
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 17 February 2025
Sec. Thoracic Oncology
Volume 15 - 2025 | https://doi.org/10.3389/fonc.2025.1469072
This article is part of the Research Topic Therapeutic Mechanism of Osteosarcoma View all 10 articles
In this report, we present a case of a 32-year-old female previously diagnosed with hereditary multiple exostoses(HME) who was incidentally found to have an asymptomatic anterior mediastinal mass during a routine examination. Computed tomography imaging revealed a well-defined mass measuring approximately 2.3 cm x 4.0 cm x 4.7 cm in the anterior mediastinum with multiple nodular areas of high density within. The mass caused compression and narrowing of the right ventricle. The patient subsequently underwent intralesional resection of the tumor, and histopathological examination confirmed a diagnosis of well-differentiated chondrosarcoma. Given the patient’s medical history, the chondrosarcoma was suspected to have originated from malignant transformation of a rib osteochondroma. The patient received adjuvant radiotherapy postoperatively and has been followed up for one year with no evidence of recurrence. This case reports a highly rare costal chondrosarcoma secondary to hereditary multiple exostoses, located in the anterior mediastinum and compressing the right ventricle. To our knowledge, this is the first reported case of costal chondrosarcoma secondary to HME occurring in the anterior mediastinum, which requires differentiation from other common anterior mediastinal tumors.
Chondrosarcoma is a relatively common malignant bone tumor originating from cartilage, accounting for approximately 20-30% of all primary malignant bone tumors in the United States, with an annual incidence of 1 to 4 cases per million population, depending on geographic and population differences (1, 2). A subset of chondrosarcomas arises from pre-existing benign conditions, such as hereditary multiple exostoses (HME), and is referred to as secondary chondrosarcoma. HME, an autosomal dominant genetic disorder, is characterized by the formation of multiple osteochondromas throughout the skeleton. These osteochondromas predominantly affect the metaphyses of long bones, the scapulae, and the pelvis, while rib involvement is comparatively rare (3). The cause of HME is linked to mutations in the EXT1 and EXT2 genes. These mutations disrupt the synthesis and elongation of heparan sulfate (HS), a glycosaminoglycan essential for regulating growth factor signaling pathways such as Indian hedgehog (IHH), fibroblast growth factors (FGFs), and bone morphogenetic proteins (BMPs). The impaired HS biosynthesis affects chondrocyte differentiation and proliferation, leading to the formation of osteochondromas (4–6). The most serious complication of HME is malignant transformation into chondrosarcoma, with previous studies estimating the incidence of malignant transformation to be approximately 1-5% (7, 8). This transformation is more commonly observed in osteochondromas located in the pelvis and proximal femur, and less frequently in the ribs (9). Costal chondrosarcoma caused by HME is very rare, with only a few reports available. For example, Acharya et al. reported a large chondrosarcoma in the anterior chest wall from a rib osteochondroma, and Liu et al. described a similar case in the posterior chest wall (10, 11). To our knowledge, there are no prior reports of costal chondrosarcoma secondary to HME occurring in the anterior mediastinum, which presents significant diagnostic challenges due to its rarity and the need to differentiate it from more common mediastinal tumors. In this study, we present an extremely rare case of costal chondrosarcoma located in the anterior mediastinum secondary to HME.
The case involves a 32-year-old female patient who presented to our hospital in July 2023 after a routine health checkup revealed an anterior mediastinal mass. Despite the presence of the mass, she did not exhibit any symptoms typically associated with such findings, such as chest pain, cough, fever, or shortness of breath. A contrast-enhanced computed tomography (CT) scan of her chest demonstrated a well-defined mass measuring approximately 2.3 cm x 4.0 cm x 4.7 cm in the anterior mediastinum, causing compression of the right ventricle. The mass had multiple nodular high-density areas within it (Figures 1A, B). Bone abnormalities were observed, including a bony prominence on the right scapula, a bony prominence on the proximal left fibula, and localized bone expansion of the right fifth anterior rib, which were all considered to be potential osteochondromas (Figures 1C, D). Cardiac assessments, including electrocardiogram and echocardiogram, showed T-wave changes and mild tricuspid valve regurgitation, respectively. A whole-body bone scan ruled out distant bone metastasis, and laboratory tests including complete blood count, alkaline phosphatase (ALP), and lactate dehydrogenase (LDH) levels were unremarkable. Physical examination and abdominal ultrasound did not reveal any significant abnormalities.
