
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 20 March 2025
Sec. Radiation Oncology
Volume 15 - 2025 | https://doi.org/10.3389/fonc.2025.1475118
This article is part of the Research TopicUse of Radiation Therapy for Hematological MalignanciesView all 6 articles
 Jennifer Ma1*†
Jennifer Ma1*† Remy Daou2†
Remy Daou2† Josiane Bou Eid2
Josiane Bou Eid2 Beatrice Fregonese1
Beatrice Fregonese1 Joe El-Khoury2
Joe El-Khoury2 N. Ari Wijetunga3
N. Ari Wijetunga3 Brandon S. Imber1
Brandon S. Imber1 Joachim Yahalom1
Joachim Yahalom1 Carla Hajj1,4*
Carla Hajj1,4*Purpose/objective: Primary hepatic lymphomas (PHL) are an extremely rare form of non-Hodgkin Lymphoma (NHL) for which there are no established treatment guidelines, with available literature largely comprised of small case reports. Therefore, we evaluate our institutional experience treating PHL within the context of existing literature to better understand treatment modalities, role of radiotherapy (RT), and outcomes.
Materials/methods: We conducted a single institutional retrospective study of all patients with PHL diagnosed from 2000-2021, defined as a biopsy-proven liver lesion in the absence of other lymphomatous solid organ involvement, except for concurrently diagnosed hepatosplenic lymphomas. Subgroup analysis was performed for diffuse large B-cell lymphoma (DLBCL) and indolent lymphomas, which included marginal zone lymphoma (MZL), Grade 1-2 follicular lymphoma (FL), and low-grade B-cell lymphoma (BCL), NOS. Univariable (UVA) and multivariable analysis (MVA) for overall survival (OS) were performed using the Cox proportional hazards model. A literature review was conducted using key words “liver”, “lymphoma”, and “treatment” to identify relevant literature.
Results: We identified 30 patients with PHL within the institutional cohort and 192 patients from comprehensive literature review. Subgroup analysis of DLBCL included 15 patients. On MVA for OS, only ECOG score (p=0.02) and Lugano stage (p=0.04) remained significant. Subgroup analysis of the indolent lymphoma group included 9 patients. On MVA for OS, only age remained significant. Systemic therapy was the most common treatment modality overall (20 patients; 67%) with surgery, radiation and observation utilized in 4 patients (13%) each. Seventeen (57%) of patients were alive at the time of data collection, with 8 (27%) deceased and 5 (17%) lost to follow-up.
Conclusion: PHL are an extremely rare subtype of NHL for which there is no clear treatment consensus. Primary hepatic DLBCL appears to be treated mostly with chemotherapy with good disease control. For indolent PHL, low-dose RT appears to have good overall disease control with minimal toxicity. Our RT data is limited by the short duration of follow-up for patients receiving RT compared to those who received chemotherapy, surgery or observation. However, our results are encouraging for the use of RT for appropriate patients with indolent PHL.
Primary hepatic lymphoma (PHL) is extremely rare, and accounts for only 0.4% of all primary extra-nodal non-Hodgkin lymphomas (1). Due to the low prevalence of disease, there are no established treatment guidelines, and the existing literature mostly entails small case series or single case reports.
The treatment approach to PHL is guided essentially by the disease histology, with diffuse large B-cell lymphomas (DLBCL) (2) treated primarily with systemic therapy and indolent lymphomas treated with various modalities including observation, surgery or systemic therapy. There have been limited reports on the use of radiotherapy for PHL in literature.
In a retrospective SEER (Surveillance, Epidemiology, and End Results) database analysis published in 2020, 1372 cases of primary hepatic lymphomas were identified between 1975 and 2016. Among those, DLBCL was the most frequent histology (78.8%), followed by T/NK-cell lymphoma (4.7%), marginal zone lymphoma (MZL; 4.5%), Burkitt (4.3%), follicular (3.1%), and small lymphatic lymphomas (SLL; 2.5%). It was also noted that the annual prevalence of PHL increased with time from 1975 to 2016 (3).
Patients may present with right upper quadrant abdominal pain, nausea or vomiting, as well as the typical B symptoms including fever, weight loss and constitutional symptoms. Older age (80 years or older), male gender, black race, unmarried status, and histology of DLBCL or T/NK-cell lymphoma were associated with a significantly increased risk of cancer-related death (3, 4). A retrospective study conducted through the Rare Cancer Network on 41 patients with PHL found a median survival at 163 months among patients of mixed PHL histology, with a 10-year OS at 59%. Positive prognostic factors include: the presence of fever, the absence of weight loss, and normal hemoglobin level (5). Main characteristics identified among patients with PHL include hepatitis B or C infection, cirrhosis, or concomitant hepatocellular carcinoma (6).
Management of PHL and selection of treatment modalities is challenging given the rarity of PHL and lack of established guidelines.
Therefore, we set out to evaluate our institutional experience treating PHL within the context of existing literature to better understand the treatment modalities, role of radiotherapy (RT), and outcomes. Traditionally, radiotherapy for indolent lymphomas has involved treatment of up to 2400 cGy in 12 fractions, however the use of low-dose radiotherapy of 400 cGy in 1-2 fractions has been increasingly utilized (7). To our knowledge, use of low-dose RT for liver lymphomas has not previously been reported in the literature.
We conducted a single institutional retrospective analysis of all patients with PHL diagnosed from 2000-2021. PHL was defined as a biopsy-proven liver lesion in the absence of other active concurrent cancers or lymphomatous solid organ involvement, except for concurrently diagnosed splenic lymphomas. Patients with other cancer diagnoses within 5 years of PHL diagnosis and non-hepatic primary lymphoma were excluded. Subgroup analysis was performed for two histologic subgroups: diffuse large B-cell lymphoma (DLBCL) and indolent lymphomas, which was comprised of marginal zone (MZL), follicular (FL), and low-grade B-cell lymphoma (BCL), NOS. Response was assessed by Deauville criteria with scores of 1-2 considered as complete response (CR) per institutional practice. Univariable (UVA) and multivariable analysis (MVA) for overall survival (OS) were performed for the overall cohort and subgroup analyses using the Cox proportional hazards model and Kaplan Meier curves were generated for overall survival (OS) and progression-free survival (PFS).
Next-generation sequencing (NGS) was performed on select patients as standard of care using the Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT) solid tumor clinical assay, which includes up to 505 genes sequenced to a depth of 700X, with germline correction (8, 9). We examined the genetic profiles of available patients in our cohorts for frequency of mutated genes.
In parallel, a literature search was carried out using the PubMed/Medline database for English and French articles and abstracts. There was no date limit applied, and all relevant studies were included. Some studies’ full texts were unavailable online, and we only had access to their abstracts. The keywords and MeSH words used in the search were “Primary hepatic lymphoma” OR “Liver lymphoma” and the histology sub-type: MALT or Mucosa-associated lymphoid tissue, DLBCL or Diffuse large B-cell lymphoma, Follicular lymphoma, Burkitt lymphoma, Mantle cell lymphoma, T-cell lymphoma, and ALCL or Anaplastic large cell lymphoma. A total of 192 cases of primary hepatic lymphoma published in case reports were found and detailed below by lymphoma sub-type. Descriptive analyses were performed on the combined studies by histologic subgroup.
We identified 30 patients with PHL within our institutional cohort and 192 patients from comprehensive literature review (Table 1). Of our institutional PHL cohort, the predominant histology types included 15 (50%) patients with DLBCL, 5 (17%) MZL, 3 (10%) FL, 4 (13%) CLL/SLL, 2 (7%) T-cell lymphoma, and 1 (3%) had low-grade B-cell lymphoma, NOS.
Overall demographics, disease characteristics and response are noted in Table 2. Median follow-up was 6 years. Sixteen patients were male (53%), 25 (84%) were Caucasian, 10 (33%) had a history of smoking, 3 (10%) had prior hepatitis C infection and 2 (7%) had prior hepatitis B infection. PHL was mostly identified incidentally (9 patients; 30%), with 8 (27%) presenting with abdominal pain and 6 (20%) presenting with weight loss. Other presenting symptoms included fatigue (4 patients; 13%), jaundice (3 patients; 10%), diarrhea (3 patients; 10%), and nausea/vomiting (3 patients; 10%). At the time of diagnosis, median LDH was 261 (range 127-2116), median hemoglobin was 12.9 (range 8.4-15.9), and median platelet count was 231 (range 78-432).
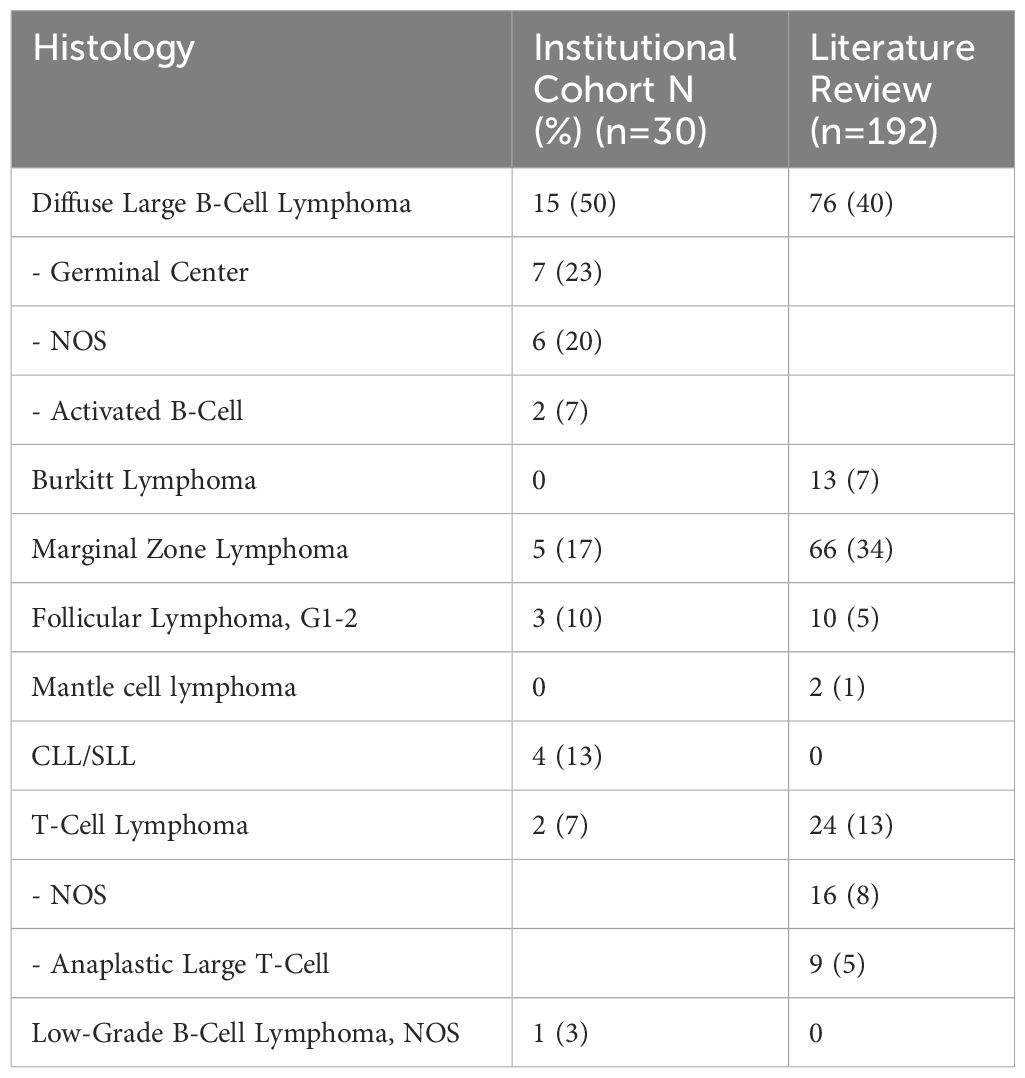
Table 2. Primary hepatic lymphomas by subtype among institutional cohort and literature review cases.
