
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 13 February 2025
Sec. Head and Neck Cancer
Volume 15 - 2025 | https://doi.org/10.3389/fonc.2025.1484002
Introduction: The lateral cervical lymph node positivity rate has been hypothesized to correlate with the recurrence risk in differentiated thyroid carcinoma (DTC) patients. However, the extent of this association within the Chinese population remains understudied. This study seeks to elucidate the potential causal link between the lymph node positivity rate and DTC recurrence.
Methods: We conducted a retrospective cohort study, examining clinical records of 4,731 DTC patients who received surgical treatment at the First Medical Center of the General Hospital of the Chinese People’s Liberation Army from January 2015 to May 2020. The study variables encompassed demographic and clinical characteristics, including sex, age, tumor size, location, laterality, capsular invasion, lymph node metastasis counts, lymph node positivity rates, histological subtypes, Hashimoto’s thyroiditis co-occurrence, and the timing of iodine-131 therapy post-surgery. After applying strict inclusion criteria, 1,074 patients were selected for analysis. Recurrence was defined as structural incomplete response (SIR), confirmed by imaging or histological means. The lymph node positivity rate was calculated as the proportion of positive lymph nodes to the total lymph node count.
Results: Multivariate analysis revealed a nonlinear association between the lateral cervical lymph node positivity rate and post-treatment recurrence, with a significant threshold at 0.5. The recurrence risk was substantially elevated with a positivity rate below this threshold (HR: 27.48, 95% CI: 7.21–104.70, P<0.0001), while no significant association was observed above it (HR: 0.17, 95% CI: 0.02–1.57, P=0.119). Subgroup analysis within the high-risk cohort did not yield a significant association between the positivity rate and recurrence risk (HR=0.43, 95% CI: 0.10–1.79, P=0.246).
Discussion: In conclusion, this study identifies a nonlinear relationship between the lateral cervical lymph node positivity rate and the risk of DTC recurrence post-treatment. A positivity rate of less than 0.5 is positively associated with recurrence, while this association diminishes in significance among high-risk patients. This differs from the results previously reported. Further studies are needed to determine the potential mechanisms of the associations observed in observational studies.
In recent years, the incidence of thyroid cancer has been increasing globally, ranking as the 9th most common cancer in 2018 (1). A similar trend is observed in China, where thyroid cancer now ranks 7th among all malignant tumors and 3rd among female malignancies, following breast cancer and lung cancer (2). Differentiated thyroid carcinoma (DTC) accounts for the majority of thyroid cancer cases. Although DTC is considered to have low aggressiveness, its 5-year mortality rate in China can exceed 10% (2). Patients experiencing structural recurrence after treatment face an even higher risk of mortality (3).
Lymph node metastasis is the most common and critical mode of spread in DTC, with a reported incidence of 20-50%, which is associated with postoperative recurrence (4–6). Notably, lateral cervical lymph node metastasis is linked to a heightened recurrence rate after treatment (7–9). Currently, the ATA risk stratification system is primarily used to predict the risk of recurrence post-treatment (10). Although this system includes factors such as histological subtype, extrathyroidal invasion, lymph node size, and number of positive lymph nodes, there remains considerable variability in treatment response among DTC patients within the same risk category. Recent studies have suggested that the positivity rate of lateral cervical lymph nodes may also correlate with the risk of recurrence following treatment in DTC patients (11–15).
To elucidate the relationship between the lateral cervical lymph node positivity rate and recurrence in differentiated thyroid carcinoma among the Chinese population, we conducted a retrospective cohort study aimed at clarifying this association.
We conducted a retrospective analysis utilizing the electronic medical record system of the First Medical Center of the PLA General Hospital. Clinical data were collected from 4,731 consecutive patients diagnosed with differentiated thyroid carcinoma who underwent surgical intervention between January 2015 and May 2020. The data collected included demographic information such as sex and age, tumor characteristics like maximum diameter and location, the presence of single or multiple lesions, capsular invasion, the number of central neck lymph node metastases, the number of lateral neck lymph node metastases, positivity rates for central and lateral neck lymph nodes, pathological type, the presence of concomitant Hashimoto’s thyroiditis, and the interval between surgery and radioactive iodine (¹³¹I) treatment (hereafter referred to as the surgical-iodine interval). Outcomes were subsequently followed up for these patients after treatment. All patients provided informed consent prior to treatment, and this study was approved by the hospital’s ethics committee.
