
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 09 April 2025
Sec. Gastrointestinal Cancers: Colorectal Cancer
Volume 15 - 2025 | https://doi.org/10.3389/fonc.2025.1429319
This article is part of the Research Topic Organ Preservation for Rectal Cancer Patients View all 7 articles
Introduction: Tumor budding (TB) is recognized as a complementary prognostic factor for colorectal cancer. However, data on its impact on the survival of patients undergoing neoadjuvant chemoradiotherapy (nCRT) remain limited. This study aims to investigate the role of TB in disease-free survival (DFS) and overall survival (OS) among patients with locally advanced rectal cancer receiving nCRT.
Methods: In this systematic review and meta-analysis, an exhaustive search of the PubMed, Scopus, Web of Science (WOS), Embase, and Cochrane databases was conducted, ultimately leading to the extraction of eight studies in the qualitative assessment and meta-analysis.
Results: All the included studies were of high quality. The total sample size comprised 1,941 individuals. Although eight studies were included, nine datasets were extracted, as some studies reported multiple outcome measurements. TB positivity was statistically associated with decreased overall survival of 3.24 (95% confidence interval [CI]: 1.71–6.16) and disease-free survival of 2.54 (95% CI: 1.56–4.15) in patients with locally advanced rectal cancer undergoing nCRT.
Discussion: Based on the findings of this study, TB negativity was statistically and directly associated with better OS and DFS in patients with locally advanced rectal cancer undergoing nCRT.
Malignancies have become a major public health challenge, representing the second leading cause of mortality worldwide (1). Among men, lung, prostate, and colorectal cancer (CRC) contribute significantly to cancer-related deaths, while in women, breast, lung, and CRC account for more than half of all malignancies. In 2020, CRC was ranked as the third most prevalent malignancy and the second leading cause of cancer-related mortality. Additionally, a gradual increase in CRC-associated deaths was observed between 2005 and 2020 across both age groups—those under and over 50 years old (2).
The histopathological analysis of CRC specimens indicates that adenocarcinoma is the most prevalent type, accounting for 95% of all CRC cases, originating from cellular proliferation and dysplasia of polyps (3). The most widely used and robust classification system applied for assessing the extent and clinical outcome of CRC is TNM staging, which plays a crucial role in determining the appropriate treatment approach, including local excision, neoadjuvant therapy, and major surgical resection (4–6).
The preferred approach for treating locally advanced rectal cancers classified as T3–T4 and/or N+, M0 according to TNM staging is total mesorectal excision (TME) combined with neoadjuvant chemoradiotherapy (nCRT) (4–6). Neoadjuvant chemoradiotherapy is generally defined as either long-course chemoradiotherapy, long-course chemoradiotherapy following primary chemotherapy, or short-course radiotherapy (7, 8).
Nevertheless, the variability in outcomes among CRC patients within the same TNM stage, even after undergoing complete radical surgery, has led to the hypotheses that additional factors may play a crucial role in assessing treatment response beyond tumor staging and the chosen therapeutic approach. Tumor budding (TB) is one of the pathological characteristics suspected to contribute to this variability. Furthermore, since TNM staging is based on pathological examination, it can only be applied to resected specimens, limiting its usage in planning and adjusting neoadjuvant therapy (9, 10).
TB is a morphological marker of epithelial–mesenchymal transition (EMT) (11) and is defined as a single cancer cell or a cluster of fewer than five cells located at the invasive front of the tumor (peritumoral budding) or within the tumor mass (intratumoral budding). These cells tend to lose adhesion, making the tumor more invasive (12). A review of the literature indicates that TB is associated with adverse tumoral characteristics, including higher tumor grade, higher TNM stage, lymphovascular invasion, lymph node involvement, distant metastasis, and overall shorter survival (13–17). The prognostic significance of TB is so pronounced that it has been suggested to be a stronger predictor of survival than ypT and ypN staging (18).
