
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 14 February 2025
Sec. Genitourinary Oncology
Volume 15 - 2025 | https://doi.org/10.3389/fonc.2025.1436015
Solitary fibrous tumor (SFT) is widely present in human connective tissues. It is mostly found in the pleura and other extrapleural sites. There is limited literature on malignant SFT in the retroperitoneum. In this study, we retrospectively reviewed the clinical data of a case of bilateral malignant SFT patient admitted to our hospital in May 2021.The patient had a huge tumor load and multiple bone metastases at the first diagnosis. Due to the need for lifelong hemodialysis after double nephrectomy and multiple bone metastases, the patient gave up surgical treatment, underwent drug treatment, and finally survived for 30 months.
Solitary fibrous tumor (SFT) is a rare spindle cell tumor that originates from dendritic interstitial cells expressing the CD34 antigen, accounting for less than 2% of all soft tissue tumors. First reported by Klemperer and Rabinz in 1931, SFT is predominantly found in human connective tissues, particularly in the pleura and other extrapleural sites. Despite its rarity, SFT has been increasingly recognized in recent years, with a growing body of literature focusing on its clinical presentation, pathological features, and management. However, literature on malignant SFT, especially in the retroperitoneum, remains limited. This study aims to contribute to the existing body of knowledge by presenting a detailed case report of a patient with bilateral malignant SFT, highlighting the diagnostic challenges and treatment outcomes. The main question addressed in this study is the clinical and pathological characteristics of bilateral malignant SFT, with a focus on the novelty of the case in terms of its presentation and management.
The patient, a 67-year-old man, was admitted to our hospital in May 21, 2021 due to low back pain. The physical examination results showed that a mass of about 20 cm × 10 cm was found in the right kidney area and a mass of about 5 cm × 5 cm was found in the left kidney area. The masses on both sides are hard, fixed, and have no obvious tenderness. There was mild tenderness in each vertebra. The tumor markers at the initial diagnosis of the patient were as follows—CEA: 3.64 ng/mL, CA19-9: 20.35 U/mL, and CA125: 18.42 U/mL, and no abnormalities were detected. The thoraco-abdominal pelvic CT image showed multiple space-occupying lesions in both renal areas as well as dilatation of the pelvis calyces and the upper ureter. Multiple low-density shadows can be seen in the left adnexa of T5, the right posterior part of the T8 vertebra, and the right adnexa of T12 and S1. Upon double renal MRI, it was noted that the volume of both kidneys increased and the shape was irregular, multiple round and irregular masses were seen, and the enhancement was uneven and enhanced. The larger one was located in the right kidney, the size was about 179 × 121 × 135 mm, and the bilateral renal pelvis was deformed under pressure (Figure 1A). At the thoracic 12 and right adnexal region, sacrum partial right side bone metastasis is possible (Figure 1B). Based on the patient’s symptoms, signs, and imaging findings, the possible differential diagnosis of bilateral giant renal masses includes biphasic renal tumors, renal cell carcinoma, renal pelvis carcinoma, renal lymphoma, etc. To further clarify the diagnosis, ultrasound-guided percutaneous double kidney and sternal vertebra-occupying biopsy was performed on May 27, 2021, and the pathology combined with immunohistochemical staining (Figures 2A–F) was consistent with isolated fibrous tumors. The immunohistochemical staining results were as follows: CKpan (-), vimentin (+), EMA (partial +), Ki-67 (about 10%+), SSTR2 (-), S100 (-), HMB45 (-), desmin (-), SMA (partial +), PAX-8 (-), CD34 (partial +), P16 (partial +), MDM2 (-), PR (-), GFAP (-), CD99 (+), actin (-), STAT6 (+), and Bcl-2 (+). The final pathological diagnosis was SFT of the kidney with multiple bone metastases. In June 2021, sorafenib (400 mg bid) was given, and the condition was stable after regular reexamination. In October 2023, he was readmitted to the hospital due to recurrent hypoglycemic coma, considered paraneoplastic syndrome (non-islet cell neoplastic hypoglycemia), and received continued glucose pumping. A reexamination by abdominal CT showed that the mass of both kidneys was larger than before and the disease was progressing. On October 20, 2023 and November 11, 2023, he was treated with temozolomide 200 mg d1-2, 300 mg d3-7 + bevacizumab 300 mg d8 Q2w. The patient developed sudden abdominal pain with hematuria on December 18, 2023. Relevant laboratory tests showed bilateral renal tumor rupture, bleeding, and renal failure, for which he received hemodialysis, renal vascular embolization, and other treatments. His condition was critical, and he eventually died of a lung infection in December 2023.