Most patients had an ECOG performance status of 0 (25 patients; 83%), and Lugano Stage I (25 patients; 83%), with 3 patients (10%) with Stage III, and 2 (7%) with Stage IV disease. Six (20%) patients had bulky disease measuring ≥7.5cm and 3 (10%) presented with nodal involvement. One (3%) patient had concurrent splenic disease. Systemic therapy was the most common treatment modality overall (20 patients; 67%) with surgery, radiation and observation utilized in 4 patients (13%) each. Seventeen (57%) of patients were alive at the time of data collection, with 8 (27%) deceased and 5 (17%) lost to follow-up. Cause of death for the 8 patients included 5 disease-related deaths and 3 unrelated to PHL. The 5 disease-related deaths included deaths from infection while on immunosuppression after transplant, 1 from infection while receiving systemic therapy, 1 from liver failure, and 1 from widespread progression of disease. The 3 patients with deaths unrelated to PHL included 2 deaths from complications of other unrelated medical comorbidities and 1 patient with no evidence of disease 12 years after last cancer treatment. On univariable analysis, only age (p=0.009; 95% CI 0.030, 0.190) and ECOG score (p=0.048; 95% CI 0.616, 1.000) were found to be significantly associated with overall survival. No variables were found to be significant on multivariable analysis.
In the paired literature review, 96 patients (50%) received systemic therapy, 69 (36%) underwent surgery, 10 (5%) received radiation and 11 (6%) underwent observation (Table 2). Seventeen (57%) of patients were alive and 5 (17%) lost to follow-up within our institutional cohort, compared with 97 alive patients (51%) and 3 (2%) lost to follow-up in the paired literature review.
We performed a subgroup analysis of DLBCL patients (n=15; Table 3), including 7 (44%) with Germinal Center B-cell lymphoma (GCB), 2 (13%) with Activated B-cell Lymphoma (ABC), and 6 (38%) with NOS. Patients were predominantly male (63%) and white (81%). Patients had overall good performance status with 73% of patients with ECOG 0 and 20% of patients with ECOG 1. Lymphomas were generally early stage (73% with Lugano Stage I) including 38% of patients with bulky disease. Almost all patients received systemic therapy at some point (93%) and one patient was observed (7%). Two patients underwent surgery (13%) and none received radiotherapy at any point. ECOG score and Lugano stage were associated with OS on UVA but did not remain significant on MVA.
Six DLBCL patients with available targeted sequencing data were identified at our institution, with 4 mutations mutated more than once: TP53 (50%), MEF2B (33%), P2RY8 (33%), and IRF8 (33%). Pathway mapping of genetic mutations was notable for alterations in cell cycle pathway genes TP53 and ATM (17%). No other pathways had more than one genetic alteration.
Subgroup analysis of the indolent lymphoma group included a total of 9 patients total, including 5 (56%) with MZL, 3 with FL, and 1 with low-grade BCL, NOS (Table 4). Among them, 4 were male, 8 were of white ethnicity, and the mean age was 67 years (range 42-77). All patients were ECOG 0, 89% had Lugano Stage I disease and no patients had bulky disease. Initial treatment modalities were varied including 2 patients each (22%) who received radiation therapy, systemic therapy, surgery or observation (Figure 1). Both patients who underwent radiotherapy monotherapy received a low-dose regimen of 4Gy in 2 fractions with complete response and no evidence of disease at 15 and 18 months respectively. Three patients were observed (33%). No patients received combined chemotherapy and radiation as first-line treatment, however one patient received combined chemoradiotherapy as a second-line treatment for lung recurrence. Complete response was seen in 6 patients, progression in 2 patients, and 1 patient was lost to follow-up. Both patients who progressed were initially treated with systemic therapy. Of those, one patient was treated with 6 cycles of R-CHOP with CR, followed by transformation to DLBCL 22 months later for which they received systemic therapy. The other patient was initially treated with rituximab with short-interval recurrence 4 months later for which they received 2 additional systemic therapy regimens as well as radiotherapy to the lungs bilaterally (Figure 1).
Median survival in our cohort was 6 years, compared with 2 years among the literature review cohort.
To build upon our institutional experience, we conducted a literature review to contextualize our experience within the broader literature. Existing literature consists largely of small patient cohorts or individual patients with heterogenous treatment approaches based on primary histology. Below we describe the basic demographics, clinical presentations, treatment approaches and outcomes, when available, categorized by lymphoma histology.
DLBCL is the predominant histology observed among primary hepatic lymphomas (6, 10). We found 75 cases of primary hepatic DLBCL in 64 studies, with a predominance among male gender (male to female ratio: 1.27), and an mean age of 60.6 years (range 19-82).
Some patients had concomitant cancers including esophageal stromal tumor (11), tumor forming pancreatitis (12) and HCC with gastric adenocarcinoma (13). One patient had a synchronous DLBCL lymphoma in the colon (14). Viral infections such as HCV (13, 15–24), HBV (11, 15, 18, 25–28), HIV (29) and EBV (28, 30, 31) were the most common predisposing factors. Other factors such as auto-immune and hepatic disease were also observed in some cases, including alcoholic cirrhosis (32), primary biliary cirrhosis (33), recurrent hepatic IPT (12), Sjogrens syndrome (33–35), sarcoidosis (36), and polymyositis (30, 37). Five patients received methotrexate for treatment of polyarthritis (27, 28, 36, 38, 39). One patient was noted to have a history of IV drug use (19).
The main clinical presentation varied among patients. Many reported GI symptoms such as right upper quadrant pain (14, 18, 19, 26, 28, 29, 34, 36–38, 40–56), abdominal discomfort (57–60), jaundice (40, 41, 47, 57, 61–63), melena (14), and left upper quadrant pain (64).
Nineteen patients presented with fever (11, 21, 30, 36–40, 46, 48, 50, 51, 53, 63, 65–68), 11 with weight loss (11, 14, 18, 19, 39, 43, 46, 56, 59, 62, 69), one with night sweats (34), and only 4 cases had all B symptoms (29, 50, 53, 60). Other systemic symptoms such as loss of appetite (70) and altered mental status (66, 67) were present. The hepatic mass was incidentally found on imaging for routine follow-up of a chronic disease in 10 cases (13, 17, 23–25, 27, 31, 32, 50, 71), and, in one case, during a workup of a pancreatic mass (12).
On physical examination, one study described a right upper quadrant mass bulge in 7 patients (15) and 2 cases were found to have hepatomegaly (61, 65). One patient presented with generalized edema (72). In 2 studies, patients were diagnosed after presenting with abnormal laboratory results (elevated LFTs (67) and cytopenia (30)).
In 2013, Kashimura M. et al. reported 2 cases where the diagnosis was made on autopsy. Both cases presented initially with fever and elevated LFTs with normal imaging: One was treated with corticosteroids for hypercalcemia, and the other with antibiotics for cholecystitis and peritonitis (67).
First-line treatment strategies were heterogeneous but were mainly chemotherapy-based. Few patients underwent partial hepatectomy alone (11, 15, 43, 52, 73). Methotrexate was discontinued in patients who were receiving it as the only intervention (28, 30), or as adjunct to other treatment such as surgery (27), R-THP-COP (rituximab, pirarubicin, cyclophosphamide, vincristine, and prednisolone) (38), R-CHOP (39), and one month of prednisolone (36).
CHOP regimen was used alone (14, 22), or preceded by 1 cycle of BACOP (bleomycin, driamycin, cyclophosphamide, vincristine, and prednisone) (17) or CEOP (cyclophosphamide, etoposide, vincristine, and prednisone) (23), or in combination with partial hepatectomy (20, 46, 53), or radiation therapy (one patient received 30.6Gy/Fr (65), another received 5x1.8Gy per week with 18Mv IMRT for a total dose of 31Gy (31)). The most commonly used chemotherapy regimen was R-CHOP (13, 15, 25–27, 30, 33, 35, 46, 48, 51, 58, 60, 63, 65). In some cases, it was given prior to resection, without{sp}69 {/sp}or with radiofrequency ablation (44) or radiation therapy (19) (1.5Gy in 4 fractions using a 2 field 3D conformal technique with 10MV photon beam therapy on day 1, 2, 9 and 12). In other instances, it was combined with partial hepatectomy (18, 48, 49), or as part of other regimens. One patient received R-CHOP and was maintained on cyclophosphamide alone for 1 year (58), while another patient received 1 cycle of R-CHOP followed by 5 cycles of R-MegaCHOP and intrathecal chemotherapy (56). Three patients were treated with R-THP-COP alone (55, 61), or with surgery (13). One patient underwent partial hepatectomy followed by COP therapy (60), another received rituximab with bendamustine (70), and one patient was treated with cyclophosphamide and methylprednisolone (59). Only one patient underwent autologous stem cell transplant, for which they received induction COP, R-CHOP then MCE (ranimustine, cyclophosphamide, etoposide) (71).
Six patients were not treated for several reasons: being clinically unstable (33, 68), lost to follow-up (41), patient refusal due to pregnancy (42, 72), and premature death (37).
