
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 03 April 2025
Sec. Cancer Immunity and Immunotherapy
Volume 15 - 2025 | https://doi.org/10.3389/fonc.2025.1485386
 Daniela A. Ferraro1*
Daniela A. Ferraro1* Bettina Bisig2,3†
Bettina Bisig2,3† David C. Rotzinger4
David C. Rotzinger4 Fresia Pareja5
Fresia Pareja5 Edoardo Missiaglia2,3†
Edoardo Missiaglia2,3† Ioannis Voutsadakis6,7
Ioannis Voutsadakis6,7 Krisztian Homicsko1,3
Krisztian Homicsko1,3 Antonia Digklia1,3
Antonia Digklia1,3Sarcomas are traditionally considered “cold” tumors with poor response to immunotherapy. However, evidence accumulating over the last years shows that immune checkpoint inhibitors (ICIs) may have a role in selected sarcoma patients according to predictive markers. Here, we report the case of a woman diagnosed with a primary cardiac undifferentiated sarcoma. Following failure of standard first line chemotherapy, high-throughput sequencing (HTS) revealed a high tumor mutational burden (TMB), pathogenic mutations in FAT1 and NOTCH2 and a microsatellite instability (MSI)-associated signature. Immunohistochemistry confirmed mismatch repair-deficiency (MMRd) and abundant CD8+ tumor-infiltrating lymphocytes (TILs), in the absence of tertiary lymphoid structures. The patient was, therefore, treated with the ICI pembrolizumab, reaching a complete response that continues to persist at last follow-up, more than seven years from initial diagnosis and nearly six years from initiation of ICI treatment. This case illustrates the importance of performing HTS in rare sarcomas given the availability of efficient therapies, such as those for tumors displaying high TMB or MMRd/MSI. In agreement with other reports, it supports the contention that MMRd/MSI status and high numbers of TILs are valuable predictive markers of response to immunotherapy in sarcomas.
Immune check-point inhibitors (ICIs) have shown remarkable results in the treatment of various cancer types, but their efficacy in soft tissue sarcomas (STS) is low except for some subtypes, including undifferentiated pleomorphic sarcoma (UPS), alveolar soft part sarcoma, clear cell sarcoma, dedifferentiated liposarcoma (DDLPS) and angiosarcoma (1, 2). Similarly, in a recently published series of primary cardiac sarcomas the response to ICI was overall poor, although it was particularly dismal for patients with angiosarcoma, the most frequent primary cardiac sarcoma subtype (3, 4).
Tumor-infiltrating lymphocytes (TILs), tumor mutational burden (TMB), and PD-L1 expression are the most important predictive markers of response to ICI therapy in epithelial tumors (5, 6). Compared to highly immunogenic tumors such as melanoma or lung cancer, sarcomas usually display a low TMB (average of 2 mutations/Mb), low extent of TILs and low PD-L1 expression (7, 8).
Another more recently described predictive marker for ICIs is the presence of tertiary lymphoid structures (TLS) within the tumor or in its immediate proximity. TLS are lymphoid aggregates with features similar to B-cell follicles in lymph nodes, believed to have a relevant role in eliciting an antitumor immune response. Patients with abundant TLS have shown an increased survival and better response to ICIs in different cancer types, including STS. However, their prevalence in STS is low, in the range of 10% (9, 10). The genetic landscape of sarcomas is markedly heterogeneous, and rare sarcoma cases harbor a high TMB and/or high numbers of TILs. This is more common in UPS and leiomyosarcoma (LMS) and correlates with response to ICIs. Indeed, tumors exhibiting a high TMB are considered more immunogenic and more likely to respond to ICIs due to a greater abundance of tumor neo-antigens able to activate the immune system (11). High TMB may result from mismatch repair deficiency (MMRd), translating into a characteristic mutational signature consisting of high levels of microsatellite instability (MSI) across the genome. MSI signature, however, is rare in sarcomas, and has been reported in only 2% of patients (12, 13).
Pembrolizumab was the first PD-1 inhibitor approved by the American Food and Drug Administration (FDA) for MMRd/MSI tumors, initially in colon cancer and later in a tissue-agnostic manner (14–17).
Here, we present the case of a 73-year-old woman with an MMRd/MSI primary cardiac undifferentiated sarcoma who achieved a complete and durable response with the anti-PD1 inhibitor pembrolizumab after failure of standard first-line treatment.
A 73-year-old woman presented in September 2016 with progressive dyspnea of several months of duration. Magnetic resonance imaging (MRI) revealed a left atrial tumor mass. A surgical resection of the lesion was performed one month later necessitating pulmonary valve and artery replacement. Histopathologic examination of the specimen led to the diagnosis of primary cardiac undifferentiated sarcoma, after exclusion of an intimal sarcoma in the absence of MDM2 gene amplification (18). Post-operatively, the patient received no adjuvant therapy and was placed on radiologic surveillance. A cardiac MRI nine months later showed disease relapse with the presence of four solid intra-cardiac lesions in the inferior vena cava, in the right and left atria, and in the superior vena cava, without vascular compression and with a preserved cardiac function (left ventricular ejection fraction (LVEF) 51%). Positron emission tomography/computed tomography (PET/CT) confirmed the presence of hypermetabolic cardiac masses without evidence of metastasis. Brain MRI did not show metastatic brain disease.