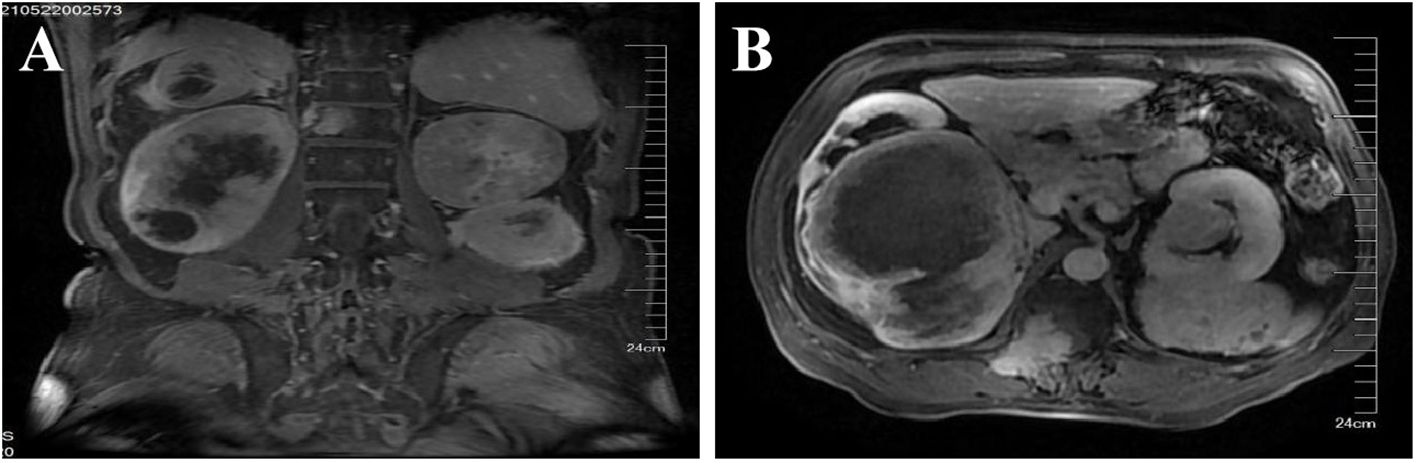
Figure 1. MRI of both kidneys. Both kidneys are enlarged in volume and irregular in shape, and multiple round and irregular masses can be seen, with uneven enhancement. The larger one is located in the right kidney, with a size of about 179 × 121 × 135 mm, and the bilateral renal pelvis is deformed by compression. Bone metastasis of chest 12, right adnexal area, and right sacrum may occur. (A) Sagittal position. (B) Coronal position.
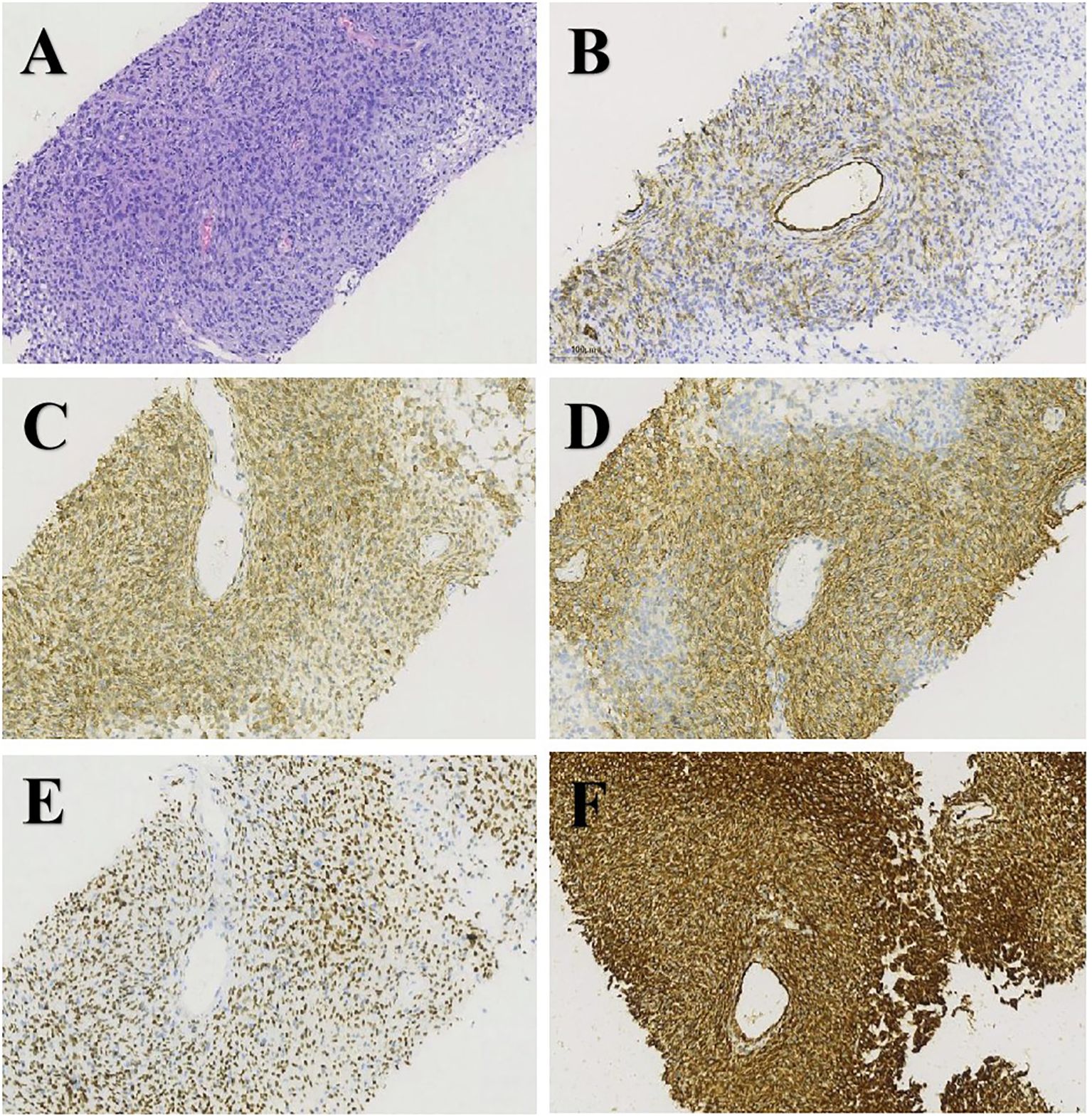
Figure 2. H&E staining and immunohistochemistry of biopsy specimens: (A) H&E (×200), (B) CD34 (×200), (C) Bcl-2 (×200), (D) CD99 (×200), (E) STAT6 (×200), and (F) vimentin (×200).
SFT are typically diagnosed in adults over 40 years of age, with no significant difference observed between male and female individuals. Approximately 15% to 20% of SFTs exhibit invasive behavior, potentially involving both systemic soft tissues and the central nervous system (1). According to the 2013 WHO classification, soft tissue tumors characterized by excessive cellularity, active mitotic activity (>4 mitotic figures per 10 high-power fields), cellular atypia, necrosis, and/or marginal infiltrative growth are classified as malignant (2).
Malignant retroperitoneal SFT often presents as painless abdominal masses, most of which have inert growth and generally have no obvious clinical symptoms. When the masses are large, they can press on adjacent structures and cause corresponding symptoms (3). Kidney SFT may have hematuria, back pain, back mass, and other symptoms. Moreover, SFT is associated with paraneoplastic syndromes, such as Doege–Potter syndrome and Pierre-Marie–Bamberger syndrome (4). These syndromes are systemic symptoms caused