Nevertheless, a comprehensive investigation into the value and utility of TB as a predisposing factor for adverse outcomes in locally advanced rectal cancer patients undergoing neoadjuvant therapy is lacking. This meta-analysis aims to address this gap.
We aimed to conduct a systematic review and meta-analysis following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (19). To ensure a comprehensive search, we utilized the PubMed, Scopus, Web of Science (WOS), Embase, and Cochrane databases up to 12 August 2023. The databases were searched using keywords derived from Medical Subject Heading (MeSH) terms extracted from MeSH on Demand. These keywords were then entered into the databases according to their respective protocols, followed by a reference search. Our initial search yielded 480 articles.
The searched keywords included: (“tumour budding”) OR (“tumor budding”) OR (“high-grade tumor budding”) OR (“low-grade tumor budding”) OR (“budding”) OR (“tumor-cell dissociation”) and (colorectal neoplasms) OR (colorectal neoplasm) OR (colorectal tumors) OR (colorectal tumor) OR (colorectal cancer) OR (colorectal cancers) OR (colorectal carcinoma) OR (colorectal carcinomas) OR (rectal carcinomas) OR (rectal carcinoma) OR (rectal neoplasms) OR (rectal neoplasm) OR (rectal tumors) OR (rectal tumor) OR (rectal cancers) OR (rectal cancer) OR (“CRC”) and (neoadjuvant therapy) OR (neoadjuvant radiotherapy) OR (neoadjuvant radiation treatment) OR (neoadjuvant radiation therapy) OR (neoadjuvant radiation) OR (neoadjuvant systemic therapy) OR (neoadjuvant systemic treatment) OR (neoadjuvant chemotherapy) OR (neoadjuvant chemotherapy treatment) OR (neoadjuvant chemoradiotherapy) OR (neoadjuvant chemoradiation therapy) OR (neoadjuvant chemoradiation treatment) OR (neoadjuvant chemoradiation).
The studies included in this meta-analysis met the following criteria: (1) written in English, (2) assessed relapse-free survival (RFS) or disease-free survival (DFS) or overall survival (OS) in patients with locally advanced rectal adenocarcinoma, (3) involved patients who received any neoadjuvant chemoradiotherapy treatments, and (4) provided histopathological reports of TB.
Studies were excluded from this meta-analysis if they lacked sufficient data for analysis, reported patients with tumors other than rectal adenocarcinoma, had inaccessible full texts, were classified as low-quality studies, were review articles, were written in languages other than English, or were individual case reports.
The authors (A.R., P.N., and P.K.) compiled and reviewed the topics of the manuscripts. The topics and names of the first authors were then checked. Next, EndNote software was used to eliminate duplicate manuscripts. Following this, the three authors independently reviewed the abstracts and selected relevant articles for inclusion. In cases where there was disagreement regarding the inclusion of a particular manuscript, another author (A.M.) made the final decision. Finally, the full text of the selected manuscripts was assessed for eligibility, evaluated for quality and risk of bias, and included in the meta-analysis.
This study focuses on patients diagnosed with locally advanced rectal cancer who have undergone neoadjuvant therapy.
Tumor budding assessment was performed to evaluate its prognostic significance in patients receiving neoadjuvant therapy.
Patients were compared based on different levels of tumor budding to determine its impact on clinical outcomes.
The study examines overall survival, disease-free survival, and relapse-free survival in patients with locally advanced rectal cancer in relation to tumor budding status.
The authors independently extracted data from the included papers, including the first author, year of publication, studied population, study type, applied protocol for nCRT, and TB reporting system.