A first line therapy consisting of doxorubicine (75mg/m2 every 3 weeks) together with olaratumab (15 mg/kg, at day 1 and 8 every 21 days) (19) was administered starting in August 2017. Given an anaphylactic shock following the first dose, no antineoplastic agents were administered for 8 months. Progressive disease developed, and a cardiac MRI showed a partial occlusion of the inferior vena cava, masses in the right ventricular outflow tract, and atria (Figure 1).
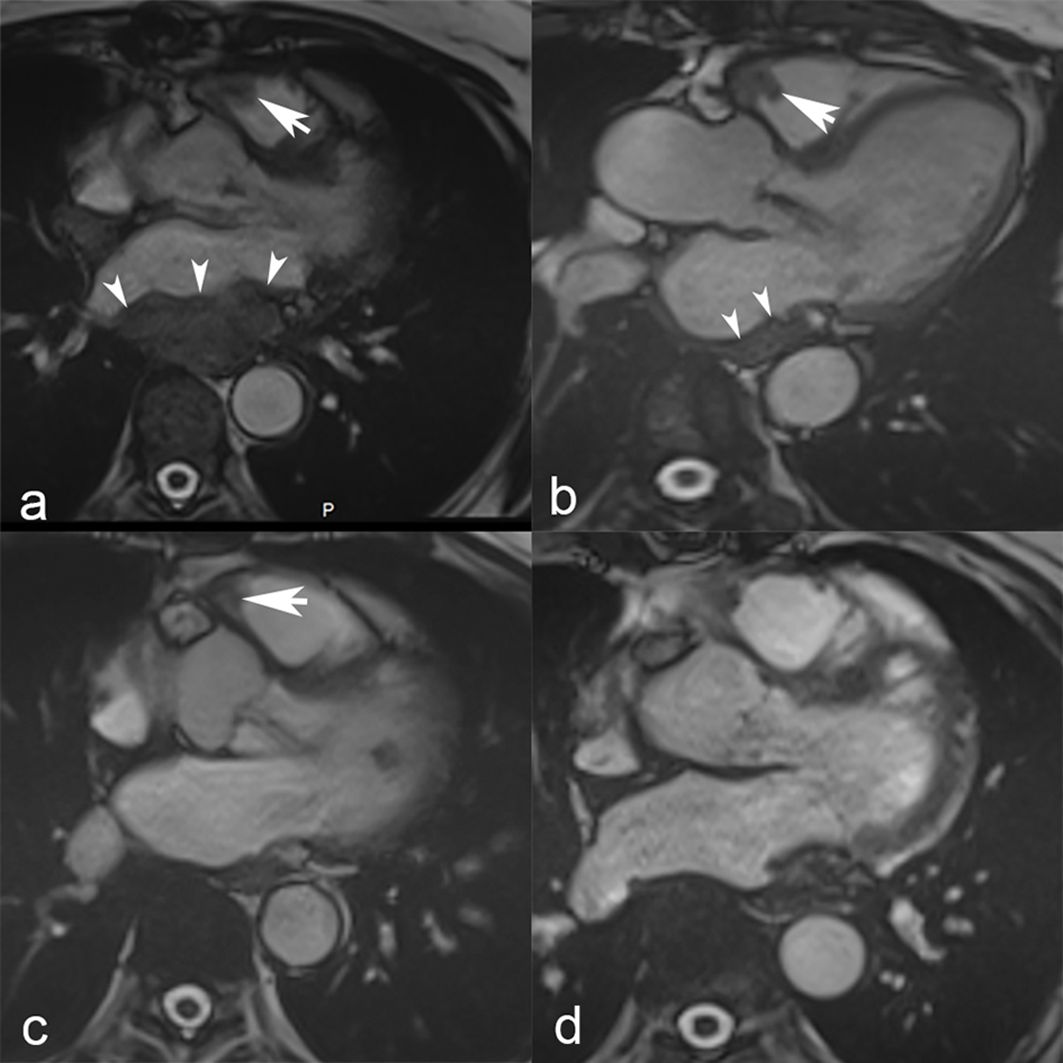
Figure 1. Cardiac MRI. Steady-state free precession cardiac MRI scans show masses in the right ventricular outflow tract (white arrow) and left atrium (white arrowheads) at baseline in March 2018 (a). Nine months later, in September 2018 (b), a follow-up MRI showed a slight decrease of the mass located in the right ventricular outflow tract (white arrow) and notable shrinkage of the left atrial mass that is merely visible as an atrial wall thickening (white arrowheads). Further tumor shrinkage of the right ventricular outflow tract mass (white arrow) was observed in May 2019 (c), and ultimately complete response with no residual tumor in October 2020 (d).