by biologically active substances secreted by tumors and are not related to direct invasion or metastasis of the tumor. Doege–Potter syndrome is mainly characterized by severe hypoglycemic symptoms, which are caused by the secretion of insulin-like growth factor II (IGF-II) by SFT. Pierre-Marie–Banberger syndrome is mainly characterized by white patches on the genital skin, which may be accompanied by itching or pain, and is caused by skin lesions due to abnormal immune system reactions triggered by SFT. The patient was admitted to the hospital due to the discovery of lumps in both kidney areas during a physical examination. Later on, symptoms such as hematuria, lower back pain, recurrent hypoglycemia, and itching of the skin around the genitals appeared, but no obvious white spot lesions were observed. It can be seen that the patient’s clinical manifestations are basically consistent with the abovementioned clinical characteristics.
The differential diagnosis for bilateral large renal masses includes biphasic renal tumors, renal cell carcinoma (RCC), urothelial carcinoma, and renal lymphoma. The biphasic tumors show imaging heterogeneity with irregular borders and may have necrosis and cystic changes; CK7 and CK20 are often positive (5). RCC, the most common renal malignancy, appears as irregular solid masses with heterogeneous enhancement on CT/MRI; PAX8, CAIX, and CD10 are positive. Urothelial carcinoma presents as masses in the renal pelvis or ureter, often with hydronephrosis and filling defects; CK7, CK20, and p53 are markers. Renal lymphoma features multiple low-density masses with mild to moderate enhancement; CD20, CD3, and BCL-2 are positive. Each condition has distinct symptoms and imaging characteristics (6).
