
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 06 March 2025
Sec. Gastrointestinal Cancers: Gastric and Esophageal Cancers
Volume 15 - 2025 | https://doi.org/10.3389/fonc.2025.1432596
Objectives: To report the latest systematic review and meta-analysis of randomized controlled trials (RCT) to compare perioperative versus adjuvant chemotherapy for resectable gastric cancer.
Methods: We conducted a systematic literature retrieval via PubMed, Embase, Web of Science, and Cochrane until April, 2024 for RCT which compared perioperative versus adjuvant chemotherapy for resectable gastric cancer. Outcomes measured were overall survival (OS) and progression-free survival (PFS).
Results: 5 RCTs including 2,735 patients were included for meta-analysis. Meta-analysis revealed a significant longer PFS in the neoadjuvant chemotherapy (NAC) group (HR: 0.77; 95% CI: 0.69, 0.85; P<0.00001) compared with adjuvant chemotherapy (AC) group. Subgroup analysis found that there was still a significant superiority of NAC in female (HR: 0.53; 95% CI: 0.40, 0.70; P<0.0001) and cN+ (HR: 0.77; 95% CI: 0.67, 0.89; P=0.0005) patients, while the superiority disappeared in male (HR: 0.87; 95% CI: 0.74, 1.01; P=0.07) and cN- patients (HR: 0.91; 95% CI: 0.46, 1.78; P=0.77). In addition, meta-analysis observed a trend towards improved OS with NAC (HR: 0.86; 95% CI: 0.70, 1.07; P = 0.17), and sensitivity analysis demonstrated instability in OS.
Conclusions: NAC can significantly prolong PFS in patients with resectable gastric cancer compared to AC, and the benefit is more significant in women and cN+ patients. Besides, our analysis indicated that NAC has a potential to improve OS compared with AC.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42024546165.
Gastric cancer is the fifth most common malignancy worldwide and the third cause of cancer death (1). China is a country with a high incidence of gastric cancer. In 2015, the incidence and mortality rate of gastric cancer in China ranked second among all malignant tumors, second only to lung cancer (2). At present, the treatment of gastric cancer mainly includes surgery, chemotherapy, radiotherapy, targeted drug therapy and immunotherapy. For locally advanced gastric cancer, D2 radical gastrectomy is the standard operation (3–6), and D2 operation plus adjuvant chemotherapy (AC) is the standard treatment in Asian country, including China, Korea and Japan (7). The ACTS-GC study conducted in Japan in 2007 (8) fully demonstrated the superiority of postoperative AC in prolongating the survival of patients. In this study, 1059 patients with locally advanced gastric cancer who received D2 radical resection were enrolled, and these patients were divided into S-1 single-agent AC group and operation group. The results of this study showed that the 3-year survival rate of the two groups was different, with the 3-year survival rate of the AC group being 72.2% and that of the surgery group being 59.6%. The 3-year survival rate was 12.6% higher in the AC group (HR: 0.62, 95%CI: 0.50-0.77).
At the end of the 20th century, the term neoadjuvant chemotherapy (NAC) was first proposed by Frie (9). NAC refers to chemotherapy after a patient is diagnosed with cancer, before surgery or radiation therapy, also known as preoperative chemotherapy. In this context, perioperative chemotherapy refers to NAC combined with adjuvant chemotherapy. MAGIC study (10) applied perioperative ECF protocol to enrolled patients with resectable gastric cancer and esophagogastric junctional adenocarcinoma, and the results showed that perioperative chemotherapy group significantly improved the long-term survival rate of patients, and the R0 resection rate increased to 79.3%. PRODIGY study (11) included a total of 484 patients with gastric or gastroesophageal junction adenocarcinoma, who were randomly divided into NAC (docetaxel+oxaliplatin+S-1) combined with radical gastrectomy combined with S-1 single-drug AC group (intervention group) and postoperative S-1 single-drug AC group (control group). The results showed that: The 3-year PFS rates in the two groups were 66.3% and 60.2%, respectively (HR: 0.70, 95%CI: 0.52-0.95), which indicated that addition of NAC DOS regimen on the basis of D2 gastrectomy and adjuvant S-1 therapy could improve progression-free survival in patients with advanced gastric cancer. The RESOLVE study (12) published at the same time showed that neoadjuvant SOX chemotherapy could improve the disease-free survival of patients compared with postoperative XELOX chemotherapy. In the other group, the disease-free survival of postoperative SOX chemotherapy regimen was no worse than postoperative XELOX chemotherapy regimen.
The meta-analysis published by Wei et al. (13) included 18 studies, including RCTs and non-randomized clinical trials. The results showed that gastric cancer patients treated with NAC had a longer OS (HR: 0.77, 95%CI: 0.69-0.87) and PFS (HR: 0.76, 95% CI: 0.69-0.84) compare with those receiving AC. Following this, Wang et al. (14) published an RCT with a larger sample size (756 patients), the results of which may change the status quo of NAC and AC treatment for gastric cancer. Therefore, the aim of this paper was to conduct a systematic review and meta-analysis of all existing RCTs to assess the difference in survival benefit between perioperative chemotherapy and AC for patients with resectable gastric cancer.