All patients underwent total thyroidectomy with central and lateral neck lymph node dissection; Pathological diagnosis must be confirmed as differentiated thyroid carcinoma; Patients met the criteria for ¹³¹I treatment and subsequently received ¹³¹I therapy; Postoperatively, patients received TSH suppression therapy, maintaining treatment except during the peri-radioactive iodine period when therapy was temporarily halted; Patients demonstrated high compliance and were able to complete follow-up evaluations.
Presence of other significant comorbidities that could adversely affect survival (e.g., other malignancies or severe trauma); Incomplete primary clinical data (e.g., unclear documentation of lymph node metastasis); Loss to follow-up during the study period.
This study received approval from the hospital’s ethics committee. After applying the inclusion and exclusion criteria, 1,074 subjects remained for data analysis from the original cohort of 4,731 participants (Figure 1).
All study patients underwent total thyroidectomy with central and lateral neck lymphadenectomy, followed by radioactive iodine (¹³¹I) ablation within 12 months post-operation. Prior to iodine treatment, patients adhered to a low-iodine diet (<50 µg/day) for 2 to 4 weeks, and any iodine-containing contrast agents or medications were avoided. L-T4 was discontinued for at least 2 to 4 weeks before iodine treatment to elevate serum TSH levels to >30 mU/L. A fixed dose of 150 mCi was administered for the single iodine treatment (10). Postoperatively, patients commenced TSH suppression therapy (excluding the 2 to 4 weeks prior to the initial iodine treatment), adhering to the suppression standards outlined in relevant guidelines (10).
The first follow-up evaluation was conducted 3 to 6 months after iodine treatment, with routine clinical assessments performed annually in the outpatient setting. These assessments included cervical ultrasound, chest CT scans, measurement of serum free thyroxine (fT4), thyrotropin (TSH), thyroglobulin (Tg), and anti-thyroglobulin (anti-Tg) antibody concentrations. Additionally, whole-body scans were performed 3 to 4 days post-iodine treatment to evaluate focal or diffuse uptake in the thyroid bed, cervical lymph nodes, and other sites. Any lesions suggestive of local recurrence were assessed via fine-needle aspiration cytology or imaging studies. Follow-up was conducted through telephone calls, text messages, and outpatient visits, starting from the date of iodine-131 treatment and concluding on December 31, 2021, resulting in follow-up durations of 18 to 81 months, with a median follow-up time of 43 months. Follow-up data were independently evaluated by two experienced attending physicians, and consensus was reached in case of any discrepancies.
Recurrence was defined as structural incomplete response (SIR), with the standard for determination based on imaging or histological findings, regardless of serum Tg levels (10, 16) The lateral lymph node positivity rate was calculated as the number of positive lymph nodes divided by the total number of harvested lymph nodes (12).
Continuous variables are expressed as means ± standard deviation (for normally distributed data) or medians (with minimum-maximum values) for skewed distributions. Categorical variables are presented as frequencies or percentages. To assess any statistical differences in means and proportions among groups, one-way ANOVA (for normally distributed data), Kruskal-Wallis H test (for skewed distributions), and Chi-square tests (for categorical variables) were employed. A univariate Cox proportional hazards regression model was utilized to evaluate the association between the rate of positive lateral lymph nodes and recurrence. Both unadjusted and multivariable-adjusted models are presented in this study. In accordance with STROBE guidelines, we simultaneously displayed the results of unadjusted, minimally adjusted, and fully adjusted analyses. Whether covariates were adjusted was determined by the principle that the odds ratio changed by at least 10% upon inclusion in the model (17). Additionally, we employed generalized additive models (GAM) to identify any nonlinear relationships. If a nonlinear association was observed, segmented linear regression models were conducted to calculate the threshold effect of the rate of positive lateral lymph nodes on recurrence based on the smoothed graphs. When the odds ratio between the rate of positive lateral lymph nodes and recurrence was significant on the smoothed curve, a recursive method automatically calculated the inflection point, and maximum likelihood estimation was used at the inflection point (18). Subgroup analyses were performed using stratified linear regression models. Likelihood ratio tests were utilized to evaluate modifications and interactions within subgroups. All statistical analyses were conducted using EasyFit statistical software (www.empowerstats.com) and R software. A p-value of less than 0.05 (two-tailed) was considered statistically significant.