To demonstrate effect size as a standardized mean difference between the tumor-budding-positive and tumor-budding-negative individuals receiving neoadjuvant chemoradiotherapy, overall survival or disease-free survival/relapse-free survival was used as the effect size measure in all studies and represented in a forest plot. The meta-analysis was conducted using declared generic, precomputed effect sizes based on mean for two-group comparisons of continuous or binary outcomes. All effect sizes, including relative risk, odds ratio, beta regression, and correlation, were converted to standardized mean difference (SMD). Also, the random-effects restricted maximum-likelihood (REML) model was applied. Substantial heterogeneity was indicated by an I2 value > 50% and a Cochran’s square test, H2, with a corresponding p-value of < 0.05. Galbraith plots were used to evaluate the sources of heterogeneity among studies. Subgroup analysis was performed using RFS/DFS or OS. Since DFS and RFS have similar definitions, they were analyzed as a subgroup (10, 20). A sensitivity analysis test was performed to assess the robustness of the associations. Publication bias was evaluated using a funnel plot, Egger’s test, and Begg’s tests. A nonparametric trim-and-fill analysis was conducted to estimate the number of missing studies. All data analyses were performed using Stata software version 17.
The literature search strategy identified 480 records, of which 236 remained after duplicate removal. Following an initial screening of titles and abstracts, 37 full-text articles were retained and assessed. Ultimately, eight studies were included in the qualitative assessment and meta-analysis, as shown in the PRISMA flow diagram (Figure 1) and Table 1.
In the meta-analysis, separate forest plots were generated for RFS/DFS and OS outcomes to assess the role of TB in the prognosis of rectal cancer.
The quality and risk of bias assessment of the included studies indicated that all studies were of good quality (Table 2) and had a low risk of bias (Table 3).
The total sample size comprised 1,941 individuals. Although there were eight studies, we extracted data from nine studies, as one study assessed outcomes using two different staining methods (18) (Table 1).
The main characteristics and data reported in the included articles are summarized in Table 1. All the studies were cohort-based and conducted on human samples. The recruited studies were from Turkey (21), South Korea (22), the USA (23, 24), Ireland (25), Austria (10), France (26), and Germany (18) (Table 1).
Tumor budding was evaluated in patients with locally advanced rectal cancer undergoing neoadjuvant chemoradiotherapy using histopathological specimens stained with hematoxylin and eosin or immunohistochemistry. Studies defined tumor budding according to different classification systems, with some reporting TB as positive or negative, while others classified it based on a cut-off or continuous scale. TB positivity was determined using various thresholds, including TB ≥ 1 (21, 25, 26), ≥ 2 (10, 24), ≥ 5 (18, 22), and ≥ 10 (23). The final assessment of the studies included 3-year (26) or 5-year (10, 18, 21–25) DFS/RFS and OS.
The National Heart, Lung, and Blood Institute (NIH) Study Quality Assessment Tool was used to evaluate the study quality, categorizing all included studies as good quality, as shown in Table 2. Moreover, the Quality In Prognosis Studies (QUIPS) tool was applied to assess validity and risk of bias, considering six bias domains: study participation, study attrition, prognostic factor measurement, outcome measurement, study confounding, statistical analysis, and reporting. Each domain was rated as low, moderate, or high risk of bias based on prompting items and considerations (27). Following the evaluation, all included studies were categorized as having a low risk of bias (Table 3).
Data from six studies on DFS, including 1,623 patients, were incorporated into this meta-analysis. Based on the definition of TB positivity, the overall hazard ratio for DFS in TB-negative patients was 2.54 times higher than in TB-positive individuals, as estimated using a random-effects model (95% confidence interval [CI]: 1.56–4.15) (Figure 2a). Moreover, no publication bias was observed in any of the analyses. The gathered data were generally homogeneous, with an I2 value of 0%. The assessment of group differences showed no significant statistical differences (p-value = 0.86) (Figure 2b). The figure illustrates the distribution of studies in the assessment of DFS, indicating that none fell outside the 95% confidence interval limits. The small-study effect was evaluated using Begg’s and Egger’s tests. In the Egger’s test, a beta value of 3.15 was calculated with a corresponding p-value of 0.19. The Begg’s test yielded a Kendall’s of 11, with a two-tailed p-value of 0.06. Finally, the nonparametric trim-and-fill analysis indicated no missing studies affecting specificity measurements. Galbraith plot of RFS/DFS is shown in Figure 2c.