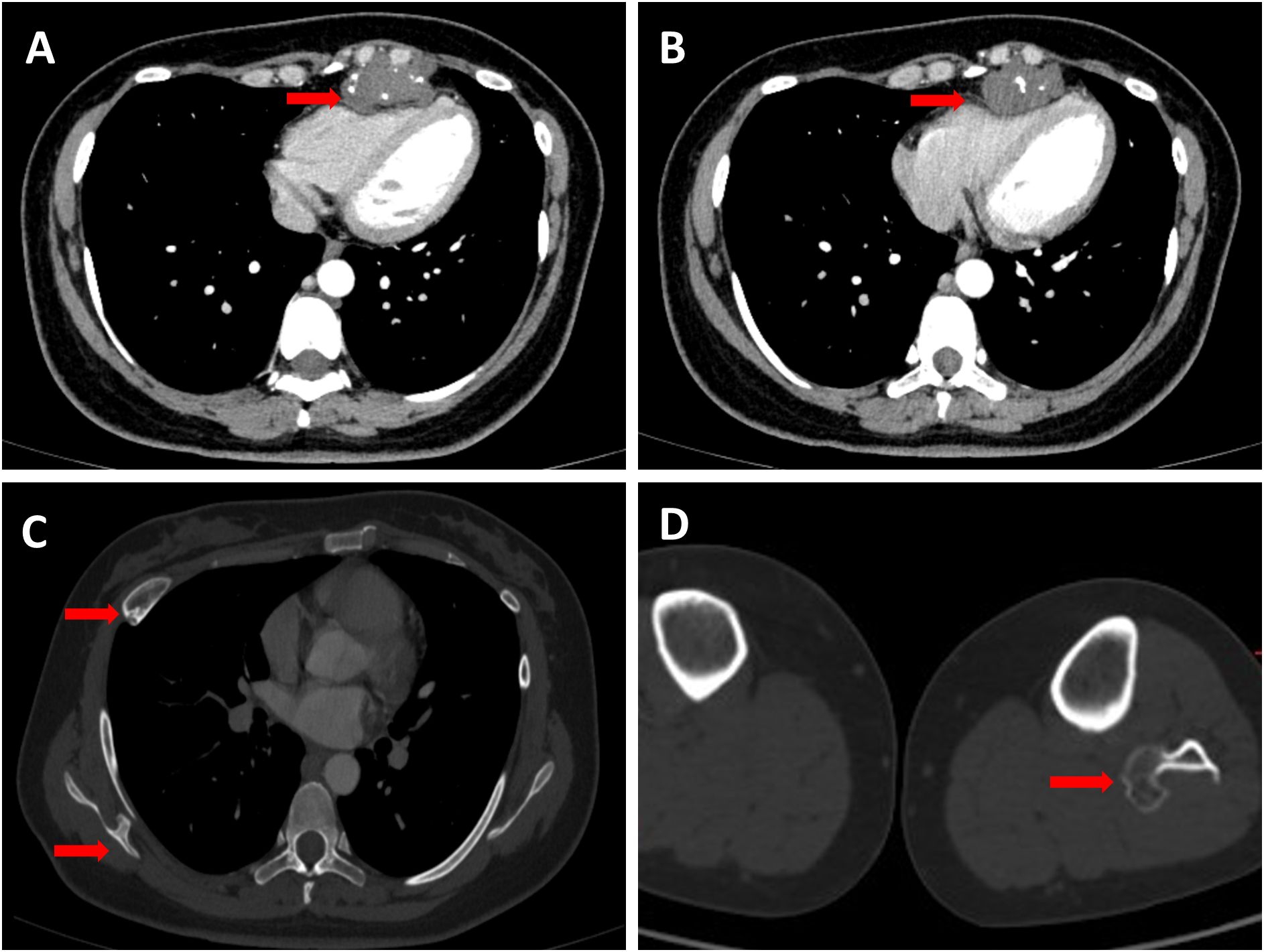
Figure 1. (A,B) Red arrows indicate the tumor in the anterior mediastinum with visible calcifications and clear edges. The tumor is pressing on the right ventricle, making it smaller. (C) Two red arrows highlight significant findings: one indicates a focal bony expansion on the right fifth anterior rib, suggesting a potential osteochondroma; the other points to a typical osteochondroma located on the right scapula. (D) The red arrow highlights an osteochondroma situated at the proximal end of the left fibula.