This meta-analysis was performed according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta Analysis) 2020 statement (15) and has been registered in the PROSPERO (CRD42024546165). We conducted a systematic literature search via PubMed, Embase, Web of Science, and Cochrane up to April, 2024 for RCT that compared perioperative versus adjuvant chemotherapy for resectable gastric cancer. We searched the literature through the following terms: “neoadjuvant”, “perioperative”, “preoperative”, “gastric cancer”, and “chemotherapy”. The detailed search strategies are as follows: (((Neoadjuvant OR Perioperative OR Preoperative) AND ((“Drug Therapy”[Mesh]) OR (((((Drug Therapies) OR (Chemotherapy)) OR (Chemotherapies)) OR (Pharmacotherapy)) OR (Pharmacotherapies)))) AND ((“Stomach Neoplasms”[Mesh]) OR (((((((((Stomach Neoplasm) OR (Gastric Neoplasms)) OR (Gastric Neoplasm)) OR (Cancer of Stomach)) OR (Stomach Cancers)) OR (Gastric Cancer)) OR (Gastric Cancers)) OR (Stomach Cancer)) OR (Cancer of the Stomach)))) AND (random*). Furthermore, we manually screened the bibliography lists of all included RCTs. Two authors (HYO and JMZ) retrieved and assessed eligible articles independently. Any differences in literature retrieval were resolved by discussion with the third author (RQ).
Articles were eligible when meeting the following standards:
P: patients diagnosed with resectable gastric cancer.
I: perioperative or NAC combined with surgery.
C: postoperative AC combined with surgery.
O: survival outcome, such as overall survival (OS), progression-free survival (PFS), and relapse-free survival (RFS), etc.
S: randomized controlled trials.
We excluded study protocols, unpublished studies, non-original studies (including meeting abstracts, correction, and reply), non-RCT studies, studies without sufficient data (survival data cannot be obtained directly or through data transformation), and reviews.
Two authors independently conducted data abstraction, with any differences resolved by a third author. The following information was abstracted from eligible RCTs: first author name, publication year, research period, study region, study design, registration number, population, intervention, control, sample size, age, gender, follow-up time, OS, PFS and subgroup outcomes. If research data were insufficient, corresponding authors were contacted for complete data when available.
The evaluation of the quality of eligible RCT was performed according to the Cochrane Handbook for Systematic Reviews of Interventions 5.1.0, considering seven domains: sequence generation randomization, allocation concealment, blinding of participants and personnel, outcome assessment blinding, incomplete outcome data, selective outcome reporting, and other potential sources of bias (16). Each study aspect was assigned one of three evaluation outcomes: low risk, high risk, or unclear risk. Studies with more “low risk” bias evaluations were considered superior. Two authors independently assessed the quality of all included studies, resolving any disagreements through discussion.
The synthesis of data was performed utilizing Review Manager 5.4.1. For the evaluation of survival outcomes, hazard ratios accompanied by 95% confidence intervals were employed. The assessment of heterogeneity across outcomes was conducted through the application of the chi-squared (χ2) test (Cochran’s Q) and the inconsistency index (17). Substantial heterogeneity was characterized by a χ2 P value below 0.1 or an I2 exceeding 50%. The computation of the overall HR was performed utilizing the random-effects model. When data were adequate, subgroup analyses based on gender, cT stage, and cN stage were conducted for survival outcomes to assess potential confounding factors. For results encompassing more than two included studies, a sensitivity analysis was carried out to evaluate the impact of each individual RCT on the overall HR. The assessment of publication bias was performed through Egger’s regression tests (18) through Stata 15.1 edition (Stata Corp, College Station, Texas, USA). P value < 0.05 was considered as statistically significant publication bias.
Figure 1 shows the flowchart of the literature retrieval and selection process. A total of 3,953 related studies in PubMed (n = 850), Embase (n = 1,260), Web of Science (n = 1,087), and Cochrane (n = 756) were identified via systematically literature search. After removing duplicate studies, a total of 2,971 titles and abstracts were evaluated. Of these, two studies were excluded during rescreening due to non-randomized controlled study design and the wrong population (19, 20). Eventually, 5 RCTs including 2,735 patients were included for meta-analysis. Table 1 presents the characteristics of each eligible RCT. Details of the quality evaluation for all included RCTs are shown in Figure 2.