Patients were followed for an average of 24 months (range 1-80, median: 22). Death occurred in three patients from aplasia and sepsis after R-CHOP (47, 50) or cyclophosphamide and methylprednisone (59). Partial regression was noted in one of the patients who received R-THP-COP (61), and in one of the methotrexate withdrawal group treated with corticoids alone, who experienced complete remission (CR) after R-CHOP regimen (36). An absence of response with tumor progression was reported in one patient treated with R-CHOP alone. However, the patient maintained complete remission for 3 years (25) after receiving second-line R-HyperCVAD (rituximab, cyclophosphamide, vincristine, doxorubicin, methotrexate, cytarabine and dexamethasone)/R-HD MTX ara-C regimen (high dose methotrexate and cytarabine) with radiation therapy (40Gy for 28 fractions). Tumor recurrence was noted in 3 cases. The first patient received R-CHOP and suffered multiple organ failure (45), the second one received autologous stem cell transplant followed by allogeneic bone marrow transplant second-line (71), and the third patient received rituximab and bendamustine and had lymph node involvement. This patient died after receiving R-miniCHOP (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone) and 2 cycles of R-EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin) (70). Some articles did not provide information regarding outcomes (15, 18, 52, 64, 73). The remainder of the described cases were associated with CR (Table 5).
Primary hepatic follicular lymphoma is very rare and limited to only 10 cases cited in 9 papers. Patients included 6 women and 4 men and were of advanced age averaging 65.7 years (range 52-85).
Symptoms upon presentation included right upper quadrant pain (74, 75), jaundice (76) or liver failure (77), and elevated LFTs (78). Tumors were incidentally diagnosed on imaging during a follow up for hepatitis C or EBV (79–81), or a work-up for prostate cancer (82). As for comorbidities, some patients had a history of hepatitis B (78) or C (79, 80), EBV (77, 81), Crohn disease (80), and alcoholic cirrhosis (77).
Almost all patients received systemic therapy. Radiation therapy was not used in any patient. Death occurred in four patients. One of them received rituximab (80), the other one received rituximab combined with melfalan (77), and no details were found for the other 2 cases (76). Complete remission was reported in the remaining cases. Among them, one patient received R-CHOP regimen (75), and four patients underwent partial hepatectomy, alone (79) or with chemotherapy (CHOP regimen (78), rituximab – fludarabine –mitoxantone (81)) or with R-CHOP and microwave ablation (74). The average follow-up was 21.5 months (range 1-48, median: 18).
Primary hepatic MALT lymphoma is a rare subtype with only 53 papers found and around 70 cases reported in the literature. In fact, according to a retrospective study conducted in 2003 on 180 extragastric MALT lymphomas from 20 institutions, only 3% had a primary located in the liver (83).
In our literature review, there was no gender predominance noted, and the average age at diagnosis was 60 years (range 30-89). The most common forms of presentation included incidental findings from unrelated complaints (84–92), on routine imaging for chronic hepatic disease follow-up (93–110), or during liver transplant evaluation (111–113). Very few patients presented with abdominal pain (unspecified (110, 114, 115), epigastric (116, 117), or in the right upper quadrant (118–120)), nausea (99), or weight loss (99, 110). Some patients had abnormal findings on physical examination (hepatomegaly (115, 120, 121), or ascites (122)), or elevated LFTs (110, 123–127). In a few cases, concomitant hepato-cellular carcinoma (93, 120) was found. In other cases, prior malignancies were present (gastric carcinoma (89, 91), colonic carcinoma (107), hepatic tumor (119), infiltrating duct breast and thyroid papillary carcinoma (96)). We also noted one case with a previously treated gastric MALT lymphoma (117). Among all the cases, associated diseases were present in the majority of the papers, with the main co-factors being chronic hepatitis B (84, 93, 95, 97, 98, 100, 101, 105, 108, 110, 113, 116, 119, 120, 128), chronic hepatitis C (94, 102, 104, 106, 110, 112, 114, 118, 126, 129, 130), and chronic H.pylori gastritis (85, 91, 94, 102, 110, 116, 117, 123, 124, 131). A single case of hepatitis A infection was published in 2002 (89). Six cases of secondary cirrhosis (103, 109, 112, 113, 126, 132) and four cases of primary biliary cholangitis (111, 122, 125, 133) were cited. We found a limited number of cases with other auto-immune or hepatic diseases such as rheumatoid polyarthritis (98), auto-immune hepatitis (99, 109), Castleman syndrome (125), hemochromatosis (103), non-alcoholic steatohepatitis (124), and Buerger disease (85).
While all reported cases were classified as stage IE Ann-Arbor except for 2 cases, which were stage II (87) and IV (110), treatment modalities differed. Mistaken at first for an hepatocellular carcinoma, almost half of the patients underwent partial hepatectomy or tumor resection, alone or with H.pylori eradication (123), hepatitis C treatment (118) or chemotherapy with regimens such as CHOP (105), Rituximab (124), R-CHOP (86, 101, 102, 131), cladribine (131), and bendamustine with Rituximab (126). Patients with advanced cirrhosis who were eligible for liver transplant underwent total hepatectomy (109, 111–113, 132).
In two cases, a combination of antibiotics for H.pylori eradication and R-CHOP was used (94, 116). Chemotherapy alone (110) was the treatment of choice in some patients, with regimens including Rituximab (88, 99), R-CHOP (95, 119, 127, 131), R-CVP (110), R-THP-COP (122) and Etoposide (133).
Two patients were treated with radiation therapy (110, 121), with one receiving a total dose of 41.4 Gy (19.8 Gy to the entire liver and an additional 21.6 Gy to the tumor (121). One patient received Rituximab and underwent percutaneous radiofrequency ablation (RFA) (134).
Four patients did not receive treatment due to patient refusal or physician’s preference. The first case showed progression after 12 months following Rituximab (99), the second progressed after 32 months (100), the third died from sepsis one month later (117), and there was no mention of follow-up or outcome for the fourth case (114).
As for the two cases with advanced disease, both achieved CR after the first treatment, 6 cycles of chemotherapy for stage IVA lymphoma (110) and partial hepatectomy for the stage II patient (87).
Patients were followed for an average of 23 months (range 1-96) after completion of first-line treatment with a median follow-up of 16 months. Almost all patients achieved CR after first-line treatment. Two deaths were caused by surgical complications (132), and one death occurred due to fulminant hepatitis B and D 3 months after R-CHOP treatment (127).
Recurrence of disease was reported in the following cases: 51 months after a treatment with cladribine followed by R-CHOP{sp}131 {/sp}as a second line, 3 years after R-CVP plus 40 Gy radiation therapy (110), 18 months after R-THP-COP and Rituximab as salvage therapy (122), 14 months after resection followed by Rituximab for 1 month (128), and 30 months after partial hepatectomy with radiation therapy and chemotherapy (98).
Two patients were lost to follow-up (103, 132), and approximately half of the studies did not mention follow-up duration of the patients (Table 6).
Only thirteen cases were found in the literature. Half of the patients were females, and the majority were less than 60 years of age except for one 75-year-old patient (mean age 38.5 years). The patients had past medical history of viral infections [EBV (135, 136), HCV (136, 137), HBV (138–140), HIV (135, 140–143)], and one had a history of kidney transplant (136).
The chief complaints were mainly gastrointestinal such as weakness with vomiting and diarrhea (136), nausea (144, 145), abdominal mass (145), and jaundice (140, 142). Abdominal discomfort and pain (135, 139–141, 144, 146, 147), and hepatomegaly (135, 136, 139, 142, 144, 146, 147) were among the most common presentations. B symptoms such as fever (141, 142, 146), night sweats (140), and weight loss (145, 147) were reported in a few patients.
As for treatment and outcomes, two patients underwent surgery with chemotherapy (138) based on hyper-CVAD with methotrexate-Ara-C THP and Rituximab. Death from septic shock was reported in 3 patients who received chemotherapy (141, 143, 145), including a patient who received cyclophosphamide-prednisone-methotrexate-dexamethasone (136). The other cases were treated with regimens including the m-BACOD protocol (methotrexate, leucovorin, bleomycin, doxorubicin, cyclophosphamide, vincristine, and dexamethasone) resulting in tumor lysis syndrome (142), rituximab with cytarabine and dexamethasone with complete remission (135), CHOP with methotrexate and intrathecal cytosine arabinoside and methotrexate with cure (139), and LMB-96 protocol (three doses of high-dose methotrexate, 8 g/m2/dose, and eight doses of HD cytarabine, 3 g/m2/dose) with remission (144). One patient refused treatment and was lost to follow-up (140).
Two cases were found in the literature.
The first paper described a 54-year-old man, known to have a history of HBV infection, who presented with weight loss, abdominal distention and hepatomegaly. After treatment with chlorambucil, partial remission was reported with a follow-up of 47 months (148). The second article reported the detailed clinical and pathological presentation of a primary hepatic lymphoma in a 73-year-old woman. Therapies used were not specified for this patient (149).
Sixteen cases on T-cell hepatic lymphomas were found in the literature. Patients included three women and 13 men with a mean age of 48 years (range 30 to 71 years). Chief complaints were either related to liver disease such as jaundice (150, 151), hepatomegaly (151–153), right upper quadrant pain (150, 154, 155) or systemic symptoms such as fatigue (156), edema (151), B symptoms (150, 152, 154, 155, 157, 158). Two patients presented with cytopenia (155, 159) and one patient with toxic hepatitis (158). History of viral infection such as EBV (150, 157), HAV (155), HBV (158, 160), and HCV{sp}153 {/sp}were noted in some cases. One paper cited two patients suffering from chronic alcoholism and chronic drug use (155). Two patients had cirrhosis secondary to hepatitis (153, 160), and one patient suffered from systemic lupus erythematosus (159).
The primary hepatic T-cell lymphomas were almost all treated with chemotherapy based on the CHOP regimen, either alone (151, 155, 158, 160) or adjuvant therapy after surgery (156, 161) coupled with radiation therapy (154, 159) (45 Gy in 15 fractions) (154) or with another chemotherapy regimen (DHAP) (155). One patient received only 20 mg of oral prednisone as short course therapy followed by 5 mg as maintenance (152). Most patients achieved complete remission except for two. One of them progressed after surgery and then treatment was withdrawn (156), and the other received ECHAP as a second-line treatment then died (158). As for the rest of the cases, all ended in death of the patient including esophageal hemorrhage following radiation and chemotherapy in one patient (159), and two patients who received supportive therapy only and died a few days after diagnosis (155, 158). One patient underwent partial hepatectomy and died from post-operative complications (153). Average follow-up was 23 months, with a maximum of 7 years (median: 12 months).
