
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 05 March 2025
Sec. Breast Cancer
Volume 15 - 2025 | https://doi.org/10.3389/fonc.2025.1459444
 Yingbo Shao1,2
Yingbo Shao1,2 Huijuan Guan3,4
Huijuan Guan3,4 Zhifen Luo5,6
Zhifen Luo5,6 Yang Yu1,2
Yang Yu1,2 Yaning He1,2
Yaning He1,2 Qi Chen1,2
Qi Chen1,2 Chaojun Liu1,2
Chaojun Liu1,2 Fangyuan Zhu1,2
Fangyuan Zhu1,2 Hui Liu1,2*
Hui Liu1,2*Objective: The present study aimed to evaluate the predictive factors that predict outcomes of HER2-low breast cancer patients who did not achieve pathological complete response(pCR) after neoadjuvant chemotherapy (NAC).
Methods: This study included patients with HER2-low breast cancer who received NAC from January 2017 to December 2020. Analysis of the clinicopathological features, NAC response and outcome of the patients were retrospectively analyzed. Univariate and multivariable Cox analysis were used to determine factors that predict outcomes of HER2-low breast cancer patients who did not exhibit pCR.
Results: 293 Asian patients were included. The proportion of patients with hormone receptor (HR) positive and triple negative breast cancer (TNBC) among HER2-low patients was 75.8% and 24.2%, respectively. The pCR rate of HR positive cases was significantly lower than TNBC (27.5% vs. 53.5%, P=0.000). The patients who obtained pCR after NAC showed better disease-free survival(DFS) (5-year DFS 93.9% vs. 83.1%, p=0.039). For patients not achieving pCR, multivariable analysis showed that Miller/Payne (MP) grading system (hazard ratio: 0.094; 95% CI: 0.037-0.238; p=0.000) and HR status (hazard ratio: 2.561; 95% CI: 1.100-5.966; p=0.029) were significant independent predictors for DFS. Additionally, The MP grading system was also an independent predictor of overall survival (OS) (hazard ratio: 0.071; 95% CI: 0.019-0.260; p=0.000).
Conclusions: The results of our study show that pathological assessment following NAC offers valuable insights into the survival outcome of HER2-low breast cancer. According to these findings, responses to NAC should be considered when choosing systemic treatment for patients with HER2-low breast cancer.
On the basis of differences in gene expression patterns, breast cancer can be divided into four clinical subtypes that have significant implications for treatment and prognosis: luminal A, luminal B, human epidermal growth factor receptor 2 (HER2) overexpression, and basal-like subtypes (1, 2). Twenty to twenty-five percent of these cases are HER2 positive breast cancers. The HER2 oncogene is a member of the ErbB tumor gene family, and it plays a crucial role in the biological behavior of breast cancer. The development of anti-HER2 targeted therapies, including trastuzumab, lapatinib, pertuzumab, and trastuzumab emtansine (T-DM1), has markedly improved the treatment outcomes of HER2-positive breast cancer patients (3–7). In 2018, the American Society of Clinical Oncology (ASCO) and the College of American Pathologists (CAP) clinical practice guideline proposed that patients whose immunohistochemistry (IHC) results show 0/1+ or IHC 2+ with negative in situ hybridization (ISH) should be diagnosed as having HER2-negative breast cancer, and those with IHC 3+ or IHC 2+ and ISH positive results should be diagnosed as having HER2-positive breast cancer (8).
With the advent of novel antibody-drug conjugate (ADC) drugs, the concept of HER2-low breast cancer has been proposed (9). HER2-low breast cancer refers to cancers with HER2 IHC staining results of 1+ or 2+ and negative ISH, results, and this kind of cancers accounts for 45% to 55% of breast cancer cases (10). HER2-low breast cancer is predominantly classified as HR positive/HER2-negative or TNBC. However, HER2-low and HER2-zero breast cancer differ not only in terms of HER2 protein expression level, but also in terms of estrogen receptor (ER) status, primary tumor volume, lymph node involvement, pathological complete response (pCR) rate after neoadjuvant chemotherapy (NAC), and disease-free survival (DFS) (11, 12). Furthermore, there is a notable difference in OS between HER2-low and HER2-zero breast cancer patients (13, 14). Although HER2-low breast cancer is not yet considered an independent molecular subtype, this does not affect the use of HER2-low breast cancer as a therapeutic target or exploration of the biological behavior of HER2-low breast cancer.