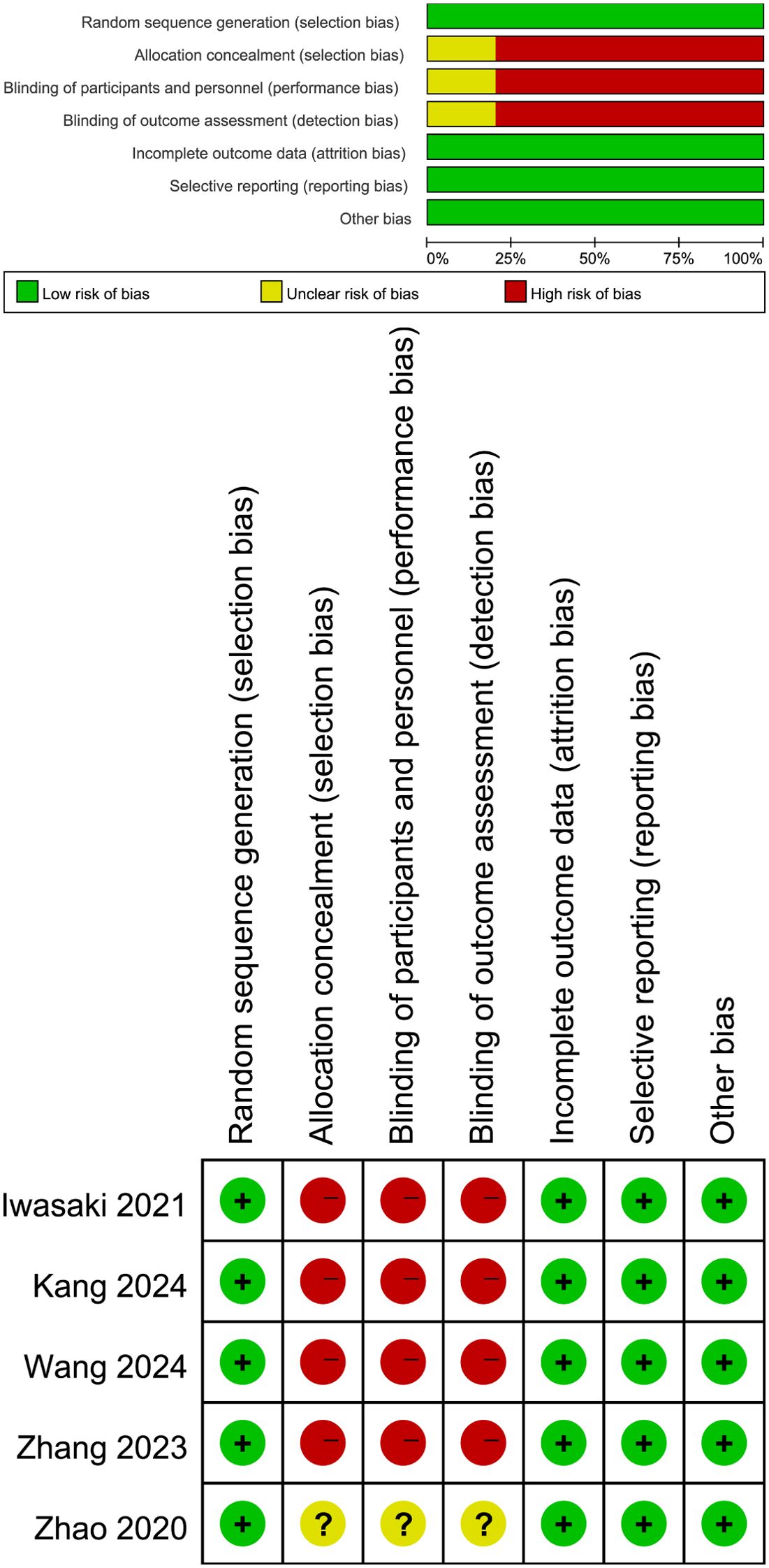
Figure 2. Details of the quality evaluation for included RCTs. Green (+) represents high risk, yellow ()? represents unclear risk, and red (-) represents high risk.
Results of PFS were synthesized from 5 RCTs including 2,735 patients (12, 14, 21–24). Meta-analysis revealed a significant longer PFS in the NAC group (HR: 0.77; 95% CI: 0.69, 0.85; P<0.00001) without significant heterogeneity (I2 = 33%, P=0.20) (Figure 3A). Subgroup analysis found that there was still a significant superiority of NAC in female (HR: 0.53; 95% CI: 0.40, 0.70; P<0.0001) and cN+ (HR: 0.77; 95% CI: 0.67, 0.89; P=0.0005) patients, while the superiority disappeared in male (HR: 0.87; 95% CI: 0.74, 1.01; P=0.07) and cN- patients (HR: 0.91; 95% CI: 0.46, 1.78; P=0.77) (Figure 4) (Table 2).
Data synthesis OS was performed in 3 RCTs including 1,466 patients (11, 21, 23). Meta-analysis observed a similar OS between the NAC and AC group (HR: 0.86; 95% CI: 0.70, 1.07; P = 0.17) without significant heterogeneity (I2 = 45%, P = 0.16) (Figure 3B). Subgroup analysis found that the difference of OS in the two groups remained non-significant in male, female, cN+, cN- and cT1-T3 stage patients, but changed to significant in the cT4 stage patients (Table 2).
We performed sensitivity analysis for the results of PFS and OS to assess the effect of each RCT on the total HR via excluding eligible RCTs one by one. Sensitivity analysis found that the new total HR kept stable after removing of each RCT for PFS (Figure 5A). However, when Iwasaki’s (21) data were excluded, the difference of OS changed from nonsignificant to significant (HR: 0.78; 95% CI: 0.65, 0.94; P = 0.008), and the heterogeneity decreased to 0%, suggesting that perioperative chemotherapy can significantly prolong the OS of patients compared with adjuvant chemotherapy (Figure 5B). In addition, the Egger’s test of PFS (P=0.184) and OS (P=0.620) did not detect a potential publication bias.