Nine cases were found in the literature. Patients included two women and seven men with a mean age of 47 years (range 33-62). Five of the patients had underlying liver disease associated with the following conditions: HIV (162), HIV and HCV (163), HBV and chronic alcoholism (164), celiac disease (165), hereditary hemochromatosis and cirrhosis (166).
The chief complaints included RUQ pain (148, 162, 163, 165–167) or one of the B symptoms such as fever (148, 162–165), weight loss (148, 162–166) or night sweats (166–168). Complete remission after 20 months of follow-up was noted in one patient who underwent hepatectomy followed by 4 cycles of CHOP (165). Partial remission was reported in another patient after 6 cycles of CHOP and etoposide (167). Diagnosis was made on autopsy (163, 164)in two patients who died within a few days of symptom onset. In one patient who experienced hepatic failure, death occurred after a treatment of 60mg of corticosteroid per day (166). Two patients underwent resection of the tumor followed by chemotherapy. In the first case, death occurred 15 months after 6 cycles of CHOP (168). The second patient showed progression after receiving IMVP16 (ifosfamide, methotrexate, and VP-16) and CHOP and autologous peripheral blood stem cell transplantation after induction with ESHAP (etoposide, methylprednisolone, high-dose cytarabine and cisplatin) and CCEP (cyclophosphamide, lomustine, etoposide and prednisolone). Death was reported 3.5 years after surgery (148). Therapies used were not specified in two papers (162, 169).
PHL are a rare subtype of NHL without clear treatment consensus. The treatment approach is driven by the treatment paradigms of the primary lymphoma tumor histology; however, our histology-based literature review demonstrates that treatment approaches are heterogenous and appropriately guided by tumor histology.
DLBCL is the most common subtype of GI lymphomas and is predominantly treated with chemotherapy in both our institutional experience as well as reported literature, with reasonable disease control. This is consistent with the established treatment paradigm for DLBCL due to its more aggressive and diffuse nature (170).
The approach to treatment for indolent PHL is less well established, with various modalities employed in both our institutional cohort as well as the literature review. Although there are limited case reports regarding the use of standard doses of radiotherapy of 30-41.4 Gy for PHL, to our knowledge this is the first case series reporting low dose radiotherapy for the treatment of PHL. Low-dose radiotherapy has been shown to have good local control in select patients with indolent lymphomas (2). At our institution, we use an adaptive approach when treating patients with indolent lymphomas. Appropriate patients are treated with low dose radiotherapy upfront of 4Gy. The option to receive additional radiation is based on PET-guided response assessment 3 months post-RT. Although our cohort data is limited by short follow-up duration, and a very limited number of the patients receiving RT, the data is concordant with local control outcomes of other indolent lymphoma sites.
Indolent lymphomas are slow growing and can often be observed for many months or years before requiring intervention. In our cohort, 33% of patients required intervention beyond observation whereas literature review revealed that all 8 cases of indolent lymphomas were treated with systemic therapy, and none were observed or treated with local therapies such as surgery or radiotherapy. Therefore, our institutional experience is encouraging for the consideration of low dose radiotherapy following an adaptive approach in select patients for effective local control and minimal toxicity, as an alternative to observation, surgery, or systemic therapy.
PHL are an extremely rare subtype of NHL for which there is no clear treatment consensus. Primary hepatic DLBCL appears to be treated mostly with chemotherapy with good disease control. For indolent PHL, low-dose RT appears to have good overall disease control with minimal toxicity. Our results are encouraging for the use of RT for appropriate patients with indolent PHL, using the adaptive approach of 400 cGy in 1-2 fractions, with the option to receive an additional 20Gy based on PET-guided response assessment 3 months post-RT. Despite the limited number of patients reported in our institutional experience, these promising results should prompt more extensive studies on the use of low dose radiation therapy in indolent PHL.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Department of Radiation Oncology, MSKCC, New York. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
JM: Writing – original draft, Writing – review & editing, Data curation, Validation. RD: Writing – review & editing, Validation, Writing – original draft. JB: Writing – original draft, Writing – review & editing, Resources, Validation. BF: Data curation, Writing – review & editing. JE-K: Validation, Writing – review & editing. NW: Validation, Writing – review & editing. BI: Validation, Writing – review & editing. JY: Validation, Writing – review & editing. CH: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing.
The author(s) declare that no financial support was received for the research and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Noronha V, Shafi NQ, Obando JA, Kummar S. Primary non-Hodgkin’s lymphoma of the liver. Crit Rev Oncol Hematol. (2005) 53:199–207. doi: 10.1016/j.critrevonc.2004.10.010
2. Bautista-Quach MA, Ake CD, Chen M, Wang J. Gastrointestinal lymphomas: Morphology, immunophenotype and molecular features. J Gastrointest Oncol. (2012) 3:209–25. doi: 10.3978/j.issn.2078-6891.2012.024
3. Qiu MJ, Fang XF, Huang ZZ, Li QT, Wang MM, Jiang X, et al. Prognosis of primary hepatic lymphoma: A US population-based analysis. Transl Oncol. (2020) 14:100931. doi: 10.1016/j.tranon.2020.100931
4. El-Fattah MA. Non-hodgkin lymphoma of the liver: A US population-based analysis. J Clin Transl Hepatol. (2017) 5:83–91. doi: 10.14218/JCTH.2017.00015
5. Ugurluer G, Miller RC, Li Y, Thariat J, Ghadjar P, Schick U, et al. Primary hepatic lymphoma: A retrospective, multicenter rare cancer network study. Rare Tumors. (2016) 8(3):6502. doi: 10.4081/rt.2016.6502
6. Zhao Q, Liu H, Gu Y, Cong W. Clinicopathological and survival features of primary hepatic lymphoma: an analysis of 35 cases. Zhonghua Zhong Liu Za Zhi. (2013) 35:689–92.
7. Imber BS, Chau KW, Lee J, Lee J, Casey DL, Yang JC, et al. Excellent response to very-low-dose radiation (4 Gy) for indolent B-cell lymphomas: is 4 Gy suitable for curable patients? Blood Adv. (2021) 5:4185–97. doi: 10.1182/bloodadvances.2021004939
8. Cheng DT, Mitchell TN, Zehir A, Shah RH, Benayed R, Syed A, et al. Memorial sloan kettering-integrated mutation profiling of actionable cancer targets (MSK-IMPACT): A hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. (2015) 17:251–64. doi: 10.1016/j.jmoldx.2014.12.006
9. Zehir A, Benayed R, Shah RH, Syed A, Middha S, Kim HR, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. (2017) 23:703–13. doi: 10.1038/nm.4333
10. Kikuma K, Watanabe J, Oshiro Y, Shimogama T, Honda Y, Okamura S, et al. Etiological factors in primary hepatic B-cell lymphoma. Virchows Arch. (2012) 460:379–87. doi: 10.1007/s00428-012-1199-x
11. Pan B, Wang CS, Han JK, Zhan LF, Ni M, Xu SC. 18F-fluorodeoxyglucose PET/CT findings of a solitary primary hepatic lymphoma: a case report. World J Gastroenterol. (2012) 18:7409–12. doi: 10.3748/wjg.v18.i48.7409
12. Kaneko R, Mitomi H, Nakazaki N, Yano Y, Ogawa M, Sato Y. Primary hepatic lymphoma complicated by a hepatic inflammatory pseudotumor and tumor-forming pancreatitis. J Gastrointestin Liver Dis. (2017) 26:299–304. doi: 10.15403/jgld.2014.1121.263.eko
13. Tajiri H, Sugimachi K, Kinjo N, Ikebe M, Tanaka J, Tanaka K, et al. Repeat hepatectomies for hepatic Malignant lymphoma and hepatocellular carcinoma associated with chronic hepatitis C: report of a case. Surg Today. (2014) 44:188–91. doi: 10.1007/s00595-013-0502-z
14. Peng J-X, Wang LZ, Tan ZJ, Zhong XS, Huang YX, Diao JF, et al. Concomitant non-Hodgkin’s lymphoma in colon and liver: report of a rare case and review of literature. Int J Clin Exp Pathol. (2015) 8:3257–61.
15. Zheng J, Hou Y, Zhou R, Zhong D. Clinicopathological features of primary hepatic diffuse large B-cell lymphoma: a report of seven cases and a literature review. Int J Clin Exp Pathol. (2015) 8:12955–60.
16. Condat B, Bonnet J, Hauuy MP, Collot V, Charlier A, Dugue L, et al. Hepatitis C virus infection and primary hepatic large B-cell lymphoma: a non-fortuitous association. Case report and review of literature. Gastroenterol Clin Biol. (2003) 27:1157–9.