The patient had a significant surgical history. Four years prior, she presented to our orthopedic department with bilateral knee pain. X-rays of the knee joints revealed bone protrusions at the distal femur, right proximal tibia, consistent with osteochondromas. The lesions caused significant knee pain, which led to the decision to surgically excise them. Histopathological examination of the excised tissue confirmed the diagnosis of osteochondroma (Figures 2A, B). The patient’s father had hereditary multiple exostoses, suggesting a genetic predisposition. The diagnosis of HME is typically based on clinical, radiological, and histological findings. Although genetic testing was not performed at that time, the patient was diagnosed with HME given her family history, imaging results, and postoperative pathological findings, which provided sufficient evidence for the diagnosis (4, 7).
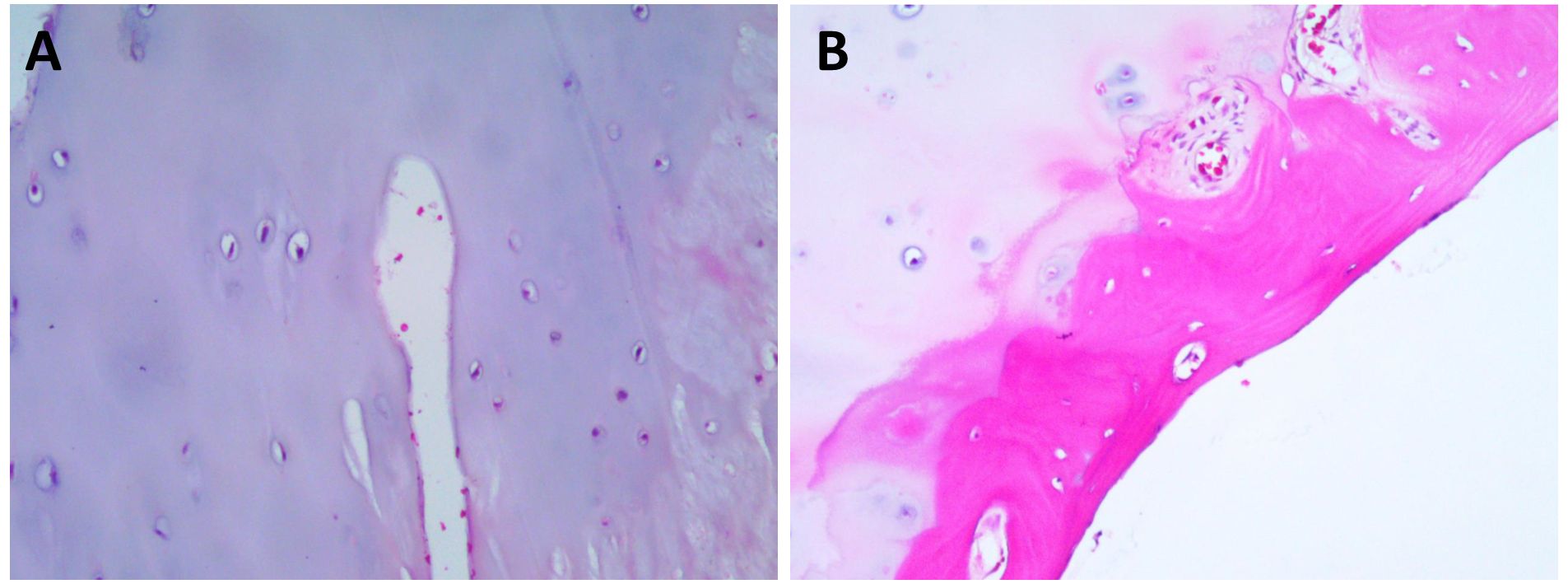
Figure 2. Hematoxylin and Eosin (HEx200) staining results. (A) HE Staining reveals the characteristic cartilage cap. (B) HE Staining delineates the cartilaginous cap and the adjacent fibrous layer, highlighting the distinct histological attributes of the tumor.