Gastric cancer is one of the most common malignant tumors in clinical practice, which seriously endangers human health. Although radical surgical resection is an important measure in the treatment of gastric cancer, quite a few patients are still likely to have tumor recurrence after D2 radical resection, which makes the prognosis of advanced gastric cancer patients unsatisfactory (25). Therefore, over the past 20 years, people have been trying new comprehensive treatment options for gastric cancer. At present, according to the results of ACTS GC and CLASSIC studies, AC after radical D2 surgery can prolong the overall survival of patients with advanced gastric cancer (8, 26–29). However, several studies have shown that NAC can also improve the overall survival rate of patients with advanced gastric cancer compared with surgery alone (30, 31). Based on the MAGIC study (10), perioperative chemotherapy has become the standard in European countries, and based on the recent FLOT4 study, the fluorouracil + leucovorin + oxaliplatin + docetaxel (FLOT) regimen is currently the standard for Western populations (32). In East Asian countries, adjuvant chemotherapy after D2 gastrectomy including S-1 or capecitabine plus oxaliplatin is currently the standard regimen based on the ACTS-GC (8) and CLASSIC (33) trials. In addition, docetaxel + S-1 is also the standard treatment for Japanese patients with stage III gastric cancer based on the JACCRO GC-07 trial (34). Through systematic evaluation and summary analysis of existing RCTs, this study explored the effects of perioperative chemotherapy and postoperative AC on the survival benefits of patients with resectable gastric cancer, and provided a certain theoretical basis for further improving the treatment level of gastric cancer, with a view to prolonging the survival period of patients.
The results of this study showed that NAC significantly prolonged PFS in gastric cancer patients compared to AC, and sensitivity analysis did not detect significant instability. Combined with the results of previous studies, it is suggested that NAC has definite advantages in PFS. However, results of subgroup analysis suggest that female patients are more sensitive to NAC, and male patients may not benefit from NAC. Research results of Xu et al. (35) showed that gender and age may be factors that independently predict the effect of NAC in patients with locally advanced gastric cancer. However, most studies took age and gender as baseline data for comparison, and accurate conclusions could not be drawn. In this study, only 3 RCTs reported gender subgroup data, so the study conclusion may have selective bias, and the effect of gender on the effect of NAC needs to be confirmed by further studies.
In addition, subgroup analysis based on lymph node staging found that cN+ patients were more sensitive to NAC, and cN- patients may not benefit from NAC. Kim et al. (36) followed up 108,731 patients with gastric cancer and found that radical surgery, depth of tumor invasion and lymph node metastasis were three important prognostic factors for gastric cancer. Therefore, if early diagnosis can be made clearly and corresponding regional lymph node dissection can be performed at the same time of radical surgery, the survival rate of patients can be significantly improved, especially the long-term survival rate of patients with stage III gastric cancer can be effectively improved (37). Another prospective study conducted by Siewert et al. (38) also showed that lymph node metastasis is one of the important factors affecting the long-term prognosis of gastric cancer. However, according to an exploratory analysis of PRODIGY, patients with cT4 disease were the ones who benefited most from neoadjuvant chemotherapy, regardless of whether they were lymph node positive or not (39). Therefore, the subgroup analysis of this study found an effect of lymph node on PFS, which may be due to different patient inclusion criteria. RESONANCE (14) included II-III disease regardless of lymph node status, the PRODIGY (22) study included patients with cT2/3 disease only when they were clinically lymph node positive, and RESOLVE (23) included patients with cT4a disease only when they were clinically lymph node positive. This difference suggests that these results need to be further verified. In addition, it should be considered that NAC may cause regression of the tumor itself, which may help relieve symptoms such as abdominal pain and dysphagia that may occur in patients with cT2 or cT3, even if they are cN-.
In addition, this study found that NAC had no significant advantage over AC in OS. This finding is consistent with previous research. Reddavid et al. (40) carried out a systematic review on whether patients with locally advanced gastric cancer could benefit from NAC. The study included 16 RCTs. Results showed that of the 6 well-designed RCTs, only 2 RCTs showed a survival advantage of NAC in the esophagogastric junction tumor subgroup. The efficacy of standardized surgery and appropriately expanded lymph node dissection is even better than that of neoadjuvant therapy. Cai et al. (41) conducted a network meta-analysis that included 33 RCTs (8989 patients) published after 1997. The results showed that perioperative NAC had no survival advantage compared with postoperative chemoradiotherapy, postoperative chemoradiotherapy and preoperative chemoradiotherapy. However, it is worth noting that the sensitivity analysis found significant instability in OS. When the data of Iwasaki (21) were excluded, the difference in OS changed from insignificant to significant, the heterogeneity decreased to 0%, and the conclusion suggested that perioperative chemotherapy had a significant effect in prolonging OS. The reason for this result may be that the two latest long-term follow-up trials, PRODIGY (22) and RESOLVE (23), both found the advantages of perioperative chemotherapy in OS. This result is worth reconsidering the efficacy of perioperative chemotherapy on OS in gastric cancer patients, but it also needs to be confirmed by more RCTs with large sample sizes.