At present, neoadjuvant therapy has emerged as a crucial component of the systemic treatment of breast cancer (15, 16). Guided by the efficacy of neoadjuvant therapy, it is possible to identify patients with suboptimal clinical response, thereby allowing for the optimization of treatment protocols or the enhancement of adjuvant therapy, which may lead to improved prognosis in patients with breast cancer. Previous clinical trials on NAC have established that HER2-low breast cancer had a significantly lower pCR rate than HER2-zero patients (17–19). Further analysis demonstrated that among HR positive breast cancer patients, HER2-low patients typically presents a lower incidence of grade III tumors, a lower Ki-67 proliferation index, and fewer TP53 mutations compared with HER2-zero patients. Currently, few clinical studies have focused on prognostic factors specific to HER2-low breast cancer after NAC. The present study aimed to evaluate factors that predict outcomes in individuals with HER2-low breast cancer who did not achieve pCR after NAC.
In this retrospective analysis, we screened women who were diagnosed with HER2-low breast cancer at Henan Provincial People’s Hospital (Zhengzhou, China) between January 2017 and December 2020. Patient participation in the analysis was contingent upon meeting the following criteria: 1) invasive breast cancer with histological confirmation; 2) HER2-low status as determined by IHC or fluorescence in situ hybridization (FISH); 3) received NAC; and 4) underwent subsequent breast surgery following NAC. The primary exclusion criteria were as follows: 1) lack of surgical intervention following NAC, which precluded any assessment of treatment efficacy on the basis of pathological evaluation; and 2) lack of available follow-up data.
Clinical and pathological data were collected for each patient, including information on therapy, HER2 status, Ki-67 levels, nodal involvement, age at tumor diagnosis, histological type, clinical tumor size, clinical stage, and HR status. The clinical stage was determined using the seventh edition of the tumor−node−metastasis (TNM) staging system established by the American Joint Committee on Cancer (AJCC. A Ki-67 threshold of 30% was selected on the basis of several considerations. First, according to the 2024 guidelines of the Chinese Society of Clinical Oncology (CSCO), a Ki-67 index greater than or equal to 30% is classified as high. Additionally, the International Ki-67 Breast Cancer Working Group (IKWG) noted out that when the Ki-67 index is ≥30%, this index is reliable for the evaluation of prognosis. Furthermore, the major of patients included in this analysis presented with locally advanced breast cancer, with a limited number exhibiting a Ki-67 index less than 20%. IHC was utilized to assess progesterone receptor (PR) and ER, with a cut-off value of ≥1%. Positive ER and/or PR scores were considered to indicate a HR-positive status, whereas negative ER and PR scores were considered to indicate a HR-negative status. The HER2 status was evaluated according to the criteria set forth by the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP), with HER2-low was described as IHC 1+, or IHC 2+ and negative FISH results. HER2 expression data was retrieved from medical records, and reviewed by a pathologist. Currently, there is no global consensus regarding the age threshold for defining young breast cancer patients, with some studies setting this threshold at 35 years of age and some studies setting it at 40 years of age. In this study, we used 35 years old as the threshold for patients with young breast cancer.
NAC was administered to all subjects who were included in this study. The chemotherapy regimens utilized included anthracycline and taxane-based protocols, taxane combined with platinum regimens, and other regimens that aligned with established guidelines. After every two cycles of treatment, all the patients underwent imaging examinations, including MRI and ultrasounds, to assess clinical efficacy of the treatment. The vast majority of these patients received surgical intervention upon completion of all the NAC cycles. However, a small number may have undergone surgery prior to completing the full chemotherapy regimen due to chemical toxicity and other factors. The pathological evaluation criterion for pathological complete response (pCR) following NAC was defined as ypTis/0ypN0, indicating the absence of invasive cancer in the breast (regardless of ductal carcinoma in situ) and axillary lymph nodes.
For patients who did not achieve pCR, the HER2 status of residual disease was determined. The evolution rate means the overall rate of HER2 discordance from primary breast cancer to residual breast cancer, including HER2-low transitioned to HER2-zero (HER2 loss) and HER2-low transitioned to HER2-positive (HER2 gain).
The MP grading system was also used to evaluate the pathological response to NAC. Grade 1: no change or some change in individual malignant cells but no reduction in overall cellularity; Grade 2: a minor loss of tumor cells but overall cellularity remaining high; accounting for up to 30% loss; Grade 3: an estimated reduction in tumor cells between 30% and 90%; Grade 4: a marked disappearance of tumor cells such that only small clusters or widely dispersed individual cells remain; more than 90% loss of tumor cells; Grade 5: no malignant cells identifiable in sections from the site of the tumor, but ductal carcinoma in situ (DCIS) may be present (20).