To remove the tumor and relieve its compression on the heart, the patient underwent resection of the mediastinal tumor after obtaining informed consent: a left subxiphoid incision was made, with a 4 cm incision along the rib arch, assisted by thoracoscopy. The tumor was excised without extensive resection, preserving adjacent ribs and sternum. Intraoperatively, a hard, popcorn-like mass was observed in the left anterior mediastinum, with unclear demarcation from the rib cartilage and anterior chest wall but relatively clear relation to the pericardium and surrounding structures, suggesting that the tumor likely originated from the rib rather than from the mediastinum itself (Figure 3A). Postoperative histopathological examination suggested a diagnosis of well-differentiated chondrosarcoma, this was consistent with the tumor features we observed during surgery. (Figures 3B, C). The patient recovered and was discharged on the third postoperative day. One month after the surgery, the patient consulted the oncology department at our hospital for a follow-up and to determine the next steps for treatment. A PET-CT scan was performed to assess for potential distant metastases, and fortunately, it revealed no evidence of distant metastasis (Figure 3D). There was visible soft tissue swelling and increased glucose metabolism in the surgical area, with a standardized uptake value (SUV) of 8.8, indicative of postoperative changes (Figure 3E). Follow-up echocardiography and electrocardiogram showed no evidence of tricuspid valve regurgitation or T-wave changes, possibly due to relief of right ventricular compression post-surgery (12). CT scans confirmed that the tumor had been successfully removed, with visible improvement in right ventricular compression (Figure 3F). According to the NCCN guidelines, for low-grade chondrosarcomas, either wide resection or intralesional resection followed by adjuvant radiotherapy is recommended (13). Therefore, the patient received adjuvant intensity-modulated radiotherapy at our hospital. Currently, the patient has been regularly followed up for one year without evidence of tumor recurrence.