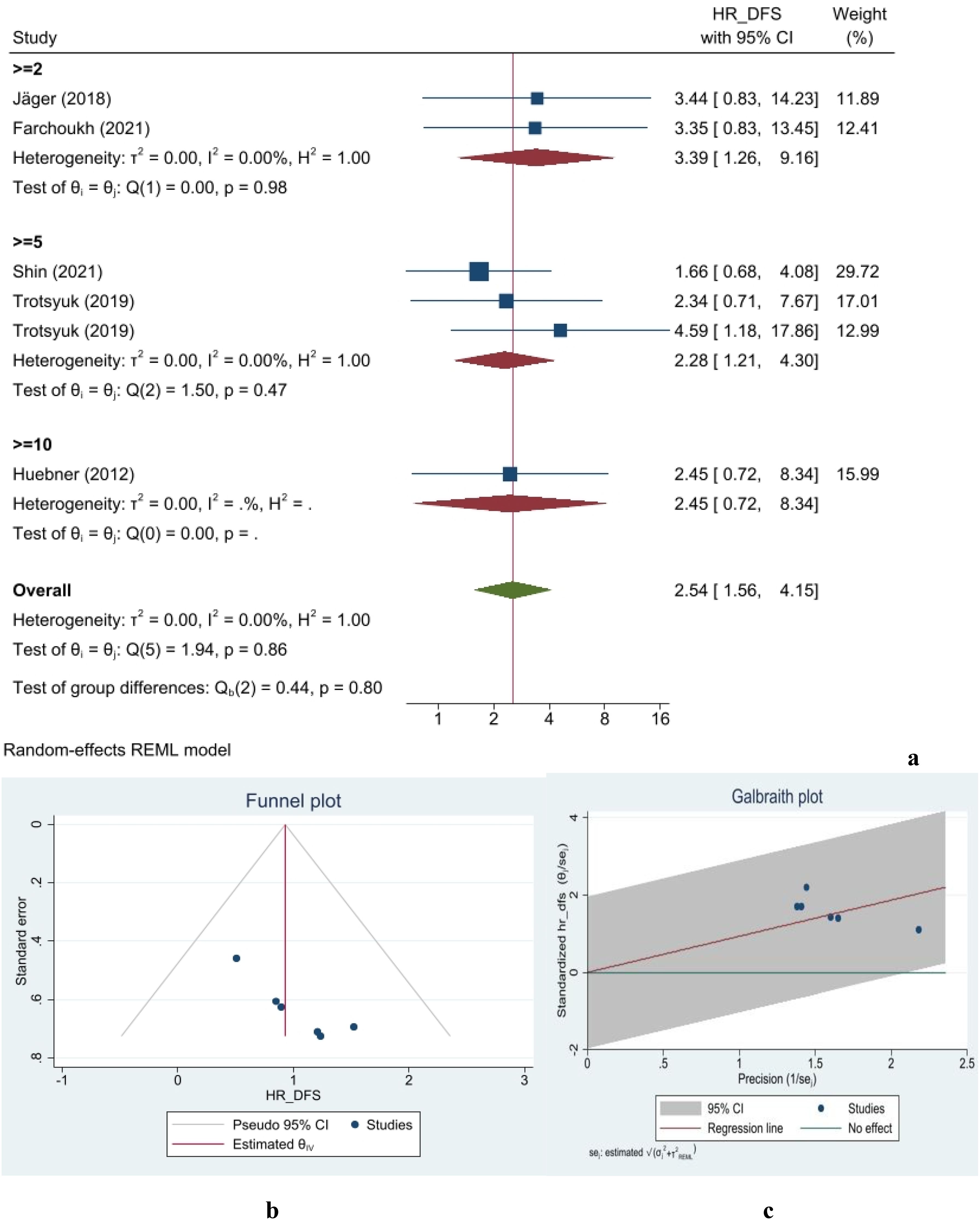
Figure 2. Forest (a), funnel (b), and Galbraith (c) plots for disease-free survival/relapse-free survival.