The clinical characteristics of the groups were compared using descriptive statistics. Fisher’s exact test or Pearson’s chi-squared test were used to compare the differences between the groups. The deadline for a follow-up was set to December 31, 2023. The median follow-up duration of the study patients was 49 months. From the date of surgery to the date of a distant relapse, locoregional relapse, death, or the last follow-up, was defined as disease free survival (DFS). From the date of surgery to the date to the patient’s death or the last follow-up, was defined as overall survival (OS). The Kaplan-Meier method was used to estimate the patient survival curves, and the log-rank test was used to compare them. Univariate and multivariable Cox analysis were used to identify characteristics predictive of survival result in patients without pCR, including age, cT, cN, clinical stage, HR, pre Ki-67, surgery, pT, pN, pathological stage, HER2, post Ki-67 and MP. Software from SPSS 22.0(SPSS Inc., IL, US) was used for all statistical descriptive analyses. P<0.05 was considered significant.
In this investigation, 293 patients with HER2-low breast cancer who received NAC were retrospectively screened. Table 1 presents the characteristics of the patients and their treatments. The proportion of patients with pure invasive breast cancer of no particular type (IBC-NST) in the whole population was 82.3%. The majority of patients included in this analysis had locally advanced breast cancer, the percentages of T3-4 and N2-3 patients were 30.0% and 41.3%, respectively. Among these patients, clinical stage II was observed in 137 individuals (46.8%), whereas stage III was observed in 156 individuals (53.2%). In total, 71 patients were diagnosed with TNBC, whereas HR-positive breast cancer was present in 222 patients (75.8%). A total of 75.8% of the population exhibited a Ki-67 percentage exceeding 30%. In this study, anthracycline- and taxane-based NAC therapy was administered to 96.6% of the patients. Following NAC, mastectomy was the most prevalent surgical procedure, the remaining 18.8% of patients underwent immediate breast reconstruction (IBR) or breast conserving surgery.
The biological activity associated with HER2-low breast cancer is significantly influenced by its HR status. The percentage of clinical stage III breast cancer in TNBC cases was considerably greater than that in their in HR positive counterparts (64.8% vs. 40.5%, P=0.025). Concurrently, there was a significant increase in the percentage of TNBC with Ki-67 > 30% compared with HR positive breast cancer (94.4% vs. 69.8%, P=0.000). A total of 5.6% of TNBC patients received platinum-based treatment. Other patient demographics and therapeutic characteristics were similar between TNBC patients and HR-positive breast cancer.
Ninety-nine patients in the total population attained pCR, yielding a 33.8% pCR rate. Pathological response after NAC are summarized in Table 2. According to the MP grading system, 10 (3.4%) patients had a grade 1 response, 57 (19.5%) patients had a grade 2 response, 80 (27.3%) patients had a grade 3 response, 42 (14.3%) patients had a grade 4 response, and 104 (35.5%) patients had a grade 5 response. The pCR rate in TNBC was significantly higher than in HR positive breast cancer patients (53.5% vs. 27.5%, P=0.000, Figure 1). With respect to the MP grading system, the proportion of MP grade 1 and grade 2 tumors in TNBC was significantly lower than in HR positive breast cancer (12.7% vs. 26.1%, P=0.001). In this study, HER2 status was compared between residual disease after NAC and tumor biopsy specimen in patients who did not achieve pCR. Compared with HR positive patients, patients with TNBC exhibited high instability after NAC. The evolution rate means the overall rate of HER2 discordance from primary breast cancer to residual breast cancer. In patients with TNBC and HR positive disease, the evolution rate of HER2-low disease following NAC was 21.1% and 14.9%, respectively.
Compared with HR positive patients, patients diagnosed with TNBC were more likely to achieve pCR, and the proportion of MP1-2 was also lower.
As shown in this study, the patients who achieved pCR had a more favorable prognosis. Specifically, the 5-year DFS rate for patients who achieved pCR was considerably greater than that for those who did not achieve pCR (93.9% vs. 83.1%, P=0.039, Figure 2a). Conversely, no statistically significant differences were observed in OS survival were observed (89.9% vs. 88.1%, P=0.296, Figure 2b).
Survival analysis was conducted on the 194 patients who did not achieve pCR. For DFS, in univariate analysis, MP grade 3–4 (HR: 0.090; 95% CI: 0.035–0.234; p=0.000) and HR negative status(HR: 2.479; 95% CI: 1.075-5.712; p=0.033) were identified as variables associated with survival outcome among patients who did not achieve pCR (Table 3). Furthermore, MP grade 3–4 (HR: 0.094; 95% CI: 0.037–0.238; p=0.000) and HR negative status (HR: 2.561; 95% CI: 1.100–5.966; p=0.029) were shown to be significant independent predictors of DFS by multivariable analysis (Figure 3, Supplementary Figure S1). The multivariate analysis revealed that the sole significant independent predictor of OS was MP grade 3–4 (HR: 0.071; 95% CI: 0.019–0.260; p=0.000) (Table 4).