At that time, in search for alternative treatment options, the initial tumor resection specimen was subjected to further predictive biomarker analyses.
Targeted high-throughput sequencing (HTS), covering the coding region of 394 cancer-related genes (1.24 megabases (Mb)), was performed in parallel on DNA extracted from the tumor tissue and matched constitutional DNA, using the MiSeq platform (Illumina). This analysis identified 17 somatic mutations (10 missenses, 6 frameshifts and 1 splice site mutation) (Table 1), providing an estimated TMB of 12.9 non-synonymous somatic mutations per Mb (splice site mutation excluded). Although this latter was not very high, the presence of a large proportion of transitions and small indels in homopolymeric sequences was suggestive of an MSI-associated mutational signature (23). Interestingly, a likely pathogenic somatic splice variant was detected at the intron 1/exon 2 boundary of MLH1 (c.117-3C>G), and was associated with a copy gain of the mutated allele and a loss of heterozygosity (loss of the wild-type allele).
Immunostains for MMR proteins confirmed a loss of nuclear expression of MLH1 and PMS2 in the tumor cells (Figures 2a–c), with retained MSH2 and MSH6. The MLH1 gene promoter, evaluated by pyrosequencing after bisulfite conversion, did not show hypermethylation. No tertiary lymphoid structures (TLS) were detected based on standard hematoxylin & eosin-stained slides. TILs were abundant, with a mean of >20 intratumor CD8+ T cells per high power field (400x magnification) (Figure 2d). The tumor cells were negative for PD-L1 (tumor proportion score (TPS) <1%), while a subset of tumor-associated immune cells were positive (combined positive score (CPS) >20) (Figure 2e).
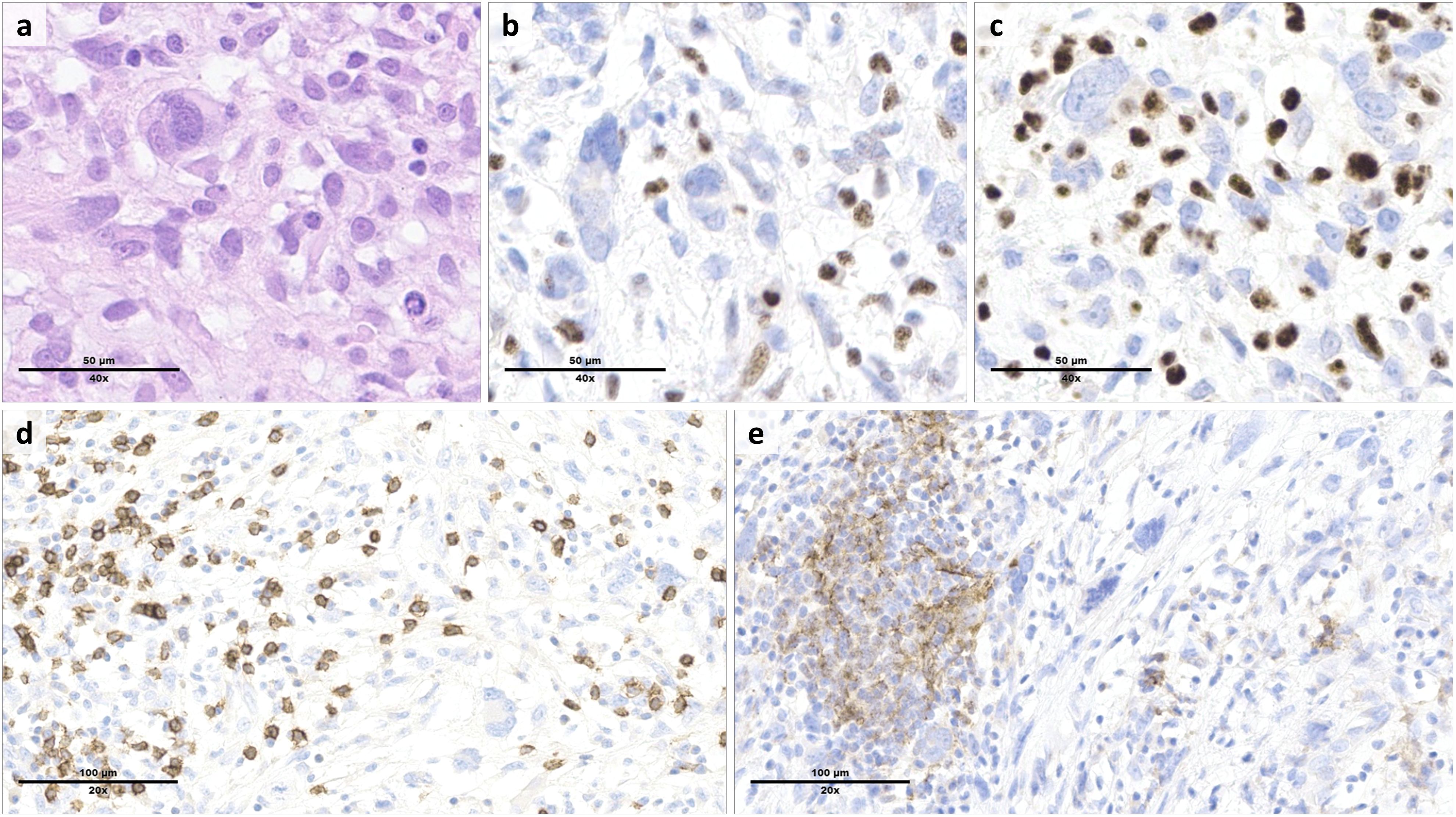
Figure 2. Morphologic and immunophenotypic features of the tumor resection specimen. Histologic examination of the cardiac mass showed a high-grade proliferation of tumor cells ranging from spindle-shaped to pleomorphic ((a), hematoxylin & eosin). By immunohistochemistry, the tumor cells were characterized by a loss of MLH1 (b) and PMS2 (c) expression, which was preserved in the reactive background. Tumor infiltrating lymphocytes included numerous CD8+ T cells (d). PD-L1 staining was negative in the tumor cells, but positive in a subset of tumor-associated immune cells (e).