Data from four studies on OS, including 1,257 patients, were incorporated into this meta-analysis. The overall measured hazard ratio indicated a 3.24-fold increase in overall survival for TB-negative patients compared with TB-positive cases (95% CI: 1.71–6.16) (Figure 3a). Moreover, no publication bias was observed in any of the analyses. The data showed no heterogeneity, with an I2 of 0%. The assessment of group differences revealed no statistical significant differences (p-value = 0.70). Figure 3b illustrates the dispersion of studies in the assessment of OS, showing that none fell outside the 95% confidence interval limits. The small-study effect was evaluated using Begg’s and Egger’s tests. The Egger’s test yielded a beta value of 4.54 with a corresponding p-value of 0.33. In the Begg’s test, Kendall’s was 2, with a two-tailed p-value of 0.73. Finally, a nonparametric trim-and-fill analysis indicated no missing studies affecting specificity measurements. Galbraith plot of OS is shown in Figure 3c.
Despite significant progress in colorectal cancer treatment, patient responses to therapeutic approaches vary, suggesting the influence of additional factors. Recent studies have highlighted TB as a potential determinant colorectal cancer invasion and response to nCRT, given its notable prognostic value in lymph node involvement, distant metastasis, local recurrence, and 5-year cancer-associated mortality in patients undergoing primary surgical resection without nCRT (17). TB is often an under-recognized pathological factor in colorectal cancer, but its importance is underscored by studies showing that high-grade TB correlates with upregulation of negative regulatory immune checkpoints (PD-L1, TIM-3) and chemokine receptors (CXCR2, CXCR4) (28), which are associated with poor prognosis in patients with colorectal cancer liver metastasis undergoing neoadjuvant chemotherapy (29). Given its significance, TB has been recommended for inclusion in future CRC reporting guidelines/protocols and the next TNM staging system as a prognostic factor for colorectal cancers (12).
Despite a few studies assessing the prognostic value of TB in patients with locally advanced rectal cancer undergoing nCRT, to the best of our knowledge, the current investigation is the first systematic review and meta-analysis assessing evaluating this parameter in these cases. Our study revealed that TB negativity, regardless of its variable scoring systems in the studies, was associated with a 2.54- and 3.24-fold increase in DFS and OS, respectively.
In more detailed information, Huebner and colleagues were the first to evaluate the prognostic value of TB in assessing response to nCRT. While they did not specify the intensity of radiation used in their chemoradiotherapy protocol, they reported administering radiotherapy in combination with 5-fluorouracil (5-FU) as the chemotherapy regimen for patients with stages I–III rectal cancer undergoing nCRT. Their assessment of TB’s role in RFS demonstrated a 2.46-fold increase in RFS among TB-negative patients (23).
Research on TB continued with two studies in 2014, conducted by Sannier et al. in France (26) and Rogers et al. in Ireland (25). Rogers reported a 5-year DFS of 33% for TB-positive subjects vs. 77% for TB-negative subjects. They further indicated that TB predicted a poor pathological response to nCRT, as it was associated with adverse conditions such as higher ypT stage, lymph node involvement, lymphovascular invasion, and poorly differentiated tumors (25). Similarly, Sannier identified TB as a prognostic factor for failure to respond to nCRT in patients with types III–IV rectal cancer who had positive node involvement (26).
Similarly, Jäger and colleagues reported significantly better outcomes for TB-negative patients, with a 5-year RFS of 90% and a distant recurrence rate of 2%, compared to 71% and 12% for TB-positive individuals. Furthermore, TB positivity was identified as a negative predictive factor for RFS (HR: 3.44). However, their results did not show a significant association between BD-1 and OS. Notably, they classified TB as negative and mild TB (BD-0) vs. moderate and severe TB (BD-1) (10).
The latter study by Şirin et al. categorized TB into four groups: none (0), mild (1–5 buds), moderate (6–10 buds), and severe (> 10 buds). Their findings on the association between TB and OS were consistent with previous studies, showing a 4.28-fold decrease in OS. However, TB was not identified as an independent prognostic factor for DFS. Notably, they did not provide HR details in their analysis (21).
