
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Oncol. , 06 February 2025
Sec. Gastrointestinal Cancers: Gastric and Esophageal Cancers
Volume 15 - 2025 | https://doi.org/10.3389/fonc.2025.1475153
This article is part of the Research Topic Use of Radiation Therapy for Hematological Malignancies View all 6 articles
 Joe El Khoury1†
Joe El Khoury1† Remy Daou1†
Remy Daou1† Neal Kim2*†
Neal Kim2*† Josiane Bou Eid1†
Josiane Bou Eid1† Brandon Imber3†
Brandon Imber3† Joachim Yahalom3†
Joachim Yahalom3† Carla Hajj3,4†
Carla Hajj3,4†Primary esophageal lymphoma is a rare malignancy that is difficult to diagnose and treat. While there have been significant advances in understanding the pathogenesis, clinical features, and treatment options, there is a lack of consensus on the most effective treatment approach. This literature review provides a comprehensive overview of the use of available treatment options for primary esophageal lymphoma, including surgery, radiotherapy, and chemotherapy. The review also highlights the current knowledge gaps that need to be addressed through further research. While no single treatment modality has emerged as a clear front-runner, a combination of these treatments may be the most effective approach in managing primary esophageal lymphoma, tailored to the histological subtypes.
Primary esophageal lymphoma is a rare malignancy that accounts for less than 1% of all esophageal neoplasms and only 0.3% of all primary non-Hodgkin’s lymphomas (1). This disease is challenging to diagnose and treat due to its nonspecific clinical presentation and variable histological features. As a result, the optimal management of primary esophageal lymphoma remains unclear, and there is no standard treatment approach. In recent years, there have been significant advances in the understanding of the pathogenesis, clinical features, and treatment options for primary esophageal lymphomas. However, the literature on this topic is limited, and there is a lack of consensus on the most effective treatment approach.
To date, various treatment modalities have been used, including surgery, radiotherapy, and chemotherapy. Each approach has its benefits and limitations, and the choice of treatment depends on the stage, histological type, and the patient’s overall health status. Given the rarity of this malignancy, there is a lack of large randomized controlled trials to establish the most effective treatment approach. As a result, the literature on the management of primary esophageal lymphoma consists primarily of case reports and small case series. In this literature review, we aimed to provide an overview of the use of available treatment options for primary esophageal lymphoma. We reviewed the existing evidence on the use of surgery, radiotherapy, chemotherapy, and immunotherapy and discuss their respective outcomes and limitations.
This literature review aimed to identify relevant studies related to primary esophageal lymphoma using the PubMed/Medline database. A comprehensive search was conducted using a combination of keywords and MeSH terms, including “primary esophageal lymphoma” OR “esophagus lymphoma” and various histology subtypes. No date or regional restrictions were applied, and all relevant analyses were included. The search yielded studies in several languages, and those in English, French, and Chinese were included in the analysis.
However, due to some studies being unavailable online, we only had limited access to their abstracts, resulting in some missing data. Despite this limitation, we included all available studies in our analysis to provide a comprehensive overview of the treatment options for primary esophageal lymphoma. The histological subtypes included in this study were MALT or mucosa-associated lymphoid tissue, DLBCL or diffuse large B-cell lymphoma, follicular lymphoma, Burkitt lymphoma, mantle cell lymphoma, T-cell lymphoma, ALCL or anaplastic large cell lymphoma, plasmablastic lymphoma, and Hodgkin lymphoma.
Overall, this review provides a comprehensive summary of the available literature on the management of primary esophageal lymphoma and highlights the current knowledge gaps that need to be addressed through further research.
A total of 62 studies were included in this review. We found that most gastrointestinal tract lymphomas arise submucosally. They may be beyond the reach of endoscopic biopsy forceps, which can pose a problem for identifying histopathology and determining the best course of treatment (2). Reported reasons for choosing surgery, in indolent and less aggressive lymphomas like MALT lymphoma, included preliminary imaging, diagnosis, and a missing biopsy.