Other relevant findings of HTS included a pathogenic missense mutation in exon 7 of TP53, and likely pathogenic frameshift deletions in exon 10 of FAT1 and exon 34 of NOTCH2.
Based on these results, the patient started treatment with the anti-PD-1 antibody pembrolizumab (200 mg every 3 weeks), in May 2018. Pembrolizumab was administrated for 14 cycles, when it was discontinued due to autoimmune colitis grade 3 and adrenal insufficiency grade 3 secondary to hypophysitis, which needed the introduction of steroids. Under steroid therapy the immune adverse events resolved after a few weeks. Given the grade 3 of the immune-adverse events, the treatment with pembrolizumab was definitively stopped. The adrenal insufficiency was substituted with hydrocortisone.
The radiological follow-up during treatment showed a partial response (PR) according to RECIST criteria 6 months after the initiation of pembrolizumab. Following discontinuation of pembrolizumab, the radiological follow-up was performed with cardiac MRI every three months. Complete response (CR) was achieved several months after discontinuation in October 2020, and no progressive disease was observed until the latest follow-up (March 2024) (Figure 3).
STS are a heterogeneous group of over 100 different entities with distinct clinical and biological behaviors also due to the very complex genetic landscape of these diseases (20).
Currently, the main approach guiding decisions on sarcoma treatments continues to be histology-based, and no predictive biomarkers are taken into consideration, mainly due to the vast histologic and biologic heterogeneity and the lack of targetable oncogenic drivers in the most common subtypes. Chemotherapy remains the main systemic treatment with, however, a low response rate (around 12% to 25%). Low response rates result in an overall survival of patients with metastatic disease being limited to a median of about 18 months (21, 22). This dismal prognosis calls for a change of approach aiming not only to discover new therapeutic agents, but also to validate predictive biomarkers for a more rational and effective therapeutic choice.
In the past 10 years, immunotherapy revolutionized cancer treatment both by improving the outcomes in several types of advanced cancer, and by imposing a new way of thinking in oncology (24). In addition, the new approach underlined the need to identify new biomarkers to efficiently select patients for these treatments.
Traditionally, sarcomas are considered non-immunogenic tumors (1), although, due to their extensive molecular heterogeneity between different subtypes, some variability is observed. The SARC-028 trial has shown the effectiveness of ICI therapy in a subset of sarcoma patients, and identified histologies of STSs with the highest responses. These included UPS with an overall response rate (ORR) of 40% (4 of 10 patients), and DDLPS with an ORR of 20% (2 of 10). Notably, one patient with UPS reached a complete response (1, 2).
The discovery and validation of predictive biomarkers are crucial for personalizing therapy, and the field of immunotherapy is no exception. Traditionally, the biomarker field for immunotherapies has focused on single cancer intrinsic factors or immune-specific markers, although given the complexity of the interaction between tumor and stroma, a combinatorial approach could be more advantageous. Predictive biomarkers for ICI immunotherapy proposed in clinical practice include PD-L1 expression, TILs, TMB and MMRd/MSI status and more recently TLS (24, 25).
For the patient we report here, who had a severe reaction to chemotherapy and refused further treatment, the choice of using ICI therapy was biomarker-driven. Extensive analysis of the cardiac tumor specimen displayed several markers known to favor responses to ICIs, despite the absence of TLS. The tumor harbored a high TMB (> 10 mutations/Mb) and a MMRd/MSI profile. Moreover, the tumor displayed an extensive infiltrate by CD8+ T cells, and PD-L1, although negative in the tumor cells, was expressed in the tumor immune microenvironment. Based on these biomarkers and given the paucity of other treatment options acceptable to the patient, she was treated with pembrolizumab monotherapy and obtained a durable response. This response is likely entirely attributable to the immunotherapy without any contribution by the previous aborted doxorubicin/olaratumab therapy, as this treatment was only given for one dose several months before the start of immunotherapy.