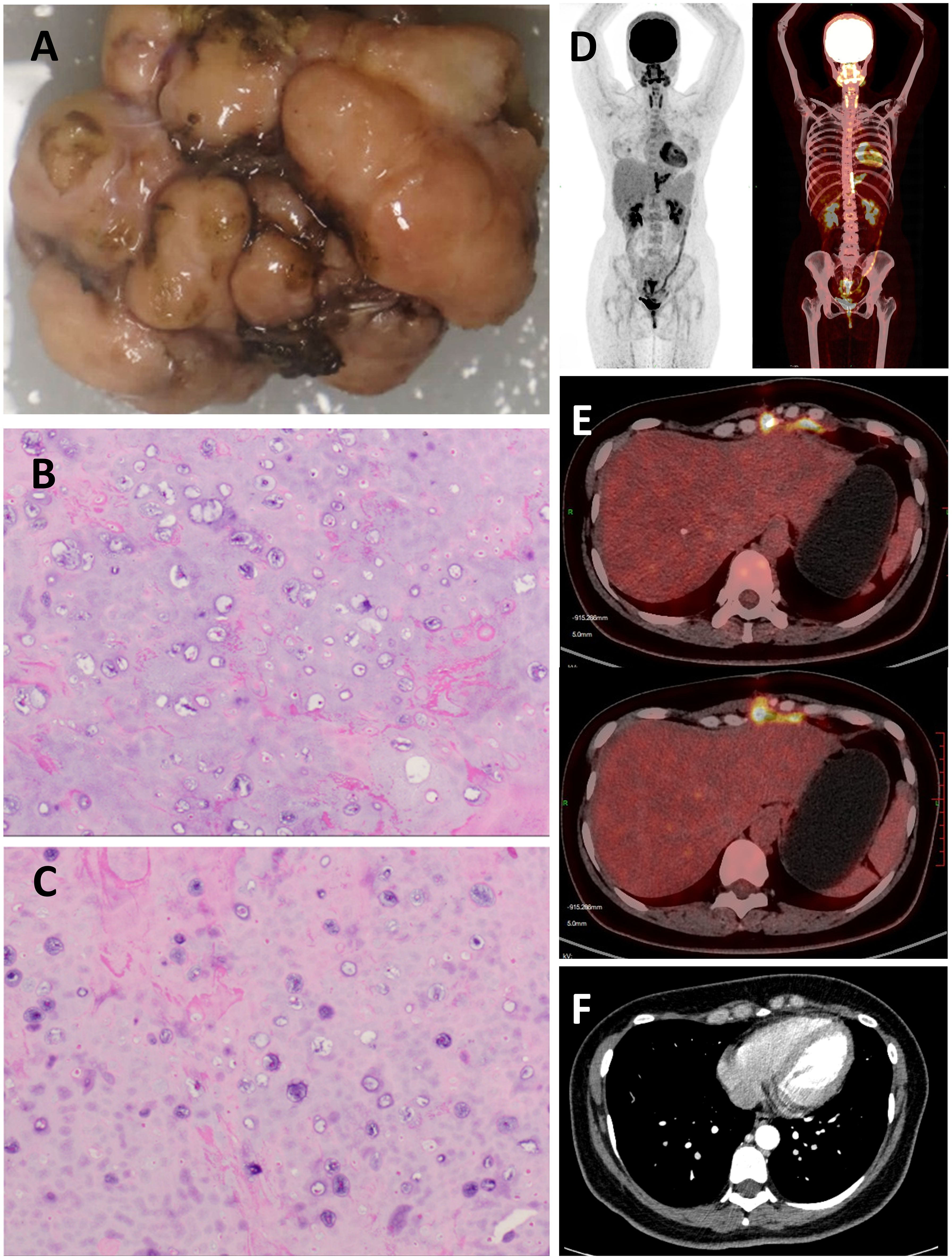
Figure 3. (A) Macroscopic Examination: The excised tumor, exhibiting a multinodular surface and a hard consistency, measured 2.3 cm × 4.0 cm × 4.7cm. (B,C) Histopathological Examination (Hematoxylin and Eosin Stain): Micrographs at high magnification revealed chondrocyte hyperplasia within the cartilaginous matrix, indicative of a malignant process. (D) No distant metastatic lesions were identified upon PET-CT examination. (E) Increased glucose metabolism was observed in the surgical site on PET-CT imaging, consistent with postoperative changes. (F) Contrast-enhanced chest CT during postoperative follow-up.
Chondrosarcoma is a relatively common malignant bone tumor originating from cartilage and is the second most common primary malignant bone tumor after osteosarcoma (2). Chondrosarcomas can be classified into conventional chondrosarcoma and special subtypes, with the conventional type comprising approximately 85% of all cases. Conventional chondrosarcoma can be further divided into primary chondrosarcoma and secondary chondrosarcoma, which arises from benign conditions such as hereditary multiple exostoses, Ollier disease (multiple enchondromatosis), and Maffucci syndrome.