MALT lymphoma of the esophagus is a rare entity. In 2017, there were less than 20 reported cases of esophageal MALT lymphomas, most of which have been diagnosed in Japan (3). They are usually localized, but we found one case report of metastasis to the lungs and stomach (4) and one report of recurrence in the stomach and lung after initial radiotherapy (5). All treatment modalities have been used with generally good results, chemotherapy (CHOP-based) and resection being the most frequent (3, 4, 6–11). The main results of the available studies are summarized in Table 1.
Despite the limited number of patients treated with radiotherapy in our review, extrapolation from other tumor sites would encourage the use of this modality, as MALT lymphomas are usually sensitive to radiation. In gastric lymphomas, radiotherapy can lead to 85-100% remission rates (4-5 years of disease-free survival), according to multiple small studies, especially in low-grade stages I and IIE (12–14). For this reason, since surgery—often indicated due to inconclusive biopsy results—and radiation therapy are effective treatment options with high remission rates, the safety profiles and tolerability of both modalities should be considered when treating patients with low-grade esophageal MALT lymphomas. Low doses of radiation therapy, compared to surgical resection, can be curative and associated with minimal side effects.
We found a single case of follicular lymphoma, described by Taal et al. in 1976. Based on the Working Formulation classification, it was diagnosed as a “small cleaved cell non-Hodgkin’s lymphoma” associated with extensive fistula formation, mainly to the left main bronchus. No manifestations of lymphoma were found outside of the esophagus. As dyspnea developed, the patient received a single dose of emergency chemotherapy but died three weeks later from sepsis (15).
DLBCL predominantly involves immunocompromised patients with HIV infection as a potential risk factor (16). All treatment modalities combined, outcomes were generally poor, and robust data is still lacking. While the results summarized in Table 2 show a 50% complete response rate, in most case reports, follow-up duration was either lacking or was very short, thus masking early disease progression, which would have been attributed to treatment failure. However, combined modalities, including radiation therapy, seem to yield better results, as all 5 patients who received either chemotherapy plus radiation therapy or surgery followed by chemotherapy and radiation therapy had a complete response, with the reports covering a follow-up period of up to 3 years.
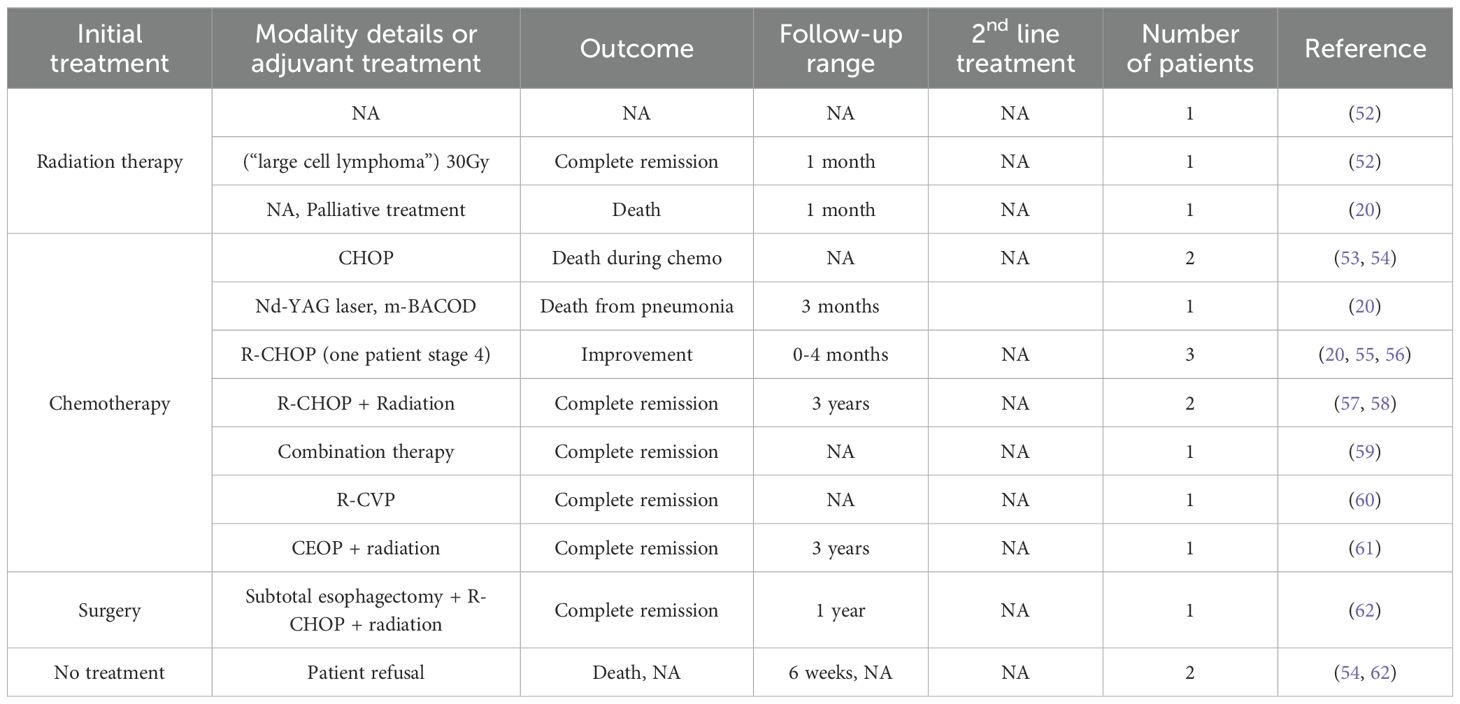
Table 2. Treatment modalities and outcomes in primary esophageal Diffuse Large B-Cell Lymphomas (DLBCL).