The expression of different immune-checkpoint receptors varies widely in sarcoma patients depending on the histology. For instance, PD-1/PD-L1 is only expressed in 10% to 20% of cases. The highest expression rate is noticed in non-translocation associated sarcomas, such as DDLPS, UPS or LMS (26, 27).
In SARC 028, expression of PD-L1 by tumor cells was present only in 2 of 40 tumors and both of them responded to treatment, although among responders there were also tumors not expressing PD-L1 (2). Moreover, a report of 2 patients with different types of STS (one with DDLPS and the other with myxofibrosarcoma) who did not express PD-L1 showed responses to immunotherapy with nivolumab and ipilimumab (28). These results suggest that using a single biomarker may not be sufficient to identify tumors destined to respond to ICIs accurately. Multiple biopsies obtained before and during treatment in SARC 028 trial have allowed the identification of immune features of the responders during therapy and shown a correlation of response to treatment with the presence of CD8+ infiltrating lymphocytes (5). Consistent with the critical role of T lymphocytes in curtailing tumor growth, in a preclinical model of osteosarcoma and chondrosarcoma, the depletion of T cells resulted in a markedly accelerated tumor growth and reduced survival, demonstrating that T cells play a role in controlling cancer progression (29, 30). Using immunohistochemistry, Pollack et al. identified UPS and LMS as the most lymphocyte-infiltrated subtypes. Moreover, the authors were able to correlate PD-1/PD-L1 expression with the degree of immune cell infiltration, suggesting that the higher the TILs, the more likely a response to ICIs (31).
TILs infiltration, expression of immune-marker such as PD-L1, TMB and MMRd/MSI status are well-known markers suggesting response to treatment in epithelial cancer or in very immunogenic cancers like melanoma (10–14) (32–34).
Most STSs have a low TMB with a median of 2.5 mutations/Mb, a small subset of STSs (around 5%), however, harbor a high TMB (>20 mutations/Mb). This association is histotype-dependent, with 10-20% of angiosarcomas, LMS and UPS displaying high TMB (8). Rosembaum et al. (35) analyzed 35 angiosarcomas who received ICIs therapy. Among them, 28% were harboring a TMB >10 mutations/Mb. However, genomic and immunohistochemical analyses showed no correlation of TMB or PD-L1 expression, nor the infiltration of lymphocytes with the response to ICIs. Validation in prospective meta-analysis showed that ≥2 predictive biomarkers used together may have more power than a single biomarker (36, 37).
TLS represent one of the most promising biomarkers predicting response to ICIs in different cancer types, including STS (10, 25). In our patient, no TLS were identified, while TILs were abundant and PD-L1 was expressed in the tumor immune microenvironment. Our observations suggest that even in the absence of TLS a robust antitumor immune response can be risen, and are in line with the literature showing that the predictive value of TLS is independent of the presence of CD8+ T cells and other recognized markers (25, 38).
Identification of recurrent mutations by genomic analysis can be added to the existing toolbox of predictive immunotherapy biomarkers (39). One of the mutations present in our patient’s tumor was a loss-of-function mutation in FAT1, which encodes a tumor suppressor proto cadherin involved in regulating several key pathways in cancer (40–42), including in sarcomas (43, 44). Interestingly, somatic mutations in FAT1 have been shown to be positively correlated with high TMB and response to ICI therapy in melanoma and non-small cell lung cancer (45, 46). Although its role as a predictive marker in sarcoma has not been investigated, the durable response of our patient to pembrolizumab and the correlation with other more established predictive markers suggests that FAT1 could serve as a potential marker to predict response to ICIs in sarcoma patients.
HTS also revealed a loss-of-function mutation in NOTCH2. Interestingly, there is evidence in the literature correlating dysregulation of Notch signaling pathway with enhanced immunogenicity and increased infiltration of TILs and CD8+ T cells in different cancer types (47, 48), although its role in the immunogenicity of sarcomas still needs to be investigated.
Moreover, a widespread multi-omics analysis in more than 32 cancer types from The Cancer Genome Atlas (TCGA) dataset identified a genomic signature of 11 genes, correlated with TMB, able to predict the response to immunotherapy, which included FAT1 and NOTCH2, both mutated in our patient’s tumor (49).
Here we described the case of a long-lasting complete response to pembrolizumab in a patient with an undifferentiated MMRd/MSI cardiac sarcoma.
Although immunotherapy may have a role in some STS patients, the identification of suitable candidates who can obtain a meaningful benefit from treatment requires improvement.