17. Rubbia-Brandt L, Bründler MA, Kerl K, Negro F, Nador RG, Scherrer A, et al. Primary hepatic diffuse large B-cell lymphoma in a patient with chronic hepatitis C. Am J Surg Pathol. (1999) 23:1124–30. doi: 10.1097/00000478-199909000-00017
18. Somaglino C, Pramaggiore P, Polastri R. Primary hepatic lymphoma in a patient with chronic hepatitis B and C infection: diagnostic pitfalls and therapeutic challenge. Updates Surg. (2014) 66:89–90. doi: 10.1007/s13304-013-0208-1
19. Tammana VS, Begum R, Oneal P, Karpurapu H, Muley A, Yeruva SL, et al. A novel use of early radiation therapy in the treatment of hyperbilirubinemia in a patient with primary hepatic lymphoma and chronic hepatitis C. Case Rep Gastrointest Med. (2014) 2014:724256. doi: 10.1155/2014/724256
20. Kitabayashi K, Hasegawa T, Ueno K, Saito H, Kosaka T, Takashima S, et al. Primary hepatic non-Hodgkin’s lymphoma in a patient with chronic hepatitis C: report of a case. Surg Today. (2004) 34:366–9. doi: 10.1007/s00595-003-2703-3
21. Kaneko F, Yokomori H, Sato A, Takeuchi H, Tahara K, Sekiguchi Y, et al. A case of primary hepatic non-Hodgkin’s lymphoma with chronic hepatitis C. Med Mol Morphol. (2008) 41:171–4. doi: 10.1007/s00795-008-0410-2
22. Iannitto E, Ammatuna E, Tripodo C, Marino C, Calvaruso G, Florena AM, et al. Long-lasting remission of primary hepatic lymphoma and hepatitis C virus infection achieved by the alpha-interferon treatment. Hematol J. (2004) 5:530–3. doi: 10.1038/sj.thj.6200408
23. Chen H-W, Sheu J-C, Lin W-C, Tsang Y-M, Liu K-L. Primary liver lymphoma in a patient with chronic hepatitis C. J Formos Med Assoc. (2006) 105:242–6. doi: 10.1016/S0929-6646(09)60313-2
24. De Renzo A, Perna F, Persico M, Mainolfi C, Pace L. 18F-fluorodeoxyglucose positron emission tomography/computed tomography in the evaluation of early response in a primary hepatic lymphoma. Br J Haematol. (2006) 133:580. doi: 10.1111/j.1365-2141.2006.06115.x
25. Zhang KJ, Chen S, Chen JL, Dong LH. Complete response to comprehensive treatment of a primary hepatic diffuse large B cell lymphoma: A case report. Oncol Lett. (2015) 9:1557–60. doi: 10.3892/ol.2015.2920
26. Yu J-J, Yan W-T, Li C, Yang T. An unusual primary hepatic lymphoma. Dig Liver Dis. (2018) 50:855. doi: 10.1016/j.dld.2018.02.016
27. Takei D, Abe T, Amano H, Hirano N, Kobayashi T, Ohdan H, et al. Methotrexate-associated primary hepatic Malignant lymphoma following hepatectomy: A case report. Int J Surg Case Rep. (2017) 31:5–9. doi: 10.1016/j.ijscr.2016.12.012
28. Matsumoto R, Numata K, Doba N, Hara K, Chuma M, Fukuda H, et al. A case of multiple hepatic lesions associated with methotrexate-associated lymphoproliferative disorder. J Med Ultrason (2001). (2016) 43:545–51. doi: 10.1007/s10396-016-0740-y
29. Widjaja D, AlShelleh M, Daniel M, Skaradinskiy Y. Complete remission of primary hepatic lymphoma in a patient with human immunodeficiency virus. World J Clin cases. (2015) 3:186–90. doi: 10.12998/wjcc.v3.i2.186
30. Tsuji H, Yoshifuji H, Shindo T, Hishizawa M, Ishida A, Fujimoto M, et al. Primary hepatic lymphoma as other iatrogenic immunodeficiency-related lymphoproliferative disorders: a case report and review of the literature. Mod Rheumatol Case Rep. (2021) 5:172–7. doi: 10.1080/24725625.2020.1826627
31. Nasr Ben Ammar C, Chaari N, Kochbati L, Besbes M, Maalej M. Primary non-Hodgkin lymphoma of the liver: case report and review of the literature. Cancer Radiother. (2006) 10:595–601. doi: 10.1016/j.canrad.2006.09.117
32. Dantas E, Santos J, Coelho M, Sequeira C, Santos I, Cardoso C, et al. Primary hepatic lymphoma in a patient with cirrhosis: a case report. J Med Case Rep. (2020) 14:168. doi: 10.1186/s13256-020-02471-0
33. Sato S, Masuda T, Oikawa H, Satoh T, Suzuki Y, Takikawa Y, et al. Primary hepatic lymphoma associated with primary biliary cirrhosis. Am J Gastroenterol. (1999) 94:1669–73. doi: 10.1111/j.1572-0241.1999.01160.x
34. Gorodetskiy V, Klapper W, Probatova N, Vasilyev V. Primary diffuse large B-cell lymphoma of the liver in a patient with sjogren syndrome. Case Rep Oncol Med. (2016) 2016:2053257. doi: 10.1155/2016/2053257
35. Gorodetskiy VR, Probatova NA, Vasilyev VI, Vardaev LI, Ipatkin RV, Gabunia ZR, et al. Primary hepatic lymphoma in a female patient with Sjögren’s disease: A case report and literature review. Ter Arkh. (2015) 87:90–4. doi: 10.17116/terarkh201587590-94
36. Kawahara A, Tsukada J, Yamaguchi T, Katsuragi T, Higashi T. Reversible methotrexate-associated lymphoma of the liver in rheumatoid arthritis: a unique case of primary hepatic lymphoma. biomark Res. (2015) 3:10. doi: 10.1186/s40364-015-0035-2
37. Mlika M, Zidi-Moaffak Y, Farah F, Kourda N, Cherif E, Baltagi-Ben Jilani S, et al. An exceptional hepatic tumor. Tunis Med. (2010) 88:954–6.
38. Tatsumi G, Ukyo N, Hirata H, Tsudo M. Primary hepatic lymphoma in a patient with rheumatoid arthritis treated with methotrexate. Case Rep Hematol. (2014) 2014:460574. doi: 10.1155/2014/460574
39. Miyagawa K, Shibata M, Noguchi H, Hayashi T, Oe S, Hiura M, et al. Methotrexate-related primary hepatic lymphoma in a patient with rheumatoid arthritis. Intern Med. (2015) 54:401–5. doi: 10.2169/internalmedicine.54.3361
40. El Nouwar R, El Murr T. Primary hepatic diffuse large B-cell lymphoma mimicking acute fulminant hepatitis: A case report and review of the literature. Eur J Case Rep Intern Med. (2018) 5:000878. doi: 10.12890/2018_000878
41. Dhingra R, Winter MW, Yilmaz OH, Jaiswal S, Sterling M. An unusual presentation of primary hepatic diffuse large B-cell lymphoma of the liver. Cureus. (2018) 10:e2242. doi: 10.7759/cureus.2242
42. Dhamija E, Madhusudhan KS, Shalimar Das P, Srivastava DN, Gupta AK. Primary hepatic diffuse large B-cell lymphoma: unusual presentation and imaging features. Curr Probl Diagn Radiol. (2015) 44:290–3. doi: 10.1067/j.cpradiol.2014.12.002
43. Mehta N, Jayapal L, Goneppanavar M, Nelamangala Ramakrishnaiah VP. Primary hepatic lymphoma: A rare case report. JGH Open. (2019) 3:261–3. doi: 10.1002/jgh3.12131
44. Wang L, Dong P, Hu W, Tian B. 18F-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography in the diagnosis and follow-up of primary hepatic diffuse large B-cell Lymphoma: A clinical case report. Med (Baltimore). (2020) 99:e18980. doi: 10.1097/MD.0000000000018980
45. Myoteri D, Dellaportas D, Arkoumani E, Marinis A, Zizi-Sermpetzoglou A. Primary hepatic lymphoma: a challenging diagnosis. Case Rep Oncol Med. (2014) 2014:212598. doi: 10.1155/2014/212598
46. Zentar A, Tarchouli M, Elkaoui H, Belhamidi MS, Ratbi MB, Bouchentouf SM, et al. Primary hepatic lymphoma. J Gastrointest Cancer. (2014) 45:380–2. doi: 10.1007/s12029-013-9505-7
47. Ma Y-J, Chen E-Q, Chen X-B, Wang J, Tang H. Primary hepatic diffuse large B cell lymphoma: A case report: Primary hepatic diffuse large B cell lymphoma. Hepat Mon. (2011) 11:203–5.
48. Oshita K, Itamoto T, Oshita A, Nakahara H, Nishisaka T. A rare case of a spontaneously ruptured secondary hepatic Malignant lymphoma. Surg Case Rep. (2018) 4:44. doi: 10.1186/s40792-018-0451-2
49. Abdelrahim WE, Mohamed KE, Mekki SO, Saad EA. Primary hepatic lymphoma presenting as an acute abdomen in a young female patient: A case report and literature review. Case Rep Surg. (2019) 2019:6784325. doi: 10.1155/2019/6784325
50. Mezzano G, Rojas R, Morales C, Gazitúa R, Díaz JC, Brahm J. Primary hepatic lymphoma: An infrequent focal liver tumour. Gastroenterol Hepatol. (2016) 39:674–6. doi: 10.1016/j.gastrohep.2015.09.008
51. Zafar MSH, Aggarwal S, Bhalla S. Complete response to chemotherapy in primary hepatic lymphoma. J Cancer Res Ther. (2012) 8:114–6. doi: 10.4103/0973-1482.95187
52. Valladolid G, Adams LL, Weisenberg E, Maker VK, Maker AV. Primary hepatic lymphoma presenting as an isolated solitary hepatic cyst. J Clin Oncol. (2013) 31:e21–23. doi: 10.1200/JCO.2012.44.9728
53. Eidt S, Nebeling M, Pohl C, Siedek M. Neoadjuvant chemotherapy of primary hepatic non-Hodgkin’s lymphoma. Med Klin (Munich). (2003) 98:96–9. doi: 10.1007/s00063-003-1232-6
54. Kang KM, Chung WC, Lee KM, Hur SE, Nah JM, Kim GH, et al. A case of primary hepatic lymphoma mimicking hepatitis. Korean J Hepatol. (2005) 11:284–8.
55. Takauchi K, Kono H, Ito T, Zaima K, Okumoto S. A case of primary hepatic lymphoma successfully treated by THP-COP therapy. Nihon Ronen Igakkai Zasshi. (2003) 40:65–8. doi: 10.3143/geriatrics.40.65
56. Serrano-Navarro I, Rodríguez-López JF, Navas-Espejo R, Pérez-Jacoiste MA, Martínez-González MA, Grande C, et al. Primary hepatic lymphoma - favorable outcome with chemotherapy plus rituximab. Rev Esp Enferm Dig. (2008) 100:724–8. doi: 10.4321/s1130-01082008001100011
57. Kaneko K, Nishie A, Arima F, Yoshida T, Ono K, Omagari J, et al. A case of diffuse-type primary hepatic lymphoma mimicking diffuse hepatocellular carcinoma. Ann Nucl Med. (2011) 25:303–7. doi: 10.1007/s12149-010-0460-0
58. Park J-I, Jung B-H. Primary hepatic lymphoma treated with liver resection followed by chemotherapy: a case report. Ann Hepatobiliary Pancreat Surg. (2017) 21:163–7. doi: 10.14701/ahbps.2017.21.3.163
59. Balduzzi C, Yantorno M, Mosca I, Apraiz M, Velázquez MJ, Puente Mdel C, et al. Primary hepatic lymphoma: an infrequent cause of focal hepatic lesion. Acta Gastroenterol Latinoam. (2010) 40:361–6.
60. Franchi R, Abbiati C, Signaroldi A, Quadrelli G, Maggioni M, Sbalzarini G. Primary lymphoma of the liver - a case-report and a review of the literature. Oncol Rep. (1995) 2:1017–9. doi: 10.3892/or.2.6.1017
61. Kawakami H, Kubota Y, Ban T. Primary hepatic diffuse large B-cell lymphoma mimicking intrahepatic cholangiocarcinoma. Intern Med. (2019) 58:143–4. doi: 10.2169/internalmedicine.1436-18
62. Laroia ST, Rastogi A, Panda D, Sarin SK. Primary hepatic non-hodgkin’s lymphoma: an enigma beyond the liver, a case report. World J Oncol. (2015) 6:338–44. doi: 10.14740/wjon900w
63. Kheyri Z, Ali Asgari A, Zare Mehrjerdi A, Zamani F, Ajdarkosh H. Fulminant hepatic failure due to primary hepatic lymphoma: a case report. Middle East J Dig Dis. (2013) 5:168–70.