Hereditary multiple exostoses (HME), also known as hereditary multiple osteochondromas (HMO), is a rare autosomal dominant genetic disorder with an incidence of approximately 1 in 50,000 among Caucasians (14). Current research indicates that mutations in the EXT1 and EXT2 tumor suppressor genes from the EXT gene family are responsible for both sporadic and syndromic osteochondromas, including hereditary multiple exostoses (HME) (5, 15–17). The condition is characterized by multiple benign bone outgrowths covered with cartilage caps, primarily affecting the metaphyses of long bones in the limbs, as well as the scapula and pelvis, with relatively rare involvement of the ribs.
HME can lead to various complications, with the most serious being malignant transformation into chondrosarcoma. Previous studies have estimated the malignant transformation rate of HME to be approximately 1-5%. However, the timing of symptom development can vary depending on the tumor’s location. Early signs of malignant transformation of HME can be detected through imaging techniques such as CT and MRI (18, 19). Fortunately, secondary chondrosarcomas are generally low-grade and have a lower rate of metastasis, which aligns with the condition of the patient we report here (20, 21). The risk of malignant transformation is related to the location of the osteochondromas; those located in the pelvis and proximal femur are more prone to malignant transformation, while malignant transformation of rib osteochondromas into chondrosarcoma is exceedingly rare, with only a few cases reported in the literature. Malignant transformation is often initially detected by clinical symptoms, such as growth and pain occurring after puberty. However, the timing of symptom development can vary depending on the tumor’s location. Metesh Acharya et al. reported a case of a large chondrosarcoma primarily located in the anterior chest wall originating from a rib (10). Similarly, Wenliang Liu et al. reported a case of a large chondrosarcoma primarily located in the posterior chest wall, both believed to have arisen from malignant transformation of HME (11). In contrast, our patient benefited from early detection, with a relatively small tumor size. However, the growth pattern of the tumor in this case extended into the thoracic cavity, directly compressing the heart and significantly narrowing the right ventricle. Additionally, due to the tumor’s location in the anterior mediastinum, the diagnosis was challenging as it needed to be differentiated from other common anterior mediastinal tumors. These include teratomas, lymphomas, and other cartilage-origin tumors such as chondroblastomas (22).
The treatment strategy for chondrosarcoma depends on the tumor’s histological grade and location. Well-differentiated (low-grade) chondrosarcomas typically grow slowly and have a relatively low recurrence rate. Due to the rarity of costal chondrosarcoma, there is no standardized treatment protocol for this condition. According to the NCCN guidelines, the preferred approach for low-grade chondrosarcomas is either wide resection or intralesional resection followed by adjuvant therapy to minimize recurrence risk. However, wide resection often requires extensive removal of adjacent chest wall structures, such as the sternum and rib cartilage, which necessitates complex reconstruction. In this case, intralesional resection was chosen to preserve the structural integrity of the chest wall, as wide resection would have caused substantial disruption to surrounding structures. Recognizing the increased risk of local recurrence with intralesional resection, the patient underwent adjuvant intensity-modulated radiotherapy (IMRT). While chondrosarcomas are traditionally considered radioresistant, recent advancements in radiotherapy, including IMRT, have shown improved potential for local control (23, 24).