Considering the genomic variability between sarcomas and the heterogeneous response to ICIs treatment, validation of new predictive markers is essential to improve the efficacy of immunotherapy in sarcomas. The use of a combination of existing biomarkers may improve their predictive power. This might allow for a more personalized approach and an increased response rate to immunotherapy in diseases that are still considered resistant.
To the best of our knowledge, complete response to immunotherapy in cardiac sarcoma patients is rare. This case report provides the evidence on how a correct histopathologic and molecular characterization of such tumors can lead to a real benefit in sarcoma patient outcome.
The data analyzed in this case report contains confidential patient information but can be made available upon request.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
DF: Writing – original draft, Conceptualization, Writing – review & editing. BB: Writing – original draft, Data curation, Formal Analysis, Writing – review & editing. DR: Data curation, Writing – original draft. FP: Writing – review & editing. EM: Writing – review & editing, Data curation, Formal analysis. IV: Writing – review & editing. KH: Writing – review & editing. AD: Conceptualization, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. F.P. is partially funded by an NIH/NCI P50 CA24779 01 grant. This work as supported in part by a Cancer Center Support Grant of the NIH/NCI (P30CA008748; MSK).
FP serves on the advisory board and reports receiving consultancy fees from AstraZeneca. In addition, F.P. was a member of the scientific advisory board of MultiplexDx.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Kerrison WGJ, Lee ATJ, Thway K, Jones RL, Huang PH. Current status and future directions of immunotherapies in soft tissue sarcomas. Biomedicines. (2022) 10. doi: 10.3390/biomedicines10030573
2. Tawbi HA, Burgess M, Bolejack V, Van Tine BA, Schuetze SM, Hu J, et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. (2017) 18:1493–501. doi: 10.1016/S1470-2045(17)30624-1
3. Diamond MS. Immune checkpoint inhibitors in cardiac sarcoma: reason to take heart? JACC CardioOncol. (2024) 6:80–2. doi: 10.1016/j.jaccao.2024.01.002
4. Nassar AH, El-Am E, Denu R, Abou Alaiwi S, El Zarif T, Macaron W, et al. Clinical outcomes among immunotherapy-treated patients with primary cardiac soft tissue sarcomas: A multicenter retrospective study. JACC CardioOncol. (2024) 6:71–9. doi: 10.1016/j.jaccao.2023.11.007
5. Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. (2015) 21:938–45. doi: 10.1038/nm.3909
6. D’Angelo SP, Shoushtari AN, Agaram NP, Kuk D, Qin LX, Carvajal RD, et al. Prevalence of tumor-infiltrating lymphocytes and PD-L1 expression in the soft tissue sarcoma microenvironment. Hum Pathol. (2015) 46:357–65. doi: 10.1016/j.humpath.2014.11.001
7. Inaguma S, Wang Z, Lasota J, Sarlomo-Rikala M, McCue PA, Ikeda H, et al. Comprehensive immunohistochemical study of programmed cell death ligand 1 (PD-L1): analysis in 5536 cases revealed consistent expression in trophoblastic tumors. Am J Surg Pathol. (2016) 40:1133–42. doi: 10.1097/PAS.0000000000000653
8. Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. (2017) 9:34. doi: 10.1186/s13073-017-0424-2
9. Wood GE, Meyer C, Petitprez F, D’Angelo SP. Immunotherapy in sarcoma: current data and promising strategies. Am Soc Clin Oncol Educ Book. (2024) 44:e432234. doi: 10.1200/EDBK_432234
10. Petitprez F, de Reynies A, Keung EZ, Chen TW, Sun CM, Calderaro J, et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature. (2020) 577:556–60. doi: 10.1038/s41586-019-1906-8
11. Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. (2020) 21:1353–65. doi: 10.1016/S1470-2045(20)30445-9
12. Doyle LA, Nowak JA, Nathenson MJ, Thornton K, Wagner AJ, Johnson JM, et al. Characteristics of mismatch repair deficiency in sarcomas. Mod Pathol. (2019) 32:977–87. doi: 10.1038/s41379-019-0202-3
13. Campanella NC, Penna V, Ribeiro G, Abrahao-MaChado LF, Scapulatempo-Neto C, Reis RM. Absence of microsatellite instability in soft tissue sarcomas. Pathobiology. (2015) 82:36–42. doi: 10.1159/000369906
14. Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. (2017) 357:409–13. doi: 10.1126/science.aan6733
15. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. (2015) 372:2509–20. doi: 10.1056/NEJMoa1500596
16. Latham A, Srinivasan P, Kemel Y, Shia J, Bandlamudi C, Mandelker D, et al. Microsatellite instability is associated with the presence of lynch syndrome pan-cancer. J Clin Oncol. (2019) 37:286–95. doi: 10.1200/JCO.18.00283
17. Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De-Jesus-Acosta A, Delord JP, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. (2020) 38:1–10. doi: 10.1200/JCO.19.02105
18. Neuville A, Coindre JM. Cardiac sarcoma with MDM2 amplification. Am J Surg Pathol. (2014) 38:1449. doi: 10.1097/PAS.0000000000000275
19. Tap WD, Jones RL, Van Tine BA, Chmielowski B, Elias AD, Adkins D, et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet. (2016) 388:488–97. doi: 10.1016/S0140-6736(16)30587-6
20. Cancer Genome Atlas Research Network. Electronic address edsc, Cancer Genome Atlas Research N. Comprehensive and Integrated Genomic Characterization of Adult Soft Tissue Sarcomas. Cell. (2017) 171:950–65 e28. doi: 10.1016/j.cell.2017.10.014
21. Leahy M, Garcia Del Muro X, Reichardt P, Judson I, Staddon A, Verweij J, et al. Chemotherapy treatment patterns and clinical outcomes in patients with metastatic soft tissue sarcoma. The SArcoma treatment and Burden of Illness in North America and Europe (SABINE) study. Ann Oncol. (2012) 23:2763–70. doi: 10.1093/annonc/mds070
22. Phillips E, Jones RL, Huang P, Digklia A. Efficacy of eribulin in soft tissue sarcomas. Front Pharmacol. (2022) 13:869754. doi: 10.3389/fphar.2022.869754
23. Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature. (2013) 500:415–21. doi: 10.1038/nature12477
24. Sharma P, Siddiqui BA, Anandhan S, Yadav SS, Subudhi SK, Gao J, et al. The next decade of immune checkpoint therapy. Cancer Discovery. (2021) 11:838–57. doi: 10.1158/2159-8290.CD-20-1680
25. Teillaud JL, Houel A, Panouillot M, Riffard C, Dieu-Nosjean MC. Tertiary lymphoid structures in anticancer immunity. Nat Rev Cancer. (2024) 24:629–46. doi: 10.1038/s41568-024-00728-0
26. Dancsok AR, Setsu N, Gao D, Blay JY, Thomas D, Maki RG, et al. Expression of lymphocyte immunoregulatory biomarkers in bone and soft-tissue sarcomas. Mod Pathol. (2019) 32:1772–85. doi: 10.1038/s41379-019-0312-y
27. Yan L, Wang Z, Cui C, Guan X, Dong B, Zhao M, et al. Comprehensive immune characterization and T-cell receptor repertoire heterogeneity of retroperitoneal liposarcoma. Cancer Sci. (2019) 110:3038–48. doi: 10.1111/cas.v110.10
28. Zhou M, Bui N, Lohman M, van de Rjin M, Hwang G, Ganjoo K. Long-term remission with ipilimumab/nivolumab in two patients with different soft tissue sarcoma subtypes and no PD-L1 expression. Case Rep Oncol. (2021) 14:459–65. doi: 10.1159/000512828
29. Simard FA, Richert I, Vandermoeten A, Decouvelaere AV, Michot JP, Caux C, et al. Description of the immune microenvironment of chondrosarcoma and contribution to progression. Oncoimmunology. (2017) 6:e1265716. doi: 10.1080/2162402X.2016.1265716
30. Sorbye SW, Kilvaer TK, Valkov A, Donnem T, Smeland E, Al-Shibli K, et al. Prognostic impact of peritumoral lymphocyte infiltration in soft tissue sarcomas. BMC Clin Pathol. (2012) 12:5. doi: 10.1186/1472-6890-12-5
31. Pollack SM, He Q, Yearley JH, Emerson R, Vignali M, Zhang Y, et al. T-cell infiltration and clonality correlate with programmed cell death protein 1 and programmed death-ligand 1 expression in patients with soft tissue sarcomas. Cancer. (2017) 123:3291–304. doi: 10.1002/cncr.v123.17
32. Mogg R, Ayers M, Albright A, Murphy E, Yearley J, Sher X, et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science. (2019) 363. doi: 10.1126/science.aar3593
33. Goodman AM, Kato S, Bazhenova L, Patel SP, Frampton GM, Miller V, et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther. (2017) 16:2598–608. doi: 10.1158/1535-7163.MCT-17-0386
34. Klempner SJ, Fabrizio D, Bane S, Reinhart M, Peoples T, Ali SM, et al. Tumor mutational burden as a predictive biomarker for response to immune checkpoint inhibitors: A review of current evidence. Oncologist. (2020) 25:e147–e59. doi: 10.1634/theoncologist.2019-0244