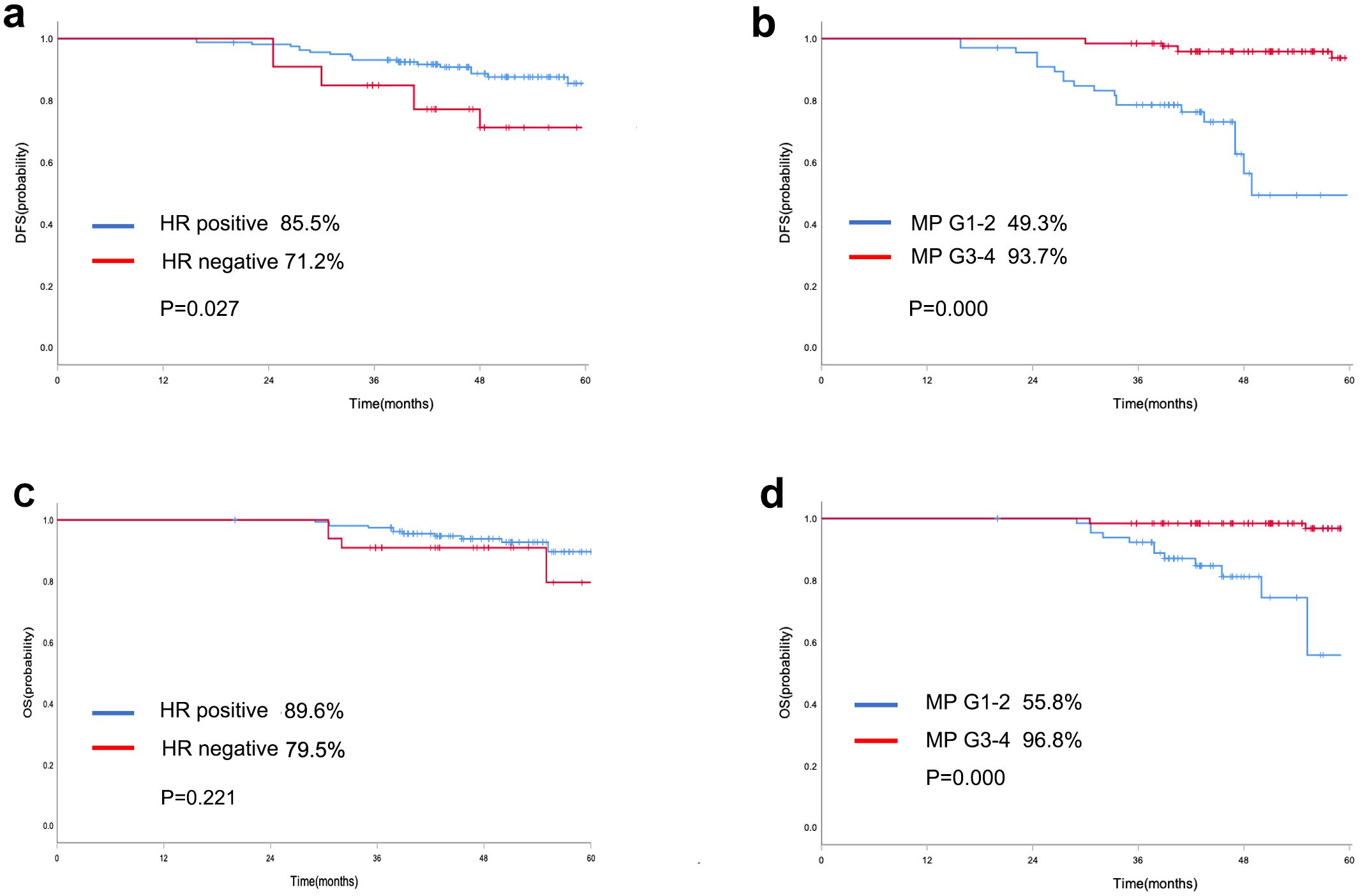
Figure 3. Kaplan-Meier curve of DFS in patients who did not achieve pCR with different HR status (a) and MP grading system (b); Kaplan-Meier curve of OS in patients who did not achieve pCR with different HR status (c) and MP grading system (d).