Out of 4,731 participants, 3,657 were excluded from this study. Among the 3,657 excluded subjects, 1,402 underwent unilateral thyroidectomy, 1,583 were classified as low recurrence risk, 619 did not receive ^131I treatment at our hospital, 18 had unclear records of lymph node metastasis, 15 had comorbid malignancies or diseases affecting survival, 16 were lost to follow-up, and 4 died due to other causes. Consequently, a total of 1,074 subjects were included for data analysis.
There were no statistically significant differences between patients with recurrence and those without recurrence regarding length of hospital stay, interval between surgery and ^131I treatment, sex, tumor location, capsular invasion, histological type, and comorbidities. However, statistical differences were observed in age, primary lesion size, central zone positive lymph node ratio, lateral zone positive lymph node ratio, whether the lesions were single or multiple, the number of central zone positive lymph nodes (≥5), the number of lateral zone positive lymph nodes (≥5), and recurrence risk stratification in Table 1.
The results of the univariate analysis are presented in Table 2. The findings indicate that age, primary lesion size, central zone positive lymph node ratio, lateral zone positive lymph node ratio, presence of single/multiple lesions, the number of central zone positive lymph nodes (≥5), the number of lateral zone positive lymph nodes (≥5), and recurrence risk stratification were significantly associated with recurrence following comprehensive treatment. Conversely, variables such as sex, length of hospital stay, interval between surgery and ^131I treatment, tumor location, capsular invasion, histological subtype, and comorbidities were not associated with recurrence after comprehensive treatment.
We utilized a univariate linear COX regression model to evaluate the relationship between the lateral zone positive lymph node ratio and recurrence. The findings are presented in Table 3, including both unadjusted and adjusted models. In the crude model, a positive association was observed between the lateral zone positive lymph node ratio and recurrence (HR=8.96, 95% confidence interval (CI): 5.00-16.06, P<0.001). In the minimally adjusted model (controlling for age and sex), the results remained largely unchanged (HR=8.25, 95% CI: 4.55-14.96, P<0.001). This association was still detected in the fully adjusted model (HR=4.02, 95% CI: 1.87-8.64, P=0.0004). For sensitivity analysis, we also treated the lateral zone positive lymph node ratio as a categorical variable (binary classification); however, no association was detected in the fully adjusted model when the ratio was >0.5 (HR=1.28, 95% CI: 0.72-2.28, P=0.405).
Given that the lateral zone positive lymph node ratio is a continuous variable, it is essential to analyze potential nonlinear relationships. In this study (Figure 2), we identified a nonlinear relationship between the lateral zone positive lymph node ratio and recurrence following comprehensive treatment, after adjusting for sex, age, primary lesion size, single/multiple lesions, the number of central zone positive lymph nodes, the number of lateral zone positive lymph nodes, central zone positive lymph node ratio, length of hospital stay, interval between surgery and ^131I treatment, tumor location, capsular invasion, histological subtype, comorbidities, and risk stratification. Utilizing two piecewise linear regression models, we calculated the inflection point to be 0.5. To the left of this inflection point, we found the hazard ratio (HR), 95% confidence interval (CI), and P value to be as follows: HR=27.48, 95% CI: 7.21-104.70, P<0.0001. However, no relationship between the lateral zone positive lymph node ratio and recurrence was observed to the right of the inflection point (HR=0.17, 95% CI: 0.02-1.57, P=0.119) (Table 4).

Figure 2. Smooth Curve Fitting SIR vs. Positive rate of lateral lymph nodes; Outcome: SIR; Exposure: Positive rate of lateral lymph nodes; Cox model time variable: Follow-up time.
As shown in Table 5, the interaction test indicated a significant association with risk stratification (interaction P<0.0001). In contrast, the interaction tests for age, sex, primary lesion size, single/multiple lesions, capsular invasion, tumor location, and co-occurrence of Hashimoto’s disease were not statistically significant (interaction P values were 0.293, 0.438, 0.789, 0.544, 0.217, 0.322, and 0.746, respectively). We observed an interaction between the lateral zone positive lymph node ratio and risk stratification, revealing that the relationship between the lateral zone positive lymph node ratio and recurrence differed significantly across various risk strata. In patients with moderate recurrence risk, the lateral zone positive lymph node ratio was positively correlated with recurrence (HR=13.61, 95% CI: 5.68-32.57). However, in patients with high recurrence risk, there was no significant association between the lateral zone positive lymph node ratio and recurrence (HR=0.43, 95% CI: 0.10-1.79, P=0.246).