Similarly to other types of esophageal lymphomas, T-cell lymphoma reports are scarce, and detailed treatment outcomes are missing in the literature. While two cases of primary NK/T-cell lymphoma were treated with chemotherapy only and achieved complete remission with a follow-up of up to 24 months (17, 18), a 40-year-old man in stage IE was also treated with CHOP but died due to complications (19).
Chemotherapy should be the mainstay of treatment, with a possible benefit when combined with radiation therapy. A review including Chinese case reports noted that systemic chemotherapy combined with concurrent radiotherapy might be an effective treatment for esophageal NK/T-cell lymphoma (17). Our literature search tends to validate this conclusion, as we noted a few cases of diffuse large T-cell Lymphomas treated with radiation therapy followed by either the HADAP regimen (patient survival of 14 months) or the CHOP regimen (2 patients, one with a survival of 7 months who died of sepsis despite evidence of early anti-tumor response, and one who survived ten months of follow-up) (20, 21). A fourth patient received two cycles of systemic chemotherapy with concurrent radiotherapy, but his general condition gradually worsened, and he passed away four months after admission (22).
Additionally, in 1999, 65 Gy irradiation was used to treat a primary esophageal T-cell lymphoma in clinical stage I(E)B with complete remission (23). The role of radiation therapy in T-cell lymphomas warrants further study, as radiotherapy has been considered of paramount importance for patients with clinically localized nasal disease, with approximately 70% of patients achieving complete remission after treatment (24).
Multifocal gut involvement has been described in mantle cell lymphoma (MCL) (25). However, esophageal involvement is uncommon. A retrospective study collecting data from 35 MCL patients with GI involvement found that the esophagus was affected in only 2 (5.7%) cases (26). All published cases were treated with chemotherapy, as lesions were usually seen in multiple locations in the GI tract. Of the few available cases, one died before receiving treatment (27); one was lost to follow-up, two had a good response to chemotherapy (28, 29), while one had no response to treatment (30). A “mantle cell lymphoma-like tumor” was treated with rituximab postoperatively and has been disease-free for more than 28 months after surgery (31).
ALCL was first described in 1985 as a large-cell neoplasm with an expression of CD30 in all neoplastic cells (32). It is an uncommon T-cell lymphoma, defined as a separate entity since the 2008 WHO lymphoma classification (33). ALCL can be ALK+ or ALK-. Radiotherapy in esophageal ALCL has not yet been reported. Only one case report in 1992 mentioned a treatment plan including chemotherapy followed by radiotherapy, but the patient was lost to follow-up (34).