With the emergence of novel ADC drugs and the release of the DESTINY-Breast 04 and the ASCENT clinical trials results, the concept of HER2-low breast cancer has attracted widespread attention (21–23). In metastatic breast cancer with low HER2 expression, the DESTINY-Breast 04 study assessed the clinical efficacy of trastuzumab deruxtecan (T-DXd) in comparison with that of conventional chemotherapy. The progression free survival (PFS) of the T-DXd group was substantially better than that of the control group (10.1 months vs. 5.4 months; HR 0.51, P < 0.001). This pivotal phase III clinical study, DESTINY-Breast 04, proved that low HER2 expression can serve as a therapeutic category for breast cancer. Do HER2-low breast cancers differ biologically from HER2-zero tumors, making them a separate molecular subtype? The biological properties of HER2-low breast cancer, which is identified by an IHC score of 1+ or 2+/ISH-, have been the subject of numerous investigations. Nevertheless, there have not been consistent findings across studies (24–26). These results suggest that breast cancers with low HER2 status constitute a physiologically diverse group of tumors.
Our previous study indicated that the proportion of HR positive patients in HER2-low breast cancer was higher than in HER2-zero breast cancer patients, which is consistent with findings from several other studies (27, 28). In HER2-low tumors, the major determinant of the gene expression profile is HR status, with the majority of HR positive tumors belonging to the luminal A or B subtype, and the majority of HR-negative tumors classified as the basal-like subtype. After adjusting for HR status, only marginal differences in gene expression between HER2-low and HER2-zero tumors were found, highlighting that these entities are not substantially different in terms of biology (29). Similarly, after adjusting for HR status, no discernible and regular variations in the genomic profiles of HER2-low and HER2-zero tumors were discovered in large-scale genomic investigations. HER2-low breast cancer should not be considered a distinct molecular subtype, but HER2-low can be used as a therapeutic target and the biological behavior of HER2-low breast cancer need further exploration (30).
In a pooled analysis of four prospective neoadjuvant clinical trials (GeparSepto, GeparOcto, GeparX, Gain-2 neoadjuvant), HER2-low breast cancer presented a significantly lower pCR rate than HER2-zero patients did (17). However, there are also studies that have yielded inconsistent results. The differences in pCR rate between HER2-low and HER2-zero patients may be associated with the definition of pCR and HR status. In the present study, 33.8% of patients achieved pCR, and the pCR rate in TNBC was significantly higher than that observed in HR positive breast cancer patients. Specifically, the pCR rate of HR-positive patients was only 27.5%; thus, optimizing the NAC regimen is challenging, and unmet clinical needs remain. ADC drugs are expected to have potential application in the use of neoadjuvant therapy to treat early HER2 positive and HER2-low breast cancer patients.
T-DXd monotherapy or T-DXd sequential THP compared with ddAC-THP is being actively studied in the clinical trial DESTINY-Breast11 (DB11) for high-risk HER2-positive early breast cancer. This novel neo-adjuvant therapy scheme is expected to further increase the pCR rate of patients and reduce the treatment burden and overall toxicity of patients. A phase II study of TALENT (TRIO-USB-12) aimed to evaluate the clinical efficacy and safety of T-DXd monotherapy or T-DXd combined with endocrine therapy in the treatment of early HR positive HER2-low breast cancer patients. By the data cut-off date, the objective response rate (ORR) of T-DXd monotherapy group was 68%(17/25), whereas that of T-DXd combined with endocrine therapy group was 58%(14/24). The results showed that the ORR of T-DXd monotherapy are equivalent to the existing data of aromatase inhibitor neoadjuvant therapy for HR+/HER2 negative breast cancer. Although the data about the pCR rate remain immature at this stage, the existing data of efficacy and safety data show that T-DXd neoadjuvant therapy is worth exploring.
Currently, few clinical studies have focused on prognostic factors for HER2-low breast cancer after NAC. In this study, we assess the predictors of outcomes in patients with HER2-low breast cancer following NAC. Better DFS is associated with achieving a pathological complete response to NAC. However, no statistically significant differences in OS were observed. pCR has been proposed as a surrogate endpoint for prediction of long‐term survival for breast cancer receiving NAC (31). However, the issue remains controversial (32–34). According to the CTNeoBC pooled analysis of NAC clinical trials, among patients with TNBC, the strongest correlation was observed between pCR and long-term outcomes(EFS: HR 0.24, 95% CI 0.18-0.33; OS: 0.16, 0.11-0.25), particularly among patients with HR-negative/HER2-positive cancers. Our present study explored the relationship between pCR and survival outcomes in HER2-low breast cancer patients. With pCR defined as ypTis/0ypN0, HER2-low breast cancer patients who achieved pCR had better DFS. Thus, pCR may be serve as a predictor for DFS in HER2-low breast cancer. The question of whether the pCR obtained after NAC can be transformed into the long-term survival benefit of the whole population of patients with breast cancer is still controversial.