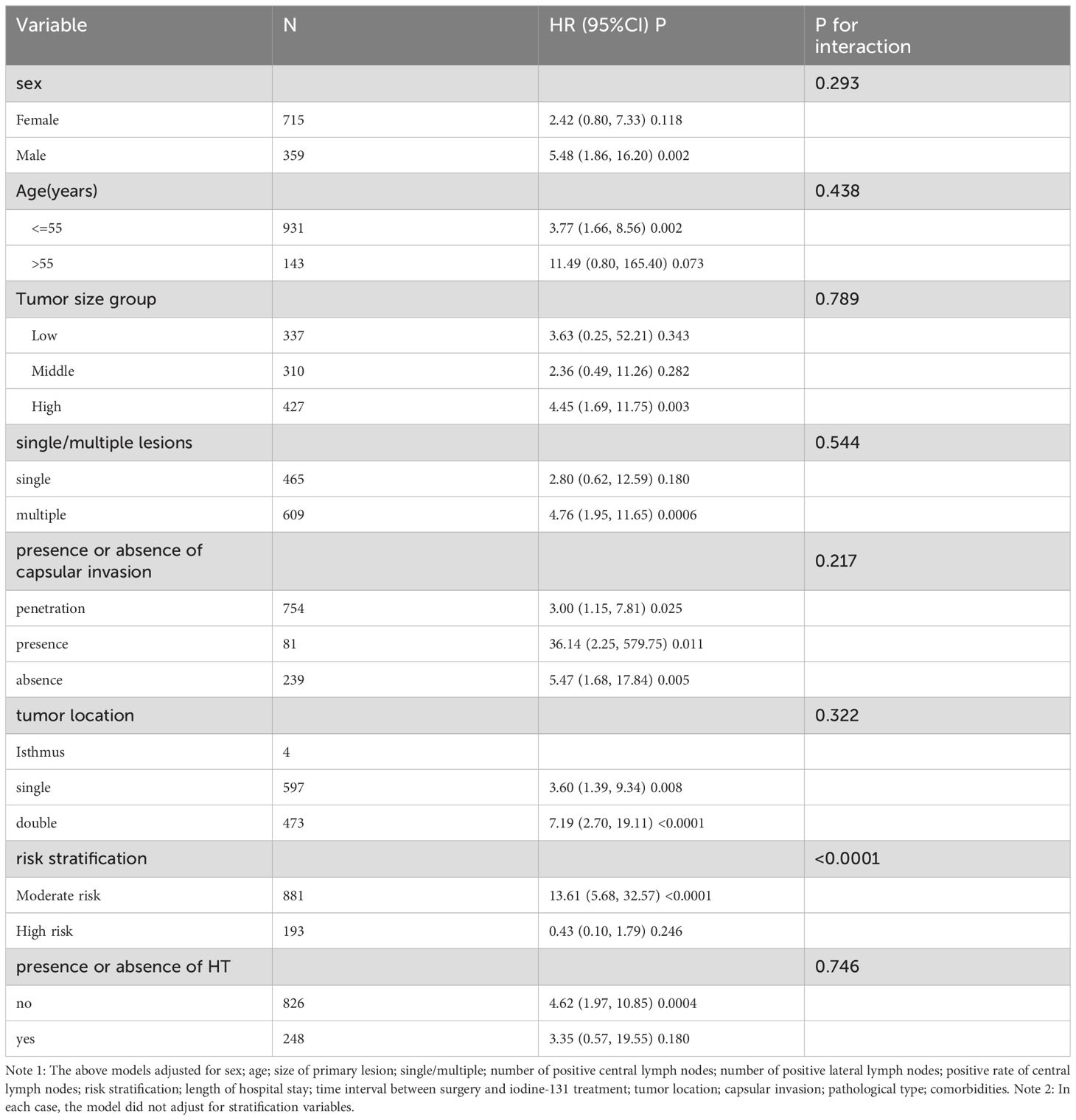
Table 5. Effect size of positive rate of lateral lymph nodes on recurrence in pre-specified and exploratory subgroups.