The few other published cases were treated with chemotherapy (usually CHOP), sometimes followed by autologous peripheral blood hematopoietic stem cell transplantation, with partial (2 cases) or complete (2 cases) remission (2, 35–37). Also, one patient was diagnosed post-mortem (38). Usually, ALK+ lymphoma is very sensitive to chemotherapy; thus, if the diagnosis is made without the need for thoracotomy and resection, chemoradiotherapy may be sufficient.
In other types and locations of ALCL, such as limited-stage systemic ALCL or breast implant-associated ALCL, radiation therapy was used in association with chemotherapy (39): for example, following doxorubicin-based chemotherapy and radiotherapy, in early-stage primary systemic anaplastic large-cell lymphoma, the 5-year overall survival (OS), progression-free survival (PFS), and local control rates for all patients were 84.4%, 63.6%, and 90.8%, respectively (40).
No case of adult esophageal Burkitt lymphoma was found in our database search. However, although it does not match our initial inclusion criteria, we found a case report of a 17-year-old patient in India with stage II intermediate risk, Group-B disease by Murphy staging system managed with LMB-96 protocol for intermediate-risk BL. She had a complete response and no recurrence after nine months (41).
We found a single case of plasmablastic lymphoma treated in 2016 with resection followed by chemotherapy, without further details (42).
In conclusion, primary esophageal lymphoma, though rare, is a potentially life-threatening condition, requiring careful evaluation and management. The literature review explored various treatment options, including surgery, chemotherapy, and radiotherapy, with varying degrees of success. While no single treatment has emerged as a clear front-runner, a combination of those treatment modalities may be the most effective approach in managing primary esophageal lymphoma, and the choice should be tailored to the histological subtypes. Radiation therapy should be considered in patients with low-grade esophageal lymphomas as a definitive therapy, and as a consolidation therapy to chemotherapy in patients with high-grade esophageal lymphomas. Additional research is crucial to determine the most effective treatment strategies that could improve patient outcomes for this disease.
JK: Writing – original draft, Writing – review & editing. RD: Writing – original draft, Writing – review & editing. NK: Writing – original draft, Writing – review & editing. JE: Writing – original draft, Writing – review & editing. BI: Writing – original draft, Writing – review & editing. JY: Writing – original draft, Writing – review & editing. CH: Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Okerbloom JA, Armitage JO, Zetterman R, Linder J. Esophageal involvement by non-Hodgkin’s lymphoma. Am J Med. (1984) 77:359–61. doi: 10.1016/0002-9343(84)90721-6
2. Yaakup H, Sagap I, Fadilah SA. Primary oesophageal Ki (CD30)-positive ALK+ anaplastic large cell lymphoma of T-cell phenotype. Singapore Med J. (2008) 49:e289–292.
3. Ma Q, Zhang C, Fang S, Zhong P, Zhu X, Lin L, et al. Primary esophageal mucosa-associated lymphoid tissue lymphoma. Med (Baltimore). (2017) 96:e6478.