Our findings show that the dichotomization of patients is a significant limitation of pCR. The response of NAC varied greatly for patients who did not achieve pCR. The vast majority of DFS events occur in patients who have not obtained pCR. Therefore, a prognostic study was carried out in patients who did not achieve pCR. The pathology evaluation system that is currently most widely utilized in China is the Miller−Payne grading system. Previous studies confirmed that MP grading system was a useful supplement and could provide more prognostic information besides pCR (35, 36). However, the prognostic value of MP grading system in HER2-low breast cancer have not yet been reported. In this study, multivariable analysis demonstrated that MP grading system was significant independent predictor of DFS and OS, indicating that pathological evaluation after NAC provide important information of survival outcome for HER2-low breast cancer. Neoadjuvant therapy allows clinicians to optimize intensive adjuvant therapy based on the patient’s response to therapy. The CREATE-X and KATHERINE studies showed that capecitabine and T-DM1 improved 5-year DFS and 3-year iDFS rates by 13.7% and 11.3%, respectively, in TNBC and HER2-positive breast cancer patients who did not achieve pCR (37, 38). Similarly, unmet clinical needs remain in HER2-low breast cancer patients who do not achieve pCR, and intensive adjuvant therapy may also be necessary.
In the context of neoadjuvant therapy, HR-positive breast cancer is less sensitive to treatment, and the PCR rate is usually lower than that of triple-negative breast cancer. However, many clinical studies have shown that a high expression of HR is related to a better prognosis. This may be attributed to the use of endocrine therapy for HR-positive breast cancer patients.
The present study does have some limitations. First, this was a retrospective study. Second, the number of cases included in this study was not very large.
Prospective clinical research is needed to verify the value of MP grading system in HER2-low breast cancer. Third, HER2 expression data was retrieved from medical records, and reviewed by the pathologist, these data were not evaluated by multiple pathologists. Nevertheless, to our knowledge our study provides information on factors that predict outcomes of HER2-low breast cancer patients after NAC for the first time.
The results of our study show that pathological assessment following NAC offers valuable insights into the survival outcome of HER2-low breast cancer. On the basis of these findings, patients with HER2-low breast cancer should have their response to NAC taken into account when choosing a systemic treatment.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the ethics committee of the Henan Provincial People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
YS: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. HG: Investigation, Methodology, Writing – review & editing. ZL: Data curation, Investigation, Writing – review & editing. YY: Data curation, Formal analysis, Writing – review & editing. YH: Formal analysis, Writing – review & editing. QC: Formal analysis, Resources, Writing – review & editing. CL: Data curation, Methodology, Writing – review & editing. FZ: Data curation, Methodology, Writing – original draft, Writing – review & editing. HL: Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Medical Science and Technique Foundation of Henan Province (No. LHGJ20210055) and Beijing Medical Award Foundation Project (No.YXJL-2020-0941-0748).
We gratefully acknowledge the follow-up team for their contribution to this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1459444/full#supplementary-material
Supplementary Figure 1 | The nomogram to predict DFS of patients based on HR and MP grading system.