This study investigated the relationship between the positive lymph node ratio in the lateral neck region and recurrence after comprehensive treatment in a Chinese population with differentiated thyroid cancer (DTC). The fully adjusted model and sensitivity analysis showed that a lateral zone lymph node positive ratio greater than 0.5 was not associated with recurrence. However, a nonlinear relationship was identified, with the ratio positively correlating with recurrence on the left side of the inflection point (0.5) but not on the right. Notably, in patients with moderate recurrence risk, the lateral zone lymph node positive ratio was positively associated with recurrence, while this association was absent in those with high recurrence risk.
Our findings indicate that the positive lymph node ratio in the lateral neck region is associated with recurrence following comprehensive treatment in the Chinese population, which is partially consistent with previous studies but also shows some discrepancies (11–15). A comparative analysis reveals several differences between our study and prior research: first, the selection of study participants varied, as previous studies predominantly focused on patients with TNM staging, whereas our participants were those with moderate to high recurrence risk following total thyroidectomy. Furthermore, while earlier studies employed regression analyses to explore linear relationships, they did not examine potential nonlinear relationships. Lastly, subgroup analyses are crucial in scientific research (19). Unfortunately, the aforementioned studies used only sex and age as stratification factors for subgroup analyses and did not perform interaction tests, impeding a comprehensive exploration of the true relationship between the lateral zone lymph node positive ratio and recurrence.
In our study, we utilized various stratification variables including age, sex, tumor size, single/multiple lesions, tumor location, capsular invasion, risk stratification, and the presence of concomitant Hashimoto’s disease. We observed differing relationships between the lateral zone lymph node positive ratio and recurrence based on risk stratification. Compared to the high-risk group, the positive association between the lateral zone lymph node positive ratio and recurrence was more pronounced in the moderate risk group. This finding has not been previously reported. Analyzing the potential reasons, we believe that the positive lymph node ratio is closely related to the number of positive lymph nodes, with more than five positive nodes being an important criterion for inclusion in the moderate recurrence risk group. In contrast, the high recurrence risk group was categorized primarily based on the size of metastatic lymph nodes and extrathyroidal invasion, resulting in the absence of a association between the lateral zone lymph node positive ratio and recurrence.
Previous studies have indicated that chronic autoimmune thyroiditis (Hashimoto’s thyroiditis, HT), a prevalent thyroid disorder, is often associated with alterations in thyroid function and an increased risk of thyroid cancer (20, 21). HT may influence the development of papillary thyroid carcinoma by modifying the tumor microenvironment. Chronic inflammation is recognized as a significant risk factor for tumorigenesis, and the inflammatory response associated with HT may contribute to genetic mutations and the malignant transformation of thyroid cells (22). Furthermore, the potential infiltration of lymphocytes in the thyroid tissue of HT patients may create a supportive microenvironment for tumor cells, thereby facilitating tumor growth and metastasis (23). However, this phenomenon was not observed in our study.
Our study has several strengths. Firstly, we employed not only generalized linear models to evaluate the linear relationship between the lateral zone lymph node positive ratio and recurrence but also generalized additive models (GAM) to elucidate nonlinear relationships. GAM has distinct advantages in addressing nonlinear relationships, allowing for nonparametric smoothing and fitting regression splines to the data. The use of GAM will aid us in better elucidating the true relationship between exposure and outcomes. Secondly, this retrospective study incorporated unavoidable potential confounding factors; therefore, we applied rigorous statistical adjustments to minimize residual confounding. Despite previous research reporting a linear relationship between the lateral zone lymph node positive ratio and recurrence, we did not observe such a relationship in populations where the lateral zone lymph node positive ratio was greater than 0.5. Thirdly, the effect modification analysis enhanced the utility of the data, revealing that no such relationship was found between the lateral zone lymph node positive ratio and recurrence in the high-risk subgroup.
Although the current American Thyroid Association (ATA) guidelines do not yet incorporate the positive rate of cervical lymph nodes in differentiated thyroid cancer into recurrence risk stratification, our study further elucidates the relationship between the positive rate of lateral cervical lymph nodes in papillary thyroid carcinoma and recurrence following comprehensive treatment. As additional evidence-based medical data accumulates and the biological mechanisms linking lymph node positivity to recurrence risk are explored, more optimized recurrence risk assessment models are expected to be integrated into clinical practice. This will provide a solid foundation for postoperative monitoring and treatment strategies for patients.