One of the most notable confirmatory studies in this area was conducted by Trotsyuk et al., who evaluated this hypothesis using two methods of staining: hematoxylin and eosin and immunohistochemistry. While both methods produced consistent results, immunohistochemistry assessments higher OS and DFS for TB-negative individuals. Additionally, they emphasized that TB was a superior predictor of overall survival compared to traditional parameters such as ypT and ypN status (18).
The latest studies in this area have indicated that TB positivity is associated with a poor response to nCRT, with a 5-year DFS of 87% in patients without TB compared to 39% in those with TB (24). Shin et al. (22) supported these findings, emphasizing an earlier theory that TB status not only determines the response to nCRT but also independently predicts disease outcome, OS, and DFS, regardless of nCRT treatment.
Despite the value of the findings in the current study, several notable challenges should not be overlooked. Accordingly, the limitations of this meta-analysis, which may also introduce potential sources of bias, should be considered. Primarily, the number of studies assessing the prognostic value of TB for DFS, OS, and response to nCRT in locally advanced rectal cancer is limited. Secondly, the TB scoring system varies considerably between studies. Although the prognostic significance of TB remains largely independent of the scoring system used, establishing a single international standard for TB assessment is necessary for consistency in reporting (12). Most studies recommended the hot spot method (a single field with the highest number of TB), while others used multiple field methods (e.g., 5 HPF and 10 HPF) (13, 15, 16). However, efforts have been made to establish a standardized definition, as outlined in the International Tumor Budding Consensus Conference (ITBCC) published in 2016 (12). The ITBCC recommended the hot spot method for counting TB, in which the invasive front is scanned at 10 medium power fields (× 10 objective)to identify the hot spot (the area containing the highest number of TB) in the initial step. Next, TB should be counted in a single × 20 objective field within the hot spot area, and the TB count is then calculated in an area measuring 0.785 mm2 using a normalization factor (12). To minimize the risk of bias in TB counting, the ITBCC recommends a continuous scale, which is more precise than the cut-off method (30). Furthermore, the ITBCC suggests a three-tier scoring system to categorize TB as low (BD-1: 0–4), intermediated (BD-2: 5–9, and severe (BD-3: ≥ 10) budding (12). The third point of discussion concerns the different staining methods used to assess tumor budding. Although only one of the studies used immunohistochemistry to assess tumor budding, variations in staining methods might influence the outcomes. However, it has been suggested that the prognostic power of H&E and IHC staining methods in evaluating TB does not differ (13, 15–17, 31). Furthermore, the ITBCC has noted that H&E is comparably favored over the methods (12).
Our study also provides new insights into the association between the presence of TB and a reduced response to nCRT in locally advanced rectal adenocarcinoma.
Based on the findings of the current study, TB negativity was statistically and directly associated with better OS and DFS in patients with locally advanced rectal cancer undergoing nCRT. If further studies confirm the role of TB in reducing the response to nCRT and decreasing OS and DFS in these patients, TB may be serve as an indication for modifying and individualizing nCRT regimens for locally advanced rectal adenocarcinoma.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
AR: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. PN: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AM: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. PK: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Maresso KC, Basen-Engquist K, Hawk E. Cancer epidemiology, prevention, and survivorship. In: Perioperative care of the cancer patient. Amsterdam: Elsevier (2023). p. 3–14.
2. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. (2023) 73:17–48. doi: 10.3322/caac.21763
3. Mattiuzzi C, Sanchis-Gomar F, Lippi G. Concise update on colorectal cancer epidemiology. Ann Trans Med. (2019) 7, 609. doi: 10.21037/atm.2019.07.91
4. Wang Y, Shen L, Wan J, Zhang H, Wu R, Wang J, et al. Neoadjuvant chemoradiotherapy combined with immunotherapy for locally advanced rectal cancer: A new era for anal preservation. Front Immunol. (2022) 13. doi: 10.3389/fimmu.2022.1067036
5. Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen Y-J, Ciombor KK, et al. Rectal cancer, version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Network. (2018) 16:874–901. doi: 10.6004/jnccn.2018.0061
6. Edge SB, Byrd DR, Carducci MA, Compton CC, Fritz A, Greene F. AJCC cancer staging manual. New York: Springer; (2010).
7. Fichtner-Feigl S. Biology-and location-oriented precision treatment of rectal cancer: present and future. Visceral Med. (2020) 36:381–7. doi: 10.1159/000510488
8. Zehir A, Benayed R, Shah RH, Syed A, Middha S, Kim HR, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. (2017) 23:703–13. doi: 10.1038/nm.4333
9. Puppa G, Sonzogni A, Colombari R, Pelosi G. TNM staging system of colorectal carcinoma: a critical appraisal of challenging issues. Arch Pathol Lab Med. (2010) 134:837–52. doi: 10.5858/134.6.837
10. Jäger T, Neureiter D, Fallaha M, Schredl P, Kiesslich T, Urbas R, et al. The potential predictive value of tumor budding for neoadjuvant chemoradiotherapy response in locally advanced rectal cancer. Strahlentherapie Und Onkologie. (2018) 194:991. doi: 10.1007/s00066-018-1340-0
11. De Smedt L, Palmans S, Andel D, Govaere O, Boeckx B, Smeets D, et al. Expression profiling of budding cells in colorectal cancer reveals an EMT-like phenotype and molecular subtype switching. Br J cancer. (2017) 116:58–65. doi: 10.1038/bjc.2016.382
12. Lugli A, Kirsch R, Ajioka Y, Bosman F, Cathomas G, Dawson H, et al. Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Modern pathology. (2017) 30:1299–311. doi: 10.1038/modpathol.2017.46
13. Mitrovic B, Schaeffer DF, Riddell RH, Kirsch R. Tumor budding in colorectal carcinoma: time to take notice. Modern Pathology. (2012) 25:1315–25. doi: 10.1038/modpathol.2012.94
14. Lugli A, Karamitopoulou E, Zlobec I. Tumour budding: a promising parameter in colorectal cancer. Br J cancer. (2012) 106:1713–7. doi: 10.1038/bjc.2012.127
15. Van Wyk H, Park J, Roxburgh C, Horgan P, Foulis A, McMillan DC. The role of tumour budding in predicting survival in patients with primary operable colorectal cancer: a systematic review. Cancer Treat Rev. (2015) 41:151–9. doi: 10.1016/j.ctrv.2014.12.007
16. De Smedt L, Palmans S, Sagaert X. Tumour budding in colorectal cancer: what do we know and what can we do? Virchows Archiv. (2016) 468:397–408. doi: 10.1007/s00428-015-1886-5
17. Rogers A, Winter D, Heeney A, Gibbons D, Lugli A, Puppa G, et al. Systematic review and meta-analysis of the impact of tumour budding in colorectal cancer. Br J cancer. (2016) 115:831–40. doi: 10.1038/bjc.2016.274
18. Trotsyuk I, Sparschuh H, Müller AJ, Neumann K, Kruschewski M, Horst D, et al. Tumor budding outperforms ypT and ypN classification in predicting outcome of rectal cancer after neoadjuvant chemoradiotherapy. BMC cancer. (2019) 19:1–12. doi: 10.1186/s12885-019-6261-5
19. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. (2010) 8:336–41. doi: 10.1016/j.ijsu.2010.02.007
20. Bhangu A, Wood G, Brown G, Darzi A, Tekkis P, Goldin R. The role of epithelial mesenchymal transition and resistance to neoadjuvant therapy in locally advanced rectal cancer. Colorectal Disease. (2014) 16:0133–43. doi: 10.1111/codi.2014.16.issue-4