1. Perou CM, Sorlie T, Eisen MB, Rijn M, Jeffrey S, Rees C, et al. Molecular portraits of human breast tumours. Nature. (2000) 406:747–52. doi: 10.1038/35021093
2. Cancer Genome Atlas N. Comprehensive molecular portraits of human breast tumours. Nature. (2012) 490:61–70. doi: 10.1038/nature11412
3. Perez EA, Romond EH, Suman VJ, Jeong JH, Sledge S, Geyer CE, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. (2014) 32:3744–52. doi: 10.1200/JCO.2014.55.5730
4. Gianni L, Pienkowski T, Im YH, Tseng LM, Liu MC, Lluch A, et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol. (2016) 17:791–800. doi: 10.1016/S1470-2045(16)00163-7
5. Tolaney SM, Guo H, Pernas S, Barry WT, Dillon DA, Ritterhouse L, et al. Seven-year follow-up analysis of adjuvant paclitaxel and trastuzumab trial for node-negative, human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. (2019) 37:1868–75. doi: 10.1200/JCO.19.00066
6. Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. (2006) 355:2733–43. doi: 10.1056/NEJMoa064320
7. Modi S, Saura C, Yamashita T, Park YH, Kim SB, Tamura K, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. (2020) 382:610–21. doi: 10.1056/NEJMoa1914510
8. Wolff AC, Hammond MEH, Allison KH, Allison KH, Harvey BE, Mangu PB, et al. Human epidermal growth factor receptor 2 testing in breast cancer: american society of clinical oncology/college of american pathologists clinical practice guideline focused update. J Clin Oncol. (2018) 36:2105–22. doi: 10.1200/JCO.2018.77.8738
9. Modi S, Park H, Murthy RK, Iwata H, Tamura K, Tsurutani J, et al. Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low-expressing advanced breast cancer: results from a phase ib study. J Clin Oncol. (2020) 38:1887–96. doi: 10.1200/JCO.19.02318
10. Shao Y, Yu Y, Luo Z, Guan H, Zhu F, He Y, et al. Clinical, pathological complete response, and prognosis characteristics of HER2-low breast cancer in the neoadjuvant chemotherapy setting: A retrospective analysis. Ann Surg Oncol. (2022) 29:8026–34. doi: 10.1245/s10434-022-12369-4
11. Schettini F, Chic N, Braso-Maristany F, Paré L, Pascual T, Conte B, et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer. (2021) 7:1. doi: 10.1038/s41523-020-00208-2
12. Zhang G, Ren C, Li C, Li C, Wang Y, Chen B, et al. Distinct clinical and somatic mutational features of breast tumors with high-, low-, or non-expressing human epidermal growth factor receptor 2 status. BMC Med. (2022) 20:142. doi: 10.1186/s12916-022-02346-9
13. Li C, Yuan Q, Deng T, Xu G, Hou J, Zhang L, et al. Prognosis difference between HER2-low and HER2-zero breast cancer patients: a systematic review and meta-analysis. Breast Cancer. (2023) 30:965–75. doi: 10.1007/s12282-023-01487-w
14. Molinelli C, Jacobs F, Agostinetto E, Nader-Marta G, Ceppi M, Bruzzone M, et al. Prognostic value of HER2-low status in breast cancer: a systematic review and meta-analysis. ESMO Open. (2023) 8:101592. doi: 10.1016/j.esmoop.2023.101592
15. Esteva FJ, Katz E. Tailoring neoadjuvant therapy in human epidermal growth factor receptor 2-positive early breast cancer: recent advances and strategies. JCO Oncol Pract. (2024), OP2300563. doi: 10.1200/OP.23.00563
16. Cantini L, Trapani D, Guidi L, Bielo LB, Scafetta R, Koziej M, et al. Neoadjuvant therapy in hormone Receptor-Positive/HER2-Negative breast cancer. Cancer Treat Rev. (2024) 123:102669. doi: 10.1016/j.ctrv.2023.102669
17. Denkert C, Seither F, Schneeweiss A, Link T, Blohmer JU, Just M, et al. Clinical and molecular characteristics of HER2-low-positive breast cancer: pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol. (2021) 22:1151–61. doi: 10.1016/S1470-2045(21)00301-6
18. Guan D, Shi Q, Zheng Y, Zheng C, Meng X. Real-world data on pathological response and survival outcomes after neoadjuvant chemotherapy in HER2-low breast cancer patients. Clin Breast Cancer. (2024). doi: 10.1016/j.clbc.2024.04.006
19. Zhong G, Song D, Lou W, Wei B, Chen YM, Cui H, et al. Pathological complete response rate and clinical outcome after neoadjuvant therapy of HER2-low breast cancer: A National Cancer Database Analysis. Eur J Surg Oncol. (2023) 49:106970. doi: 10.1016/j.ejso.2023.06.022
20. Ogston KN, Miller ID, Payne S, Sarkar TK, Smith I, Schofield A, et al. A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast. (2003) 12:320–7. doi: 10.1016/S0960-9776(03)00106-1
21. Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. (2022) 387:9–20. doi: 10.1056/NEJMoa2203690