64. Patel SK, Shera IA. A rare tumor with unusual clinical presentation detected by positron emission tomography-computed tomography. Indian J Nucl Med. (2015) 30:331–3. doi: 10.4103/0972-3919.164024
65. Ozaki K, Ikeno H, Koneri K, Higuchi S, Hosono N, Kosaka N, et al. Primary hepatic diffuse large B-cell lymphoma presenting unusual imaging features. Clin J Gastroenterol. (2020) 13:1265–72. doi: 10.1007/s12328-020-01203-7
66. Farag F, Morcus R, Ramachandran P, Pasrija UR, Wang JC. Fever of unknown origin due to primary hepatic diffuse large B-cell lymphoma: A case report. Cureus. (2019) 11:e4220. doi: 10.7759/cureus.4220
67. Kashimura M, Murayama K, Kojima M. Primary hepatic and hepatosplenic diffuse large B-cell lymphomas with intrasinusoidal and interstitial lymphomatous infiltration. Int J Surg Pathol. (2013) 21:531–4. doi: 10.1177/1066896913483897
68. Takeuchi N, Naba K. Primary hepatic lymphoma is difficult to discriminate from a liver abscess. Case Rep Gastrointest Med. (2014) 2014:925307. doi: 10.1155/2014/925307
69. Skulimowski A, Hogendorf P, Poznańska G, Smolewski P, Strzelczyk J, Durczynski A. Successful hemihepatectomy following chemotherapy for primary liver lymphoma: case report and review of literature. Pol Przegl Chir. (2017) 89:54–8. doi: 10.5604/01.3001.0010.5609
70. Liao S-H, Chen YK, Yu SC, Wu MS, Wang HP, Tseng PH. An unusual case of primary hepatic lymphoma with dramatic but unsustained response to bendamustine plus rituximab and literature review. SAGE Open Med Case Rep. (2017) 5:2050313X17709190. doi: 10.1177/2050313X17709190
71. Ishiguro K, Hayashi T, Aoki Y, Maruyama Y, Ikeda H, Ishida T, et al. Allogeneic bone marrow transplantation in a patient with primary hepatic diffuse large B-cell lymphoma that relapsed after autologous stem cell transplantation. Nihon Shokakibyo Gakkai Zasshi. (2014) 111:1798–804.
72. Lee J-A, Jeong WK, Min JH, Kim J. Primary hepatic lymphoma mimicking acute hepatitis. Clin Mol Hepatol. (2013) 19:320–3. doi: 10.3350/cmh.2013.19.3.320
73. Avilés-Salas A, Garduño-López AL, Soberanes-Cerino C, Mondragón-Sánchez R. Primary non-Hodgkin’s lymphoma of the liver. Report of a case and review of the literature. Rev Gastroenterol Mex. (2002) 67:43–6.
74. Xu J, Hu S, Li S, Gao Y, Wang W, Zhou X, et al. Primary hepatic follicular lymphoma: a case report and literature review. Int J Clin Exp Pathol. (2019) 12:3671–4.
75. Gomyo H, Kagami Y, Kato H, Kawase T, Ohshiro A, Oyama T, et al. Primary hepatic follicular lymphoma : a case report and discussion of chemotherapy and favorable outcomes. J Clin Exp Hematop. (2007) 47:73–7. doi: 10.3960/jslrt.47.73
76. Ohsawa M, Aozasa K, Horiuchi K, Kataoka M, Hida J, Shimada H, et al. Malignant lymphoma of the liver. Report of five cases and review of the literature. Dig Dis Sci. (1992) 37:1105–9. doi: 10.1007/BF01300294
77. Williams M-JO, Akhondi H, Khan O. Primary hepatic follicular lymphoma presenting as sub-acute liver failure: A case report and review of the literature. Clin Pathol. (2019) 12:2632010X19829261. doi: 10.1177/2632010X19829261
78. Matano S, Nakamura S, Annen Y, Hattori N, Kiyohara K, Kakuta K, et al. Primary hepatic lymphoma in a patient with chronic hepatitis B. Am J Gastroenterol. (1998) 93:2301–2. doi: 10.1111/j.1572-0241.1998.00648.x
79. Shimagaki T, Maeda T, Kinjo N, Imai D, Wang H, Ohama N, et al. Primary hepatic follicular lymphoma 5 years post sustained virological response from hepatitis C viral infection. J Clin Pathol. (2020) 74(2):e3. doi: 10.1136/jclinpath-2020-206469
80. Scucchi L, Neri B, Argirò R, Nasso D, Provenzano I, Potenza S, et al. Hepatic follicular lymphoma in an old patient with Crohn’s disease: a rare case and review of the literature. Eur Rev Med Pharmacol Sci. (2020) 24:10045–50. doi: 10.26355/eurrev_202010_23219
81. Raimondo L, Ferrara I, Stefano AD, Cella CA, D'Armiento FP, Ciancia G, et al. Primary hepatic lymphoma in a patient with previous rectal adenocarcinoma: a case report and discussion of etiopathogenesis and diagnostic tools. Int J Hematol. (2012) 95:320–3. doi: 10.1007/s12185-012-1025-x
82. Ryan J, Straus DJ, Lange C, Filippa DA, Botet JF, Sanders LM, et al. Primary lymphoma of the liver. Cancer. (1988) 61:370–5. doi: 10.1002/1097-0142(19880115)61:2<370::AID-CNCR2820610228>3.0.CO;2-I
83. Zucca E, Conconi A, Pedrinis E, Cortelazzo S, Motta T, Gospodarowicz MK, et al. Nongastric marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue. Blood. (2003) 101:2489–95. doi: 10.1182/blood-2002-04-1279
84. Xie H, Lv J, Ji Y, Du X, Yang X. Primary hepatic mucosa-associated lymphoid tissue lymphoma: A case report and literature review. Med (Baltimore). (2019) 98:e15034. doi: 10.1097/MD.0000000000015034
85. Koubaa Mahjoub W, Chaumette-Planckaert MT, Murga Penas EM, Dierlamm J, Leroy K, Delfau MH, et al. Primary hepatic lymphoma of mucosa-associated lymphoid tissue type: a case report with cytogenetic study. Int J Surg Pathol. (2008) 16:301–7. doi: 10.1177/1066896907312671
86. Betianu CI, Dima A, Pavaloiu G. Primary hepatic mucosa-associated lymphoid tissue lymphoma in a patient with no chronic liver disease: Case report. Radiol Case Rep. (2017) 12:715–9. doi: 10.1016/j.radcr.2017.08.004
87. Choi S, Kim JH, Kim K, Kim M, Choi HJ, Kim YM, et al. Primary hepatic extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue. J Pathol Transl Med. (2020) 54:340–5. doi: 10.4132/jptm.2020.03.18
88. Dong S, Chen L, Chen Y, Chen X. Primary hepatic extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue type: A case report and literature review. Med (Baltimore). (2017) 96:e6305. doi: 10.1097/MD.0000000000006305
89. Murakami J, Fukushima N, Ueno H, Saito T, Watanabe T, Tanosaki R, et al. Primary hepatic low-grade B-cell lymphoma of the mucosa-associated lymphoid tissue type: a case report and review of the literature. Int J Hematol. (2002) 75:85–90. doi: 10.1007/BF02981985
90. Kirk CM, Lewin D, Lazarchick J. Primary hepatic B-cell lymphoma of mucosa-associated lymphoid tissue. Arch Pathol Lab Med. (1999) 123:716–9. doi: 10.5858/1999-123-0716-PHBCLO
91. Iida T, Iwahashi M, Nakamura M, Nakamori M, Yokoyama S, Tani M, et al. Primary hepatic low-grade B-cell lymphoma of MALT-type associated with Helicobacter pylori infection. Hepato-gastroenterology. 54(78):1898–901.
92. Shiozawa K, Watanabe M, Ikehara T, Matsukiyo Y, Kikuchi Y, Kaneko H, et al. A case of contiguous primary hepatic marginal zone B-cell lymphoma and hemangioma ultimately diagnosed using contrast-enhanced ultrasonography. Case Rep Oncol. (2015) 8:50–6. doi: 10.1159/000375118
93. Takeshima F, Kunisaki M, Aritomi T, Osabe M, Akama F, Nakasone T, et al. Hepatic mucosa-associated lymphoid tissue lymphoma and hepatocellular carcinoma in a patient with hepatitis B virus infection. J Clin Gastroenterol. (2004) 38:823–6. doi: 10.1097/01.mcg.0000139058.43414.a1
94. Hamada T, Kakizaki S, Koiso H, Irisawa H, Nobusawa S, Mori M. Primary hepatic mucosa-associated lymphoid tissue (MALT) lymphoma. Clin J Gastroenterol. (2013) 6:150–5. doi: 10.1007/s12328-013-0362-5
95. Dong A, Xiao Z, Yang J, Zuo C. CT, MRI, and 18F-FDG PET/CT findings in untreated pulmonary and hepatic B-cell lymphoma of mucosa-associated lymphoid tissue (MALT) over a five-year period: A case report. Med (Baltimore). (2016) 95:e3197. doi: 10.1097/MD.0000000000003197
96. Khurana. A, Mukherjee U, Patil N. An unusual case of hepatic lymphoma with multiple epithelial Malignancies. Indian J Pathol Microbiol. (2018) 61(4):585–6. doi: 10.4103/IJPM.IJPM_509_17
97. Lee SQW, Azhar R, Goh BKP. An unusual cause of an arterial enhancing liver mass. Gastroenterology. (2017) 152(5):e5–6. doi: 10.1053/j.gastro.2016.09.014
98. Fujiwara Y, Sakamoto K, Tokuhisa Y, Tokumitsu Y, Nakajima M, Matsukuma S, et al. Two cases of primary hepatic mucosa-associated lymphoid tissue(MALT) lymphoma. Gan To Kagaku Ryoho. (2016) 43:1794–6.