4. Hayashi M, Ueda K, Tanaka T, Enoki T, Tanaka N, Gondo T, et al. Mucosa-associated lymphoid tissue (MALT) lymphoma arising in the esophagus, stomach, and lung. Gen Thorac Cardiovasc Surg. (2011) 59:826–30. doi: 10.1007/s11748-010-0761-1
5. Byun SJ, Kang HW, Cha JK, Ryoo SR, Lee JH, Kim DY, et al. Primary mucosa-associated lymphoid tissue lymphoma metachronously involving esophagus and stomach. Korean J Gastroenterol. (2016) 67:257–61. doi: 10.4166/kjg.2016.67.5.257
6. Kudo K, Ota M, Narumiya K, Shirai Y, Ohki T, Yamamoto M. Primary esophageal mucosa-associated lymphoid tissue lymphoma treated by endoscopic submucosal dissection. Dig Endosc. (2014) 26:478–81. doi: 10.1111/den.2014.26.issue-3
7. Jung JG, Kang HW, Hahn SJ, Kim Jsc and EJ. Primary mucosa-associated lymphoid tissue lymphoma of the esophagus, manifesting as a submucosal tumor. Korean J Gastroenterol. (2013) 62:117–21. doi: 10.4166/kjg.2013.62.2.117
8. Lee DS, Ahn YC, Eom DW, Lee SJ. Primary esophageal mucosa-associated lymphoid tissue lymphoma diagnosed by using stacked forceps biopsy. Dis Esophagus Off J Int Soc Dis Esophagus. (2016) 29:887–90. doi: 10.1111/dote.2016.29.issue-7
9. Musquer N, Zidane-Marinnes M, Dib M. Primary mucosa-associated lymphoid tissue lymphoma of the entire esophagus. Gastrointest Endosc. (2017) 86:560–1. doi: 10.1016/j.gie.2017.02.029
10. Tabibian JH, Kalani A, Moran AM, Panganamamula K. Extranodal marginal zone B cell (MALT) lymphoma of the esophagus. J Gastrointest Cancer. (2019) 50:1034–6. doi: 10.1007/s12029-018-00187-5
11. Ishida M, Hodohara K, Furuya A, Okuno H, Yoshii M, Horinouchi A, et al. Sarcoidal granulomas in the mediastinal lymph nodes after treatment for marginal zone lymphoma of the esophagus: report of a case with review of the concept of the sarcoidosis-lymphoma syndrome. Int J Clin Exp Pathol. (2014) 7:4428–32.
12. Yahalom J, Xu AJ, Noy A, Lobaugh S, Chelius M, Chau K, et al. Involved-site radiotherapy for Helicobacter pylori-independent gastric MALT lymphoma: 26 years of experience with 178 patients. Blood Adv. (2021) 5(7):1830–6. doi: 10.1182/bloodadvances.2020003992
13. Aleman BMP, Haas RLM, van der Maazen RWM. Role of radiotherapy in the treatment of lymphomas of the gastrointestinal tract. Best Pract Res Clin Gastroenterol. (2010) 24:27–34. doi: 10.1016/j.bpg.2009.12.002
14. Olszewska-Szopa M, Wróbel T. Gastrointestinal non-Hodgkin lymphomas. Adv Clin Exp Med Off Organ Wroclaw Med Univ. (2019) 28:1119–24. doi: 10.17219/acem/94068
15. Taal BG, Van Heerde P, Somers R. Isolated primary oesophageal involvement by lymphoma: a rare cause of dysphagia: two case histories and a review of other published data. Gut. (1993) 34:994–8. doi: 10.1136/gut.34.7.994
16. Pasqualucci L, Dalla-Favera R. Genetics of diffuse large B-cell lymphoma. Blood. (2018) 131:2307–19. doi: 10.1182/blood-2017-11-764332
17. Liang P, Ren XC, Gao JB. Radiological and clinical features of primary NK/T-cell lymphoma involving the whole length of the esophagus: A case report. Oncol Lett. (2017) 14:2147–52. doi: 10.3892/ol.2017.6456
18. George MK, Ramachandran V, Ramanan SG, Sagar TG. Primary esophageal T-cell non-Hodgkin’s lymphoma. Indian J Gastroenterol Off J Indian Soc Gastroenterol. (2005) 24:119–20.
19. Ye ZY, Cao QH, Liu F, Lu XF, Li SR, Li CZ, et al. Primary esophageal extranasal NK/T cell lymphoma with biphasic morphology. Med (Baltimore). (2015) 94:e1151.