35. Rosenbaum E, Antonescu CR, Smith S, Bradic M, Kashani D, Richards AL, et al. Clinical, genomic, and transcriptomic correlates of response to immune checkpoint blockade-based therapy in a cohort of patients with angiosarcoma treated at a single center. J Immunother Cancer. (2022) 10. doi: 10.1136/jitc-2021-004149
36. Cristescu R, Mogg R, Ayers M, Albright A, Murphy E, Yearley J, et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science. (2018) 362. doi: 10.1126/science.aar3593
37. Litchfield K, Reading JL, Puttick C, Thakkar K, Abbosh C, Bentham R, et al. Meta-analysis of tumor- and T cell-intrinsic mechanisms of sensitization to checkpoint inhibition. Cell. (2021) 184:596–614 e14. doi: 10.1016/j.cell.2021.01.002
38. Vanhersecke L, Brunet M, Guegan JP, Rey C, Bougouin A, Cousin S, et al. Mature tertiary lymphoid structures predict immune checkpoint inhibitor efficacy in solid tumors independently of PD-L1 expression. Nat Cancer. (2021) 2:794–802. doi: 10.1038/s43018-021-00232-6
39. Nacev BA, Sanchez-Vega F, Smith SA, Antonescu CR, Rosenbaum E, Shi H, et al. Clinical sequencing of soft tissue and bone sarcomas delineates diverse genomic landscapes and potential therapeutic targets. Nat Commun. (2022) 13:3405. doi: 10.1038/s41467-022-30453-x
40. Sade-Feldman M, Jiao YJ, Chen JH, Rooney MS, Barzily-Rokni M, Eliane JP, et al. Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nat Commun. (2017) 8:1136. doi: 10.1038/s41467-017-01062-w
41. Martin D, Degese MS, Vitale-Cross L, Iglesias-Bartolome R, Valera JLC, Wang Z, et al. Assembly and activation of the Hippo signalome by FAT1 tumor suppressor. Nat Commun. (2018) 9:2372. doi: 10.1038/s41467-018-04590-1
42. Hu X, Zhai Y, Kong P, Cui H, Yan T, Yang J, et al. FAT1 prevents epithelial mesenchymal transition (EMT) via MAPK/ERK signaling pathway in esophageal squamous cell cancer. Cancer Lett. (2017) 397:83–93. doi: 10.1016/j.canlet.2017.03.033
43. Moukarzel LA, Ferrando L, Da Cruz Paula A, Brown DN, Geyer FC, Pareja F, et al. The genetic landscape of metaplastic breast cancers and uterine carcinosarcomas. Mol Oncol. (2021) 15:1024–39. doi: 10.1002/1878-0261.12813
44. Griewank KG, Wiesner T, Murali R, Pischler C, Muller H, Koelsche C, et al. Atypical fibroxanthoma and pleomorphic dermal sarcoma harbor frequent NOTCH1/2 and FAT1 mutations and similar DNA copy number alteration profiles. Mod Pathol. (2018) 31:418–28. doi: 10.1038/modpathol.2017.146
45. Zhang W, Tang Y, Guo Y, Kong Y, Shi F, Sheng C, et al. Favorable immune checkpoint inhibitor outcome of patients with melanoma and NSCLC harboring FAT1 mutations. NPJ Precis Oncol. (2022) 6:46. doi: 10.1038/s41698-022-00292-6
46. Feng Z, Yin Y, Liu B, Zheng Y, Shi D, Zhang H, et al. Prognostic and immunological role of FAT family genes in non-small cell lung cancer. Cancer Control. (2022) 29:10732748221076682. doi: 10.1177/10732748221076682
47. Long J, Wang D, Yang X, Wang A, Lin Y, Zheng M, et al. Identification of NOTCH4 mutation as a response biomarker for immune checkpoint inhibitor therapy. BMC Med. (2021) 19:154. doi: 10.1186/s12916-021-02031-3
48. Wang M, Yu F, Zhang Y, Li P. Novel insights into Notch signaling in tumor immunity: potential targets for cancer immunotherapy. Front Immunol. (2024) 15:1352484. doi: 10.3389/fimmu.2024.1352484
Keywords: cardiac sarcoma, immune-checkpoint inhibitors (ICI), complete response (CR), predictive markers, mismatch repair deficiency (MMRd), microsatellite instability (MSI)
Citation: Ferraro DA, Bisig B, Rotzinger DC, Pareja F, Missiaglia E, Voutsadakis I, Homicsko K and Digklia A (2025) Case Report: Lasting complete response to pembrolizumab in mismatch repair-deficient cardiac sarcoma: a genomic characterization. Front. Oncol. 15:1485386. doi: 10.3389/fonc.2025.1485386
Received: 23 August 2024; Accepted: 10 March 2025;
Published: 03 April 2025.
Edited by:
Paulo Rodrigues-Santos, University of Coimbra, PortugalReviewed by:
Howard Streicher, National Cancer Institute at Frederick (NIH), United StatesCopyright © 2025 Ferraro, Bisig, Rotzinger, Pareja, Missiaglia, Voutsadakis, Homicsko and Digklia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniela A. Ferraro, ZGFuaWVsYS1hbGVpZGEuZmVycmFyb0BjaHV2LmNo
†ORCID: Bettina Bisig, orcid.org/0000-0002-6840-375X
Edoardo Missiaglia, orcid.org/0000-0001-9221-0117
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.