99. Obiorah IE, Johnson L, Ozdemirli M. Primary mucosa-associated lymphoid tissue lymphoma of the liver: A report of two cases and review of the literature. World J Hepatol. (2017) 9:155–60. doi: 10.4254/wjh.v9.i3.155
100. Takata N, Terasaki S, Iwata A, Harada K. Hepatic mucosa-associated lymphoid tissue lymphoma in a patient with chronic hepatitis B. Nihon Shokakibyo Gakkai Zasshi. (2015) 112:880–7. doi: 10.11405/nisshoshi.112.880
101. Zhong Y, Wang X, Deng M, Fang H, Xu R. Primary hepatic mucosa-associated lymphoid tissue lymphoma and hemangioma with chronic hepatitis B virus infection as an underlying condition. Biosci Trends. (2014) 8:185–8. doi: 10.5582/bst.2014.01057
102. Doi H, Horiike N, Hiraoka A, Koizumi Y, Yamamoto Y, Hasebe A, et al. Primary hepatic marginal zone B cell lymphoma of mucosa-associated lymphoid tissue type: case report and review of the literature. Int J Hematol. (2008) 88:418–23. doi: 10.1007/s12185-008-0153-9
103. Bohlok A, De Grez T, Bouazza F, De Wind R, El-Khoury M, Repullo D, et al. Primary hepatic lymphoma mimicking a hepatocellular carcinoma in a cirrhotic patient: case report and systematic review of the literature. Case Rep Surg. (2018) 2018:9183717. doi: 10.1155/2018/9183717
104. Yasuda T, Nakagawa S, Imai K, Okabe H, Hayashi H, Yamashita YI, et al. A case of primary hepatic mucosa-associated lymphoid tissue lymphoma incidentally found in the sustained virological response state of chronic hepatitis C: review of the literature of this rare disease. Int Cancer Conf J. (2020) 9:59–65. doi: 10.1007/s13691-019-00397-z
105. Yu Y-D, Kim DS, Byun GY, Lee JH, Kim IS, Kim CY, et al. Primary hepatic marginal zone B cell lymphoma : a case report and review of the literature. Indian J Surg. (2013) 75:331–6. doi: 10.1007/s12262-012-0695-1
106. Yago K, Shimada H, Itoh M, Ooba N, Itoh K, Suzuki M, et al. Primary low-grade B-cell lymphoma of mucosa-associated lymphoid tissue (MALT)-type of the liver in a patient with hepatitis C virus infection. Leuk Lymphoma. (2002) 43:1497–500. doi: 10.1080/1042819022386734
107. Chatelain D, Maes C, Yzet T, Brevet M, Bounicaud D, Plachot JP, et al. Primary hepatic lymphoma of MALT-type: a tumor that can simulate a liver metastasis. Ann Chir. (2006) 131:121–4. doi: 10.1016/j.anchir.2005.07.006
108. Charton-Bain MC, Lelong B, Bouabdallah R, Dubus P, Merlio JP, Hassoun J, et al. Hepatic MALT lymphoma disclosing a nodal extension. Ann Pathol. (2000) 20:137–41.
109. Liu J, Guo RR, Fang JC, Zhong L. Primary hepatic mucosa-associated lymphoid tissue lymphoma with hepatocellular carcinoma: A case report and literature review. J Dig Dis. (2020) 21:526–8. doi: 10.1111/1751-2980.12917
110. Albano D, Giubbini R, Bertagna F. 18F-FDG PET/CT and primary hepatic MALT: a case series. Abdom Radiol (NY). (2016) 41:1956–9. doi: 10.1007/s00261-016-0800-1
111. Ye MQ, Suriawinata A, Black C, Min AD, Strauchen J, Thung SN. Primary hepatic marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue type in a patient with primary biliary cirrhosis. Arch Pathol Lab Med. (2000) 124:604–8. doi: 10.5858/2000-124-0604-PHMZBC
112. Orrego M, Guo L, Reeder C, De Petris G, Balan V, Douglas DD, et al. Hepatic B-cell non-Hodgkin’s lymphoma of MALT type in the liver explant of a patient with chronic hepatitis C infection. Liver Transpl. (2005) 11:796–9. doi: 10.1002/lt.20384
113. Nart D, Ertan Y, Yilmaz F, Yüce G, Zeytunlu M, Kilic M. Primary hepatic marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue type in a liver transplant patient with hepatitis B cirrhosis. Transplant Proc. (2005) 37(10):4408–12. doi: 10.1016/j.transproceed.2005.10.109
114. Bao C, Wei J, Zhao X, Lin L, Chen D, Liu K, et al. Prognostic value of fluorine-18-fluorodeoxyglucose positron emission tomography/computed tomography in primary hepatic mucosa-associated lymphoid tissue lymphoma: A case report and review of the literature. Med (Baltimore). (2018) 97:e9877. doi: 10.1097/MD.0000000000009877
115. Mehrain S, Schima W, Ba-Ssalamah A, Kurtaran A, Raderer M. Primary MALT-lymphoma of the liver: multimodality imaging. Crit Rev Comput Tomogr. (2003) 44:347–55. doi: 10.3109/bctg.44.6.347.355
116. Cabassa P, Morone M, Matricardi L. An unusual liver mass. Gastroenterology. (2010) 138:e7–9. doi: 10.1053/j.gastro.2009.05.063
117. Chung YW, Sohn JH, Paik CH, Jeong JY, Han DS, Jeon YC, et al. High-grade hepatic mucosa-associated lymphoid tissue (MALT) lymphoma probably transformed from the low-grade gastric MALT lymphoma. Korean J Intern Med. (2006) 21:194–8. doi: 10.3904/kjim.2006.21.3.194
118. Li LX, Zhou ST, Ji X, Ren H, Sun YL, Zhang JB, et al. Misdiagnosis of primary hepatic marginal zone B cell lymphoma of mucosa-associated lymphoid tissue type, a case report. World J Surg Oncol. (2016) 14:69. doi: 10.1186/s12957-016-0817-5
119. Chen Y-Y, Chen Y-F, Chen C-H. A long-term follow-up of primary hepatic mucosa-associated lymphoid tissue lymphoma. Dig Liver Dis. (2020) 52:1365–6. doi: 10.1016/j.dld.2020.04.024
120. Chan RCK, Chu CM, Chow C, Chan SL, Chan AWH. A concurrent primary hepatic MALT lymphoma and hepatocellular carcinoma. Pathology. (2015) 47:178–81. doi: 10.1097/PAT.0000000000000220
121. Shin SY, Kim JS, Lim JK, Hahn JS, Yang WI, Suh CO. Longlasting remission of primary hepatic mucosa-associated lymphoid tissue (MALT) lymphoma achieved by radiotherapy alone. Korean J Intern Med. (2006) 21:127–31. doi: 10.3904/kjim.2006.21.2.127
122. Igarashi T, Yokoyama Y, Ikeda T, Tsujisaki M, Yawata A. Primary biliary cholangitis complicated by primary hepatic extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue. Rinsho Ketsueki. (2019) 60:1532–7. doi: 10.11406/rinketsu.60.1532
123. Nagata S, Harimoto N, Kajiyama K. Primary hepatic mucosa-associated lymphoid tissue lymphoma: a case report and literature review. Surg Case Rep. (2015) 1:87. doi: 10.1186/s40792-015-0091-8
124. Haefliger S, Milowich D, Sciarra A, Trimeche M, Bouilly J, Kaiser J, et al. Primary hepatic marginal B cell lymphoma of mucosa-associated lymphoid tissue (MALT) and non-alcoholic steatohepatitis (NASH): more than a coincidence? Ann Hematol. (2019) 98:1513–6. doi: 10.1007/s00277-018-3565-5
125. Prabhu RM, Medeiros LJ, Kumar D, Drachenberg CI, Papadimitriou JC, Appelman HD, et al. Primary hepatic low-grade B-cell lymphoma of mucosa-associated lymphoid tissue (MALT) associated with primary biliary cirrhosis. Mod Pathol. (1998) 11:404–10.
126. Gherlan GS, Stoia R, Enyedi M, Dobrea C, Calistru PI. Primary hepatic marginal zone lymphoma in a patient with chronic hepatitis C. Maedica (Bucur). (2016) 11:250–4.
127. Klimko A, Toma GA, Bejinariu N, Secareanu S-M, Andreiana I. Acute kidney injury in a patient with cryoglobulinemia secondary to hepatic mucosa-associated lymphoid tissue lymphoma: case report and literature review. Cureus. (2020) 12:e10451. doi: 10.7759/cureus.10451
128. Gockel HR, Heidemann J, Lugering A, Mesters RM, Parwaresch R, Domschke W, et al. Stable remission after administration of rituximab in a patient with primary hepatic marginal zone B-cell lymphoma. Eur J Haematol. (2005) 74:445–7. doi: 10.1111/j.1600-0609.2005.00419.x
129. Clarke G, MacMathuna P, Fenlon H, Callagy G, O'Keane JC, Carney D, et al. Primary hepatic lymphoma in a man with chronic hepatitis C. Eur J Gastroenterol Hepatol. (1997) 9:87–90. doi: 10.1097/00042737-199701000-00020
130. Ascoli V, Lo Coco F, Artini M, Levrero M, Martelli M, Negro F. Extranodal lymphomas associated with hepatitis C virus infection. Am J Clin Pathol. (1998) 109:600–9. doi: 10.1093/ajcp/109.5.600
131. Kiesewetter B, Müllauer L, Streubel B, Agis H, Hirschl A, Makristathis A, et al. Primary mucosa-associated lymphoid tissue (MALT) lymphoma of the liver: clinical, molecular, and microbiological aspects. Ann Hematol. (2012) 91:1817–8. doi: 10.1007/s00277-012-1459-5
132. Isaacson PG, Banks PM, Best PV, McLure SP, Muller-Hermelink HK, Wyatt JI. Primary low-grade hepatic B-cell lymphoma of mucosa-associated lymphoid tissue (MALT)-type. Am J Surg Pathol. (1995) 19:571–5. doi: 10.1097/00000478-199505000-00009
133. Nakayama S, Yokote T, Kobayashi K, Hirata Y, Akioka T, Miyoshi T, et al. Primary hepatic MALT lymphoma associated with primary biliary cirrhosis. Leuk Res. (2010) 34:e17–20. doi: 10.1016/j.leukres.2009.07.031
134. Hamada M, Tanaka Y, Kobayashi Y, Takeshita E, Joko K. A case of MALT lymphoma of the liver treated by RFA and Rituximab. Nihon Shokakibyo Gakkai Zasshi. (2006) 103:655–60.