Although this study was unable to conduct subgroup analysis through NAC regimen and cycle number due to insufficient data, it is worth noting that the impact of NAC cycle number and regimen on postoperative survival of gastric cancer patients is still controversial. On the one hand, some people believe that increasing the number of chemotherapy cycles may further shrink the tumor and lower the stage, thereby improving the postoperative survival rate (42). However, on the other hand, some studies have pointed out that too long a chemotherapy cycle may lead to a decrease in the patient’s physical tolerance and an increase in postoperative complications, which may adversely affect postoperative survival (43). SOX regimen has received widespread attention in neoadjuvant chemotherapy for gastric cancer due to its significant efficacy and relatively low toxicity. The results of the RESOLVE study (12) show that for patients with locally advanced gastric cancer, 3 cycles of SOX neoadjuvant chemotherapy before surgery can significantly improve the 3-year DFS and increase the R0 resection rate. Therefore, the SOX regimen is listed as the preferred regimen for distal advanced gastric cancer by China’s gastric cancer-related diagnosis and treatment guidelines and consensus. The XELOX regimen is another commonly used neoadjuvant chemotherapy regimen for gastric cancer. Although it did not show better efficacy than the SOX regimen in some studies, the XELOX regimen is still widely used in clinical practice (44). For patients with gastric cancer whose pathological stage is pII/pIII after D2 radical surgery, the XELOX regimen is recommended as an option for postoperative adjuvant chemotherapy (45, 46). The ECF/ECX regimen (epirubicin + cisplatin + fluorouracil/capecitabine) also has a place in neoadjuvant chemotherapy for gastric cancer (47). However, due to the toxicity of the anthracyclines in the ECF regimen and the limited efficacy of the regimen itself, the ECF regimen is no longer recommended by the Chinese gastric cancer guidelines (48, 49). Despite this, the MAGIC study still confirmed that three courses of ECF before and after surgery can further improve the OS and DFS of patients with locally advanced gastric cancer (50). FLOT regimen (docetaxel, oxaliplatin, and fluorouracil) has been a research hotspot in the field of neoadjuvant chemotherapy for gastric cancer in recent years (51). The results of the FLOT4-AIO study show that compared with the ECF/ECX regimen, the FLOT regimen can further improve the R0 resection rate and pathological response rate, thereby improving the patient’s 5-year OS rate and DFS rate (52). Therefore, the FLOT regimen is also regarded as an effective option for neoadjuvant chemotherapy for gastric cancer (42, 53). In addition, the DOS regimen (docetaxel, oxaliplatin, and Tigeol) showed good efficacy and safety in the Korean PRODIGY study (11), and can be used as one of the recommended regimens for neoadjuvant chemotherapy for locally advanced gastric cancer. Considering that the effect of NAC is affected by many factors, including NAC regimen, number of cycles, tumor type, stage, chemotherapy drugs and doses, postoperative complications of patients, etc., patients should be fully considered when determining the number of cycles of neoadjuvant chemotherapy. Based on the specific situation, a personalized treatment plan can be developed. In addition, studies have shown that the application of the multidisciplinary diagnosis and treatment model (MDT) can also help provide patients with more accurate and effective NAC solutions (54, 55).
However, we must acknowledge several limitations of this meta-analysis. Firstly, none of 7 included RCTs had low risk in the allocation concealment, blinding of participants, personnel, and outcome assessment. Secondly, the RCTs included in our study had different intervention (different NAC strategies and AC strategies), which may be one of the sources of heterogeneity. On the other hand, the definition of PFS varies among different studies, which may also be one of the sources of heterogeneity in this study. Although it was not clearly stated whether positive resection margins was defined as a PD event in the MAGIC, FLOT4, and RESOLVE studies, positive resection margins was listed as a PD event in the FNCLCC and FFCD (56) and PRODIGY (11) studies on PFS, resulting in an early decline in their survival curves. Considering the issue of subsequent treatment after positive resection margins and the fact that the PFS benefit was converted into an OS benefit in the FNCLCC, FFCD, and PRODIGY studies, it seems reasonable to define positive resection margins (including distant metastases diagnosed during or after surgery) as a PD event in the neoadjuvant setting (6, 57). In addition, differences in populations may also be a potential source of heterogeneity in this article, especially differences in tumor stages, which may also affect the therapeutic effect of NAC to some extent.
Thirdly, due to the small number of literature, this study could not obtain enough data to combine surgery-related outcomes and chemotherapy response outcomes. At the same time, due to data limitations, this study did not analyze subgroups of patients with different perioperative chemotherapy regimens, number of cycles, age, and pathological types of tumors. Fourthly, all of the included studies were from Asian countries (including Japan, Korea and China) and the data of European populations were still deficient. Another unavoidable limitation is that the proportion of patients for whom NAC might be recommended who actually receive it is often greater than the proportion of patients who actually start and complete adjuvant chemotherapy in patients for whom NAC is recommended postoperatively because of postoperative complications and decreased performance status. This is not reflected in randomized controlled trials that include patients after surgery because patients who are not suitable for adjuvant therapy due to the above factors would not be included in the trial population. Despite several limitations of this meta-analysis, we conducted the latest meta-analysis of RCTs to compare perioperative versus adjuvant chemotherapy for resectable gastric cancer. Results of this meta-analysis validated the superiority of the NAC for PFS of gastric cancer compared with AC. More large-scale, multi-center, double-blind RCTs are needed to further confirm our findings.
The results of this study demonstrate that NAC can significantly prolong PFS in patients with resectable gastric cancer compared to AC, and the benefit is more significant in women and cN+ patients. Besides, our analysis indicated that NAC has a potential to improve OS compared with AC. Considering the limitations of this paper, such as small sample size, missing data and regional selectivity bias, more large-scale, multi-center, double-blind RCTs are needed to further compare the efficacy of perioperative versus adjuvant chemotherapy for resectable gastric cancer.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
HO: Conceptualization, Data curation, Formal Analysis, Methodology, Writing – original draft, Writing – review & editing. JZ: Resources, Software, Supervision, Writing – original draft. MJ: Data curation, Formal Analysis, Investigation, Writing – original draft. XZ: Formal Analysis, Investigation, Validation, Writing – original draft. TW: Investigation, Resources, Supervision, Writing – original draft. HC: Software, Supervision, Validation, Writing – original draft. RQ: Conceptualization, Data curation, Formal Analysis, Methodology, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA: Cancer J Clin. (2015) 65:87–108. doi: 10.3322/caac.21262
2. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA: Cancer J Clin. (2016) 66:115–32. doi: 10.3322/caac.21338
3. Ajani JA, Bentrem DJ, Besh S, D’Amico TA, Das P, Denlinger C, et al. Gastric cancer, version 2.2013. J Natl Compr Cancer network. (2013) 11:531–46.
4. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018. Gastric cancer: Off J Int Gastric Cancer Assoc Japanese Gastric Cancer Assoc. (2021) 24:1–21. doi: 10.1007/s10120-020-01042-y
5. Fong C, Johnston E, Starling N. Neoadjuvant and adjuvant therapy approaches to gastric cancer. Curr Treat options Oncol. (2022) 23:1247–68. doi: 10.1007/s11864-022-01004-9
6. Kim HD, Ryu MH, Kang YK. Adjuvant treatment for locally advanced gastric cancer: an Asian perspective. Gastric cancer: Off J Int Gastric Cancer Assoc Japanese Gastric Cancer Assoc. (2024) 27:439–50. doi: 10.1007/s10120-024-01484-8
7. Noh SH, Park SR, Yang H-K, Chung HC, Chung I-J, Kim S-W, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. (2014) 15:1389–96. doi: 10.1016/S1470-2045(14)70473-5
8. Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. New Engl J Med. (2007) 357:1810–20. doi: 10.1056/NEJMoa072252
9. Frei Iii E. Clinical cancer research: an embattled species. Cancer. (1982) 50:1979–92. doi: 10.1002/1097-0142(19821115)50:10<1979::AID-CNCR2820501002>3.0.CO;2-D
10. Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJH, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. New Engl J Med. (2006) 355:11–20. doi: 10.1056/NEJMoa055531
11. Kang YK, Yook JH, Park YK, Lee JS, Kim YW, Kim JY, et al. PRODIGY: A phase III study of neoadjuvant docetaxel, oxaliplatin, and S-1 plus surgery and adjuvant S-1 versus surgery and adjuvant S-1 for resectable advanced gastric cancer. J Clin oncology: Off J Am Soc Clin Oncol. (2021) 39:2903–13. doi: 10.1200/JCO.20.02914
12. Zhang X, Liang H, Li Z, Xue Y, Wang Y, Zhou Z, et al. Perioperative or postoperative adjuvant oxaliplatin with S-1 versus adjuvant oxaliplatin with capecitabine in patients with locally advanced gastric or gastro-oesophageal junction adenocarcinoma undergoing D2 gastrectomy (RESOLVE): an open-label, superiority and non-inferiority, phase 3 randomised controlled trial. Lancet Oncol. (2021) 22:1081–92. doi: 10.1016/S1470-2045(21)00297-7
13. Wei C, Du X, Hu J, Dong Y, Chen Y, Cao B. Perioperative Chemotherapy versus Adjuvant Chemotherapy in Patients with Resectable Gastric Cancer: A Systematic Review with meta-analysis. Crit Rev oncology/hematology. (2023) 104082.
14. Wang X, Lu C, Wei B, Li S, Li Z, Xue Y, et al. Perioperative versus adjuvant S-1 plus oxaliplatin chemotherapy for stage II/III resectable gastric cancer (RESONANCE): a randomized, open-label, phase 3 trial. J Hematol Oncol. (2024) 17:17. doi: 10.1186/s13045-024-01536-7
15. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical Res ed). (2021) 372:n71.
16. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database systematic Rev. (2019) 10:Ed000142. doi: 10.1002/14651858.ED000142
17. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.v21:11
18. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical Res ed). (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
19. Nio Y, Koike M, Omori H, Hashimoto K, Itakura M, Yano S, et al. A randomized consent design trial of neoadjuvant chemotherapy with tegafur plus uracil (UFT) for gastric cancer–a single institute study. Anticancer Res. (2004) 24:1879–87.