21. Şirin A, Sökmen S, Ünlü S, Ellidokuz H, Sarioğlu S. The prognostic value of tumor budding in patients who had surgery for rectal cancer with and without neoadjuvant therapy. Techniques Coloproctology. (2019) 23:333–42. doi: 10.1007/s10151-019-01959-2
22. Shin JK, Park YA, Huh JW, Yun SH, Kim HC, Lee WY, et al. Tumor budding as a prognostic marker in rectal cancer patients on propensity score analysis. Ann Surg Oncol. (2021) 28:8813–22. doi: 10.1245/s10434-021-10286-6
23. Huebner M, Wolff BG, Smyrk TC, Aakre J, Larson DW. Partial pathologic response and nodal status as most significant prognostic factors for advanced rectal cancer treated with preoperative chemoradiotherapy. World J Surg. (2012) 36:675–83. doi: 10.1007/s00268-011-1409-8
24. Farchoukh L, Hartman DJ, Ma C, Celebrezze J, Medich D, Bahary N, et al. Intratumoral budding and automated CD8-positive T-cell density in pretreatment biopsies can predict response to neoadjuvant therapy in rectal adenocarcinoma. Modern Pathology. (2021) 34:171–83. doi: 10.1038/s41379-020-0619-8
25. Rogers AC, Gibbons D, Hanly AM, Hyland JM, O’connell PR, Winter DC, et al. Prognostic significance of tumor budding in rectal cancer biopsies before neoadjuvant therapy. Modern Pathology. (2014) 27:156–62. doi: 10.1038/modpathol.2013.124
26. Sannier A, Lefèvre JH, Panis Y, Cazals-Hatem D, Bedossa P, Guedj N. Pathological prognostic factors in locally advanced rectal carcinoma after neoadjuvant radiochemotherapy: analysis of 113 cases. Histopathology. (2014) 65:623–30. doi: 10.1111/his.2014.65.issue-5
27. Hayden JA, van der Windt DA, Cartwright JL, Cote P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Internal Med. (2013) 158:280–6. doi: 10.7326/0003-4819-158-4-201302190-00009
28. Guil-Luna S, Mena R, Navarrete-Sirvent C, Lopez-Sanchez LM, Khouadri K, Toledano-Fonseca M, et al. Association of tumor budding with immune evasion pathways in primary colorectal cancer and patient-derived xenografts. Front Med. (2020) 7:264. doi: 10.3389/fmed.2020.00264
29. D’Alterio C, Nasti G, Polimeno M, Ottaiano A, Conson M, Circelli L, et al. CXCR4-CXCL12-CXCR7, TLR2-TLR4, and PD-1/PD-L1 in colorectal cancer liver metastases from neoadjuvant-treated patients. Oncoimmunology. (2016) 5:e1254313. doi: 10.1080/2162402X.2016.1254313
30. Zlobec I, Hädrich M, Dawson H, Koelzer VH, Borner M, Mallaev M, et al. Intratumoural budding (ITB) in preoperative biopsies predicts the presence of lymph node and distant metastases in colon and rectal cancer patients. Br J cancer. (2014) 110:1008–13. doi: 10.1038/bjc.2013.797
Keywords: rectal neoplasm, neoadjuvant therapy, tumor budding, prognosis, colon cancer
Citation: Rafiee A, Nasri P, Moradi A and Karimian P (2025) Tumor budding as an indicator of prognosis in locally advanced rectal cancer after neoadjuvant chemoradiotherapy: a systematic review and meta-analysis. Front. Oncol. 15:1429319. doi: 10.3389/fonc.2025.1429319
Received: 07 May 2024; Accepted: 24 February 2025;
Published: 09 April 2025.
Edited by:
Sharon R. Pine, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Alessandro Ottaiano, IRCCS Istituto Nazionale per lo Studio e la Cura dei Tumori “Fondazione Giovanni Pascale” - Napoli, ItalyCopyright © 2025 Rafiee, Nasri, Moradi and Karimian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Azita Rafiee, YXoucmFmaWVlQGdtYWlsLmNvbQ==
†ORCID: Azita Rafiee, orcid.org/0000-0002-2568-0175
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.