20. Chadha KS, Hernandez-Ilizaliturri FJ, Javle M. Primary esophageal lymphoma: case series and review of the literature. Dig Dis Sci. (2006) 51:77–83. doi: 10.1007/s10620-006-3088-0
21. Wagner PL, Tam W, Lau PY, Port JL, Paul S, Altorki NK, et al. Primary esophageal large T-cell lymphoma mimicking esophageal carcinoma: A case report and literature review. J Thorac Cardiovasc Surg. (2008) 135:957–958.e1. doi: 10.1016/j.jtcvs.2007.12.010
22. Fujihara S, Mori H, Kobara H, Nishiyama N, Kobayashi M, Masaki T. Esophageal natural killer (NK)/T cell lymphoma of true natural killer cell origin. Endoscopy. (2014) 46:E77–8. doi: 10.1055/s-0032-1309852
23. Fujisawa S, Motomura S, Fujimaki K, Tanabe J, Tomita N, Hara M, et al. Primary esophageal T cell lymphoma. Leuk Lymphoma. (1999) 33:199–202. doi: 10.3109/10428199909093743
24. Li YX, Yao B, Jin J, Wang WH, Liu YP, Song YW, et al. Radiotherapy as primary treatment for stage IE and IIE nasal natural killer/T-cell lymphoma. J Clin Oncol Off J Am Soc Clin Oncol. (2006) 24:181–9. doi: 10.1200/JCO.2005.03.2573
26. Iwamuro M, Okada H, Kawahara Y, Shinagawa K, Morito T, Yoshino T, et al. Endoscopic features and prognoses of mantle cell lymphoma with gastrointestinal involvement. World J Gastroenterol. (2010) 16:4661–9. doi: 10.3748/wjg.v16.i37.4661
27. Nopjaroonsri P, Mankongpaisarnrung C. Mantle cell lymphoma presenting as diffuse esophageal, gastric, and duodenal bulb polyposis. Ann Gastroenterol. (2019) 32:317. doi: 10.20524/aog.2019.0356
28. Toyonaga H, Fukushima M, Inokuma T, Imai Y. Mantle cell lymphoma involving the esophagus evaluated by magnifying endoscopy with narrow-band imaging. Gastrointest Endosc. (2018) 87:305–6. doi: 10.1016/j.gie.2017.07.016
29. Zullo A, Cerro P, Chios A, Andriani A, Balsamo G, Francesco VD, et al. A very unusual cause of dysphagia: mantle cell lymphoma. Ann Gastroenterol Q Publ Hell Soc Gastroenterol. (2016) 29:383–5.
30. Saito M, Mori A, Irie T, Tanaka M, Morioka M, Ozasa M, et al. Endoscopic follow-up of 3 cases with gastrointestinal tract involvement of mantle cell lymphoma. Intern Med Tokyo Jpn. (2010) 49:231–5. doi: 10.2169/internalmedicine.49.2766
31. Mori T, Komeno T, Ohtani H. Mantle cell lymphoma-like solitary polypoid tumor of the esophagus: a case report. cases J. (2009) 2.
32. Stein H, Mason DY, Gerdes J, O’Connor N, Wainscoat J, Pallesen G, et al. The expression of the Hodgkin’s disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic Malignancies are derived from activated lymphoid cells. Blood. (1985) 66:848–58. doi: 10.1182/blood.V66.4.848.848
33. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO classification of tumours of haematopoietic and lymphoid tissues . Available online at: https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/WHO-Classification-Of-Tumours-Of-Haematopoietic-And-Lymphoid-Tissues-2017.
34. Ross CW, Hanson CA, Schnitzer B. CD30 (Ki-1)-positive, anaplastic large cell lymphoma mimicking gastrointestinal carcinoma. Cancer. (1992) 70:2517–23. doi: 10.1002/1097-0142(19921115)70:10<2517::AID-CNCR2820701021>3.0.CO;2-U
35. Azarpira N, Safaie A, Monabati A, Hosseinzadeh M, Noori S, Moini M, et al. PAX-5 positive anaplastic large cell lymphoma presenting by dysphagia; a case report. Gastroenterol Hepatol Bed Bench. (2017) 10:332–6.