20. Yonemura Y, Sawa T, Kinoshita K, Matsuki N, Fushida S, Tanaka S, et al. Neoadjuvant chemotherapy for high-grade advanced gastric cancer. World J surgery. (1993) 17:256–61; discussion 61-2. doi: 10.1007/BF01658939
21. Iwasaki Y, Terashima M, Mizusawa J, Katayama H, Nakamura K, Katai H, et al. Gastrectomy with or without neoadjuvant S-1 plus cisplatin for type 4 or large type 3 gastric cancer (JCOG0501): an open-label, phase 3, randomized controlled trial. Gastric cancer: Off J Int Gastric Cancer Assoc Japanese Gastric Cancer Assoc. (2021) 24:492–502. doi: 10.1007/s10120-020-01136-7
22. Kang YK, Kim HD, Yook JH, Park YK, Lee JS, Kim YW, et al. Neoadjuvant docetaxel, oxaliplatin, and S-1 plus surgery and adjuvant S-1 for resectable advanced gastric cancer: updated overall survival outcomes from phase III PRODIGY. J Clin oncology: Off J Am Soc Clin Oncol. (2024) 42:2961–5. doi: 10.1200/JCO.23.02167
23. Zhang X, Li Z, Liang H, Xue Y, Wang Y, Zhou Z, et al. LBA78 overall survival of perioperative or postoperative adjuvant oxaliplatin with S-1 versus adjuvant oxaliplatin with capecitabine in locally advanced gastric or gastro-oesophageal junction adenocarcinoma undergoing D2 gastrectomy: an updated analysis of RESOLVE trial. Ann Oncol. (2023) 34:S1318–S9. doi: 10.1016/j.annonc.2023.10.079
24. Zhao Q, Lian C, Huo Z, Li M, Liu Y, Fan L, et al. The efficacy and safety of neoadjuvant chemotherapy on patients with advanced gastric cancer: A multicenter randomized clinical trial. Cancer Med. (2020) 9:5731–45. doi: 10.1002/cam4.v9.16
25. Koerner AS, Moy RH, Ryeom SW, Yoon SS. The present and future of neoadjuvant and adjuvant therapy for locally advanced gastric cancer. Cancers. (2023) 15. doi: 10.3390/cancers15164114
26. Paoletti X, Oba K, Burzykowski T, Michiels S, Ohashi Y, Pignon J-P, et al. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. Jama. (2010) 303:1729–37. doi: 10.1001/jama.2010.534
27. Lavacchi D, Fancelli S, Buttitta E, Vannini G, Guidolin A, Winchler C, et al. Perioperative tailored treatments for gastric cancer: times are changing. Int J Mol Sci. (2023) 24. doi: 10.3390/ijms24054877
28. Marano L, Carbone L, Poto GE, Restaino V, Piccioni SA, Verre L, et al. Extended lymphadenectomy for gastric cancer in the neoadjuvant era: current status, clinical implications and contentious issues. Curr Oncol (Toronto Ont). (2023) 30:875–96. doi: 10.3390/curroncol30010067
29. Shoji Y, Koyanagi K, Kanamori K, Tajima K, Ogimi M, Yatabe K, et al. Current status and future perspectives for the treatment of resectable locally advanced esophagogastric junction cancer: A narrative review. World J gastroenterology. (2023) 29:3758–69. doi: 10.3748/wjg.v29.i24.3758
30. Taieb J, Bennouna J, Penault-Llorca F, Basile D, Samalin E, Zaanan A. Treatment of gastric adenocarcinoma: A rapidly evolving landscape. Eur J Cancer (Oxford England: 1990). (2023) 195:113370. doi: 10.1016/j.ejca.2023.113370
31. Tong X, Zhi P, Lin S. Neoadjuvant chemotherapy in asian patients with locally advanced gastric cancer. J gastric cancer. (2023) 23:182–93. doi: 10.5230/jgc.2023.23.e12
32. Al-Batran S-E, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. (2019) 393:1948–57. doi: 10.1016/S0140-6736(18)32557-1
33. Bang Y-J, Kim Y-W, Yang H-K, Chung HC, Park Y-K, Lee KH, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. (2012) 379:315–21. doi: 10.1016/S0140-6736(11)61873-4
34. Yoshida K, Kodera Y, Kochi M, Ichikawa W, Kakeji Y, Sano T, et al. Addition of docetaxel to oral fluoropyrimidine improves efficacy in patients with stage III gastric cancer: interim analysis of JACCRO GC-07, a randomized controlled trial. J Clin Oncol. (2019) 37:1296–304. doi: 10.1200/JCO.18.01138
35. Xu W, Fan Z, Wang L, He C, Ni Z, Hua Z, et al. Prediction model of objective response after neoadjuvant chemotherapy in patients with locally advanced gastric cancer. Am J Trans Res. (2021) 13:1568.
36. Kim J-P, Lee J-H, Kim S-J, Yu H-J, Yang H-K. Clinicopathologic characteristics and prognostic factors in 10 783 patients with gastric cancer. Gastric cancer: Off J Int Gastric Cancer Assoc Japanese Gastric Cancer Assoc. (1998) 1:125–33. doi: 10.1007/s101200050006
37. Yeh JH, Yeh YS, Tsai HL, Huang CW, Chang TK, Su WC, et al. Neoadjuvant chemoradiotherapy for locally advanced gastric cancer: where are we at? Cancers. (2022) 14. doi: 10.3390/cancers14123026
38. Siewert JR, Böttcher K, Stein HJ, Roder JD, German Gastric Carcinoma Study G. Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann surgery. (1998) 228:449–61. doi: 10.1097/00000658-199810000-00002
39. Kim HD, Lee JS, Yook JH, Ryu MH, Park YK, Kim JY, et al. Radiological criteria for selecting candidates for neoadjuvant chemotherapy for gastric cancer: an exploratory analysis from the PRODIGY study. Gastric cancer: Off J Int Gastric Cancer Assoc Japanese Gastric Cancer Assoc. (2022) 25:170–9. doi: 10.1007/s10120-021-01243-z
40. Reddavid R, Sofia S, Chiaro P, Colli F, Trapani R, Esposito L, et al. Neoadjuvant chemotherapy for gastric cancer. Is it a must or a fake? World J gastroenterology. (2018) 24:274. doi: 10.3748/wjg.v24.i2.274
