: a 185-kilodalton glycoprotein with tyrosine kinase activity. Science. (1986) 232:1644–6. doi: 10.1126/science.3012781
34. Catalano OA, Horn GL, Signore A, Iannace C, Lepore M, Vangel M, et al. PET/MR in invasive ductal breast cancer: correlation between imaging markers and histological phenotype. Br J Cancer. (2017) 116:893–902. doi: 10.1038/bjc.2017.26
35. Li H, Jiang X, Xie J, Gore JC, Xu J. Impact of transcytolemmal water exchange on estimates of tissue microstructural properties derived from diffusion MRI. Magn Reson Med. (2017) 77:2239–49. doi: 10.1002/mrm.26309
36. Jiang X, Li H, Devan SP, Gore JC, Xu J. MR cell size imaging with temporal diffusion spectroscopy. Magnetic Resonance Imaging. (2021) 77:109–23. doi: 10.1016/j.mri.2020.12.010
37. Black R, Prescott R, Bers K, Hawkins A, Stewart H, Forrest P. Tumor cellularity index, estrogen receptors and prognosis in breast cancer. Clin Oncol. (1983) 9:311–18.
38. Ludovini V, Sidoni A, Pistola L, Bellezza G, De Angelis V, Gori S, et al. Evaluation of the prognostic role of vascular endothelial growth factor and micro vessel density in stages I and II breast cancer patients. Breast Cancer Res Treat. (2003) 81:159–68. doi: 10.1023/A:1025755717912
39. Makkat S, Luypaert R, Stadnik T, Bourgain C, Sourbron S, Dujardin M, et al. Deconvolution-based dynamic contrast-enhanced MR imaging of breast tumors: correlation of tumor blood flow with human epidermal growth factor receptor 2 status and clinicopathologic findings-preliminary results. Radiology. (2008) 249:471–82. doi: 10.1148/radiol.2492071147
40. Yoshikawa MI, Ohsumi S, Sugata S, Kataoka M, Takashima S, Mochizuki T, et al. Relation between cancer cellularity index and apparent diffusion coefficient values using diffusion weighted magnetic resonance imaging in breast cancer. Radiat Med. (2008) 26:222–6. doi: 10.1007/s11604-007-0218-3
Keywords: microstructural diffusion MRI, breast tumor, benign and malignant, molecular prognostic biomarker, IMPULSED method
Citation: Wang X, Zhang Y, Cheng J, Lin L, Hu Y, Wang A, Zhang Y, Wang R, Li Y, Zhang K and Zhang W (2025) Microstructural diffusion MRI for differentiation of breast tumors and prediction of prognostic factors in breast cancer. Front. Oncol. 15:1498691. doi: 10.3389/fonc.2025.1498691
Received: 19 September 2024; Accepted: 06 January 2025;
Published: 05 March 2025.
Edited by:
Chao Li, University of Cambridge, United KingdomReviewed by:
Junzhong Xu, Vanderbilt University Medical Center, United StatesCopyright © 2025 Wang, Zhang, Cheng, Lin, Hu, Wang, Zhang, Wang, Li, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Zhang, emhhbmd5YW5oeUAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.