22. Bardia A, Hurvitz SA, Tolaney SM, Loirat D, Punie K, Oliveira M, et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med. (2021) 384:1529–41. doi: 10.1056/NEJMoa2028485
23. Bardia A, Rugo HS, Tolaney SM, Loirat D, Punie K, Oliveira M, et al. Final results from the randomized phase III ASCENT clinical trial in metastatic triple-negative breast cancer and association of outcomes by human epidermal growth factor receptor 2 and trophoblast cell surface antigen 2 expression. J Clin Oncol. (2024) 42:1738–44. doi: 10.1200/JCO.23.01409
24. Brevet M, Li Z, Parwani A. Computational pathology in the identification of HER2-low breast cancer: Opportunities and challenges. J Pathol Inform. (2024) 15:100343. doi: 10.1016/j.jpi.2023.100343
25. Khalil MA, Habibian L, Martin C, Semaan K, Khaddage A, Kassis NE, et al. Landscape of HER2-low breast cancer: Insights from a six-year study on prevalence and clinicopathological characteristics. Ann Diagn Pathol. (2024) 72:152326. doi: 10.1016/j.anndiagpath.2024.152326
26. Han BY, Chen C, Luo H, Lin CJ, Han XC, Nasir J, et al. Clinical sequencing defines the somatic and germline mutation landscapes of Chinese HER2-Low Breast Cancer. Cancer Lett. (2024) 588:216763. doi: 10.1016/j.canlet.2024.216763
27. Check DK, Jackson BE, Reeder-Hayes KE, Dinan MA, Faherty E, Kwong J, et al. Characteristics, healthcare utilization, and outcomes of patients with HER2-low breast cancer. Breast Cancer Res Treat. (2024) 203:329–38. doi: 10.1007/s10549-023-07142-4
28. Won HS, Ahn J, Kim Y, Kim JS, Song JY, Kim HK, et al. Clinical significance of HER2-low expression in early breast cancer: a nationwide study from the Korean Breast Cancer Society. Breast Cancer Res. (2022) 24:22. doi: 10.1186/s13058-022-01519-x
29. Agostinetto E, Rediti M, Fimereli D, Debien V, Piccart M, Aftimos P, et al. HER2-low breast cancer: molecular characteristics and prognosis. Cancers (Basel). (2021) 13. doi: 10.3390/cancers13112824
30. Tarantino P, Viale G, Press MF, Hu X, Llorca FP, Bardia A, et al. ESMO expert consensus statements (ECS) on the definition, diagnosis, and management of HER2-low breast cancer. Ann Oncol. (2023) 34:645–59. doi: 10.1016/j.annonc.2023.05.008
31. Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. (2014) 384:164–72. doi: 10.1016/S0140-6736(13)62422-8
32. De-Mattos-Arruda L, Shen R, Reis-Filho JS, Cortes J. Translating neoadjuvant therapy into survival benefits: one size does not fit all. Nat Rev Clin Oncol. (2016) 13:566–79. doi: 10.1038/nrclinonc.2016.35
33. Baselga J, Bradbury I, Eidtmann H, Cosimo SD, Azambuja ED, Aura C, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. (2012) 379:633–40. doi: 10.1016/S0140-6736(11)61847-3
34. Piccart-Gebhart M, Holmes E, Baselga J, Azambuja ED, Dueck AC, Viale G, et al. Adjuvant lapatinib and trastuzumab for early human epidermal growth factor receptor 2-positive breast cancer: results from the randomized phase III adjuvant lapatinib and/or trastuzumab treatment optimization trial. J Clin Oncol. (2016) 34:1034–42. doi: 10.1200/JCO.2015.62.1797
35. Wang L, Luo R, Lu Q, Jiang K, Hong R, Lee K, et al. Miller-payne grading and 70-gene signature are associated with prognosis of hormone receptor-positive, human epidermal growth factor receptor 2-negative early-stage breast cancer after neoadjuvant chemotherapy. Front Oncol. (2021) 11:735670. doi: 10.3389/fonc.2021.735670
36. Hou W, Yao Q, Niu DF, Xue WC. Clinicopathological characteristics related to Miller/Payne grading system of breast carcinoma after neoadjuvant therapy and establishment of novel prediction models. Zhonghua Bing Li Xue Za Zhi. (2022) 51:743–8. doi: 10.3760/cma.j.cn112151-20220413-00277
37. Masuda N, Lee SJ, Ohtani S, Im YH, Lee ES, Yokota I, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. (2017) 376:2147–59. doi: 10.1056/NEJMoa1612645
Keywords: breast cancer, neoadjuvant chemotherapy, HER2-low expression, pathological complete response, prognosis
Citation: Shao Y, Guan H, Luo Z, Yu Y, He Y, Chen Q, Liu C, Zhu F and Liu H (2025) Predictive factors for outcome in HER2-low breast cancer patients after neoadjuvant chemotherapy. Front. Oncol. 15:1459444. doi: 10.3389/fonc.2025.1459444
Received: 04 July 2024; Accepted: 14 February 2025;
Published: 05 March 2025.
Edited by:
Xiaowei Qi, Army Medical University, ChinaReviewed by:
Chengxin Li, The University of Hong Kong, Hong Kong SAR, ChinaCopyright © 2025 Shao, Guan, Luo, Yu, He, Chen, Liu, Zhu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Liu, cnhsaXVodWlAenp1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.