41. Cai Z, Yin Y, Shen C, Wang J, Yin X, Chen Z, et al. Comparative effectiveness of preoperative, postoperative and perioperative treatments for resectable gastric cancer: a network meta-analysis of the literature from the past 20 years. Surg Oncol. (2018) 27:563–74. doi: 10.1016/j.suronc.2018.07.011
42. Rencuzogullari A, Karahan SN, Selcukbiricik F, Lacin S, Taskin OC, Saka B, et al. The new era of total neoadjuvant FLOT therapy for locally advanced, resectable gastric cancer: A propensity-matched comparison with standard perioperative therapy. J Surg Oncol. (2024). doi: 10.1002/jso.27934
43. Sah BK, Xu W, Zhang B, Zhang H, Yuan F, Li J, et al. Feasibility and safety of perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel for locally advanced gastric cancer patients in China. Front Oncol. (2020) 10:567529. doi: 10.3389/fonc.2020.567529
44. He J, Zhang B, Zhou S, Yang Y, Han Z, Wu T, et al. Phase II study of perioperative camrelizumab and XELOX for locally advanced gastric or gastroesophageal junction adenocarcinoma. Cancer Sci. (2024). doi: 10.1111/cas.16425
45. Yun JH, Song GJ, Son MW, Lee MS. Global leadership initiative on malnutrition criteria and immunonutritional status predict chemoadherence and survival in stage II/III gastric cancer treated with XELOX chemotherapy. Nutrients. (2024) 16. doi: 10.3390/nu16203468
46. Zhou J, Wang J, Wang W, Sun L, Zhao S, Sun Q, et al. Pathological complete response achieved with XELOX chemotherapy, HIPEC, and anti-PD-1 immunotherapy in stage IV gastric adenocarcinoma with peritoneal metastasis: A case report and review of the literature. J gastrointestinal cancer. (2024) 55:1441–7. doi: 10.1007/s12029-024-01056-0
47. Egebjerg K, Andersen TS, Bæksgaard L, Garbyal R, Siemsen M, Achiam M, et al. Implementation of perioperative FLOT compared to ECX/EOX chemotherapy regimens in resectable esophagogastric adenocarcinomas: an analysis of real-world data. Acta Oncol (Stockholm Sweden). (2024) 63:322–9. doi: 10.2340/1651-226X.2024.35431
48. Forouhari A, Moghaddas A, Darakhshandeh A. Outcome evaluation of ECF, DCF, FOLFOX, and FLOT chemotherapy regimens as perioperative treatment in elderly patients with resectable gastric cancer; A retrospective comparative study. J Res Med sciences: Off J Isfahan Univ Med Sci. (2023) 28:79. doi: 10.4103/jrms.jrms_417_22
49. Zeng H, Wang C, Song LY, Jia SJ, Zeng X, Liu Q. Economic evaluation of FLOT and ECF/ECX perioperative chemotherapy in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma. BMJ Open. (2022) 12:e060983. doi: 10.1136/bmjopen-2022-060983
50. Okines AF, Thompson LC, Cunningham D, Wotherspoon A, Reis-Filho JS, Langley RE, et al. Effect of HER2 on prognosis and benefit from peri-operative chemotherapy in early oesophago-gastric adenocarcinoma in the MAGIC trial. Ann oncology: Off J Eur Soc Med Oncol. (2013) 24:1253–61. doi: 10.1093/annonc/mds622
51. Kee W, Ng KYY, Liong SZ, Zhou S, Chee SK, Lim CW, et al. Real-world outcomes for localised gastro-oesophageal adenocarcinoma cancer treated with perioperative FLOT and prophylactic GCSF support in a single asian centre. Cancers. (2024) 16. doi: 10.3390/cancers16213697
52. Al-Batran SE, Hofheinz RD, Pauligk C, Kopp HG, Haag GM, Luley KB, et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol. (2016) 17:1697–708. doi: 10.1016/S1470-2045(16)30531-9
53. Osminin S, Vetshev F, Bilyalov I, Astaeva M, Yeventyeva Y. PERIOPERATIVE FLOT CHEMOTHERAPY FOR GASTRIC CANCER: A RETROSPECTIVE SINGLE-CENTER COHORT TRIAL. Georgian Med News. (2024) 354:75–81.
54. Zhang Q, Zhou Y, Song L, Fang W, Qiu M, Gu Y, et al. China special issue on gastrointestinal tumors-Improved survival after multidisciplinary team decision for patients with advanced gastrointestinal cancer: A multicenter, noninterventional, controlled study. Int J cancer. (2023) 153:1885–93. doi: 10.1002/ijc.v153.11
55. Zhao J, Liu T. Complete clinical response in neoadjuvant treatment of advanced gastric cancer under multidisciplinary treatment mode. Asian J Surg. (2023) 46:1896–7. doi: 10.1016/j.asjsur.2022.10.084
56. Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin oncology: Off J Am Soc Clin Oncol. (2011) 29:1715–21. doi: 10.1200/JCO.2010.33.0597
Keywords: neoadjuvant chemotherapy, adjuvant chemotherapy, gastric cancer, meta-analysis, NAc
Citation: Ou H, Zhuang J, Jian M, Zheng X, Wu T, Cheng H and Qian R (2025) Perioperative versus adjuvant chemotherapy for resectable gastric cancer: a meta-analysis of randomized controlled trials. Front. Oncol. 15:1432596. doi: 10.3389/fonc.2025.1432596
Received: 14 May 2024; Accepted: 17 February 2025;
Published: 06 March 2025.
Edited by:
Sharon R Pine, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Sacheen Kumar, Royal Marsden NHS Foundation Trust, United KingdomCopyright © 2025 Ou, Zhuang, Jian, Zheng, Wu, Cheng and Qian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rui Qian, MTAwOTU0NTE4OEBxcS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.