36. Joshi A, Fields P, Simo R. Anaplastic lymphoma of the cervical esophagus presenting as a tracheoesophageal fistula. Head Neck. (2008) 30:1264–8. doi: 10.1002/hed.20774
37. Wu N, Pang L, Chen Z, Wang Y, Ma Q, Chen G, et al. Primary esophageal CD30-positive ALK-positive anaplastic large cell lymphoma: a case report and literature review. J Gastrointest Cancer. (2011) 42:57–60. doi: 10.1007/s12029-010-9147-y
38. Pearson JM, Borg-Grech A. Primary Ki-1 (CD 30)–positive, large cell, anaplastic lymphoma of the esophagus. Cancer. (1991) 68:418–21. doi: 10.1002/1097-0142(19910715)68:2<418::AID-CNCR2820680234>3.0.CO;2-1
39. Vu K, Ai W. Update on the treatment of anaplastic large cell lymphoma. Curr Hematol Malig Rep. (2018) 13:135–41. doi: 10.1007/s11899-018-0436-z
40. Zhang XM, Li YX, Wang WH, Jin J, Wang SL, Liu YP, et al. Favorable outcome with doxorubicin-based chemotherapy and radiotherapy for adult patients with early stage primary systemic anaplastic large-cell lymphoma. Eur J Haematol. (2013) 90:195–201. doi: 10.1111/ejh.2013.90.issue-3
41. Madabhavi I, Patel A, Revannasiddaiah S, Choudhary M, Anand A, Das P, et al. Primary esophageal Burkitt’s lymphoma: a rare case report and review of literature. Gastroenterol Hepatol Bed Bench. (2014) 7:230–7.
42. Mihara K, Sasaki N, Mamoru O, Kanbe M, Ichinohe T, Suehiro S. Solitary plasmablastic lymphoma in the esophagus. Ann Hematol. (2016) 95:845–6. doi: 10.1007/s00277-016-2626-x
43. Bardisi ES, Alghanmi N, Merdad AA. Primary mucosa-associated lymphoid tissue lymphoma of the esophagus masquerading as a benign tumor. Ann Med Surg 2012. (2014) 3:39–42. doi: 10.1016/j.amsu.2014.05.001
44. Hosaka S, Nakamura N, Akamatsu T, Fujisawa T, Ogiwara Y, Kiyosawa K, et al. A case of primary low grade mucosa associated lymphoid tissue (MALT) lymphoma of the oesophagus. Gut. (2002) 51:281–4. doi: 10.1136/gut.51.2.281
45. Kobayashi S, Iwamuro M, Nishida K, Tanaka T, Kawano S, Kawahara Y, et al. Primary localized esophageal mucosa-associated lymphoid tissue lymphoma treated by endoscopic submucosal dissection. Intern Med Tokyo Jpn. (2018) 57:2347–52. doi: 10.2169/internalmedicine.0487-17
46. Baek DH, Kim GH, Song GA, Ryu KD, Cho EJ, Shin MJ, et al. Primary esophageal mucosa-associated lymphoid tissue lymphoma treated with endoscopic resection. Gastrointest Endosc. (2012) 75:1282–3. doi: 10.1016/j.gie.2011.06.003
47. Yano S, Usui N, Dobashi N, Yahagi Y, Takahara S, Sugiyama K, et al. A case of primary esophageal mucosa-associated lymphoid tissue lymphoma with a numerical abnormality of 18q21 detected by fluorescence in situ hybridization. Ann Hematol. (2009) 88:703–4. doi: 10.1007/s00277-008-0653-y
48. Kitamoto Y, Hasegawa M, Ishikawa H, Saito Ji, Yamakawa M, Kojima M, et al. Mucosa-associated lymphoid tissue lymphoma of the esophagus: A case report. J Clin Gastroenterol. (2003) 36:414–6. doi: 10.1097/00004836-200305000-00011
49. Kishi K, Maeda H, Nakamura Y, Shirai S, Sato M. Radiotherapy for mucosa-associated lymphoid tissue (MALT) lymphoma of the esophagus: a case report with a diagnostic and therapeutic discussion. Int J Clin Oncol. (2012) 17:174–80. doi: 10.1007/s10147-011-0265-8
50. Malik AO, Baig Z, Ahmed A, Qureshi N, Malik FN. Extremely rare case of primary esophageal mucous associated lymphoid tissue lymphoma. World J Gastrointest Endosc. (2013) 5:446–9. doi: 10.4253/wjge.v5.i9.446
51. Moriya K, Tamura H, Nakamura K, Hosone M, Inokuchi K. A primary esophageal MALT lymphoma patient with Helicobacter pylori infection achieved complete remission after H. pylori eradication without anti-lymphoma treatment. Leuk Res Rep. (2016) 7:2–5.