Pathological diagnosis is the gold standard for disease diagnosis. Typical immunohistochemical manifestations of SFT are being positive for CD34 and nuclear STAT6 and positive for other immune markers such as Bcl-2, vimentin, and CD99. CD34 and STAT6 are usually absent in some dedifferentiated SFT (7). Ki-67 >5% is conducive to the identification of benign and malignant SFT and is considered to be an independent factor in predicting malignant potential (8). In addition, the presence of NAB2–STAT6 fusion protein detected by molecular pathology can also indicate the diagnosis of SFT, especially in cases of atypical histopathological features (9). This fusion gene promotes tumor cell proliferation and survival by activating the STAT6 signaling pathway. In normal function, NAB2 and STAT6 are involved in transcriptional regulation, cell differentiation, and immune regulation, respectively. The detection of NAB2–STAT6 fusion gene not only helps diagnose SFT but also provides potential targets for prognosis evaluation and targeted therapy. The patient’s immunohistochemical results conform to the abovementioned description, but the patient did not undergo molecular pathological testing.
At present, there are no standardized guidelines for the treatment of SFT, and multidisciplinary discussions are needed to find a better treatment plan (10). The main treatment is surgical resection, but it is prone to recurrence. Treatment for advanced and malignant cases is very limited due to poor response to conventional chemoradiotherapy, so research for systemic therapy is moving toward targeted agents and immune checkpoint inhibitors. At present, there are two main types of anti-angiogenic drugs: monoclonal antibodies (such as bevacizumab) (11) and tyrosine kinase inhibitors (such as sorafenib, pazopanib, regorafenib, etc.) (12–14). Secondly, immune checkpoint inhibitors (ICI) may have certain curative effects, but further large-scale clinical studies are needed (15, 16).
In the present case, the patient presented with a massive tumor burden and multiple bone metastases at the initial diagnosis. Given the need for lifelong hemodialysis following bilateral nephrectomy and the presence of multiple bone metastases, surgical treatment was deemed infeasible, and the patient underwent medical therapy. The patient was initially treated with sorafenib (400 mg bid), which stabilized the condition upon regular reexamination. However, the patient was readmitted in October 2023 due to recurrent hypoglycemic coma, which was considered a paraneoplastic syndrome (non-islet cell tumor hypoglycemia). A subsequent treatment with temozolomide and bevacizumab was initiated, but the patient’s condition progressed, with the renal masses increasing in size. The patient eventually succumbed to a lung infection in December 2023 after experiencing sudden abdominal pain with hematuria, which was attributed to bilateral renal tumor rupture and bleeding.
In summary, malignant retroperitoneal solitary fibrous tumors (SFTs) are rare in clinical practice, and the existing literature primarily consists of retrospective studies on the diagnosis and treatment of SFT patients as well as related case reports. The clinical manifestations of these patients lack specific characteristics, making it essential for clinicians to perform pathological and immunohistochemical examinations to ensure accurate diagnosis and treatment, thereby minimizing the risk of misdiagnosis. Complete resection of the tumor is an effective method for the treatment of malignant retroperitoneal SFT, and adjuvant chemoradiotherapy is provided after surgery if necessary. The increasing focus on targeted therapies and immunosuppressants in the management of advanced SFT highlights the need for more standardized and prospective research to address the current gaps in this field.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
YC: Writing – original draft. JL: Writing – original draft. BL: Writing – review & editing. LH: Writing – review & editing. HZ: Funding acquisition, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Supported by Hebei Youth Science and Technology Program (Department of Health), Project number: 20220882; and Hebei Provincial Government-Funded Outstanding Clinical Medical Talents Program, Project number: ZF2025031; and Hebei Natural Science Foundation, Project number: H2021307017.