In summary, we report a highly rare case of costal chondrosarcoma secondary to HME, located in the anterior mediastinum and causing compression of the right ventricle, which required differentiation from other common anterior mediastinal tumors. This case underscores the importance of routine imaging follow-up for patients with HME to enable early detection of malignant transformation. It also highlights the critical role of a multidisciplinary team, including surgeons, oncologists, radiologists, and pathologists, in improving diagnostic accuracy, surgical planning, and postoperative management (3, 25). To our knowledge, this is the first reported case of costal chondrosarcoma secondary to HME occurring in the anterior mediastinum. Our experience highlights the need to suspect secondary chondrosarcoma in HME patients when a mediastinal mass with calcified foci is detected, especially if the mass is in close proximity to the chest wall.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Ethics Committee of the Affiliated Hospital of Southwest Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
ZY: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Writing – original draft, Writing – review & editing. KW: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. JP: Conceptualization, Formal analysis, Funding acquisition, Methodology, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We would like to express our gratitude to the patient who provided written informed consent for publication and to the research personnel involved in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Thorkildsen J, Myklebust T. The national incidence of chondrosarcoma of bone; a review. Acta Oncol (Stockholm Sweden). (2023) 62:110–7. doi: 10.1080/0284186x.2023.2177975
2. Gazendam A, Popovic S, Parasu N, Ghert M. Chondrosarcoma: A clinical review. J Clin Med. (2023) 12. doi: 10.3390/jcm12072506
3. D’Arienzo A, Andreani L, Sacchetti F, Colangeli S, Capanna R. Hereditary multiple exostoses: current insights. Orthop Res Rev. (2019) 11:199–211. doi: 10.2147/ORR.S183979
4. Bukowska-Olech E, Trzebiatowska W, Czech W, Drzymała O, Frąk P, Klarowski F, et al. Hereditary multiple exostoses-A review of the molecular background, diagnostics, and potential therapeutic strategies. Front Genet. (2021) 12:759129. doi: 10.3389/fgene.2021.759129
5. Al-Zayed Z, Al-Rijjal RA, Al-Ghofaili L, BinEssa HA, Pant R, Alrabiah A, et al. Mutation spectrum of EXT1 and EXT2 in the Saudi patients with hereditary multiple exostoses. Orphanet J Rare Dis. (2021) 16:100. doi: 10.1186/s13023-021-01738-z
6. Wang Y, Zhong L, Xu Y, Ding L, Ji Y, Schutz S, et al. EXT1 and EXT2 variants in 22 chinese families with multiple osteochondromas: seven new variants and potentiation of preimplantation genetic testing and prenatal diagnosis. Front Genet. (2020) 11:607838. doi: 10.3389/fgene.2020.607838
7. Pierz KA, Stieber JR, Kusumi K, Dormans JP. Hereditary multiple exostoses: one center’s experience and review of etiology. Clin Orthopaedics Related Res. (2002) 401:49–59. doi: 10.1097/00003086-200208000-00008
8. Fei L, Ngoh C, Porter DE. Chondrosarcoma transformation in hereditary multiple exostoses: A systematic review and clinical and cost-effectiveness of a proposed screening model. J Bone Oncol. (2018) 13:114–22. doi: 10.1016/j.jbo.2018.09.011
9. Gnoli M, Gambarotti M, Righi A, Staals EL, Evangelista A, Tremosini M, et al. Secondary peripheral chondrosarcoma in multiple osteochondromas: a retrospective single-institution case series. Orphanet J Rare Dis. (2024) 19:63. doi: 10.1186/s13023-023-03006-8
10. Acharya M, Jamali A, Rao J. Giant costal chondrosarcoma in a patient with hereditary multiple exostoses. Ann Thorac Surgery. (2014) 98:1848. doi: 10.1016/j.athoracsur.2014.06.081