However, our study has certain limitations. First, being an analytical retrospective study, it provides only weak evidence regarding the exposure-outcome relationship, making it challenging to distinguish causal relationships. Secondly, the study population comprised only Chinese individuals, which may limit the generalizability of the findings to other populations. Thirdly, data on genetic testing, particularly the status of BRAF mutations (24, 25), were not included in the study. Moreover, due to sample size limitations, certain pathology subtypes associated with a high risk of recurrence, such as high cellular variant, columnar cell variant, and tall-cell variant of papillary thyroid carcinoma (PTC), were not included in this study (26). Therefore, the conclusions of our study warrant further validation through clinical research with larger sample sizes and diverse populations.
There exists a nonlinear relationship between the lateral zone lymph node positive ratio and recurrence following comprehensive treatment. When the lateral zone lymph node positive ratio is less than 0.5, it is positively correlated with recurrence. Additionally, no such association was observed among subjects in the high recurrence risk group.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Ethics Committee Of Chinese PLA General Hosptial. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
TY: Formal analysis, Software, Writing – original draft, Data curation. SS: Data curation, Investigation, Writing – original draft. SY: Data curation, Investigation, Writing – original draft. RW: Supervision, Writing – review & editing, Methodology.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors thank Dr. Xing-lin Chen of Yi-er college, for her kind help in data analysis.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that Generative AI was used in the creation of this manuscript. Text translation and editing.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Zheng R, Zhang S, Zeng H, Wang S, Sun K, Chen R, et al. Cancer incidence and mortality in China, 2016. J Natl Cancer Center. (2022) 2(1):1–9. doi: 10.1530/ERC-19-0213
3. Pitoia F, Jerkovich F. Dynamic risk assessment in patients with differentiated thyroid cancer. Endocr Relat Cancer. (2019) 26(10):R553–66. doi: 10.1530/ERC-19-0213
4. Scheumann GF, Gimm O, Wegener G, Hundeshagen H, Dralle H. Prognostic significance and surgical management of locoregional lymph node metastases in papillary thyroid cancer. World J Surg. (1994) 18:559–67. doi: 10.1007/BF00353765
5. Grebe SK, Hay ID. Thyroid cancer nodal metastases: biologic significance and therapeutic considerations. Surg Oncol Clin N Am. (1996) 5:43–63.
6. Maksimovic S, Jakovljevic B, Gojkovic Z. Lymph node metastases papillary thyroid carcinoma and their importance in recurrence of disease. Med Arch. (2018) 72:108–11. doi: 10.5455/medarh.2018.72.108-111
7. Roh JL, Park JY, Park CI. Total thyroidectomy plus neck dissection in differentiated papillary thyroid carcinoma patients: pattern of nodal metastasis, morbidity, recurrence, and postoperative levels of serum parathyroid hormone. Ann Surg. (2007) 245:604–10. doi: 10.1097/01.sla.0000250451.59685.67
8. Ito Y, Kudo T, Takamura Y, Kobayashi K, Miya A, Miyauchi A. Lymph node recurrence in patients with N1b papillary thyroid carcinoma who underwent unilateral therapeutic modified radical neck dissection. World J Surg. (2012) 36:593–7. doi: 10.1007/s00268-011-1391-1
9. Baek SK, Jung KY, Kang SM, Kwon SY, Woo JS, Cho SH, et al. Clinical risk factors associated with cervical lymph node recurrence in papillary thyroid carcinoma. Thyroid. (2010) 20:147–52. doi: 10.1089/thy.2008.0243
10. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. (2016) 26:1–133. doi: 10.1089/thy.2015.0020
11. Lee SH, Roh JL, Gong G, Cho KJ, Choi SH, Nam SY, et al. Risk factors for recurrence after treatment of N1b papillary thyroid carcinoma. Ann Surg. (2019) 269:966–71. doi: 10.1097/SLA.0000000000002710
12. Choi SY, Cho JK, Moon JH, Son YI. Metastatic lymph node ratio of central neck compartment has predictive values for locoregional recurrence in papillary thyroid microcarcinoma. Clin Exp Otorhinolaryngol. (2016) 9:75–9. doi: 10.21053/ceo.2016.9.1.75