The authors would like to express their gratitude to EditSprings (https://www.editsprings.cn) for the expert linguistic services provided.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sbaraglia M, Bellan E, Dei TA. The 2020 WHO Classification of Soft Tissue Tumours: news and perspectives. Pathologica. (2021) 113:70–84. doi: 10.32074/1591-951X-213
2. Rosenberg AE. WHO Classification of Soft Tissue and Bone, fourth edition: summary and commentary. Curr Opin Oncol. (2013) 25:571–3. doi: 10.1097/01.cco.0000432522.16734.2d
3. Putra LS, Santoso RB, Harahap EU, Cahyanti D, Bramono IA, Hamid ARAH. Solitary fibrous tumor in the retroperitoneum: A case report. Int J Surg Case Rep. (2024) 114:109118. doi: 10.1016/j.ijscr.2023.109118
4. Hanif M, Jaiswal V, Naz S, Patel N, Pokhrel NB, Vadiyala MR. Malignant fibroma presenting as a hypoglycemia and coma in a 45-year-old male patient: A case report. Clin Case Rep. (2022) 10:e6627. doi: 10.1002/ccr3.v10.11
5. Mudaliar KM, Mehta V, Gupta GN, Picken MM. Expanding the morphologic spectrum of adult biphasic renal tumors-mixed epithelial and stromal tumor of the kidney with focal papillary renal cell carcinoma. Int J Surg Pathology. (2014) 22:266. doi: 10.1177/1066896913488823
6. Mittal MK, Sureka B. Solid renal masses in adults. Indian J Radiology Imaging. (2016) 26:429–42. doi: 10.4103/0971-3026.195773
7. Vincek V, Kallis P, Vause A, Vincek E, Ilkovitch D, Motaparthi K. Cutaneous solitary fibrous tumor: Report of three cases with review of histopathological mimics. J Cutan Pathol. (2022) 49:167–71. doi: 10.1111/cup.14138
8. Sugita S, Segawa K, Kikuchi N, Takenami T, Kido T, Emori M, et al. Prognostic usefulness of a modified risk model for solitary fibrous tumor that includes the Ki-67 labeling index. World J Surg Oncol. (2022) 20:29. doi: 10.1186/s12957-022-02497-2
9. Nonaka H, Kandori S, Nitta S, Shiga M, Nagumo Y, Kimura T, et al. Case report: molecular characterization of aggressive Malignant retroperitoneal solitary fibrous tumor: A case study. Front Oncol. (2021) 11:736969. doi: 10.3389/fonc.2021.736969
10. Janik AM, Terlecka A, Spalek MJ, Boye K, Szostakowski B, Chmiel P, et al. Diagnostics and treatment of extrameningeal solitary fibrous tumors. Cancers (Basel). (2023) 15. doi: 10.3390/cancers15245854
11. Valentin T, Fournier C, Penel N, Trent JC, Conrad CA, Lazar AJ, et al. Sorafenib in patients with progressive Malignant solitary fibrous tumors: a subgroup analysis from a phase II study of the French Sarcoma Group (GSF/GETO). Invest New Drugs. (2013) 31:1626–7. doi: 10.1007/s10637-013-0023-z
12. Maruzzo M, Martin-Liberal J, Messiou C, Bompas E, Chaigneau L, Isambert N, et al. Pazopanib as first line treatment for solitary fibrous tumours: the Royal Marsden Hospital experience. Clin Sarcoma Res. (2015) 5:5. doi: 10.1186/s13569-015-0022-2
13. Stacchiotti S, Baldi GG, Frezza AM, Miah A, Thway K, Alvarado R, et al. Regorafenib in advanced solitary fibrous tumour: Results from an exploratory phase II clinical study. Eur J Cancer. (2023) 195:113391. doi: 10.1016/j.ejca.2023.113391
14. Boothe JT, Budd GT, Smolkin MB, Morosi C, Greco FG, Collini P, et al. Durable near-complete response to anti-PD-1 checkpoint immunotherapy in a refractory Malignant solitary fibrous tumor of the pleura. Case Rep Oncol. (2017) 10:998–1005. doi: 10.1159/000484041
15. Toulmonde M, Penel N, Adam J, Ma PC. Use of PD-1 targeting, macrophage infiltration, and IDO pathway activation in sarcomas: A phase 2 clinical trial. JAMA Oncol. (2018) 4:93–7. doi: 10.1001/jamaoncol.2017.1617
Keywords: malignant solitary fibroma tumor, CD34, STAT6, paraneoplastic syndrome, therapy
Citation: Cui Y, Li B, Liang J, He L and Zhang H (2025) Case report: A study on disease diagnosis and treatment outcome of a case of bilateral malignant solitary fibrous tumor of the kidneys. Front. Oncol. 15:1436015. doi: 10.3389/fonc.2025.1436015
Received: 19 July 2024; Accepted: 21 January 2025;
Published: 14 February 2025.
Edited by:
Gianandrea Pasquinelli, University of Bologna, ItalyCopyright © 2025 Cui, Li, Liang, He and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongzhen Zhang, emhpZHUwNjkwMDlAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.