11. Liu W, Kong D, Tang J, Yu F. Giant costal osteochondroma in a man with multiple exostoses. Ann Thorac Surgery. (2013) 96:675–7. doi: 10.1016/j.athoracsur.2012.12.007
12. Kozaki T, Fuchigami T, Nagata N. Tricuspid regurgitation caused by right ventricle compression by pectus excavatum. Asian Cardiovasc Thorac Ann. (2018) 26:582–3. doi: 10.1177/0218492318799928
13. Biermann JS, Chow W, Reed DR, Lucas D, Adkins DR, Agulnik M, et al. NCCN guidelines insights: bone cancer, version 2.2017. J Natl Compr Cancer Network: JNCCN. (2017) 15:155–67. doi: 10.6004/jnccn.2017.0017
14. Schmale GA, Conrad EU III, Raskind WH. The natural history of hereditary multiple exostoses. J Bone Joint Surg Am. (1994) 76(7):986–92. doi: 10.2106/00004623-199407000-00005
15. Fusco C, Nardella G, Fischetto R, Copetti M, Petracca A, Annunziata F, et al. Mutational spectrum and clinical signatures in 114 families with hereditary multiple osteochondromas: insights into molecular properties of selected exostosin variants. Hum Mol Genet. (2019) 28:2133–42. doi: 10.1093/hmg/ddz046
16. Pacifici M. The pathogenic roles of heparan sulfate deficiency in hereditary multiple exostoses. Matrix Biol. (2018) 71-72:28–39. doi: 10.1016/j.matbio.2017.12.011
17. Sinha S, Mundy C, Bechtold T, Sgariglia F, Ibrahim MM, Billings PC, et al. Unsuspected osteochondroma-like outgrowths in the cranial base of Hereditary Multiple Exostoses patients and modeling and treatment with a BMP antagonist in mice. PloS Genet. (2017) 13:e1006742. doi: 10.1371/journal.pgen.1006742
18. Verdegaal SH, Bovée JV, Pansuriya TC, Grimer RJ, Ozger H, Jutte PC, et al. Incidence, predictive factors, and prognosis of chondrosarcoma in patients with Ollier disease and Maffucci syndrome: an international multicenter study of 161 patients. Oncol. (2011) 16:1771–9. doi: 10.1634/theoncologist.2011-0200
19. Kwee RM, Fayad LM, Fishman EK, Fritz J. Multidetector computed tomography in the evaluation of hereditary multiple exostoses. Eur J Radiol. (2016) 85:383–91. doi: 10.1016/j.ejrad.2015.11.034
20. Ahmed AR, Tan TS, Unni KK, Collins MS, Wenger DE, Sim FH. Secondary chondrosarcoma in osteochondroma: report of 107 patients. Clin Orthopaedics Related Res. (2003) 411:193–206. doi: 10.1097/01.blo.0000069888.31220.2b
21. Tsuda Y, Gregory JJ, Fujiwara T, Abudu S. Secondary chondrosarcoma arising from osteochondroma: outcomes and prognostic factors. Bone Joint J. (2019) 101-B:1313–20. doi: 10.1302/0301-620X.101B9.BJJ-2019-0190.R1
22. Nakazono T, Yamaguchi K, Egashira R, Mizuguchi M, Irie H. Anterior mediastinal lesions: CT and MRI features and differential diagnosis. Jpn J Radiol. (2021) 39:101–17. doi: 10.1007/s11604-020-01031-2
23. Sahgal A, Chan MW, Atenafu EG, Masson-Cote L, Bahl G, Yu E, et al. Image-guided, intensity-modulated radiation therapy (IG-IMRT) for skull base chordoma and chondrosarcoma: preliminary outcomes. Neuro-oncology. (2015) 17:889–94. doi: 10.1093/neuonc/nou347
24. Gilbert A, Tudor M, Montanari J, Commenchail K, Savu DI, Lesueur P, et al. Chondrosarcoma resistance to radiation therapy: origins and potential therapeutic solutions. Cancers (Basel). (2023) 15. doi: 10.3390/cancers15071962
Keywords: hereditary multiple exostoses, malignant conversion, costal chondrosarcoma, mediastinal tumor, treatment
Citation: Yang Z, Wang K and Pu J (2025) Costal chondrosarcoma in a woman with hereditary multiple exostoses - a case report. Front. Oncol. 15:1469072. doi: 10.3389/fonc.2025.1469072
Received: 23 July 2024; Accepted: 30 January 2025;
Published: 17 February 2025.
Edited by:
Yang Wang, Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital, ChinaReviewed by:
Paulo Roberto Lacerda Leal, Federal University of Ceara, BrazilCopyright © 2025 Yang, Wang and Pu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiangtao Pu, ZHJqaWFuZ3Rhb3BAMTYzLmNvbQ==
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.