13. Sun RH, Li C, Zhou YQ, Cai YC, Shui CY, Liu W, et al. Predictive role of intraoperative clinicopathological features of the central compartment in estimating lymph nodes metastasis status. Ann Transl Med. (2019) 7:471. doi: 10.21037/atm.2019.08.01
14. Sui C, He Q, Du R, Zhang D, Li F, Dionigi G, et al. Lymph node characteristics of 6279 N1 differentiated thyroid cancer patients. Endocr Connect. (2020) 9:201–10. doi: 10.1530/EC-20-0019
15. Liu J, Guo M. Clinical analysis of cervical lymph node metastasis patterns and multivariate factors in differentiated thyroid carcinoma. Oncol Lett. (2023) 26:431. doi: 10.3892/ol.2023.14018
16. Kruijff S, Aniss AM, Chen P, Sidhu SB, Delbridge LW, Robinson B, et al. Decreasing the dose of radioiodine for remnant ablation does not increase structural recurrence rates in papillary thyroid carcinoma. Surgery. (2013) 154:1337–44. doi: 10.1016/j.surg.2013.06.034
17. Kernan WN, Viscoli CM, Brass LM, Broderick JP, Brott T, Feldmann E, et al. Phenylpropanolamine and the risk of hemorrhagic stroke. N Engl J Med. (2000) 343:1826–32. doi: 10.1056/NEJM200012213432501
18. Liu S, Wang X, Lu Y, Li T, Gong Z, Sheng T, et al. The effects of intraoperative cryoprecipitate transfusion on acute renal failure following orthotropic liver transplantation. Hepatol Int. (2013) 7:901–9. doi: 10.1007/s12072-013-9457-9
19. Vandenbroucke JP, von Elm E, Altman DG, Gotzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PloS Med. (2007) 4:e297. doi: 10.1371/journal.pmed.0040297
20. Davies L, Morris L, Haymart M, Chen A, Goldenberg D, Morris J, et al. American association of clinical endocrinologists and american college of endocrinology disease state clinical review: the increasing incidence of thyroid cancer. Endocrine Pract. (2015) 21:686–96. doi: 10.4158/ep14466.dscr
21. McLeod DSA, Bedno SA, Cooper DS, Hutfless S, Ippolito S, Jordan SJ, et al. Pre-existing thyroid autoimmunity and risk of papillary thyroid cancer: a nested case-control study of us active-duty personnel. J Clin Oncol. (2022) 40:2578–87. doi: 10.1200/jco.21.02618
22. Shi L, Wang J, Ding N, Zhang Y, Zhu Y, Dong S, et al. Inflammation induced by incomplete radiofrequency ablation accelerates tumor progression and hinders pd-1 immunotherapy. Nat Commun. (2019) 10:5421. doi: 10.1038/s41467-019-13204-3
23. Sahu A, Kose K, Kraehenbuehl L, Byers C, Holland A, Tembo T, et al. In vivo tumor immune microenvironment phenotypes correlate with inflammation and vasculature to predict immunotherapy response. Nat Commun. (2022) 13:5312. doi: 10.1038/s41467-022-32738-7
24. Pelizzo MR, Dobrinja C, Casal IE, Zane M, Lora O, Toniato A, et al. The role of BRAF(V600E) mutation as a poor prognostic factor for the outcome of patients with intrathyroid papillary thyroid carcinoma. BioMed Pharmacother. (2014) 68:413–7. doi: 10.1016/j.biopha.2014.03.008
25. Moon S, Song YS, Kim YA, Lim JA, Cho SW, Moon JH, et al. Effects of coexistent BRAF(V600E) and TERT promoter mutations on poor clinical outcomes in papillary thyroid cancer: a meta-analysis. Thyroid. (2017) 27:651–60. doi: 10.1089/thy.2016.0350
Keywords: differentiated thyroid carcinoma, lateral cervical lymph node positivity rate, post-treatment recurrence, association, structural incomplete response, iodine-131 therapy
Citation: Ye T, Shao S, Yao S and Wang R (2025) The relationship between lateral cervical lymph node positivity rate and recurrence after comprehensive treatment in differentiated thyroid carcinoma: a single-center retrospective cohort study from China. Front. Oncol. 15:1484002. doi: 10.3389/fonc.2025.1484002
Received: 09 December 2024; Accepted: 28 January 2025;
Published: 13 February 2025.
Edited by:
Erivelto Martinho Volpi, Hospital Alemão Oswaldo Cruz, BrazilReviewed by:
Denise Engelbrecht Zantut Wittmann, State University of Campinas, BrazilCopyright © 2025 Ye, Shao, Yao and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruimin Wang, d2FuZ3J1aW1pbnZpcEBzaW5hLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.