52. Bernal A, del Junco GW. Endoscopic and pathologic features of esophageal lymphoma: a report of four cases in patients with acquired immune deficiency syndrome. Gastrointest Endosc. (1986) 32:96–9. doi: 10.1016/S0016-5107(86)71765-3
53. Moses AE, Rahav G, Bloom AI, Okon E, Polliack A, Maayan S, et al. Primary lymphoma of the esophagus in a patient with AIDS. J Clin Gastroenterol. (1995) 21:327–8. doi: 10.1097/00004836-199512000-00018
54. Weeratunge CN, Bolivar HH, Anstead GM, Lu DH. Primary esophageal lymphoma: a diagnostic challenge in acquired immunodeficiency syndrome–two case reports and review. South Med J. (2004) 97:383–7. doi: 10.1097/01.SMJ.0000100120.49153.3F
55. Teerakanok J, DeWitt JP, Juarez E, Thein KZ, Warraich I. Primary esophageal diffuse large B cell lymphoma presenting with tracheoesophageal fistula: A rare case and review. World J Gastrointest Oncol. (2017) 9:431–5. doi: 10.4251/wjgo.v9.i10.431
56. Castresana D, Bansal P, Vasef MA, Kapoor V, Leone C, Quintana D. Aggressive lymphoma presenting as dysphagia: A rare cause of dysphagia. Clin Case Rep. (2017) 5:555–8. doi: 10.1002/ccr3.2017.5.issue-5
57. Sabljak P, Stojakov D, Bjelovic M, Mihaljevic B, Velickovic D, Ebrahimi K, et al. Primary esophageal diffuse large B-cell lymphoma: report of a case. Surg Today. (2008) 38:647–50. doi: 10.1007/s00595-007-3690-6
58. Inayat F, Munir A, Wahab A, Younus F, Zafar F, Ullah W. Primary esophageal diffuse large B-cell lymphoma: A comparative review of 15 cases. J Investig Med High Impact Case Rep. (2018) 6.
59. Kalogeropoulos IV, Chalazonitis AN, Tsolaki S, Laspas F, Ptohis N, Neofytou I, et al. A case of primary isolated non-Hodgkin’s lymphoma of the esophagus in an immunocompetent patient. World J Gastroenterol WJG. (2009) 15:1901–3. doi: 10.3748/wjg.15.1901
60. Mrad RA, El-Majzoub N, Shamseddine A, Soweid A. The elusive diagnosis of primary esophageal lymphoma. Turk J Hematol. (2018) 35:199.
61. Sugoor PT, Jiwnani S, Rekhi B, Karimundackal G, Purandare N, Pramesh CS. Isolated primary non-hodgkin’s lymphoma of the esophagus. Indian J Med Paediatr Oncol. (2018) 39:244.
Keywords: esophageal lymphoma, DLBCL, follicular lymphoma, radiation treatment, MALT lymphoma
Citation: El Khoury J, Daou R, Kim N, Bou Eid J, Imber B, Yahalom J and Hajj C (2025) Treatment of primary esophageal lymphomas: A review. Front. Oncol. 15:1475153. doi: 10.3389/fonc.2025.1475153
Received: 02 August 2024; Accepted: 17 January 2025;
Published: 06 February 2025.
Edited by:
Liang Qiao, The University of Sydney, AustraliaReviewed by:
Lara Hilal, American University of Beirut Medical Center, LebanonCopyright © 2025 El Khoury, Daou, Kim, Bou Eid, Imber, Yahalom and Hajj. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Neal Kim, S2ltTjRAbXNrY2Mub3Jn
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.