135. Mattar WE, Alex BK, Sherker AH. Primary hepatic burkitt lymphoma presenting with acute liver failure. J Gastrointest Cancer. (2010) 41:261–3. doi: 10.1007/s12029-010-9150-3
136. Lionaki S, Tsakonas E, Androulaki A, Liapis G, Panayiotidis P, Zavos G, et al. Primary hepatic burkitt lymphoma in a kidney transplant recipient. Case Rep Nephrol. (2018) 2018:7425785. doi: 10.1155/2018/7425785
137. Kuroda J, Omoto A, Fujiki H, Okugawa K, Tamai H, Yamagishi H, et al. Primary hepatic Burkitt’s lymphoma with chronic hepatitis C. Acta Haematol. (2001) 105:237–40. doi: 10.1159/000046571
138. Sekiguchi Y, Yoshikawa H, Shimada A, Imai H, Wakabayashi M, Sugimoto K, et al. Primary hepatic circumscribed Burkitt’s lymphoma that developed after acute hepatitis B: report of a case with a review of the literature. J Clin Exp Hematop. (2013) 53:167–73. doi: 10.3960/jslrt.53.167
139. Lee SH, Kim HJ, Mun JS, Oh HC, Lee HW, Choi CH, et al. A case of primary hepatic Burkitt’s lymphoma. Korean J Gastroenterol. (2008) 51:259–64.
140. Reyes AJ, Ramcharan K, Aboh S, Greaves W. Primary hepatic Burkitt’s lymphoma as first manifestation of HIV-AIDS in a hepatitis B seropositive adult: when defences fail. BMJ Case Rep. (2016) 2016:bcr2016217620. doi: 10.1136/bcr-2016-217620
141. Jacobs SL, Rozenblit A. HIV-associated hypervascular primary Burkitt’s lymphoma of the liver. Clin Radiol. (2006) 61:453–5. doi: 10.1016/j.crad.2005.12.007
142. Mossad SB, Tomford JW, Avery RK, Hussein MA, Vaughn KW. Isolated primary hepatic lymphoma in a patient with acquired immunodeficiency syndrome. Int J Infect Dis. (2000) 4:57–8. doi: 10.1016/S1201-9712(00)90069-9
143. Scoazec JY, Degott C, Brousse N, Barge J, Molas G, Potet F, et al. Non-Hodgkin’s lymphoma presenting as a primary tumor of the liver: presentation, diagnosis and outcome in eight patients. Hepatology. (1991) 13:870–5. doi: 10.1002/hep.1840130512
144. Modi G, Madabhavi I, Patel A, Revannasiddaiah S, Anand A, Panchal H, et al. Primary hepatic burkitt lymphoma: A bizarre site and triumph tale. J Clin Exp Hepatol. (2015) 5:159–62. doi: 10.1016/j.jceh.2015.05.002
145. Chim CS, Choy C, Ooi GC, Liang R. Primary hepatic lymphoma. Leuk Lymphoma. (2001) 40:667–70. doi: 10.3109/10428190109097665
146. Souto P, Romãozinho JM, Figueiredo P, Ferreira M, Sousa I, Camacho E, et al. Severe acute liver failure as the initial manifestation of haematological Malignancy. Eur J Gastroenterol Hepatol. (1997) 9:1113–5. doi: 10.1097/00042737-199711000-00016
147. Stemmer S, Geffen DB, Goldstein J, Cohen Y. Primary small noncleaved cell lymphoma of the liver. Report of an adult case in complete remission after treatment with combination chemotherapy. J Clin Gastroenterol. (1993) 16:65–9. doi: 10.1097/00004836-199301000-00018
148. Lei KI, Chow JH, Johnson PJ. Aggressive primary hepatic lymphoma in Chinese patients. Presentation, pathologic features, and outcome. Cancer. (1995) 76:1336–43. doi: 10.1002/1097-0142(19951015)76:8<1336::AID-CNCR2820760807>3.0.CO;2-I
149. Zhang Z, Liu Q, Wu Y, Hong C, Li X. Clinicopathologic feature of primary hepatic mantle cell lymphoma: report of a case. Zhonghua Bing Li Xue Za Zhi. (2010) 39:418–20.
150. Ramai D, Ofori E, Nigar S, Reddy M. Primary hepatic peripheral T-cell lymphoma associated with Epstein-Barr viral infection. World J Hepatol. (2018) 10:347–51. doi: 10.4254/wjh.v10.i2.347
151. Mishra S, Shukla A, Tripathi AK, Kumar A. Primary T-cell lymphoma of liver. BMJ Case Rep. (2013) 2013:bcr2012008467. doi: 10.1136/bcr-2012-008467
152. Miyashita K, Tomita N, Oshiro H, Matsumoto C, Nakajima Y, Ito S, et al. Primary hepatic peripheral T-cell lymphoma treated with corticosteroid. Intern Med. (2011) 50:617–20. doi: 10.2169/internalmedicine.50.4686
153. Kim JH, Kim HY, Kang I, Kim YB, Park CK, Yoo JY, et al. A case of primary hepatic lymphoma with hepatitis C liver cirrhosis. Am J Gastroenterol. (2000) 95:2377–80. doi: 10.1111/j.1572-0241.2000.02278.x
154. Hu H-J, Liao M-Y, Qu Y-J. Primary hepatic peripheral T-cell lymphoma: A case report. Oncol Lett. (2014) 8:258–62. doi: 10.3892/ol.2014.2119
155. Stancu M, Jones D, Vega F, Medeiros LJ. Peripheral T-cell lymphoma arising in the liver. Am J Clin Pathol. (2002) 118:574–81. doi: 10.1309/9DAQ-PWP3-XKDG-CUTG
156. Lee J, Park KS, Kang MH, Kim Y, Son SM, Choi H, et al. Primary hepatic peripheral T-cell lymphoma mimicking hepatocellular carcinoma: a case report. Ann Surg Treat Res. (2017) 93:110–4. doi: 10.4174/astr.2017.93.2.110
157. Peng Y, Cai J, Yue C, Qing X. Primary hepatic Epstein-Barr virus-associated CD30-positive peripheral T-cell lymphoma of cytotoxic phenotype. Exp Mol Pathol. (2016) 100:207–11. doi: 10.1016/j.yexmp.2016.01.002
158. Eom D-W, Huh JR, Kang YK, Lee YS, Yu E. Clinicopathological features of eight Korean cases of primary hepatic lymphoma. Pathol Int. (2004) 54:830–6. doi: 10.1111/j.1440-1827.2004.01752.x
159. Tsutsumi Y, Deng YL, Uchiyama M, Kawano K, Ikeda Y. OPD4-positive T-cell lymphoma of the liver in systemic lupus erythematosus. Acta Pathol Jpn. (1991) 41:829–33. doi: 10.1111/j.1440-1827.1991.tb01626.x
160. Leung VK, Lin SY, Loke TK, Chau TN, Leung CY, Fung TP, et al. Primary hepatic peripheral T-cell lymphoma in a patient with chronic hepatitis B infection. Hong Kong Med J. (2009) 15:288–90.
161. Taketomi A, Takenaka K, Shirabe K, Matsumata T, Maeda T, Shimada M, et al. Surgically resected primary Malignant lymphoma of the liver. Hepatogastroenterology. (1996) 43:651–7.
162. Cai G, Inghirami G, Moreira A, Sen F. Primary hepatic anaplastic large-cell lymphoma diagnosed by fine-needle aspiration biopsy. Diagn Cytopathol. (2005) 33:106–9. doi: 10.1002/dc.20307
163. Baschinsky DY, Weidner N, Baker PB, Frankel WL. Primary hepatic anaplastic large-cell lymphoma of T-cell phenotype in acquired immunodeficiency syndrome: a report of an autopsy case and review of the literature. Am J Gastroenterol. (2001) 96:227–32. doi: 10.1111/j.1572-0241.2001.03481.x
164. Saikia UN, Sharma N, Duseja A, Bhalla A, Joshi K. Anaplastic large cell lymphoma presenting as acute liver failure: A report of two cases with review of literature. Ann Hepatol. (2010) 9:457–61. doi: 10.1016/S1665-2681(19)31623-0
165. Jiménez Fuertes M, Costa Navarro D, Montalvá Orón EM, López-Andújar R, de Juan Burgueño M, Mir Pallardó J, et al. Primary hepatic anaplastic large cell ki-1 lymphoma and celiac disease: a casual association? J Gastrointest Oncol. (2013) 4:109–13. doi: 10.3978/j.issn.2078-6891.2012.059
166. Andrès E, Perrin A-E, Maloisel F, Marcellin L, Goichot B. Primary hepatic anaplastic large cell Ki-1 non-Hodgkin’s lymphoma and hereditary hemochromatosis: a fortuitous association? Clin Lab Haematol. (2003) 25:185–6. doi: 10.1046/j.1365-2257.2003.00510.x
167. Cerban R, Gheorghe L, Becheanu G, Serban V, Gheorghe C. Primary focal T-cell lymphoma of the liver: a case report and review of the literature. J Gastrointestin Liver Dis. (2012) 21:213–6.
168. Siebert S, Amos N, Williams BD, Lawson TM. Cytokine production by hepatic anaplastic large-cell lymphoma presenting as a rheumatic syndrome. Semin Arthritis Rheum. (2007) 37:63–7. doi: 10.1016/j.semarthrit.2006.12.007
169. Bronowicki J-P, Bineau C, Feugier P, Hermine O, Brousse N, Oberti F, et al. Primary lymphoma of the liver: clinical-pathological features and relationship with HCV infection in French patients. Hepatology. (2003) 37:781–7. doi: 10.1053/jhep.2003.50121
Keywords: primary hepatic lymphoma (PHL), diffuse large B cell lymphoma (DLBCL), indolent lymphoma, non-Hodgkin lymphoma (NHL), liver neoplasms, radiation therapy (radiotherapy)
Citation: Ma J, Daou R, Bou Eid J, Fregonese B, El-Khoury J, Wijetunga NA, Imber BS, Yahalom J and Hajj C (2025) Management approaches for primary hepatic lymphoma: 10 year institutional experience with comprehensive literature review. Front. Oncol. 15:1475118. doi: 10.3389/fonc.2025.1475118
Received: 02 August 2024; Accepted: 28 January 2025;
Published: 20 March 2025.
Edited by:
Youssef Zeidan, Baptist Health South Florida, United StatesReviewed by:
Omran Saifi, Mayo Clinic Florida, United StatesCopyright © 2025 Ma, Daou, Bou Eid, Fregonese, El-Khoury, Wijetunga, Imber, Yahalom and Hajj. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jennifer Ma, bWFqQG1za2NjLm9yZw==; Carla Hajj, c2Fzc2luZS5oYWpqLmNhcmxhQGdtYWlsLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.